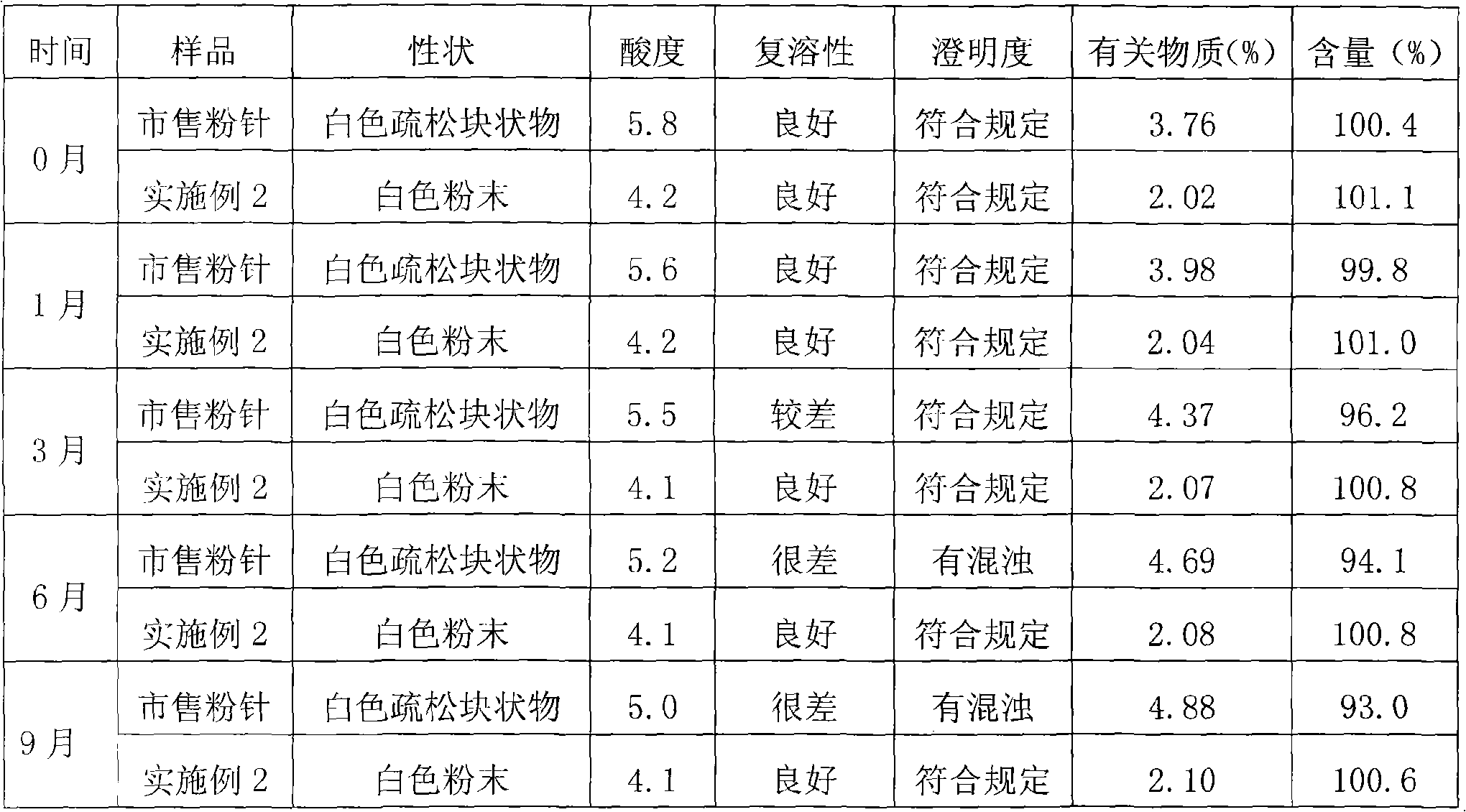
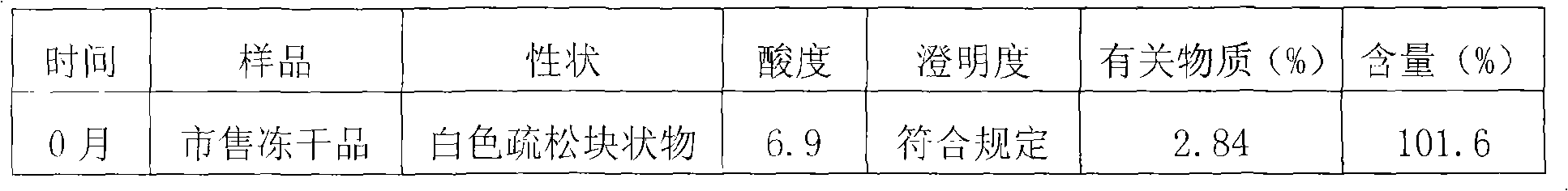
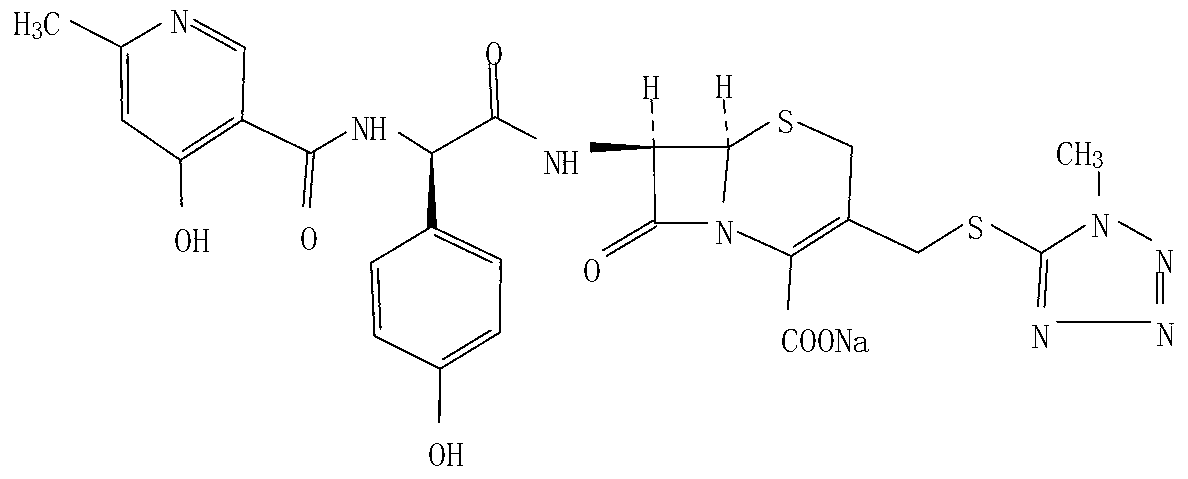
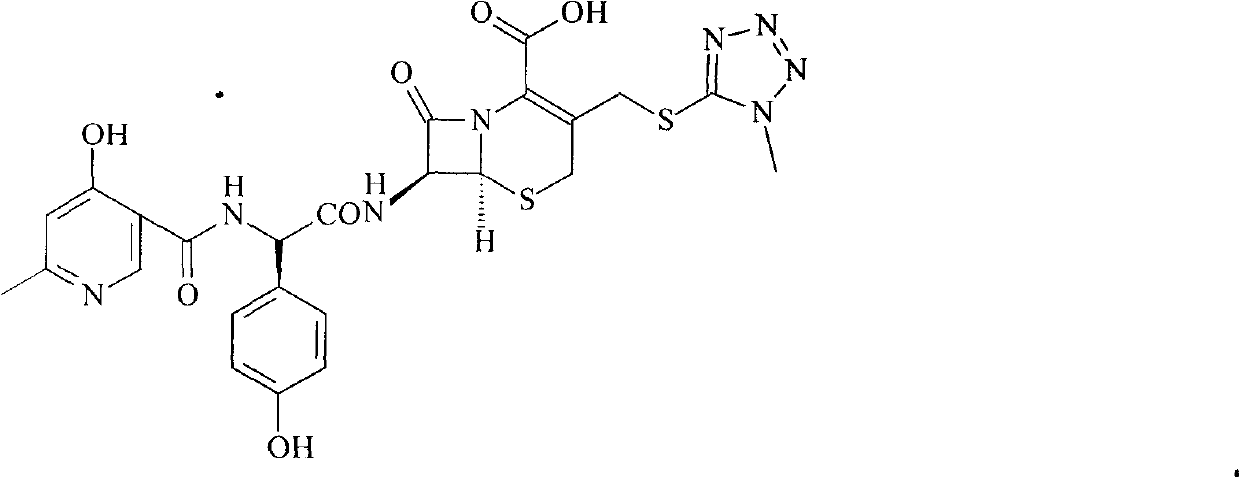
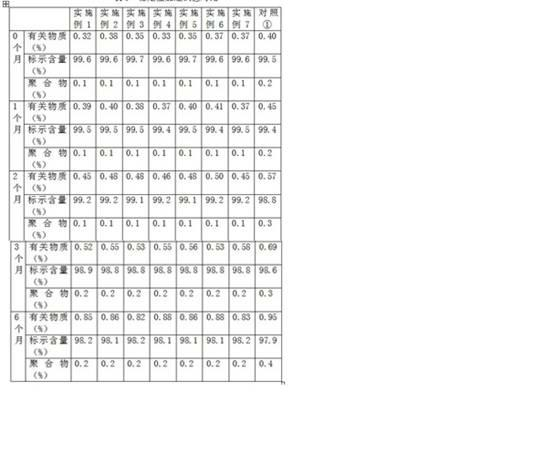
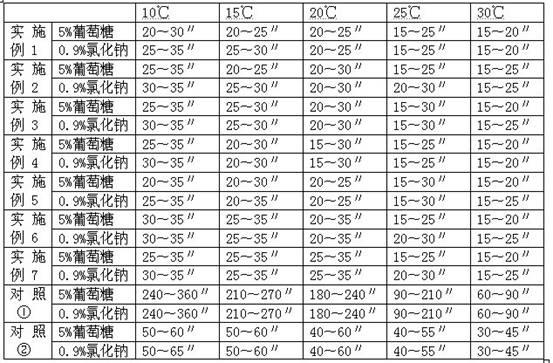
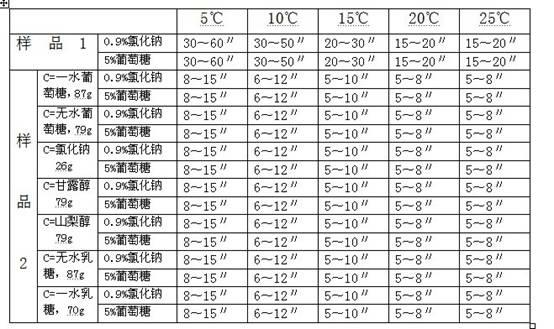
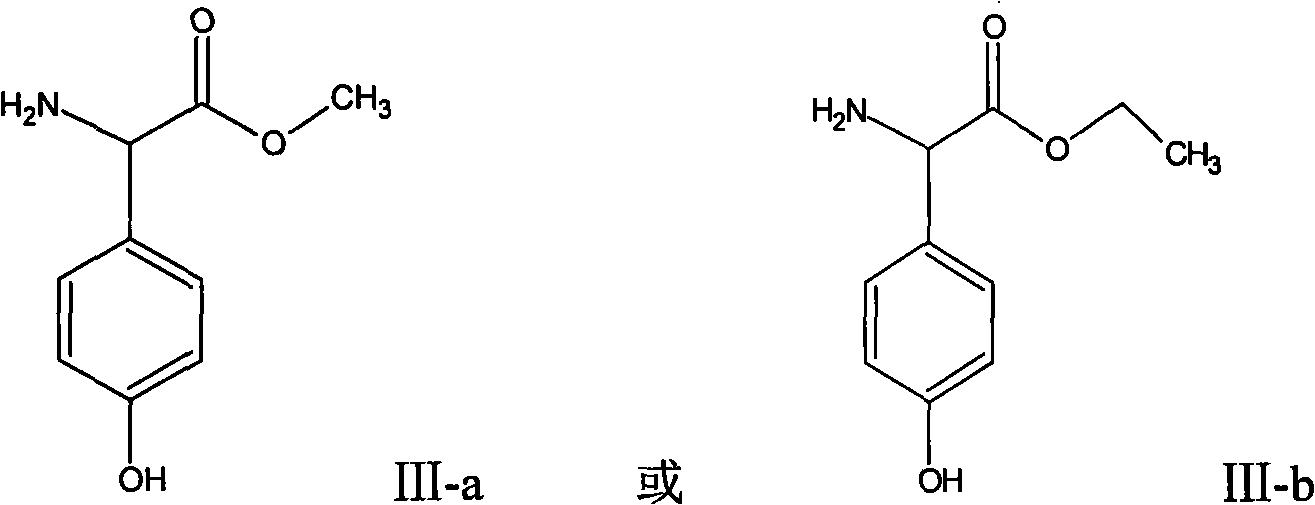
Patents
Literature
