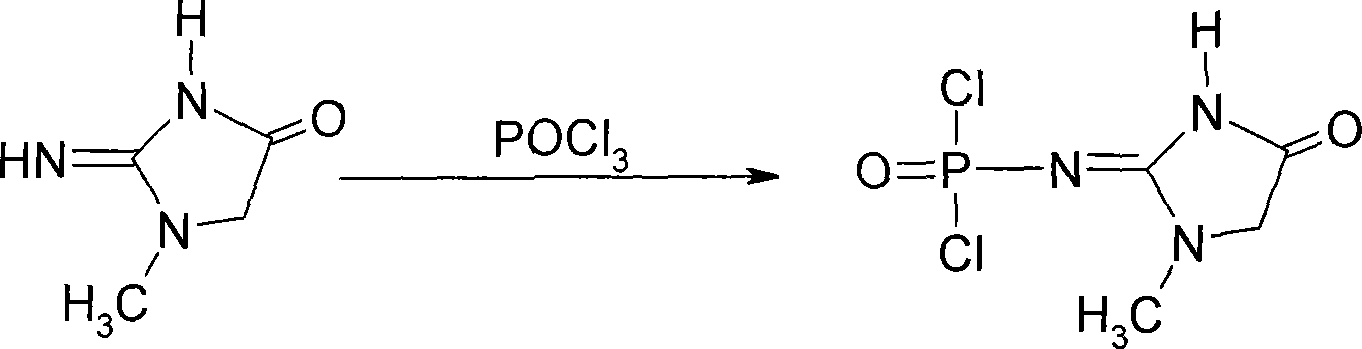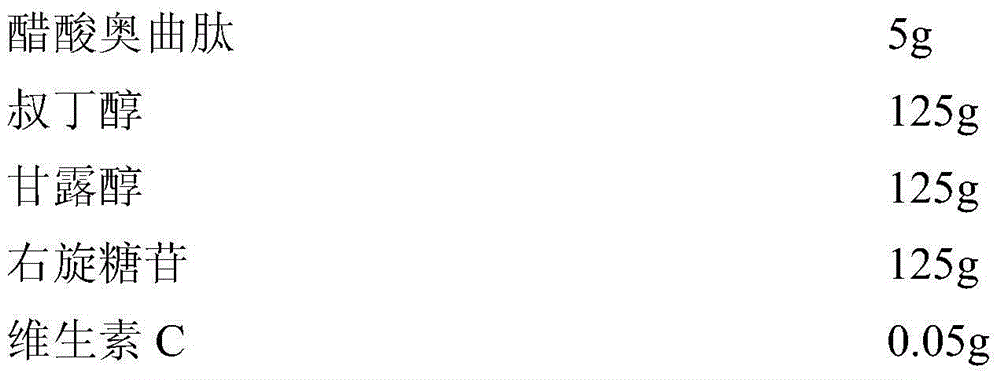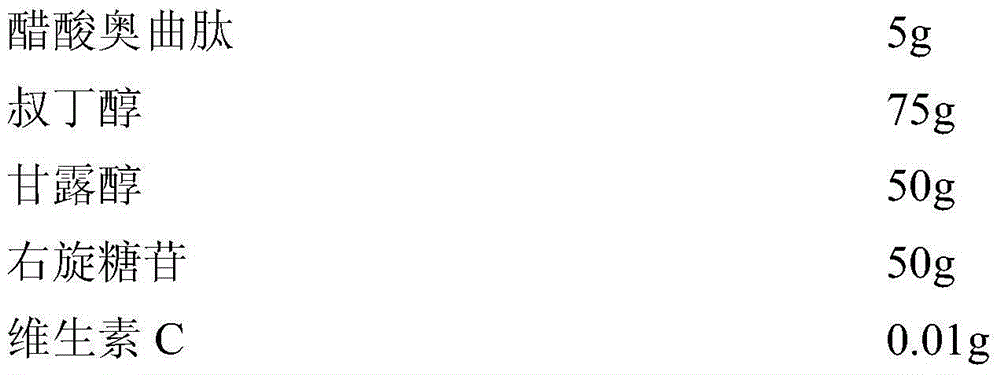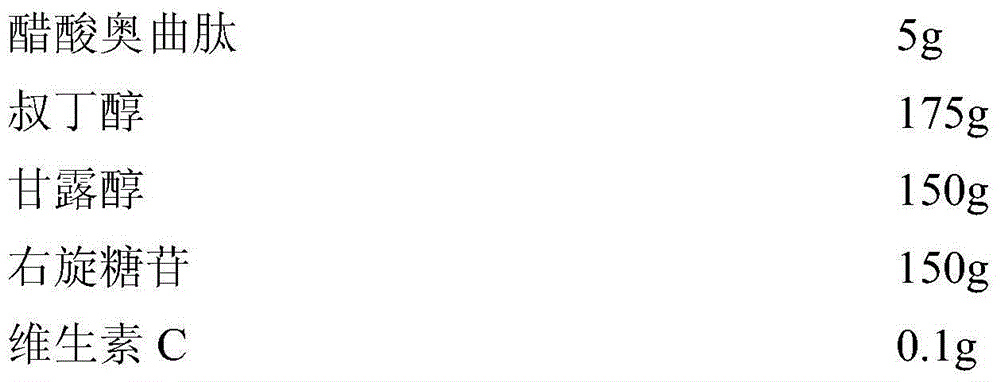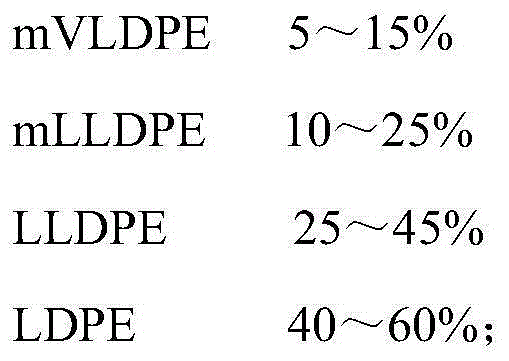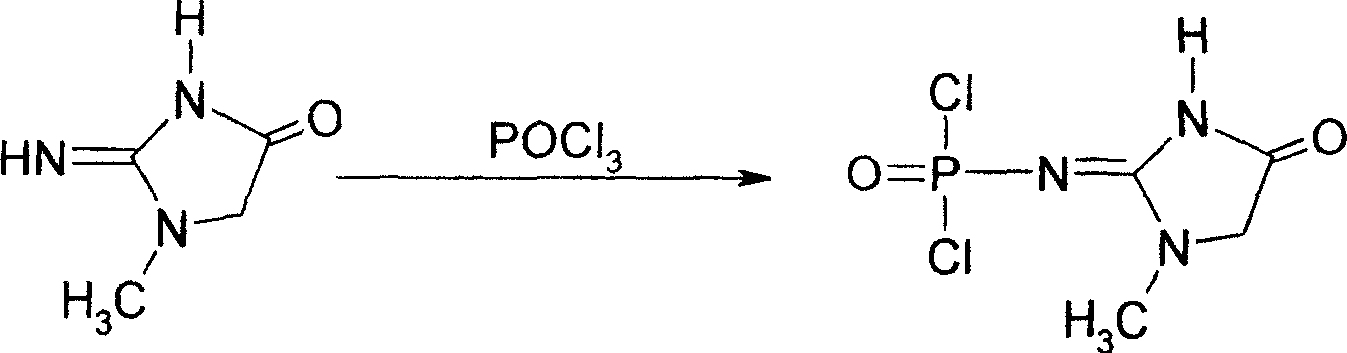Patents
Literature
86 results about "Injection powder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The powder injection moulding (PIM) process is an efficient method for the high volume production of shaped components from powders. PIM is a derivative of polymer injection moulding and uses much of the same technology, along with batch sintering processes used in powder metallurgy and ceramic processing.
Medicinal disodium creatine phosphate hexahydrate and preparing method thereof
ActiveCN101033237AHigh purityLow drying temperatureGroup 5/15 element organic compoundsCreatinine riseDistillation
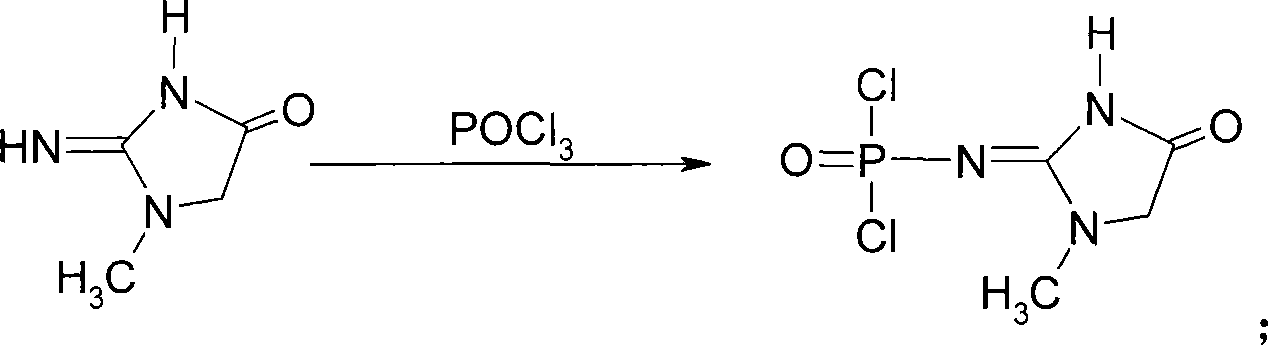
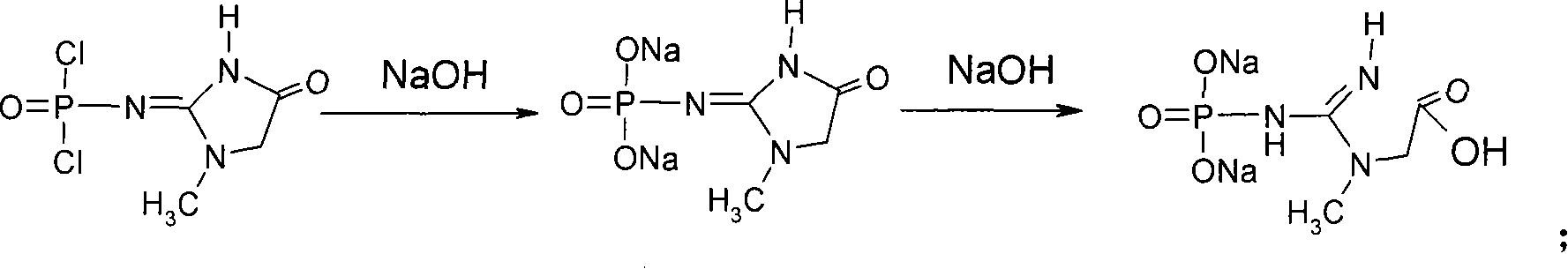
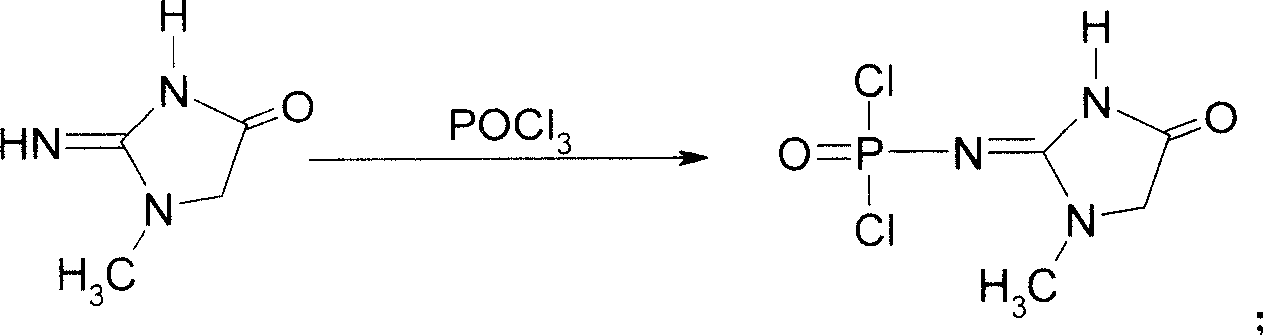
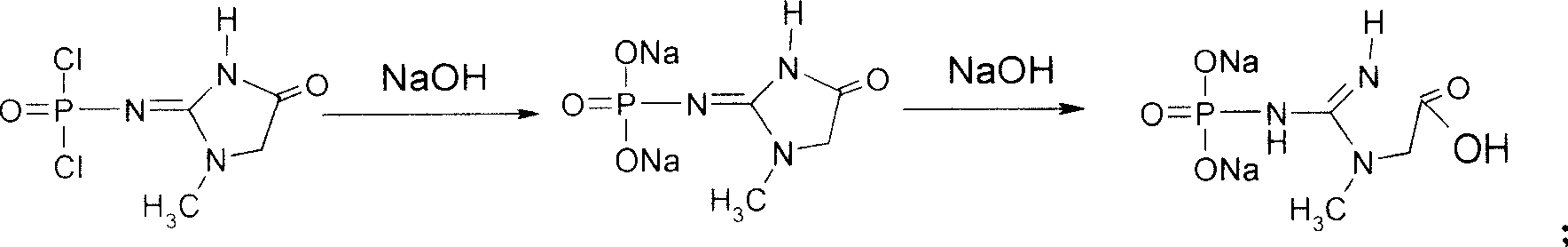
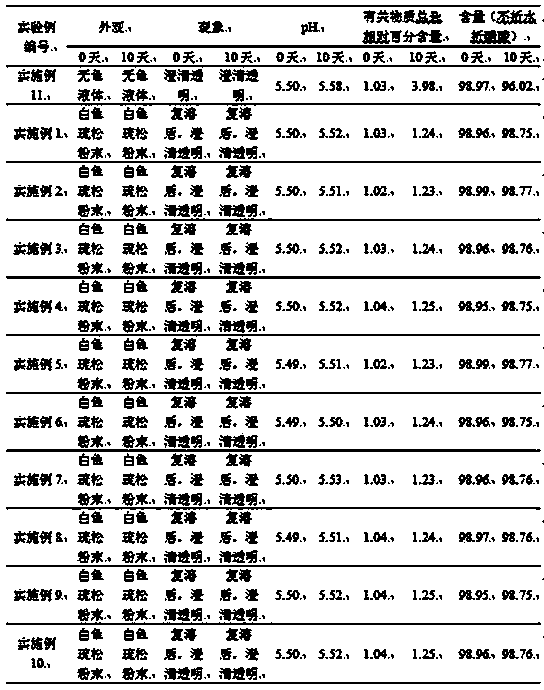
This invention discloses a phosphocreatine disodium salt six-hydrate and its preparation method. The compound is phosphocreatine disodium salt with high purity and six-crystal water, and the preparation method includes the following steps: (1) Kondens of creatinine and phosphorus oxychloride prepares creatinine phosphorus oxychloride, (2) creatinine phosphorus oxychloride is taken the ring opening reaction to get crude phosphorus disodium, (3) purification and crystallization gets the target product. This invention has the advantages of high purity suitable for the manufacture of injection powder, and it avoids the large use of solvents with high yield of products. The excessive phosphorus oxychloride is reused with distillation, and the product has high purity through intermediate and resin purification. It has no potential safety problem about heavy metal residues, and can effectively improve the clarification to reach the intravenous requirement.
