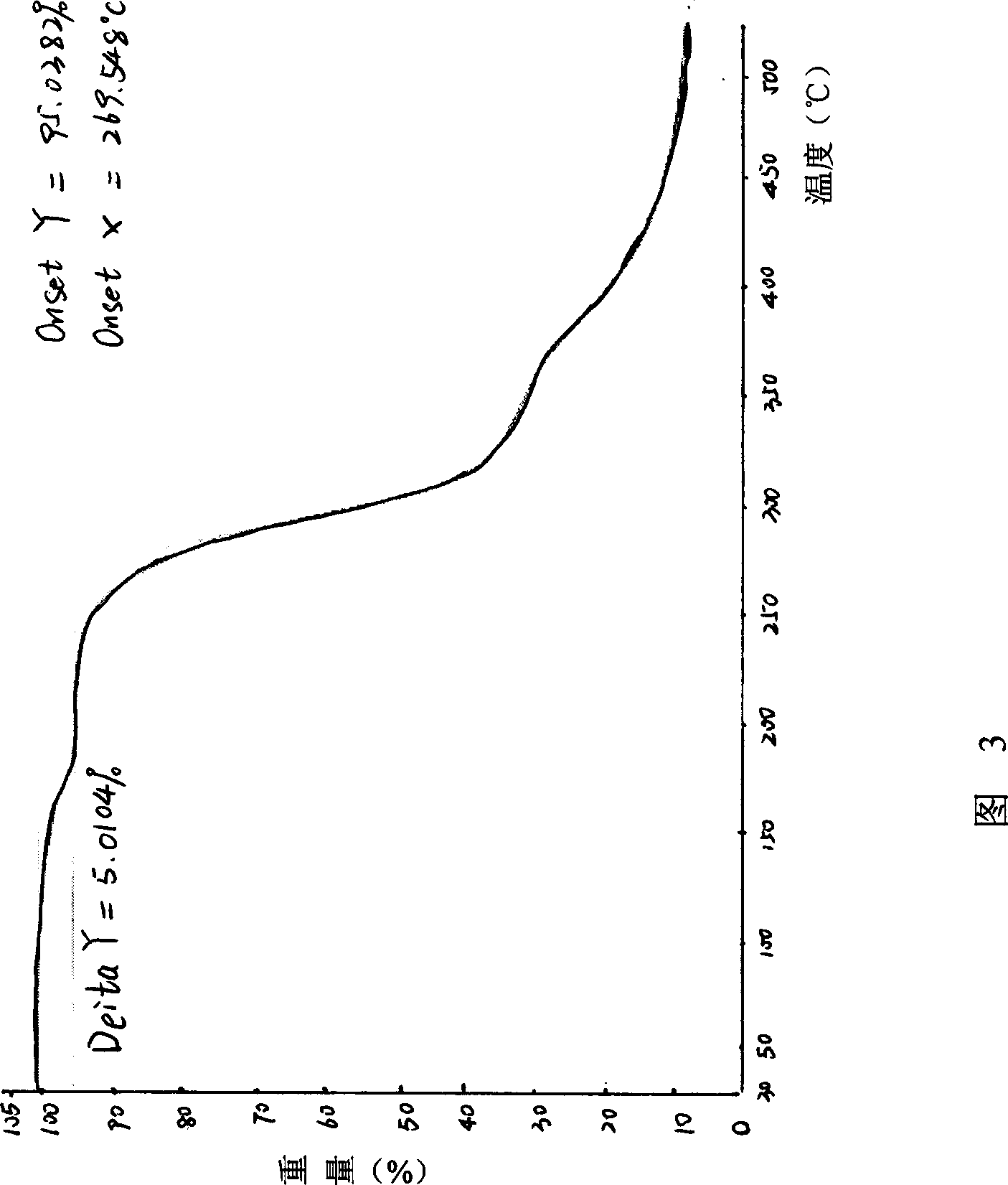
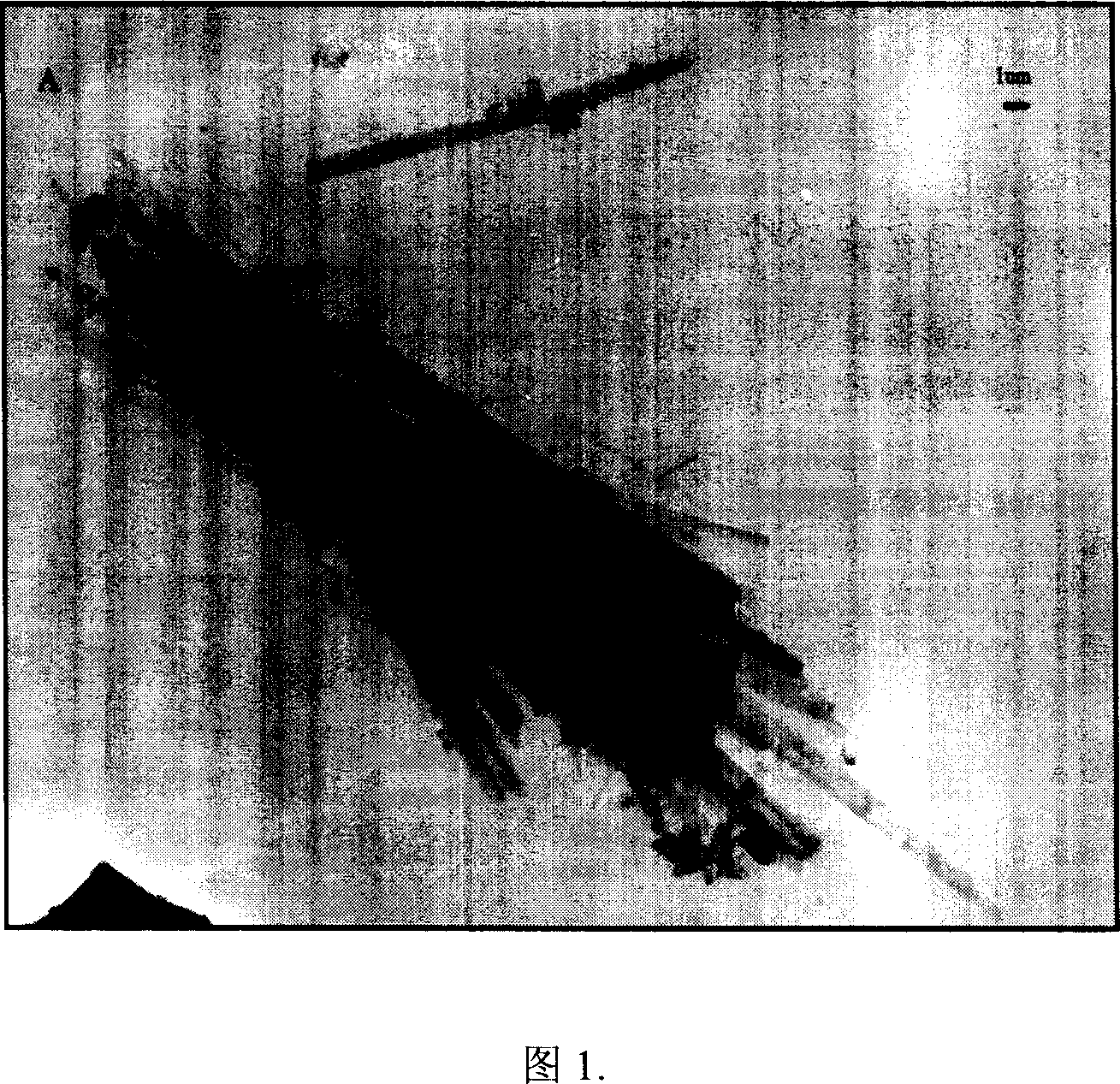
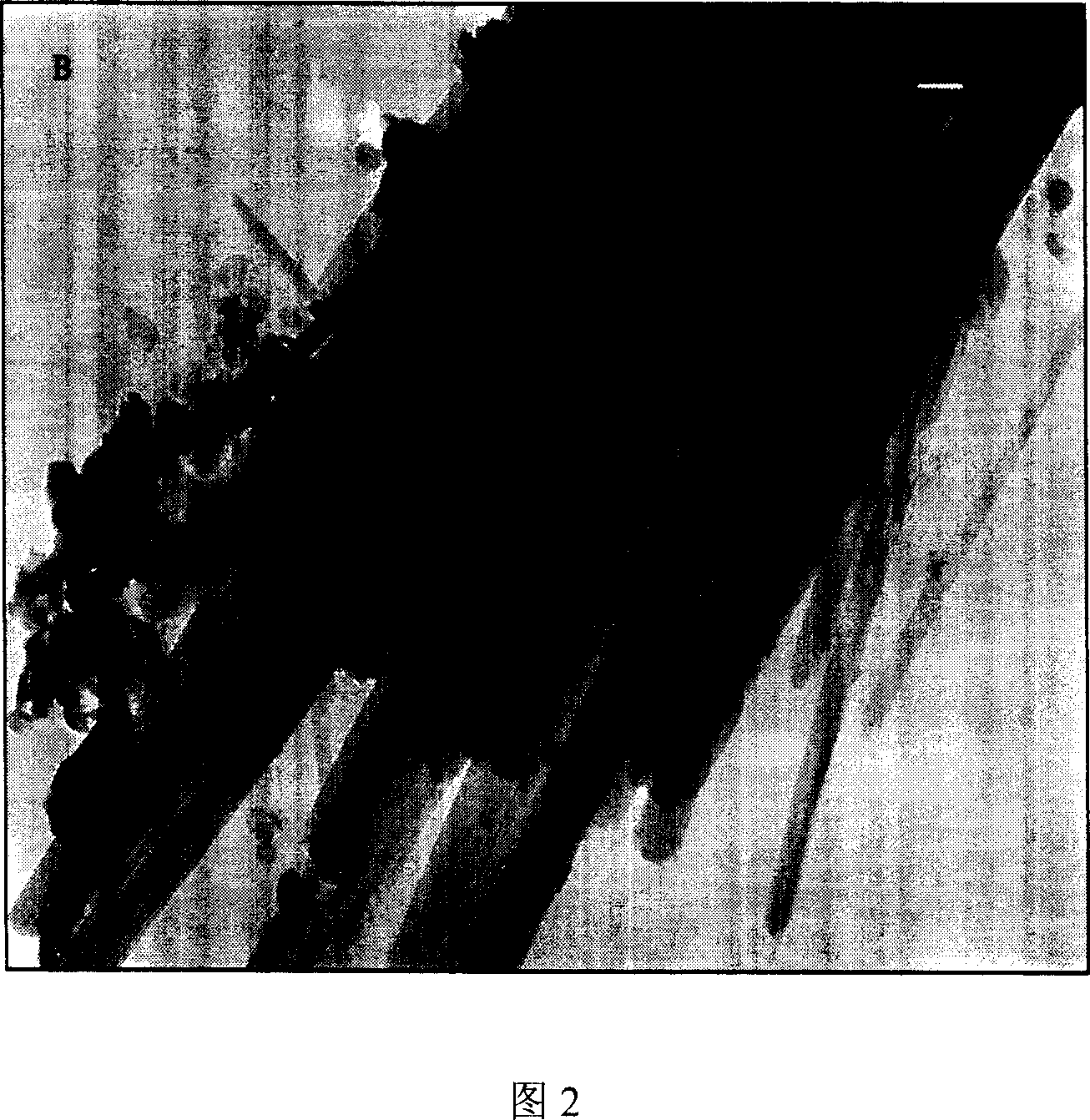
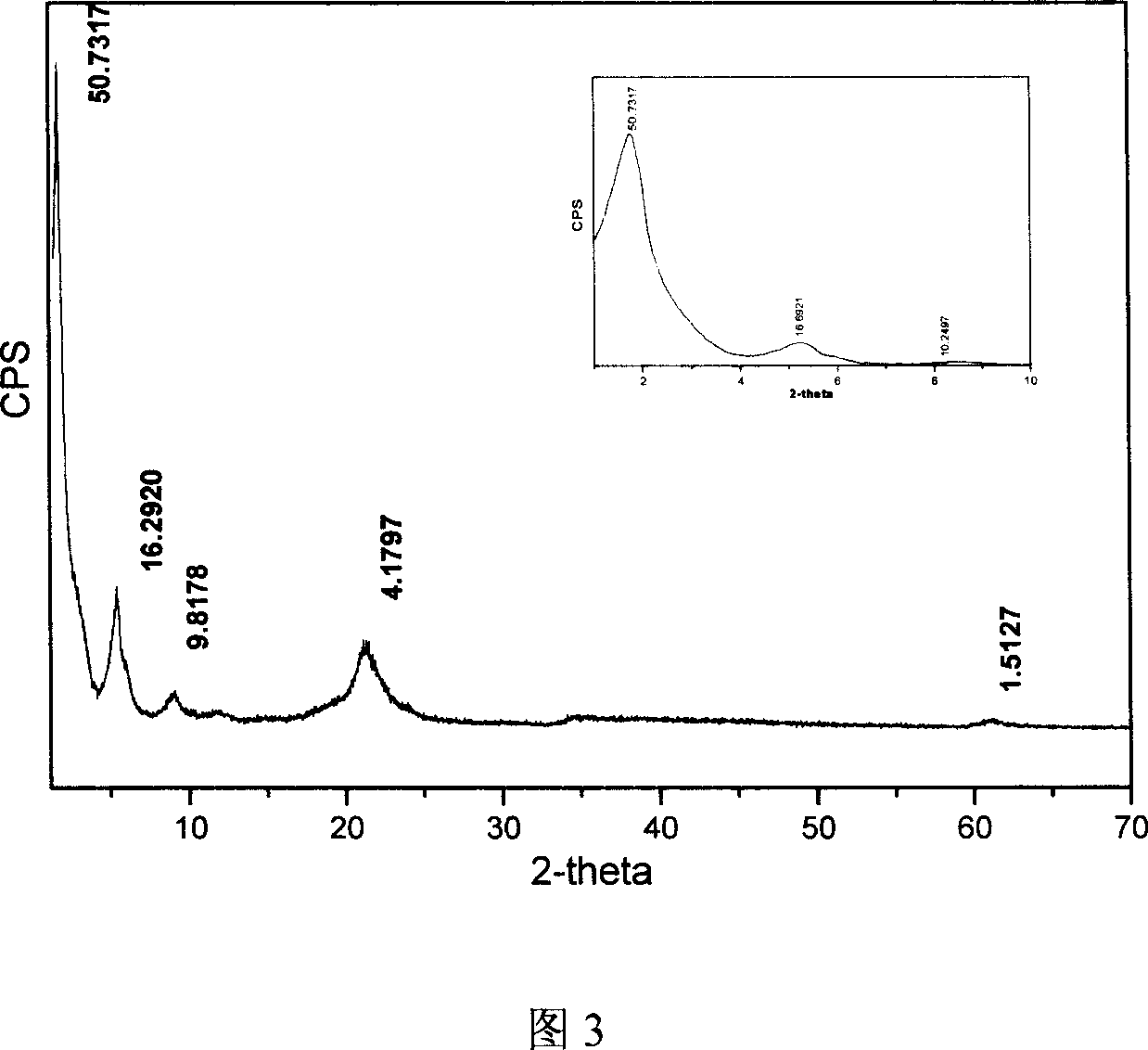
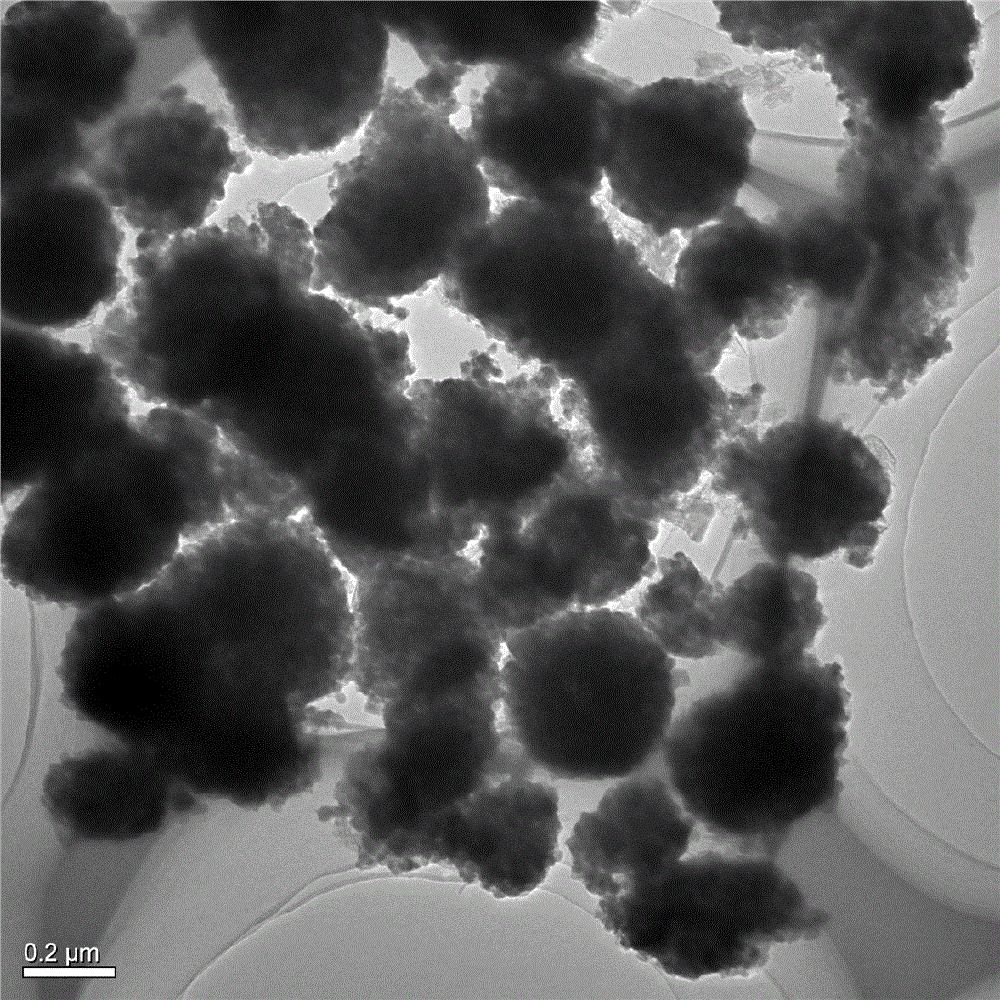
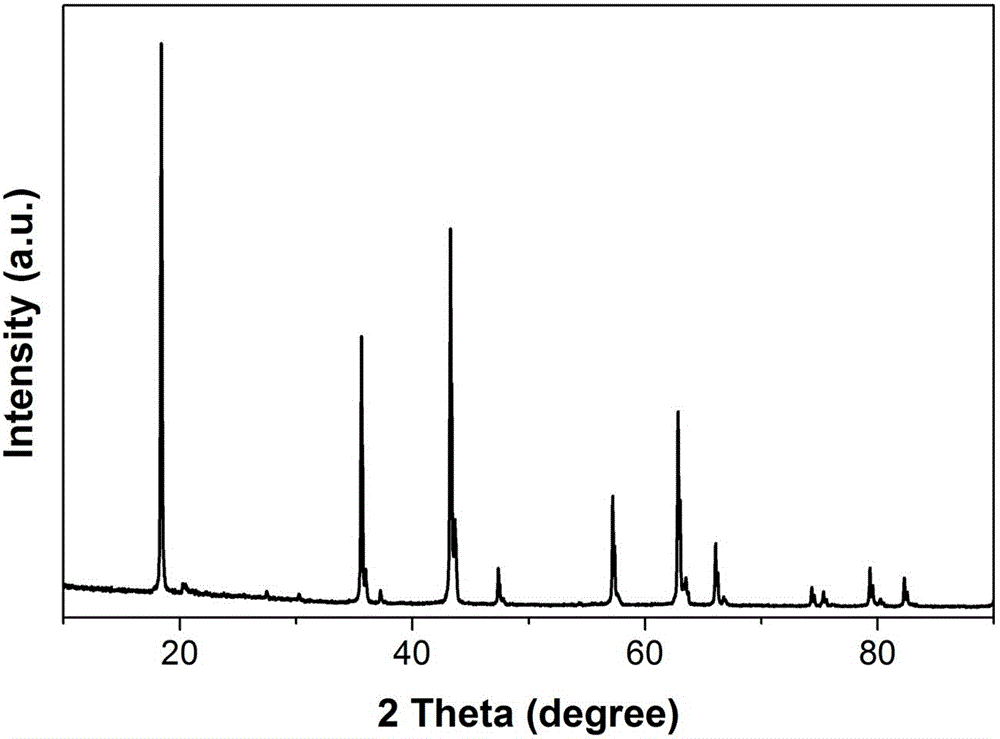
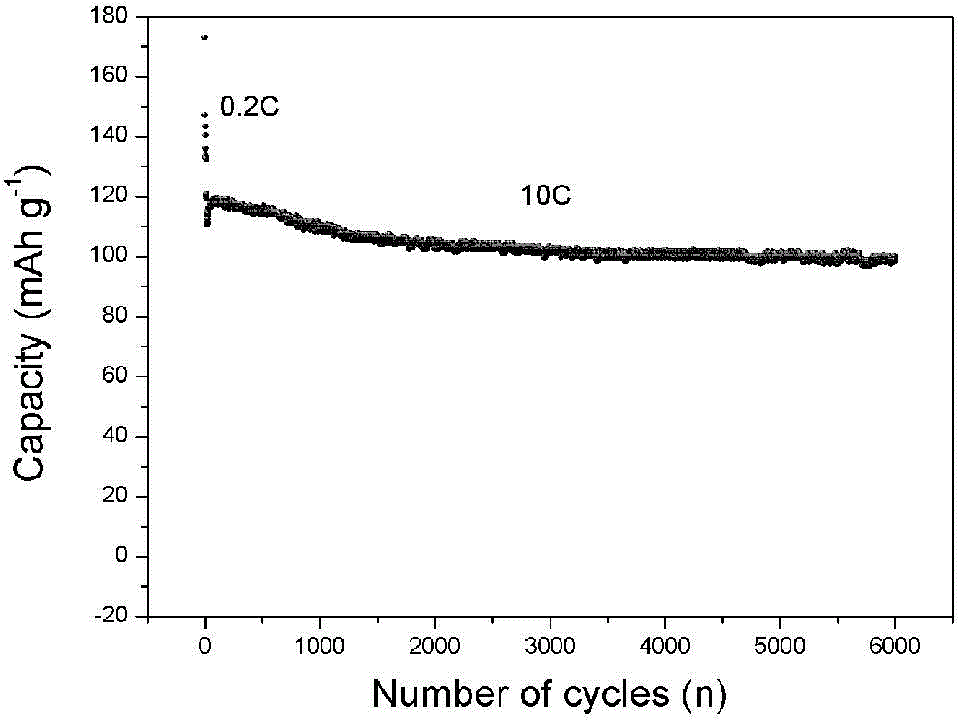
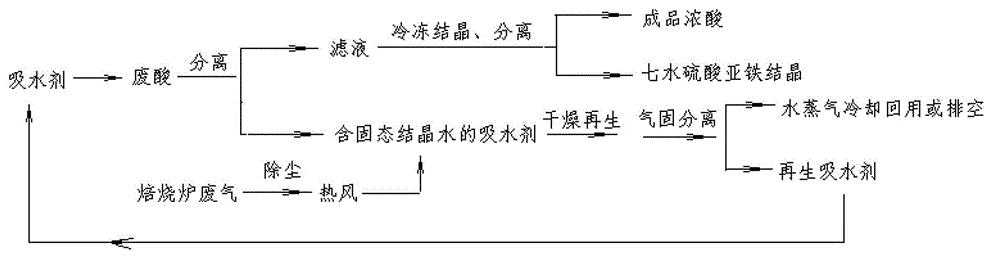
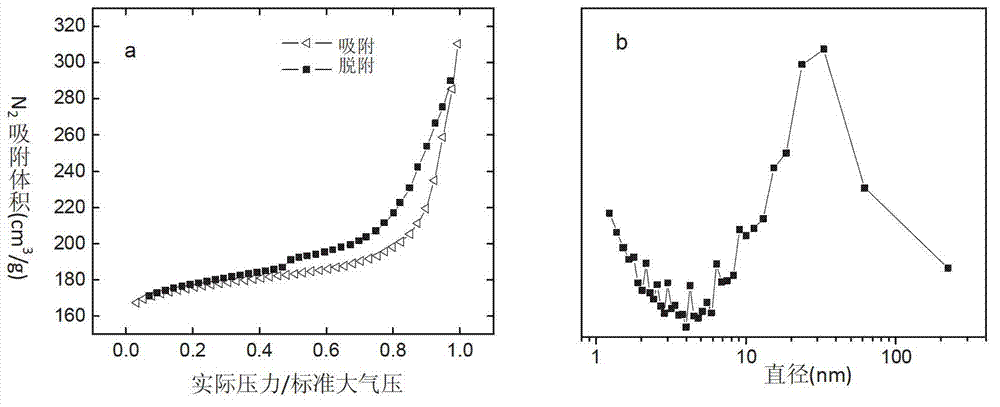
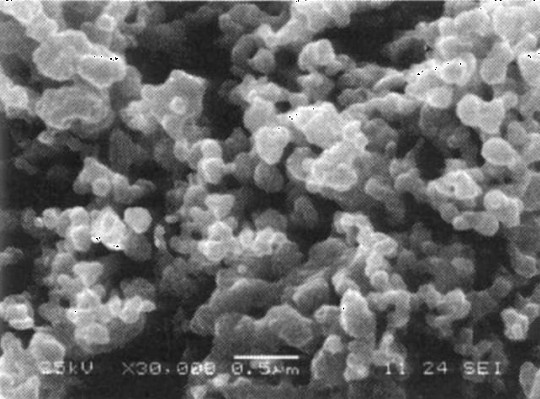