42 results about "Cefpiramide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
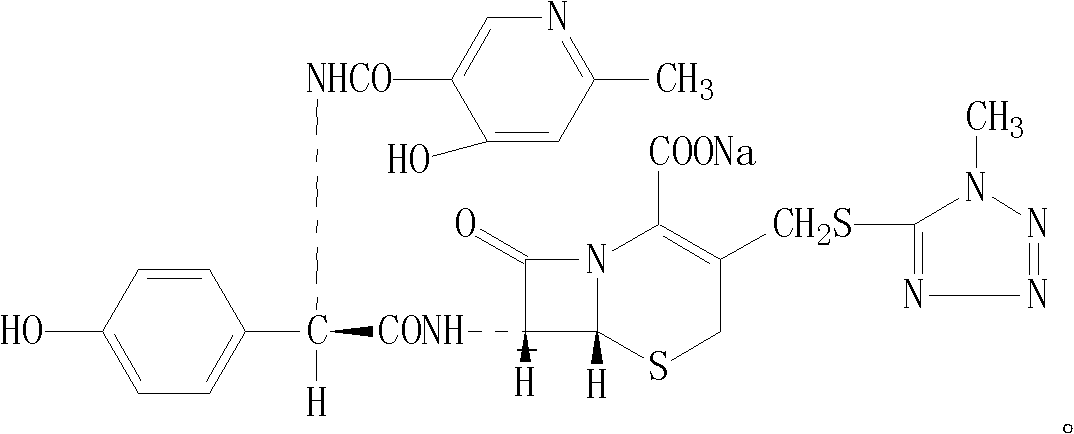
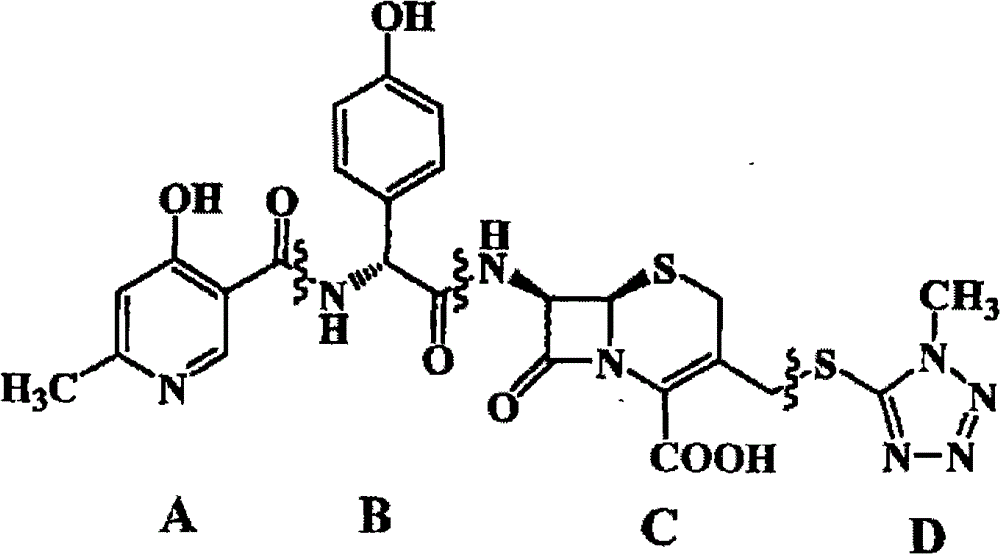
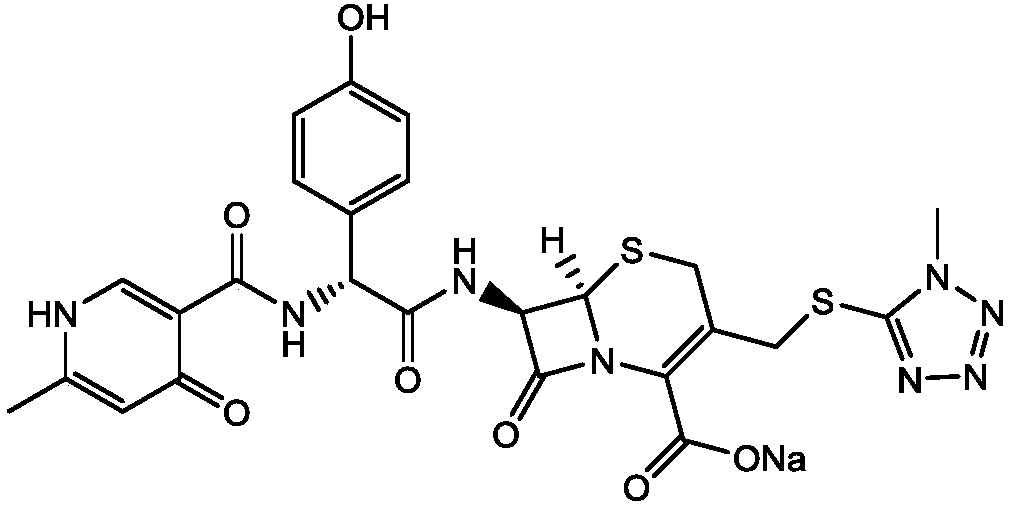
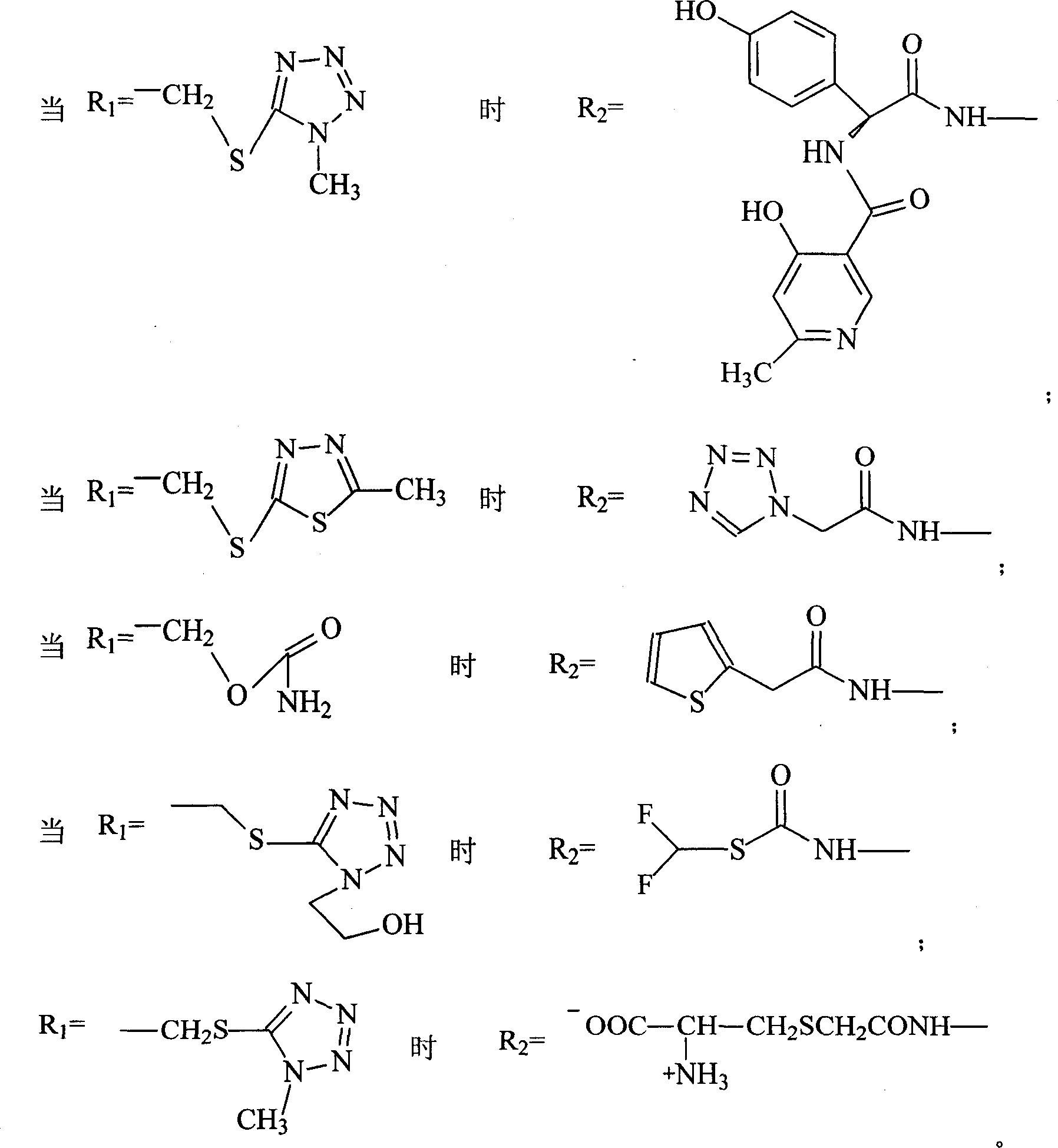
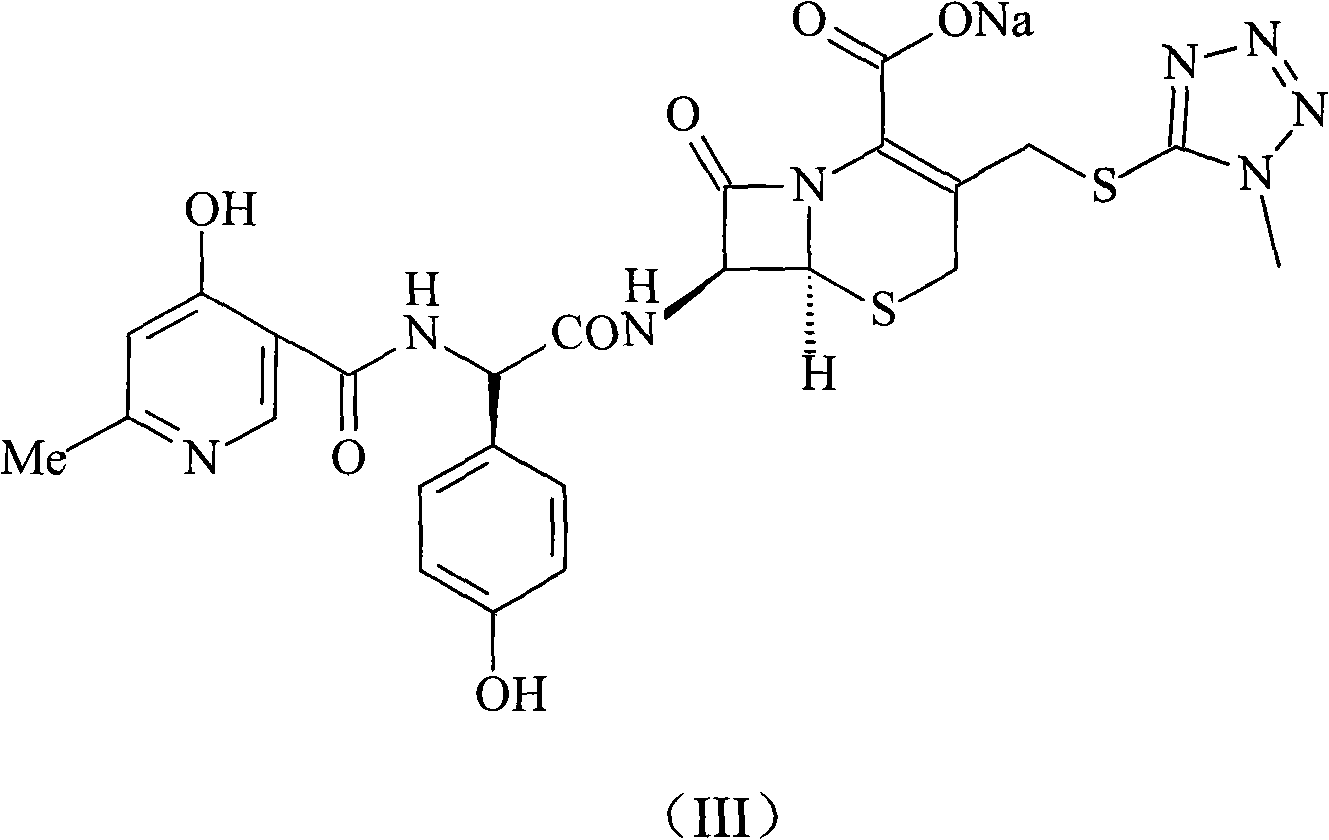
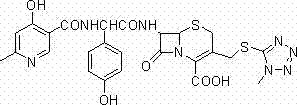
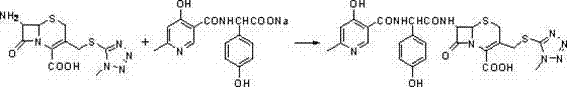
