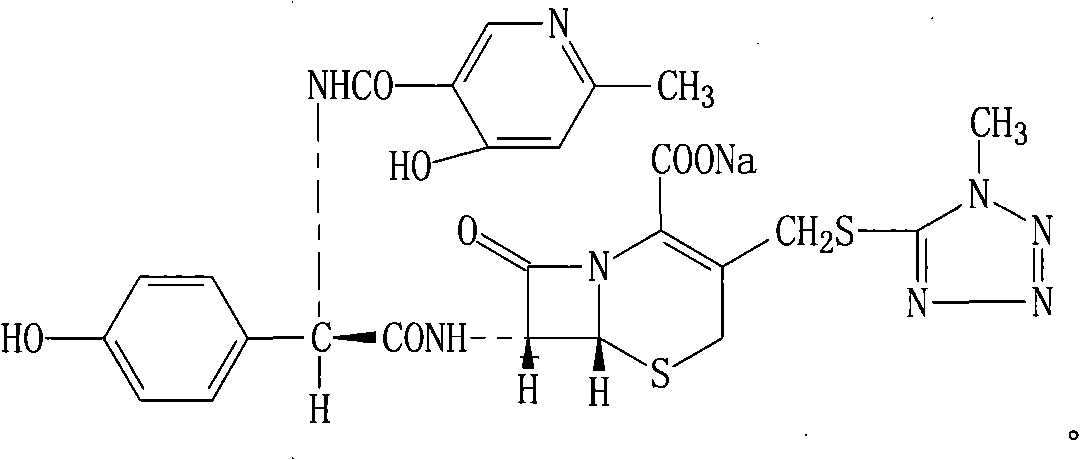
CefPiramide sodium powder for injection
A technology of cefpiramide sodium and cefpiramic acid, which is applied in the direction of powder transportation, freeze-drying transportation, antibacterial drugs, etc., can solve the problems of high corrosion of equipment, slow dissolution speed, environmental pollution, etc., and achieve low corrosion, Improved productivity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
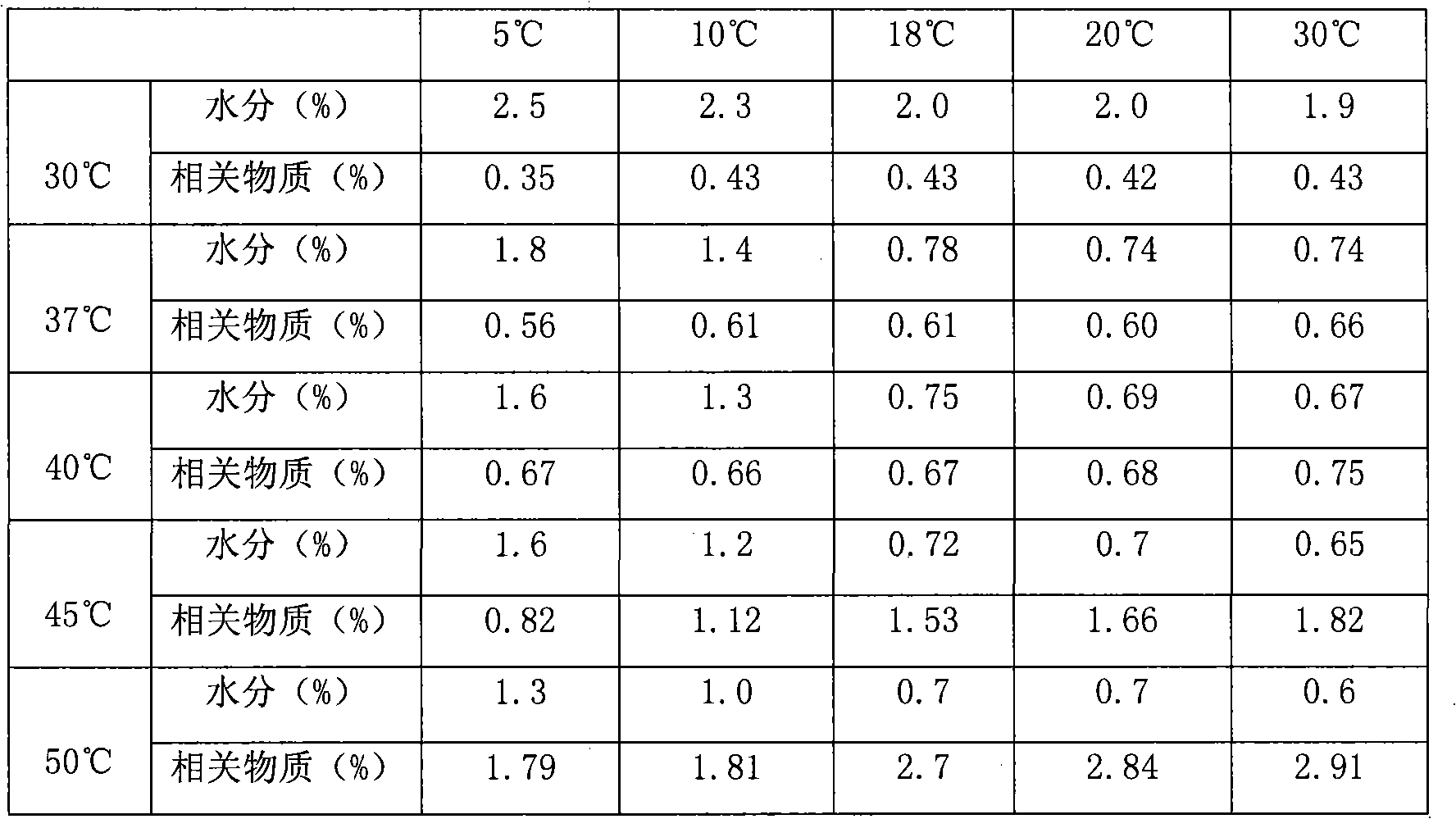
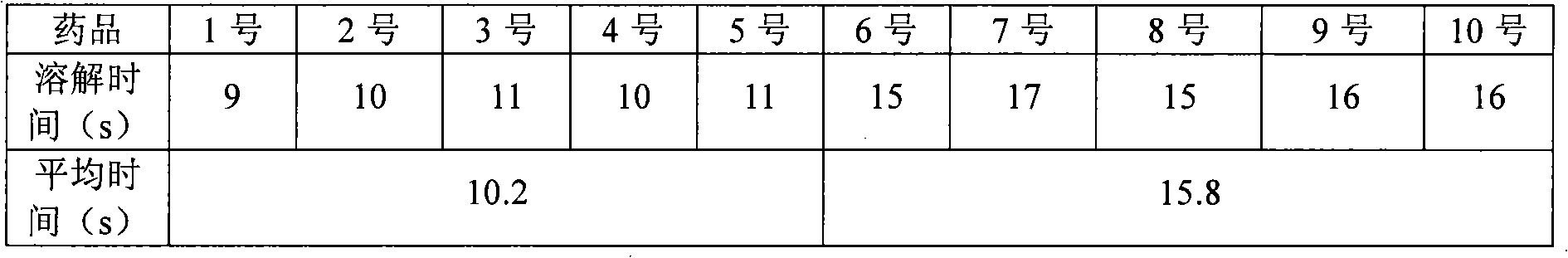
[0044] Weigh 730g of sodium benzoate, 100g of sodium dihydrogen phosphate, and 480g of disodium hydrogen phosphate in a reaction kettle, stir and dissolve with 35L of water for injection at room temperature, add 10.0kg of cefpiramic acid, and control the temperature at 20°C, stirring until it does not dissolve; Add 16L of 4% sodium hydroxide solution dropwise at a speed of 155mL / min, stir to dissolve, stop the dropwise addition and keep stirring for 13 minutes every 20 minutes, adjust the pH value to 6.5; add 200g of activated carbon, decolorize for 30 minutes; filter with suction , put the filtrate into a lyophilizer at a rate of 6°C / min to cool down and freeze to -50°C, vacuumize, heat up to -10°C within 5 hours, maintain -10°C for 12 hours, and continue to heat up to within 2.5 hours 20°C, maintain 20°C for 9 hours, then raise the temperature to 37°C within 2 hours, and maintain 37°C for 5 hours to obtain cefpiramide sodium freeze-dried powder injection. Moisture is 0.73%. ...
Embodiment 2
