Peramivir impurity M as well as preparation method and application thereof
A technology of impurities and sodium hydroxide, which is applied in the preparation of urea derivatives, chemical instruments and methods, and the preparation of test samples, etc., can solve the problems of no impurity-related reports and achieve the effect of ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
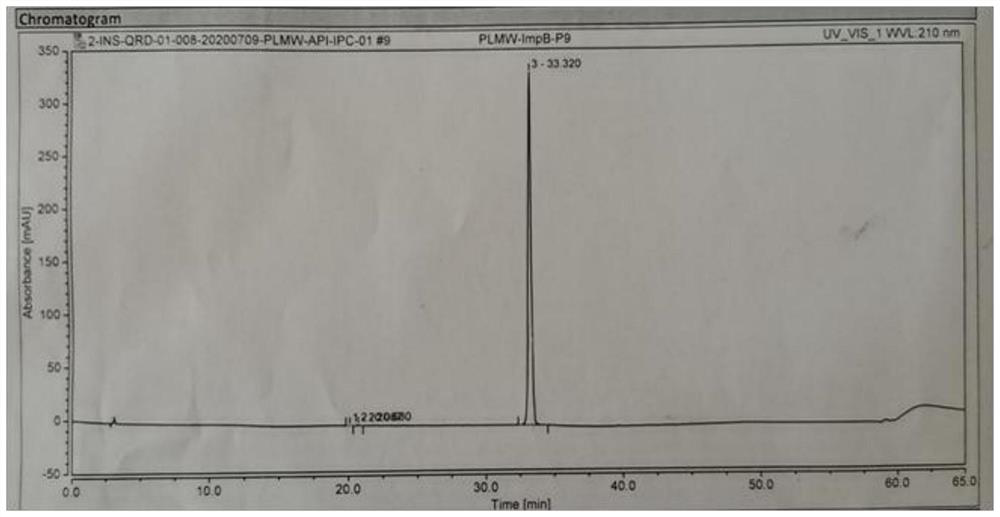
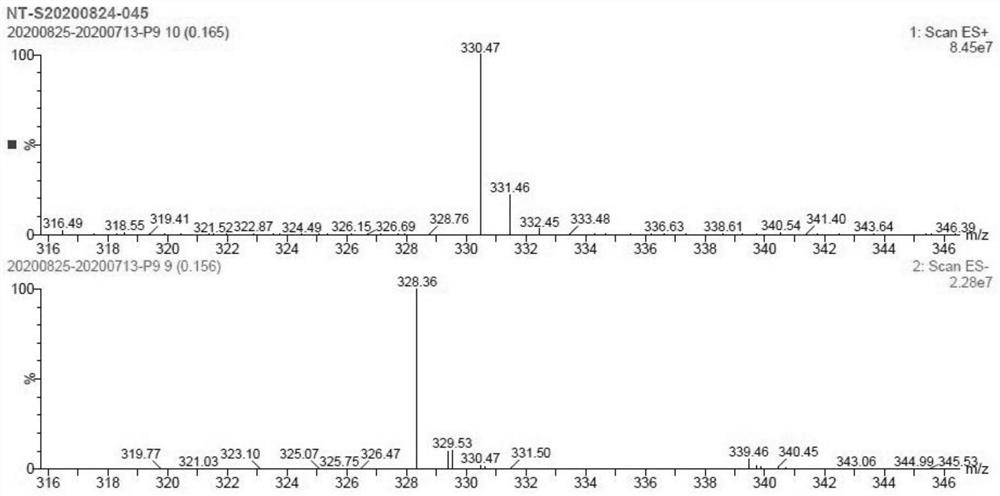
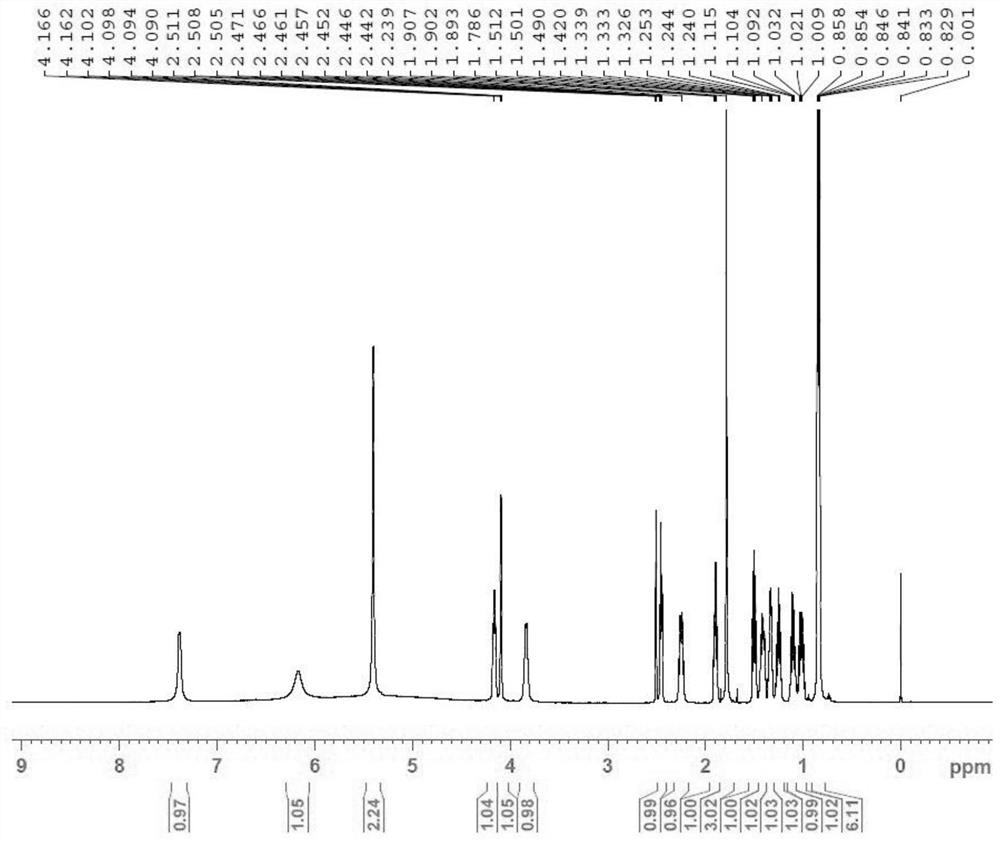
[0032] Add 5 g of peramivir to 150 mL of 1 mol / L sodium hydroxide solution, raise the temperature to 70°C and stir for 4 hours, stop stirring to obtain a reaction solution; add 250 mL of 1 mol / L hydrochloric acid to adjust the pH to about 3-4, a large amount of solids are precipitated, pump Filter to obtain peramivir impurity M. (Yield 78.1%, HPLC purity: 99.856%, see figure 1 ; [M+H]+ peak 330.47, see figure 2 ; HNMR See image 3 ).