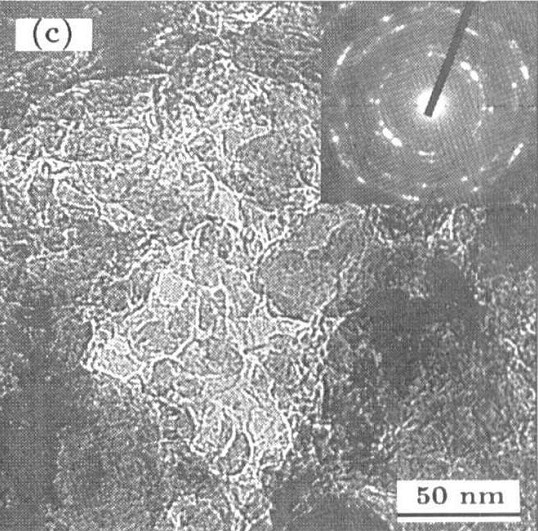
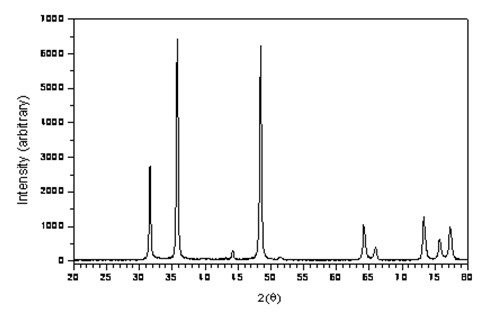
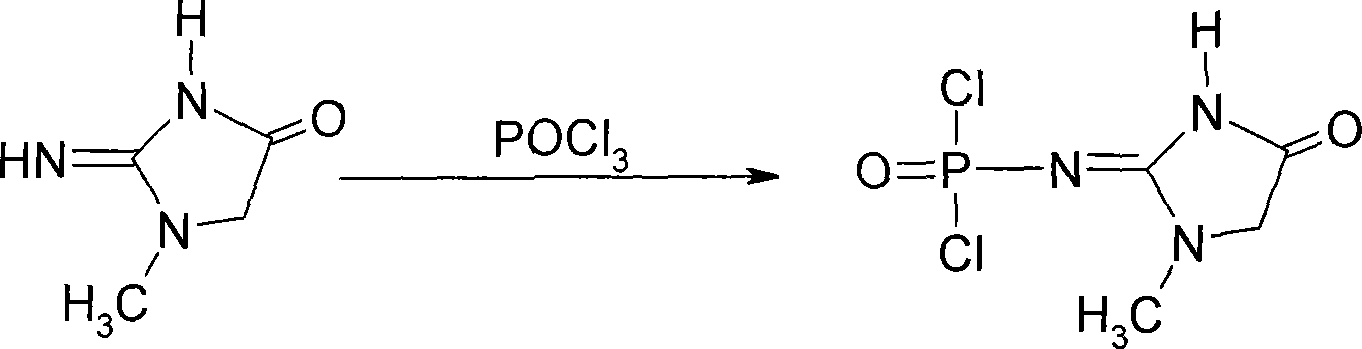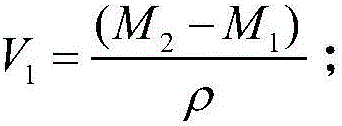Patents
Literature
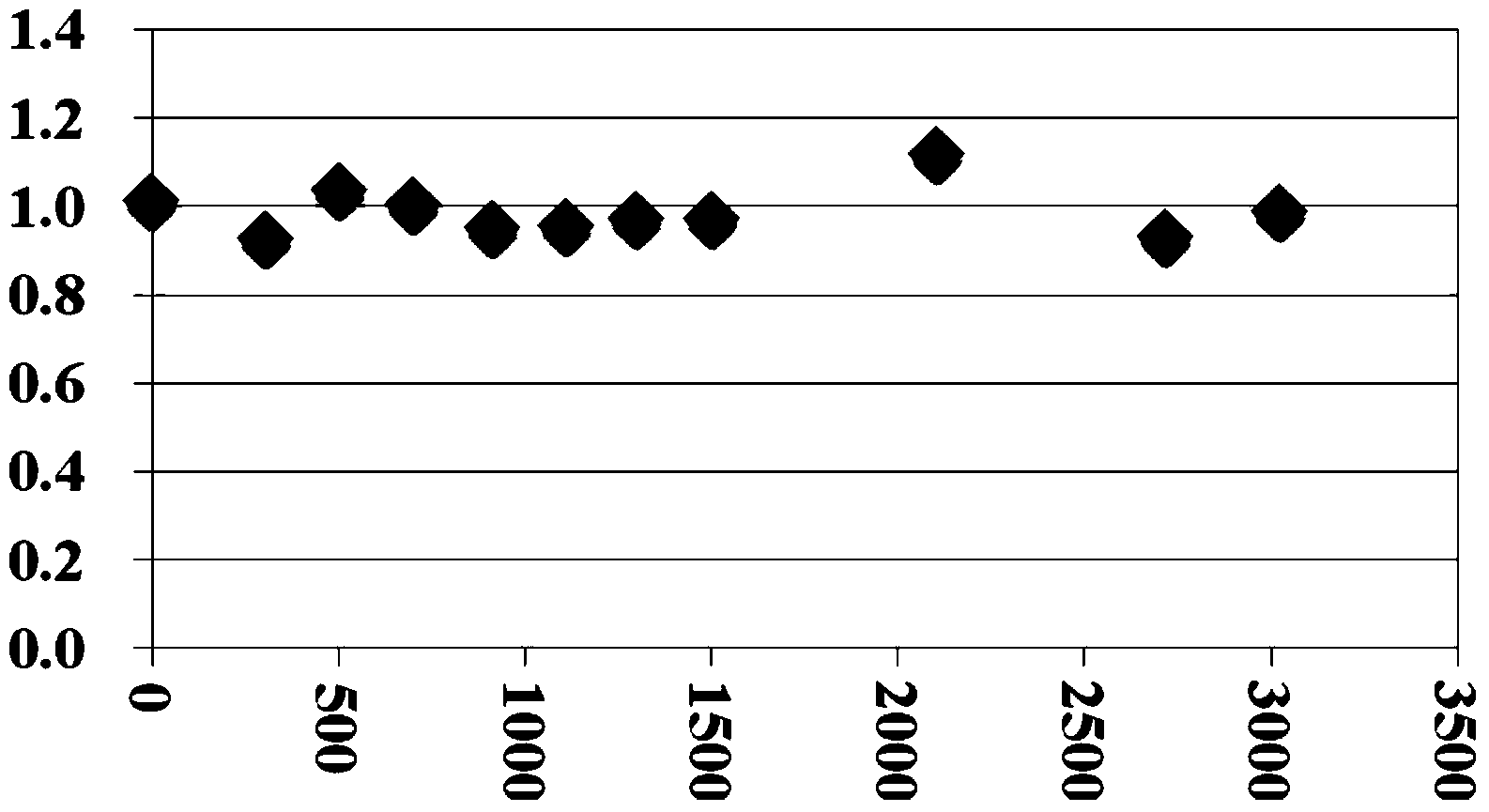
1192 results about "Water of crystallization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, water of crystallization or water of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.