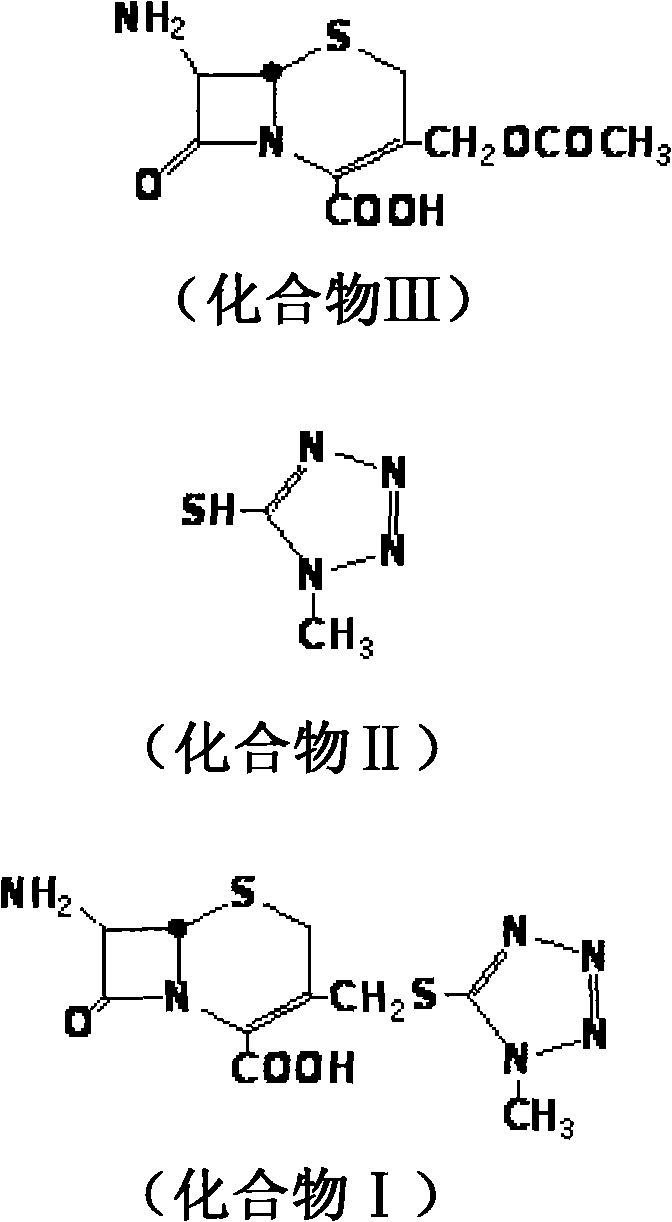
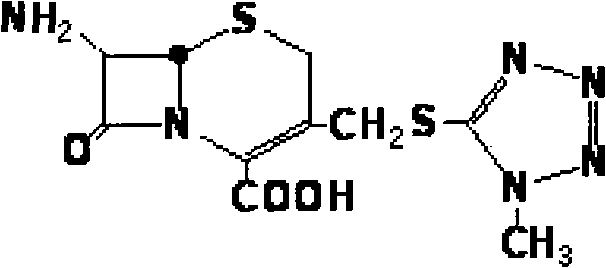
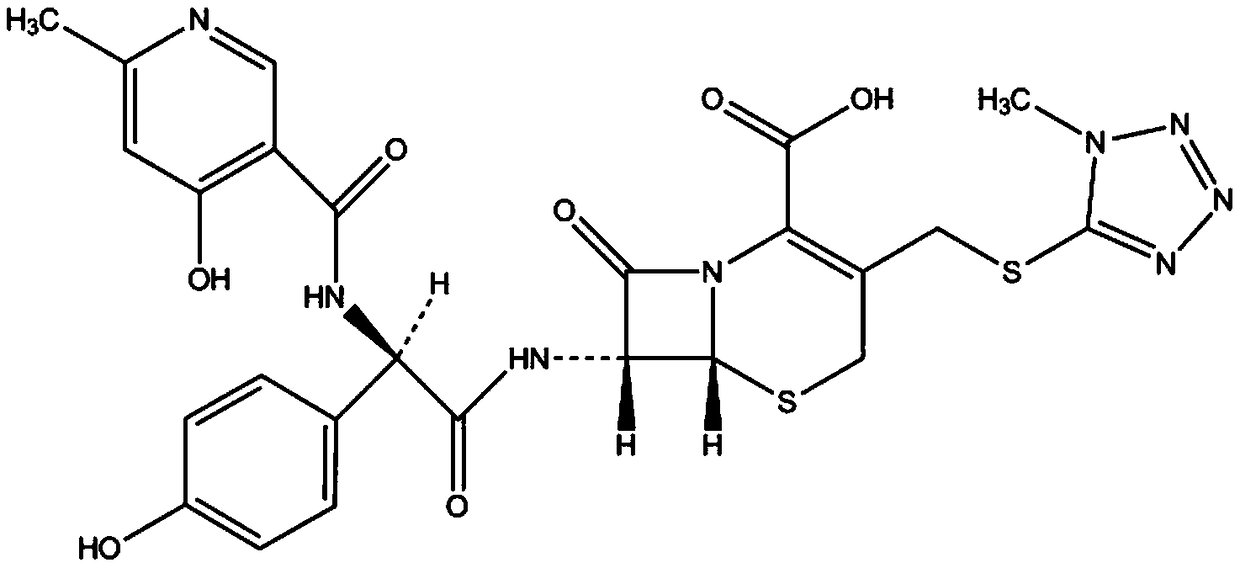
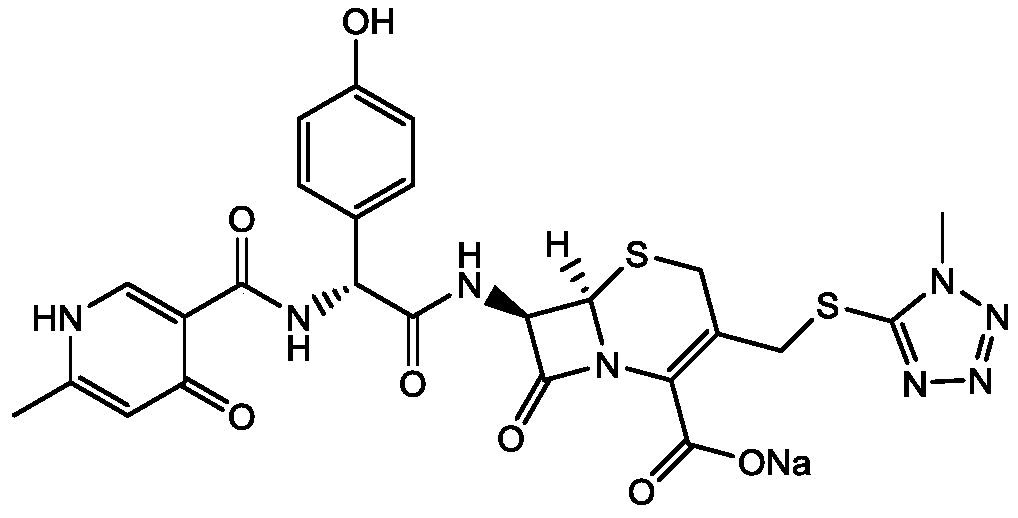
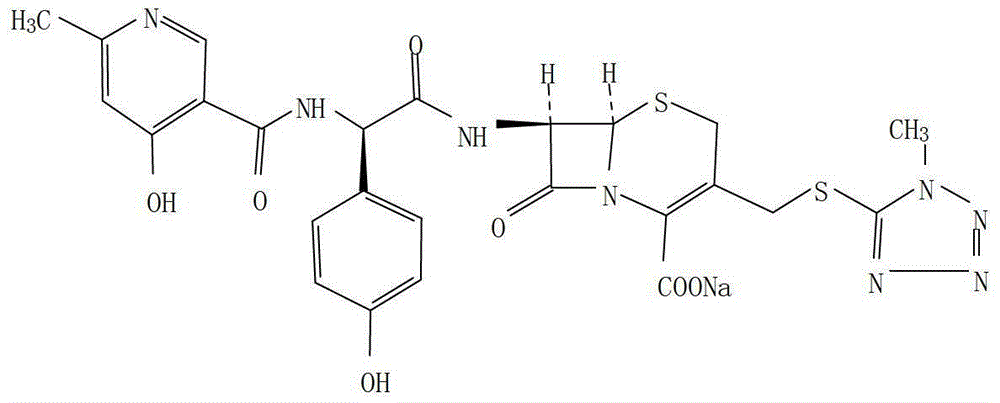
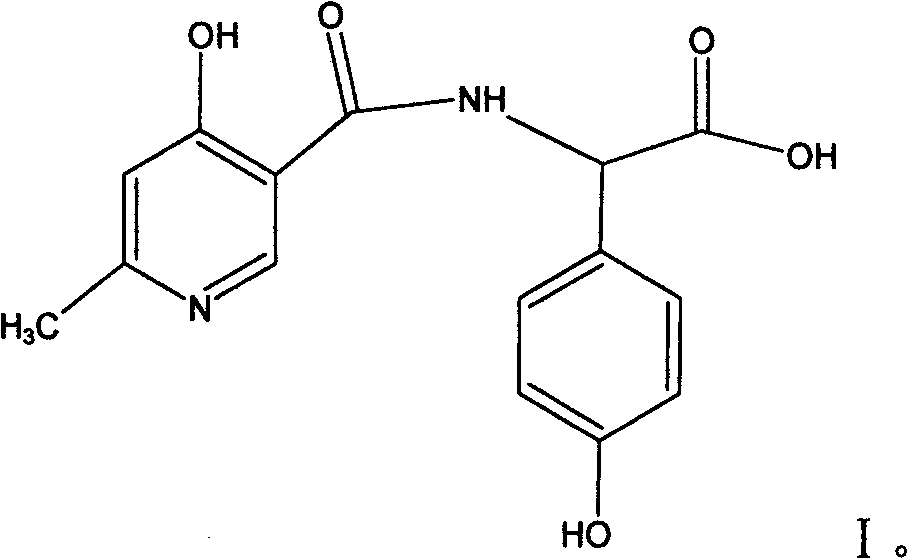
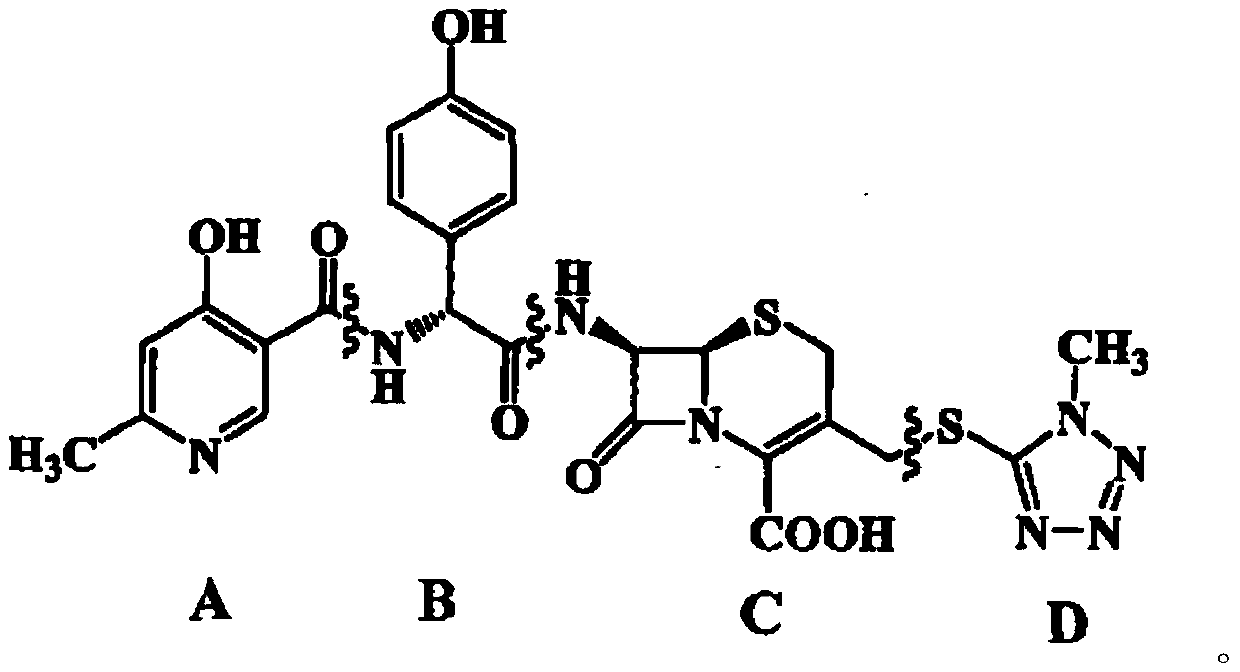
Cefpiramide is a third-generation cephalosporin antibiotic.