[0033] image 3 HNMR analysis is as follows: 1HNMR (500MHz, DMSO) δ (ppm) 0.829 ~ 0.858 (m, 1H, -CH), 0.83 ~ 0.86 (m, 6H, -CH 3 ),1.092~1.24or1.009~1.032 (m,2H,-CH 2 ),1.24~1.30or0.90~1.04(m,2H,-CH 2 ), 1.88~1.92 (m,1H,-CH), 2.22~2.27&1.46~1.52(m,2H,-CH 2 ), 2.45~2.47(m,1H,-CH), 4.09~4.10(s,1H,-CH), 3.83~3.84 (s,1H,-CH), 4.14~4.17(s,1H,-CH).
[0034] figure 1 The specific situation of the HPLC collection of illustrative plates is as table 2:
[0035] Table 2
[0036] name Peak time (min) area content(%) Peak width (...
Embodiment 2
[0038] Add 5 g of peramivir to 150 mL of 2 mol / L sodium hydroxide solution, raise the temperature to 80°C and stir for 6 hours, stop stirring to obtain a reaction solution; add 400 mL of 1 mol / L hydrochloric acid to adjust the pH to about 3-4, a large amount of solids are precipitated, pump Filter to obtain peramivir impurity M. (Yield 70.1%, HPLC purity: 94.8%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com