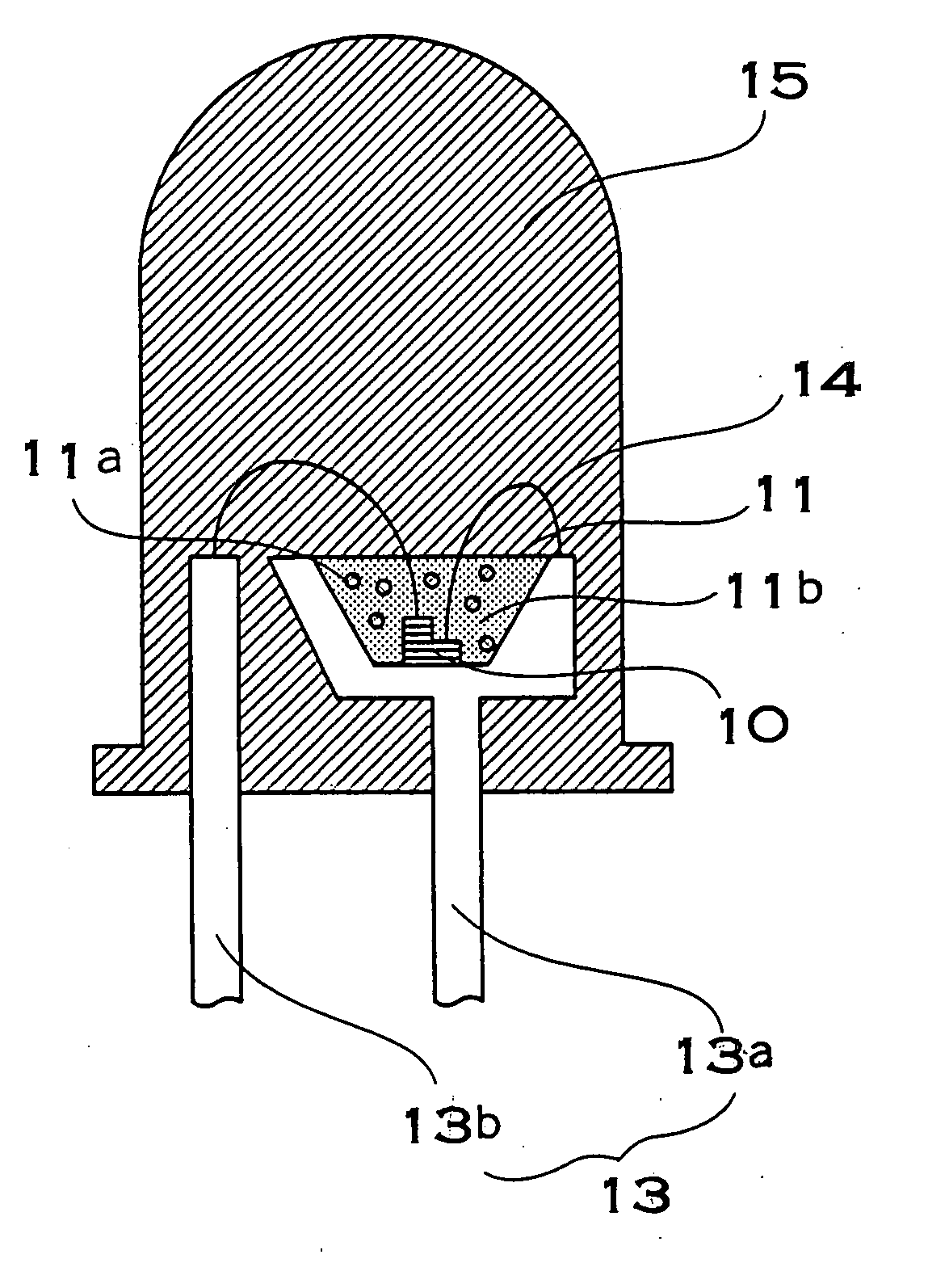
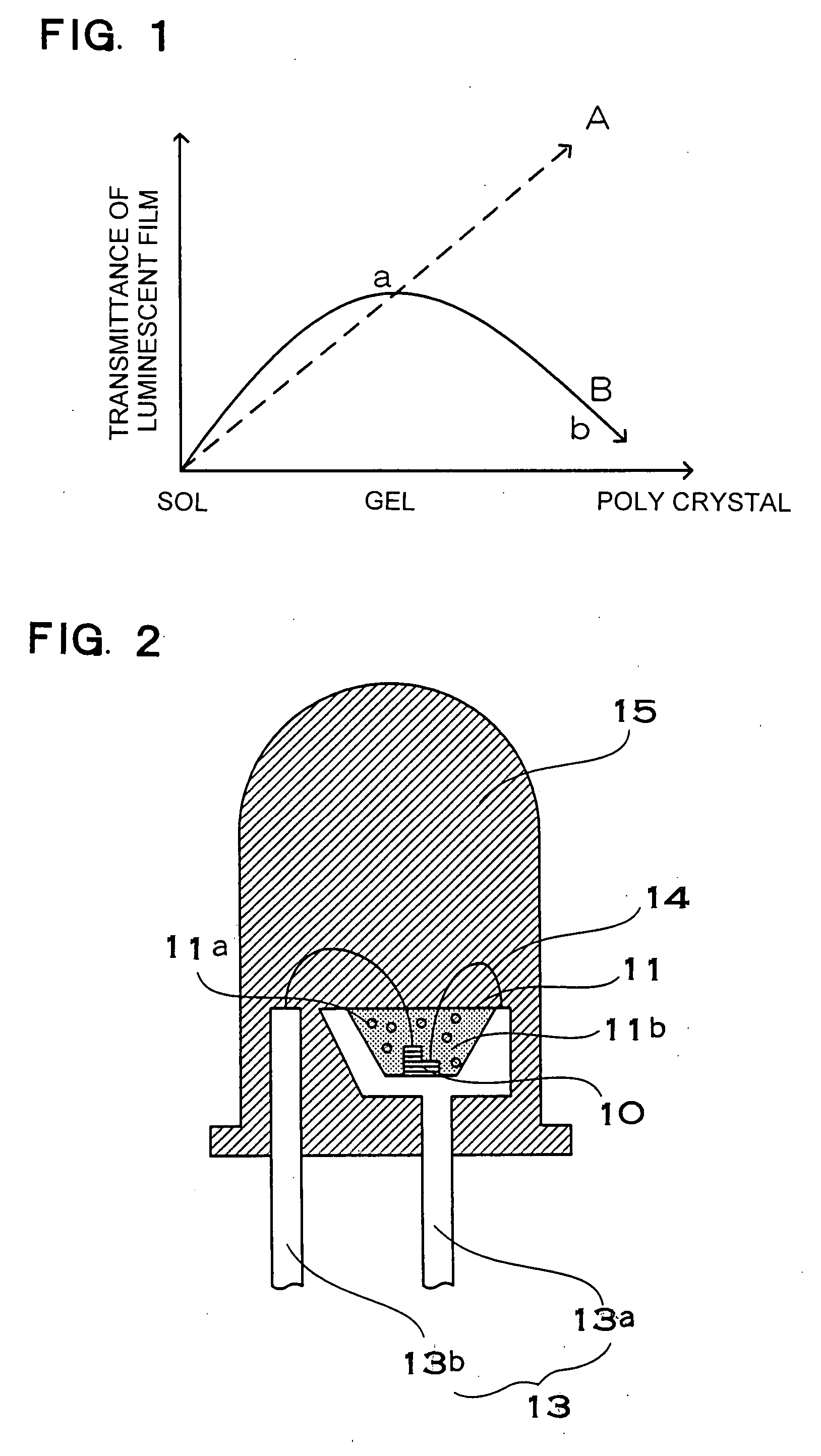
Light emitting film, luminescent device, method for manufacturing light emitting film and method for manufacturing luminescent device
InactiveUS20060170332A1High light-outgoing efficiencyHardness is easy to controlDischarge tube luminescnet screensLamp detailsPhosphorTransmittance
The present invention provides a reliable, long-life phosphor, or the like, which is prevented from darkening due to aging. A light emitting apparatus has a light emitting element and a phosphor layer. The phosphor layer has a phosphor excited by light from the light emitting element, and a binder which binds the phosphor. The binder is hydroxide oxide gel obtained by curing sol of a hydroxide oxide mixed with sol containing at least one metallic element selected from the group consisting of Al, Y, Gd, Lu, Sc, Ga, In, and B. Transmittance of hydroxide oxide in a gel state is higher than the transmittance in the polycrystal state where the sol-gel reaction is proceeded. In addition, the content of hydroxyl group or water of crystallization in the hydroxide oxide is 10% or less by weight.
Owner:NICHIA CORP
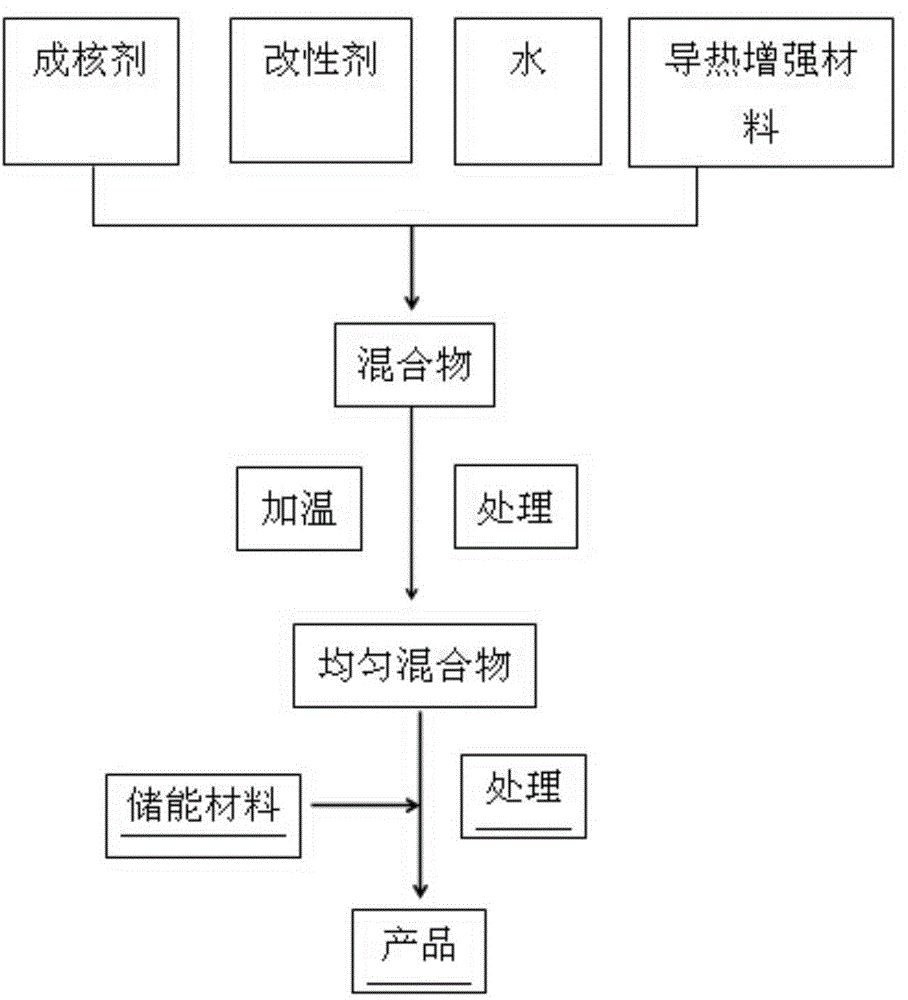
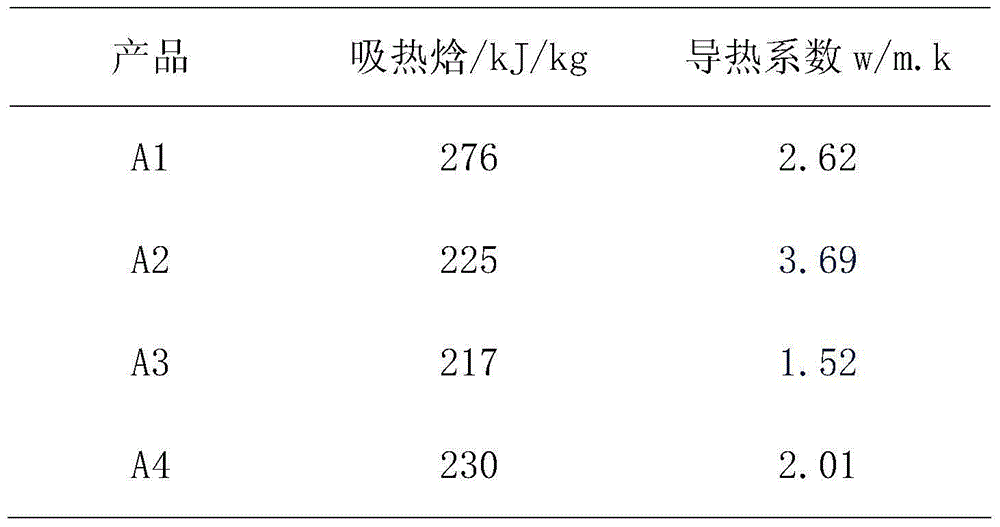
Inorganic phase change energy storage material
InactiveCN102604599AImprove liquidityPrevent Mutual AggregationHeat-exchange elementsPhase changeEnergy storage
The invention relates to the technical field of energy storage material, in particular to inorganic phase change energy storage material. The material comprises the following components by mass: 70 to 99.5 % of energy storage material, 0.25-20 % of nucleating agent, 0.1-15 % of modifying agent and 0.1-15 % of water. The bases hydrate or crystalline hydrous salt, especially the bases hydrate is adopted as energy storage material; the nucleating agent adopts carbonate; and the modifying agent is dispersant. According to the inorganic phase change energy storage material, by selecting proper nucleating agent and the ration of the nucleating agent, material phase separation can be effectively solved, especially the phase change energy storage material prepared by adding the dispersant into a system has good cycling stability, and simultaneously the potential heat value does not change after circulation for thousands of times.
