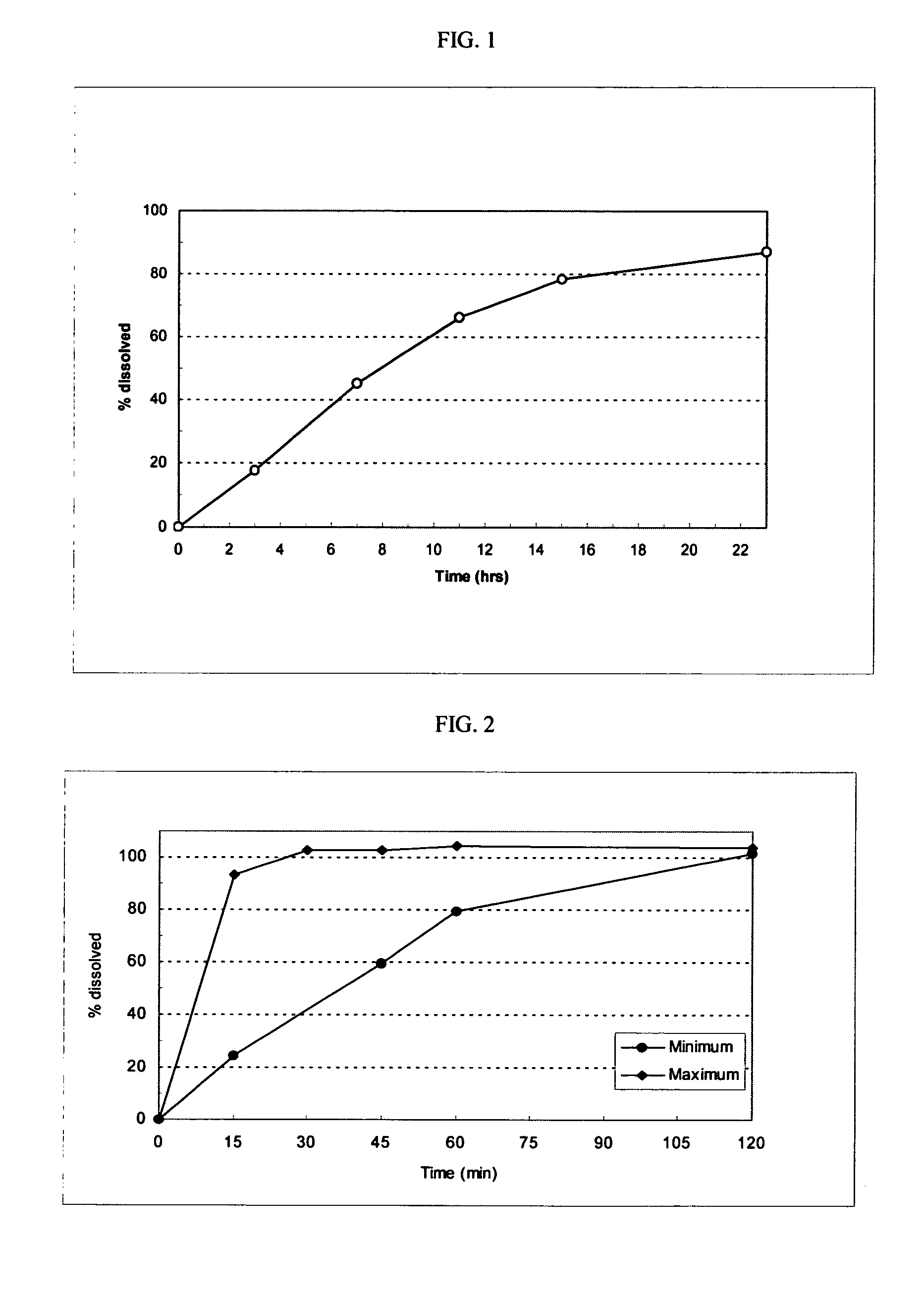
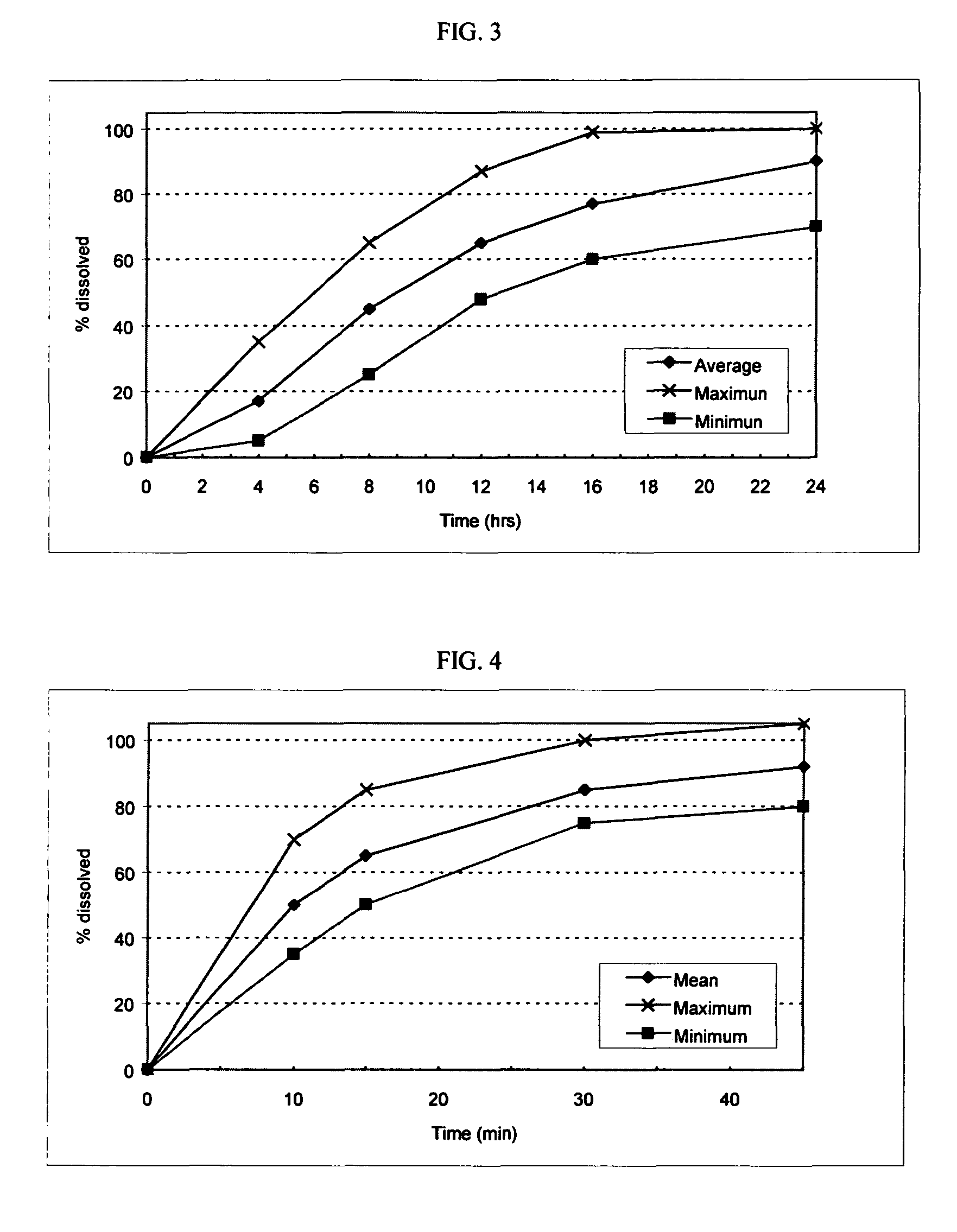
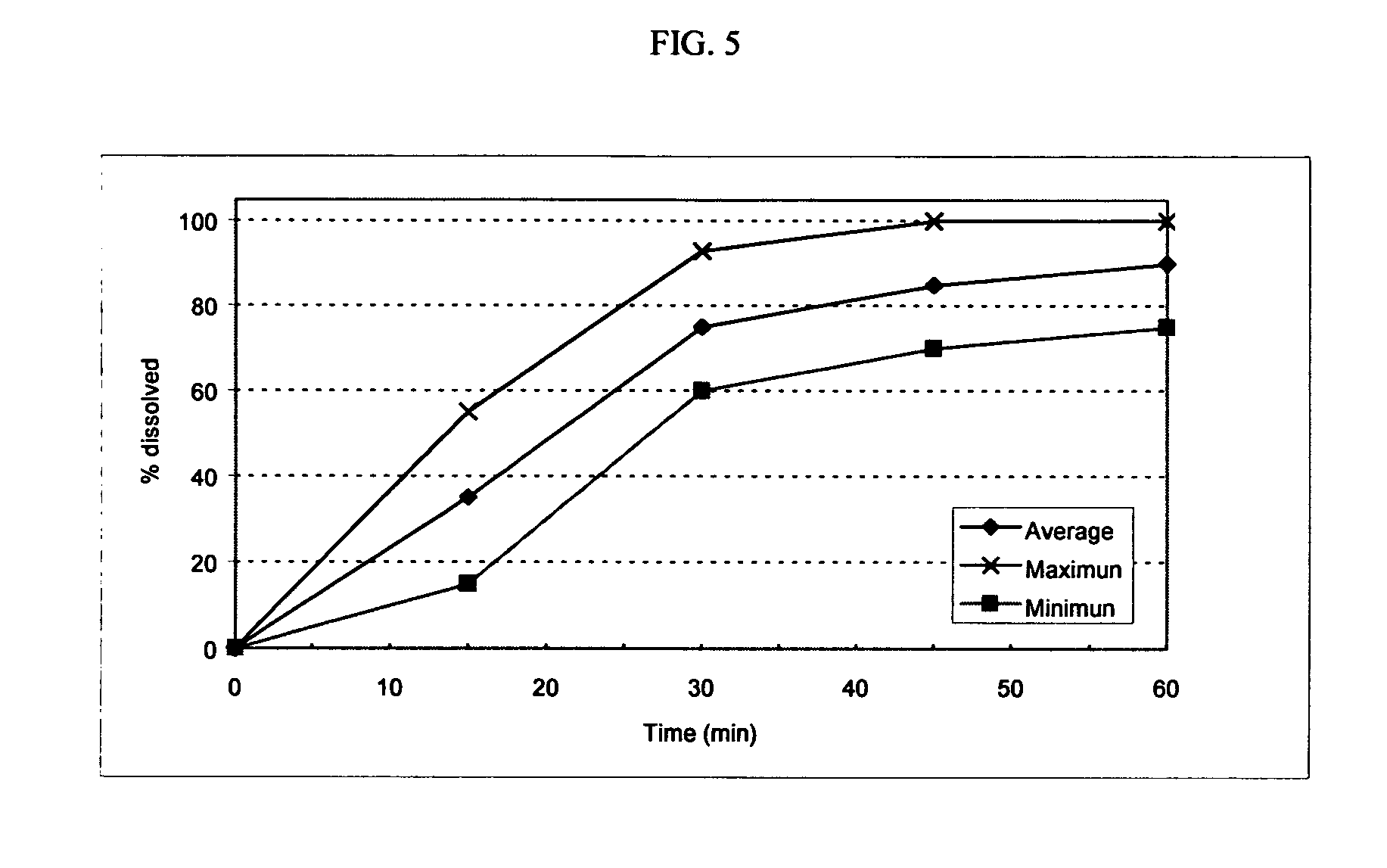
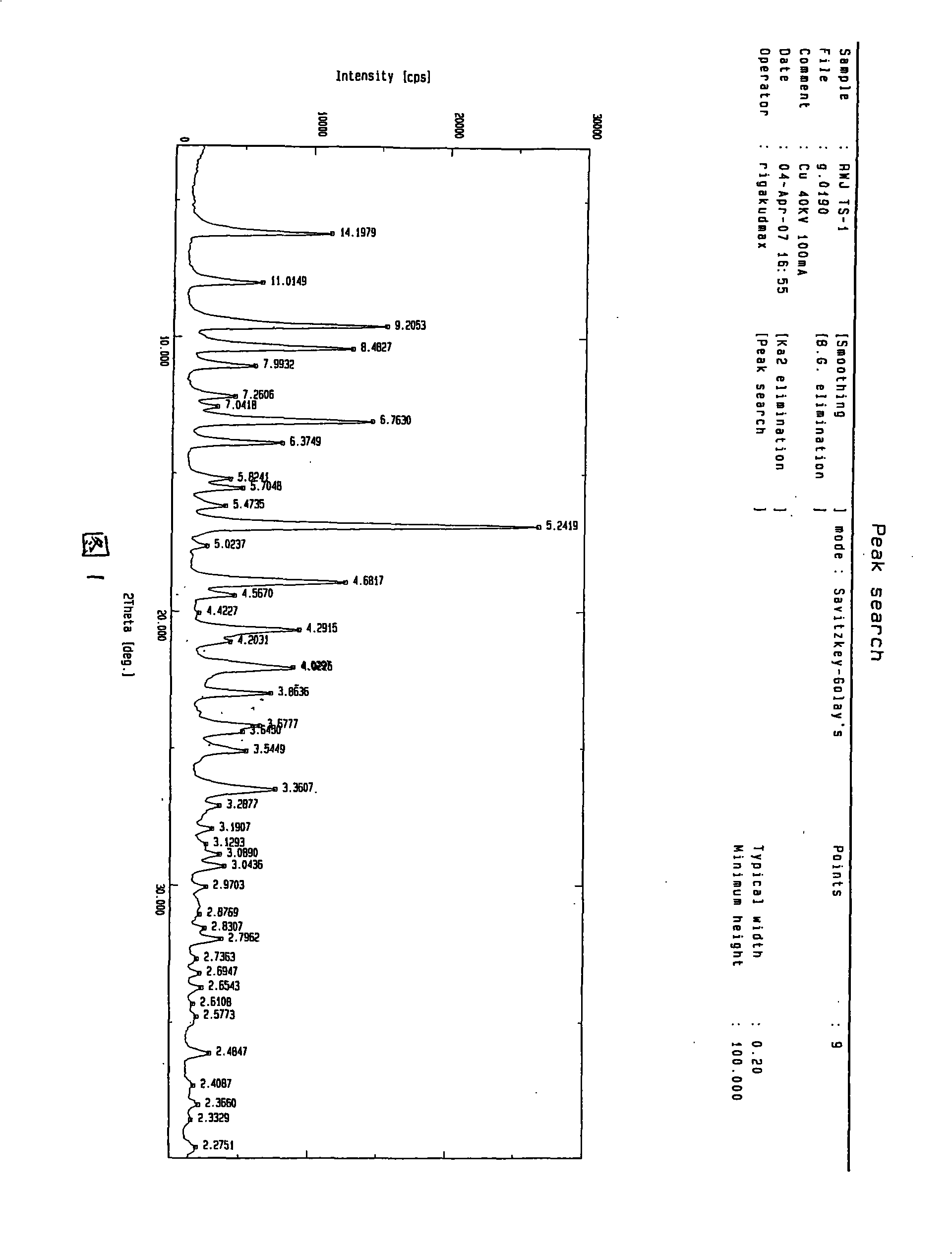
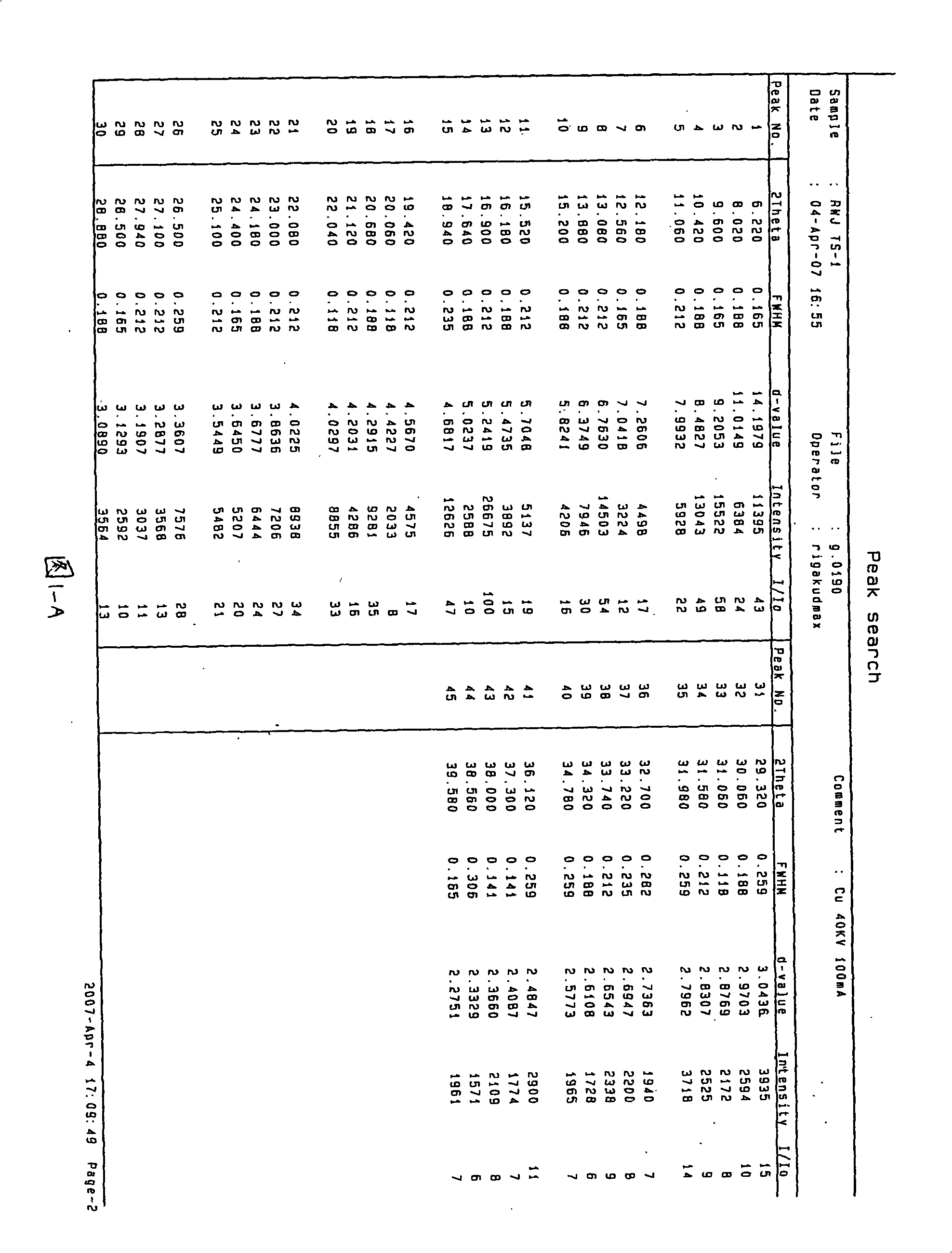
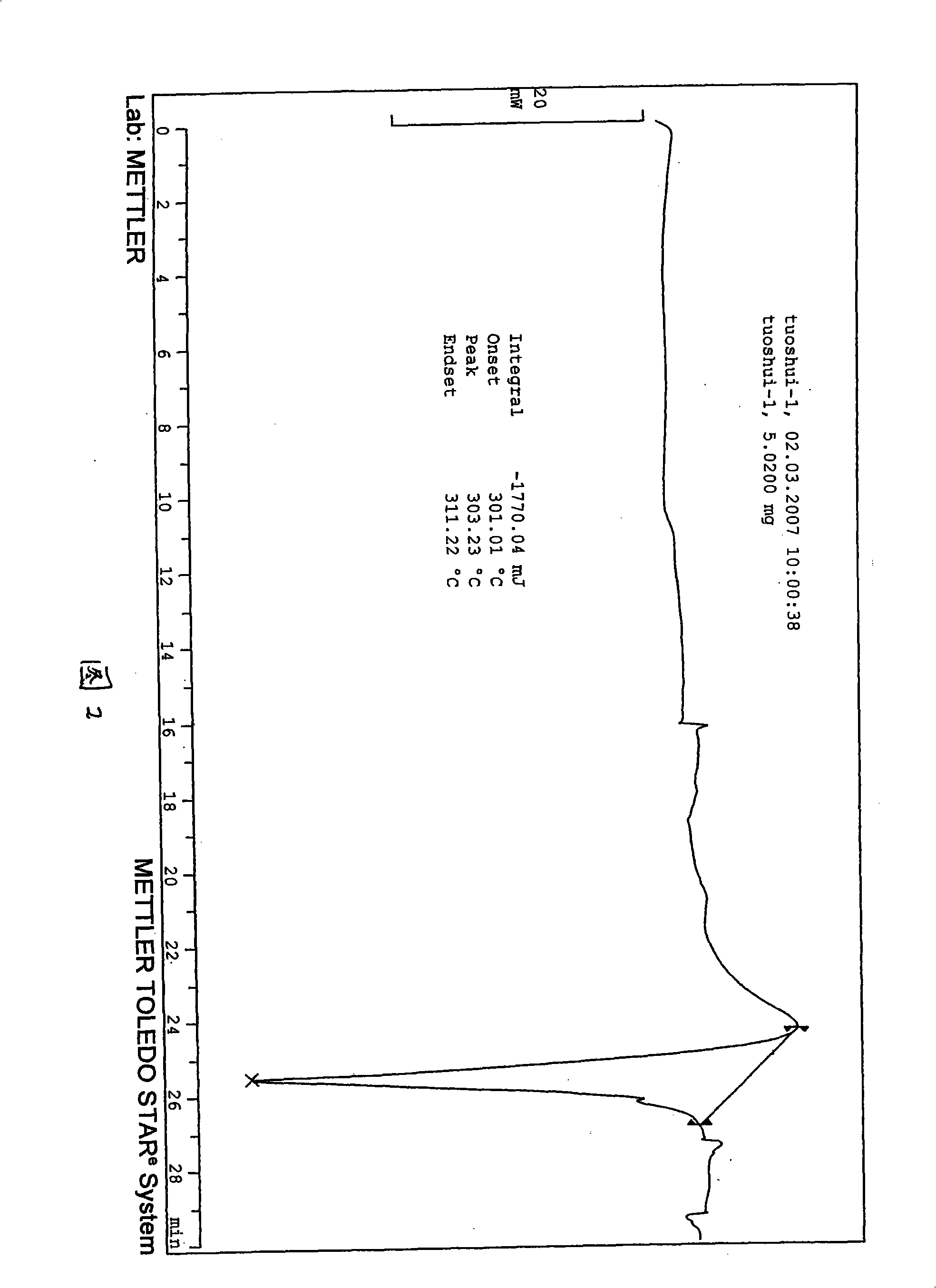
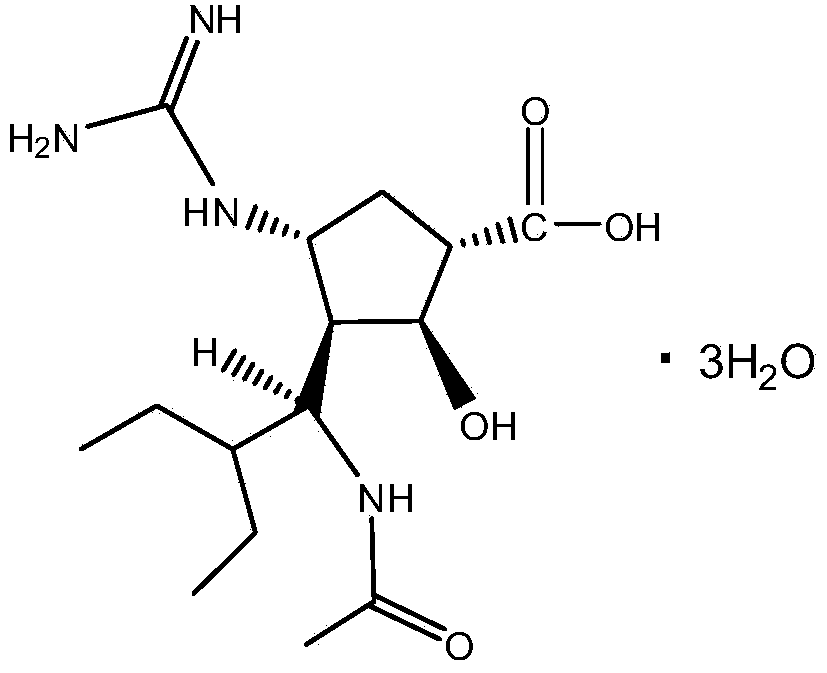
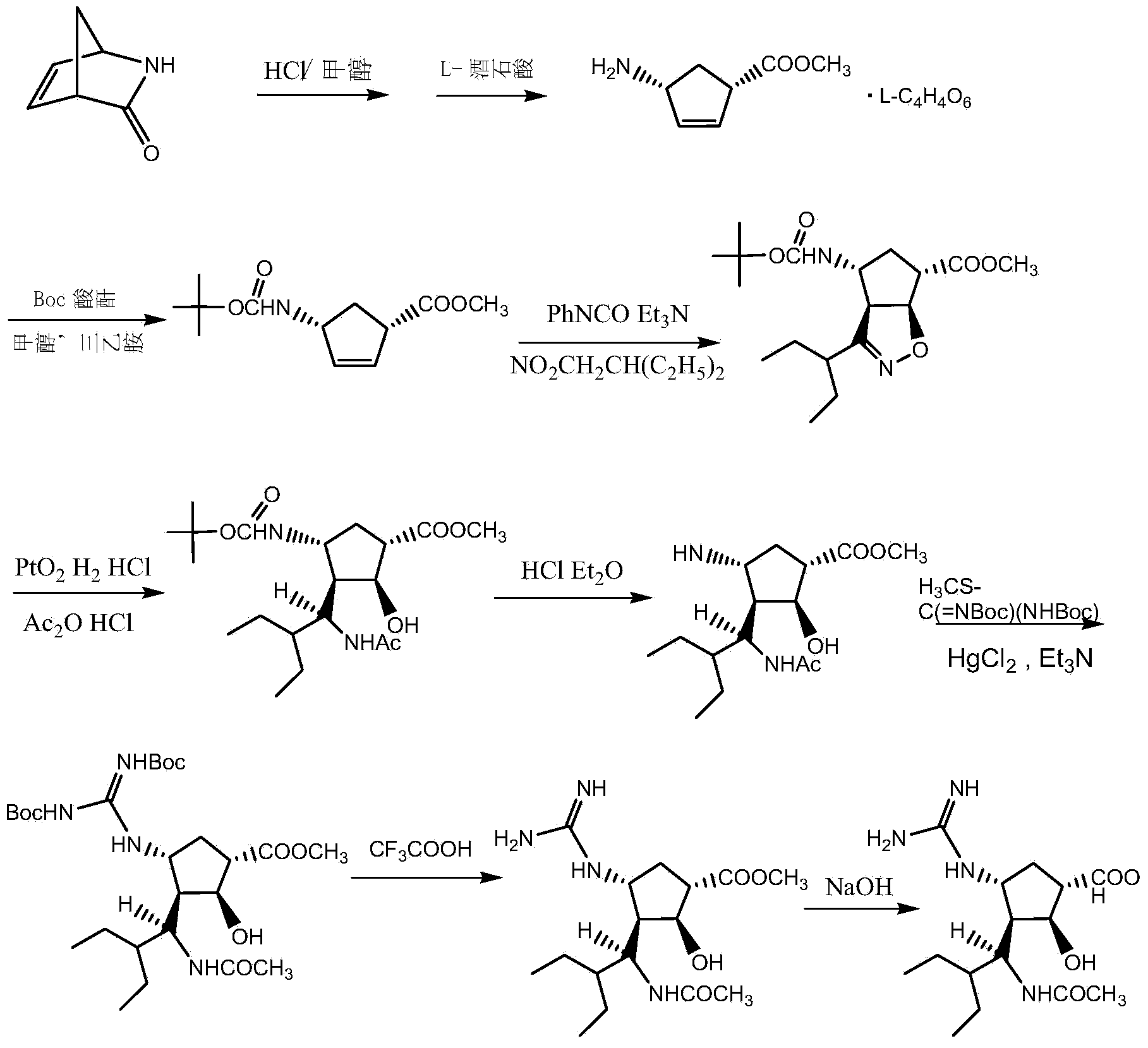
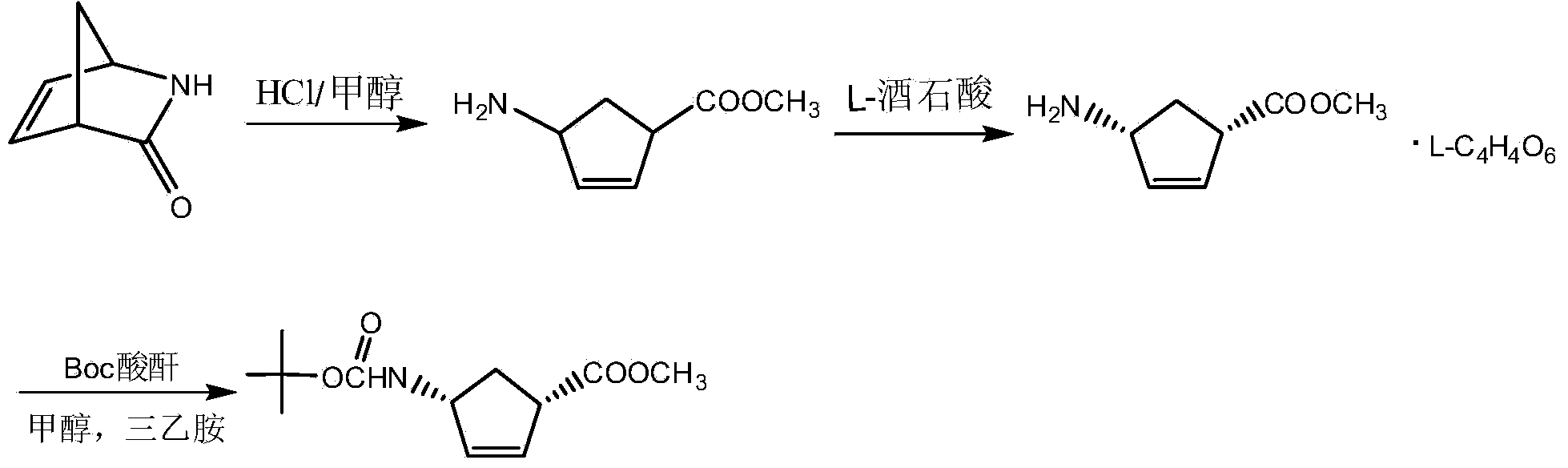
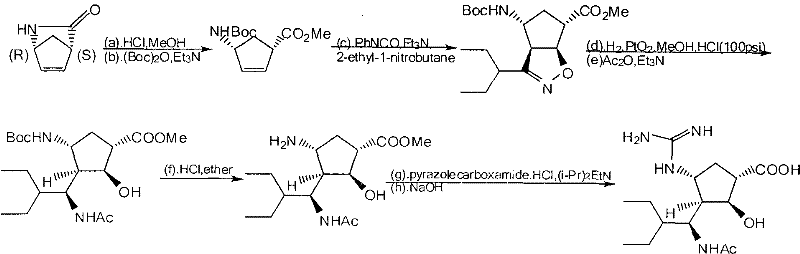
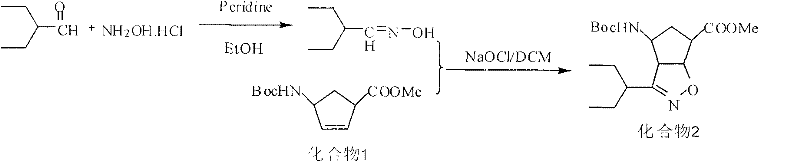
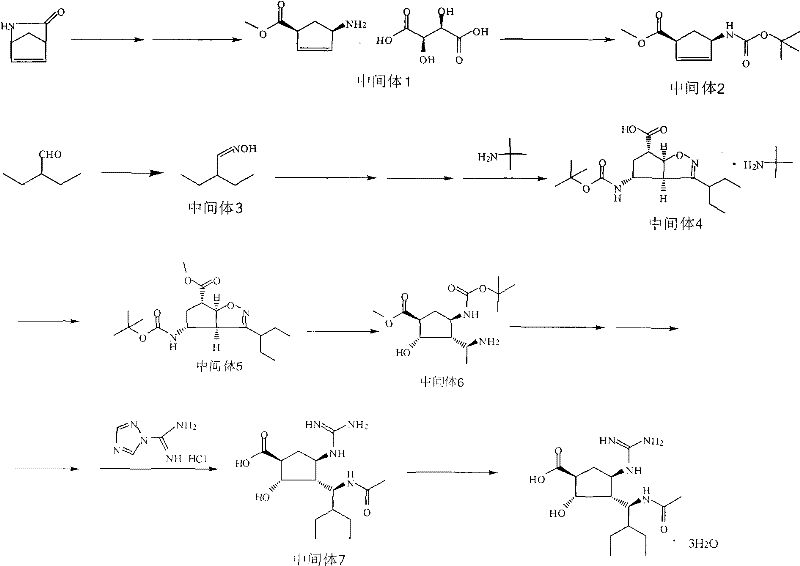
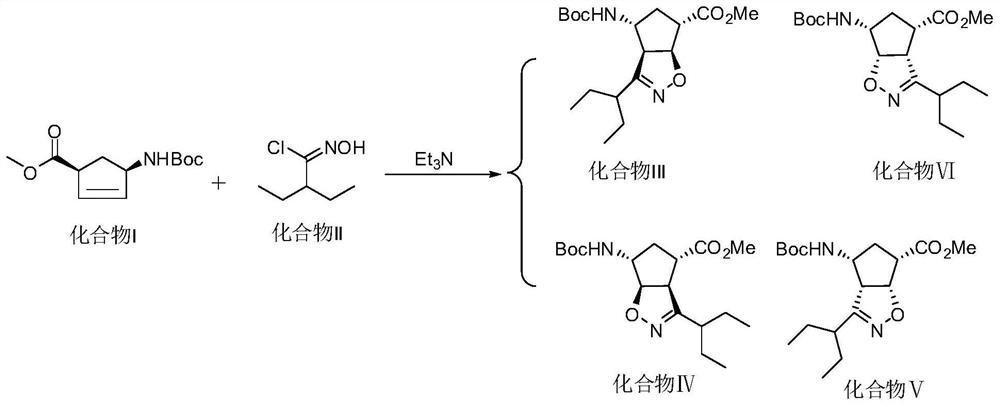
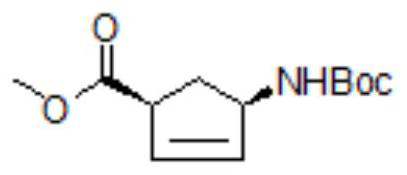
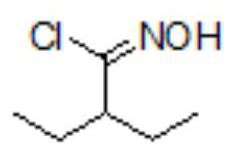
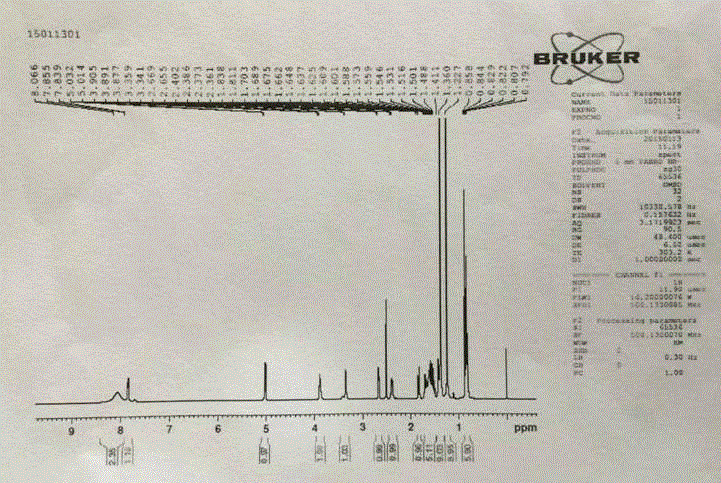
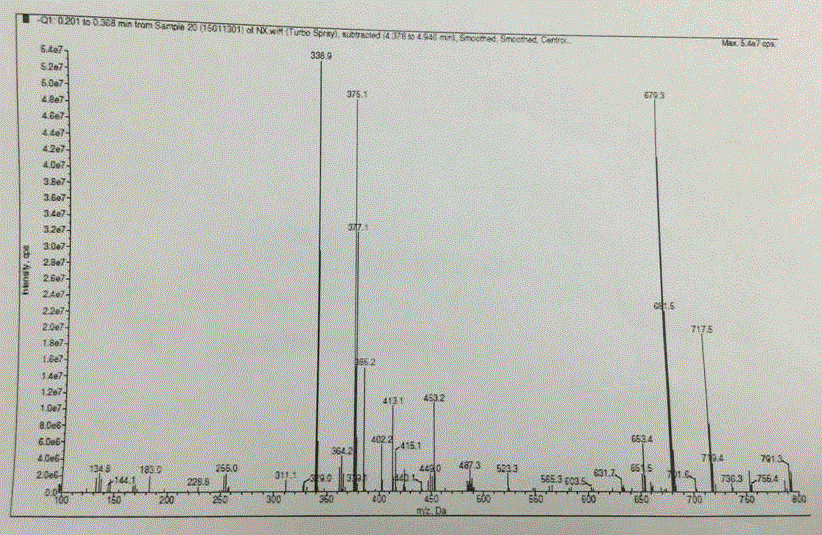
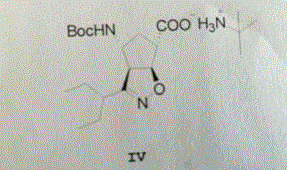
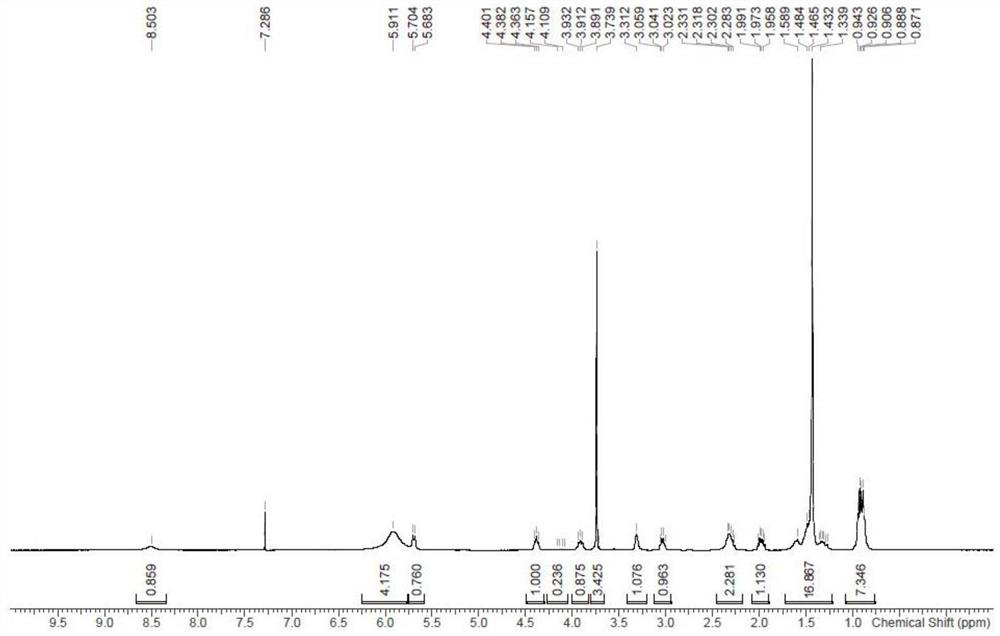
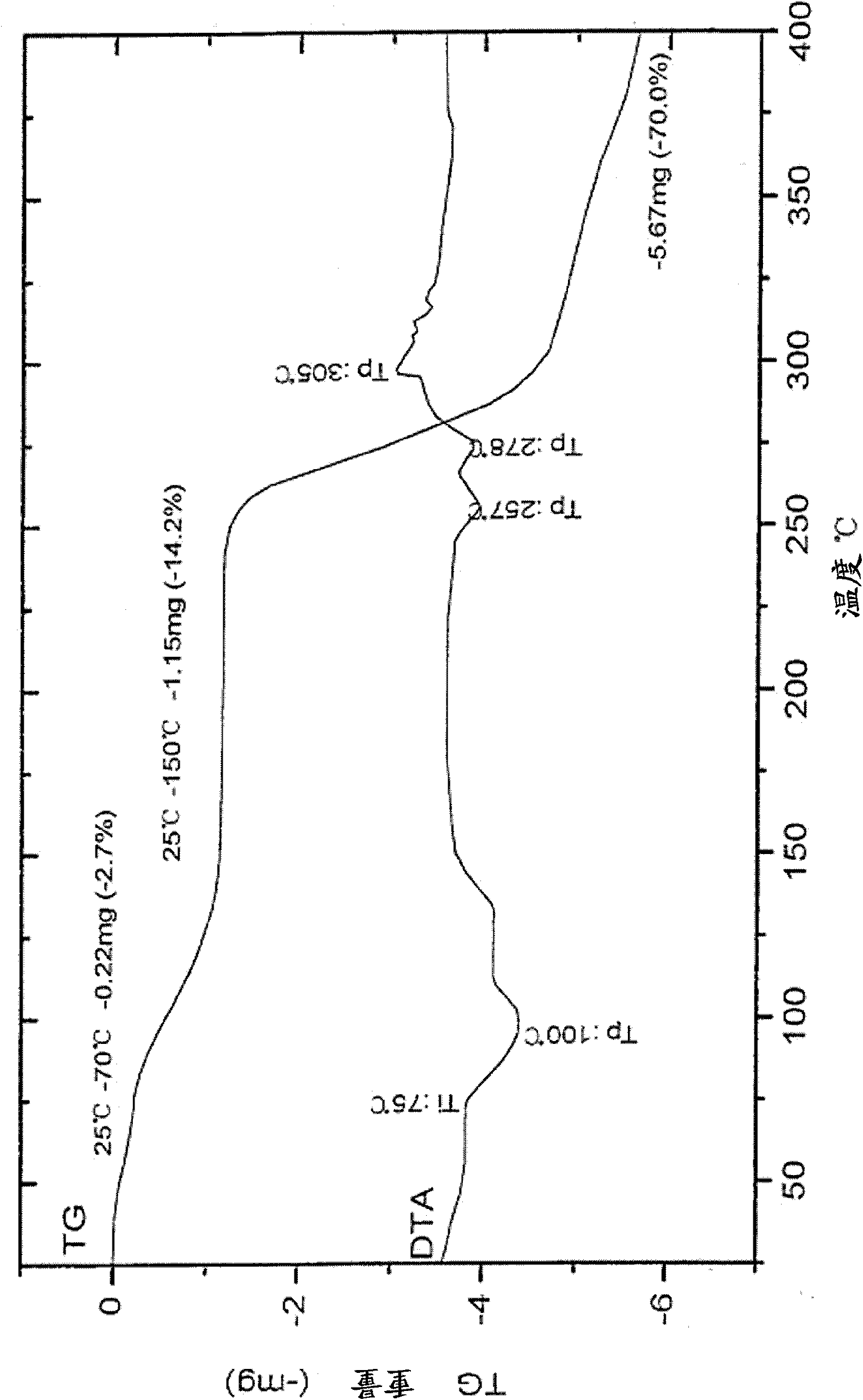
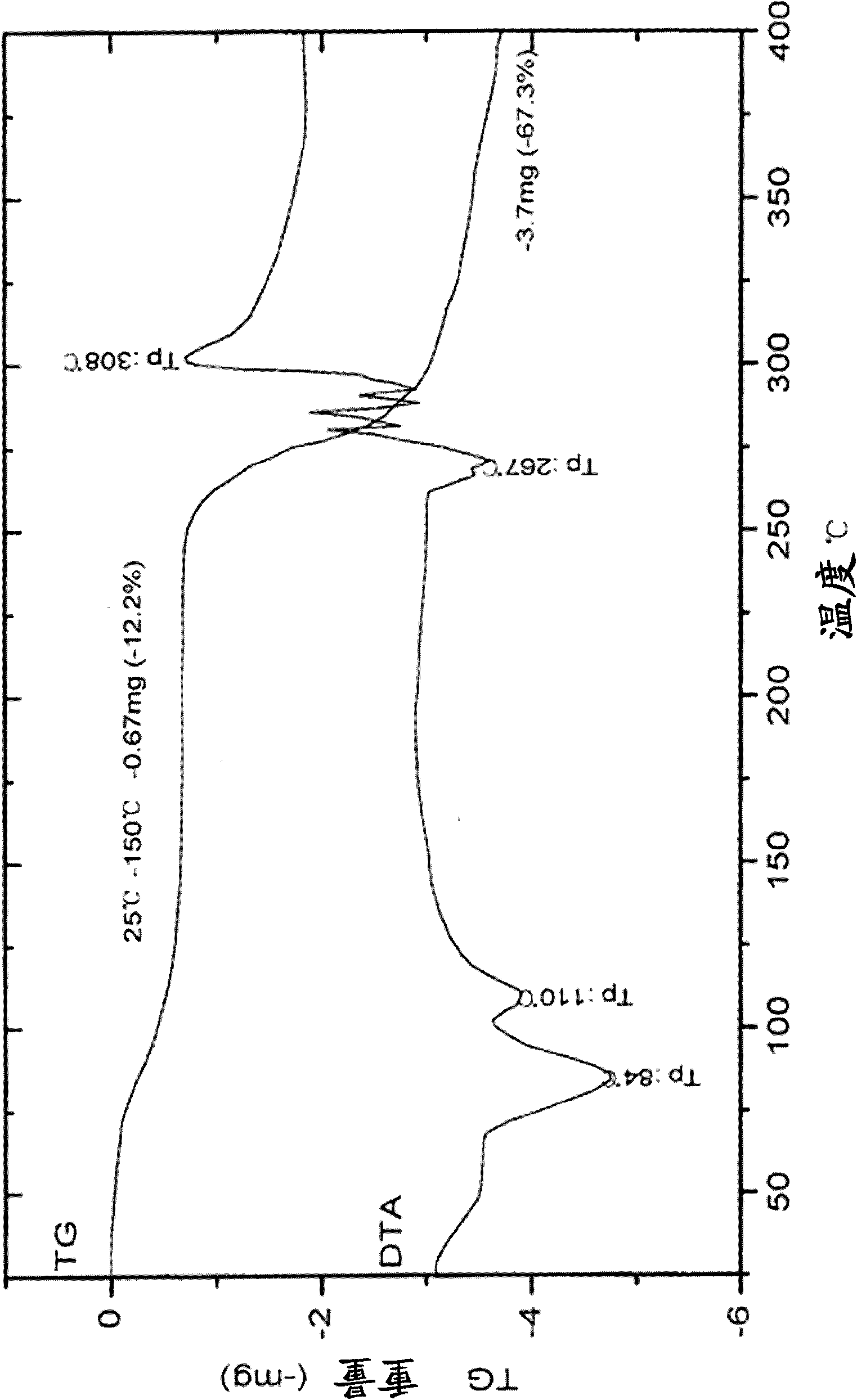
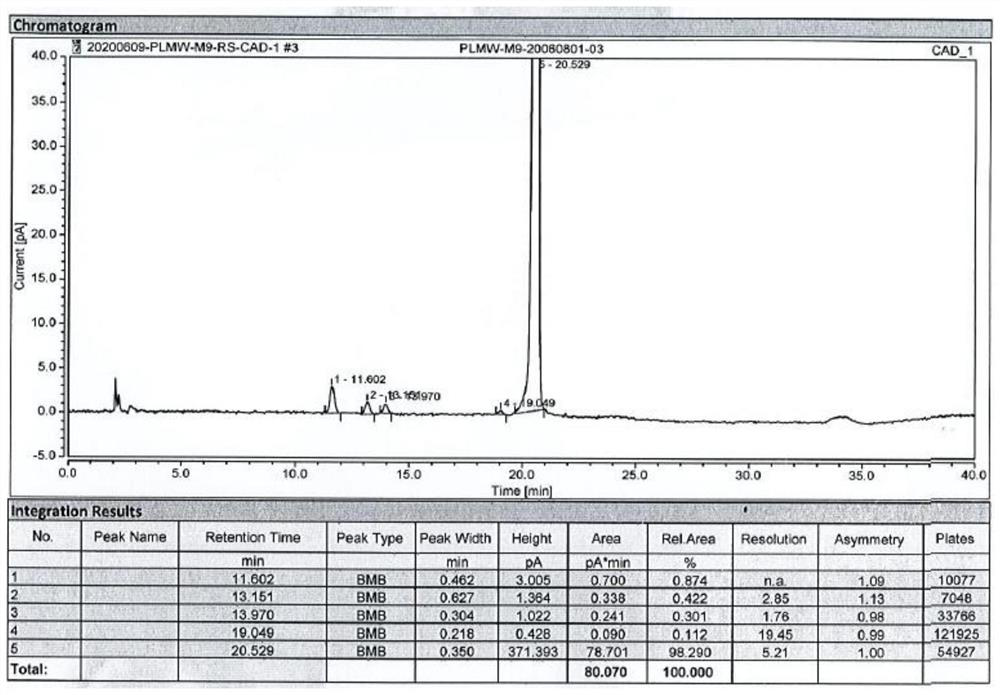
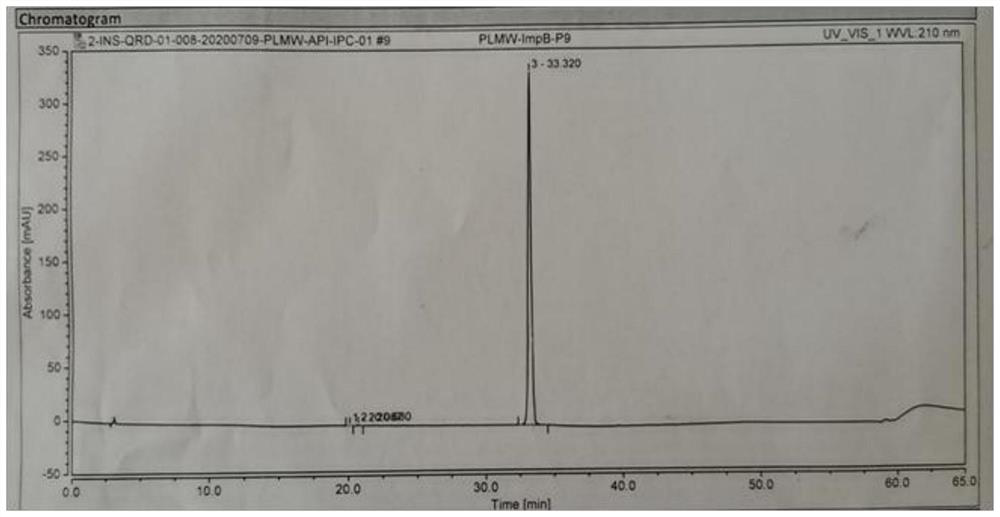
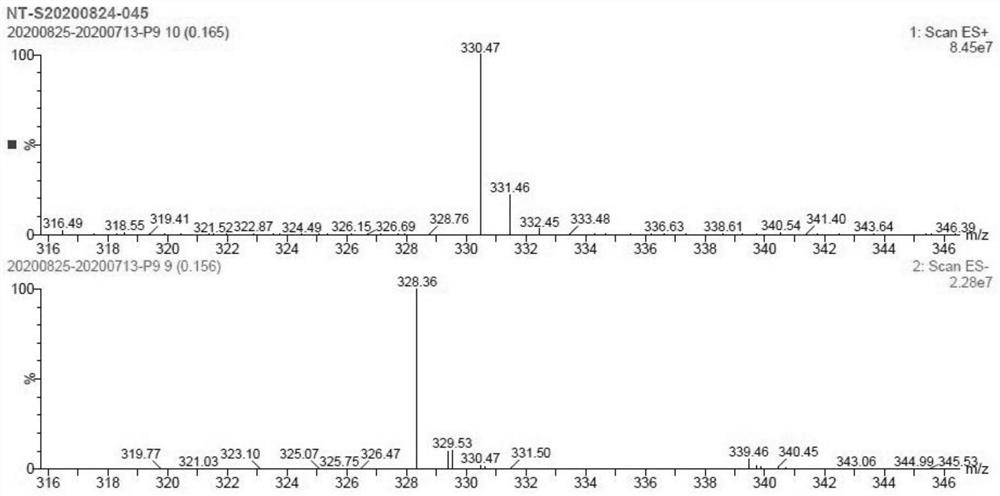
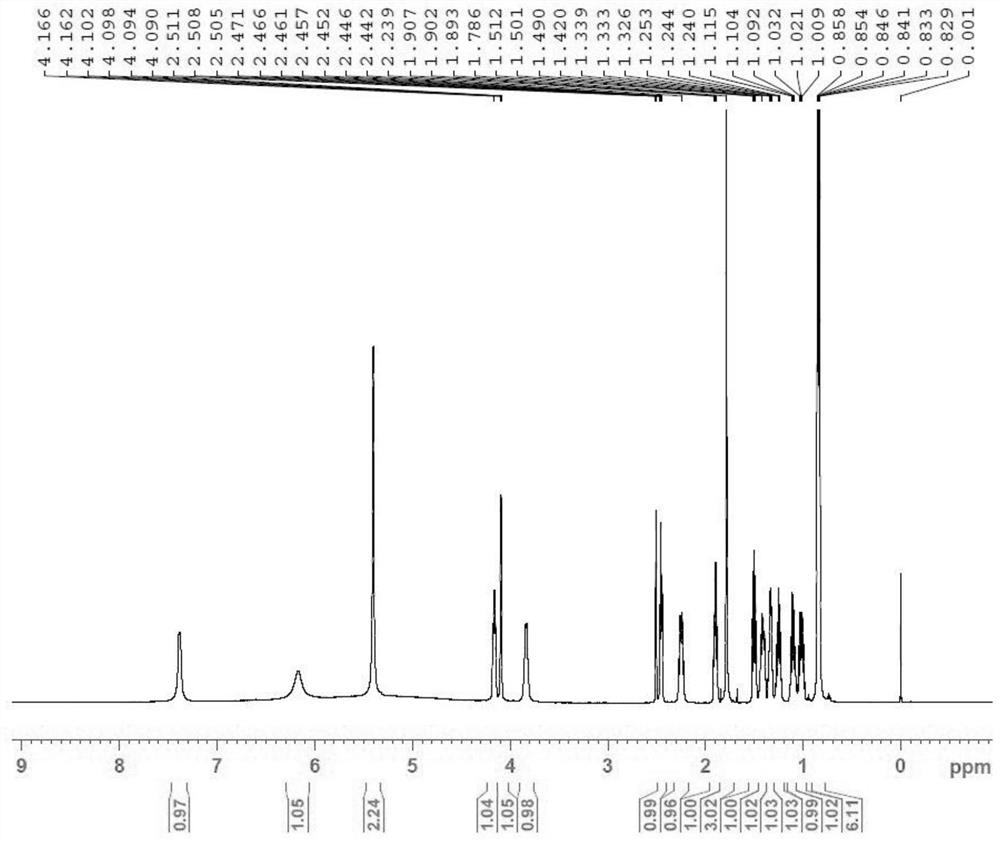
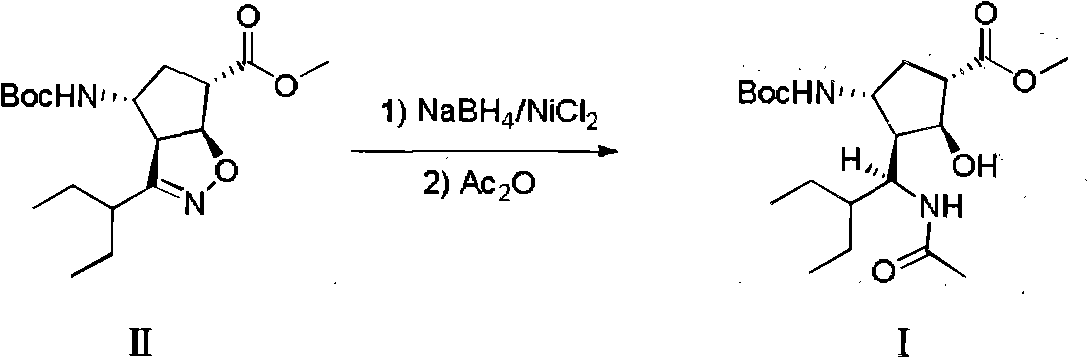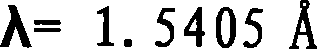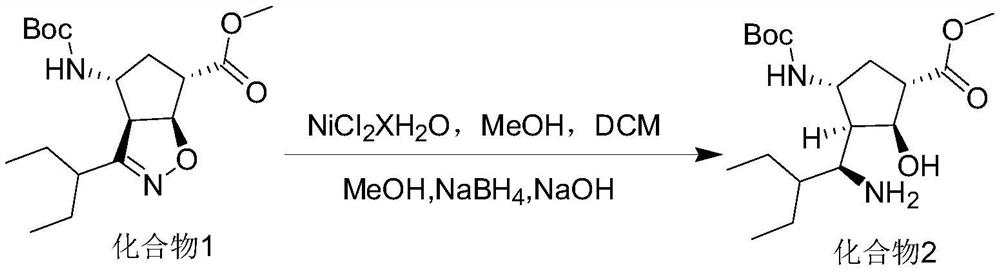Patents
