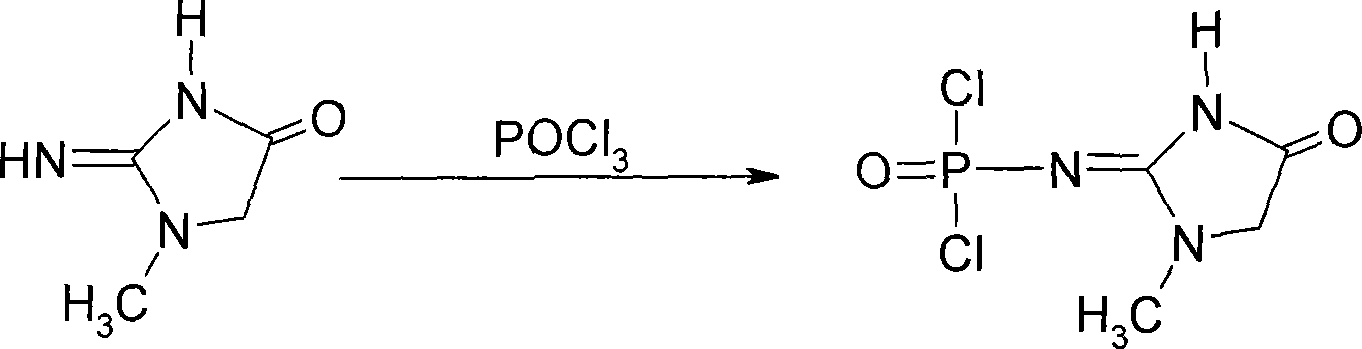Medicinal disodium creatine phosphate hexahydrate and preparing method thereof
A technology of creatine phosphate disodium salt and hexahydrate, which is applied in the field of pharmaceutical creatine phosphate disodium salt hexahydrate and its preparation, which can solve the impact on product quality, affect the clarity inspection of injections, and the results cannot meet the regulations, etc. Problems, to achieve good fluidity, avoid thermal decomposition, easy to dry the end of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
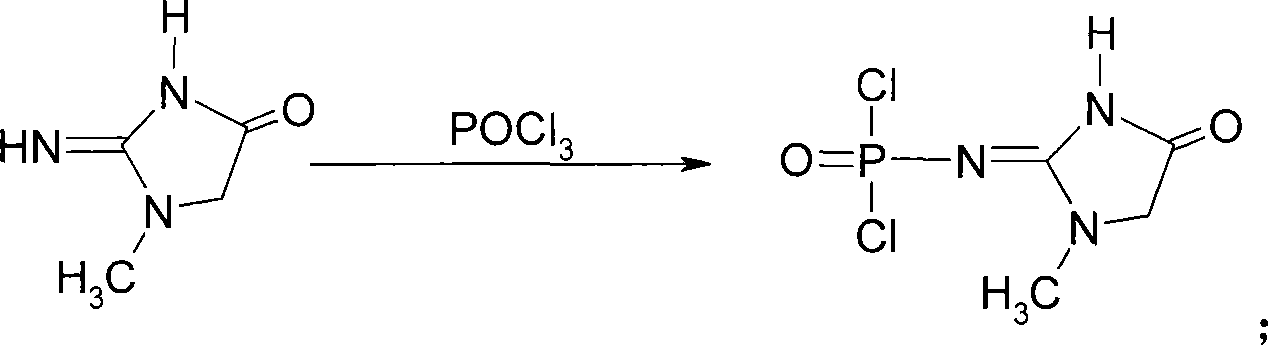
[0034] Embodiment 1: the preparation of creatinine chlorinated phosphoric acid
[0035]In a 250ml round bottom flask, put 5.0g of creatinine and 150ml of re-evaporated and dried POCl 3 . Heat to reflux under stirring for 15 minutes, then lower the temperature to 80°C, and distill out about 135ml of POCl under reduced pressure 3 , add 20ml of toluene to the remaining yellow viscous liquid, cool to room temperature under stirring, filter, and wash the filter cake with a small amount of solvent to obtain about 10g of creatinine phosphorus oxychloride with a melting point of 120-123°C.
Embodiment 2
[0036] Embodiment 2: the preparation of creatinine chlorinated phosphoric acid
[0037] In the 500L reactor, pump POCl 3 360L, creatinine 12Kg, stirred and heated to reflux for 1 hour, dissolved, cooled to 80°C and evaporated under reduced pressure to remove POCl 3 , to 40-50Kg, add 50kg of toluene, cool to room temperature, precipitate crystals, filter, wash with a small amount of toluene, and drain to obtain creatinine chloride with a wet weight of about 20kg. The melting point is 120-123°C.
Embodiment 3
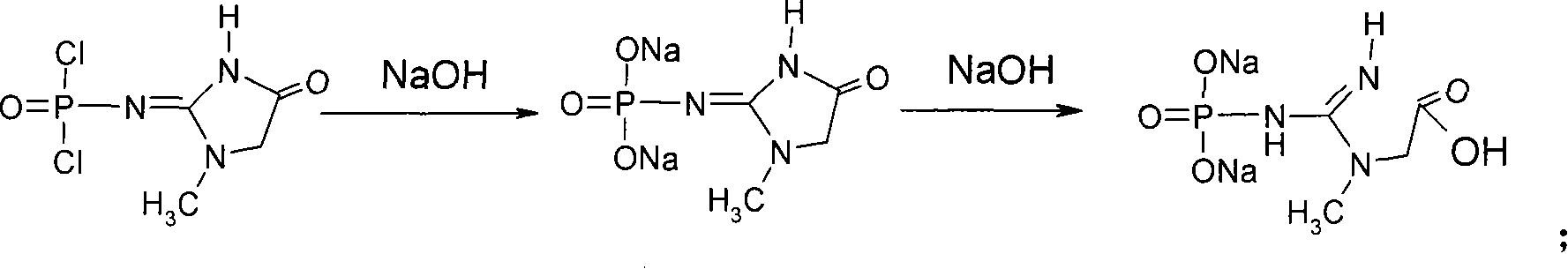
[0038] Embodiment 3: Preparation of creatine phosphate disodium salt crude product
[0039] Add 160ml of 4mol / l NaOH solution into a 500ml round bottom flask, cool to -5°C with an ice-salt bath, and slowly add 36g of creatinine chloride prepared in Example 2. The solid dissolved slowly, and kept stirring at about 0°C for 1 hour until the reaction system no longer exothermic, then raised the temperature to 45°C, and stirred for 12 hours. Cool to room temperature, adjust pH to 8.0-9.0 with concentrated hydrochloric acid, slowly add 40ml of ethanol, filter, add 600ml of ethanol to the filtrate, let it stand for 12 hours, white crystals precipitate, filter, and dry to obtain 26g of crude creatine phosphate disodium salt.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com