Owner:QIDONG HUATUO PHARMA
Method for surface treatment of an internal combustion piston and an internal combustion piston
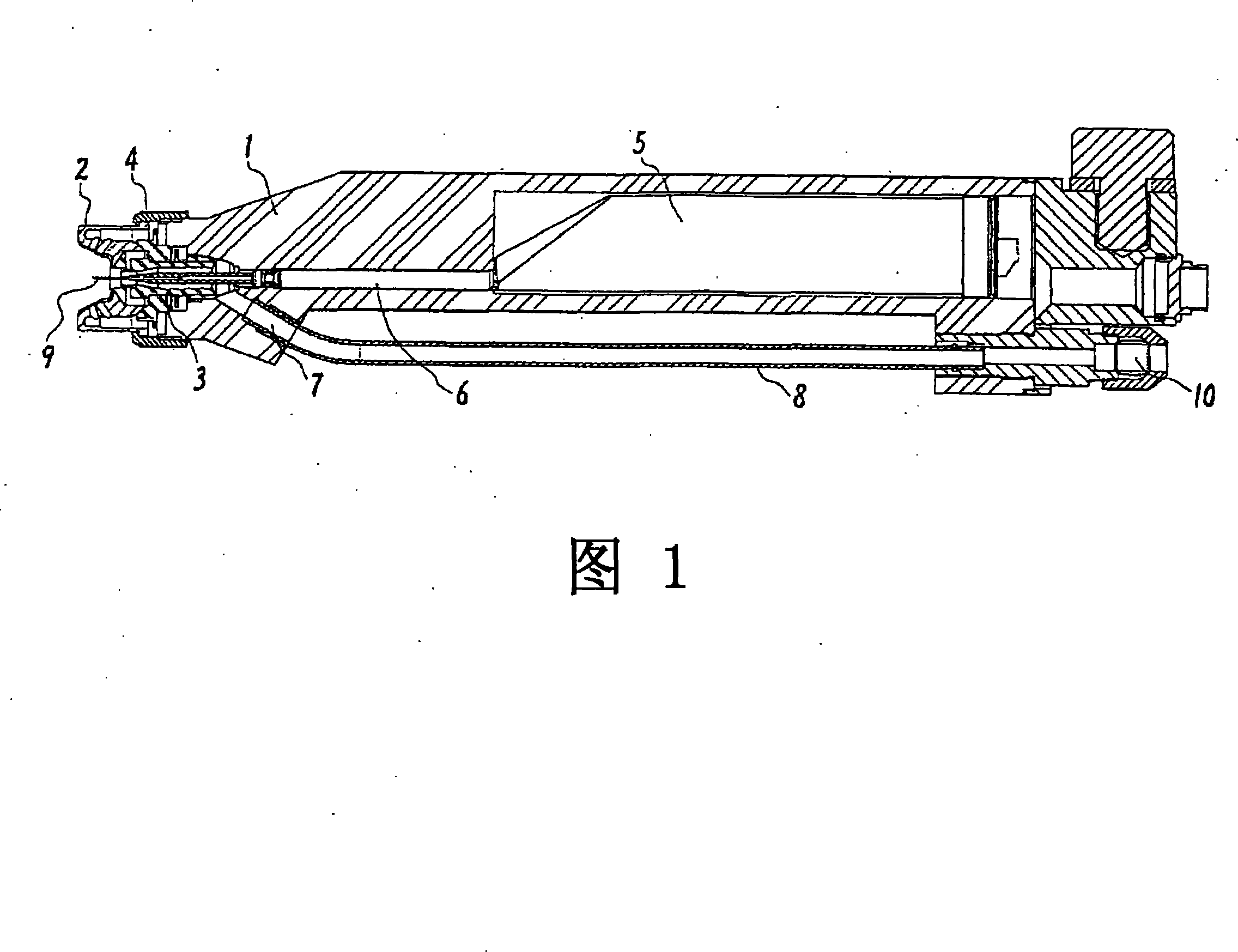
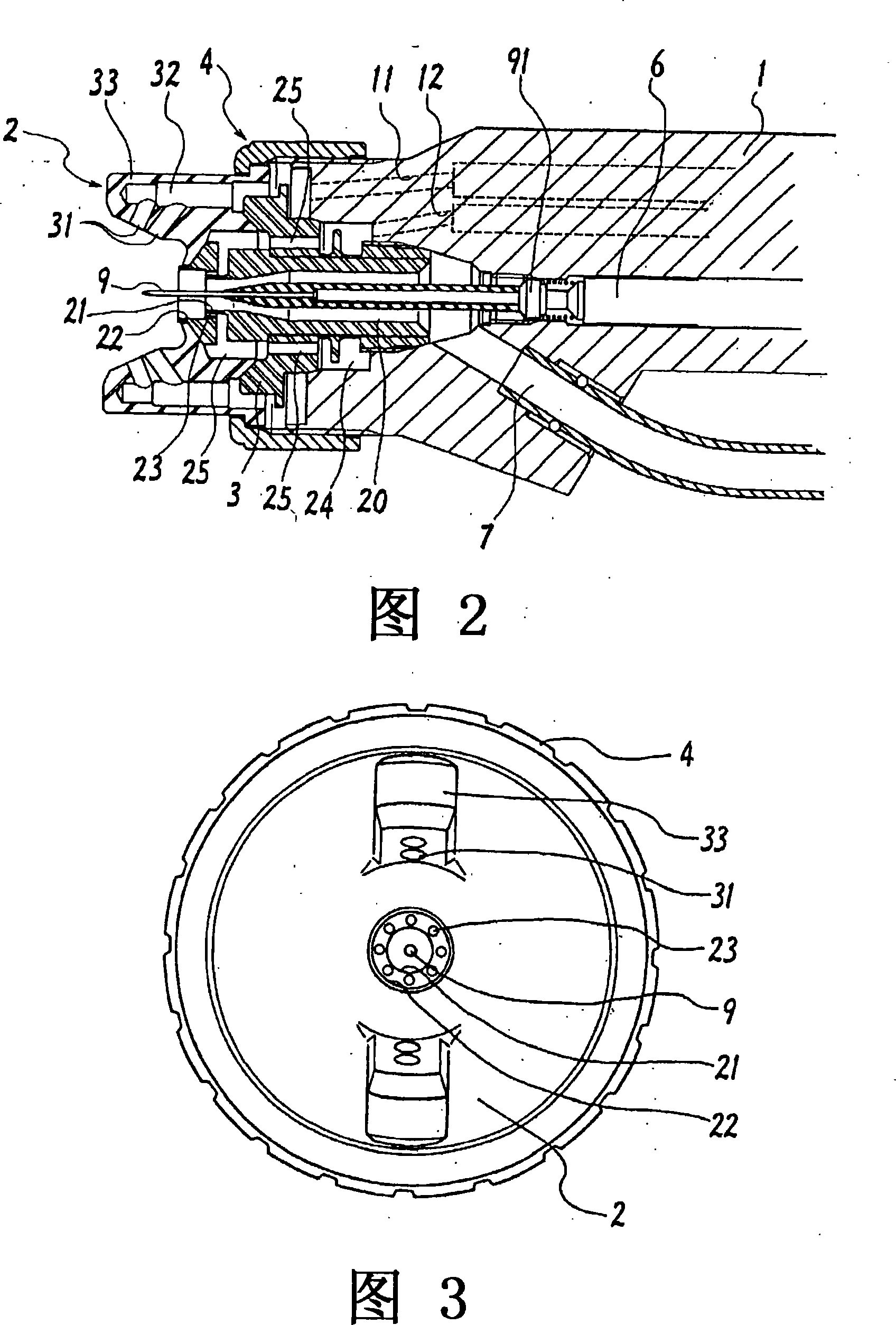
ActiveUS20080022962A1High mechanical strengthHigh strengthLiquid surface applicatorsSolid state diffusion coatingSurface layerCombustion
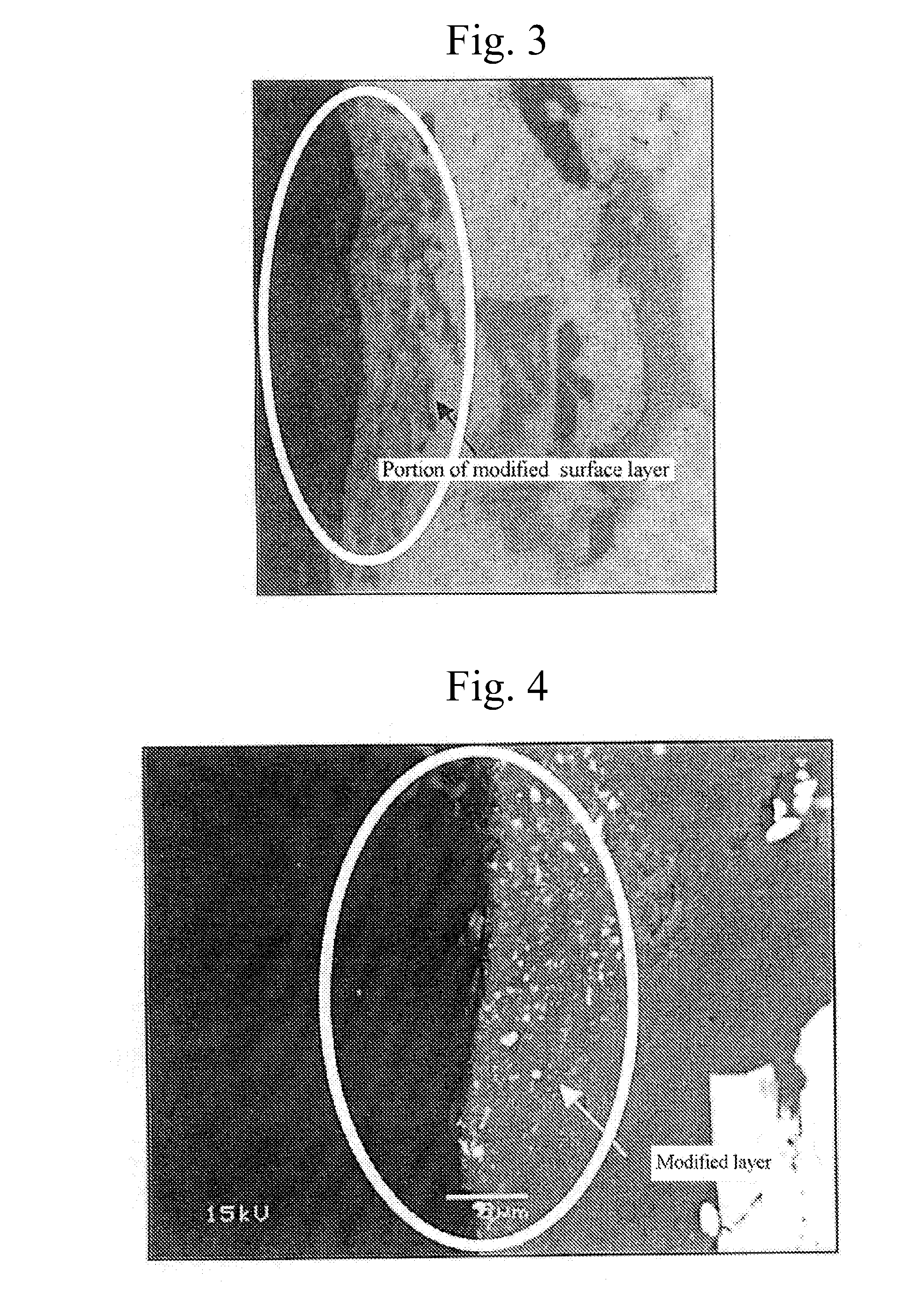
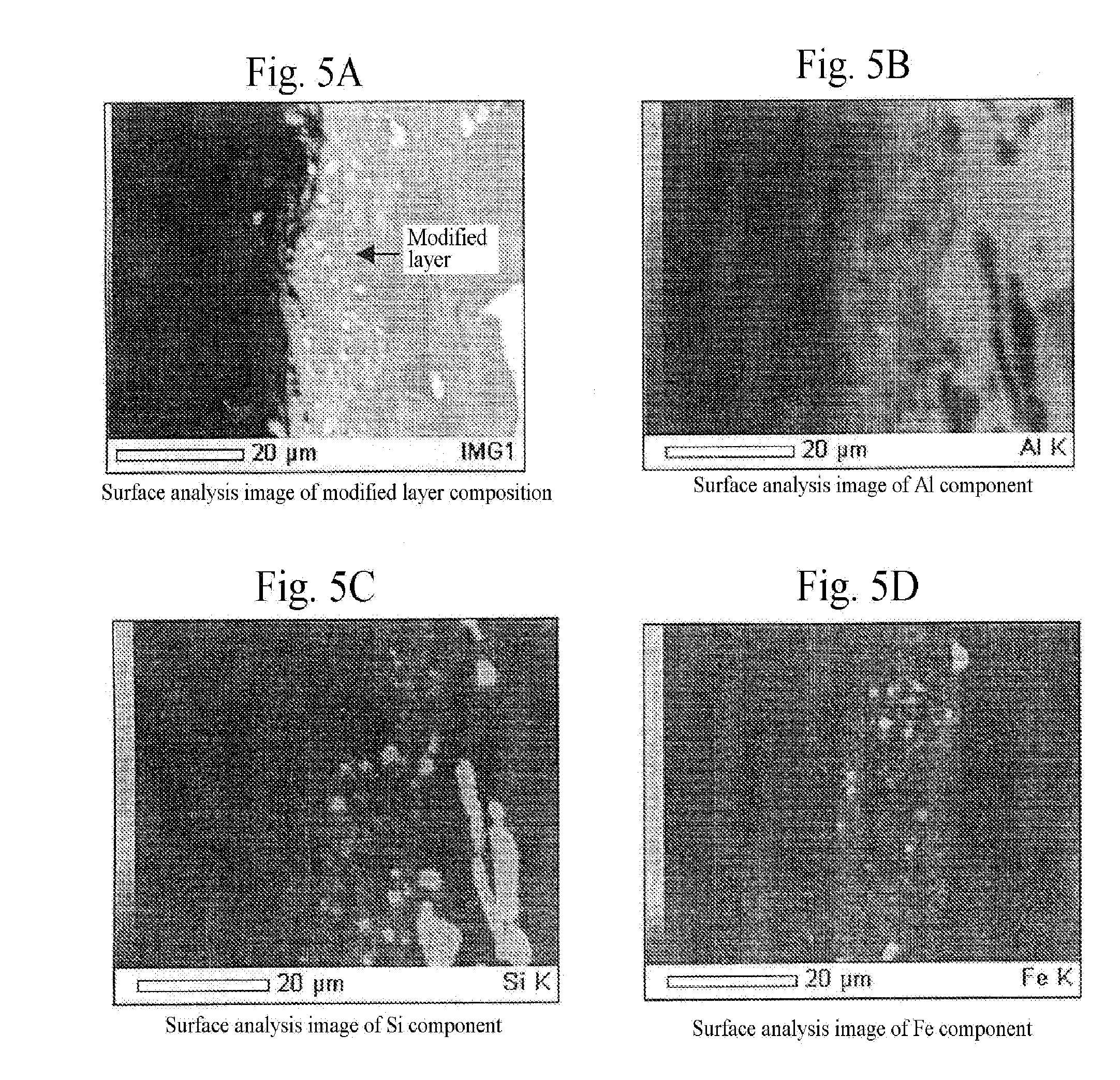
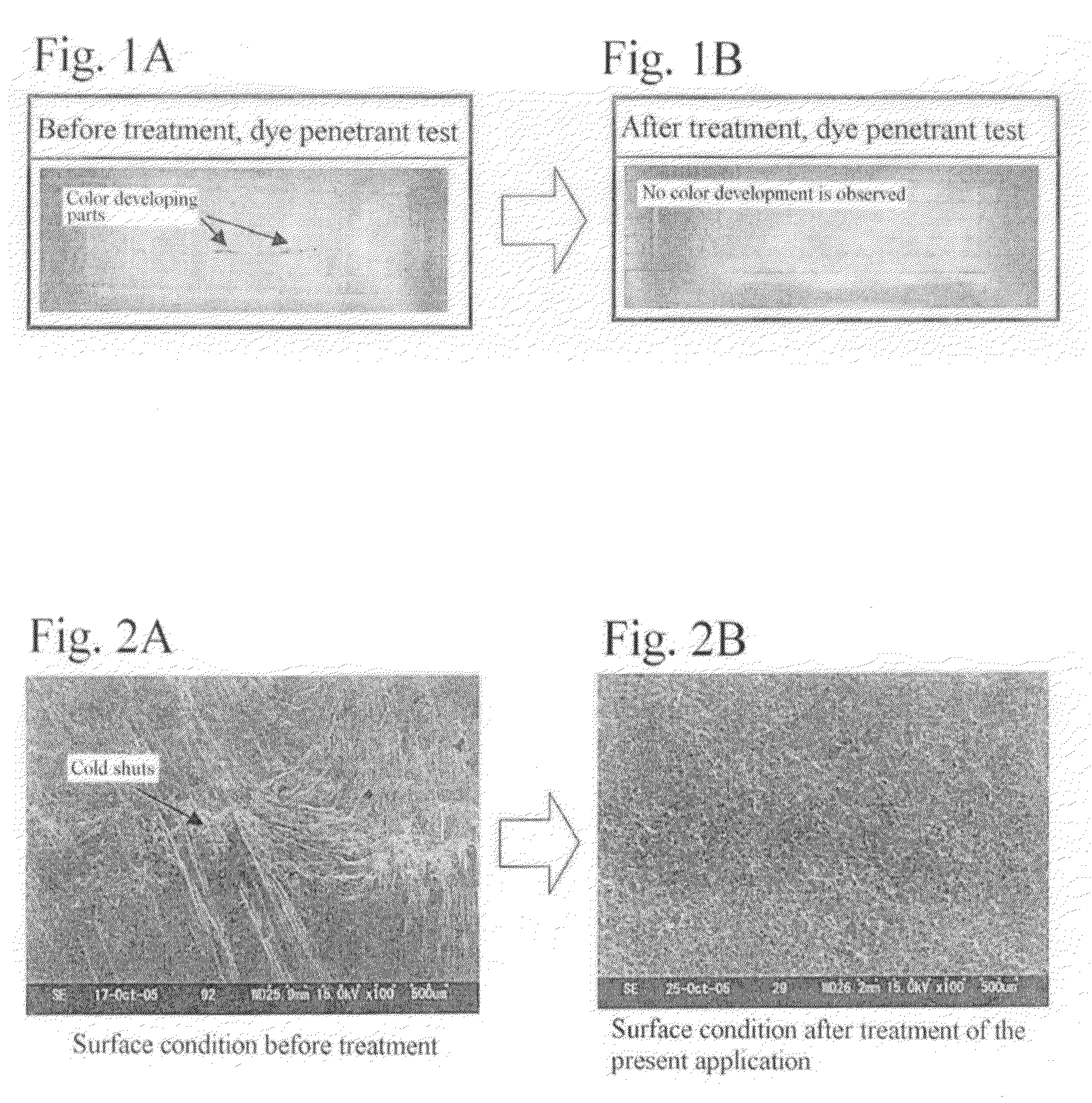
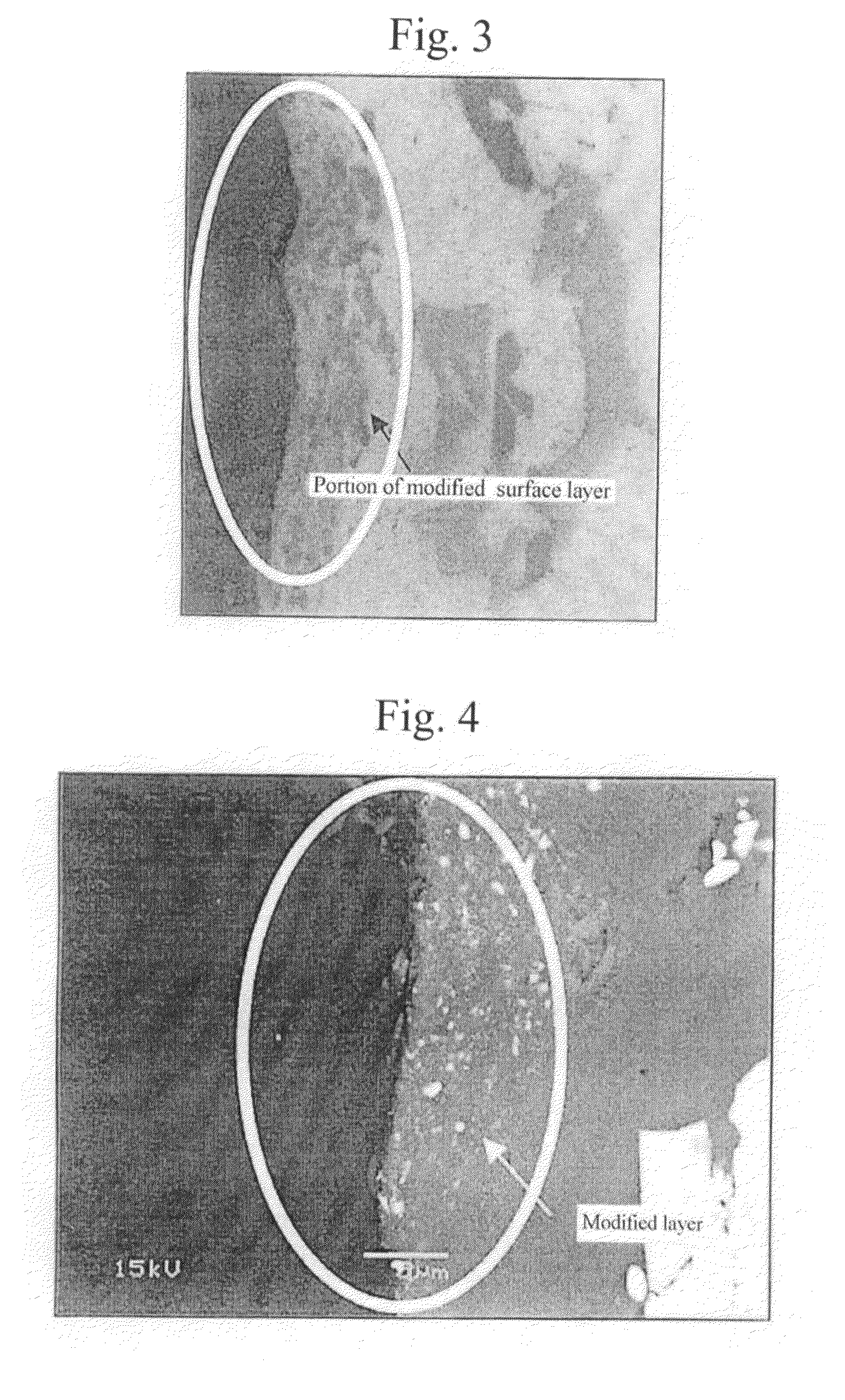
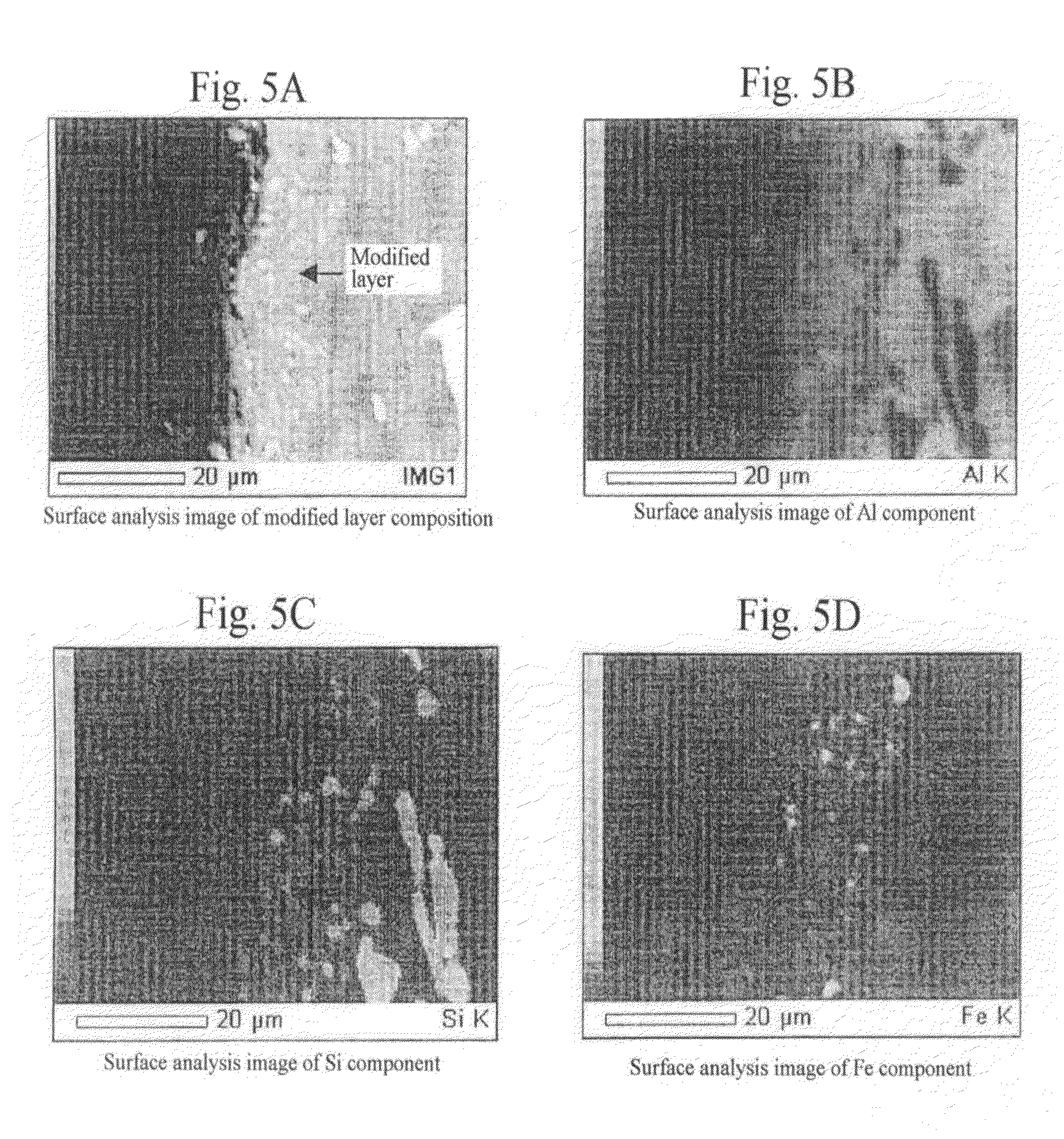
A method for surface treatment capable of easily improving a mechanical strength of an internal combustion piston at a reasonable cost is provided. A modified surface layer is formed by injecting injection powders containing a reinforcing element to be collided with an Al—Si alloy-based piston obtained by casting and forging by injecting under predetermined conditions, the reinforcing element being diffused and penetrated in the piston to improve the strength thereof. When a function, such as fuel modification, is imparted to the modified surface layer, an element exhibiting a photocatalytic function by oxidation, such as Ti, Sn, Zn, Zr, or W, is selected as the reinforcing element. By locally heating and cooling performed on the piston surface by the collision with the injection powders, alloy elements are fine-grained by recrystallization, the reinforcing element in the injection powders is diffused and penetrated in the piston surface by activated adsorption, and a modified layer having a uniformly fine-grained microstructure containing the alloy elements and the reinforcing element is formed. As a result, besides improvement in strength of the piston, by the selection of the above element, such as Ti, the photocatalytic function, such as fuel modification, can also be obtained.
Owner:ART METAL MFG +1
Kukoline intravenous transfusion preparation
ActiveCN101347408AOvercome the bias that IV route of administration is not possibleSolve the clinical problems prone to anaphylactic shockOrganic active ingredientsAntipyreticClinical efficacyRheumatism
The invention provides a sinomenine injection special for intravenous administration. The sinomenine injection is an injection which comprises 0.005-0.3wt% of sinomenine and an aqueous solvent for injection, or an injection which comprises sterile injection powder or lyophilized injection powder used for preparation just before injection to cause the sinomenine concentration to be 0.005-0.3wt% in the injection and the aqueous solvent for injection. The sinomenine injection of the invention is used for treating rheumatism, chronic pain and other chronic inflammatory diseases. Compared with other existing injection forms, the sinomenine injection special for intravenous injection has lower drug adverse reaction, and the clinical curative effect is obviously improved.