Literature
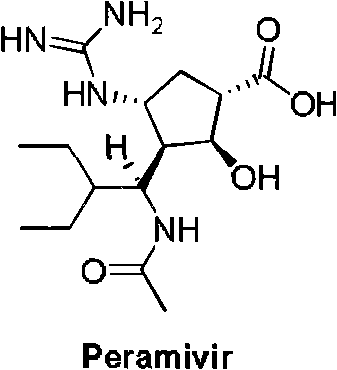
40 results about "Peramivir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
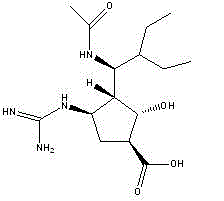
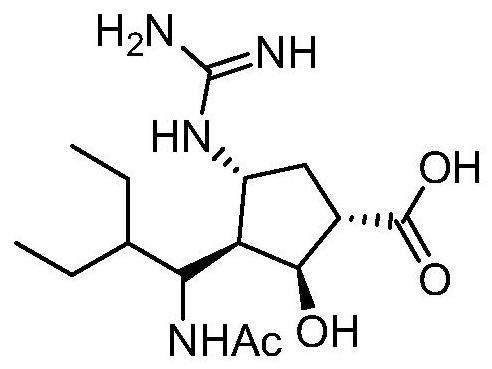
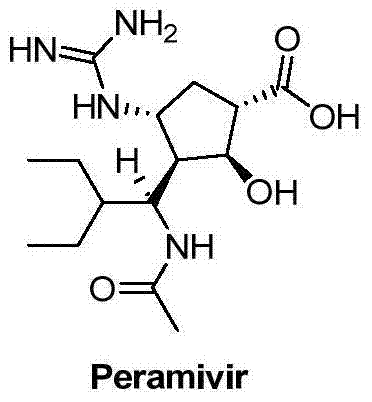
Peramivir is used to treat symptoms caused by the flu virus (influenza) if you have had symptoms for 2 days or less.
Drug delivery device containing neuraminidase inhibitor and an H1 antagonist
The present invention provides a dual release solid dosage form containing a first composition that releases a neuraminidase inhibitor, such as oseltamivir, zanamivir, or peramivir, in a controlled manner and a second composition that releases an H1 antagonist in a rapid and / or immediate manner. A wide range of H1 antagonist antihistamines, especially fexofenadine and loratadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. The device is useful for the treatment of respiratory congestion and other viral infection associated symptoms.
Owner:ACELLA HLDG LLC +1
Waterless Peramivir crystal and medicament composition thereof