Owner:SHANGHAI XUNENG NEW ENERGY TECH
Energy-saving temperature-control phase-change material
InactiveCN102827588ARaw materials are easy to getLarge latent heatScarvesHead-scarvesMaterials scienceWater of crystallization
The invention relates to an energy-saving temperature-control phase-change material. The energy-saving temperature-control phase-change material is characterized by comprising compositions in percentage by weight: 50-90% of phase-change host, 1-40% melting point control agent, 0.1-10% nucleating agent, 5-15% of crystal modifier and the balance of water. Crystallized hydrated salt phase-change material has the advantages that raw materials are easily available, the price is low, the energy density is large and the like, and the defects of being supercooling, carrying out phase separating, blocking and the like also seriously hinders the popularization and application. The phase-change host is of crystallized hydrated salt, has fixed melting point, the phase-change temperature is regulated through adding the melting point control agent so as to be capable of completing meeting the requirement of normal application. The phase-change crystal particles are fine through adding the crystal modifier, phase separation can also be prevented, attenuation is slowed down, the stability of the phase-change materials is improved, and the service life of the product is prolonged.
Owner:杨宁
Phase-change energy-storage material
InactiveCN103666381AImprove cycle stabilityRaise the ratioHeat-exchange elementsFiberSodium phosphates
The invention relates to a phase-change energy-storage material which comprises the following components in percentage by mass: 75%-99% of an energy storage main body material, 0.1%-10% of a thickening agent, 0.2%-15% of a nucleating agent and 0.01%-8% of a reinforcing material, wherein the energy storage main body material comprises crystal water and salt; the thickening agent comprises one or more of an inorganic thickening agent, fibers, polyacrylates, polyurethanes and a natural polymer thickening agent; the nucleating agent comprises one or more of sodium pyrophosphate, sodium phosphate decahydrate, calcium sulfate dihydrate, potassium sulfate, potassium borate, barium hydroxide octahydrate, sodium phosphate dibasic dodecahydrate and sodium sulfate; the reinforcing material comprises one or more of metal and metal oxide powder, carbon materials, nanoparticles and foam materials. By selecting proper materials and ratio, the supercooling degree and the phase separation phenomenon are reduced and good circulating stability is achieved; especially the reinforcing material is added into a system to form a heat-conducting network so as to improve the heat conductivity of the material by more than two times.
Owner:PIONEER ENERGY JIANGSU
Process for producing iron phosphate for producing iron lithium phosphate material
The preparation method of iron phosphate used for preparing lithium iron phosphate material in the present invention relates to heavy metal phosphate, and the steps are: dissolving analytically pure soluble iron salt in distilled water to prepare a 0.05-5M aqueous solution, and the added mass is the iron salt mass 0.01~3% of anionic surfactant, then add analytically pure phosphoric acid according to the molar ratio of Fe3+:PO43-=1:0.8~1.2 and stir evenly, and slowly add alkaline solution with a concentration of 1~9M under stirring , the feeding time is more than 1 hour, until the pH value of the solution reaches 6-7, the iron phosphate precipitate is filtered, and the filtered iron phosphate is washed 3-5 times with distilled water 2-5 times its weight. Dry at ~90°C to obtain the product FePO4·2H2O powder. The iron phosphate product with two crystal waters prepared by the method of the invention has high reactivity, and the performance of the lithium iron phosphate material made by using it is better than that of the lithium iron phosphate material made by commercially available iron phosphate products.
Owner:HEBEI UNIV OF TECH
Method for preparing high purity ferric phosphate using ferrous sulfate as by-product of white titanium pigment
InactiveCN101531355AEasy to oxidizeSolve the use problemPhosphorus compoundsLithium iron phosphateGlaze
The invention relates to a preparation method of pure ferric phosphate, characterized in that: iron vitriol, phosphorus source as by-product of white titanium pigment are used as reactant and purificant and oxidant are added into the reactant and the reaction temperature is controlled at 25-100 DEG C and the reaction time is 0.5-10 hours, thus white ferric phosphate powder (FePO[4]2-4H[2]O) with 2-4 crystal water is obtained. The technological process has features of simple process, low production cost, high product purity, increased added value of ferrous sulfate as by-product of white titanium pigment and the product can be used as ceramic metal glaze-color glaze material and raw material for producing lithium iron phosphate as lithium ionization cell anode material.
Owner:GUANGXI UNIV +1
Method for production of phosphoric acid and combined production of a-hemihydrate gypsum through dehydrate-hemihydrate wet phosphoric acid technology
ActiveCN103086335AIncrease concentrationHigh recovery rateCalcium/strontium/barium sulfatesPhosphorus compoundsO-Phosphoric AcidSulfate radicals
The invention discloses a method for the production of phosphoric acid and the combined production of a-hemihydrate gypsum through a dehydrate-hemihydrate wet phosphoric acid technology. A dehydrate part production technology is characterized in that the reaction tank temperature is 70-80DEG C, the retention time is 1.5-3h, the free sulfate radical concentration is 1-2%, and the produced wet phosphoric acid concentration omega(P2O5) is 35-39%. A hemihydrate part production technology is characterized in that the reaction tank temperature is 86-94DEG C, the retention time is 1-2h, and the mass concentration of free sulfate radicals is 6-8%. Phosphoric acid produced through the hemihydrate technology has a concentration omega(P2O5) of 10-15% and is used as a recycle acid of the dehydrate part, the crystal water content of the combined-production hemihydrate gypsum is 5-7%, the mass percentage of free P2O5 in gypsum is less than 0.4%, and the crystal form of the produced hemihydrate gypsum is a-hemihydrate gypsum. Compared with the dehydrate technology, the method disclosed in the invention improves the phosphoric acid concentration and improves the phosphorus recovery rate to above 98%, and the combined-production a-hemihydrate gypsum can be directly used as a building gypsum product, so energy consumption saving is realized.
Owner:WENGFU (GRP) CO LTD
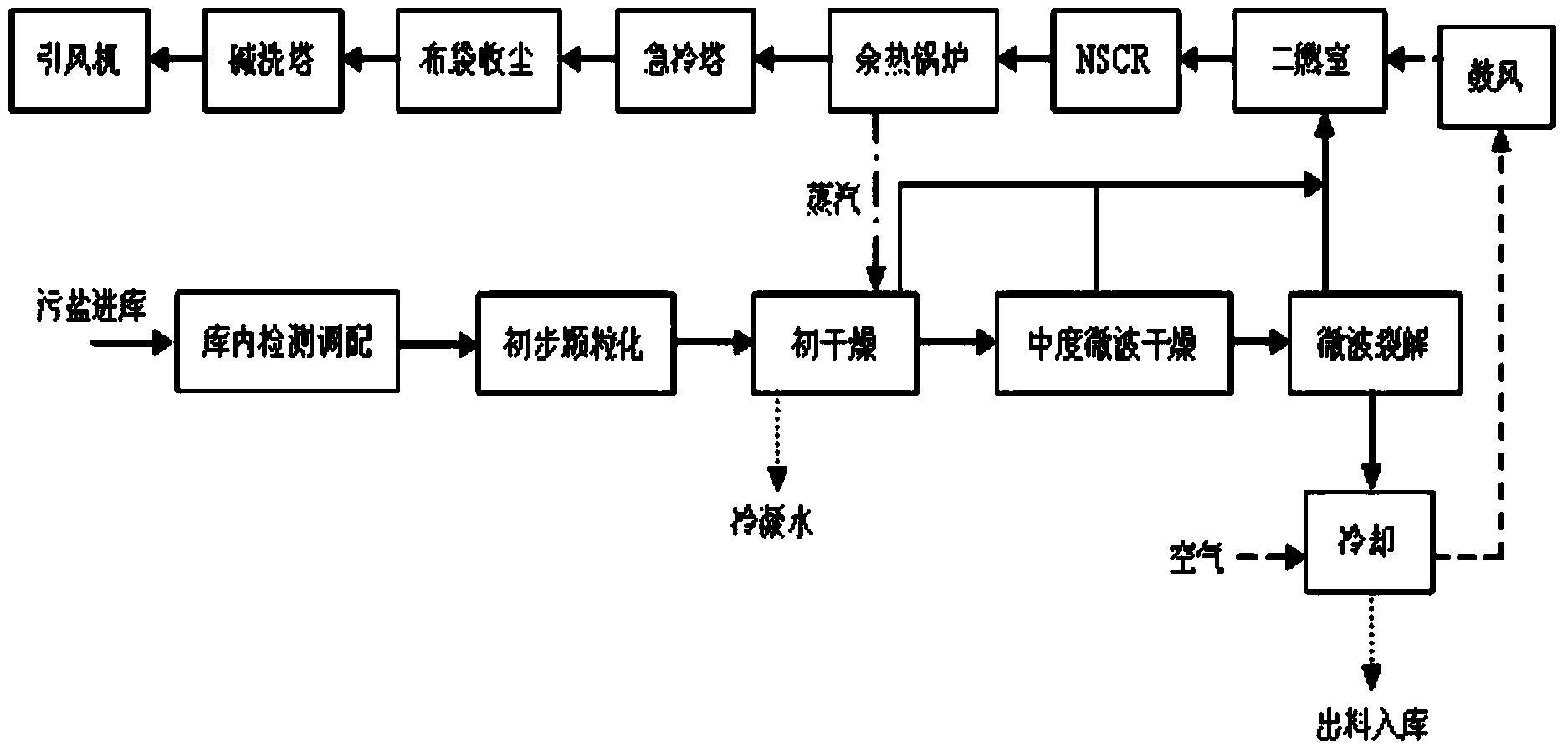
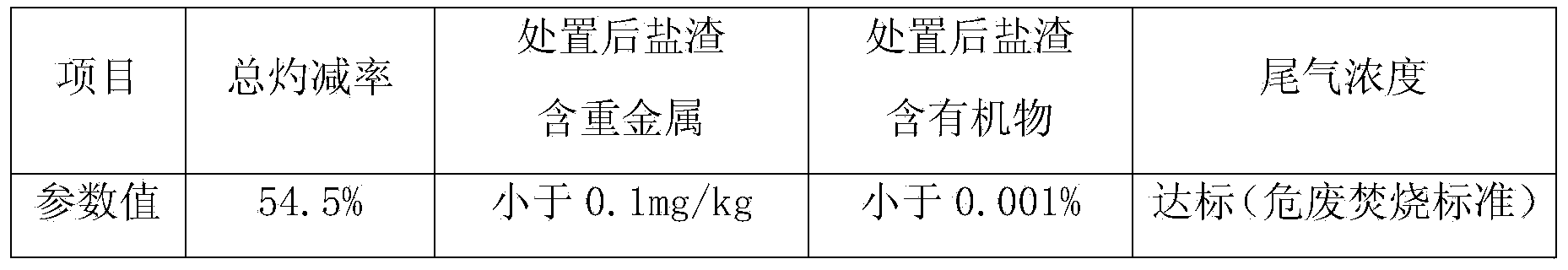
Industrial waste salt slag innocent treatment method
ActiveCN104344407AAlleviate the pressure of solid waste managementPromote sustainable developmentIncinerator apparatusSlagPetrochemical
The invention discloses an industrial waste salt slag innocent treatment method. The industrial waste salt slag innocent treatment method mainly comprises the following steps: a) burdening materials according to types of the industrial waste salt slag, and uniformly mixing the materials; 2) drying the mixture, wherein the drying process at least comprises initial drying and medium microwave drying, the initial drying process can remove the most part of free water, the medium microwave drying can remove the crystal water and gasify a part of organic matters, and the drying temperature is a continuously adjustable stable temperature gradient; c) anaerobic microwave pyrolysis: pyrolyzing the dried mixture at the temperature of 450-500 DEG C and under a nitrogen charged environment for more than 100 minutes, so as to pyrolyze the organic matters to form micromolecules to be gasified, organic salt to be split and gasified and the contained ammonium salt to be decomposed; 3) conducting air cooling to the pyrolyzed waste salt to be put in storage, and then conducting complete innocent treatment to the pyrolyzed waste salt, detecting and then selling the qualified product or reusing the treated waste salt. The industrial waste salt slag innocent treatment method has the characteristics of safety, efficiency, energy conservation and the like, has the obvious advantage in the resource recycling aspect, can greatly slow down the solid waste management stress in the industrial developed area, in particular the chemical engineering petrochemical industry park, is favorable to promote the related sustained development of the park.
Owner:ZHEJIANG DONGTIANHONG ENVIRONMENTAL PROTECTION ENG CO LTD
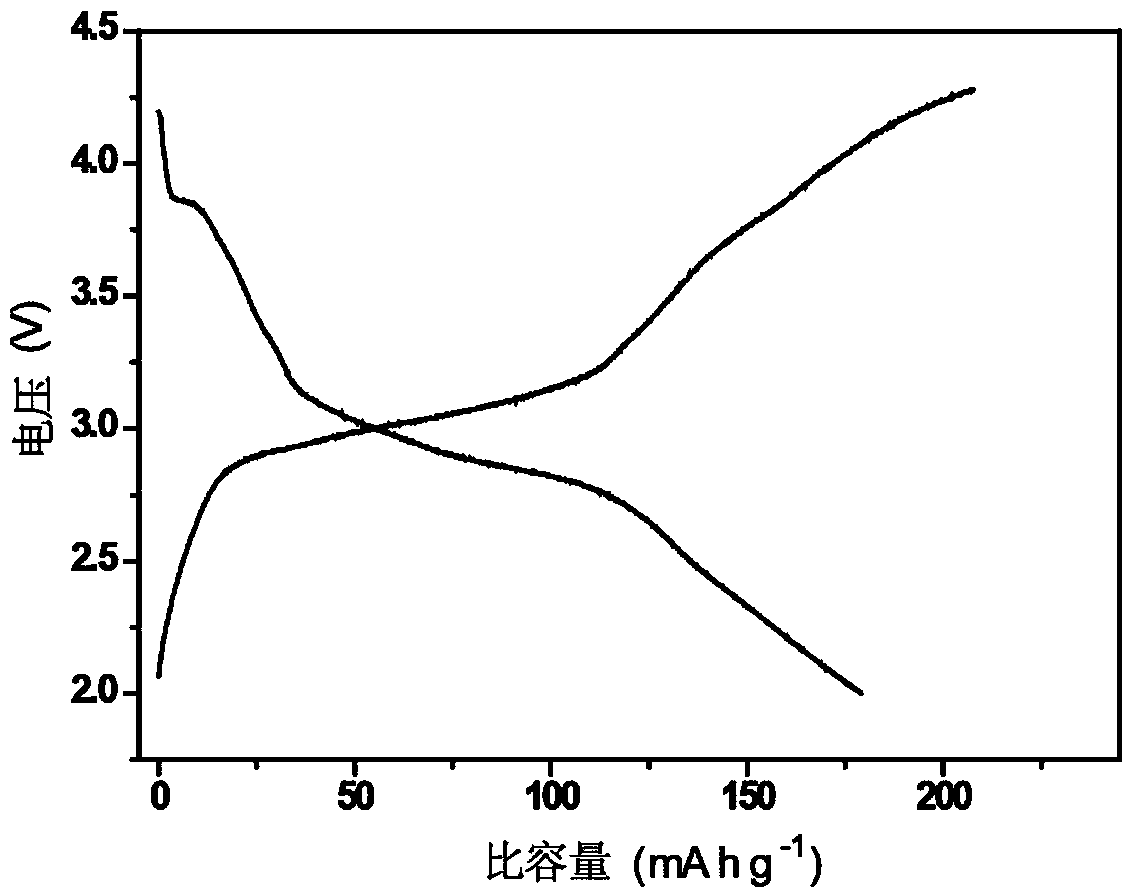
Preparation method and application of prussian blue complex/carbon composite material
ActiveCN103441241AHigh degree of practicalityPromote growthCell electrodesCarbon compositesSodium-ion battery
The invention discloses a preparation method of a prussian blue complex / carbon composite material and an application of the composite material as a positive electrode material for lithium ion and sodium ion batteries. The preparation method of the prussian blue complex / carbon composite material comprises at least the steps of uniformly dispersing a transition metal cyano complex, an inorganic acid and a carbon material in water to obtain a reaction solution; and heating the solution for a certain time to obtain the prussian blue complex / carbon composite material. The method is simple in preparation, can be controlled easily and has high practical degree. Crystal water content and coordinated water content in the obtained prussian blue complex / carbon composite material are little, so that the prussian blue complex / carbon composite material presents high capacity and excellent cycle performance when being used as the positive electrode material for the lithium ion and sodium ion batteries.