Owner:李蕴麟
Prepn process of Qingkailing injection and injection powder and its quality control method
The present invention provides a improved preparation process of Qingkailing injection and powder for injection. The improved preparation process includes high speed centrifugal treatment in extracting cape jasmine, isatis root and honeysuckle, merging extractive liquid, ultrafiltering and other technological steps. It can produce Qingkailing injection product and powder product for injection with ever higher stability and ever longer effective period. The present invention also discloses the quality control method of baicalin, cholic acid, hyodeoxycholic acid, jasminoidin and chlorogenic acid in Qingkailing injection and powder for injectino as well as identification method of Qingkailing injection and power for injection.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Medicament composition for improving stability of Ulinastatin
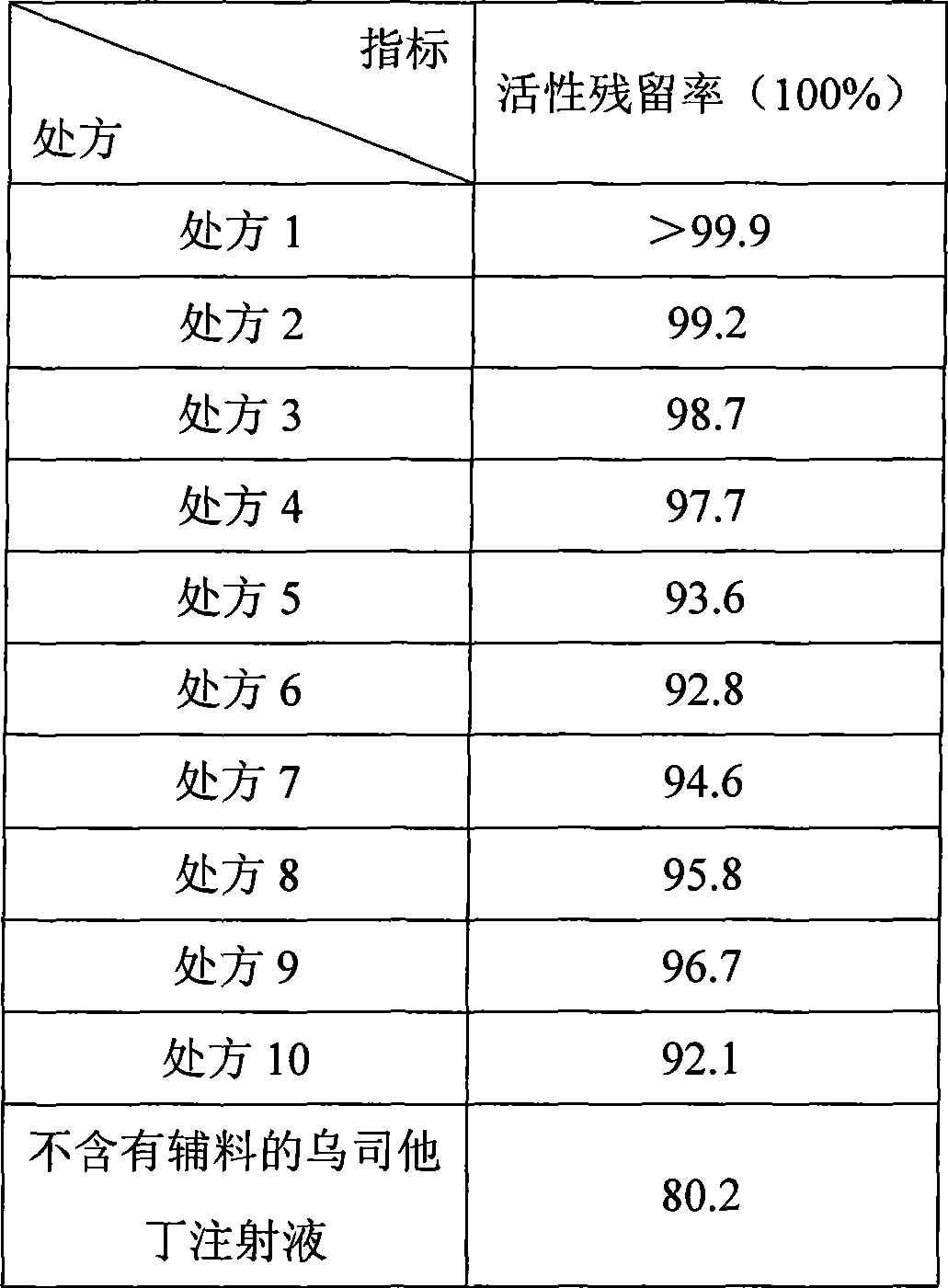
InactiveCN101439180AOvercome the problem of easy inactivation in clinical useImprove stabilityPeptide/protein ingredientsDigestive systemFreeze-dryingInjection powder
The invention discloses a stable ulinastatin sterile injection powder pharmaceutical composition which contains an effective dose of ulinastatin and a pharmaceutical excipient, and the excipient is one or the combination of a plurality of kinds of mannitol, hydrolyzed gelatin, dextran and sodium chloride. The composition is generally used in the form of the freeze-dried sterile injection powder, when in clinical application, the composition is dissolved in glucose or physiological saline and other large infusions, and the composition can significantly reduce the decrease of the activity of the ulinastatin in water solution.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Lyophilized injection powder using Lanluodier and its salt as active ingredients and preparing technique therefor
This invention is about a freeze-dried preparation using Llandeilo and its salt as active components and its making method and usages. It is a medicine composition that takes Llandeilo and its salt as active components, and is formed by mixing acceptable finding. It can be used to emergency cure tachycardia arrhythmia (containing atria fibrillation, atria flutter and sinus tachycardia) during operation. It uses Llandeilo and its salt as raw materials, adds some findings of specify kinds and proportions, and develop freeze-dried preparation according to this invention for intravenous injection.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
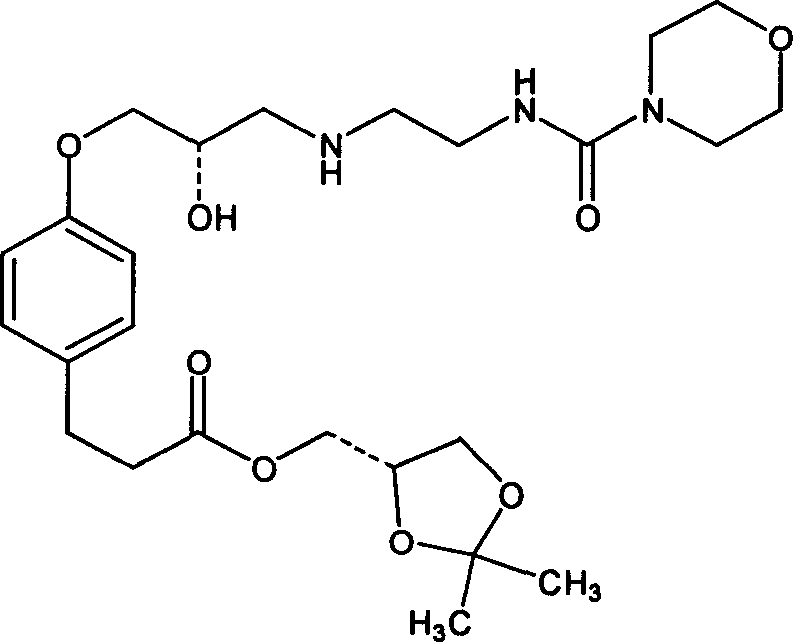
Octreotide acetate sterile injection powder preparation and preparation method thereof
InactiveCN104689297AExtended shelf lifeImprove stabilityPowder deliveryPeptide/protein ingredientsOctreotide acetateVitamin C
The invention relates to an octreotide acetate sterile injection powder preparation and a preparation method thereof. The octreotide acetate sterile injection powder preparation is prepared by octreotide acetate, TBA (Tert Butyl Alcohol), mannitol, dextran and a vitamin C. The preparation method comprises the following steps: 1) adding the vitamin C, the mannitol, the dextran, the TBA and the octreotide acetate into purified water sequentially, and stirring to completely dissolve; 2) filling a prepared liquid medicine in an ampoule bottle for freeze-drying. According to the octreotide acetate sterile injection powder preparation provided by the invention, the guarantee period can be greatly improved, the stability can be improved, and the preparation method is simple.
Owner:陈卓杰
Dispensing method used for injection powder bottle and dispensing device
ActiveCN104224537AEasy to operateSimple structureInfusion devicesPharmaceutical containersAutomatic controlAir pump
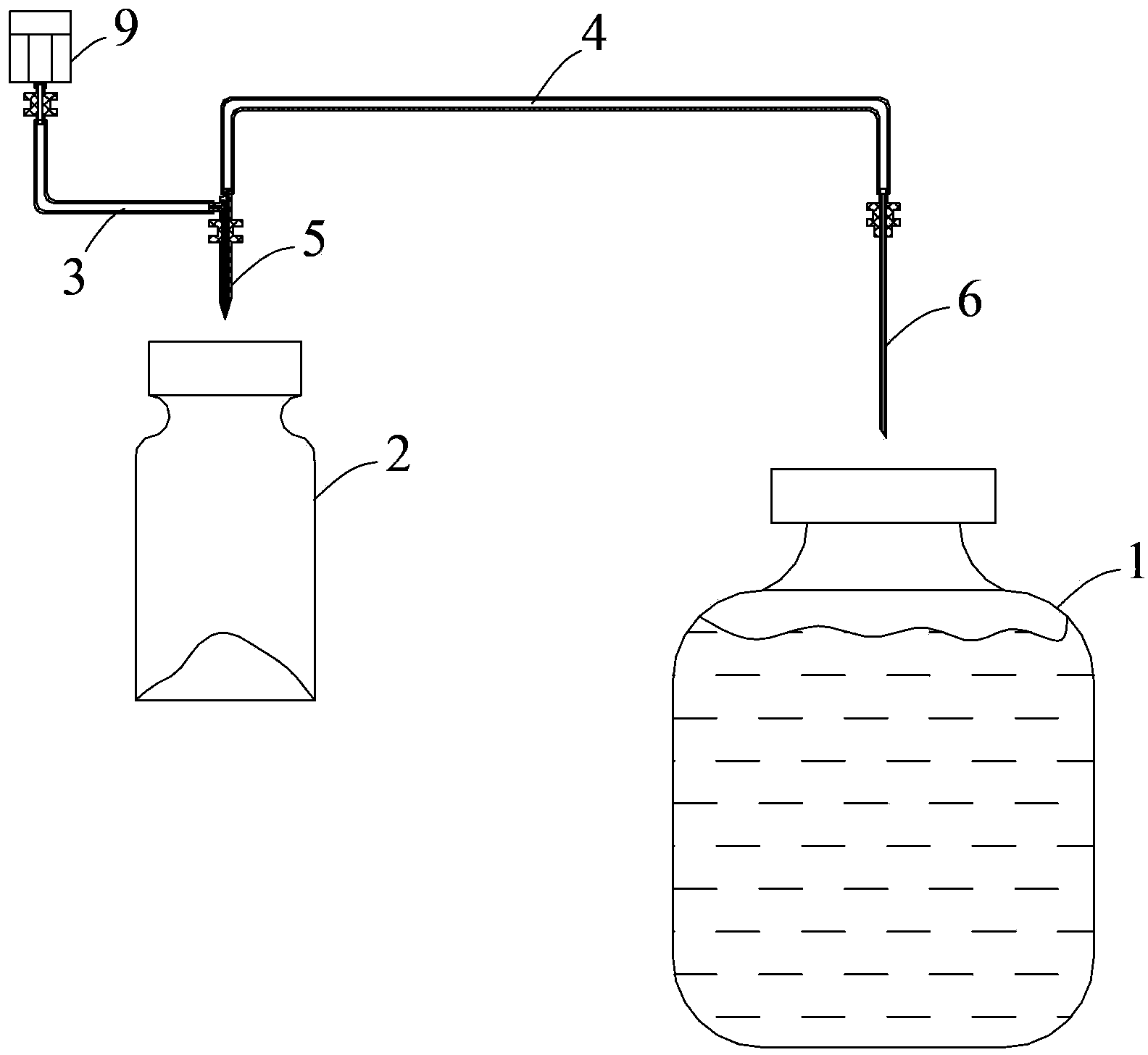
The invention discloses a dispensing method used for an injection powder bottle and a dispensing device. The dispensing method includes the steps of making preparations, inserting needles to the injection powder bottle and a transfusion bag respectively, sucking a part of liquid medicine in the transfusion bag into the injection powder bottle, pulling out the needles after sucking is finished, shaking the injection powder bottle to enable the liquid medicine and injection powder to be uniformly mixed and turning the injection needle bottle by 180 degrees, inserting the first syringe needle into the injection powder bottle upwards, injecting the liquid medicine in the injection powder bottle into the transfusion bag, pulling out the first syringe needle, and dispensing under the assistance of a first liquid level controller and a second liquid level controller. The dispensing device comprises a catheter, a first syringe needle and a second syringe needle, the first syringe needle comprises a transfusion needle hole and a ventilation needle hole, the ventilation needle hole communicates an air pipe with an air pump, the first syringe needle, the second syringe needle and the air pipe are provided with a first clamping head, a second clamping head and a third clamping head respectively and the first clamping head, the second clamping head and the third clamping head are convenient to clamp. According to the dispensing method, needle insertion is easy to carry out, operations are convenient to carry out, the first syringe needle and the second syringe needle of the dispensing device are simple in structure and low in cost, liquid medicine can be automatically dispensed when the first syringe needle and the second syringe needle are matched with a mechanical automatic control device, and dispensing efficiency is improved.