ActiveCN101314579AGood reproducibilityKeep dryOrganic active ingredientsOrganic chemistryMedicineAvian influenza virus
The invention relates to an anhydrous peramivir crystal compound and a preparation method thereof, and further discloses a pharmaceutical composition comprising the same and the application thereof in the preparation of medicaments for treating the influenza virus and the Avian influenza virus.
Owner:天津泰普制药有限公司
Method for preparing peramivir
ActiveCN103524383AImprove production efficiencyHigh purityOrganic chemistryOrganic compound preparationChemical synthesis1,3-Dipolar cycloaddition
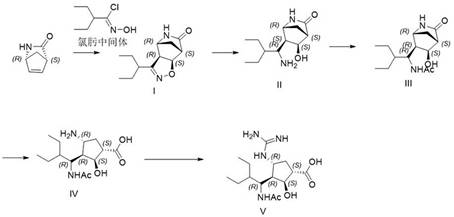
The invention relates to the field of chemical synthesis, and discloses a method for preparing peramivir. In allusion to the defects of two technical schemes of conventional patents CN1358170 and CN1367776, the method improves some steps in an amide alcoholysis opening ring reaction of 2-azabicyclo[2.2.1]hept-5-en-3-one and a 1,3-dipolar cycloaddition reaction, increases the preparation efficiency, purity and yield of the peramivir and an intermediate thereof, and has the extremely significant practical significance for industrialized production of the peramivir.