Breviscapine injection liquid and its preparing process
A technology of breviscapine and injection, which is applied in the fields of blood diseases, medical formulas, and extracellular fluid diseases, etc. It can solve problems such as inconvenient use, secondary pollution, and high production conditions, so as to avoid secondary pollution and solve stability problems , the effect of ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
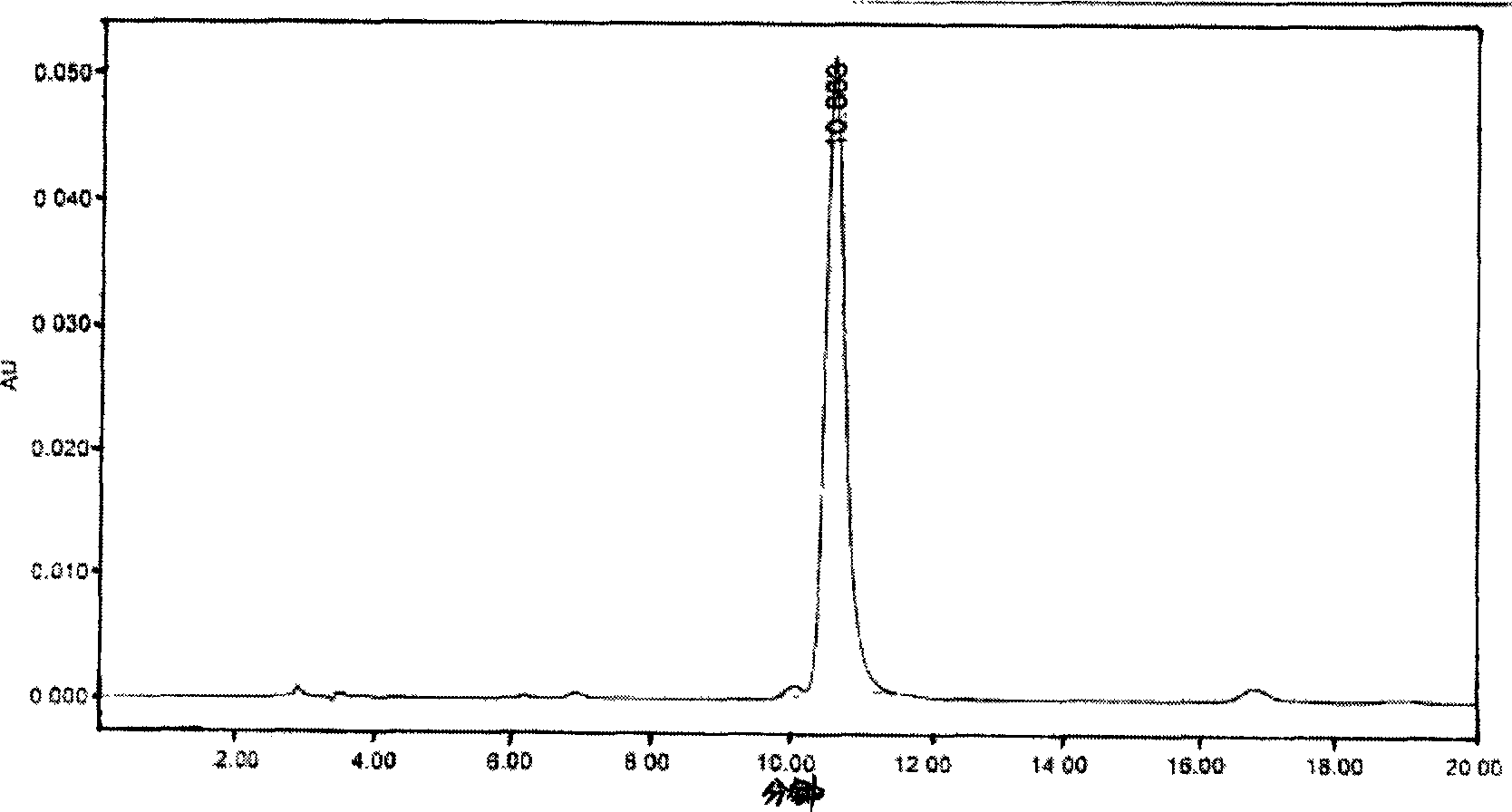
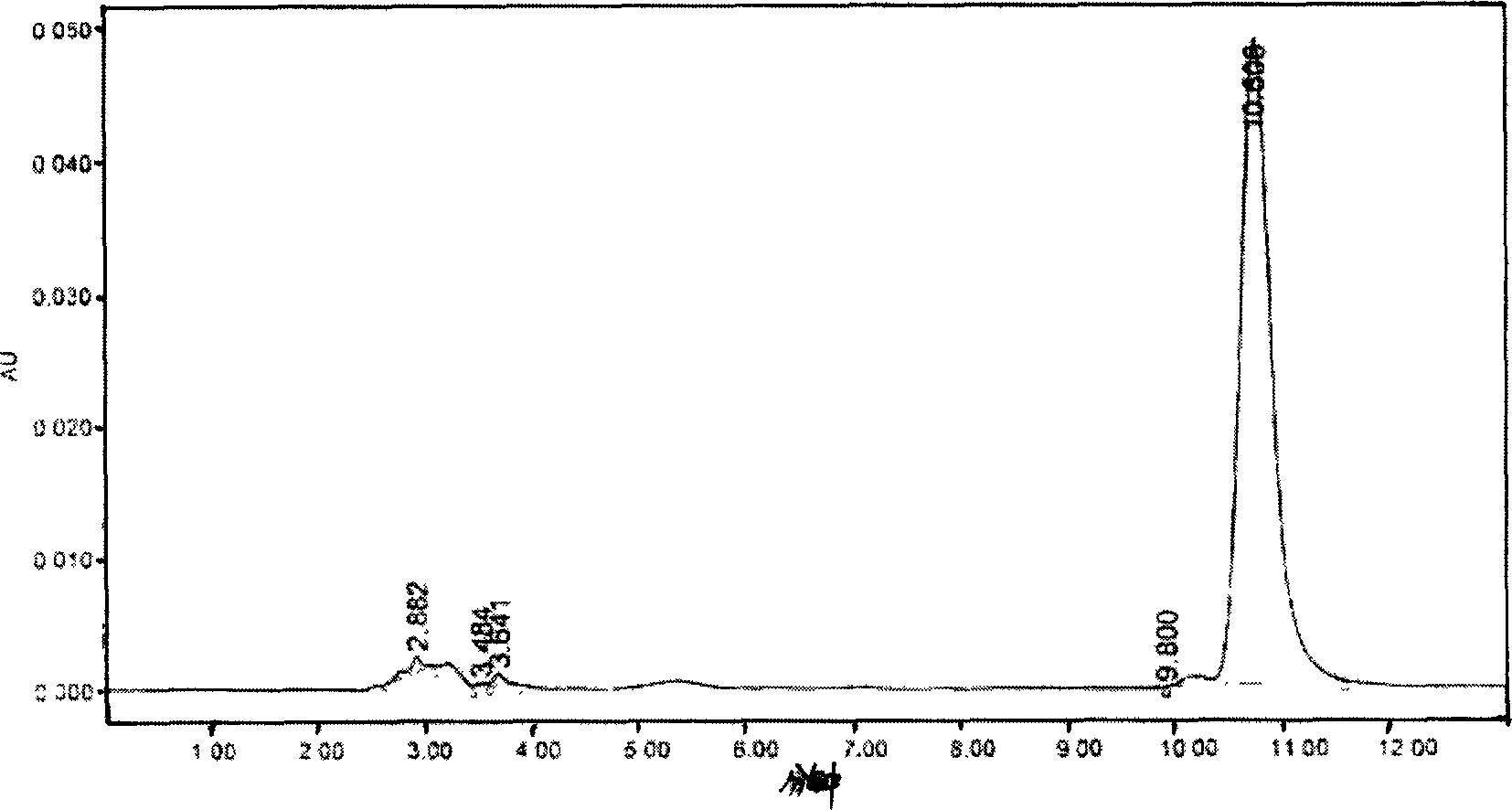
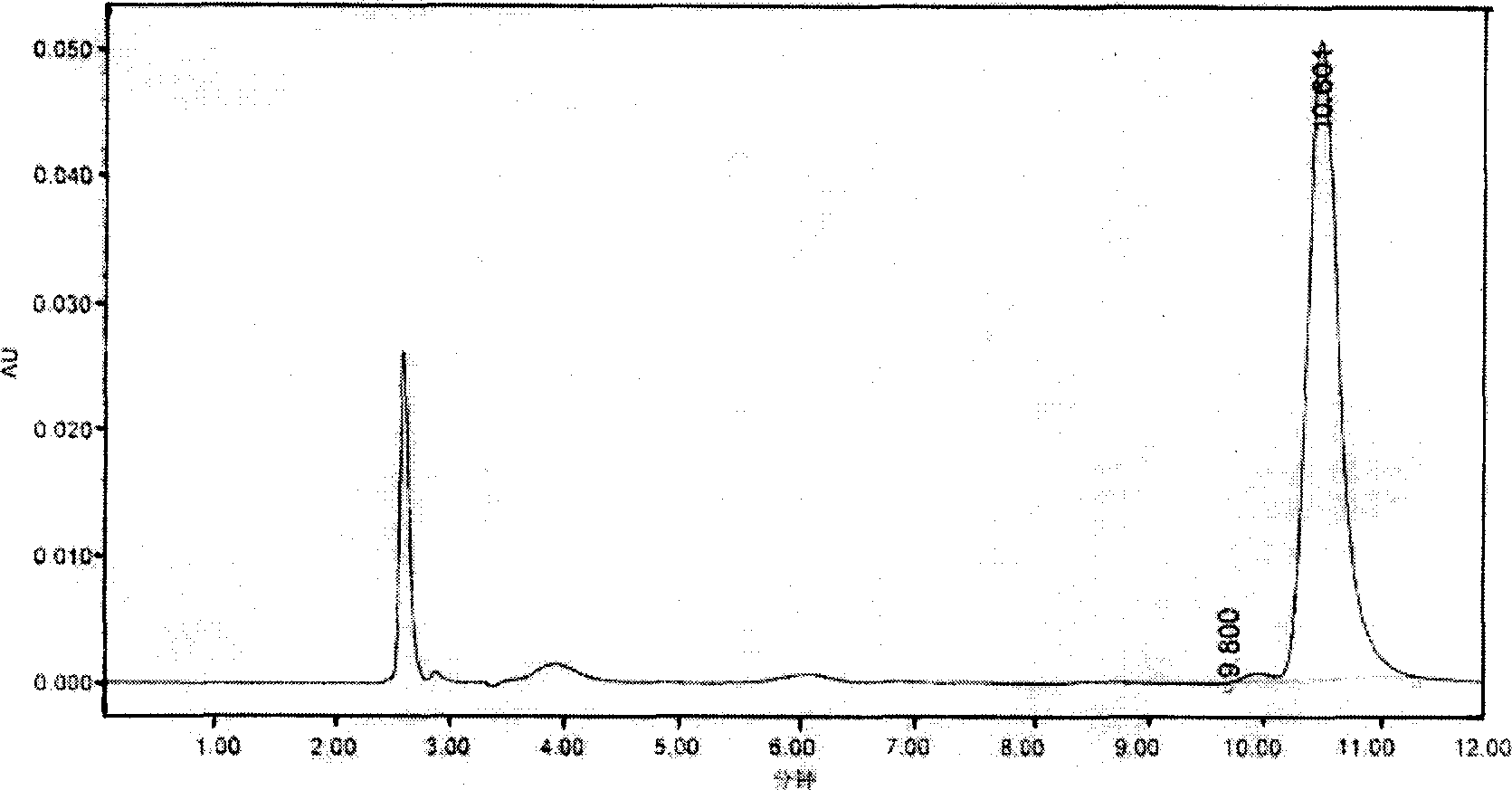
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of Breviscapine Injection
[0042] prescription:
[0043] Breviscapine 60g
[0044] Na 2 S 2 o 3 100g
[0045] EDTANa-Ca 30g
[0046] Glucose 5kg
[0047] Activated carbon for needles 20g
[0048] Make up to 100L with water for injection.
[0049] Process:
[0050] Na 2 S 2 o 3 , EDTANa-Ca was dissolved in 90L water for injection; add the raw materials, and add an appropriate amount of 1N NaOH dropwise while stirring to make