Owner:INST OF CHEM CHINESE ACAD OF SCI
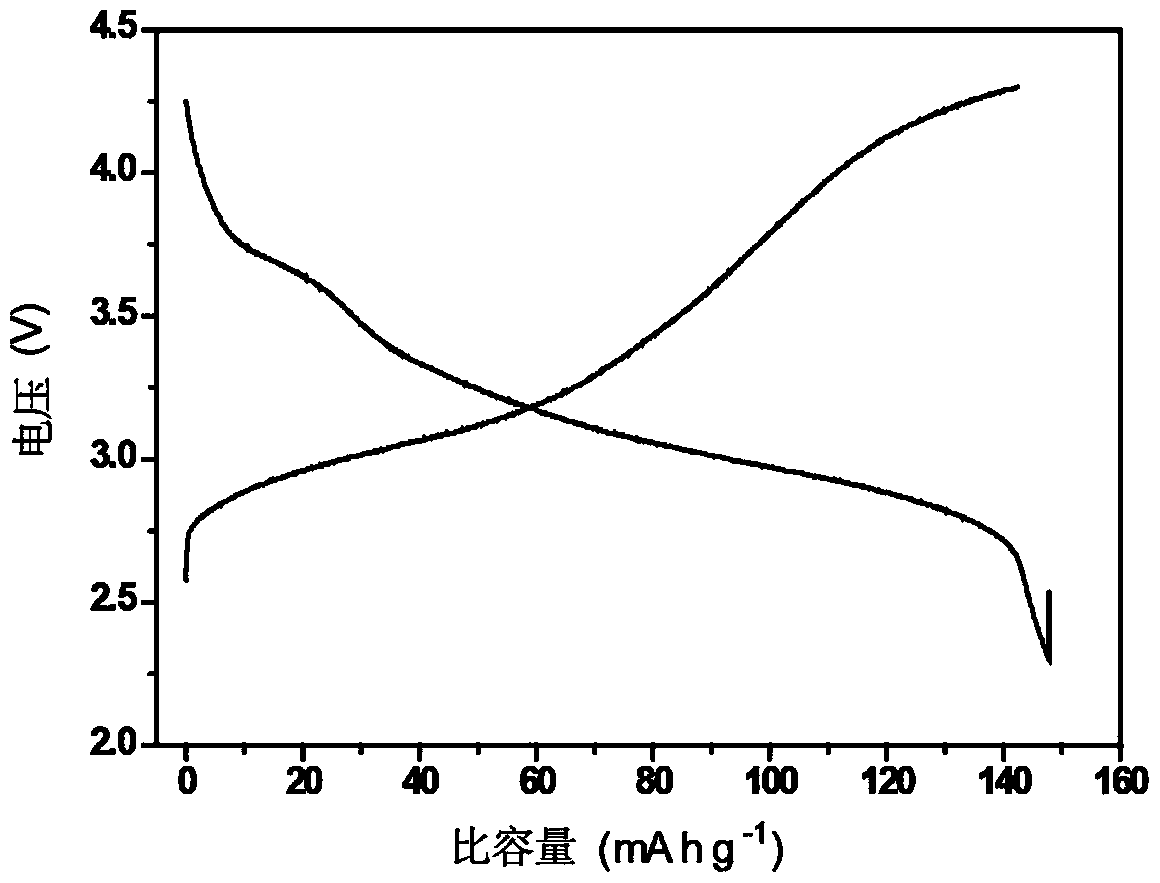
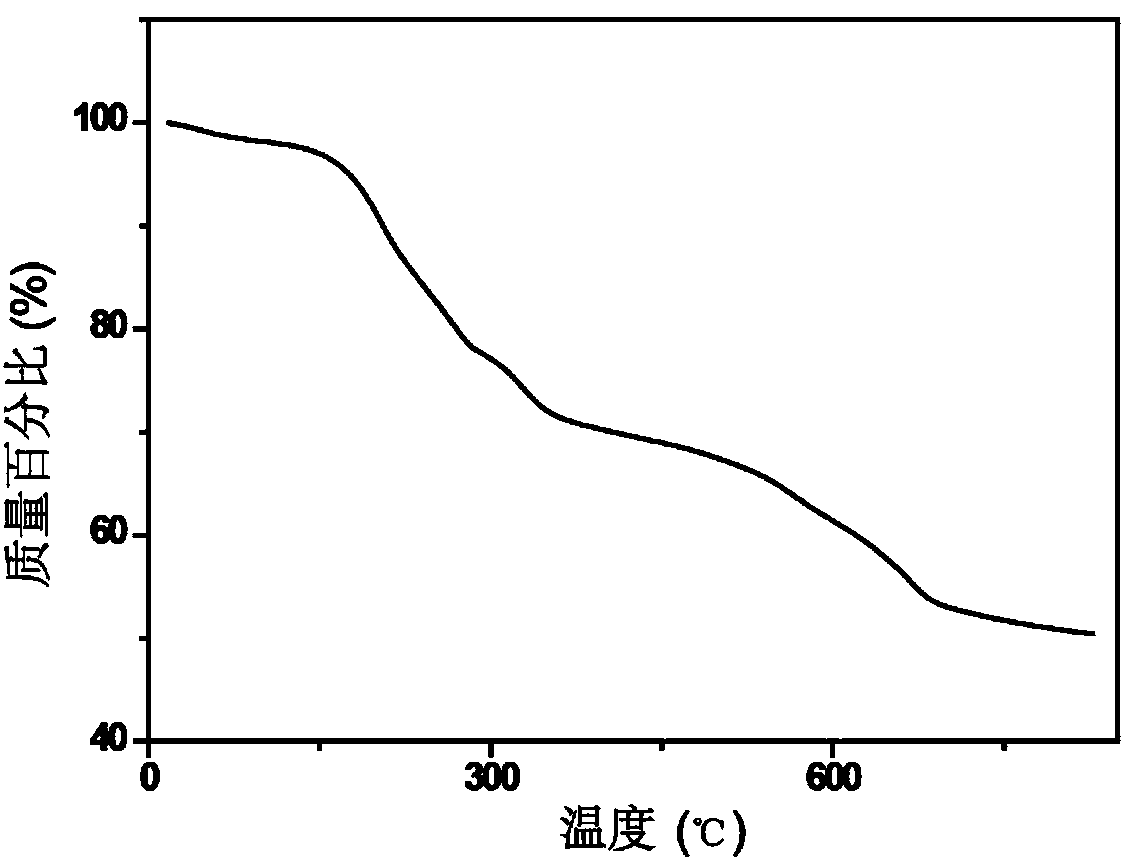
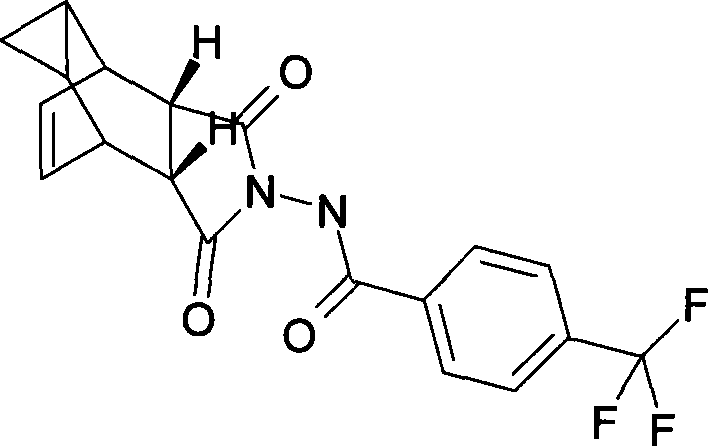
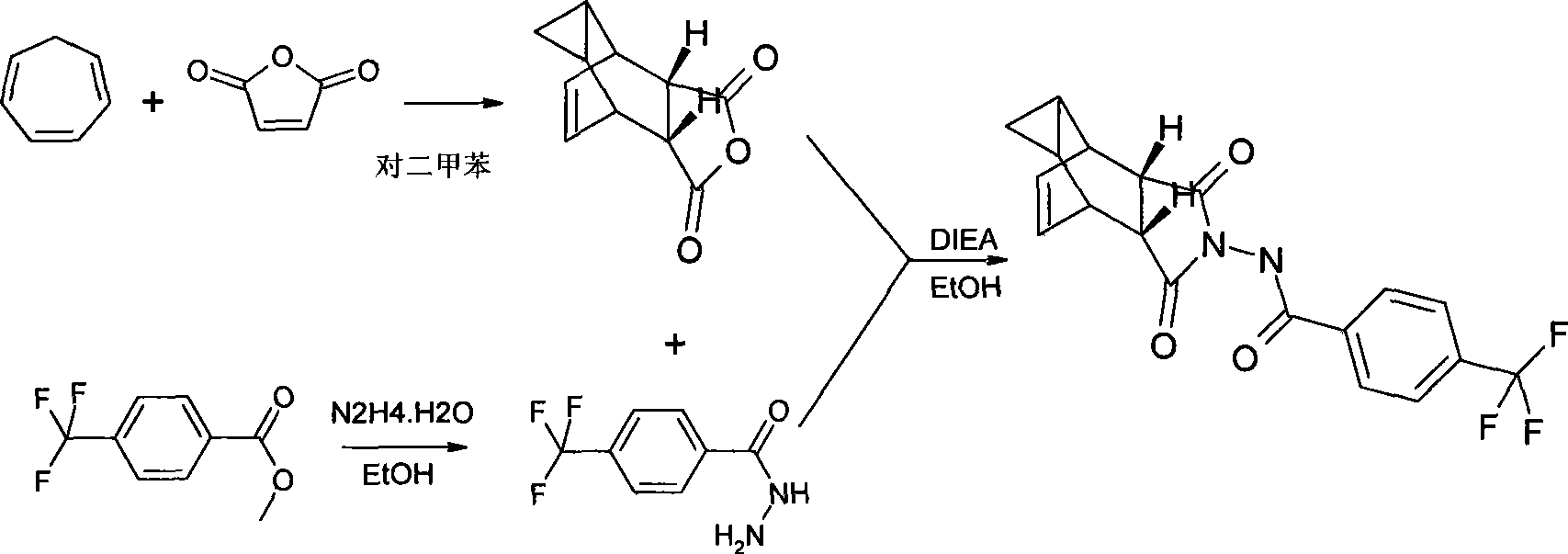
Compound ST-246 containing a crystal water, crystal thereof and preparation method thereof
ActiveCN101445478AStable structurePromote crystallizationOrganic active ingredientsOrganic chemistryOrganic baseTropane
The invention discloses a compound ST-246 containing a crystal water, known as ST-246.H2O. The ST-246.H2O is prepared according to the following method: in the presence of organic base and organic solvent and being under protection of nitrogen, tropane anhydride and p-trifluoromethyl benzoylhydrazine are heated and return flow and reaction solution is cooled and filtered, thereby obtaining the ST-246.H2O. The ST-246.H2O prepared by the method is steady at room temperature, is difficult to lose the crystal water or absorb moisture, is difficult to agglomerate after micronization and is beneficial for improving bioavailability. The compound can be used for preparing anti-poxvirus medicines.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Rod hydrotalcite-like compound and its prepn process
InactiveCN1974399ASimple processLow costCatalyst carriersOther chemical processesSodium stearateCrystal structure
The present invention discloses rod-shaped magnesia alumina hydrotalcite-like compound (HTlc) as one new shaped hydrotalcite-like compound. The rod-shaped magnesia alumina hydrotalcite-like compound has the crystal structure of hydrotalcite and chemical expression of [MII(1-x)MIIIx(OH)2]x+[An-x / n]x-mH2O, where, MII and MIII are bivalent and trivalent metal cation separately, An- is interlayer anion, x is the molar fraction of MIII in HTlc, m is the molar fraction of interlayer crystalline water. It is prepared through a traditional co-precipitation process under the induction of soft template or shape regulating agent sodium stearate. It has simple preparation process and low cost, and may be used as rubber and plastic additive, fire retardant, pesticide release controlling agent, etc.
Owner:SHANDONG UNIV
High-heat-conductivity inorganic phase-change energy storage material
InactiveCN104087254AImprove liquidityIncrease fluid volumeHeat-exchange elementsFiberHeat conducting
The invention relates to the technical field of energy storage materials, particularly a high-heat-conductivity inorganic phase-change energy storage material. The high-heat-conductivity inorganic phase-change energy storage material is composed of the following components in percentage by mass: 80-99.4% of energy storage material, 0.25-10% of nucleating agent, 0.1-15% of modifier, 0.1-15% of water and 0.15-19% of heat-conducting reinforcing material. The energy storage material is crystalline hydrated salt, the nucleating agent is carbonate or borate, the modifier is a polyacrylic acid emulsion or thickening powder, and the heat-conducting reinforcing material is one or mixture of more of graphite, carbon powder, copper powder, carbon fiber and silicon carbide powder. Compared with the prior art, the energy storage material has the advantages of high stability, low tendency to supercooling, high latent heat energy, high heat conductivity and the like. By selecting the proper modifier and proportion thereof, the heat-conducting reinforcing material and nucleating agent material can be successfully dispersed uniformly in the system, thereby solving the problem of the phenomenon of phase separation of the material due to long-term cycle, further enhancing the heat-conducting property of the material and being worthy of popularization and application.