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Preparation method of peramivir
InactiveCN102633686APrecise and controllable dosageHigh yieldOrganic chemistryOrganic compound preparationAntiviral drugKetone
The invention discloses a preparation method of an antiviral drug of peramivir, which is characterized in that 2-diazabicyclo [2.2.1] hept-5-en-3-one is used as an initial raw material for peramivir synthesis, and the overall yield is up to 35%. Compared with the prior art, the method has the advantages of high yield, fewer 'three wastes', safe and convenient operations, high purity of the obtained finished products, easy realization of industrial production, and the like.
Owner:FUAN PHARM (GRP) CO LTD
A novel process for the preparation of peramivir and intermediates thereof
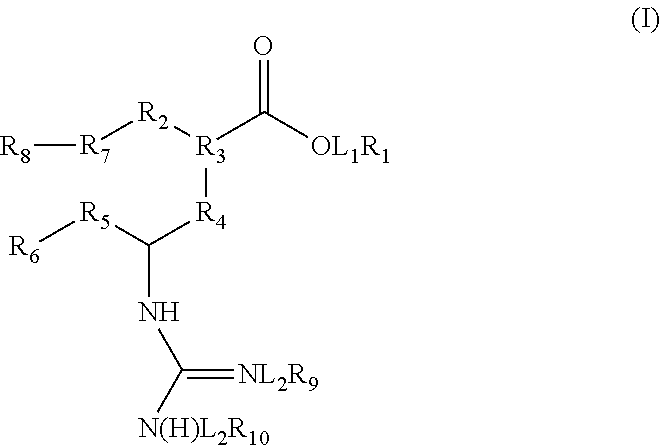
The present invention relates to a novel process for preparing peramivir formula (I) or a pharmaceutically acceptable salt thereof, and to intermediates used therein.