Method for preparing powder injection using superfine communication technique and prepared products
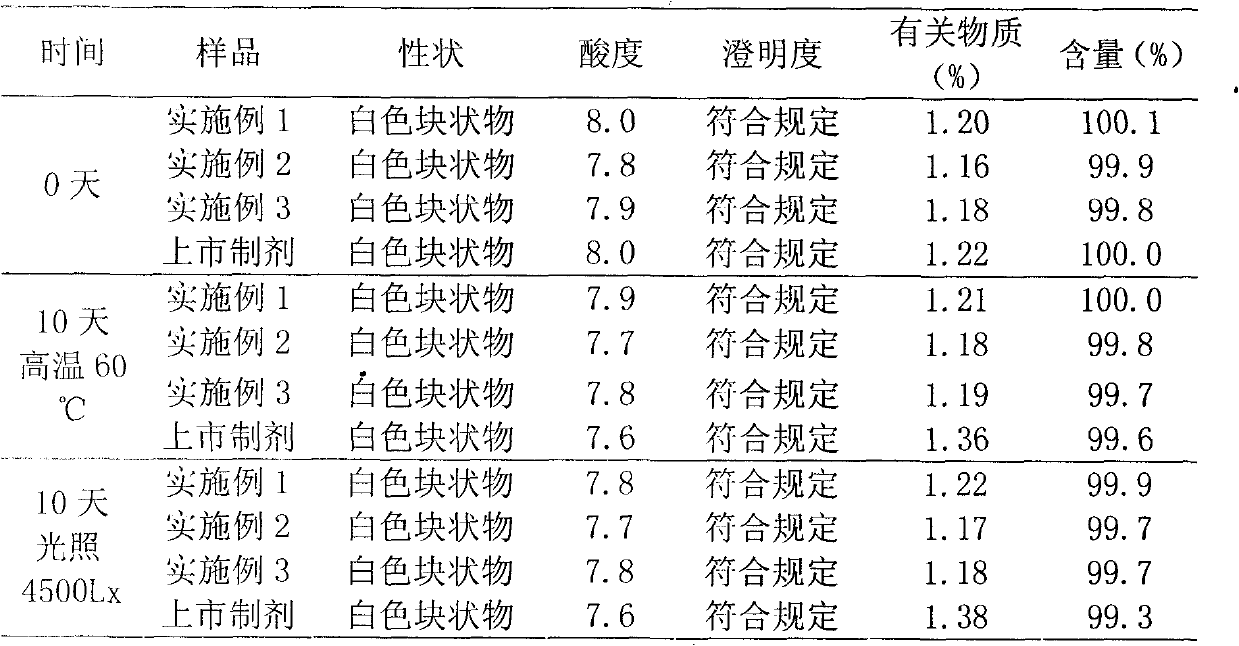
InactiveCN101332188AReduce contentUniform colorAntibacterial agentsOrganic active ingredientsLatamoxefClindamycin Phosphate
The present invention relates to a method of using a superfine pulverizing technology to prepare sterile powder for injection (powder injection) of chemical medicine and the prepared medicine powder injection. Invert sugar, clindamycin phosphate, cefpiramide sodium, cefepime hydrochloride, latamoxef sodium or cefmetazole sodium are preferable as the chemical medicine.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for crystallizing and producing cefpiramide sodium crystals
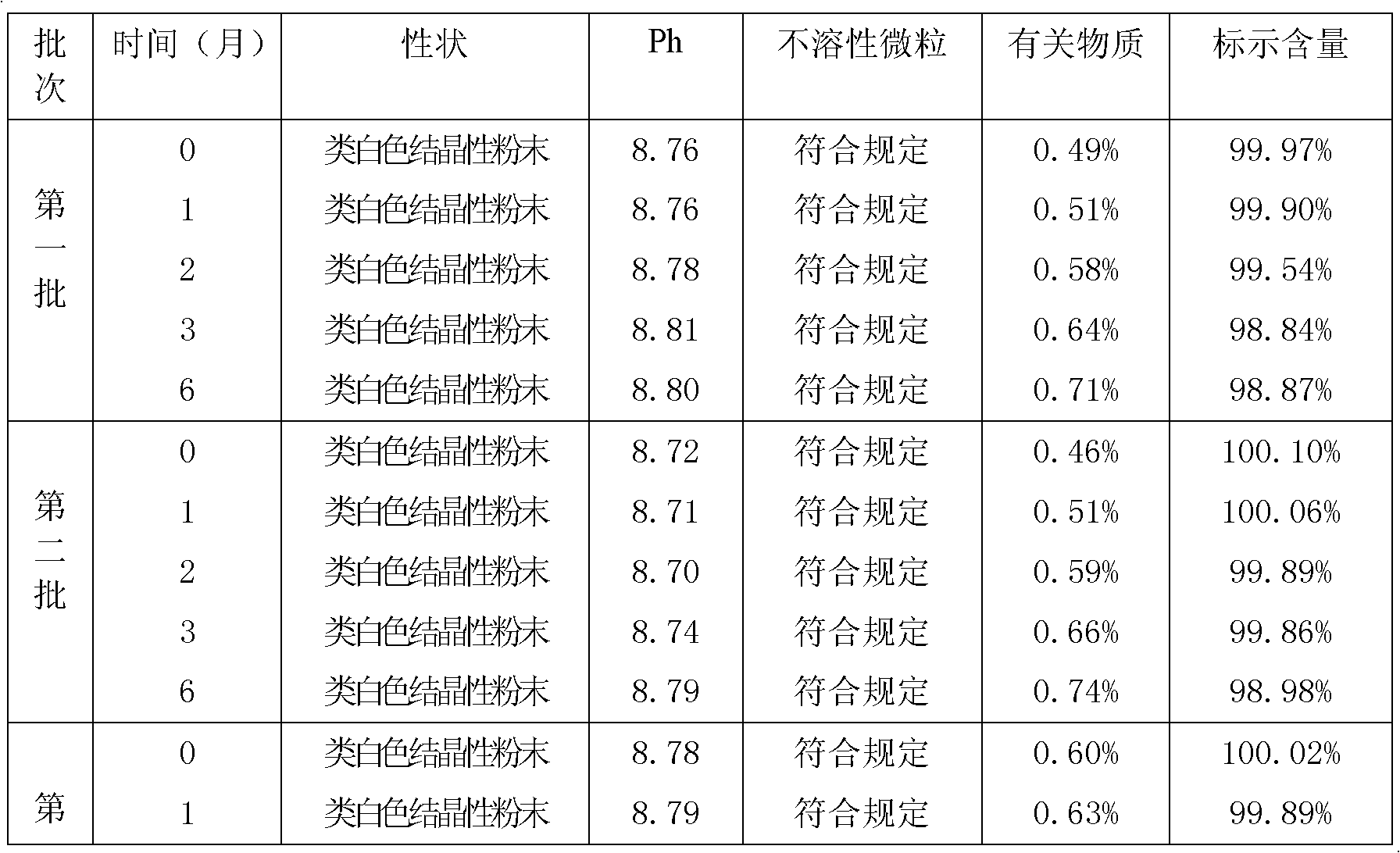
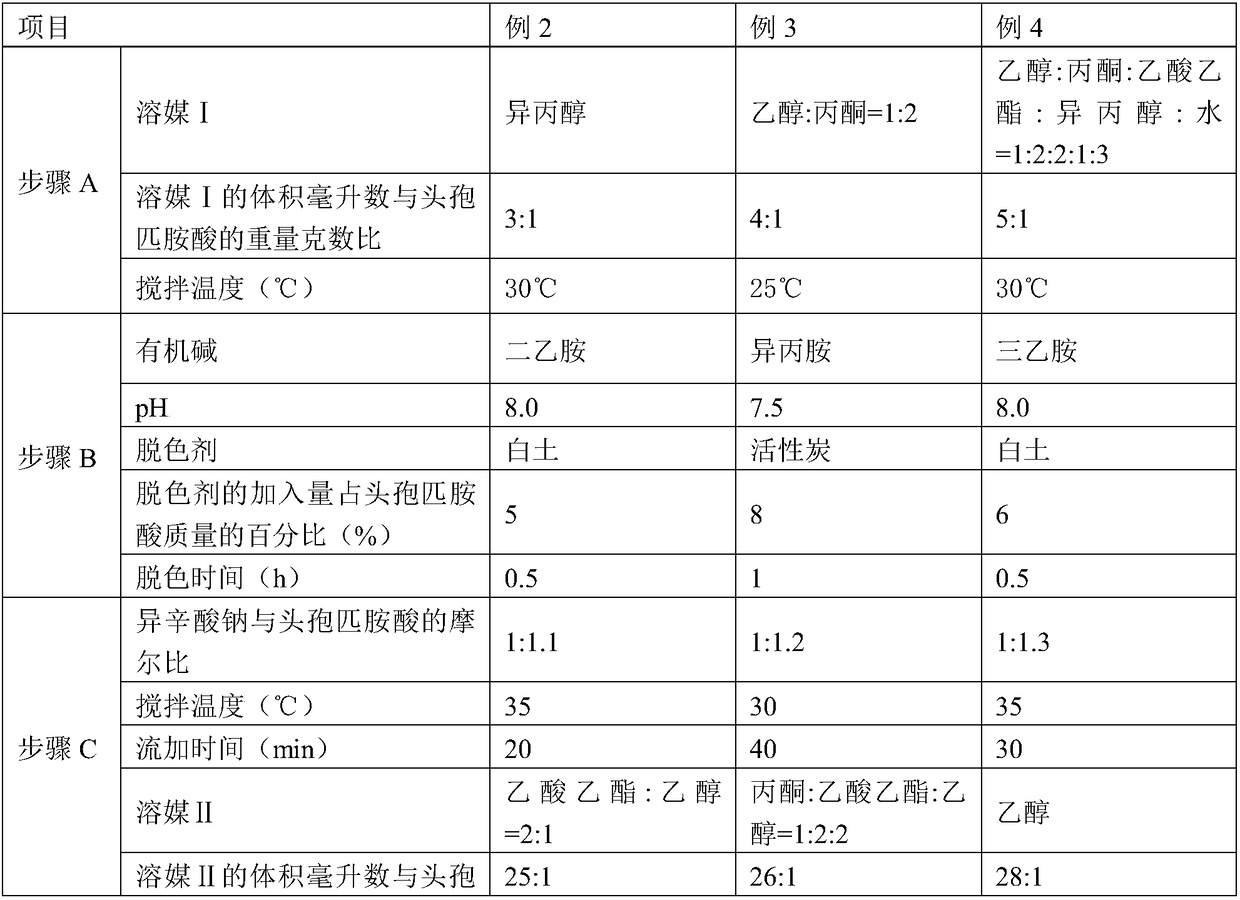
The invention discloses a method for crystallizing and producing cefpiramide sodium crystals. The method comprises the steps that: (a) cefpiramide acid and an transamination agent are dissolved in a solvent I, wherein the transamination agent is any one selected from triethylamine, diisopropylamine, and isopropylamine; and a temperature is controlled, and the materials are stirred until completely dissolved; (b) a salt-forming agent is added into a solvent II, wherein when the salt-forming agent is sodium ethylhexanoate, the solvent II is an acetone solvent, and when the salt-forming agent is sodium hydroxide, the solvent II is any one or a mixture of two selected from methanol and tetrahydrofuran; and the materials are stirred until completely dissolved; (c) the salt-forming agent solution is uniformly added into the solution of cefpiramide amine salt; the temperature is controlled, and crystal seeds are added; curing crystallization is carried out; acetone is added into the crystallization system for regulating the pH value of the crystallization system and for carrying out solvent-out crystallization; and filtering, washing, and drying are carried out, such that the cefpiramide sodium crystals are obtained. The method provided by the invention has the advantages of simple operation, uniform crystals, high purity, low impurity content, good stability, easy storage, and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Stable cephalosporin powder injection
InactiveCN1706387AImprove stabilityExtended shelf lifeAntibacterial agentsPowder deliverySodium bicarbonateArginine
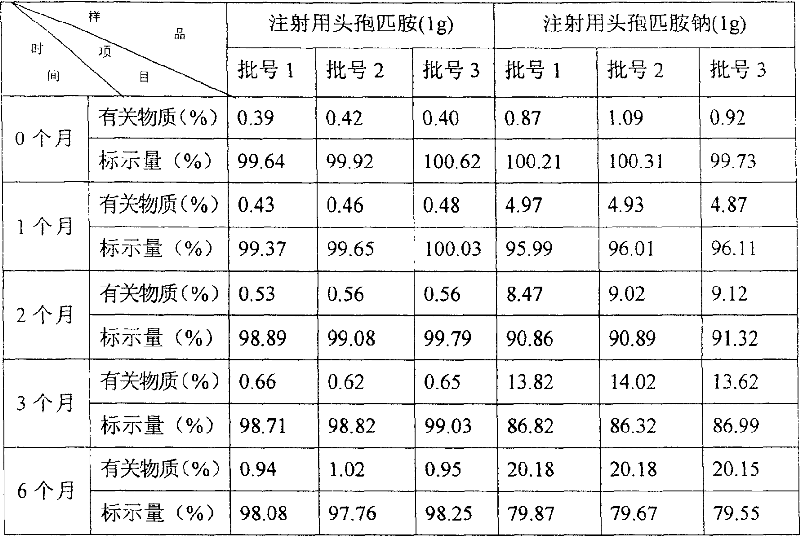
The stable cephalosporin powder for injection is mixture of bacteria-free cefpiramide and cosolvent in the weight ratio of 1 to 0.1-0.5. The cosolvent is sodium carbonate, arginine or sodium hydroxide. Compared with available cefpiramide injection, the cephalosporin powder for injection has the features of ever high stability, less impurity content and longer preservation period.
Owner:北京盛世伟唐科技有限公司
Cefpiramide, sodium benzoate, sodium bicarbonate pharmaceutical composite lipidosome injection
InactiveCN101766571AMature production processEasy to implementAntibacterial agentsOrganic active ingredientsSodium bicarbonateAntioxidant
The invention discloses a cefpiramide, sodium benzoate, sodium bicarbonate pharmaceutical composite lipidosome injection and a preparation method thereof. The cefpiramide, sodium benzoate, sodium bicarbonate pharmaceutical composite lipidosome injection includes cefpiramide, sodium benzoate, sodium bicarbonate, lipidosome matrix, osmotic pressure regulator and antioxidant, wherein, the lipidosome matrix is the mixture of phospholipids, cholesterin and Tween-80. Preferentially, the mass ratio of the phospholipids, the cholesterin and the Tween-80 in the lipidosome matrix is 1-3:2:1; the mass ratio of the cefpiramide and the lipidosome matrix is 1:2-11, preferentially as 1:2-11; the mass ratio of the cefpiramide, the sodium benzoate and the sodium bicarbonate is 1:0.05-0.06:0.6-0.8.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefpiramide sodium powder composition and preparation method thereof
ActiveCN102286002AGood water solubilityImprove solubilityAntibacterial agentsPowder deliverySolubilityMass ratio
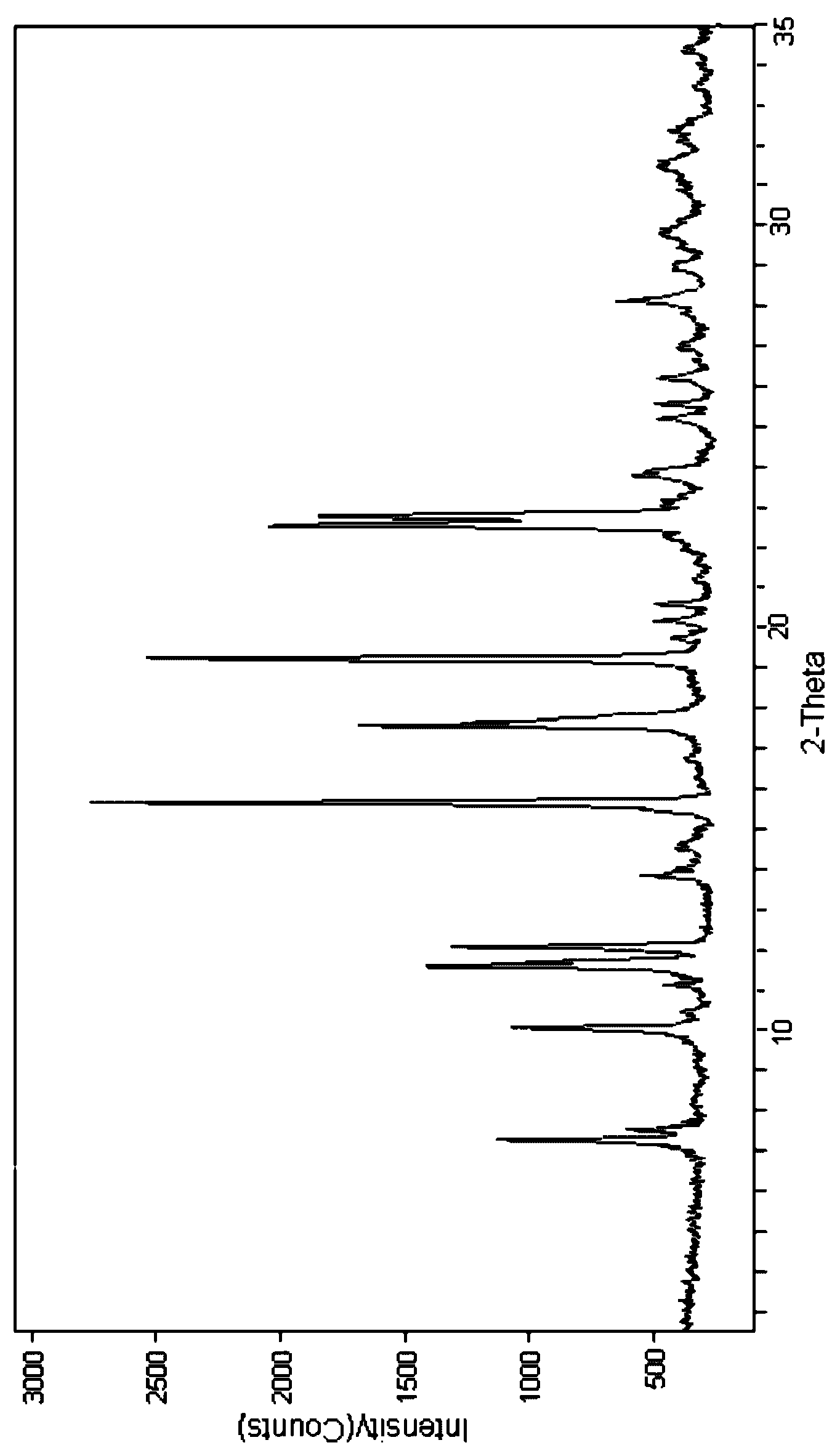
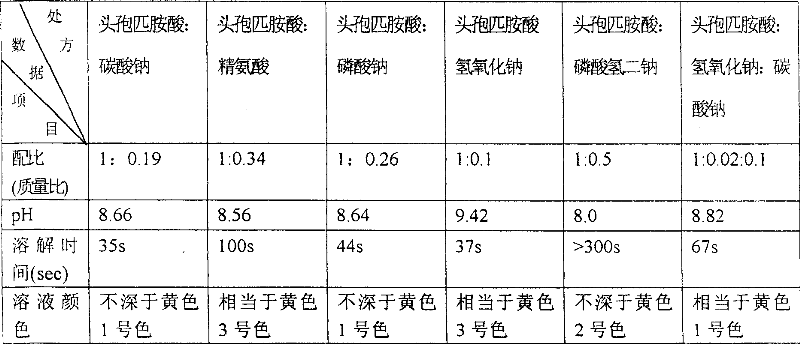
The invention relates to a cefpiramide sodium powder injection composition which contains cefpiramide crystals and anhydrous sodium carbonate, wherein the mass ratio of the cefpiramide crystals to the anhydrous sodium carbonate is (1:0.17)-(1:0.2); and in the X-ray powder diffraction, the main peaks of the cefpiramide crystals are displayed at the diffraction angles of 7.25 degrees + / -0.1 degree,10.04 degrees+ / -0.1 degree, 11.51 degrees+ / -0.1 degree, 12.04 degrees+ / -0.1 degree, 15.67 degrees+ / -0.1 degree, 17.65 degrees+ / -0.1 degree, 19.28 degrees+ / -0.1 degree, 22.52 degrees+ / -0.1 degree and 22.84 degrees+ / -0.1 degree. According to the invention, the water solubility of the cefpiramide crystals is improved to certain extent; when the cefpiramide crystals together with the anhydrous sodiumcarbonate are prepared into sterile powder injection, the amount of anhydrous sodium carbonate is greatly reduced, and the dissolution speed of the powder injection is high, that is to say, the powder injection can be completely dissolved in a short time, thereby being convenient for clinical use.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Preparation method of cephapian aseptic mixed powder injection agent
InactiveCN1823783AHigh yieldReduce processing lossAntibacterial agentsPowder deliveryBottleButyl rubber
An aseptic mixed powder injection of a cefpiramide is prepared through respectively pulverizing aseptic cefpiramide and aseptic sodium carbonate by 100 meshes in 100-class clean region, respectively loading them in antibiotic tubular bottles, adding butyl rubber plug to them and pressing aluminum-plastic covers to said bottles.