Owner:CHENGDU TECHCAL UNIV
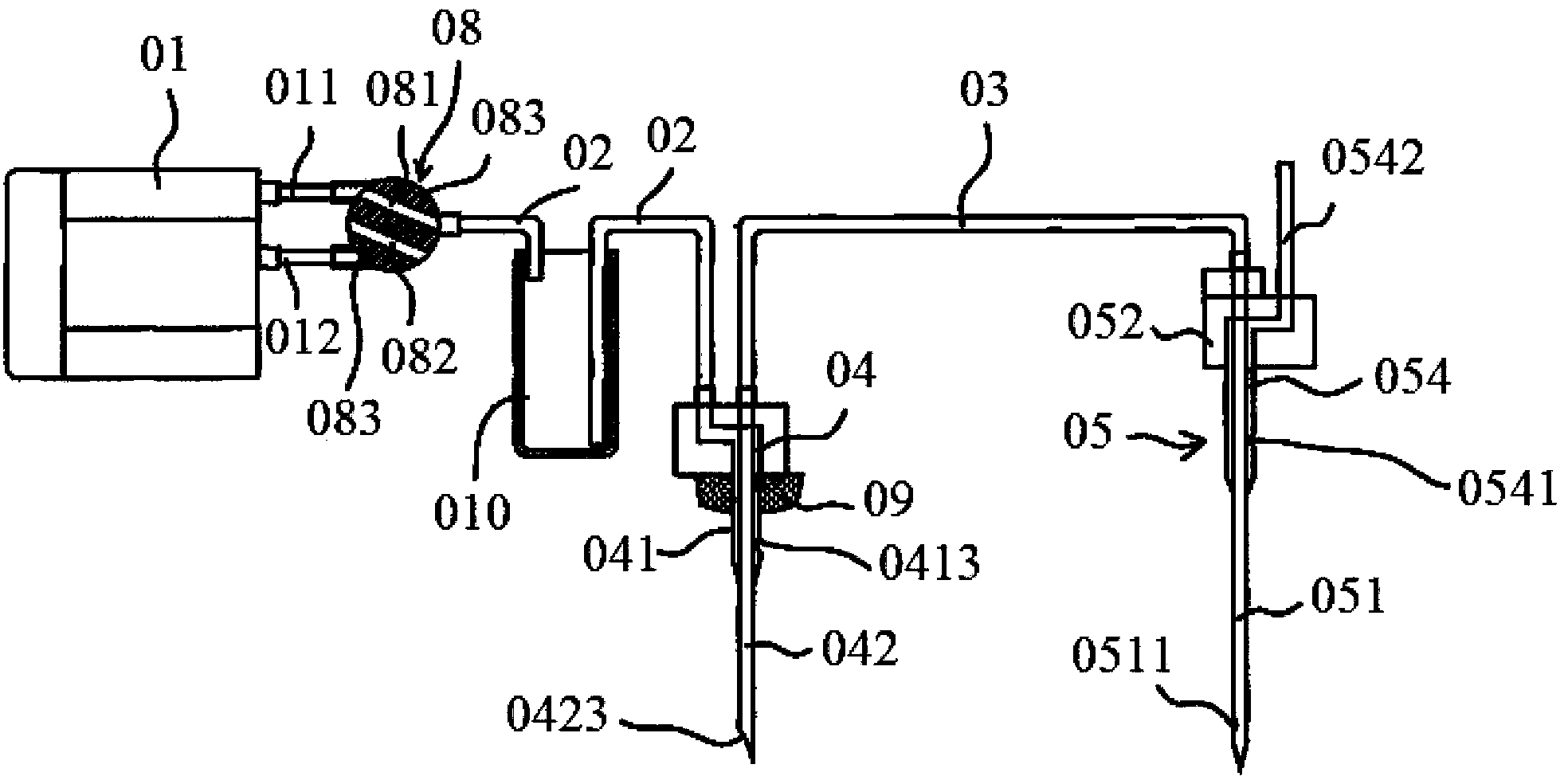
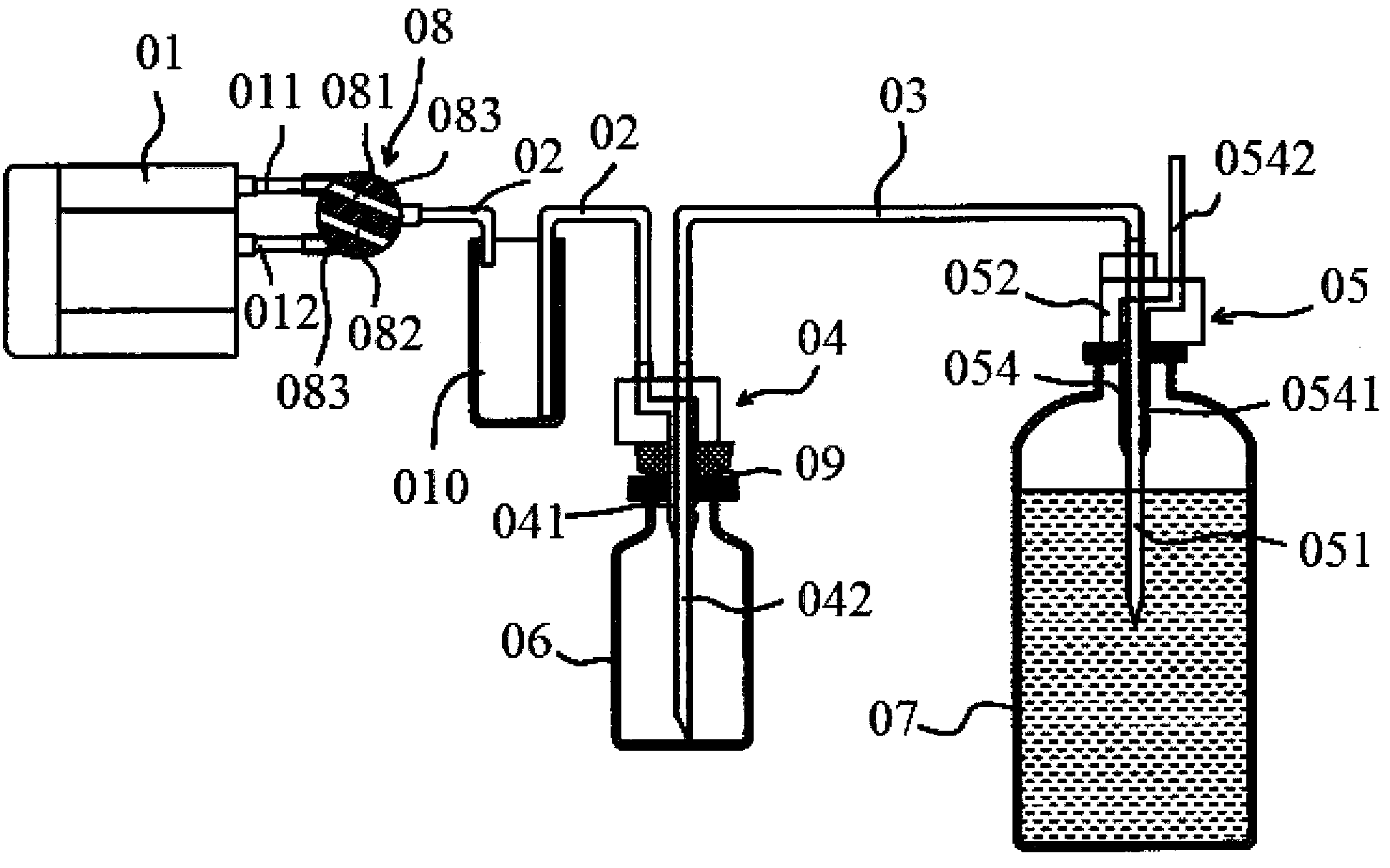
Automatic injection powder mixing syringe
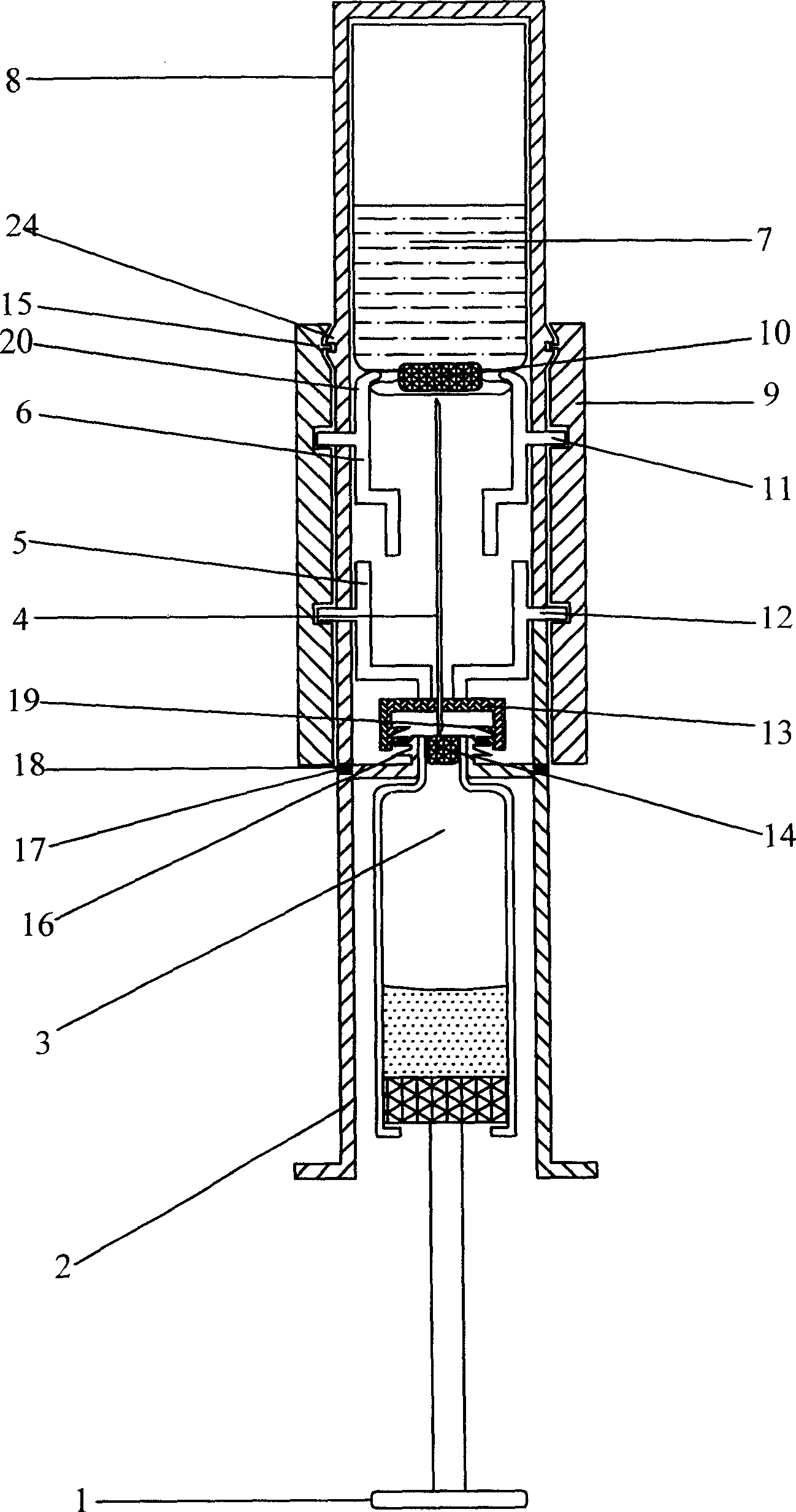
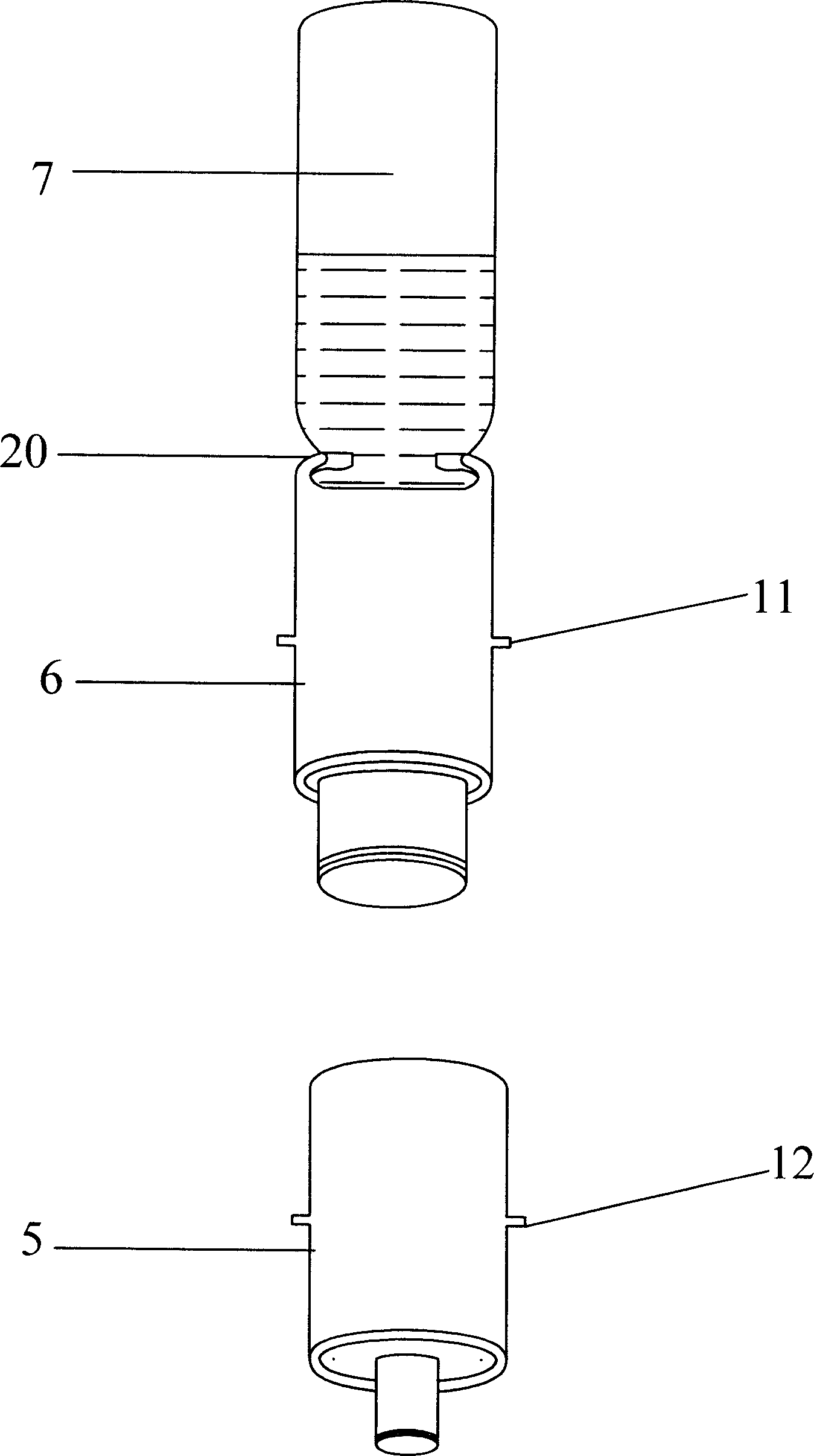
ActiveCN1772313AReduce labor intensityEasy to operateInfusion syringesPharmaceutical containersSyringe CapsBottle
The automatic injection powder mixing syringe includes solvent bottle, solute bottle and syringe cap, as well as solute bottle coating, solvent bottle coating, rotary coating, the first push unit and the second push unit. Rotating the rotary coating can drive the first push unit and the second push unit, so that the needle in the syringe cap massive pierces the rubber plug in the solute bottle and that in the solvent bottle successively and the solvent under the action of the pressure flows to the solute bottle for automatic mixing. The solvent bottle coating is then rotated off, and the solute bottle with the solute bottle coating serves as one syringe. The present invention is disposable to avoid blood contamination.
Owner:ZHONGXIN FUJIAN MEDICAL TECH CO LTD
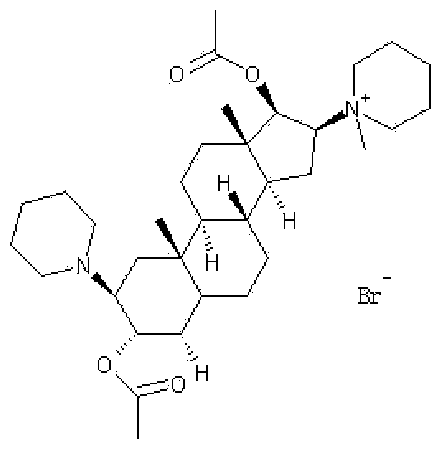
Vecuronium bromide freeze-dried powder injection for injection and preparation method thereof
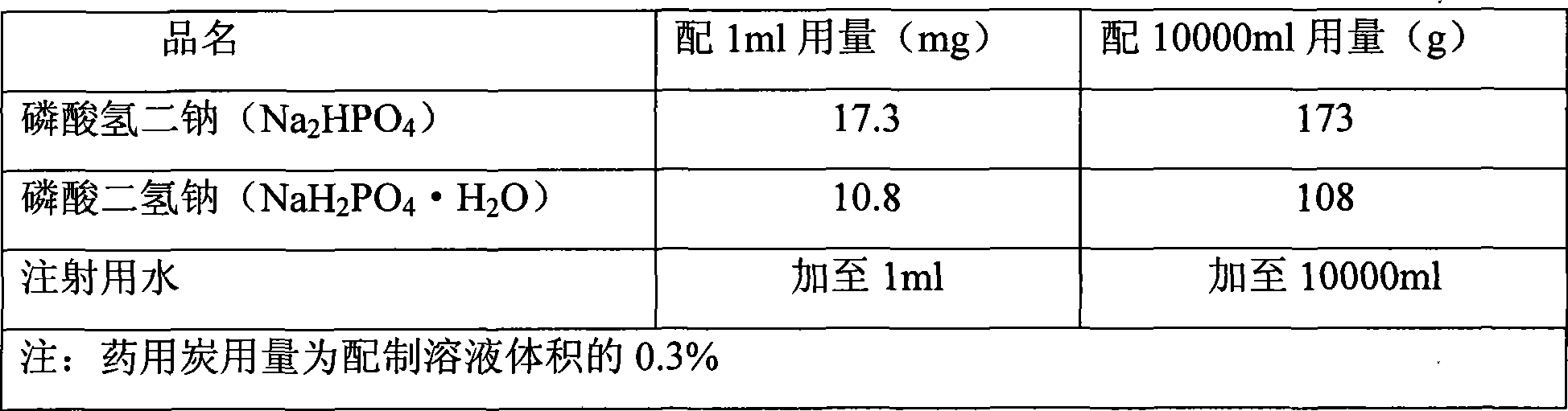
The invention discloses a vecuronium bromide freeze-dried powder injection for injection and a preparation method thereof. The vecuronium bromide freeze-dried powder injection for injection, provided by the invention, is prepared from the following raw materials: 4 g of vecuronium bromide, 5 to 7.5 g of mannitol, 5 to 7.5 g of lactose, 2.5 to 5 g of mycose, 3 to 3.5 g of citric acid, 6.5 to 7 g of disodium hydrogen phosphate and water for injection which is added until the volume is 1000 ml. The freeze-dried formula adopts combination of vecuronium bromide, mannitol, lactose, mycose, citric acid and disodium hydrogen phosphate and is combined with accurate freeze-drying process parameter control, so the appearance is full and not withered, the texture is loose and porous, the stability is high, the rehydration speed is high, and the requirement of the freeze-dried injection powder injection is met.