Owner:PIONEER ENERGY JIANGSU
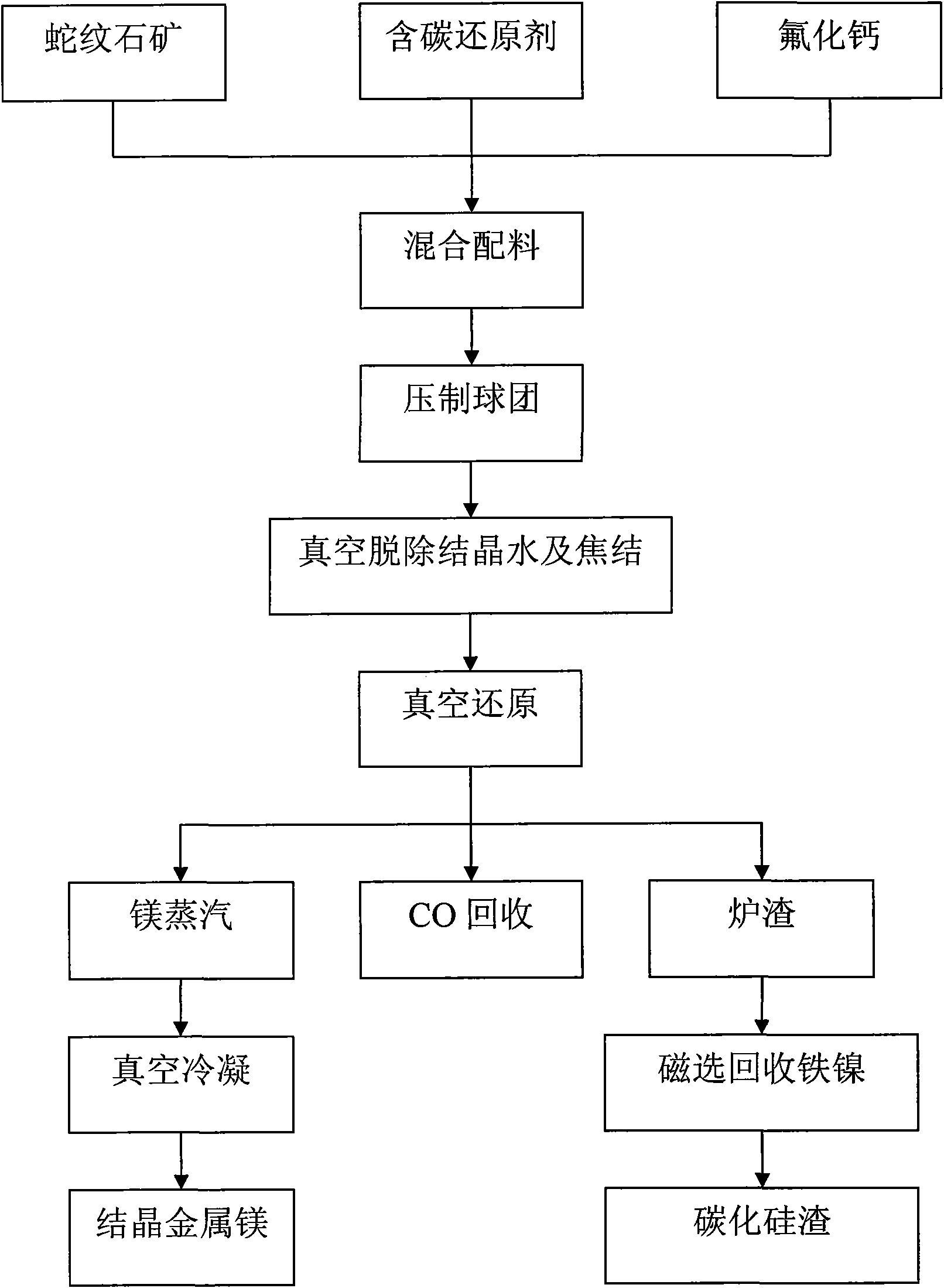
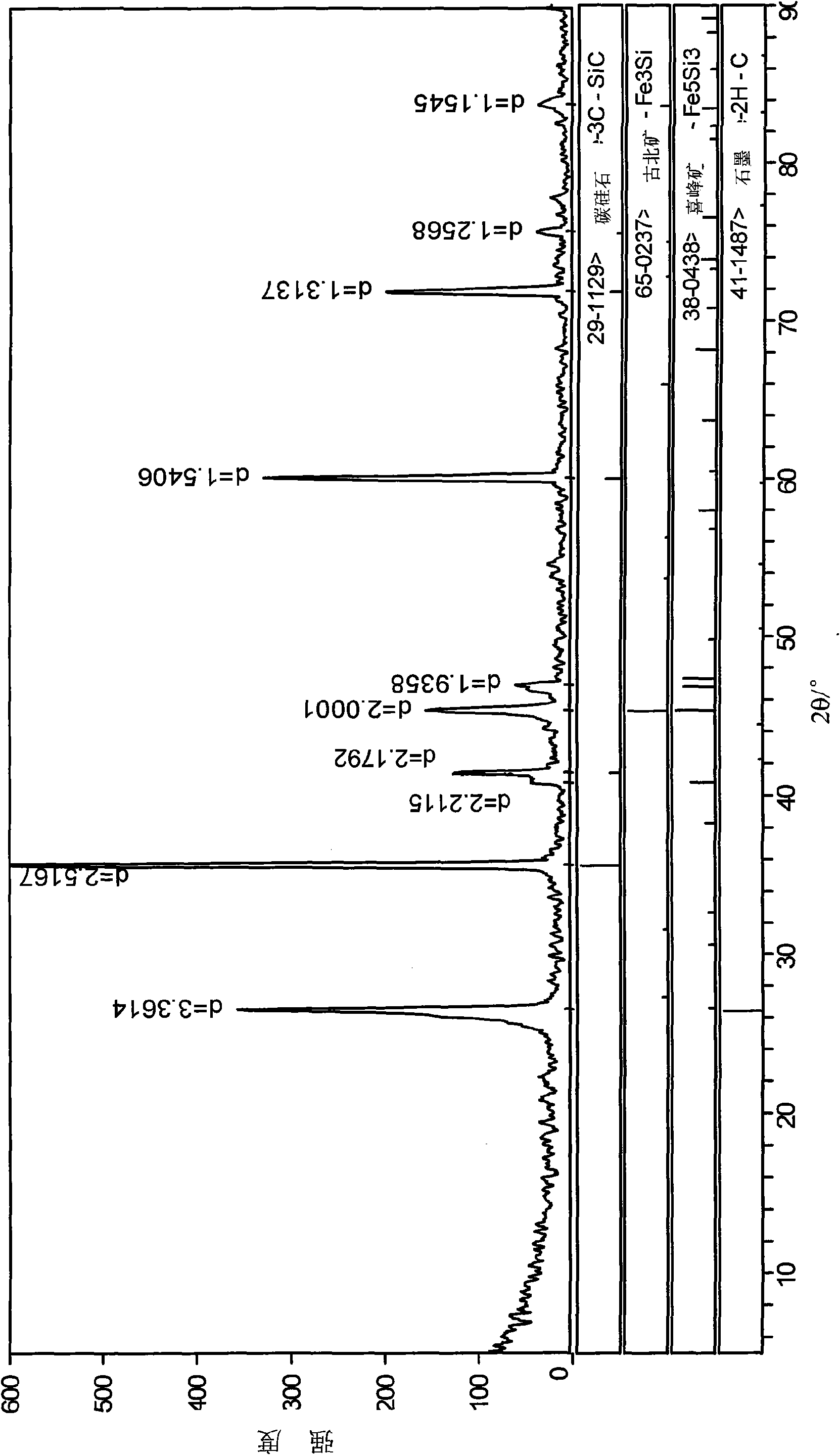
Method for preparing magnesium metal and by-product by vacuum carbothermic reduction with serpentine minerals
InactiveCN101560603AHigh in magnesiumNo need for calcination to remove carbonProcess efficiency improvementMagnetic separationSlagMassicot
The invention discloses a method for preparing a magnesium metal and a by-product by vacuum carbothermic reduction with serpentine minerals. The method comprises the following steps: using serpentine mineral powder as a raw material; adding a carbonaceous reducing agent which is 1 to 2 times of the theoretical quantity of carbon required for completely reducing magnesium silicate in the serpentine; adding a catalyst, and mixing the materials evenly to obtain a mixed raw material; pressing the mixed raw material into spherical or blocky ball agglomerations and drying the ball agglomerations; putting the dried ball agglomerations into a vacuum furnace, controlling the vacuum degree in the furnace to between 10 and 500 Pa, raising the temperature to between 500 and 700 DEG C, and keeping the temperature for 20 to 60 minutes to remove crystal water and clinker the materials; keeping the vacuum degree in the furnace, raising the temperature to between 1,200 and 1,500 DEG C, and reducing the magnesium silicate and oxides of metallic iron and nickel at a constant temperature for 30 to 60 minutes; and condensing magnesium vapor obtained from the reduction on a magnesium condenser into crystallized magnesium, recycling the metallic iron and the metallic nickel in the slag through magnetic separation, and preparing the slag after the magnetic separation into industrial silicon carbide through decarburization and purification.
Owner:北京华夏建龙矿业科技有限公司
Method for solving flatulence of lithium titanate cathode of lithium battery by using double-boundary wrapping
ActiveCN103187562AImprove cycle stabilityImprove cycle lifeCell electrodesNickel–lithium batteryFlatulence
The invention relates to a method for solving flatulence of a lithium titanate cathode of a lithium battery by using double-boundary wrapping, and belongs to the technical field of energy materials. The method is characterized in that firstly, boundary stabilizing layer wrapping is carried out on a nitride of a lithium titanate cathode material, so as to establish an electrochemistry stable boundary between an electrode material and electrolyte, and subsequently hydrophobic surface layer wrapping is carried out by adopting a nitrogen-containing compound so as to establish a hydrophobic electrode boundary and prevent the crystal water in the electrode material from entering electrolyte; and at the same time the trace moisture of an electrolyte system is prevented from diffusing to the electrode boundary to cause catalysis reaction, and the electrolyte is prevented from decomposing to generate gas in the charging and discharging process, so that the problem that a lithium ion battery is swelled is solved, and the circulation service life of the battery is prolonged.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Process for preparinbg vanadylic sulfate and use
The preparation process of vanadylic sulfate includes adding V2O3 and V2O5 into sulfuric acid, filtering and evaporating the filtrate, eliminating crystalline water to obtain light blue VOSO4 powder. Compared with available technological path, the said technological process of the present invention has less steps, mild reaction condition, simple technological process, low cost and stable product quality. The filtrate may be used as the material as electrolyte in vanadium cell.
Owner:攀枝花钢铁有限责任公司钢铁研究院
Battery grade iron and manganese phosphate and preparation method thereof
The invention relates to battery grade iron and manganese phosphate and a preparation method thereof. The chemical composition of the battery grade iron and manganese phosphate is MnxFe(1-x)PO4, wherein x is greater than or equal to 0.1 and smaller than or equal to 0.9; the preparation method comprises the steps of adopting a hydrothermal oxidation-coprecipitation technology, putting a soluble phosphorus source, iron source and manganese source solution into a reaction kettle according to the chemical composition MnxFe(1-x)PO4 of the iron and manganese phosphate, adding a surfactant and nitric acid, controlling the system pH value to be within 1 to 4, stirring for 2 to 48 h at 100 to 250 DEG C, so as to perform hydrothermal reaction, obtaining turbid liquid containing MnxFe(1-x)PO4.yH2O precipitate, naturally cooling the turbid liquid to room temperature, filtering, washing and drying, obtaining MnxFe(1-x)PO4.yH2O, performing high-temperature roasting at 250 to 700 DEG C, and then obtaining the battery grade iron and manganese phosphate not containing crystal water. The method is simple and practicable, and is easy to realize scale production, the prepared iron and manganese phosphate has the advantages that size distribution is uniform, the purity is high, and iron and manganese elements realize atomic-scale uniform distribution, and the battery grade iron and manganese phosphate a belongs to the optimal precursor for preparing lithium ferric manganese phosphate battery materials.
Owner:杨志宽
Method for concentrating waste sulfuric acid by utilizing waste heat of titanium dioxide calcinator
InactiveCN102910594ASolve manySolve process problemsSulfur compoundsEnergy inputLiquid waterOperability
The invention discloses a method for concentrating waste sulfuric acid by utilizing waste heat of a titanium dioxide calcinator, comprising the following steps: at normal temperature and pressure, proper amount of water absorbent is added to titanium dioxide waste acid, liquid water is dissolved out in the form of solid crystal water through the water absorbent, filtrate after solid-liquid separation is cooled, iron vitriol in the solution is crystallized and dissolved out, and the acid liquid is further concentrated; the two steps are repeatedly carried out until the concentration of sulfuric acid in the final filtrate reaches more than 60%, and then the final filtrate is utilized in the acid hydrolysis procedure; and the water absorbent is dehydrated and dried to be regenerated by taking dustproof tail gas of the calcinator, and the steam generated in the drying and regeneration is cooled and flows back to technological process as washing water in titanic acid rinsing or is drained. The method provided by the invention solves the problems of more equipment, long flow and easy blockage in the existing technology, and the equipment investment and operating cost are reduced; and the water absorbent is recycled after being regenerated, so that the energy is saved, the consumption is reduced, and the efficiency is obvious; no wastewater, waste gases and residues are generated in the whole technology, the operability is strong, the productivity of equipment is high, the flow is simple, the investment is less, the cost is low, energy is saved, emission is reduced, and the benefit is obvious.
Owner:黄正源
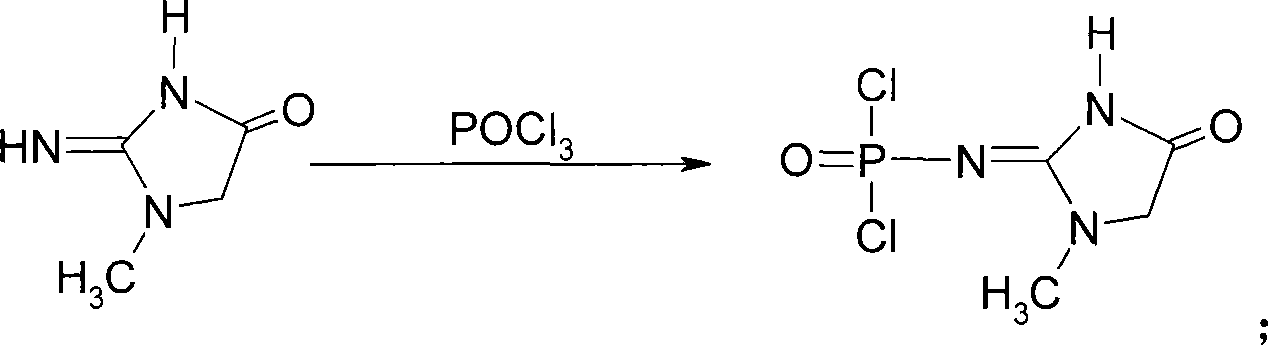
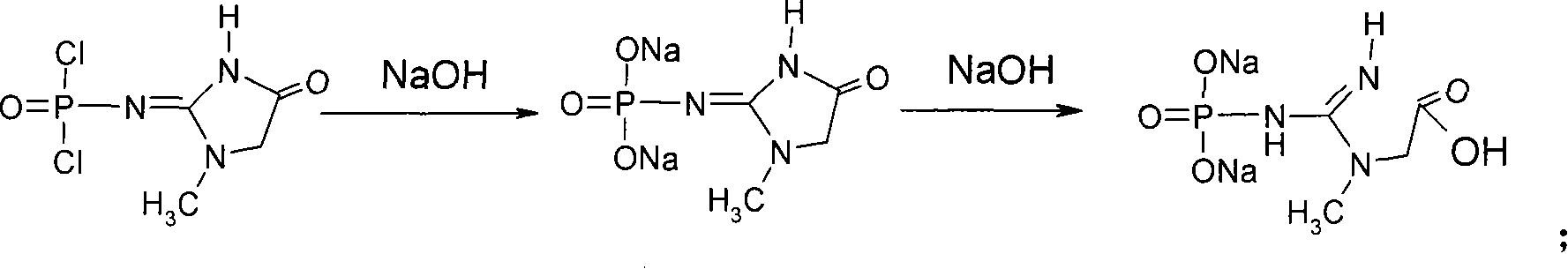
Medicinal disodium creatine phosphate hexahydrate and preparing method thereof
ActiveCN101033237AHigh purityLow drying temperatureGroup 5/15 element organic compoundsCreatinine riseDistillation
This invention discloses a phosphocreatine disodium salt six-hydrate and its preparation method. The compound is phosphocreatine disodium salt with high purity and six-crystal water, and the preparation method includes the following steps: (1) Kondens of creatinine and phosphorus oxychloride prepares creatinine phosphorus oxychloride, (2) creatinine phosphorus oxychloride is taken the ring opening reaction to get crude phosphorus disodium, (3) purification and crystallization gets the target product. This invention has the advantages of high purity suitable for the manufacture of injection powder, and it avoids the large use of solvents with high yield of products. The excessive phosphorus oxychloride is reused with distillation, and the product has high purity through intermediate and resin purification. It has no potential safety problem about heavy metal residues, and can effectively improve the clarification to reach the intravenous requirement.
Owner:QIDONG HUATUO PHARMA
Battery-grade anhydrous iron phosphate and preparation method thereof
The invention relates to a battery grade anhydrous iron phosphate and a preparation method thereof. The battery grade anhydrous iron phosphate is in an orthorhombic type. The preparation method of the battery grade anhydrous iron phosphate is an oxidation precipitation method using air as oxidant and includes steps: adding pH value modifier solution into aqueous solution of mixture of ferrous salt with phosphoric acid or phosphate, feeding air and stirring for reaction to generate a crystalline-state composite containing ammonium, hydroxyl and water of crystallization; and obtaining the battery grade anhydrous iron phosphate after solid-liquid separating, washing, drying and roasting. The battery grade anhydrous iron phosphate is an ideal material for preparation of lithium iron phosphate which is the material of anodes of lithium ion batteries. The preparation method is suitable for large-scale, economical, stable and reliable production of high-quality battery grade anhydrous iron phosphate, and has evident advantages and practical value.
