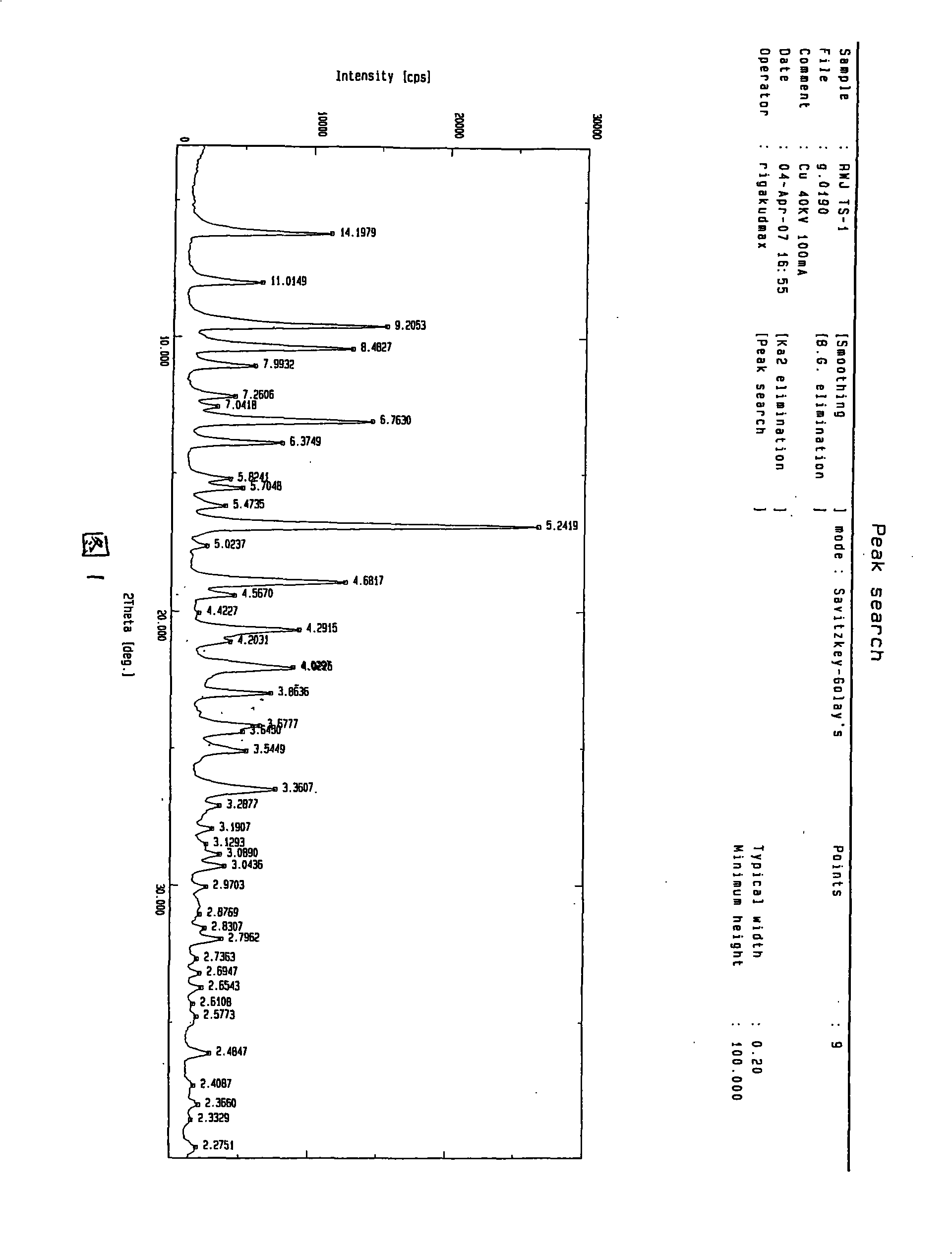
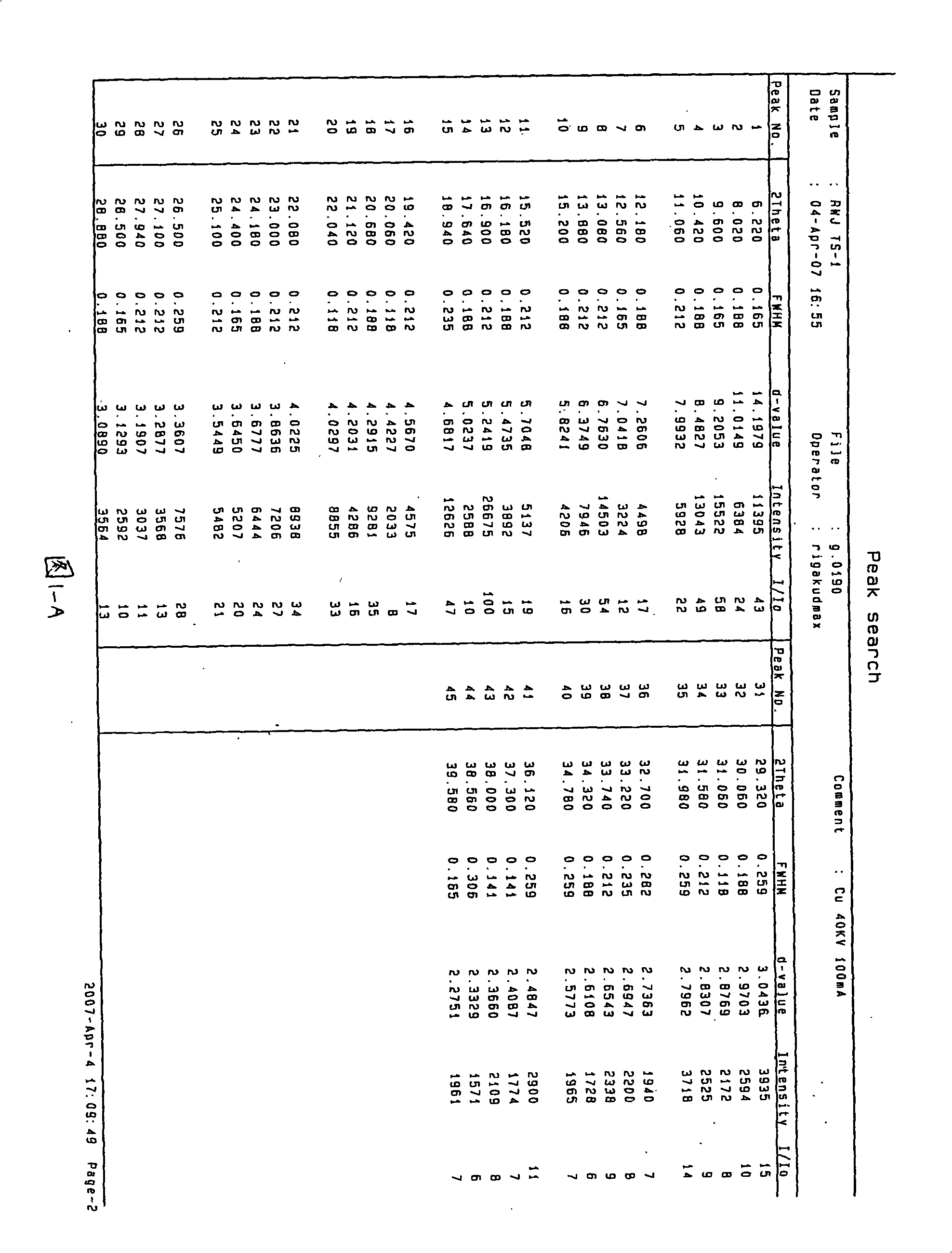
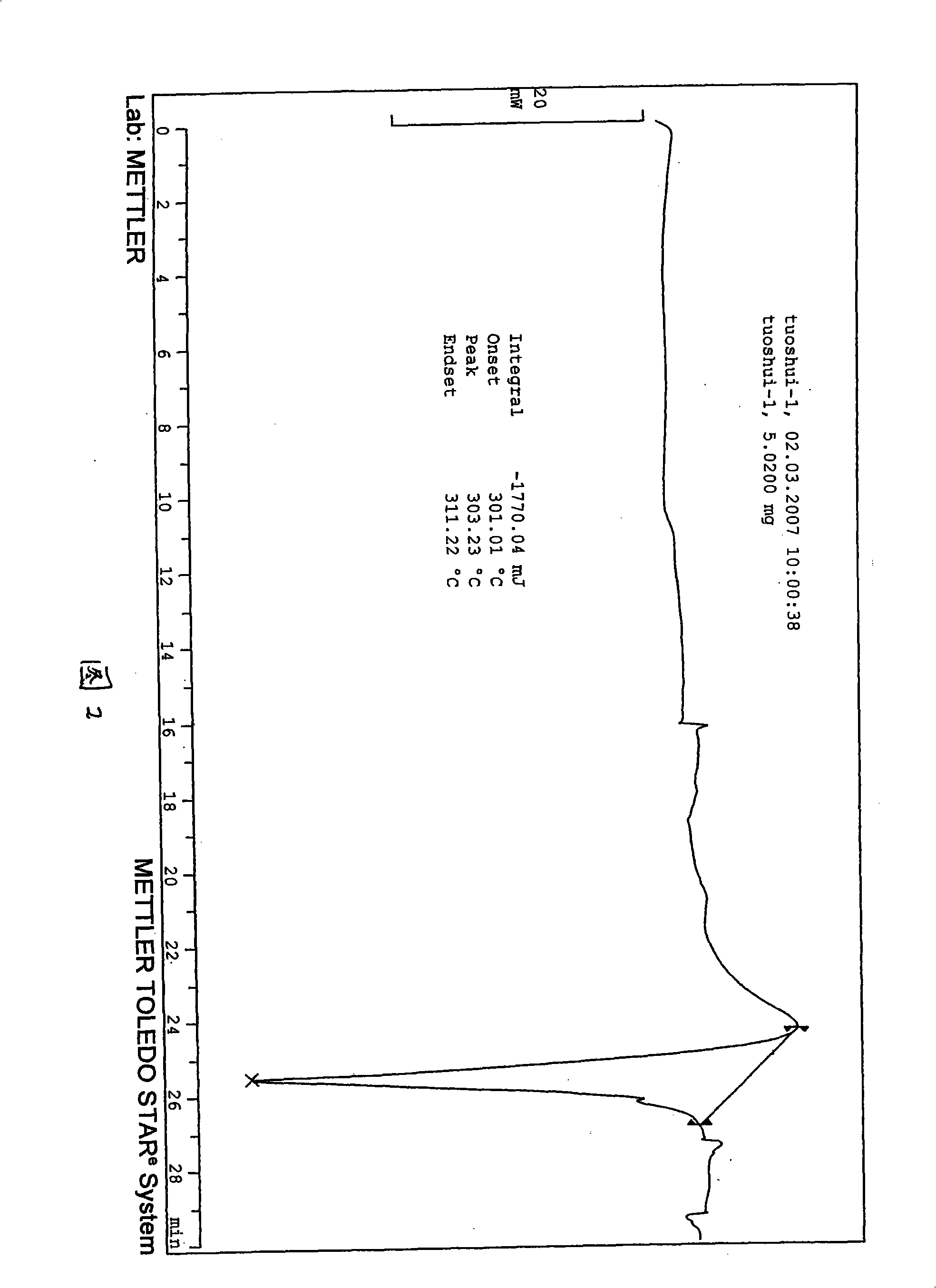
Waterless Peramivir crystal and medicament composition thereof
A technology of composition and crystal, which is applied to the crystal form of the influenza neuraminidase inhibitor anhydrous peramivir and the field of preparation of the crystal, which can solve the problems that there are no anhydrous peramivir crystals yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation of (1S, 4R) methyl 4-aminocyclopent-2-1-carboxylate tartrate
[0071] (±)-2-Azabicyclo[2.2.1]hept-5-en-3-one (100.0 g, 0.916 mol) was dissolved in methanol (83.0 g), forming a brown solution. HCl gas 37.8 g was carefully bubbled in for 20 minutes. After completion of HCl introduction, the mixture was cooled to 20 °C. Add L-tartaric acid (82.5g, 0.55mol) and water (50.0g) successively, then add triethylamine (60.0g, 0.596mol), when the pH of the solution reaches 2.0, add a small amount of L-tartaric acid (1S, 4R) Crystals of methyl 4-aminocyclopent-2-ene-1-carboxylate