Owner:HAINAN STAR PHARM CO LTD
Stable water injection medicament composition containing Ulinastatin
ActiveCN101439181AConvenient treatmentPeptide/protein ingredientsDigestive systemSodium acetateFreeze-drying
The invention discloses a stable ulinastatin pharmaceutical composition which contains an effective dose of ulinastatin and a pharmaceutical excipient with a certain proportion. Wherein, the excipient can be one or more of mannitol, sodium chloride, sodium acetate and acetic acid, and the range of pH value is 4.2-6.0. The composition is generally used in the form of injection, when in clinical application, the composition can be directly used for intravenous infusion and the composition can significantly improve the problem that the activity of an ulinastatin freeze-dried sterile injection powder preparation is easy to decrease during the preparation.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
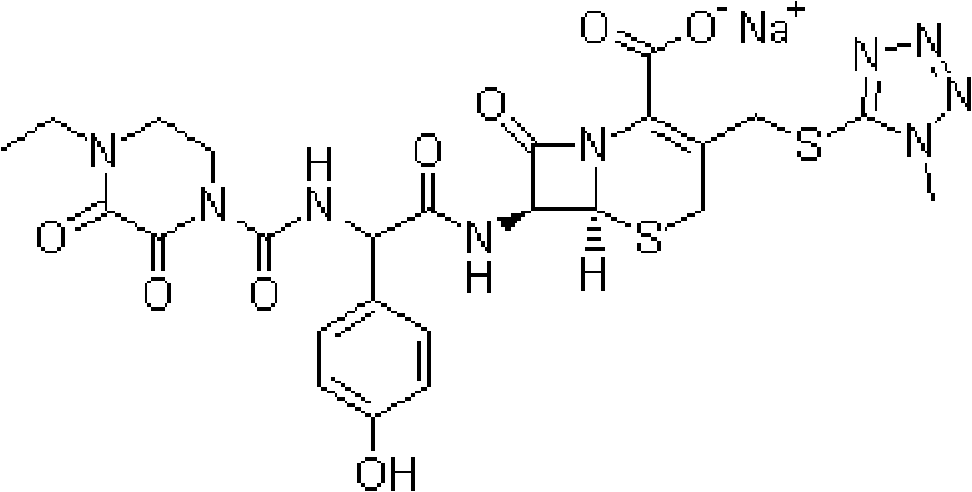
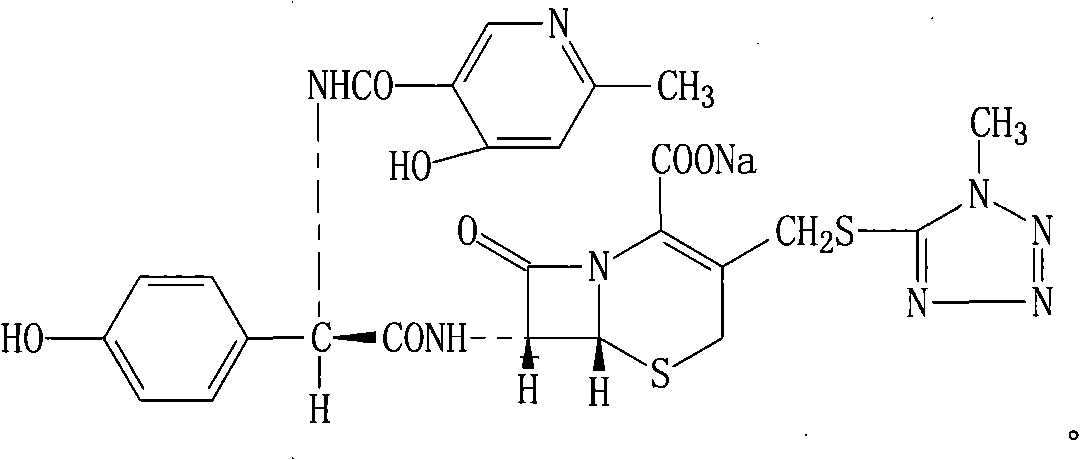
Injection powder and injection preparation of cefoperazone sodium-tazobactam combination
ActiveCN102552275AImprove efficacyAntibacterial agentsPowder deliveryCurative effectInjection powder
The invention relates to the technical field of medicine, in particular to an injection powder and injection preparation of a cefoperazone sodium-tazobactam combination. The injection powder and injection preparation comprises cefoperazone sodium, tazobactam combination and lignocaine hydrochloride, wherein the mass ratio of the cefoperazone sodium to the tazobactam combination to the lignocaine hydrochloride is 4:1:(0.01-0.05). Compared with a positive medicament control group, the injection powder and injection preparation of the cefoperazone sodium-tazobactam combination provided by the invention has the advantages of capability of relieving injection pain and remarkable improvement on the curative effect.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Plastic packaging material for injection powder and preparation process thereof
ActiveCN105175849AImprove puncture strengthHigh tear strengthSynthetic resin layered productsPlastic packagingRoom temperature
The present invention relates to a plastic packaging material for injection powder and a preparation process thereof, and belongs to the field of pharmaceutical packaging materials. The plastic packaging material has a three-layer structure comprising an inner layer, a middle layer and an outer layer. The inner layer and outer layer are made from the following components by weight: 5-15% of mVLDPE, 10-25% of mLLDPE, 25-45% of LLDPE and 40-60% of LDPE; and the middle layer is made from the following components by weight: 5-15% of mLLDPE, 20-40% of LLDPE and 45-75% of LDPE. The plastic packaging material provided by the present invention has good toughness, high transparency, low heat viscosity low heat sealing temperature and small odor, and performances superior to conventional polyethylene products; the plastic packaging material has excellent anti-impact resistance and resistance to environmental stress cracking at room temperature or even at a low temperature of -80 DEG C, excellent barrier properties and tearing strength. The invention provides a better securer choice with higher quality for aseptic pharmaceutical production and packaging.
Owner:ZIBO HUA ZHI LIN PACKING PROD CO LTD
Method for surface treatment of an internal combustion piston and an internal combustion piston
ActiveUS7767033B2High mechanical strengthHigh strengthLiquid surface applicatorsSolid state diffusion coatingCombustionSurface layer
A method for surface treatment capable of easily improving a mechanical strength of an internal combustion piston at a reasonable cost is provided. A modified surface layer is formed by injecting injection powders containing a reinforcing element to be collided with an Al—Si alloy-based piston obtained by casting and forging by injecting under predetermined conditions, the reinforcing element being diffused and penetrated in the piston to improve the strength thereof. When a function, such as fuel modification, is imparted to the modified surface layer, an element exhibiting a photocatalytic function by oxidation, such as Ti, Sn, Zn, Zr, or W, is selected as the reinforcing element. By locally heating and cooling performed on the piston surface by the collision with the injection powders, alloy elements are fine-grained by recrystallization, the reinforcing element in the injection powders is diffused and penetrated in the piston surface by activated adsorption, and a modified layer having a uniformly fine-grained microstructure containing the alloy elements and the reinforcing element is formed. As a result, besides improvement in strength of the piston, by the selection of the above element, such as Ti, the photocatalytic function, such as fuel modification, can also be obtained.
Owner:ART METAL MFG +1
Medicinal disodium creatine phosphate hexahydrate and preparing method thereof
ActiveCN100488968CHigh purityLow drying temperatureGroup 5/15 element organic compoundsCreatinine riseDistillation
This invention discloses a phosphocreatine disodium salt six-hydrate and its preparation method. The compound is phosphocreatine disodium salt with high purity and six-crystal water, and the preparation method includes the following steps: (1) Kondens of creatinine and phosphorus oxychloride prepares creatinine phosphorus oxychloride, (2) creatinine phosphorus oxychloride is taken the ring opening reaction to get crude phosphorus disodium, (3) purification and crystallization gets the target product. This invention has the advantages of high purity suitable for the manufacture of injection powder, and it avoids the large use of solvents with high yield of products. The excessive phosphorus oxychloride is reused with distillation, and the product has high purity through intermediate and resin purification. It has no potential safety problem about heavy metal residues, and can effectively improve the clarification to reach the intravenous requirement.
Owner:QIDONG HUATUO PHARMA
Steel ladle powder injection desulfuration refining process for low-sulfur steel production
The invention relates to a steel ladle powder injection desulfuration refining process for low-sulfur steel production. The process is characterized in that: 3.0 to 6.5kg / t of aluminum iron for strong deoxidation is adopted in converter tapping; 0.4 to 0.6kg / t of aluminum for slag surface deoxidation is added on the steel ladle slag surface after tapping is finished; the oxidation property (FeO%+Mn%) of station reaching top slag of steel ladles is less than or equal to 3.5 percent; the temperature of the molten steel reaching a powder injection desulfuration station is 1,610 to 1,640 DEG C, and Alt in the steel is 0.040 to 0.090 percent; the working pressure of steel ladle powder injection desulfuration delivery carrier gas is 3.0 to 4.0bar, and the flow is 40 to 60m<3> / h; and the powder injection velocity of steel ladle powder injection desulfuration is 40 to 60kg / min, and the consumption of the injection powder is 4.0 to 6.0 kg / t of steel. After the steel ladle powder injection desulfuration process is adopted, the sulfur content of the steel can be reduced from 50-80ppm to within 12ppm, and the desulfuration rate can reach 78.0 to 85.0 percent.
Owner:SHOUGANG CORPORATION
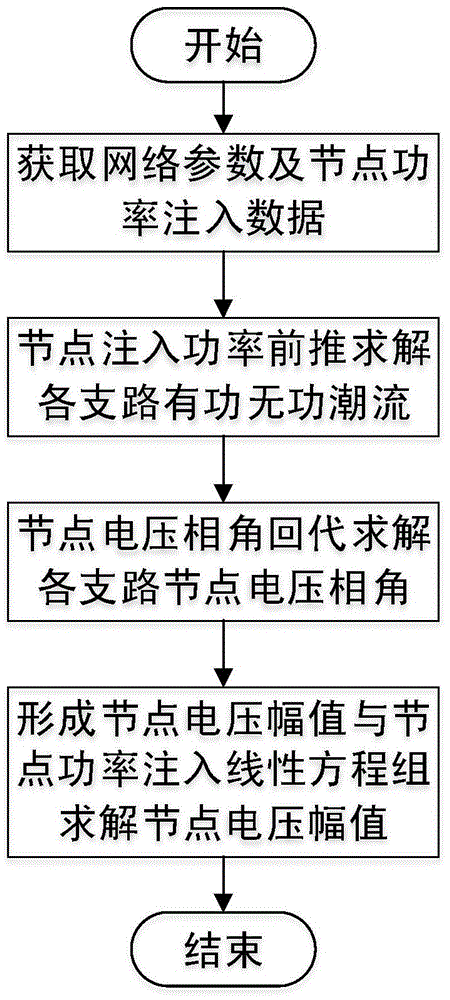
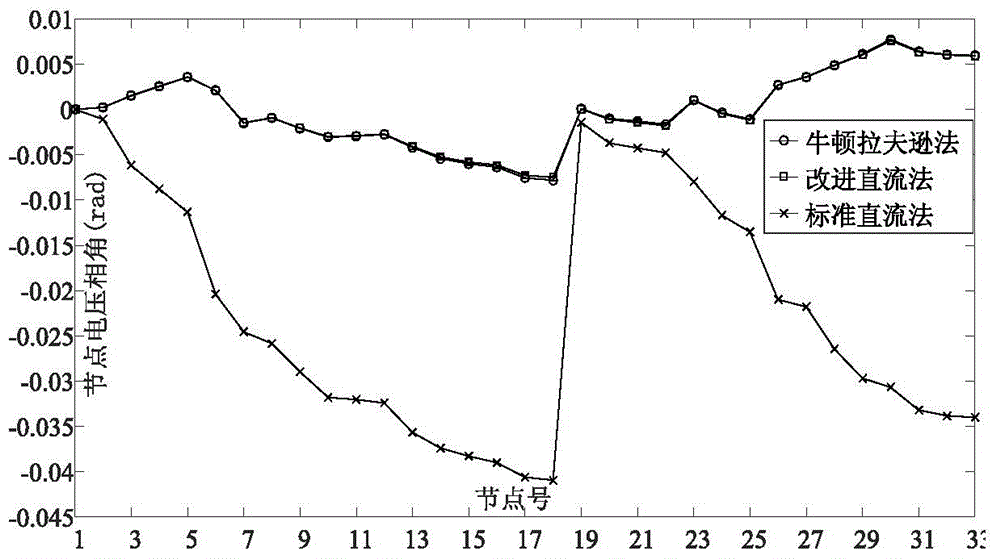
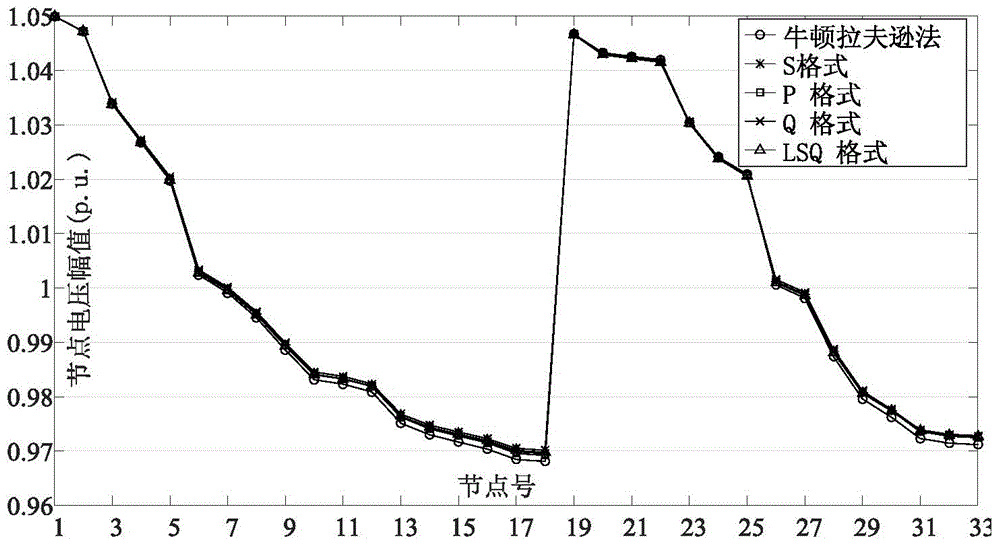
Modified direct-current power flow algorithm suitable for online analysis of distribution network
ActiveCN104573384AFast solutionHigh solution accuracySpecial data processing applicationsVoltage amplitudeNumerical stability
The invention discloses a modified direct-current power flow algorithm suitable for online analysis of a distribution network and belongs to the field of power flow analysis for power systems. The algorithm is applicable to the fields such as online analysis of the distribution network and real-time optimal control. The algorithm includes solving active and reactive power flows of branches of the distributed network by forward sweeping of node injection powder from the tail end of the distribution network to a root node; solving voltage phase angles of nodes of the distributed network by back substitution from the root node of the distributed network to a distribution terminal; acquiring linear equations of node voltage amplitudes and node injection powers by omitting deviation second-order terms of node voltage amplitudes of the distributed network, and solving the linear equations to obtain node voltage amplitudes. The algorithm has the advantages that the advantages of the standard direct-current power flow method are retained, no hypothesis is made to line parameters, the algorithm is suitable for radial power grids of any parameters, both the node voltage amplitudes and the node voltage phase angles can be computed, and the solving precision and the value stability are greatly improved as compared with those acquired by the standard direct-current power flow algorithm.