Owner:HUBEI WANRUN NEW ENERGY TECH DEV
Treatment method for circulating water sewerage and reverse osmosis concentrated water and equipment thereof
InactiveCN102942279APrevent scalingAvoid fragileGeneral water supply conservationMultistage water/sewage treatmentChemistrySaline water
The invention provides a treatment method for circulating water sewerage and reverse osmosis concentrated water and equipment thereof. The treatment method includes: firstly adding a softening medicament into sewerage, filter-pressing the sludge after precipitation, burying the sludge or recycling by resource utilization; filtering and nanofiltering the softened and precipitated sewerage; then electrodialysis treating the nanofiltered sewerage; finally, evaporating and crystallizing the collected nanofiltration concentrated water and the electrodialysis concentrated water, and discharging the crystal substance to landfill, wherein the crystal water and electrodialysis water is used as make-up water for circulating water or as pretreatment water for desalinated water. Accordint to the invention, the double membrane method superposition process of nanofiltration and electrodialysis as well as the steam crystallization technology are empolyed, scaling and wearing of the membrane modules are prevented, regular cleaning is not needed, running time is long and stable, sewerage recovery is improved, the running costs of sewerage treatment is reduced, sewerage recycling is realized, no secondary pollution occurs; and the processing equipment is set reasonably and efficiently, and is stable in running and long-lasting.
Owner:JIANGSU HUAHUI ENVIRONMENTAL PROTECTION TECH +1
Preparation method of zirconium-based microporous coordination polymer
ActiveCN105777791APromote formationGuaranteed qualityGroup 4/14 organic compounds without C-metal linkagesCarboxylic acidSolvent
The invention provides a preparation method of a zirconium-based microporous coordination polymer. The preparation method includes: utilizing organic monocarboxylic acid which is simple as a regulator. The organic monocarboxylic acid can promote forming of a Zr6O4(OH)4(CO2)12 cluster, so that influence of water contained on Zr6O4(OH)4 is avoided, forming of Zr-MOF is facilitated, and quality of Zr-MOF is guaranteed. ZrO2+salt containing crystal water, such as ZrOCl2.8H2O is selected as zirconium salt, so that cost can be lowered, water in a trace amount can be guided in to promote generation of the Zr6O4(OH)4(CO2)12 cluster. A periodic adding mode is adopted, so that generation of a reactant can be accelerated, technical process can be shortened effectively, solvent utilization rate is increased, and reaction efficiency is improved finally; more importantly, product quality is guaranteed. The zirconium-based microporous coordination polymer is uniform in grain size, high in crystallinity, complete in shape and suitable for gas storage and separation and serving as a carrier. By using the method, quality such as crystallinity, specific surface area and grain size uniformity of Zr-MOF can be guaranteed, and the zirconium-based microporous coordination polymer is suitable for large-scale production.
Owner:李亚丰
Method for preparing calcium sulfate crystal whisker in low cost
InactiveCN102965721AIncrease profitReduce manufacturing costPolycrystalline material growthFrom normal temperature solutionsAnhydrous Calcium SulfateHigh pressure
The invention discloses a method for preparing calcium sulfate crystal whisker in low cost and relates to a method for preparing the calcium sulfate crystal whisker. The method comprises the following steps of: crushing raw materials consisting of ground limestone and anhydrite, treating the crushed ground limestone and anhydrite by a sulfuric acid solution, adding a crystal growth substance to prepare a mixture solution, preparing calcium sulfate hemihydrate crystal whisker with higher length-diameter ratio by a hydrothermal synthesis method at high temperature under high pressure, and carrying out filtering, washing and high-temperature crystal water removing steps on the calcium sulfate hemihydrate crystal whisker to obtain high-quality anhydrous calcium sulfate crystal whisker products. The calcium sulfate crystal whisker prepared by the method disclosed by the invention can be applied to high polymer material such as plastic and rubber to improve the performances of polymer materials by serving as a reinforcing material. The method disclosed by the invention has the advantages of low production cost, simple operation and high additional value of products.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Foam metal composite phase-change material and preparation method thereof
InactiveCN103436240AImprove thermal performanceHigh heat storage densityHeat-exchange elementsHeat conductingImpurity
The invention relates to a foam metal composite phase-change material and a preparation method thereof, and belongs to the technical field of phase-change energy storage materials. A crystallized hydrated salt-foam metal composite phase-change energy storage material is prepared by adopting the adsorption characteristic of a porous foam metal skeleton structure, and a vacuum argon filling state is kept in a preparation process, so that impurities are prevented from entering, and the quality of a product is ensured. The phase-change material is relatively uniformly and sufficiently distributed in a substrate of the foam metal skeleton material, and the shaping characteristic of the composite material is maintained under the combined action of a capillary force and surface tension of foam metal, so that the phase-change material does not leak easily in a phase-change process, and the preparation method of the phase-change material is simple and convenient, high in recombination rate and good in operability. The composite phase-change material has the advantages of high phase-change latent heat in unit volume, high heat storage and release rates, good heat-conducting property, lower supercooling degree, and the like; and the lower heat conductivity and supercooling problems existing after the crystallized hydrated salt phase-change material is applied for a long time are solved effectively.
Owner:BEIHANG UNIV
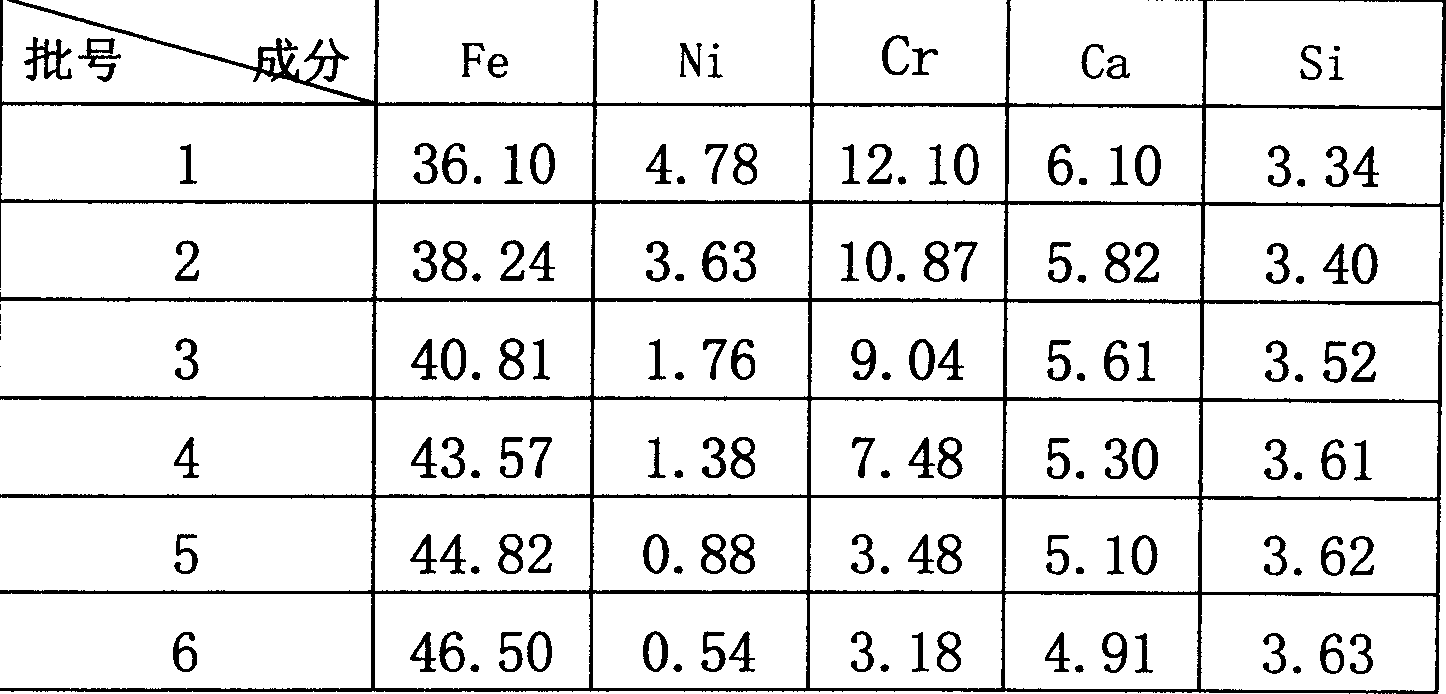
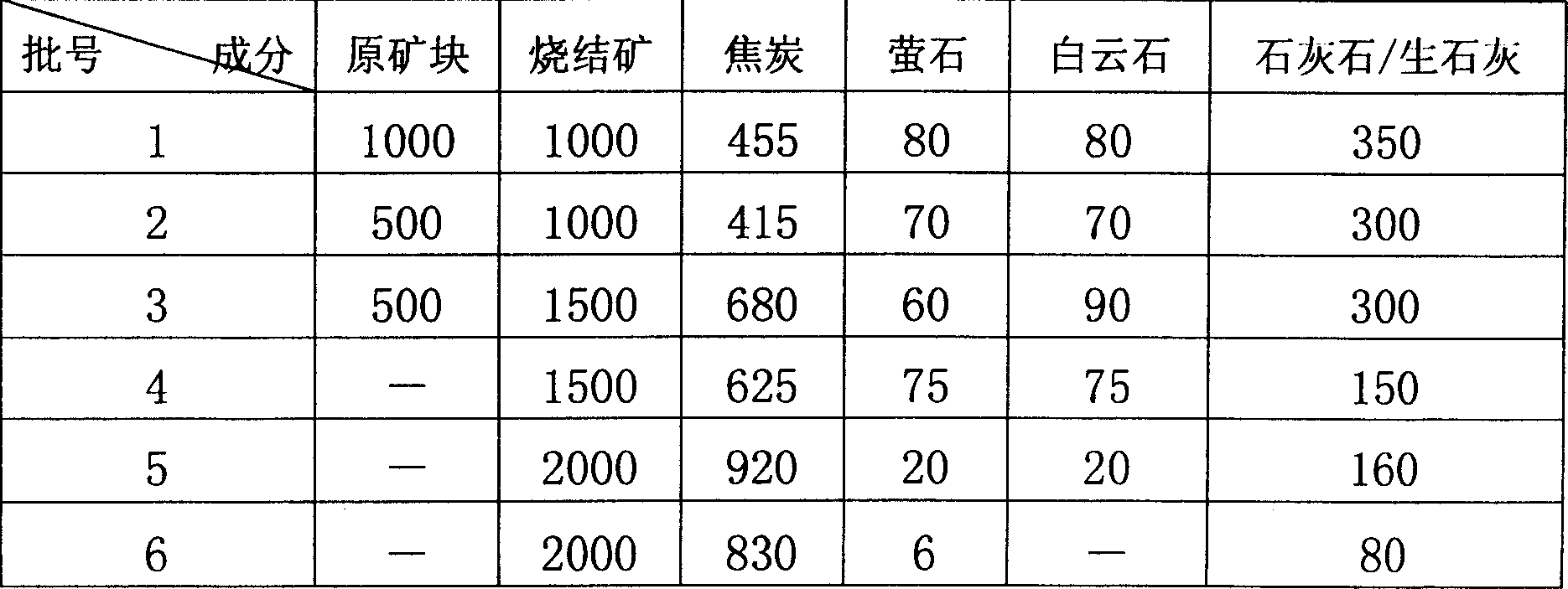
Nickel-iron smelting process from nickel oxide ore containing crystal water through blast furnace
InactiveCN1743476AImprove resource utilizationImprove liquidityBlast furnace detailsSmelting processDolomite
This invention provides a technology for smelting ferronickel of a nickel oxide ore containing water of crystallization including crushing and sieving the original ore to produce the powder to sintered ore blocks, in which, the sintered blocks, coke, limestone / calcium lime, dolomite and cand are mixed and matched to be smelted in a blast furnace to get the ferric nickel and the weight percentage of additive and the sintered ore blocks is: cand 0.3-20%, dolomite 0-8%, limestone / calcium lime 4-35% the blocks are crushed, sieved and magnetic-selected to get refined breezes to be sintered.
