A kind of peramivir solution type inhalation agent and preparation method thereof
An inhalant and solution-type technology, applied in the field of medicine, can solve problems such as poor stability, achieve good stability, uniform distribution, and reduce the production of irrelevant substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
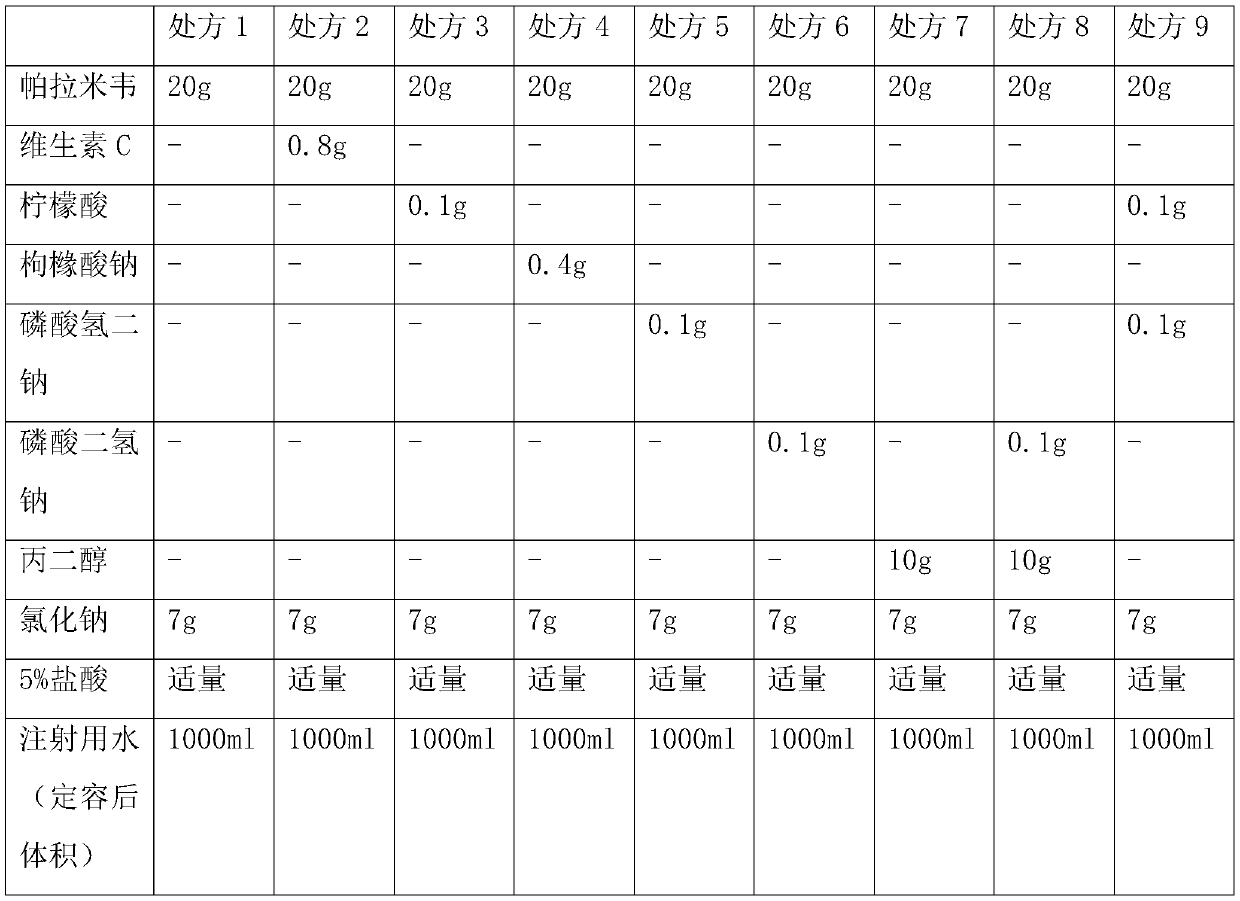
[0031] Example 1: Inhalant Prescription
[0032] According to the prescription in Table 1, mix the peramivir raw material and auxiliary materials in the liquid preparation tank, add water for injection at 70-80°C to 1000ml, stir and dissolve, then cool the solution, and dilute the solution with dilute hydrochloric acid (5% v / v) The pH value is adjusted between 5.0 and 6.0, and then the peramivir solution is pre-filtered and then aseptically filtered, and aseptically filled to obtain the product.