Owner:SOUTHEAST UNIV
CefPiramide sodium powder for injection
ActiveCN101317822AStable structureStructural influenceAntibacterial agentsPowder deliveryFreeze-dryingCefpiramide sodium
The invention provides a freeze-dried injection powder of Cefpiramide Sodium. The whole process of the preparation method of the injection powder is carried out in water solution and the obtained Cefpiramide Sodium has high purity. The water content of the product prepared by the freeze-drying technology of the invention can be controlled below 1 percent. The freeze-dried injection powder of Cefpiramide Sodium has high dissolution rate and good stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for manufacturing valve train parts using metal powder injection molding
InactiveUS9085028B2High dimensional accuracyImprove economyValve arrangementsTransportation and packagingInjection powderSolvent
Disclosed is a method for manufacturing a plurality of valve train parts using metal powder injection molding, comprising: obtaining a raw material for injection molding by mixing a metal powder and a binder; forming a formed body by injecting the obtained raw material for injection molding into a mold of a valve train part shape; solvent extracting the formed body; forming a sintered body by debinding and sintering the solvent extracted formed body; sizing processing the sintered body; vacuum carburizing the sizing processed sintered body; and polishing the vacuum carburized sintered body.
Owner:HYUNDAI MOTOR CO LTD +1
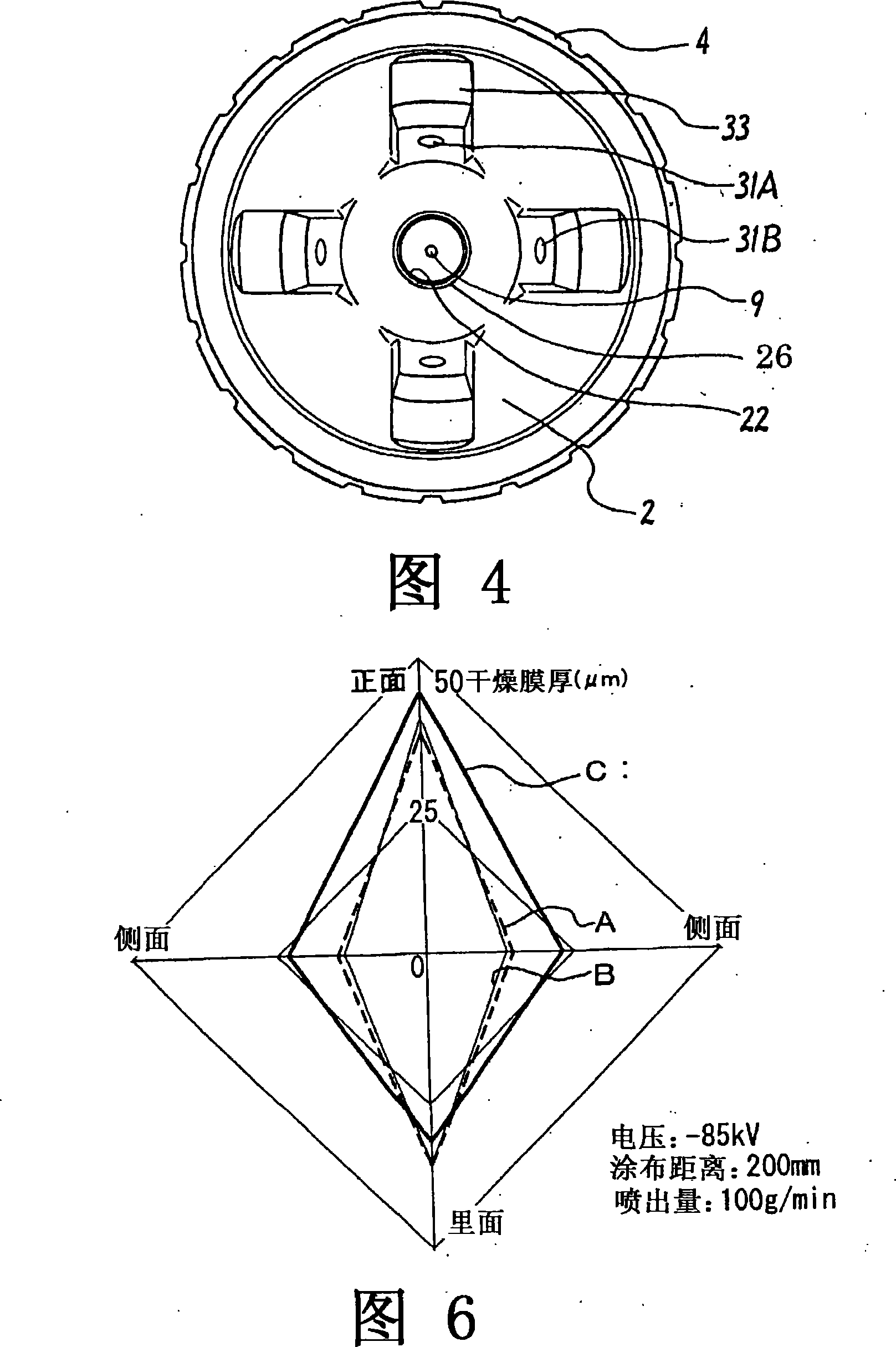
Spray gun for powder electrostatic coating
InactiveCN101184555ALess ejection operationImprove spraying efficiencyLiquid spraying apparatusSpray discharge apparatusElectrostatic coatingInjection powder
The utility model relates to a spray gun for powder electrostatic spraying, the pattern width of which can be easily adjusted to form a spray pattern with a corresponding spray speed and a corresponding width. A higher charging effect can be applied to the spray particles through the charged electrode, and the spraying efficiency can be improved. On both sides of the ejection port (22), there are adjusting air ports (31) for jetting compressed air towards the center, and the pattern width can be adjusted by adjusting the flow rate of the compressed air. An auxiliary air port (23) is provided at the ejection port (22) for ejecting compressed air along the powder jet flow around the central opening (21) where the powder is ejected. Through the compressed air from the auxiliary air port (23), the straight-forward force is exerted on the spray flow, which corresponds to the shape of the sprayed object and the sprayed part under the optimal condition. In addition, a charging electrode (9) is arranged at the center of the ejection port (22), and the tip of the electrode is placed at or in front of the collision / scattering position of the above-mentioned jet air.
Owner:ANEST IWATA CORP
Freeze-drying method of pseudo-ginseng total saponin freeze-dried injection powder with filling amount more than 500 mg
ActiveCN102429942AShorten drying timeShorten the timePowder deliveryPharmaceutical product form changeFreeze-dryingMedicine
The invention relates to a freeze-drying method of pseudo-ginseng total saponin freeze-dried injection powder with a filling amount more than 500 mg. In the method, freeze-dried is carried out mainly through specific refrigerating parameters. The freeze-drying method provided by the invention has good drying effect and significantly shortened freeze-drying time, which is 20-50% of common freeze-drying time.
Owner:GUANGXI WUZHOU PHARMA GRP
Device and method for desulfurization by rotating inverted T-shaped spray gun
InactiveCN102719590AImprove dynamic conditionsSpeed up desulfurization mass transfer processMotor driveControl system
The invention provides a device and a method for desulfurization by rotating an inverted T-shaped spray gun. A rotary motor is installed on a spray gun fixing frame, an inverted T-shaped spray gun is movably connected with a spray gun base, wherein a chain wheel is arranged on the upper part of the inverted T-shaped spray gun, the spray gun is connected with a chain wheel of the rotary motor by a chain, and the rotary motor is connected with a motor control system. A lifting mechanism drives the spray gun to insert into molten iron in an iron ladle, when powder feeding speed ratio reaches a set value, the rotary motor drives the spray gun to rotate forwardly and reversely, and when the injection powder amount reaches a set value, the motor stops rotating, and the lifting mechanism lifts the spray gun to the limit position. According to the device and the method, the dynamic condition of the desulfurization of molten iron is improved obviously, the acceleration of the desulfurization mass transfer process is facilitated, the desulfurization processing time is shortened, the magnesium floating stroke is increased due to magnesium-base desulfurization, the utilization ratio of magnesium is increased, the mixing time of sulfur and a desulfurizing agent in the iron ladle is shortened, the desulfurization effect is improved, and the phenomena of splashing and gun blocking in the blowing process are reduced.
Owner:ANGANG STEEL CO LTD
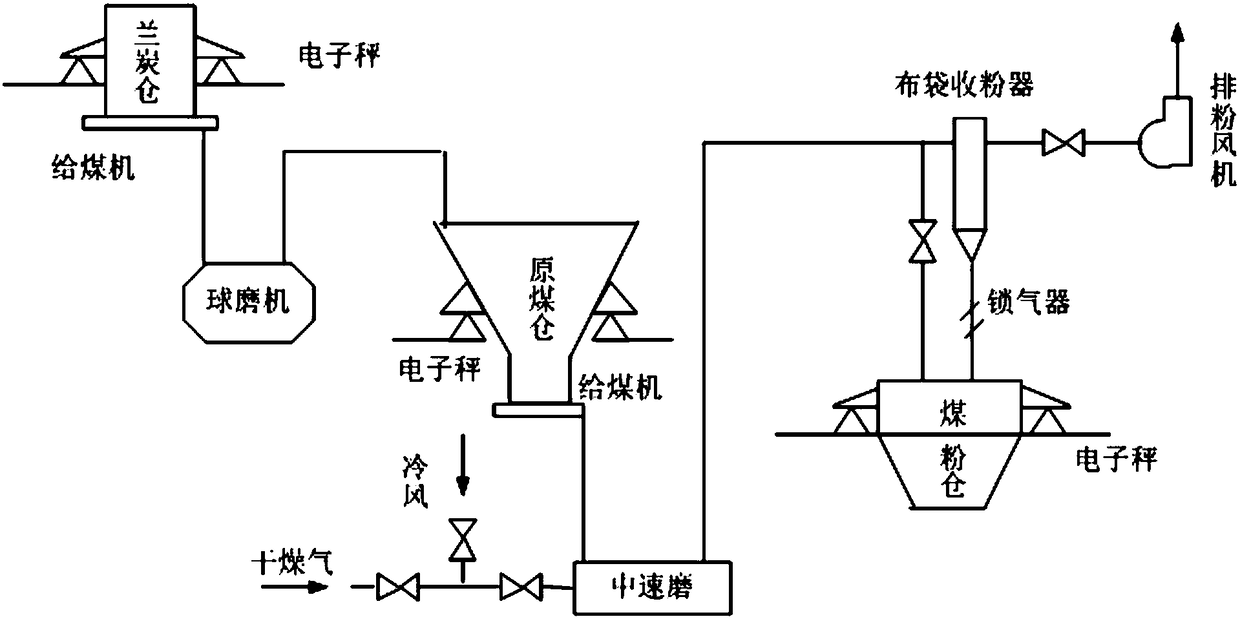
Blast furnace injection pulverized coal preparation method with semi-coke as blast furnace injection raw material
InactiveCN108531674AImprove hard-to-grind situationsIncrease usageBlast furnace detailsGrain treatmentsSize differenceInjection equipment
The invention relates to a blast furnace injection pulverized coal preparation method with semi-coke as a blast furnace injection raw material. The method adopts the semi-coke as the raw material forpreparing blast furnace injection pulverized coal; and in addition, before the semi-coke is made into pulverized coal finally through a medium-speed mill, the step that the semi-coke is separately manufactured into semi-coke prefabricated powder is included. According to the method, the step of preparing the semi-coke prefabricated powder is added based on an existing blast furnace injection powder preparation technology, thus, the particle size difference is formed between semi-coke particles and soft coal, the grinding effect of the semi-coke particles is improved, the problem that in the existing technologies, semi-coke is difficult to ground is solved, accordingly, the soft coal and the semi-coke can be ground into powder at the same time, and efficient injection of a blast furnace isachieved finally; and in addition, the corner angle sharpness of the semi-coke particles can be perfected by preparing the semi-coke prefabricated powder, the edges of the semi-coke particles are smooth, and the effect of relieving abrasion of transport injection equipment in the follow-up injection process is well achieved.
Owner:包头市国卉能源科技有限责任公司
Icatibant composition for injection and preparation method and preparation thereof
InactiveCN104043101ANo degradationGuaranteed stabilityPowder deliveryPeptide/protein ingredientsFreeze-dryingDissolution
The invention relates to the technical field of medicine, and in particular relates to an Icatibant composition for injection and a preparation method and a preparation thereof. Icatibant composition for injection includes Icatibant, a freeze-drying protective agent, a cosolvent and stabilizer, and a pH regulator, wherein the mass ratio of Icatibant, the freeze-drying protective agent and the cosolvent and stabilizer is 1:(1-100):(1-100). The freeze-drying protective agent ensures that Icatibant does not degrade in the preparation and preservation processes; the cosolvent and stabilizer not only solves the problem of easy precipitation of Icatibant in solution state, but also plays the role of accelerating the re-dissolution of the freeze-dried injection powder preparation; the pH regulator can regulate the pH value of the Icatibant composition for injection after re-dissolution, and further maintains the stability of Icatibant. The Icatibant composition for injection provided by the invention has good stability, simple and reasonable formula and good re-dissolution performance, and can be prepared into the freeze-dried injection powder without being prepared into a pre-filled syringe; and the production process is simple.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Medicine composition for treating coronary heart disease and its prepn process
ActiveCN1846762ANo adverse reactionSafe for clinical usePowder deliveryPill deliveryCoronary artery diseaseCoronary heart disease
The present invention discloses one kind of Chinese medicine composition for treating coronary heart disease and its preparation process. The Chinese medicine composition is prepared with the Chinese medicinal materials, including astragalus root, Dangshen, ophiopogon root, schisandra and southern schisandra. The preparation process includes water extracting, filtering, centrifuging, decompression concentrating, adding dextrin, drying, adding pharmaceutically acceptable excipient and other steps to prepare granule, capsule, dripping pill, tablet, slow releasing preparation, oral liquid or injection powder. The present invention also provides the quality control method for the Chinese medicine composition preparing process and the usage of the Chinese medicine composition.
Owner:ZHEJIANG XINGUANG PHARM CO LTD
Cleanliness detection method of rubber plugs for sterile powder for ultraviolet absorbance evaluative injection
InactiveCN106198426AEasy to operateImprove accuracyColor/spectral properties measurementsUltravioletSterile water
The invention provides a cleanliness detection method of rubber plugs for sterile powder for ultraviolet absorbance evaluative injection. The cleanliness detection method includes soaking the rubber plugs into an ethanol solution with the mass concentration of 15%, an isopropanol solution with the mass concentration of 50% and normal saline respectively before storing; putting the rubber plugs into a thermostat, and soaking the rubber plugs at the temperature of 60 DEG C for one month; measuring ultraviolet absorbances of extracts at the positions of 220nm, and recording absorption spectra in different solvents; according to different ultraviolet absorbances, comparing ultraviolet absorption conditions of the plug rubbers for different sterile injection powder in the same solvent, and judging the cleanness of the formula rubber plugs. The cleanliness detection method has the advantages that the cleanness of the rubber plugs for the sterile powder for ultraviolet absorbance evaluative injection can be judged quickly and simply, and the foundation is laid for research on compatibility between the rubber plugs and drugs as well as for product screening; the method is simple to operate and easy to popularize.