Owner:刘沈杰
P-Ca-V composite phosphating solution on magnesium alloy surface and chemical conversion processing method
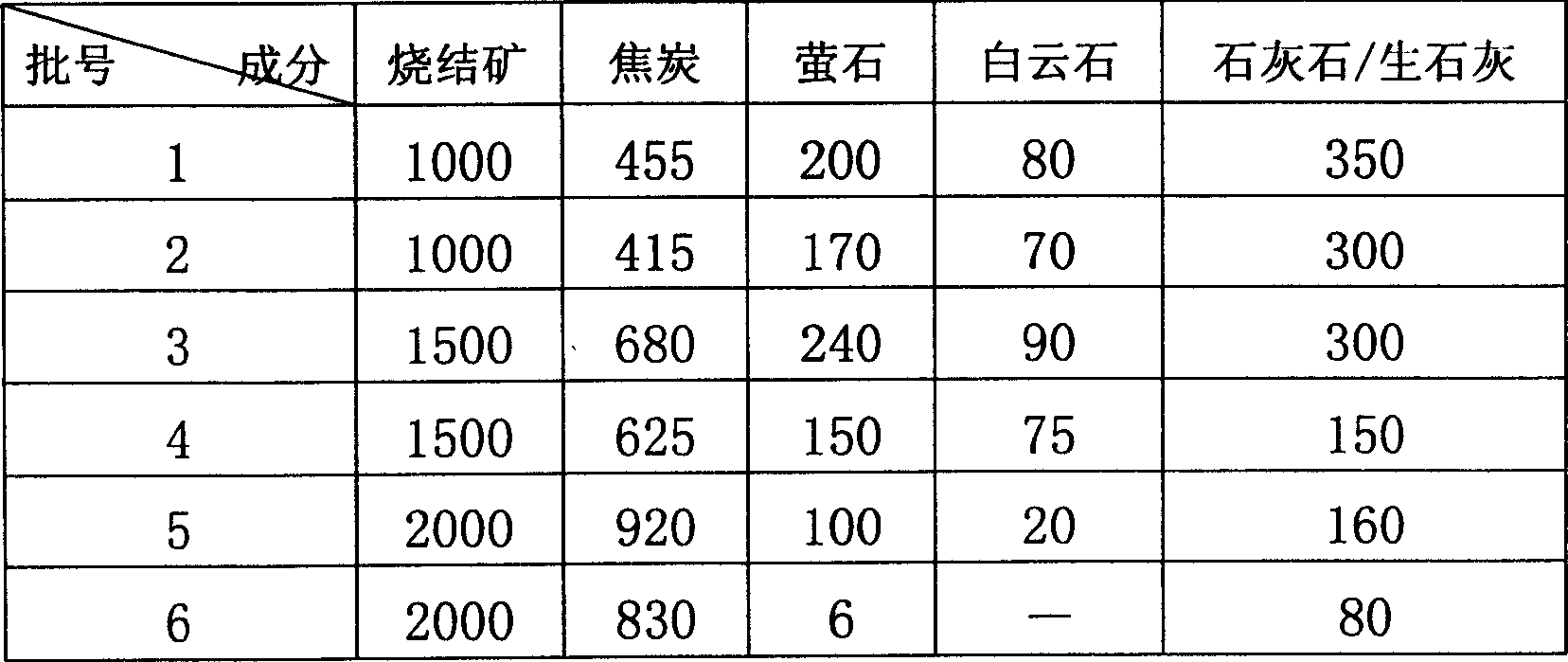
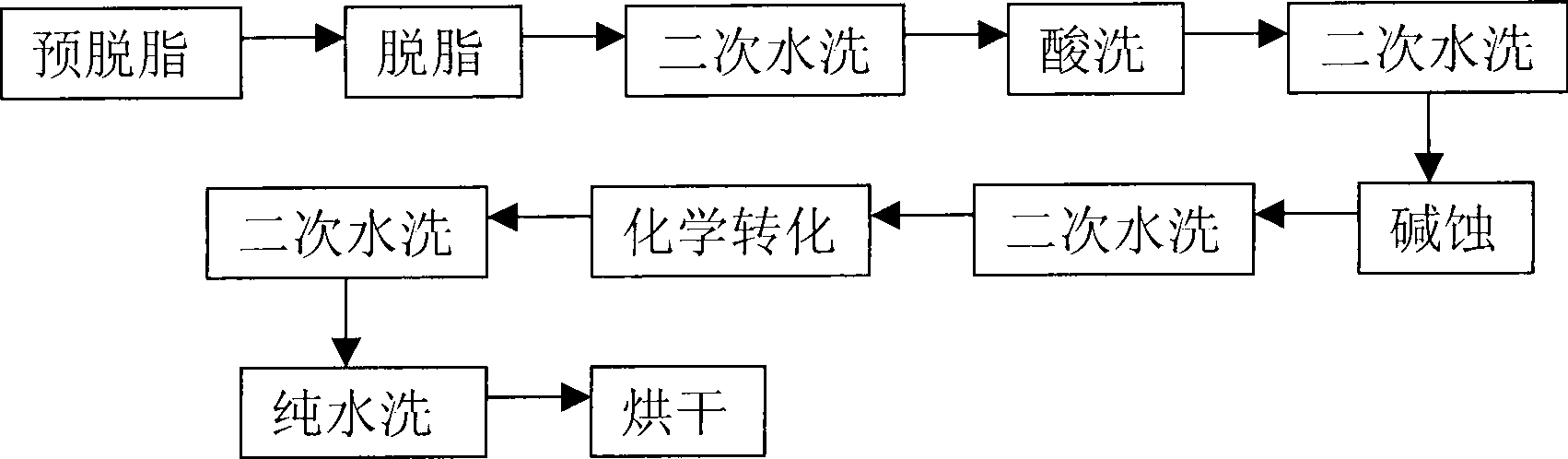
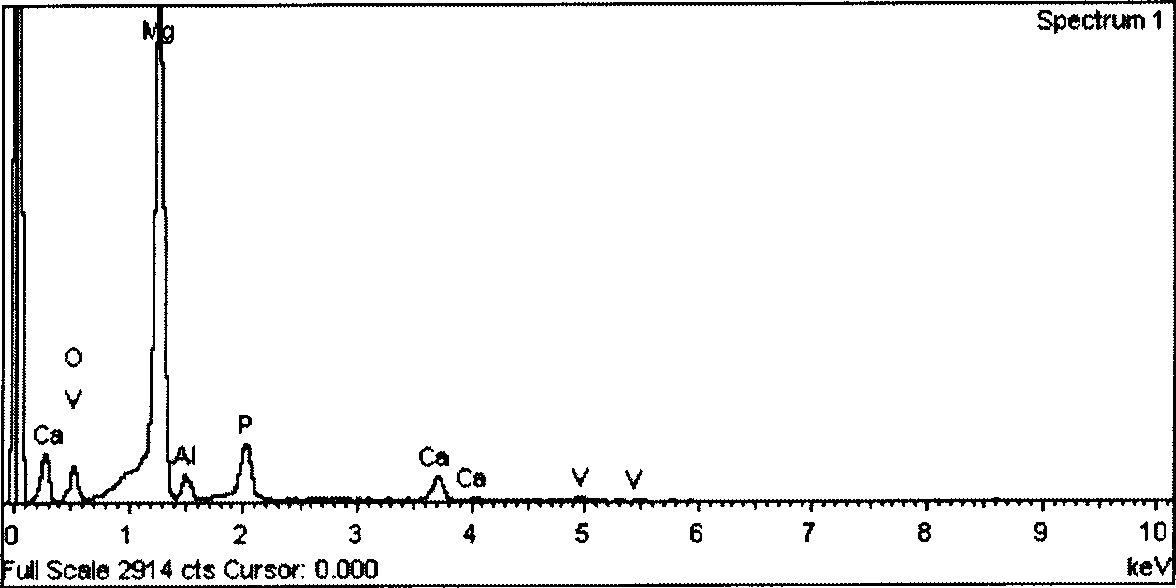
ActiveCN101418441AImprove corrosion resistanceLow resistivityMetallic material coating processesSalt spray testAcid washing
The invention relates to a composite phosphating solution of P-Ca-V on the surface of a magnesium alloy and a method for chemical conversion treatment. The solution is characterized in that each liter of the composite phosphating solution comprises the following compositions: 4 to 20 milliliters of 85 percent phosphoric acid of, 5 to 30 grams of sodium dihydrogen phosphate, 10 to 40 grams of calcium nitrate, 0.5 to 10 grams of benzene sulfonic acid sodium salt, 0.5 to 5 grams of ammonium metavanadate, and the balance being water. The method comprises the following steps: pre-degreasing, degreasing, secondary water washing, acid washing, secondary water washing, alkaline etching, secondary water washing, chemical transformation, secondary water washing, pure water washing, and drying. Taking an AZ91D magnesium alloy as an example, 48 hours after a corrosion resistance salt spray test after the treatment by the method of the invention, the corrosion area of the AZ91D magnesium alloy is less than 1 percent; the paint film adhesive force is at 0 level by a grid method and is obviously superior to the performance of a chromate conversion coating; and the formed chemical conversion coating does not contain crystal water. The composite phosphating solution has the synergistic reaction of Ca and V, as well as the functions of a corrosion inhibitor and a wetting agent of the benzene sulfonic acid sodium salt.
Owner:嘉兴中科亚美合金技术有限责任公司
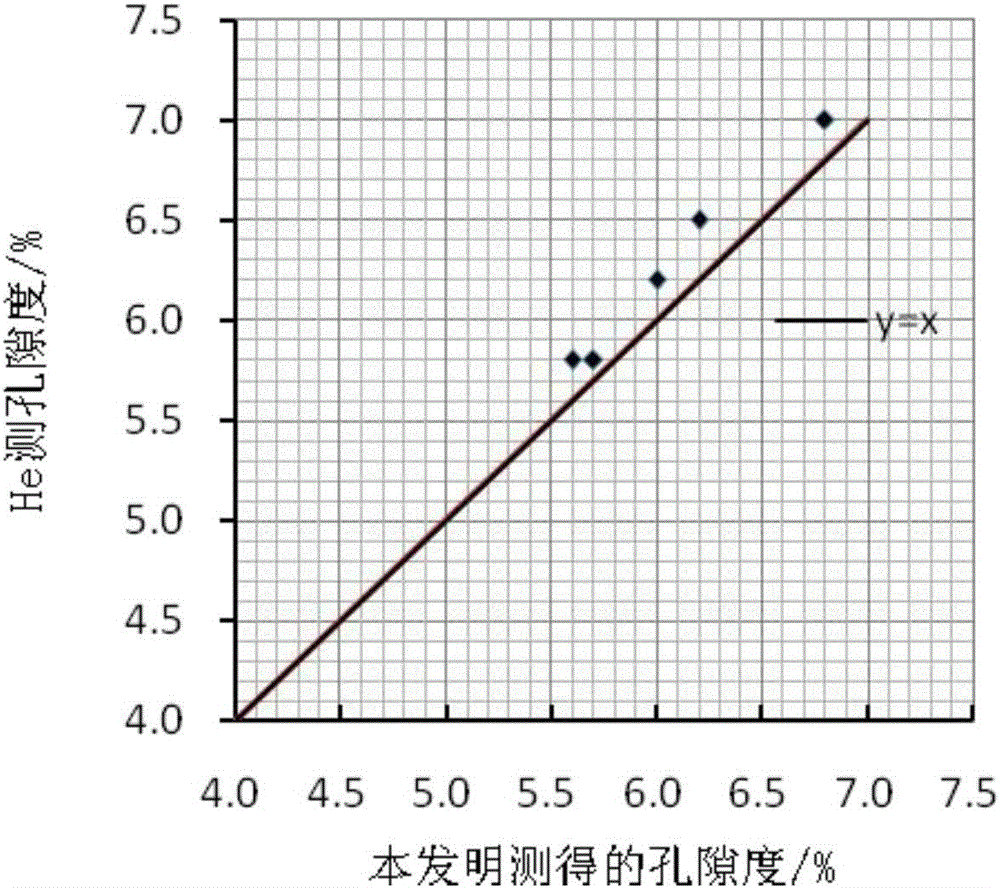
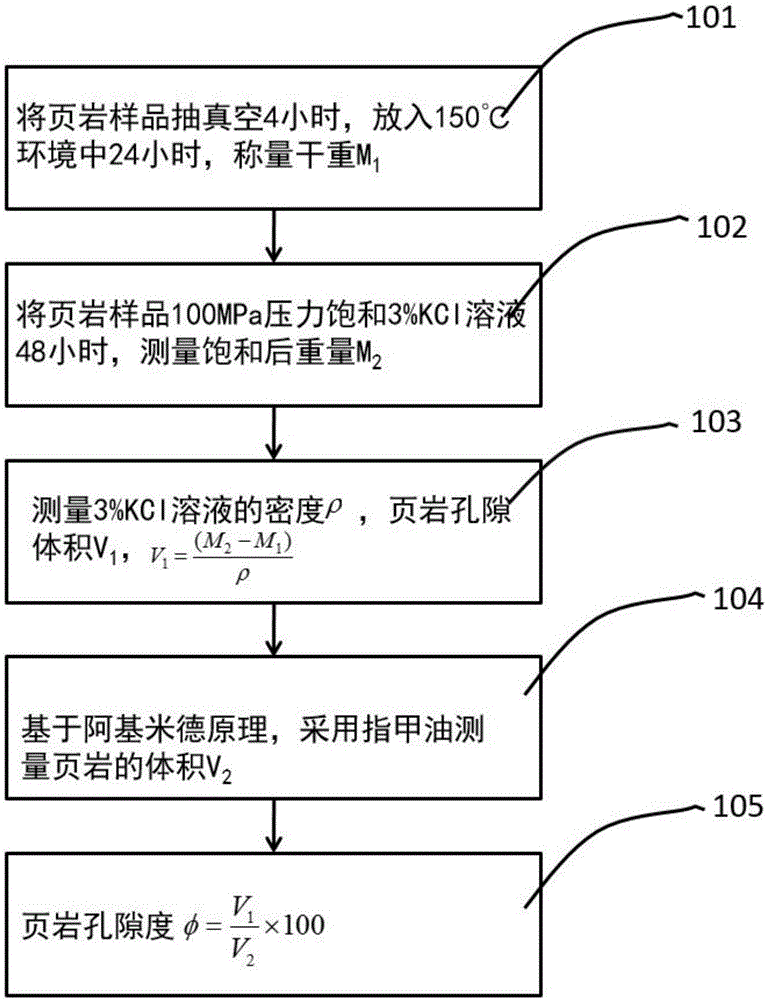
Shale porosity measurement method
PendingCN106323840AReal volumeAvoid breakingPermeability/surface area analysisWeighing by absorbing componentVacuum pumpingPorosity
The invention discloses a shale porosity measurement method with an aim to solve problems that conventional shale porosity measurement is low in precision, long in time consumption and uneconomical. The shale porosity measurement method includes: selecting a whole-piece to-be-measured shale sample, subjecting shale to vacuum pumping for 4 hours, heating to 150DEG C under the vacuum condition, taking out after 24 hours and weighing dry weight M1; adopting 3% of a KCl solution to perform pressure saturation for 48 hours, and weighing the weight M2 with a scale after the shale is saturated; measuring 3% of the KCl solution to be p in density and calculating porosity volume V1 of the sample (as is shown in the following formula) on the premise of Archimedes principle, placing the shale sample in a container with nail polish, and measuring the volume V2 of the shale sample; calculating the porosity of the sample as is shown in the following formula. With the method, influence of clay-bound water and crystal water on measurement precision of the porosity can be removed, whether the shale sample is regular or not is not required, the method is economic and practical in measurement of the porosity and high in operability.
Owner:SOUTHWEST PETROLEUM UNIV
Ferronickel smelting process of nickel oxide ore free of crystal water in blast furnace
InactiveCN1733950AImprove resource utilizationImprove liquidityBlast furnace detailsSmelting processDolomite
The invention provides a smelting ferro nickel technique with nickel oxide without crystal water by blast furnace, which comprises mainly: breaking and sieving the material, sintering the mineral powder, and matching and mixing the sintered block, coke, 4~35% lime / calcium lime to provide basicity and balance the additive, 0~8% dolomite with magnesium to overcome poor iron fluid property, and 0.3~8% fluorite. Compared with existing technique, this invention can reduce the effect to furnace temperature from chrome, avoids accidents from high fluorin content, and has high yield radio.
Owner:刘沈杰
Modified method of Y-type molecular sieve
InactiveCN102951655AIncreased mesopore volumeFaujasite aluminosilicate zeoliteMolecular sieveAluminium sulfate
The invention discloses a modified method of a Y-type molecular sieve. The modified method comprises the following steps of: dissolving the Y-type molecular sieve and aluminum sulfate or aluminum sulfate containing crystal water into water, adding alcohol, reacting for 0-10 days at the temperature of 100-200 DEG C, filtering products after reaction, cleaning and obtaining mixture of an alunite-like phase and the Y-type molecular sieve; and adopting alkali compound to treat the mixture for 0-100 hours at the temperature of 0-100 DEG C and obtaining the modified Y-type molecular sieve. The modified method disclosed by the invention is wide in material source, low in cost and simple in reaction process, and is suitable for industrial production.
Owner:EAST CHINA NORMAL UNIV
Test method of sulphate content of desulfurization gypsum
InactiveCN101625300ASimple methodFast analysisWeighing by removing componentCooking & bakingWater content
The invention provides a test method of the sulphate content of desulfurization gypsum, comprising the following steps: 3-5 grams of gypsum samples are taken to be heated and dried for 30-60 minutes in a baking oven with the temperature of 42-48 DEG C and then placed into a drier to be cooled to room temperature and weighed by a 1 / 10000 level balance, the gypsum samples are repeatedly heated and weighed till the gypsum samples have constant weight, and the weight W (g) of the gypsum samples is recorded; the gypsum samples are placed back to the baking oven to be heated and dried for 30-60 minutes under the temperature of 350-370 DEG C and then placed into the drier to be cooled to the room temperature and weighed by the 1 / 10000 level balance, the gypsum samples are repeatedly heated and weighed till the gypsum samples have constant weight, and the weight W1 (g) of the gypsum samples is recorded; the computation of the crystallization water content of the gypsum samples is as follows: the crystallization water content X(%)=(W-W1)*100 / W, and the computation of the sulphate content of the gypsum samples is as follows: the sulphate content is indicated by calcium sulphate dehydrate content, CaSO4.2H2O(%)=X*100 / 20.9275. The method is simple, convenient and fast; and in addition, compared with the prior art, the invention greatly enhances the analysis speed, simplifies the analysis steps and decreases the experimental apparatus.
Owner:HEBEI ELECTRIC POWER RES INST
Preparation method of nano-structure WC-Co composite powder
InactiveCN102350506AWell mixedOvercoming coarse compound salt crystallizationChromium CompoundsVanadium oxide
The invention discloses a preparation method of a nano-structure WC-Co composite powder, and belongs to the field of an alloy preparation method. The method comprises the following steps in sequence: adding a carbonic powder material which is excessive to tungsten, vanadium and chromium carbides into water-soluble saturated mixed aqueous solution of tungsten-containing compound, cobalt-containing compound, chromium-containing compound and vanadium-containing compound to adsorb the saturated composite salt solution; dehydrating and drying to form a nano-scale composite salt thin layer on the surface of the carbonic powder material; removing crystal water out of the composite salt at the temperature below 500 DEG C under a condition of isolating air, and decomposing the composite salt into composite oxide of tungsten oxide, chromium hemitrioxide, vanadium oxide and cobalt oxide, further heating, and reducing and carbonizing the composite oxide on the surface of the carbonic powder material to generate the WC-Co nano-structure composite powder at the temperature below 850 DEG C. The preparation is a simpler and more reliable novel preparation method of the nano WC-Co composite powder by a direct carbonization method.
Owner:SOUTHWEST PETROLEUM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com