Owner:张庆华
Method for preparing cefpiramide acid
InactiveCN103059048AHigh purityImprove responseOrganic chemistryTrimethylsilyl chlorideCarboxylic acid
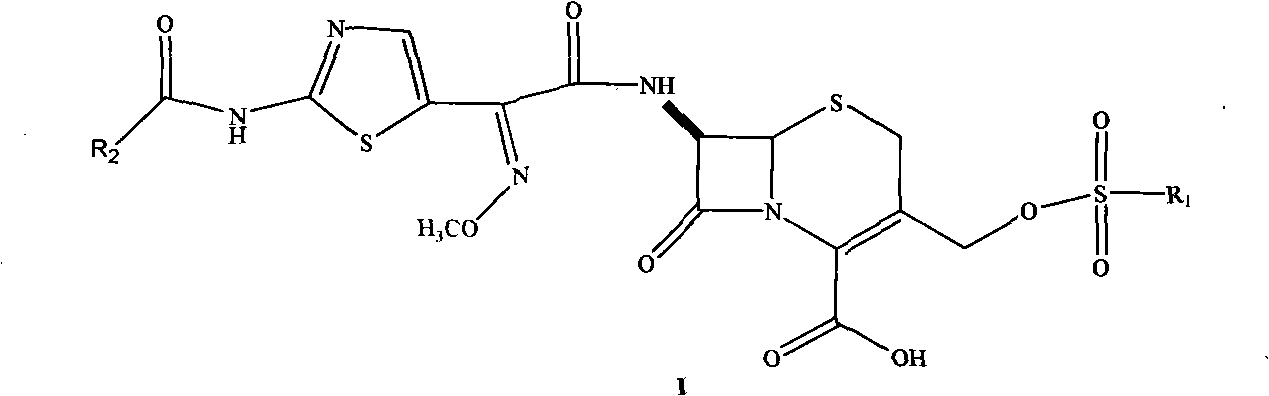
The invention provides a method for preparing cefpiramide acid. The method provided by the invention adopts (6R,R7)-3-[(1-methyl-1H-tetrazolyl-5-yl)thiomethyl]-7-amino-8-oxo-5-thia-1-azabicyclo-[4.2.0]octyl-2-alkenyl-2-carboxylic acid as a trimethylchlorosilicyl protective agent. The method provided by the invention is simple to operate, obviously enhances the product purity and yield, and can easily implement industrial production.
Owner:珠海保税区丽珠合成制药有限公司 +1
Powder injection of cefpiramide
A powder injection of cefpiramide is disclosed, which contains the cefpiramide or its acids or salts.
Owner:孙明杰 +1
Preparation method of cefpiramide sodium
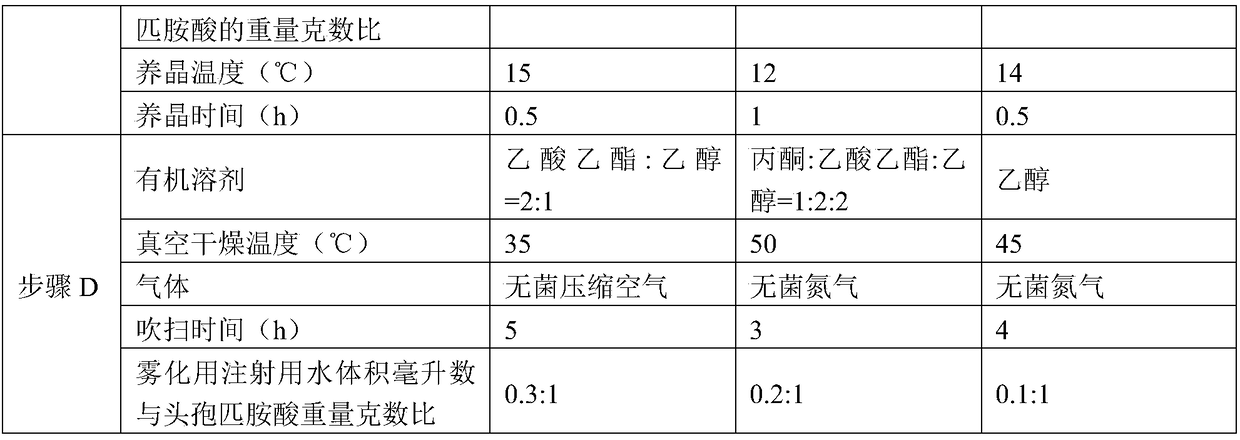
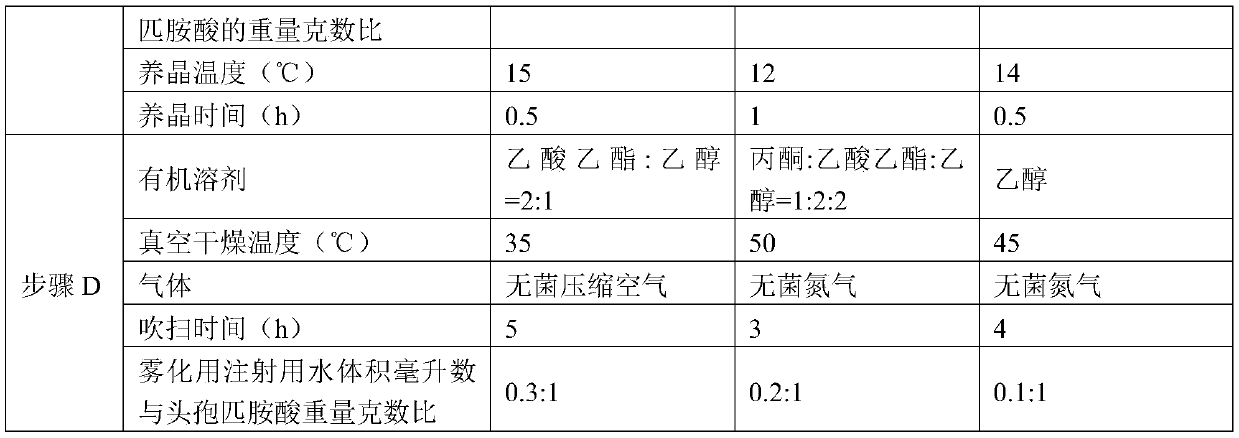
The invention discloses a preparation method of cefpiramide sodium, belonging to the field of chemical pharmacy. The preparation method comprises the following steps: preparing a cefpiramide amine salt by using a cefpiramide acid as a raw material, carrying out salt formation and crystallization, drying, and driving atomized water for injection by using gas to blow before drying. The preparation method can reduce the solvent residue in the product, is simple, energy-saving and environmentally-friendly and is suitable for large-scale industrial production; and the prepared cefpiramide sodium has the advantages of high purity and good stability.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefpiramide composition
ActiveCN100336510CStable quality when stored at room temperatureRapid dissolution in clinical useAntibacterial agentsOrganic active ingredientsSolubilitySolvent
The invention provides a Cefpiramide composition which comprises asepsis cefpiramide acid and asepsis solubility promoter, wherein the weight ratio of cefpiramide acid and solubility promoter is 1 : 0.10 to 1 : 0.6. The advantages of the invention include stabilized conserving quality and rapid dissolving in clinic application.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Cefpiramide composite
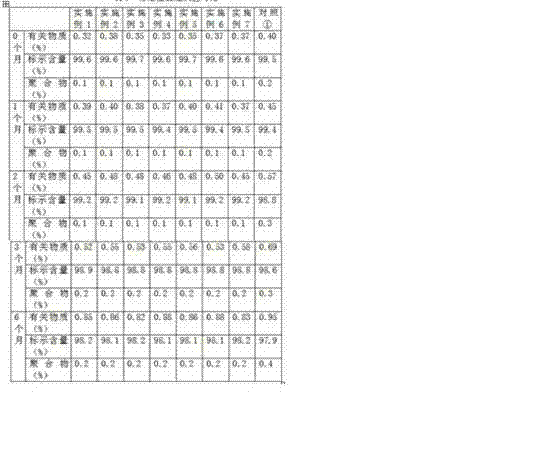
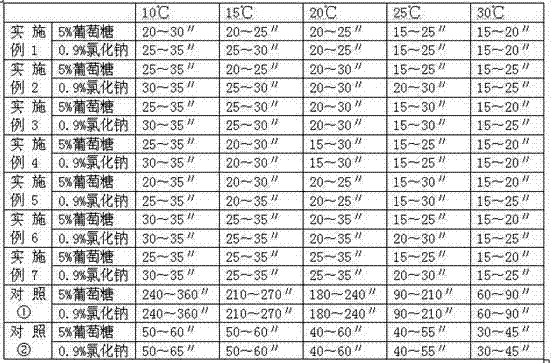
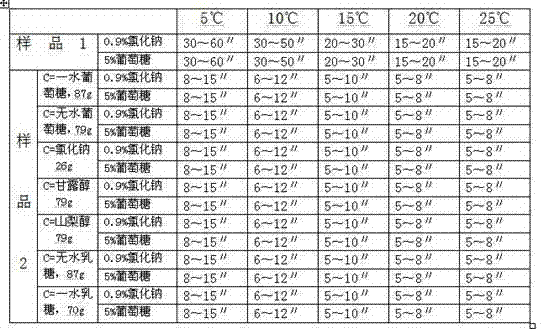