Owner:郑州翱翔医药科技股份有限公司
Cefmetazole sodium suspension injection powder and novel application thereof
InactiveCN101780053AIncrease spawn rateEasy to wrapPowder deliveryOrganic active ingredientsEmulsionWhole body
The invention provides cefmetazole sodium suspension injection powder and a preparation method thereof. The cefmetazole sodium suspension injection powder comprises the following components by weight part: 10 parts of cefmetazole sodium, 5 to 30 parts of biodegradable polymer receivable on pharmacy, 1 to 20 parts of emulsifier, 6 to 12 parts of emulsion assistant and 2 to 30 parts of skeleton agent. The cefmetazole sodium suspension injection powder has the application for treating body extensive Psoriasis Pustulosa.
Owner:HAINAN LINGKANG PHARMA CO LTD
Venous injection powder injection for preventing and treating ischemic cerebral apoplexy and preparation method thereof
InactiveCN101167733AImprove neurobehavioral disordersReduce swellingPowder deliveryOrganic active ingredientsGlycoside formationInjection powder
The invention discloses an intravenous powder injection used for preventing and curing ischemic cerebral apoplexy and a process for preparation, which is made from phenolpropanoid glycosides extracted form radix scrophulariae, wherein the content of angolamycin glycosides C arrives to 100-850mg / g. The pharmacological research indicates that the invention brings significant effects in curing ischemic cerebral apoplexy.
Owner:杨泗兴
Levoleucovorin freeze-dried injection agent and preparation method and pharmaceutical use thereof
The invention provides a freeze-dried sterile injection powder containing an active component named levorotation folinic acid; the invention contains 9-100% levorotation folinic acid, 0-91% excipients acceptable in pharmacy or / and appropriate pH moderator. The pharmaceutical excipients refer to one or a plurality of mannitol, dextran, cane sugar, lactose, gelatin and phosphate. The pH moderator refers to one or a plurality of NaOH, natronite, kalium bicarbonicum, sodium phosphate, etc. The invention also relates to the preparation of the freeze-dried sterile injection powder. The invention avoids decomposition caused by high temperature sterilization in the process of levorotation folinic acid preparation, and is suitable for mass production; the invention is low in water content and stable when placed at room temperature, so the invention is suitable for long-term storage. The sterile injection powder of the invention can be taken as a synergist or attenuation agent in tumor chemotherapy.
Owner:SHANGHAI HUILUN JIANGSU PHARM CO LTD +1
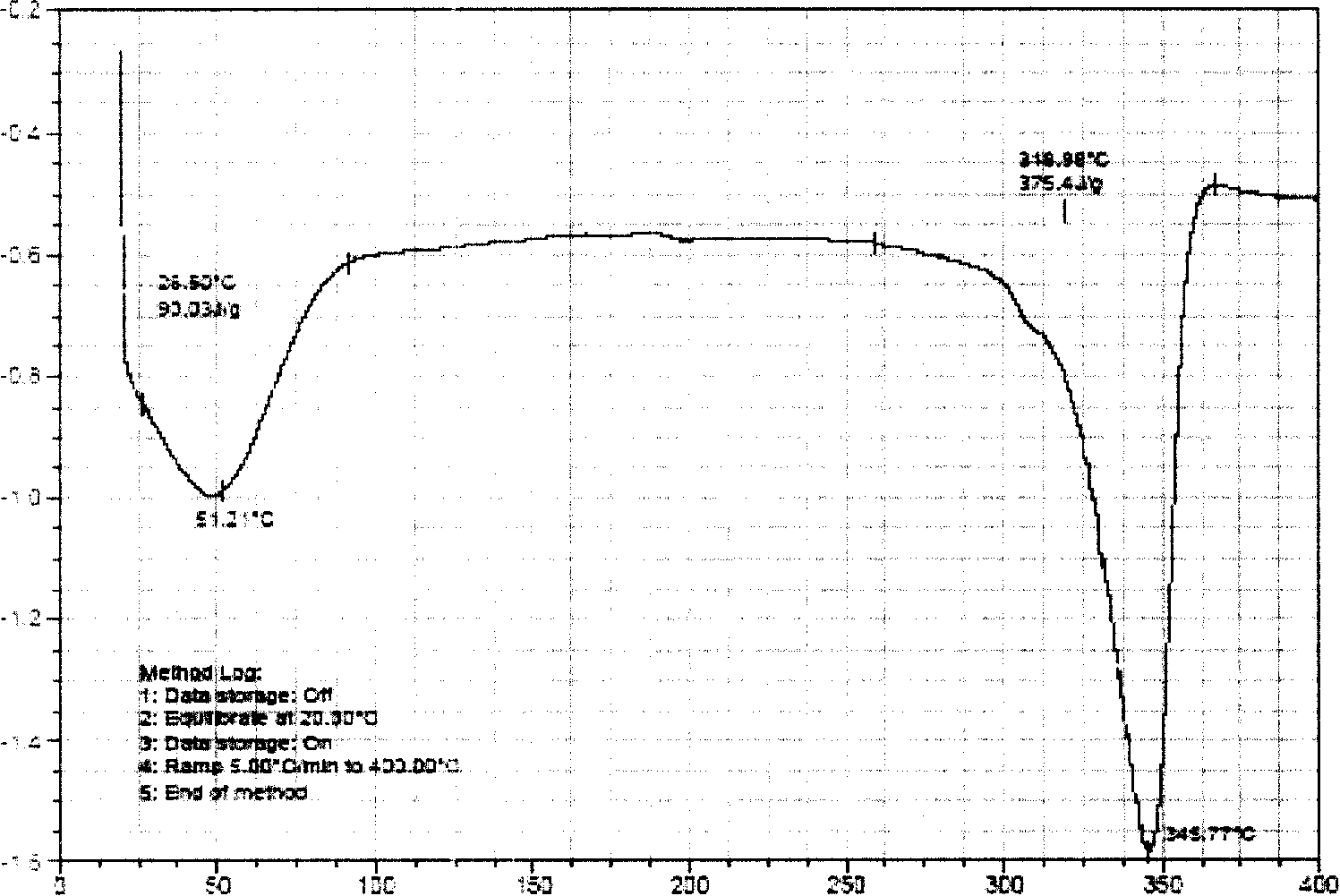
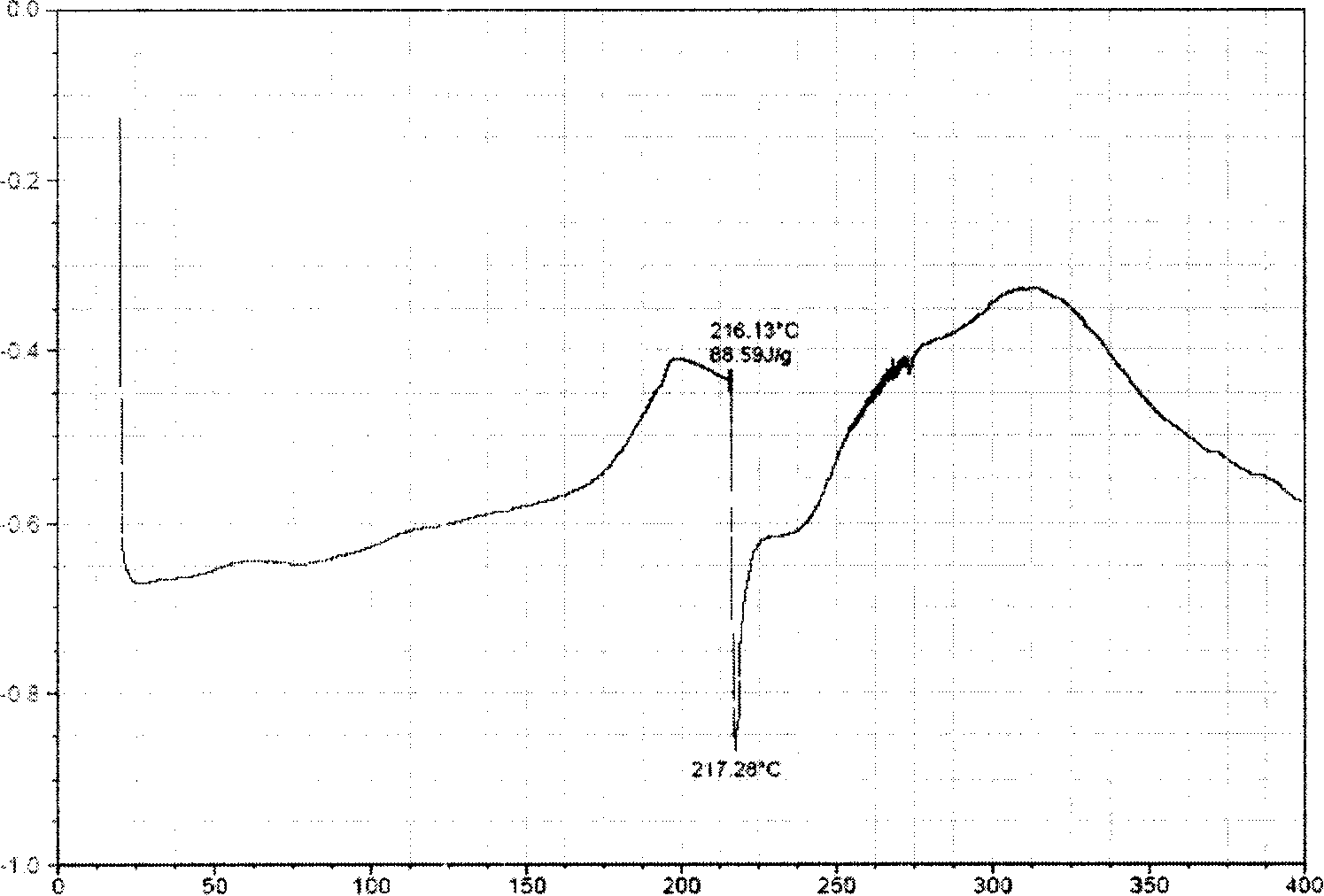
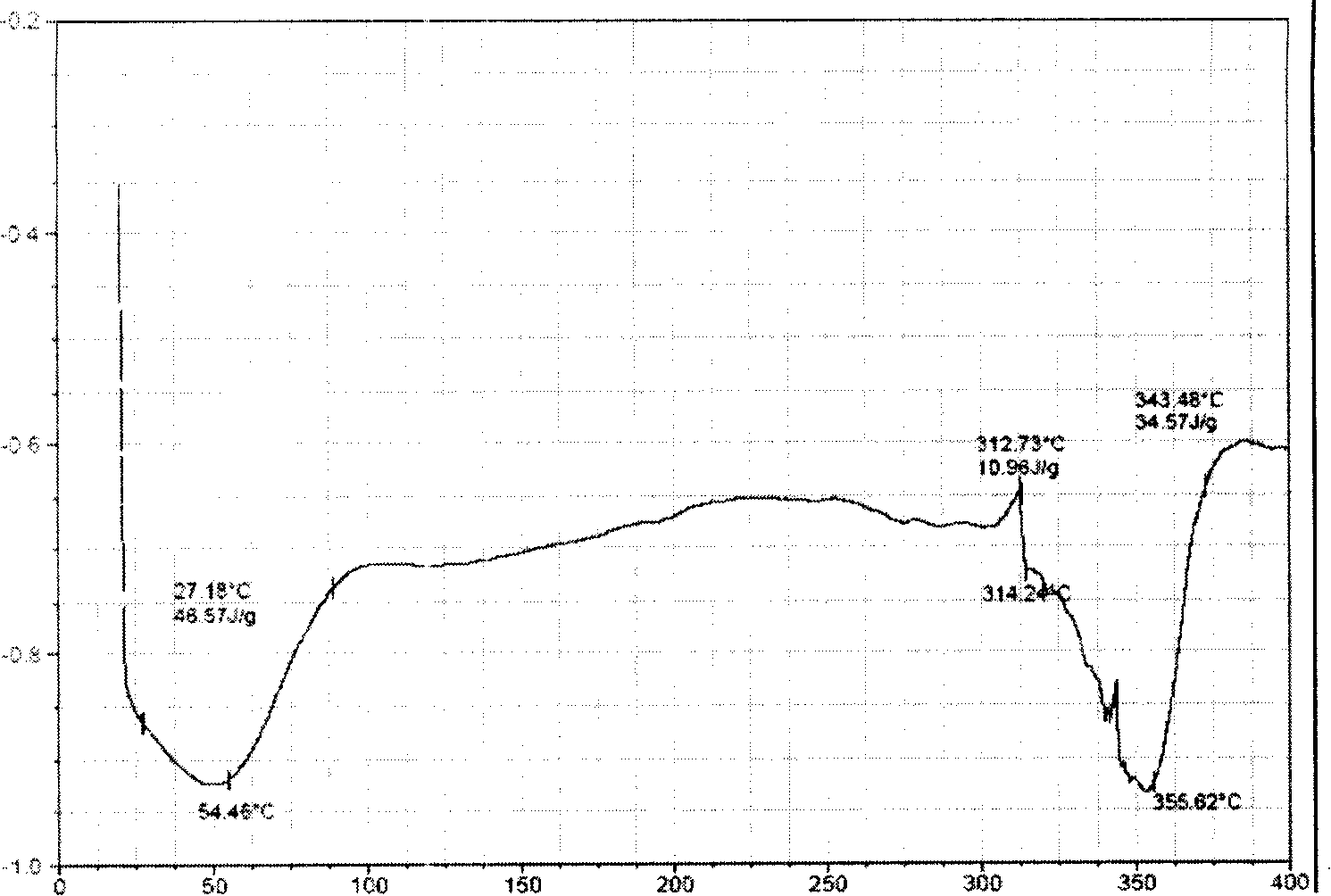
Corydalis total alkali clathrate, its preparing method and application
A rhizoma corydalis total alkaloids inclusion compound is disclosed in the present invention, the inclusion compound in the heat absorbing curve obtained by detecting with differential scanning calorimetry, in temperature 300 to 380 C DEG range has obvious heat absorbing peak, the temperature of the maximum heat absorbing peak of the heat absorbing peaks is 335 to 365 C DEG. Its preparation method is with hydroxyl-beta-cyclodextrin as subject, with rhizoma corydalis total alkaloids as object, under absolute ethanol existing by inclusion operation, to inclusion or embed the rhizoma corydalis total alkaloids into hydroxyl-beta-cyclodextrin, forming inclusion compound. Application of the rhizoma corydalis total alkaloids inclusion compound in preparing medicine preparations such as tablet, capsule, soft capsule, granule, oral rapidly disintegrable preparation, slow release preparation or freeze-dried injection powder and etc. The rhizoma corydalis total alkaloids inclusion compound of the present invention is very easy or easy to solute in water, promotes water solubility and stability, and stores conveniently.
Owner:SHANGHAI INST OF PHARMA IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com