Owner:PHARMA SHANGHAI +1
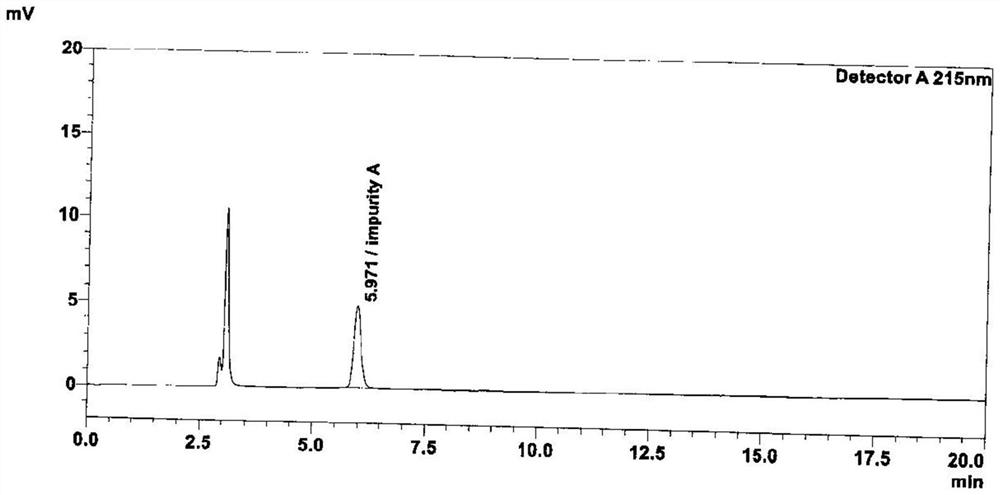
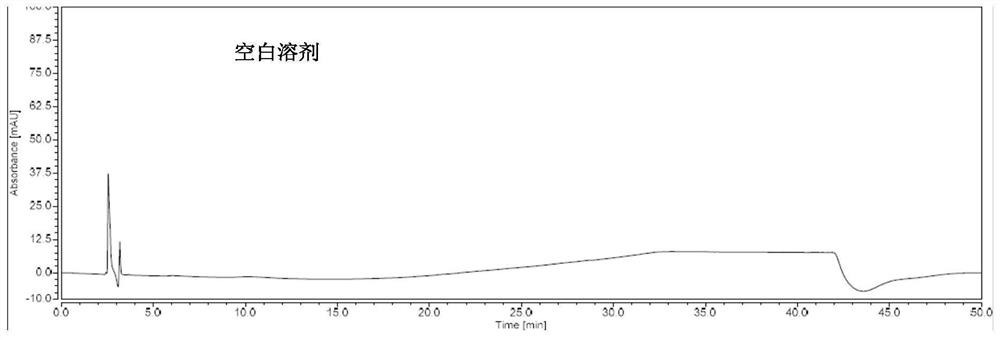
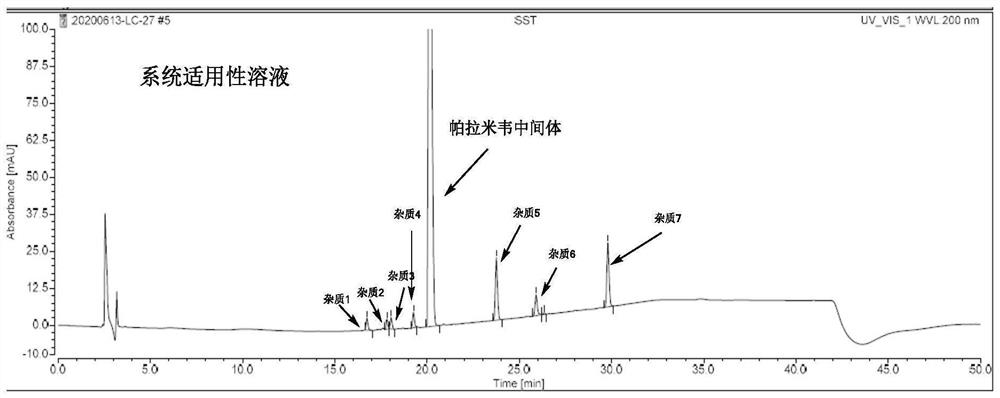
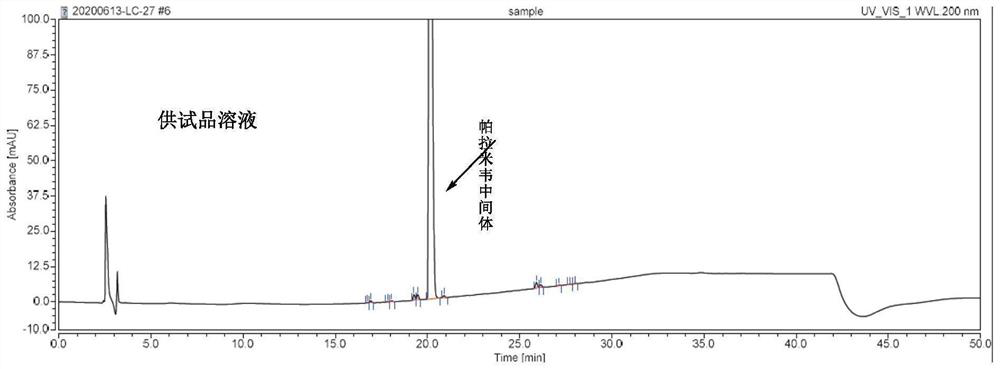
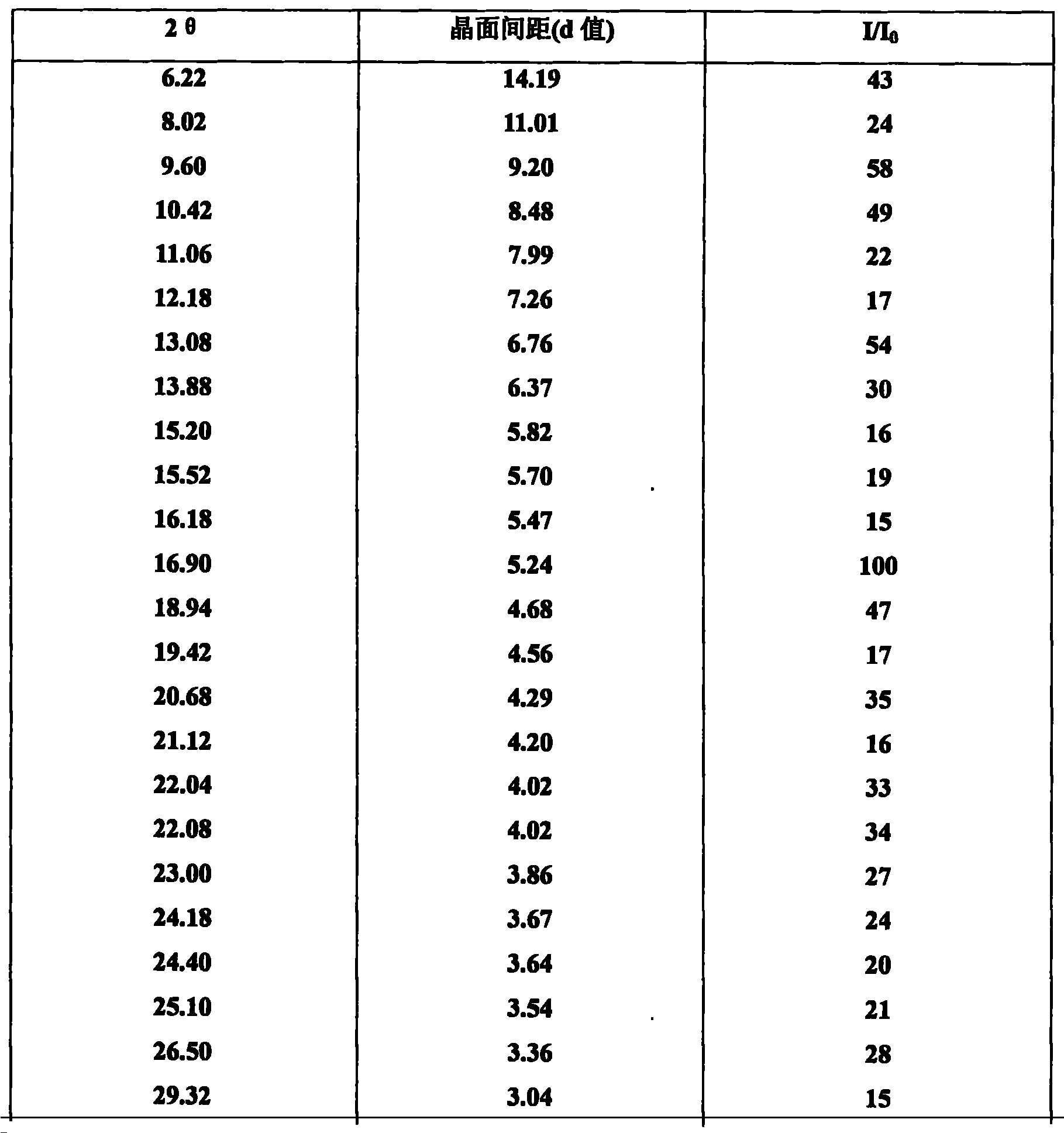
Method for determining peramivir intermediate isomers by using high performance liquid chromatography
ActiveCN111983074AEfficient separationEasy to separateComponent separationChromatography columnHplc mass spectrometry
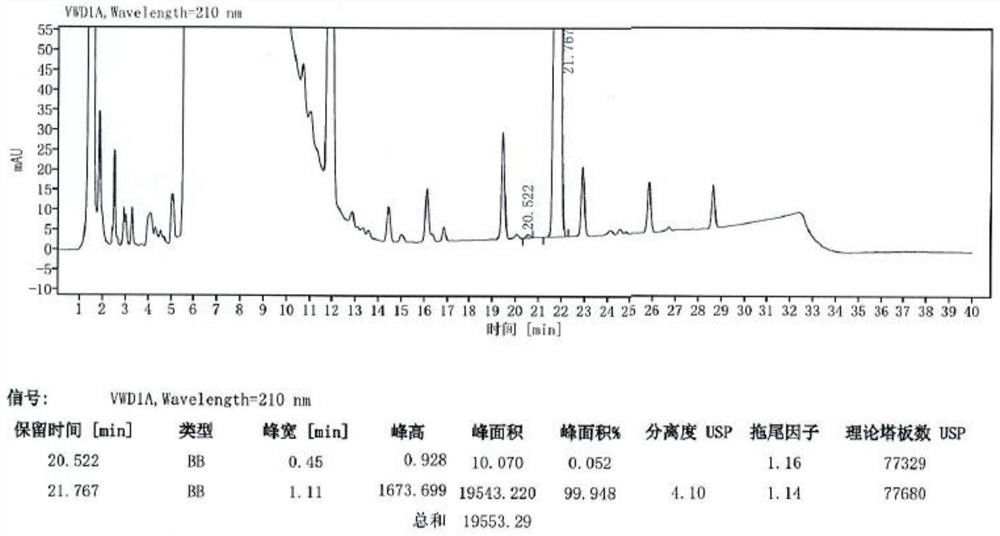
The invention relates to a method for determining peramivir intermediate isomers by using high performance liquid chromatography. The method is characterized in that an adopted chromatographic columnis a polysaccharide derivative coated chiral chromatographic column; the adopted mobile phase is a mixed solution of isopropanol and normal hexane, and isocratic elution is adopted in a high performance liquid chromatography system; in the mobile phase, the volume ratio of isopropanol to normal hexane is 10: 90 to 20: 80; the flow velocity of the mobile phase is 0.8 to 1.0 ml / min; an adopted detector is an ultraviolet detector, and the monitoring wavelength is 215 nm. The method overcomes the defects in the prior art, solves the problem of analytical determination of the peramivir intermediateisomer, can effectively control the contents of the target product and isomer impurities thereof, avoids the interference of the isomer impurities on the subsequent synthesis reaction, enhances the quality of the subsequent prepared peramivir, and ensures the medication safety. The invention provides an accurate and efficient detection method for determining the isomer of the peramivir intermediate.
Owner:苏州正济药业有限公司
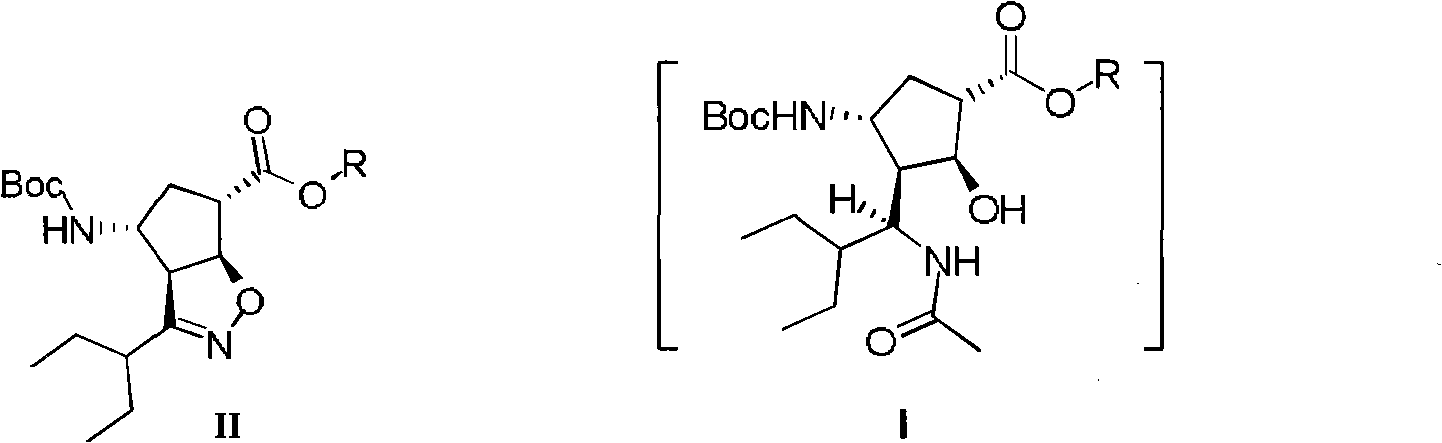
New method for preparing peramivir key intermediate
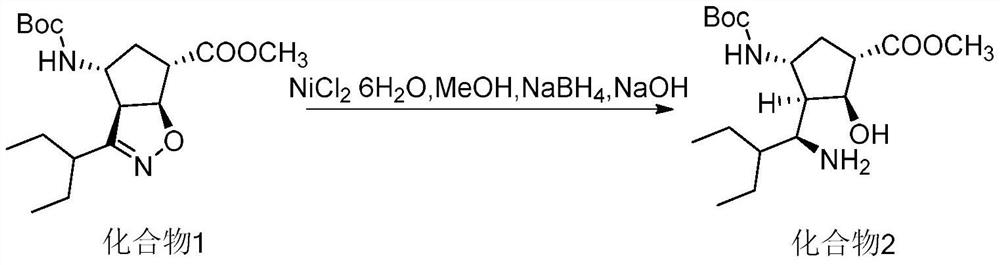
ActiveCN102757365AEasy to operateHigh yieldCarbamic acid derivatives preparationOrganic compound preparationPeramivirMedicinal chemistry
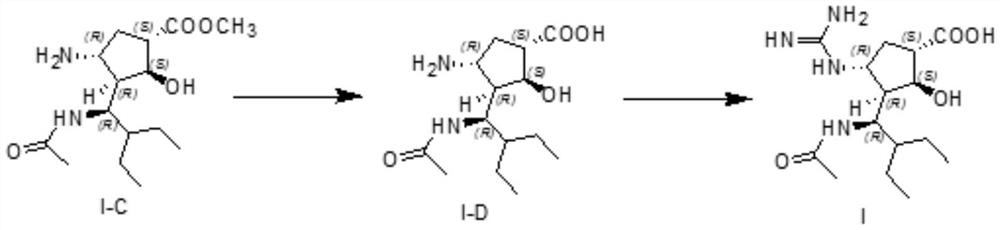
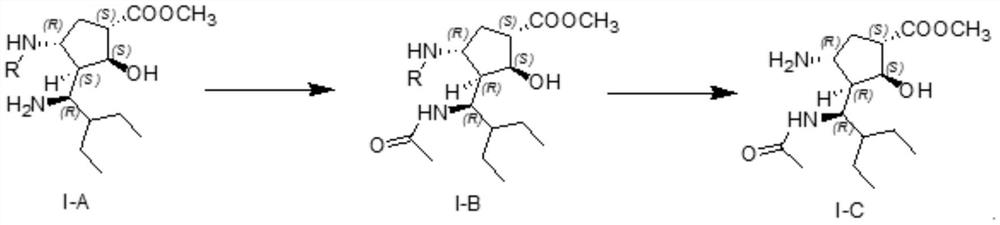
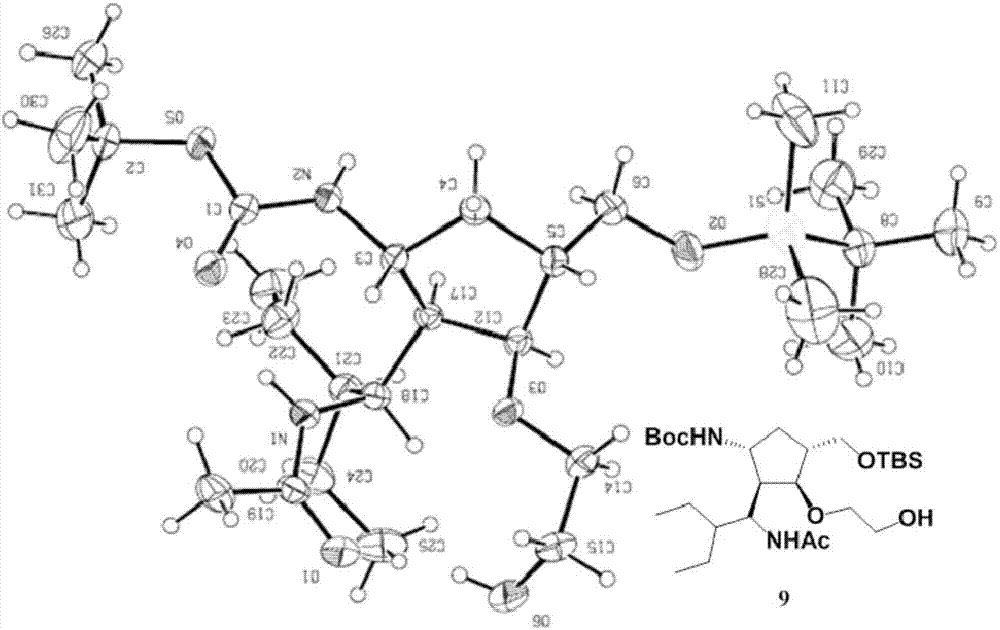
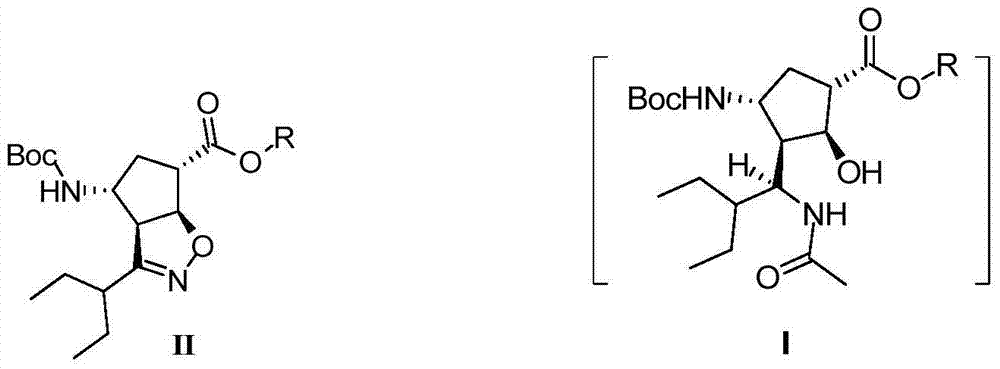
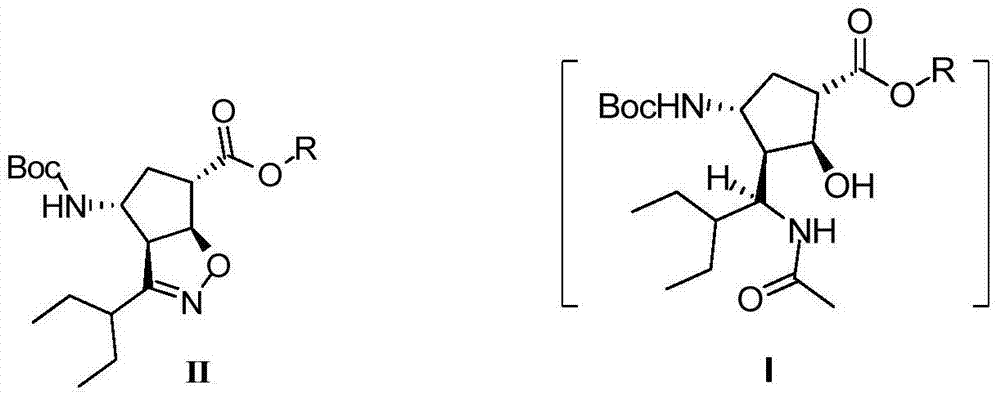
The invention discloses a method for preparing a peramivir key intermediate. According to the method for preparing the peramivir key intermediate, an intermediate (II) is taken as a raw material; and after reductive ring opening, post-processing of a reaction system is not required, and an acetylating agent is added by a one-pot method so as to obtain a peramivir intermediate (I) (1S, 2S, 3S, 4R, 1'S)-3-(1-acetamido-2-ethylbutyl)-4-(Boc)-amino-2-hydroxy-cyclopentane-1-carboxylate. Through the adoption of the method, a middle complicated post-processing process is omitted and the acetylating agent is directly used for quenching and acetylating, so that the method is simple in operation and the yield is greater than 80%.