it completely dissolved. Add glucose, stir well; add water for injection to nearly 100L, use 10% NaHCO 3 Adjust the pH to 7.8, add activated carbon for needles, stir, stand for half an hour, decarburize, and set the volume to 100L; fine filter, fill with nitrogen and seal, 100mL per bottle; sterilize at 100°C for half an hour to obtain the lamp Florin Injection.
Embodiment 2
[0051] Example 2: Preparation of Breviscapine Injection
[0052] prescription:
[0053] Breviscapine 80g
[0054] Na 2 S 2 o 3 150g
[0055] EDTANa-Ca 50g
[0056] Glucose 8kg
[0057] Activated carbon for needles 30g
[0058] Make up to 100L with water for injection.
[0059] Process:
[0060] Na 2 S 2 o 3 , EDTANa-Ca was dissolved in 90L water for injection; add the raw materials, and add an appropriate amount of 1N NaOH dropwise while stirring to make it completely dissolved. Add glucose, stir well; add water for injection to nearly 100L, use 10% NaHCO 3 Adjust the pH to 8.0, add activated carbon for needles, stir, stand for half an hour, decarburize, and set the volume to 100L; fine filter, fill with nitrogen and seal, 100mL per bottle; sterilize at 100°C for half an hour to obtain the lamp. Florin Injection.
Embodiment 3
[0061] Example 3: Preparation of Breviscapine Injection
[0062] prescription:
[0063] Breviscapine 50g
[0064] Na 2 S 2 o 3 50g
[0065] EDTANa-Ca 10g
[0066] Glucose 6kg
[0067] Activated carbon for needles 10g
[0068] Make up to 100L with water for injection.
[0069] Process:
[0070] Na 2 S 2 o 3 , EDTANa-Ca was dissolved in 90L water for injection; add the raw materials, and add an appropriate amount of 1N NaOH dropwise while stirring to make it completely dissolved. Add glucose, stir well; add water for injection to nearly 100L, use 10% NaHCO 3 Adjust the pH to 7.7, add activated carbon for needles, stir, stand for half an hour, decarburize, and set the volume to 100L; fine filter, fill with nitrogen and seal, 100mL per bottle; sterilize at 100°C for half an hour to obtain the lamp Florin Injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com