were used as seeds. The mixture was cooled to 22°C and filtered. The solid was washed with methanol and dried under vacuum (D 20℃ =-40.0° (c=1g / dL, H 2 O).
Embodiment 2
[0073] Preparation of (1S,4R)4-[[(1,1-methylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylic acid methyl ester
[0074] Methyl L-tartrate (1S,4R) 4-aminocyclopent-2-ene-1-carboxylate and di-tert-butyl dicarbonate (78.8 g, 0.361 mol) were suspended in 130 g of methanol. Triethylamine (80.0 g, 0.791 mol) was then added dropwise. The mixture solution was stirred for 1 hour, the solution was cooled to 5°C, and water was added. A small amount of (1S,4R) methyl 4-[[(1,1-methylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylate crystals were added as seed crystals to give a white precipitate. The mixture was stirred and filtered. The filtered solid was washed with water and dried in vacuo.
[0075] Yield: (1S,4R) 4-[[(1,1-methylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylate (79.0 g, 95.7%); mp: 51.9 -52.4°C; [α] D 20℃ =--52.0° (c=1g / dL, cHcl 3 ).
Embodiment 3
[0077] (3aR, 4R, 6S, 6aS)-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(1'-ethylpropyl)-3a,5,6,6a-tetra Preparation of 2,2-dimethylethylammonium hydrogen-4H-cyclopenta[d]isoxazole-6-carboxylate
[0078] Dissolve (1S,4R)-(-)-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopent-2-ene-1-carboxylic acid methyl ester (50 g, 0.207 mol) in Toluene and triethylamine (62.9 g, 0.622 mol), and the reaction mixture was heated to 70°C. To this solution was added 2-ethyl-N-hydroxybutyrimide chloride (93.0 g, 0.622 mol) in toluene, and a white solid precipitated. After the addition was complete, the reaction mixture was stirred for an additional 5 hours. The reaction mixture was cooled, the precipitate was removed by filtration, and the filter cake was washed with toluene. The combined filtrates were treated at 15°C with a solution of sodium hydroxide (12.4g) in water (37.3g). The reaction mixture was diluted with water, the organic phase was extracted with water and the combined aqueous phases wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com