Owner:PHARMA SHANGHAI
A kind of peramivir solution type inhalation agent and preparation method thereof
ActiveCN109771398BImprove stabilityReduce generationOrganic active ingredientsDispersion deliveryD-GlucoseGlucose polymers
Owner:GUANGZHOU NUCIEN PHARM CO LTD
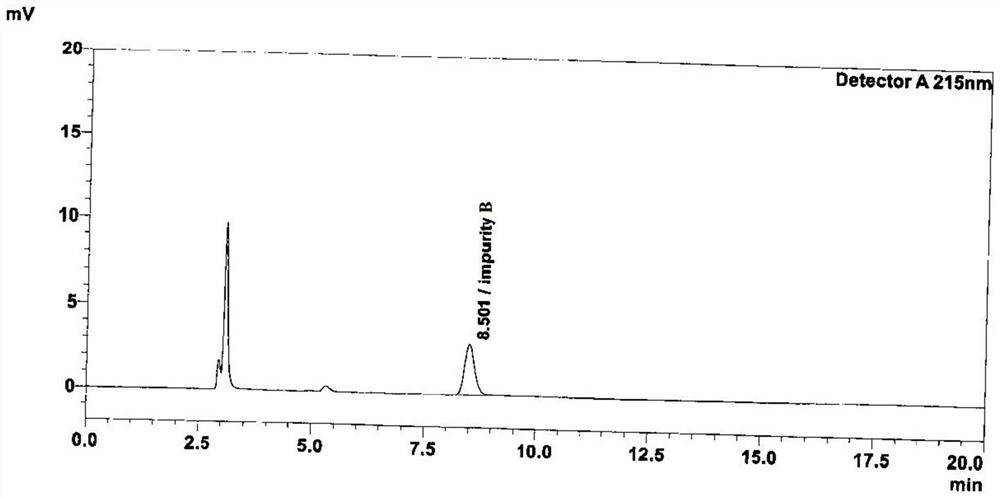
Method for detecting peramivir intermediate I by reversed-phase high performance liquid chromatography
ActiveCN112834637AEfficient separationAccurate quality inspectionComponent separationBulk chemical productionUv absorbanceFluid phase
The invention discloses a method for detecting a peramivir intermediate I by reversed-phase high performance liquid chromatography. The method comprises the following steps: preparing a test sample solution, detecting a test sample by reversed-phase high performance liquid chromatography, and calculating the contents of single impurities and total impurities in the test sample according to an area normalization method. According to the method, the intermediate I is completely separated from chromatographic peaks of various impurities by changing a mobile phase, a ratio, the wavelength of an ultraviolet absorption detector and the like; and the detection time is short, the detection specificity, accuracy and sensitivity are high, and more accurate detection and quality control can be carried out on the peramivir intermediate I.
Owner:日照正济药业有限公司
Preparation method and application of peramivir related substances
PendingCN112694421ACarbamic acid derivatives preparationOrganic compound preparationFluid phaseChemical compound
The invention discloses a preparation method and application of a peramivir related substance I. The related substance can be used as a peramivir impurity reference substance for separating and determining peramivir and the peramivir related substance I by a high performance liquid chromatography method. The preparation method provided by the invention has the advantages of mild reaction conditions and simple post-treatment, and can be used for large-scale preparation of the compound shown as the formula I with purity meeting the requirements to serve as an impurity reference substance for peramivir quality research.
Owner:日照正济药业有限公司
Antiviral drug and preparation method therefor
ActiveCN105294589AIncrease the half effective concentrationIncrease the effective concentrationOrganic active ingredientsOrganic chemistryEthyl groupPharmaceutical Substances
The invention discloses an antiviral drug and a preparation method therefor. The invention discloses a peramivir ester derivative and the preparation method therefor. The chemical name of the peramivir ester derivative is: (1S,2S,3R,4R)-1'-carboxylic acid-3'[(1S)-1''-acetamido-2''-ethyl]butyl-4'-guanidino cyclopentyl-2'-oxo-{5-[1,4,8,11-tetraazaheterocyclotetradecyl]methyl}phenyl)-1,4,8,11-tetraazaheterocyclotetradecyl]}-R-carboxylate. The peramivir ester derivative disclosed by the invention has an obviously higher activity (EC50) and a selectivity index (SI = EC50 / CC50) by comparison with the peramivir, and also is a more potent anti-flu drug.
Owner:福建龙岩龙瑆医药科技有限公司
Compound, preparation method thereof and purpose of compound in preparing drug
ActiveCN106928097APlay a role in preventioPlay a therapeutic roleOrganic chemistryOrganic compound preparationMetaboliteChemistry
The invention provides a compound, a preparation method thereof and a purpose of the compound in preparing a drug. The compound is a compound shown in a formula (I) or a stereoisomer, a geometric isomer, a tautomer, a nitrogen oxide, a hydrate, a solvate, a metabolite or a pharmaceutically acceptable salt of the compound shown in the formula (I). The compound provided by the invention can transform peramivir in an injection dosage form into an inhalant dosage form, improves the medication compliance of the peramivir and enables the peramivir to have the characteristics of convenience in use and suitability for use in a wide range; and in addition, the drug directly acts on a respiratory tract and a lung infected with an influenza virus after being inhaled, so that single administration is hopefully realized, and the drug can effectively have double actions of prevention and treatment to the influenza. A chemical formula is described in the description.
Owner:WWHS BIOTECH INC +1
Preparation method of peramivir key intermediate
The invention discloses a preparation method of a key intermediate (3aR, 4R, 6S, 6As)-4-[[(1, 2, 3, 4-triazole-3-yl)-1, 3, 4-triazole-3-yl]-1, 3, 4-triazole-3- The invention relates to a synthesis method of (1S, 4R)-(-)-[[(1, 1-dimethyl ethyoxyl) carbonyl] amino]-3-(1 '-ethyl propyl)-3a, 5, 6, 6a-tetrahydro-4H-cyclopenta [d] isoxazole-6-carboxylic acid methyl ester, which comprises the following steps: taking (1S, 4R)-(-)-[[(1, 1-dimethyl ethyoxyl) carbonyl] amino] cyclopent-2-ene-1-carboxylic acid methyl ester as an initial raw material; the preparation method comprises the following steps: under the catalysis of triethylamine, carrying out cyclization reaction on (3aR, 4R, 6S, 6As)-4-[[(1, 2, 4-triazine-2-yl)-1, 3-diketone]-1, 3-diketone and a toluene solution containing 2-ethyl-N-hydroxybutyrimidyl chloride, and directly obtaining (3aR, 4R, 6S, 6As)-4-[[(1, 2, 4-triazine-2-yl)-1, 3-diketone]-1, 3-diketone through solvent treatment, impurity removal and Compared with the prior art, the preparation method disclosed by the invention has the advantages that the yield is high, the operation is safe and simple, the purity of a finished product is high, and industrial large-scale production is convenient to realize. The preparation method disclosed by the invention has the advantages that the preparation method disclosed by the invention can be used for preparing the 3-(1, 1-dimethyl ethyoxyl) carbonyl] amino]-3-(1 '-ethyl propyl)-3a, 5, 6, 6a-tetrahydro-4H-cyclopenta [d] isoxazole-6-carboxylic acid methyl ester.
Owner:重庆恩联生物科技有限公司
Prodrugs of Neuraminidase Inhibitors
InactiveUS20120058937A9Improve oral bioavailabilityInhibition is effectiveGroup 4/14 element organic compoundsBiocideDipeptidePrimary alcohol
A new class of neuramidase inhibitor prodrugs is provided characterized by a prodrug moiety of a carboxyl group modified to form a carbonyl ethoxy amino acid, a carbonyl ethoxy dipeptide or a carbonyl ethoxy tripeptide, a guanidine group modified to form a carbonyl ethoxy amino acid, a carbonyl ethoxy dipeptide, a carbonyl ethoxy tripeptide; a primary alcohol modified to form an esterified single amino acid, dipeptide or tripeptide of zanavimir of the unaltered therapeutic agent. Exemplary therapeutic agents so modified to form prodrugs include zanavimir, oseltamivir and peramivir. The prodrug has increased oral bioavailability relative to the unaltered neuraminidase inhibitor and is effective in the inhibition of viral infections involving neuraminidase in the viral reproductive cycle.
Owner:SINEVIR THERAPEUTICS
Peramivir dry powder inhalant and preparation method thereof
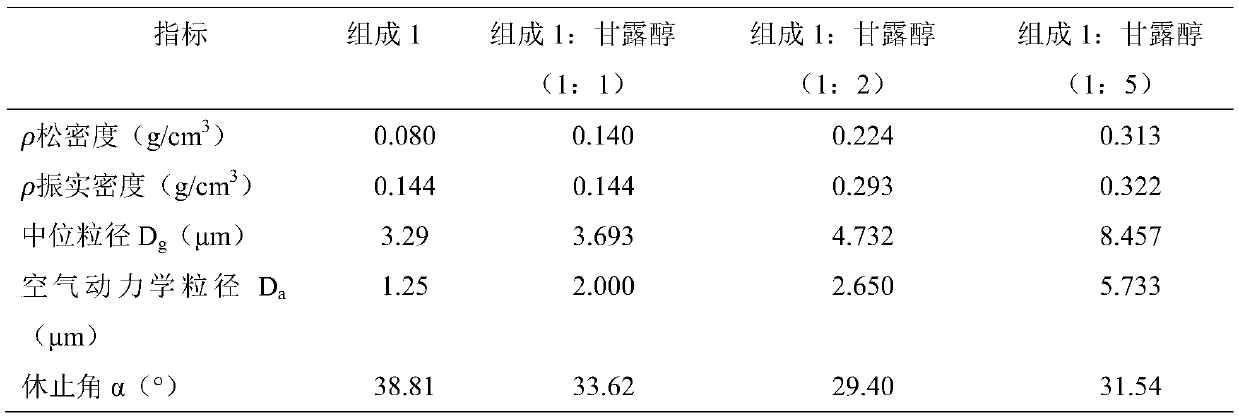
ActiveCN111358773AEasy to prepareGuaranteed validityPowder deliveryOrganic active ingredientsSodium Chloride InjectionPharmaceutical drug
The invention relates to a Peramivir dry powder inhalant, which is prepared from Peramivir or an acceptable salt or a hydrate thereof. A single-dosage preparation is 5-30 mg, the particle size D10 ofthe dry powder is 1.3-2.2 um, D50 is 3-6 um, and D90 is 6-13 um. The valence of influenza A viruses in the lungs of a mouse can be effectively lowered, the Peramivir dry powder inhalant has an obviousanti-virus function, can obviously prolong survival time and lowers a death rate, and the medicine effect function of the Peramivir dry powder inhalant is superior to the medicine effect function ofa Peramivir sodium chloride injection and Oseltamivir phosphate. The Peramivir dry powder inhalant has a specific lung targeting function, and the effectiveness and the safety of the medicine can be obviously improved.
Owner:GUANGZHOU NUCIEN PHARM CO LTD +1
Peramivir impurity F as well as preparation method and application thereof
InactiveCN112724046AGuarantee drug safetyOrganic compound preparationOrganic chemistry methodsPhysical chemistryStructural formula
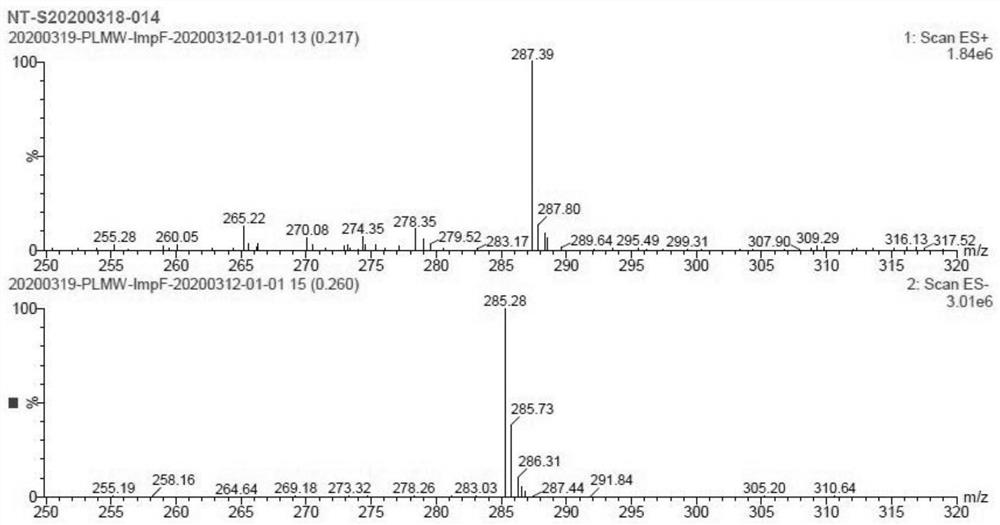
The invention provides a peramivir impurity F. The structural formula of the peramivir impurity F is shown as a formula (I). The preparation method of the peramivir impurity F comprises the following steps that A, peramivir reacts under the condition of an acid solvent, the weight-volume ratio of peramivir to the acid solvent is 1: 10-1: 100, and the unit is Kg: L; wherein the reaction temperature is 20-100 DEG C, the reaction time is 2-10 hours, and a reaction solution is obtained; and B,the reaction solution is concentrated under reduced pressure to obtain the peramivir impurity F; through research on peramivir impurities, a brand-new peramivir impurity F is obtained, the peramivir impurity F is hydrochloride, after mass spectrometric determination and hydrogen chloride deduction of the peramivir impurity F, a [M+1] peak 287.39 exists in MS, the corresponding molecular weight is 286.39, the purity reaches 95.0% or above, the peramivir impurity F can be used as a reference substance for impurity research for peramivir content determination, and the medication safety of peramivir can be effectively guaranteed.
Owner:天津应天成科技有限公司
Synthetic method of peramivir intermediate
InactiveCN105198827AReduce manufacturing costHigh reaction yieldOrganic chemistryPtru catalystAmmonium compounds
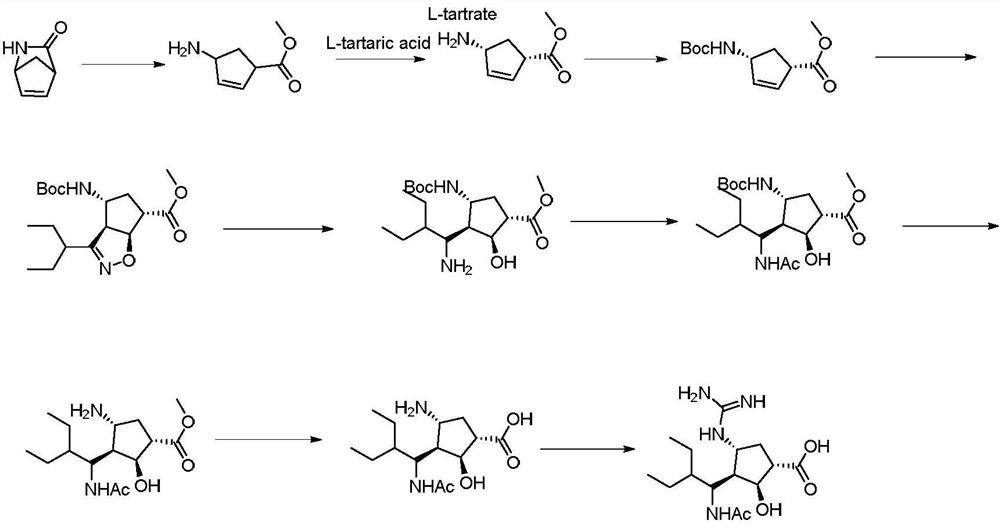
The invention relates to a synthetic method of a peramivir intermediate, in particular to a synthetic method of a key intermediate by adopting an anti-influenza drug, namely peramivir, wherein the key intermediate is (3aR,4R,6S,6aS)-4-[[(1,1-dimethyl ethoxy) carbonyl] amino]-3-(1'-ethyl propyl)-3a,5,6,6a-tetralin-tetrahydro-4H-cyclopentano[d]isoxazole-6-carboxylic acid ammonium tertiary butyl (compound IV). According to the synthetic method of peramivir intermediate, provided by the invention, (1S,4R)-(-)-[[(1,1-dimethyl ethoxy)carbonyl]amino]cyclopentyl-2-alkenyl-1- carboxylic acid methyl ester (compound I) is taken as the raw material (preparation reference patent is CN101367750B) to be subjected to ring-closure reaction with butyric imine acyl chloride under catalyzation of a metal catalyst, so that the target compound is formed. According to the synthetic method of peramivir intermediate, provided by the invention, the low-cost, easily available and efficient metal catalyst is used, in addition, compared with the conventional technological process, the synthetic method is obviously increased in the yield, the technological process is simple, and industrial large-scale production is facilitated.