ActiveCN101966152AEasy to operateRaw materials are easy to getAntibacterial agentsPowder deliveryDextrose MonohydrateMannitol
The invention relates to a cefpiramide composite which is used for the cefpiramide powder-injection in the medicinal manufacture field. The invention discloses the following characteristic of the cefpiramide composite: the composite for the cefpiramide powder-injection which is prepare by the conventional production technology of powder-injection contains two or more of the following components: (a) sterile cefpiramide, (b) sterile sodium carbonate; and (c) sterile anhydrous glucose, dextrose monohydrate, sodium chloride, mannitol, sorbitol, anhydrous lactose or lactose monohydrate, wherein the weight ratio of the component (a) to the component (b) and the component (c) is 1:0.15-0.4:0.03-4.0. The invention has the advantages that the problem that the cefpiramide for injection on the market is difficult to dissolve, especially at a low temperature is solved; the operation of the nurse is easy; and the raw materials are accessible and the drug stability is good.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
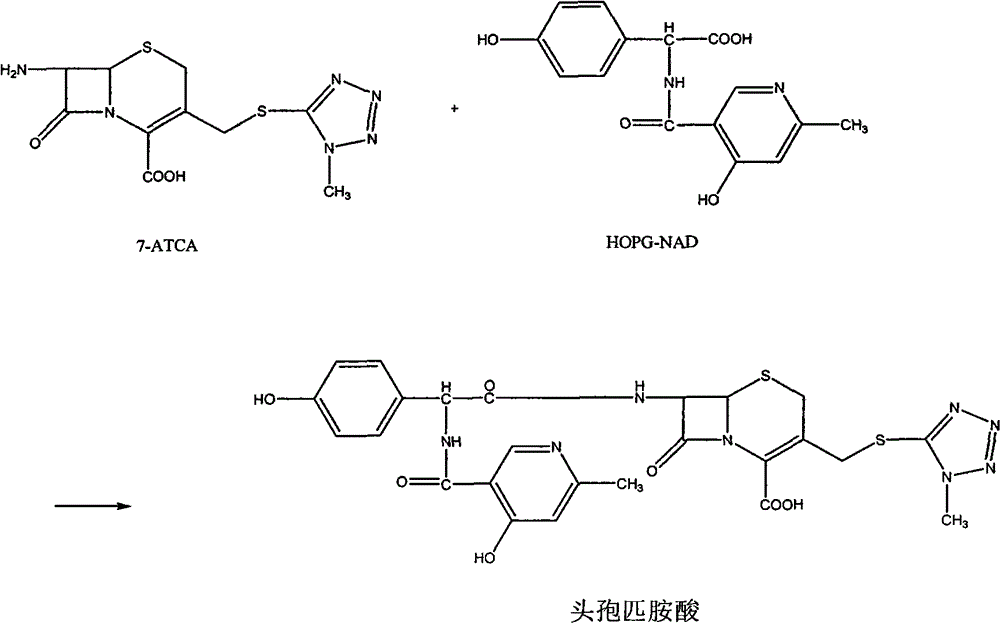
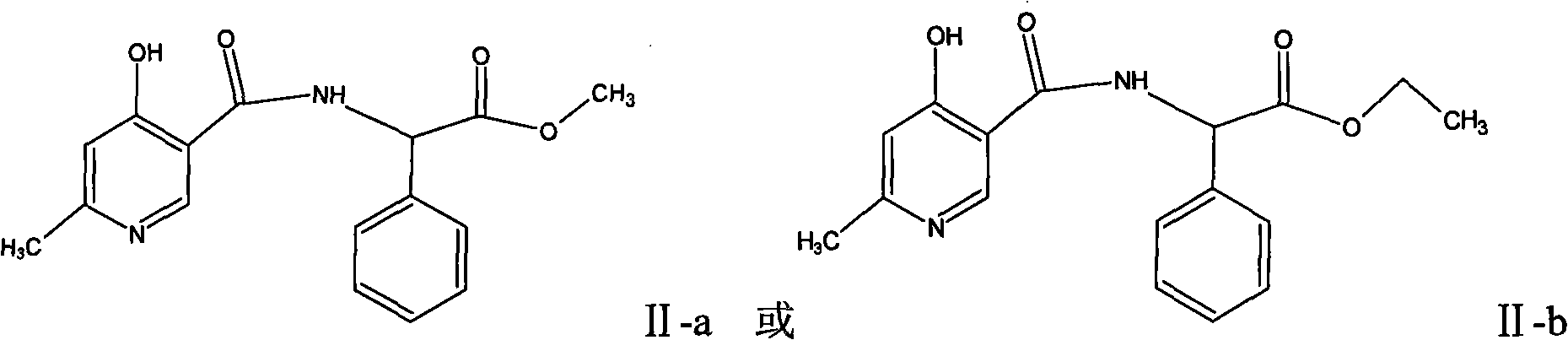
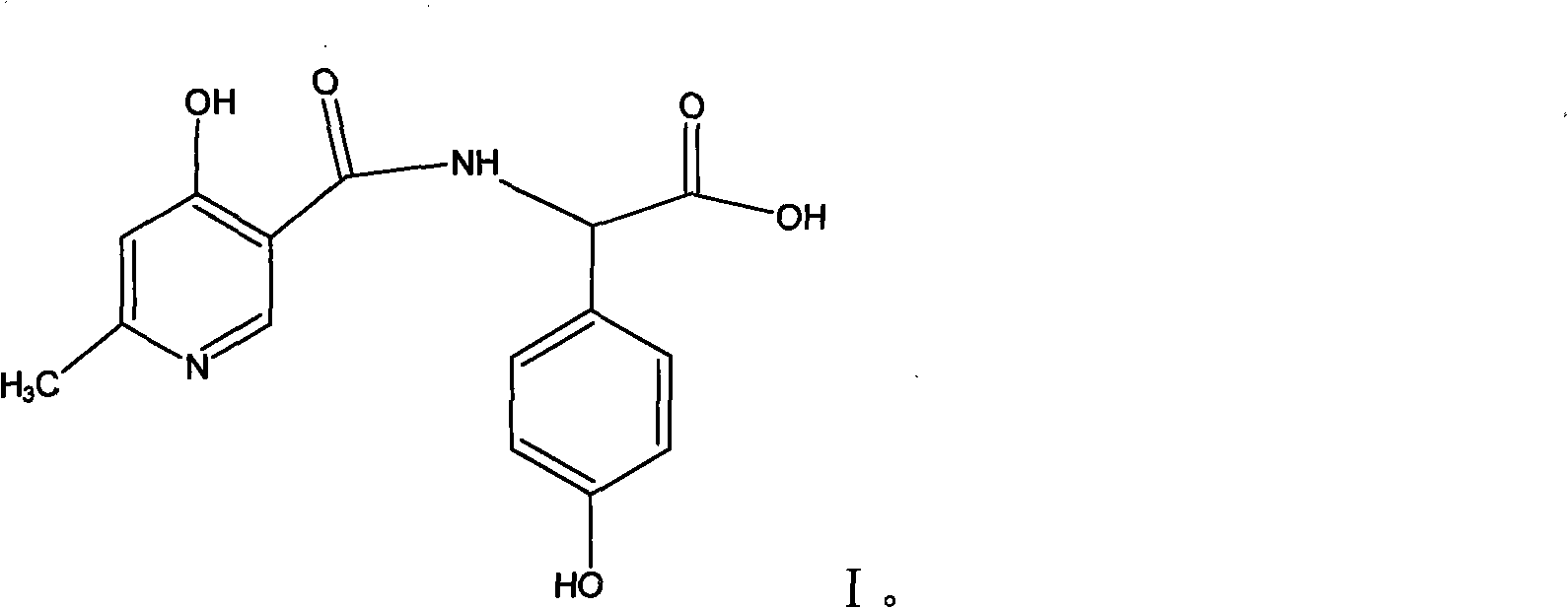
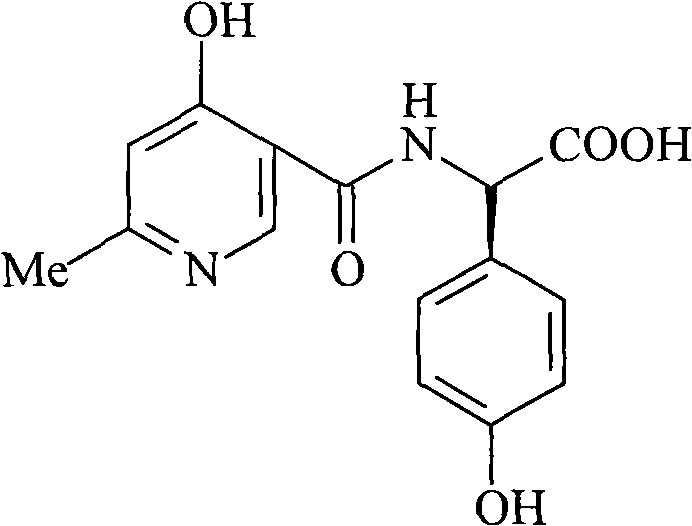
Process for synthesizing D-alpha-(6-methyl-4-hydroxyl nicotinamide base)p-hydroxyphenylacetic acid
ActiveCN101293868AProcess parameters are easy to controlNo pollution in the processOrganic chemistryBulk chemical productionAntibiotic YP-hydroxyphenylacetic acid
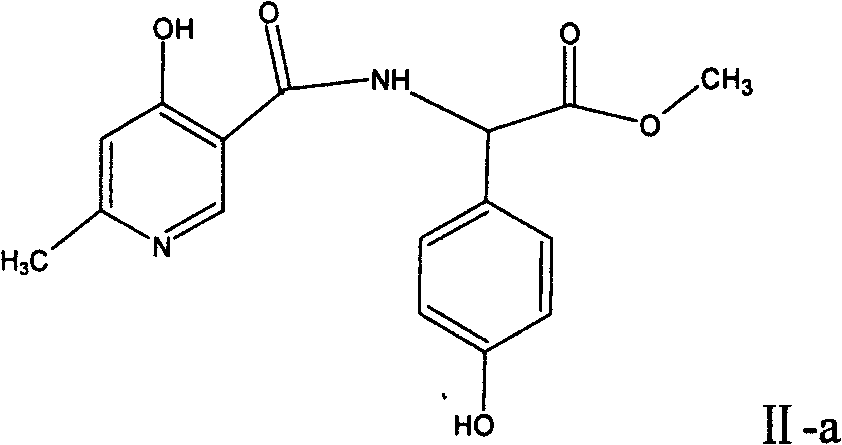
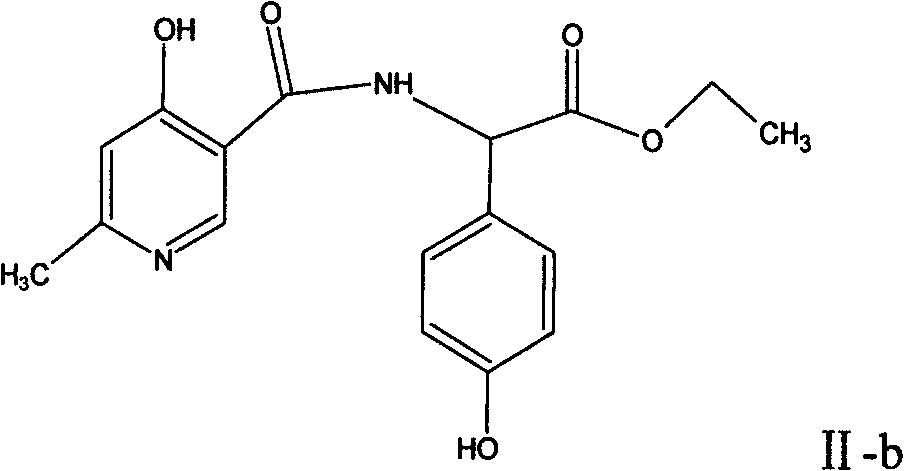
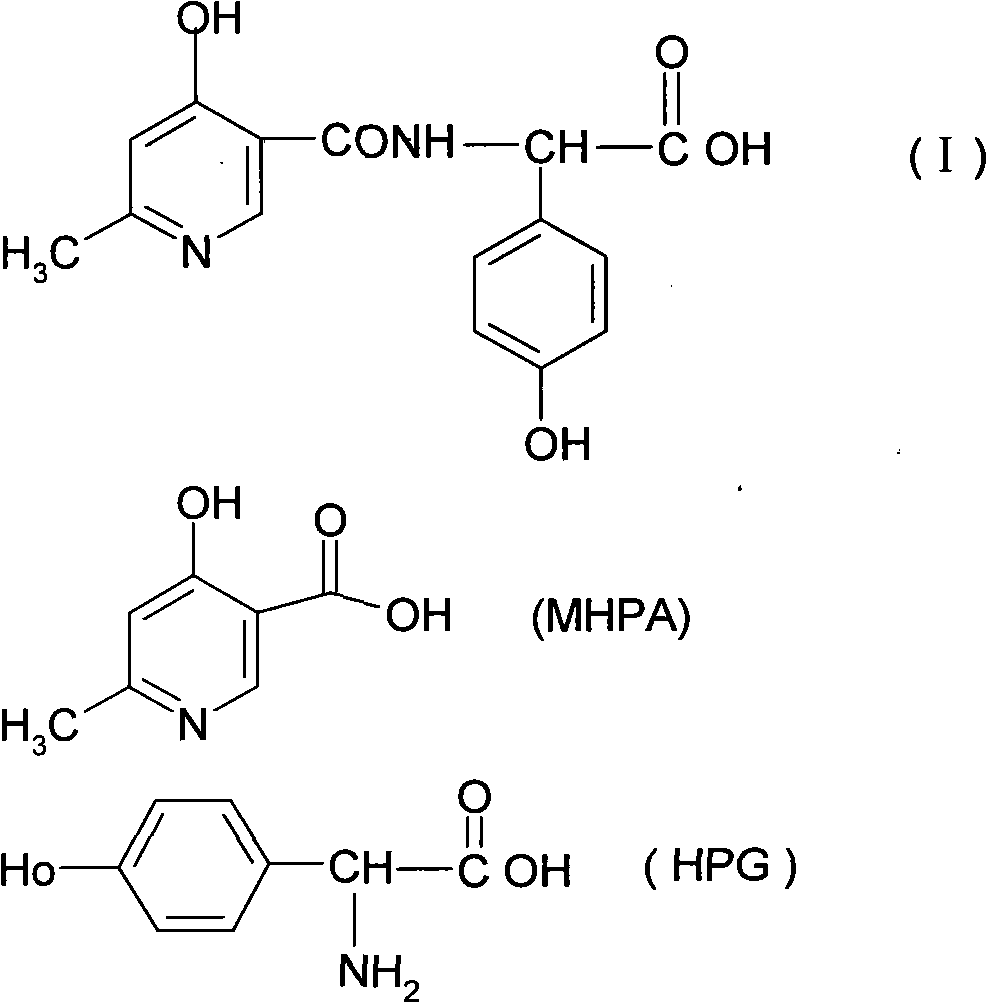
The invention relates to a method for synthesizing an intermediate of cephalosporins antibiotics cefpiramide, D-alpha-(6-methyl-4-hydroxy nicotinamido)-p-hydroxyphenyl acetic acid. The method comprises the steps of: reacting carboxyl group protected D-p-hydroxyphenyl glycine and anhydride or acyl chloride of 4-hydroxy-6-methylnicotinic acid to obtain acylated product, and hydrolyzing to remove the protecting group to obtain the target product D-alpha-(6-methyl-4-hydroxy nicotinamido)-p-hydroxyphenyl acetic acid. The method has the advantages of easy and feasible operation, and applicability to large scale production.
