Compound, preparation method thereof and purpose of compound in preparing drug
A compound and hydrate technology, which is applied in the field of medicine, can solve the problem of undeveloped drugs for influenza, and achieve the effects of being suitable for a wide range of use, improving medication compliance, and being convenient to use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] The compound represented by formula (I-1) was synthesized according to the following route.
[0108]
[0109]
Embodiment 2
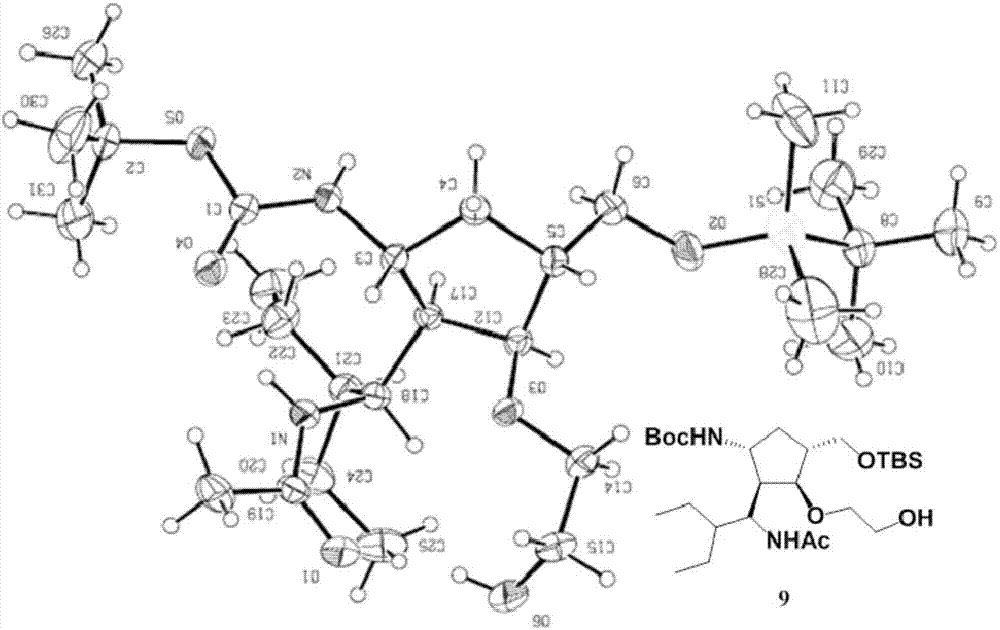
[0111] The single crystal diffraction results of compound 9 obtained in Example 1 confirmed the correctness of the structure of compound (I-1): the ethylene glycol component was successfully connected to the -OH position of the five-membered ring skeleton ( figure 1 ). The NMR data of compound 9 are: 1 H NMR (CDCl 3 ,500MHz): δ=7.72-7.70(d,J=10.3Hz,1H),4.67-4.66(d,J=9.2Hz,1H),4.33-4.30(t,J=9.3Hz,1H),4.17- 4.10(m,1H),3.56-3.51(m,5H),3.40-3.36(m,1H),3.19-3.14(m,1H),2.19-2.14(m,1H),2.10-2.07(m,1H ),1.99(s,3H),1.82-1.78(m,1H),1.41(s,9H),1.35-1.28(m,3H),1.18-1.10(m,3H),0.85(s,9H), 0.83-0.77(m,6H),0.01(s,6H); 13 C NMR (CDCl 3 ,125MHz): δ=170.3,156.2,85.9,80.1,77.6,77.2,76.7,73.6,71.3,65.1,51.6,50.6,47.7,45.2,42.9,33.5,28.4,25.9,23.6,22.2,21.6,18.3, 11.4, 10.8, -5.4, -5.5; HRMS (ESI) Calcd.forC 27 h 55 N 2 o 6 Si + [M + h + ] 531.3824, found 531.3822.