Owner:GUANGZHOU NANXIN PHARMA
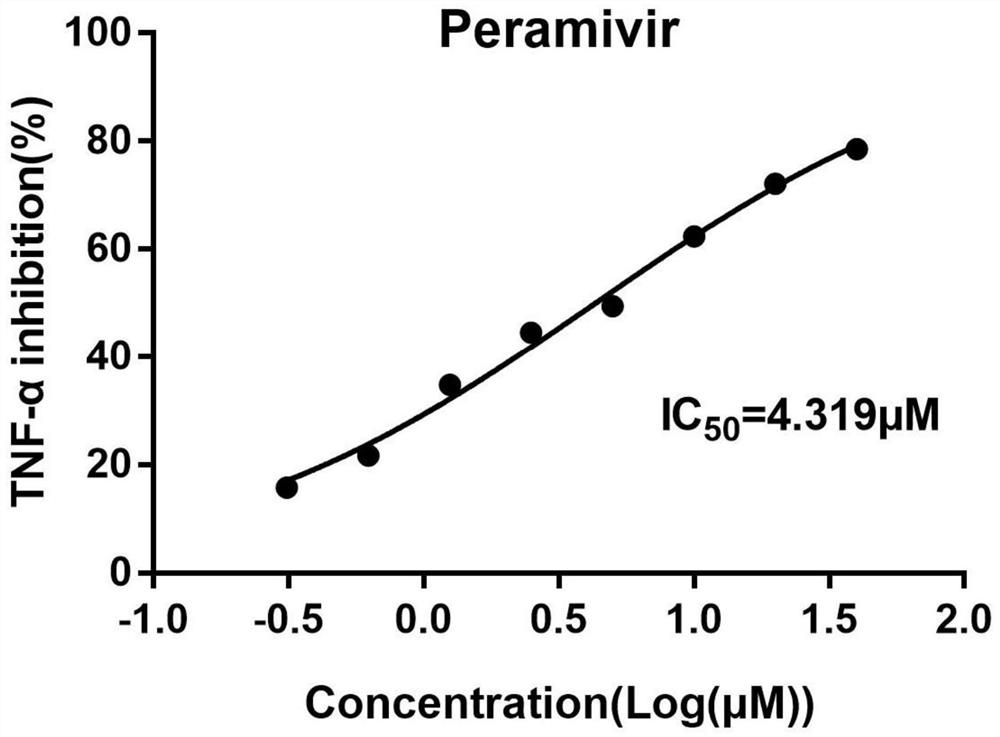
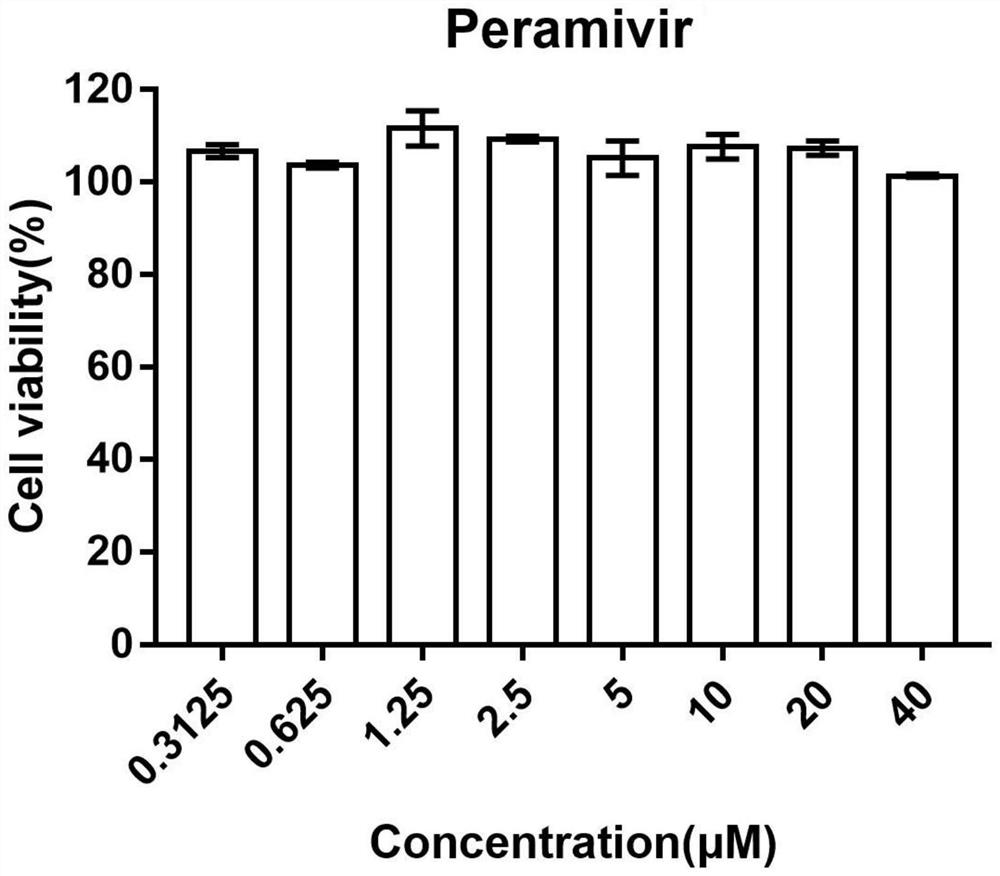
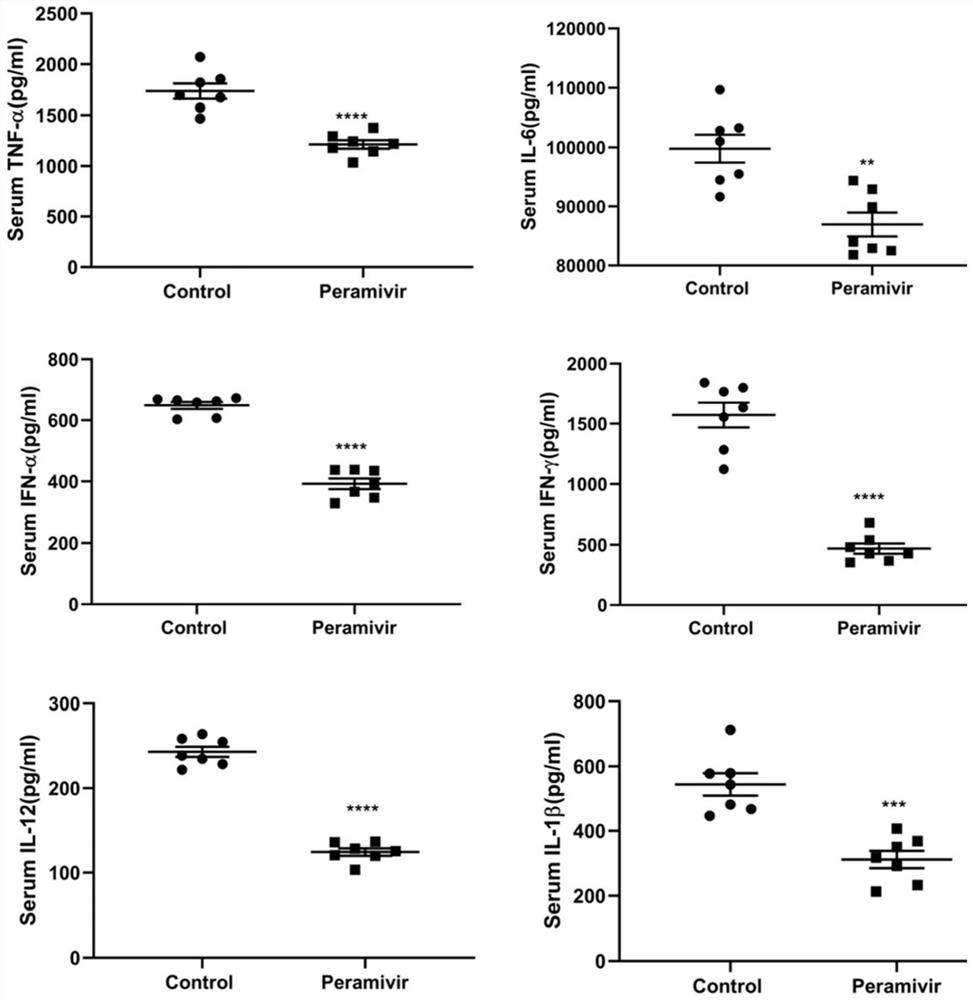
Application of peramivir in the preparation of drugs for treating inflammatory storm caused by infectious diseases
ActiveCN111249266BEnhanced inhibitory effectReduce secretionAntibacterial agentsOrganic active ingredientsCytokineInfective disorder
The invention discloses an application of peramivir in preparing a medicine for treating an inflammatory storm caused by an infectious disease, wherein the infectious disease is an infection caused by a virus, chlamydia, mycoplasma, bacteria or parasite, and the inflammatory storm is Infectious diseases cause the rapid and massive production of various cytokines in the body, and the various cytokines in the body are TNFα, IL-6, IL-1β, IL-12, IFNα and IFN-γ. The present invention proves for the first time that peramivir has the effect of treating inflammatory storm caused by infectious disease, can be used for preparing the therapeutic drug for inflammatory storm caused by infectious disease, and provides a new anti-inflammatory scheme for clinical treatment.
Owner:THE NAVAL MEDICAL UNIV OF PLA
Peramivir impurity A and impurity C, and preparation methods and application thereof
InactiveCN113880732AQuality improvementEnsure safetyOrganic active ingredientsOrganic compound preparationPharmaceutical drugActive ingredient
The invention discloses a peramivir impurity A and a peramivir impurity C with structures as shown in the specification. The invention also provides synthesis methods of the impurity A and the impurity C, and application of the impurity A and the impurity C as impurity reference substances in quality control of peramivir or a pharmaceutical composition thereof. The impurities can be stably prepared and supplied, and a basis is provided for related research and contrast. The peramivir impurity A and the peramivir impurity C disclosed by the invention have important significance in controlling peramivir raw material medicines and researching impurities of preparations of the peramivir raw material medicines.
Owner:湖南凯铂生物药业有限公司
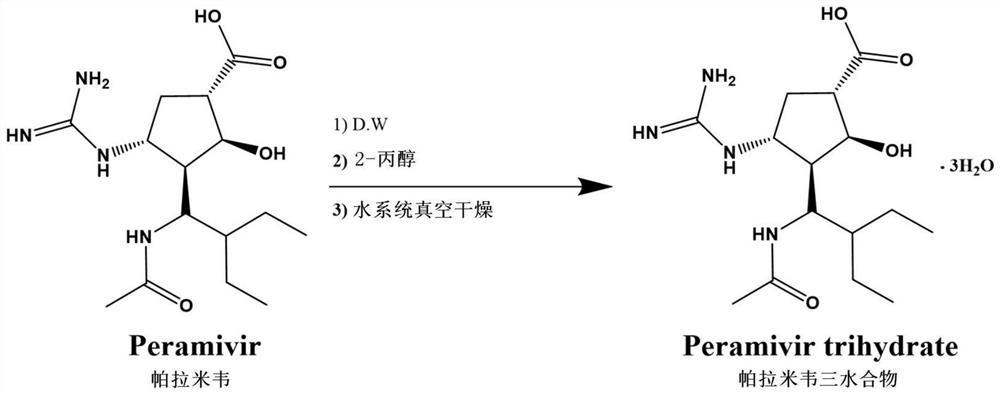
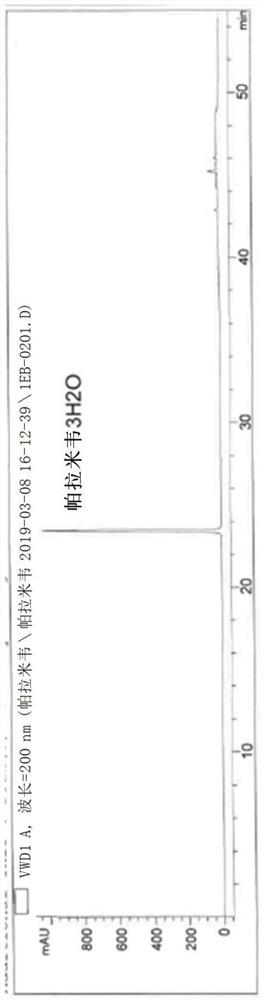
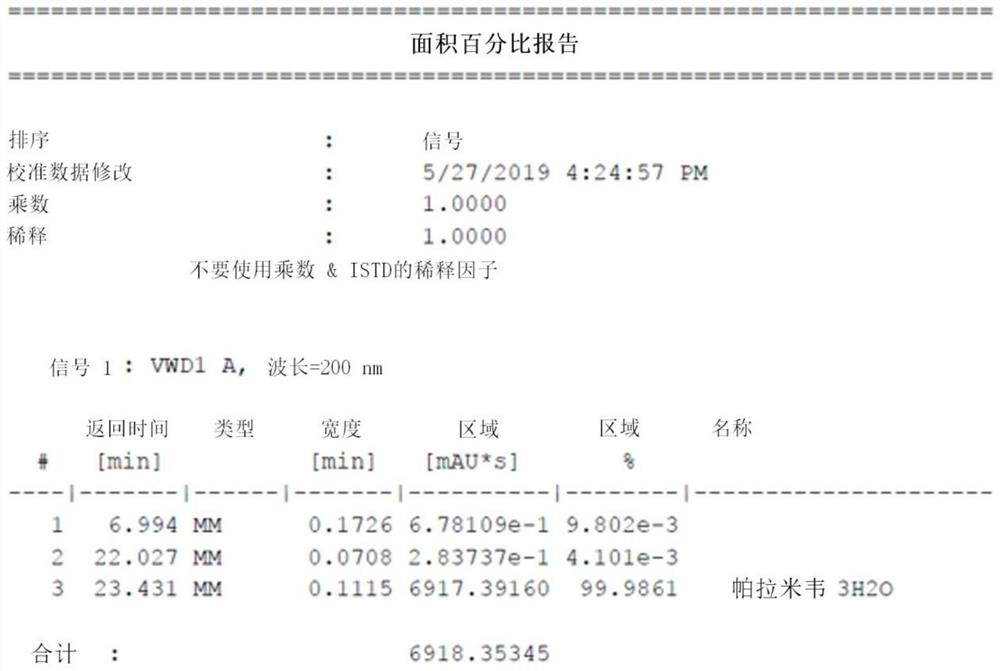
A kind of synthetic method of peramivir trihydrate
ActiveCN105085328BEmission reductionImprove "Atom Utilization Efficiency"Organic chemistryOrganic compound preparationCarboxylic acidAnti influenza drug
The invention relates to a synthesis method of peramivir trihydrate, an anti-influenza medicine. The present invention uses (3aR,4R,6S,6aS)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(1'-ethylpropyl)-3a,5, 6,6a-tetrahydro-4H-cyclopenta[d]isoxazole-6-carboxylic acid 1,1-dimethylethylammonium (compound I, prepared by referring to CN101367750B method) as raw material, through five steps Synthetic procedure Preparation of peramivir trihydrate. Compared with the methods reported in existing documents, the present invention designs a shorter, more effective and practical process route, and the total yield of finished product preparation is significantly improved.
Owner:GUANGZHOU NANXIN PHARMA
A kind of synthetic method of 3+2 ring closure
ActiveCN108997171BImprove utilization efficiencyEmission reductionOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryPeramivir
The invention discloses a synthesis method of a 3+2 closed ring. According to the method, chiral induction is enhanced under the spatial steric effect of bridge ring molecules, so that a ring closingreaction has efficient spatial selectivity. The method is applied to synthesis of peramivir and has the characteristics that the synthesis route is short, the stereoselectivity is high and the operation procedure is convenient.
Owner:苏州正济医药研究有限公司
Waterless Peramivir crystal and medicament composition thereof
ActiveCN101314579BGood reproducibilityKeep dryOrganic active ingredientsOrganic chemistryMedicineAvian influenza virus
The invention relates to an anhydrous peramivir crystal compound and a preparation method thereof, and further discloses a pharmaceutical composition comprising the same and the application thereof in the preparation of medicaments for treating the influenza virus and the Avian influenza virus.