Owner:QILU ANTIBIOTICS PHARMA
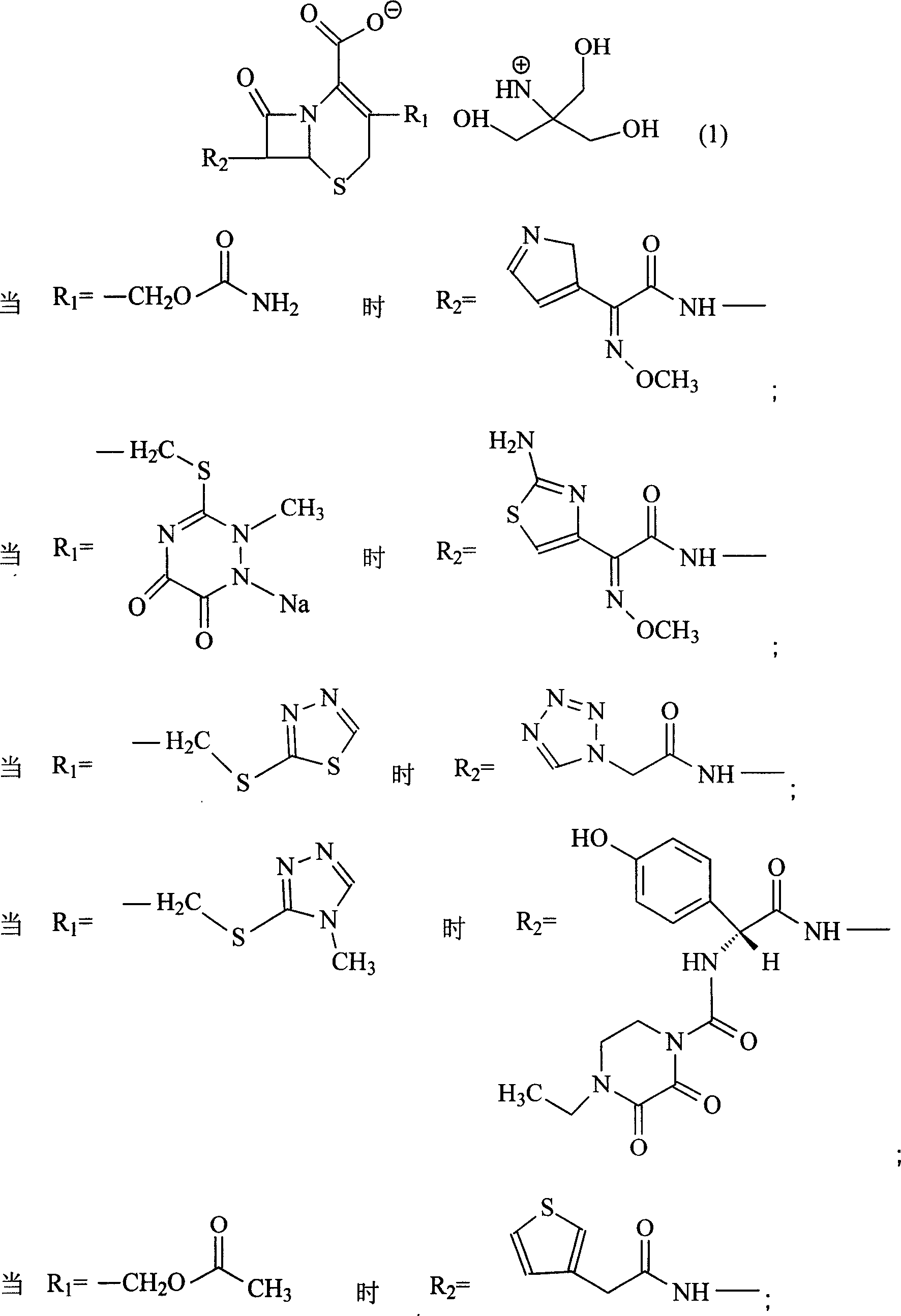
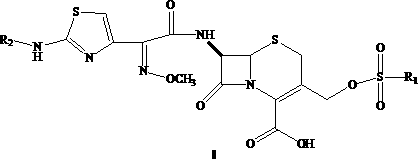
Amino butanetriol salt of cephalosporin compounds and preparing method
InactiveCN101012235AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefuroximeCefazolin
The invention discloses a pehanorm salt or hydrate with chemical formula as picture (1) and drug composition and application to treat bacterial infection, which comprises the following parts: cefuroxime oxtatromethane, cepham qusong tromethane, cepham thiotepa tromethane, cefoperazone tromethane, cephalothin tromethane, cefotaxime tromethane, cefolading tromethane, cefonixin tromethane, cefameizin tromethane, cefadizine tromethane, cefuroxime tromethane, cefazolin tromethane, cefapamine tromethane, cefazoline tromethane, cefaadid tromethane, cefaoxofluoride tromethane, cefaminol tromethane and their hydrate.
Owner:GUANGDONG ZHONGKE DRUG R&D
Cefpiramide sodium compound of new way
InactiveCN101671350AEnsure safetyHigh purityAntibacterial agentsOrganic chemistryOrganic solventNiacinamide
The invention relates to a cefpiramide sodium compound of a new way. The target product, i.e. cefpiramide sodium compound, is prepared by dissolving triphosgene, D-alpha-(6-methyl-4-hydroxy niacinamide)-p-hydroxy phenylacetic acid and triphenylphosphine oxide into an organic solvent, mixing and reacting the D-alpha-(6-methyl-4-hydroxy niacinamide)-p-hydroxy phenylacetic acid with the triphenylphosphine oxide, and then dripping reaction liquid into an aqueous 7-TMCA solution for reaction.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for preparing cefpiramide
InactiveCN102964358ASimple and fast operationEasy to handleOrganic chemistryCarboxylic acidSilylation
The invention provides a method for preparing cefpiramide. The method is characterized by comprising the following steps: (1) a compound of D-a-(4-hydroxyl-6-methyl nicotinamide)-P-sodium hydroxyphenyl acetate is dissolved in organic solvent and is activated through an activating agent under alkaline catalysis at the temperature of minus 25 to minus 20 DEG C so as to produce mixed acid anhydride solution (a); (2) a compound of 7-amino-3-(1-mehtyl-1H-tetrazole-5-sulfomethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid is dissolved in the organic solvent, is added with a silylation reagent at the temperature of 20-25 DEG C so as to be subjected to silylation protective reaction and is cooled to the temperature below minus 25 to minus 20 DEG C to obtain solution (b); and (3) the solution (a) and the solution (b) are mixed together, the obtained mixed solution is added with triethylamine to react at the temperature of minus 25 to minus 20 DEG C and is added into aqueous solution after completing the reaction to regulate the pH value, so that the cefpiramide is obtained. The method is simple to operate, the yield and purity of the produced cefpiramide are high, and the method is suitable for industrial production.
Owner:苏州盛达药业有限公司
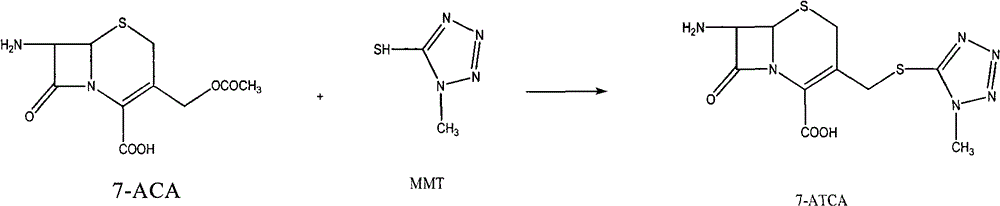
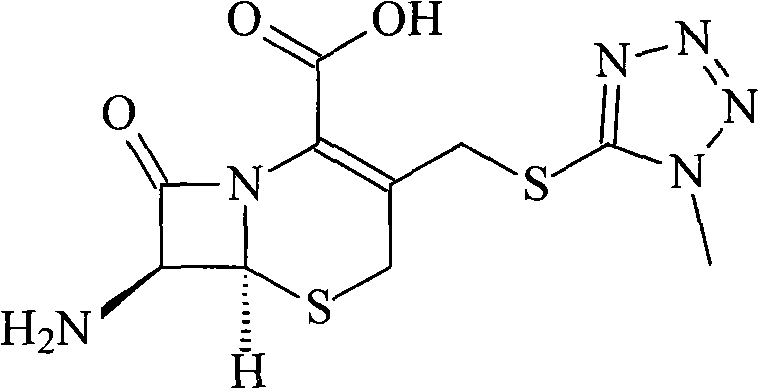
Preparation method of 7-aminocephalo-5-mercapto-1-methyltetrazole
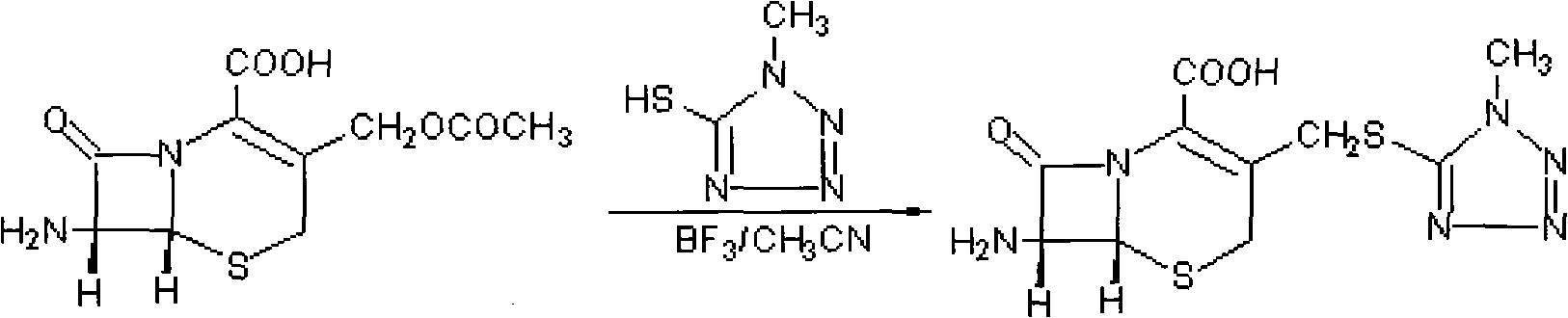
The invention provides a preparation method of 7-aminocephalo-5-mercapto-1-methyltetrazole which is a cephems intermediate. In the method, 7-ACA is taken as the raw material; a suitable catalyst is added to a proper solvent to react with 5-mercapto-1-methyltetrazole to obtain the intermediate of 7-aminocephalo-5-mercapto-1-methyltetrazole. The intermediate is applied to the synthesis of cefmenoxime, cefpiramide and the like.
Owner:徐斌
Cefpiramide sodium composition freeze-dried injection for injection
InactiveCN103550247ABroad spectrum antibacterial activity in vitroIn line with the research and development directionAntibacterial agentsOrganic active ingredientsChitosan nanoparticlesFreeze-drying
The invention provides a cefpiramide sodium composition freeze-dried injection for injection, and relates to the technical field of medicines and medicine preparation. The cefpiramide sodium composition freeze-dried injection for injection comprises the following raw material components in parts by weight: 7.26-9.17 parts of cefpiramide sodium, 5.78-7.67 parts of chitosan nanoparticles and 81.38-87.10 parts of water for injection. The cefpiramide sodium composition freeze-dried injection for injection has the advantages that 1) the in vitro antimicrobial activity spectrum is wider and covers Gram-positive bacteria and Gram-negative bacteria (comprising Pseudomonas aeruginosa), the characteristics that the G+ bacterial and Pseudomonas aeruginosa resistance of the third and fourth generation cephalosporin and the activity of anaerobic bacteria are poor, and research and development direction of cephalosporin is met; 2) the injection is good in stability, thus greatly reducing the risk that the product quality is greatly reduced in production and circulation links (particularly commercial circulation; 3) the activity is enhanced, so that the medication period of the patient is shortened, and the probability of adverse reaction caused by accumulation of cefpiramide sodium is reduced; 4) the chitosan nanoparticles can replace mannitol as a freeze-dried skeleton agent of the freeze-dried injection, so that the activity of mannitol on human body is eliminated.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
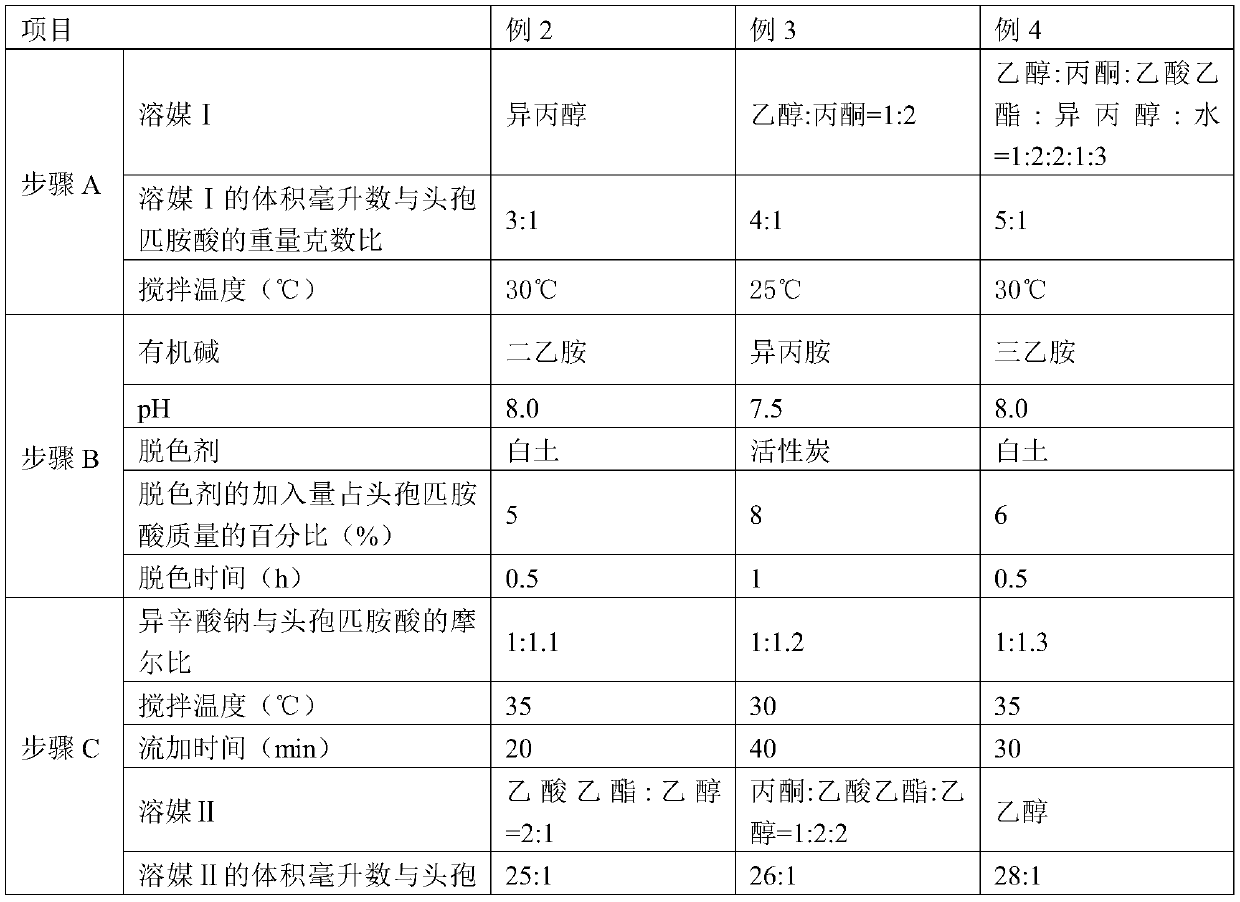
Method for refining cefpiramide acid
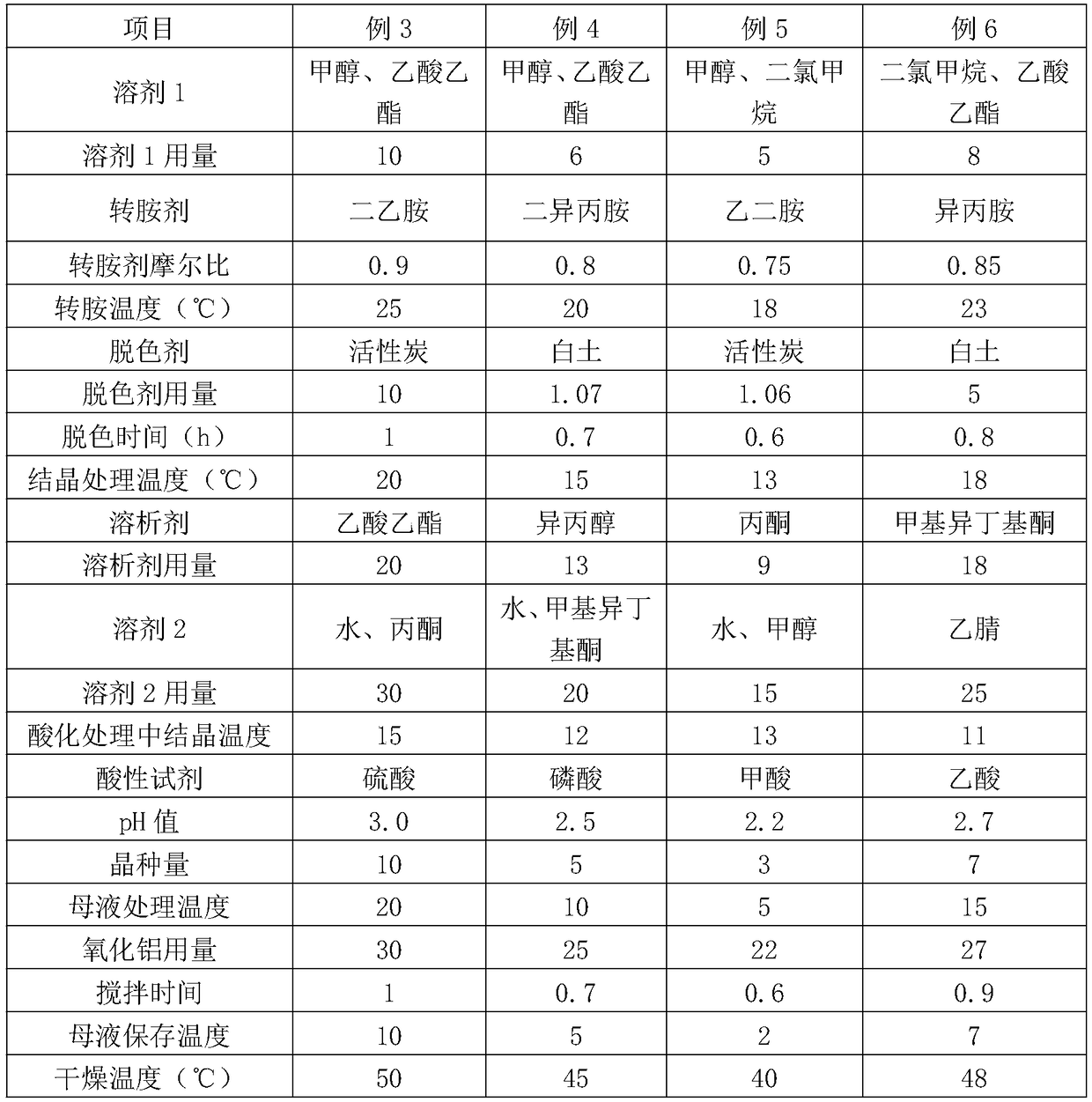
The invention discloses a method for refining cefpiramide acid, and belongs to the technical field of medicines. The method comprises the following steps: A, putting crude cefpiramide acid into a solvent 1, adding a transaminating agent, stirring and dissolving the mixture to clarification, so as to obtain a cefpiramide salt solution; B, adding a decolorizing agent into the cefpiramide salt solution, filtering and collecting filtrate; C, controlling the filtrate temperature to be 10-20 DEG C, adding a dissolution agent, filtering and collecting a solid crystal; D, putting the solid crystal into a solvent 2, controlling the temperature to be 10-15 DEG C, adding an acidic reagent to adjust the pH value to 2.0-3.0, adding a seed crystal, crystallizing, filtering and collecting the solid crystal of cefpiramide acid and mother liquor; and E, washing the solid crystal of cefpiramide acid and drying to obtain refined cefpiramide acid. The cefpiramide acid prepared with the method is high in purity, few in impurities and low in color grade; and the refining method provided by the invention has the advantages of being simple in process, environmentally friendly, and suitable for large-scaleindustrial production, saving energy, and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