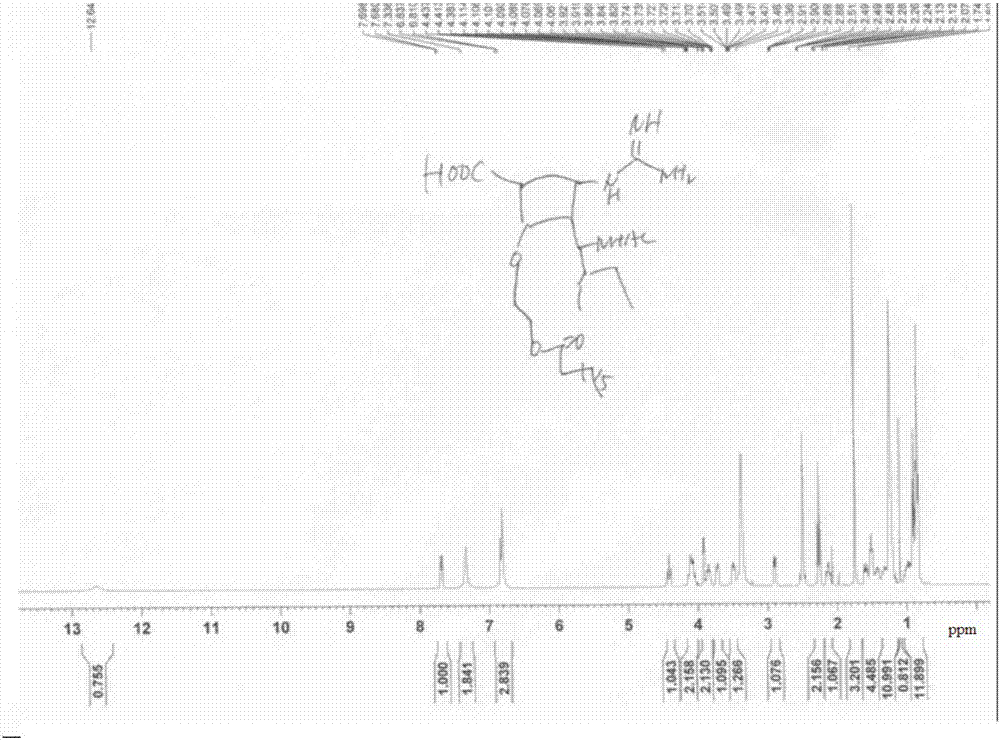
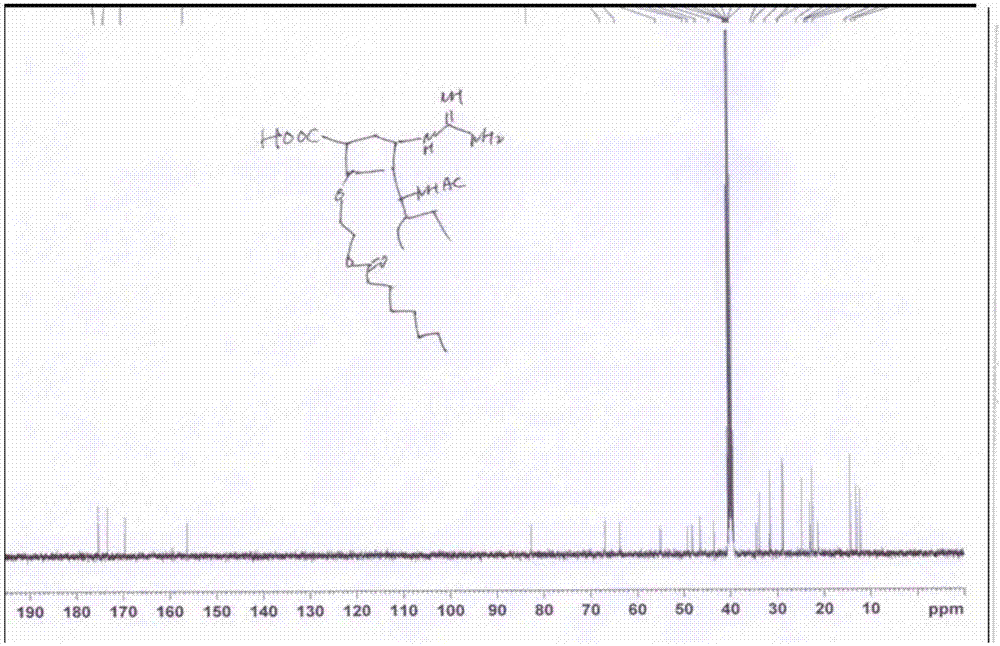
[0112] The final target molecular prodrug (compound (I-1)) was confirmed by NMR and HRMS ( Figure 2~4 ). Its NMR da...
Embodiment 3
[0115] MDCK cells were seeded on 12-well tissue culture plates (1.2 × 10 5 cells / well, DMEM containing 10% FBS), and incubated for 48 hours at 37°C in a humidified 5% carbon dioxide incubator. Cells were infected with virus (100 PFU, Udorn-4) for 1 hour in the incubator. After adsorption of virus, the virus suspension was removed and the cells were washed with DMEM. The infected cell monolayer was flattened in DMED solid medium (containing 1% agar, 1‰TCPK-trypsin and 1% antibiotics), and the medium contained 2-fold serially diluted compound (I-1), a total of 6 gradients with 2 wells per concentration. 48 hours after infection, monolayer cells were fixed and stained with 0.5% crystal violet solution to count the number of virus-induced plaques.
[0116] The result is as Figure 5 As shown, the compound (I-1) has strong anti-influenza virus activity, and its in vitro anti-influenza virus activity EC50 is about 1nM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com