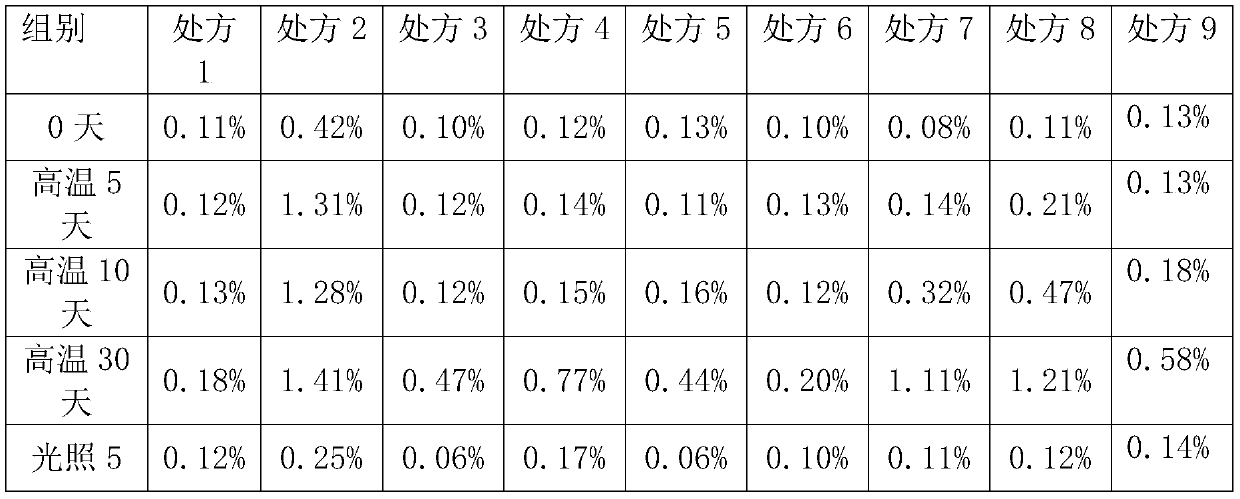
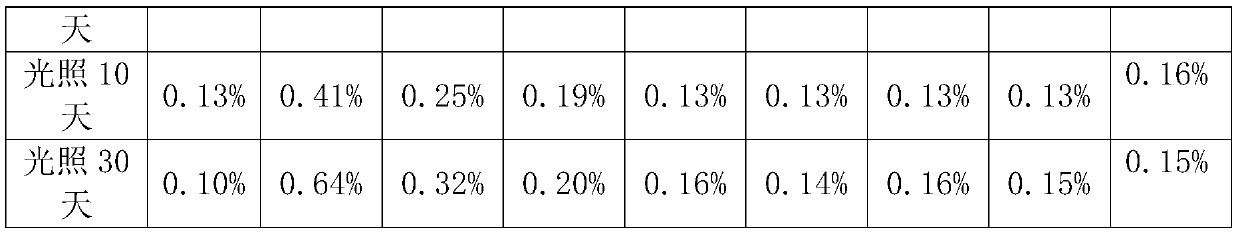
[0033] Prescriptions 1-9 in Table 1 were placed under airtight high temperature (60°C) and light (4500lx±500lx) conditions for 0 days, 5 days, 10 days, and 30 days, respectively, to investigate the situation of related substances.
[0034] Determination method of related substances: HPLC: Agilent 1260 Infinity USA; Chromatographic column: EclipsePlus C 18 (5μm, 4.6×250mm, Agilent, USA); mobile phase: gradient elution is as follows:
[0035] Table 1 Mobile phase gradient elution
...
Embodiment 2
[0044] Embodiment 2: Experiment on the influence of different pH on the solubility of peramivir
[0045] Take by weighing excessive peramivir crude drug and add in the 25ml volumetric flask, be settled to scale with 0.7% saline, be placed in shaker shaking (25 ℃, 75rpm) after 24h, with dilute hydrochloric acid (5%, V / V) adjust the pH to 4, 4.5, 5.0, 5.5 respectively, continue to stand for 48h, filter with a 0.45 μm filter membrane, and the filtrate is diluted 250 times with deionized water, and the sample adopts the HPLC method in the peramivir standard to measure the peramivir content .
[0046] Table 4 The equilibrium solubility of peramivir in different pH physiological saline solutions
[0047]
[0048] The experimental results showed that the equilibrium solubility of peramivir was pH-dependent, and its equilibrium solubility increased as the pH decreased. Combined with the requirements of preparation pH (3-8) and factors such as physiological stimulation, the pH of...
Embodiment 3
[0049] Embodiment 3: Stability experiment of peramivir inhalation solution under different pH conditions
[0050] Regulate the solution pH value at 4.5, 4.5, 5.0, 5.5 with dilute hydrochloric acid (5% v / v) with prescription 1, 6 respectively, and the solution that is not adjusted with dilute hydrochloric acid, then the peramivir solution is placed in airtight Place it under the conditions of high temperature (60°C) and light (4500lx±500lx) for 0 day and 30 days to investigate the situation of related substances.
[0051] Related substance content (%) under different pH conditions of table 5
[0052]
[0053]
[0054] The experimental results show that the peramivir inhalation solution is the most stable at a pH value of 5.5, and can still maintain good stability under high temperature conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com