Owner:天津泰普制药有限公司
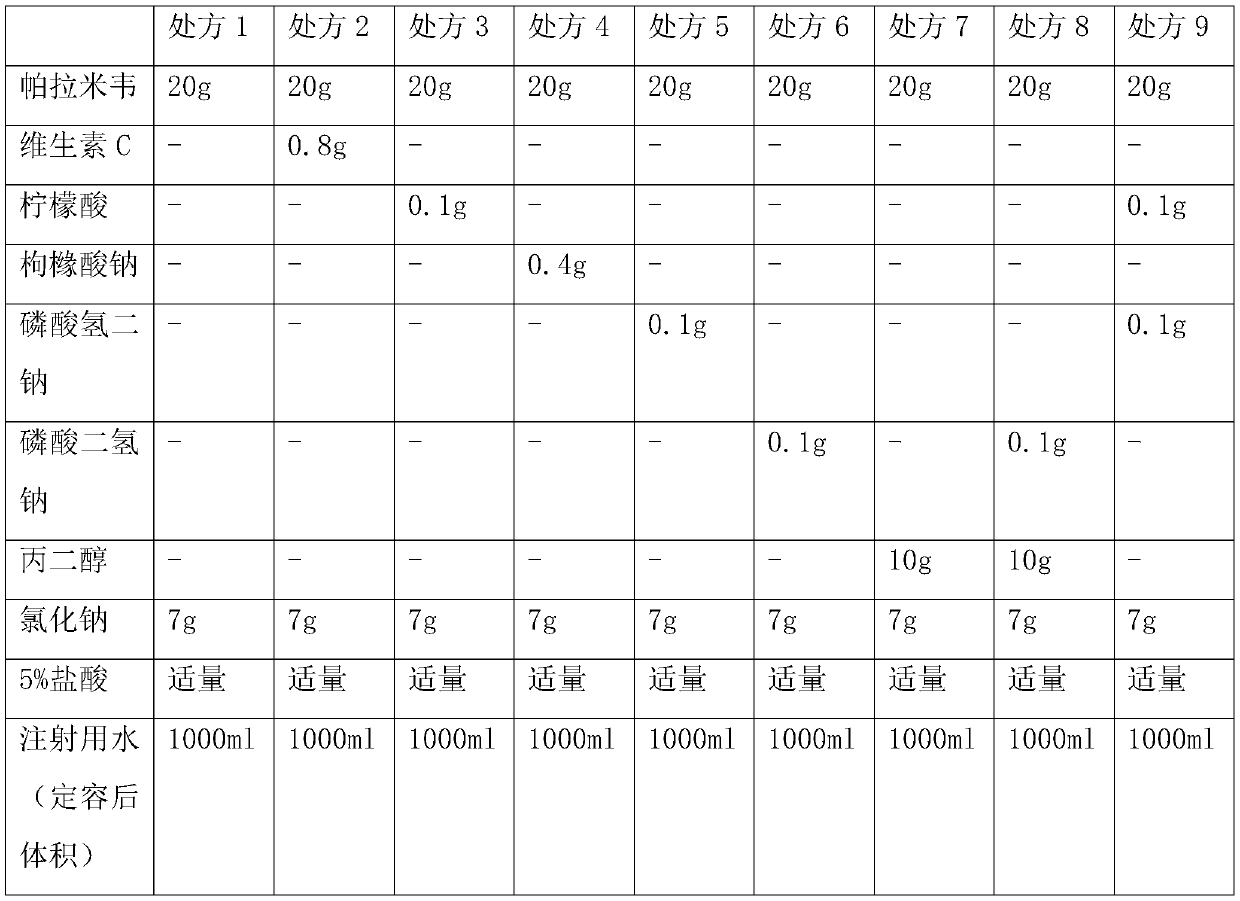
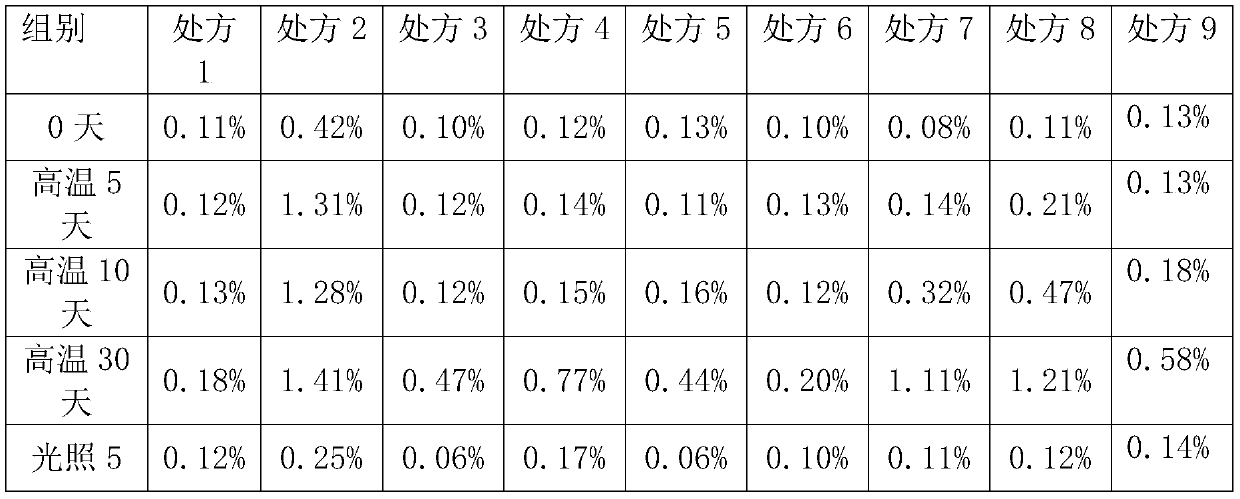
Peramivir pharmaceutical composition and preparation method thereof
ActiveCN114177135ASimple processLow costOrganic active ingredientsInorganic non-active ingredientsUltrasonic emulsificationOrganosolv
The invention discloses a peramivir pharmaceutical composition and a preparation method thereof. The preparation method comprises the following steps: S100, dissolving peramivir in an organic solvent to obtain an oil phase solution; s200, dissolving the auxiliary materials in the water for injection to obtain a water-phase solution; and S300, dropwise adding the aqueous phase solution into the oil phase solution, and carrying out ultrasonic emulsification to obtain the peramivir pharmaceutical composition. The peramivir pharmaceutical composition obtained by the invention is simple in process and low in cost.
Owner:朗天药业(湖北)有限公司 +1
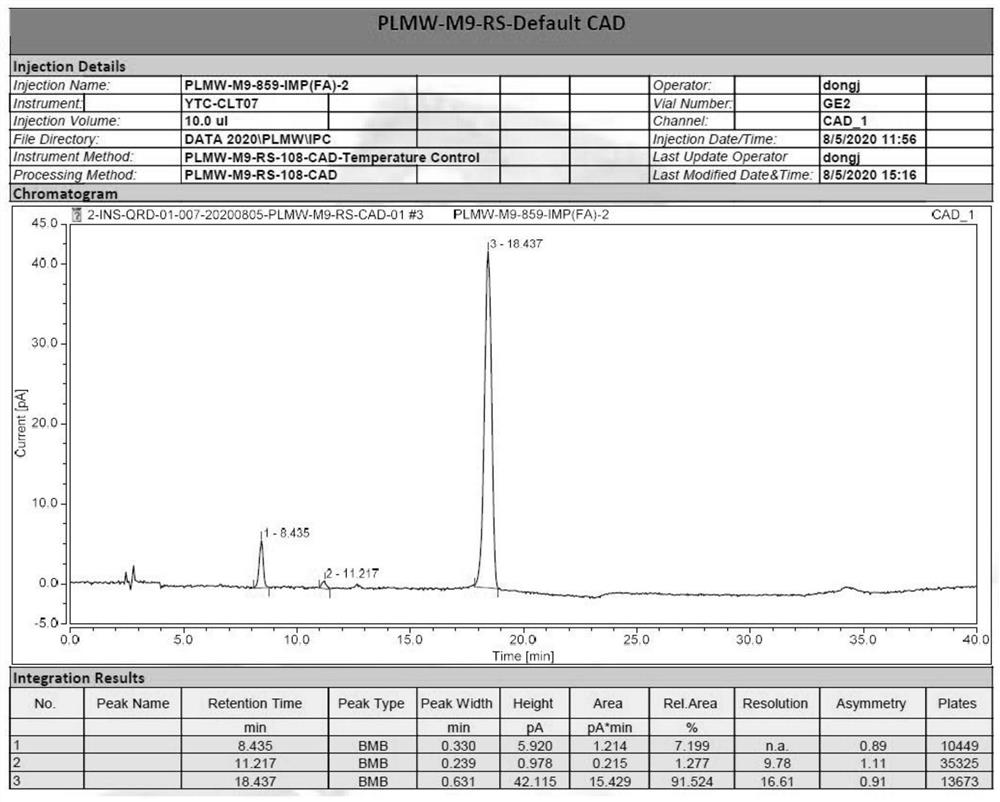
Peramivir intermediate 9 impurity F as well as preparation method and application thereof
PendingCN112266341AGuarantee drug safetyCarbamic acid derivatives preparationOrganic compound preparationPhysical chemistryMass Spectrometry-Mass Spectrometry
The invention provides a peramivir intermediate 9 impurity F. The structural formula of the peramivir intermediate 9 impurity F is shown as a formula (I). The preparation method of the peramivir intermediate 9 impurity F comprises the following steps: A, performing reduction reaction on a peramivir intermediate 8 and a reducing agent in an organic solvent and an acid solution at the reaction temperature of -20 DEG C to 100 DEG C for 2-10 hours to obtain a reaction solution; and B, concentrating the reaction solution under reduced pressure to obtain a crude product of the impurity F of the peramivir intermediate 9, and conducting purifying to obtain the impurity F of the peramivir intermediate 9. Through research on peramivir impurities, the brand-new peramivir intermediate 9 impurity F isobtained, through mass spectrometry, the MS has a [M+1] peak 359.26, the corresponding molecular weight is 358.25, the purity reaches 90.0% or above, the impurity F can be used as a reference substance for impurity research for peramivir content determination, and the medication safety of peramivir can be effectively guaranteed.
Owner:天津应天成科技有限公司
Peramivir hydrate crystal, preparation method, medical compound and usage thereof
ActiveCN102584637BOrganic active ingredientsOrganic chemistryCombinatorial chemistryStructural formula
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
Novel method for producing peramivir trihydrate, and water-based drying thereof
ActiveCN114040906AStable manufacturingHigh yieldOrganic compound preparationOrganic chemistry methodsActivated carbonNeuraminidase
The present invention relates to a method for producing peramivir trihydrate, which is an inhibitor of neuraminidase infection, as an anti-influenza agent. According to the production method of the present invention, peramivir trihydrate can be produced with high yield and stability through a process suitable for producing excellent pharmaceuticals and quality control standards (GMP) without using highly-toxic methanol and activated carbon.
Owner:CHONGKUNDANG BIO
Method for preparing peramivir key intermediate
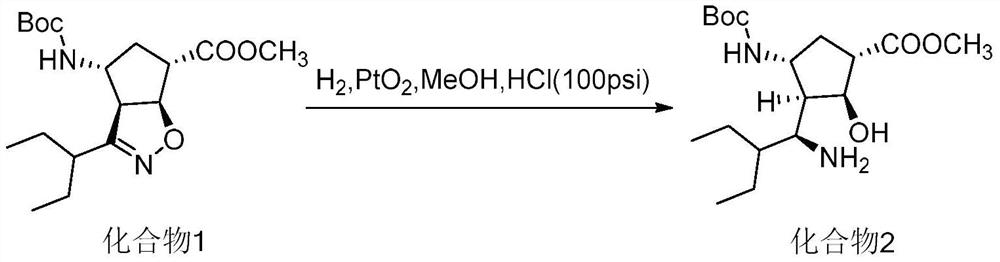
ActiveCN102757365BEasy to operateHigh yieldCarbamic acid derivatives preparationOrganic compound preparationPeramivirMedicinal chemistry
The invention discloses a new method for preparing a peramivir key intermediate. According to the new method for preparing the peramivir key intermediate, an intermediate (II) is taken as a raw material; and after reductive ring opening, post-processing of a reaction system is not required, and an acetylating agent is added by a one-pot method so as to obtain a peramivir intermediate (I) (1S, 2S, 3S, 4R, 1'S)-3-(1-acetamido-2-ethylbutyl)-4-(Boc)-amino-2-hydroxy-cyclopentane-1-carboxylate. Through the adoption of the method, a middle complicated post-processing process is omitted and the acetylating agent is directly used for quenching and acetylating, so that the method is simple in operation and the yield is greater than 80%.
Owner:PHARMA SHANGHAI
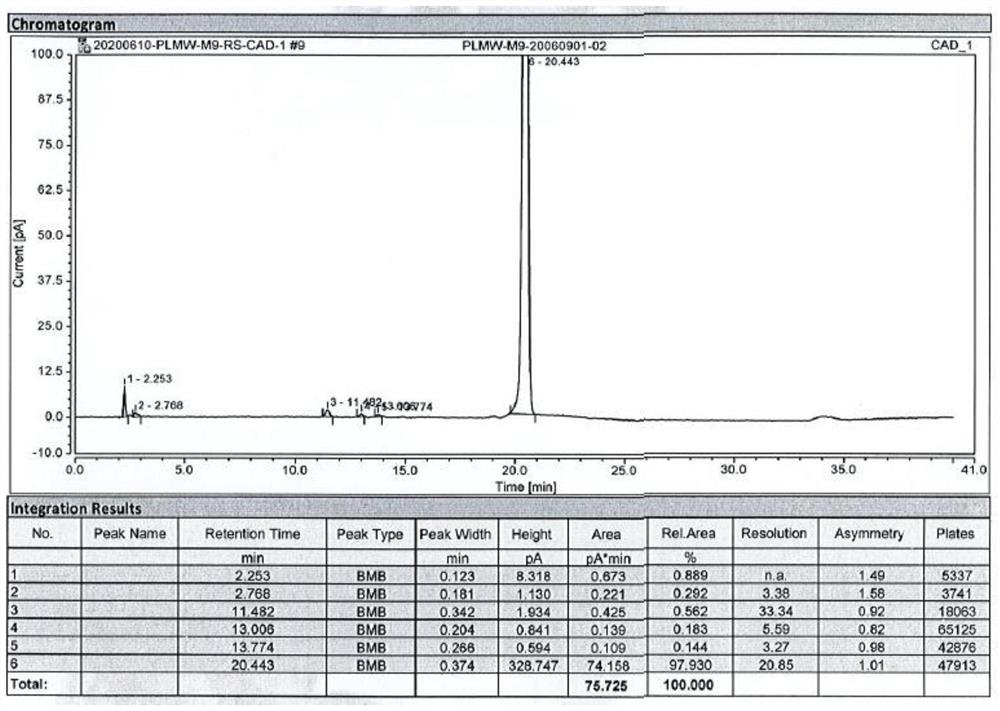
Safe and non-toxic method for removing nickel ion impurities in peramivir trihydrate intermediate M9
ActiveCN112250601AEfficient removalReduce usageCarbamic acid derivatives preparationOrganic compound preparationActivated carbonPharmaceutical drug
The invention provides a safe and nontoxic method for removing nickel ion impurities in a peramivir trihydrate intermediate M9. The method comprises the following steps: adding pure water into a reaction kettle containing a peramivir trihydrate crude product at a temperature of -5-5 DEG C; dropwise adding an acidic solution to obtain a first solution after dropwise adding is finished; adding purewater into the first solution again, heating to 15-20 DEG C, keeping the temperature for 20-40 minutes, adding activated carbon, and stirring for 20-40 minutes to obtain a second solution; centrifugally separating the second solution to obtain centrifugal filtrate and a first filter cake; leaching the first filter cake with purified water, and combining the leaching filtrate with the centrifugal filtrate to obtain a third solution; and carrying out washing, crystallizing and other treatments on the third solution to finally obtain the purified peramivir trihydrate. According to the method, nickel ion impurities in peramivir trihydrate can be effectively removed, sodium nitrite is prevented from being used in the process, generation of genotoxic impurities is avoided, and the safety of themedicine is greatly improved.
Owner:天津应天成科技有限公司
Peramivir impurity M as well as preparation method and application thereof
PendingCN112266342AGuarantee drug safetyUrea derivatives preparationComponent separationPhysical chemistryStructural formula
The invention provides a peramivir impurity M. The structural formula of the peramivir impurity M is shown as a formula (I). The preparation method of the peramivir impurity M comprises the followingsteps: A, reacting peramivir in an alkaline solution, and conducting stirring at the reaction temperature of 10-100 DEG C for 2-10 hours to obtain a reaction solution; and B, adjusting the pH value ofthe reaction solution to 3-4 by using an acid, separating out solids, and carrying out suction filtration to obtain the peramivir impurity M. By researching the peramivir impurity, the brand-new peramivir impurity M is obtained, the corresponding molecular weight of the peramivir impurity M is 329.40 and the purity of the peramivir impurity M reaches 95.0% or above through mass spectrometric determination, and the peramivir impurity M can be used as a reference substance for impurity research for peramivir content determination and can effectively guarantee the medication safety of peramivir.
Owner:天津应天成科技有限公司
Preparation method of peramivir intermediate
ActiveCN114181117AImprove conversion rateReduce contentCarbamic acid derivatives preparationOrganic compound preparationTert-Butyloxycarbonyl protecting groupEthyl group
The invention discloses a preparation method of a peramivir intermediate, which comprises the following steps: adding (3aR, 4R, 6S, 6aS)-4-((t-butyloxycarboryl) amino)-3-(pentane-3-yl)-3a, 5, 6, 6a-tetrahydro-4H-cyclopentane [d] isoxazole-6-carboxylic acid methyl ester and nickel chloride into a mixed solution of methanol and dichloromethane, then adding a methanol solution of sodium borohydride and sodium hydroxide, stirring, filtering, washing, and drying to obtain the peramivir intermediate. And after the reaction is completed, adding an aqueous solution of sodium nitrite, ethylenediamine tetraacetic acid and ammonia water, stirring, quenching, extracting, concentrating under reduced pressure, and crystallizing by using a mixed solvent of alcohol and water to obtain the peramivir intermediate (1S, 2S, 3S, 4R)-3-((S)-1-amino-2-ethylbutyl)-4-((t-butyloxycarboryl) amino)-2-hydroxycyclopentane-1-carboxylic acid methyl ester.
Owner:ZENJI RES LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com