[0046] Weigh 750g of sodium benzoate, 95g of sodium dihydrogen phosphate, and 490g of disodium hydrogen phosphate in a reaction kettle, stir and dissolve with 35L of water for injection at room temperature, add 10.0kg of cefpiramic acid, and control the temperature at 15°C, stirring until it does not dissolve; Add 15L of 4% sodium hydroxide solution dropwise at a rate of 150mL / min, stir to dissolve, stop the dropwise addition and keep stirring for 15 minutes every 25 minutes, adjust the pH value to 7.3; add 200g of activated carbon, decolorize for 30 minutes; filter with suction , put the filtrate into the freeze dryer at a rate of 6.5°C / min to cool down and freeze to -52°C, vacuumize, heat up to -11°C within 5.5 hours, maintain -11°C for 11.5 hours, and continue to heat up to within 3 hours 18°C, maintain 18°C for 8 hours, then raise the temperature to 40°C within 2.5 hours, and maintain 40°C for 5.5 hours to obtain cefpiramide sodium freeze-dried powder. Moisture is 0.69% ...
Embodiment 3
[0048] Weigh 720g of sodium benzoate, 105g of sodium dihydrogen phosphate, and 470g of disodium hydrogen phosphate in a reaction kettle, stir and dissolve with 35L of water for injection at room temperature, add 10.0kg of cefpiramic acid, and control the temperature at 20°C, stirring until it does not dissolve; Add 15.7L of 4% sodium hydroxide solution dropwise at a rate of 160mL / min, stir to dissolve, stop the dropwise addition and keep stirring for 10 minutes every 22 minutes, adjust the pH value to 7.0; add 200g of activated carbon, decolorize for 30 minutes; Filtrate, put the filtrate into a lyophilizer at a rate of 6.5°C / min to cool down and freeze to -55°C, vacuumize, heat up to -10°C within 6 hours, maintain -10°C for 12 hours, and continue to heat up within 2.8 hours to 19°C, the time for maintaining 19°C was 8.5 hours, and then the temperature was raised to 39°C within 2 hours, and the time for maintaining 39°C was 5 hours to obtain cefpiramide sodium freeze-dried powd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com