New antibiotic composition
The antibiotic medicine composition is mixture of cefpiramide and beta-lactamase inhibitor in the weight ratio of 1 / 3 to 10. The cefpiramide may be replaced with alkali metal salt of cefpiramide or the mixture of cefpiramide, sodium carbonate and arginine. The beta-lactamase inhibitor may be clavulanic acid or its derivative, sulbactam or its derivative, or tazobactum or its derivative. The antibiotic medicine composition has high antibiotic effect, especially on sensitive bacteria and zymogenic bacteria, and wide antibiotic spectrum.
Owner:北京盛世伟唐科技有限公司
Cefpiramide drug combination
InactiveCN101822680AImprove antibacterial propertiesNot be damagedAntibacterial agentsOrganic active ingredientsAspirin DL-LysineAspirin lysine
The invention discloses a cefpiramide drug combination, which consists of the following effective ingredients in part by weight: 500 to 2000 parts of cefpiramide or pharmaceutically acceptable salt thereof (by cefpiramide), 100 to 200 parts of lidocaine, 20 to 100 parts of reduced glutathione and 50 to 100 parts of aspirin-DL-lysine. The drug combination cannot cause adverse reactions, and has a high curative effect, and moreover, the preparation method is simple and environment-friendly.
Owner:邓学峰
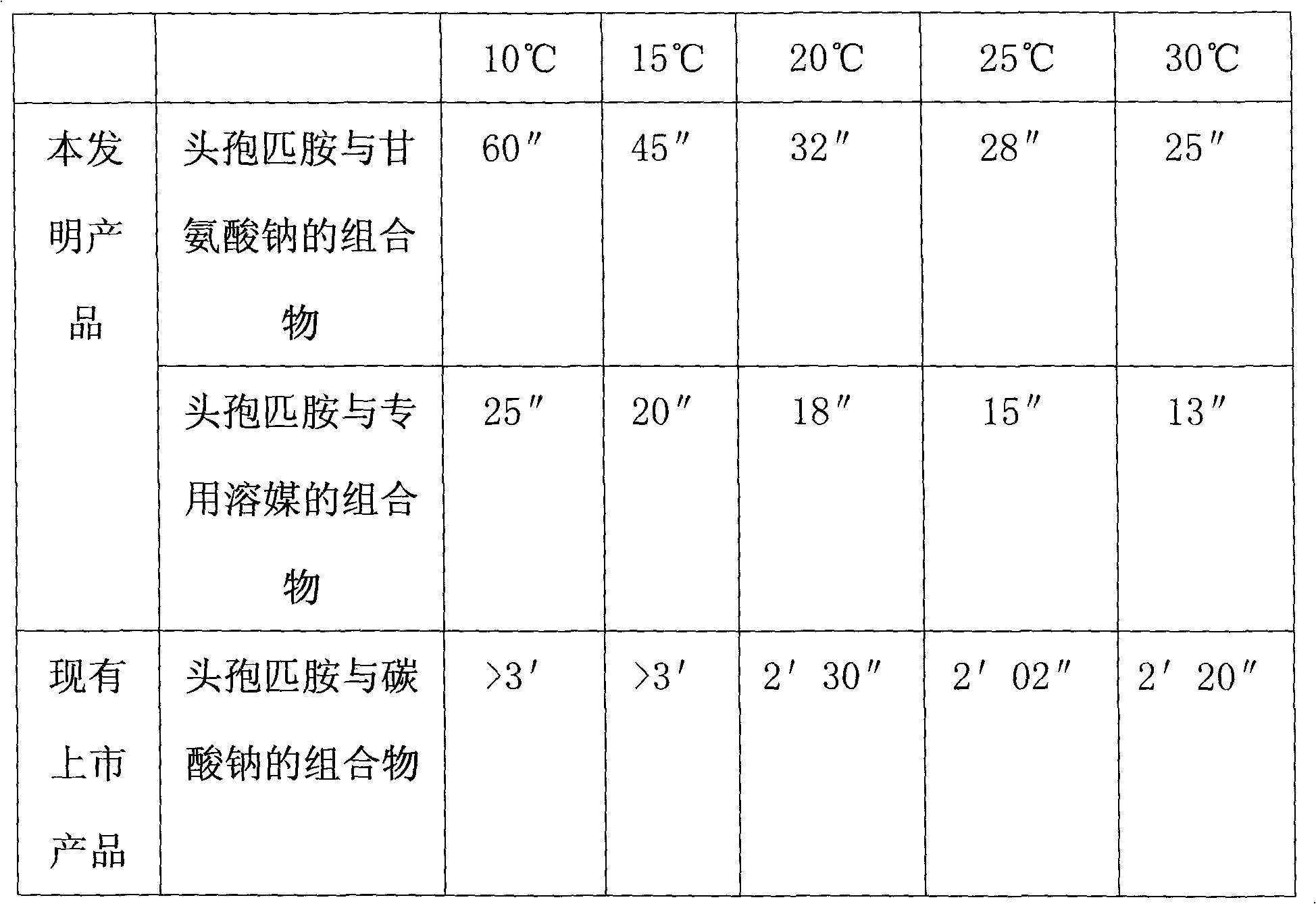
Composition of cefpiramide and sodium glycinate or special solvent composition containing sodium glycinate
ActiveCN101773506ASolve the difficulty of dissolutionLittle to no blood vesselsAntibacterial agentsOrganic active ingredientsBottleAmino acid
The invention relates to a composition of cefpiramide and sodium glycinate or a special solvent composition containing sodium glycinate. The invention is used for pharmacy. The weight ratio of the cefpiramide to the sodium glycinate is 1:0.152-0.210 and the solvent composition is specially used for injection. A bottle of 0.5g of cefpiramide is mixed with 2-10ml of solvent, and a bottle of 1g of cefpiramide is mixed with 5-20ml of solvent. The cefpiramide is easy to dissolve, and the invention is safe to inject, can provide amino acid indispensible to human body and improve the immunity of the patients, and is simple and convenient to operate.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
A kind of preparation method of cefpiramide sodium
The invention discloses a preparation method of cefpiramide sodium, which belongs to the field of chemical pharmacy, and comprises the steps of preparing cefpiramide salt by using cefpiramide as raw material, forming salt and crystallizing, drying, and using gas to drive the atomized cefpiramide before drying. Purging with water for injection; the invention can reduce the solvent residue in the product, and the prepared cefpiramide sodium has the advantages of high purity and good stability, the preparation method is simple, energy-saving and environment-friendly, and is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
The method for crystallization production cefpiramide sodium crystal
The invention discloses a method for crystallizing and producing cefpiramide sodium crystals. The method comprises the steps that: (a) cefpiramide acid and an transamination agent are dissolved in a solvent I, wherein the transamination agent is any one selected from triethylamine, diisopropylamine, and isopropylamine; and a temperature is controlled, and the materials are stirred until completely dissolved; (b) a salt-forming agent is added into a solvent II, wherein when the salt-forming agent is sodium ethylhexanoate, the solvent II is an acetone solvent, and when the salt-forming agent is sodium hydroxide, the solvent II is any one or a mixture of two selected from methanol and tetrahydrofuran; and the materials are stirred until completely dissolved; (c) the salt-forming agent solution is uniformly added into the solution of cefpiramide amine salt; the temperature is controlled, and crystal seeds are added; curing crystallization is carried out; acetone is added into the crystallization system for regulating the pH value of the crystallization system and for carrying out solvent-out crystallization; and filtering, washing, and drying are carried out, such that the cefpiramide sodium crystals are obtained. The method provided by the invention has the advantages of simple operation, uniform crystals, high purity, low impurity content, good stability, easy storage, and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefpiramide-containing medicine used for injection
InactiveCN101773505ADissolve fastShort dissolution timeAntibacterial agentsOrganic active ingredientsDiseaseMedicine
The invention relates to a cefpiramide-containing medicine used for injection, which is used for an injection used for treating diseases. The invention discloses the medicine used for the injection, which comprises a component A of cefpiramide and a component B of a solvent which contains organic base and is exclusively used for the cefpiramide used for the injection, wherein the cefpiramide and the solvent are separately packaged, and the weight ratio of the component A to the component B is 1:5 to 20. The invention is easy to dissolve, is simple and convenient to operate and is safe during injection.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Process for synthesizing D-alpha-(6-methyl-4-hydroxyl nicotinamide base)p-hydroxyphenylacetic acid
ActiveCN101293868BProcess parameters are easy to controlProcess parameter controlOrganic chemistryBulk chemical productionAntibiotic YP-hydroxyphenylacetic acid
The invention relates to a method for synthesizing an intermediate of cephalosporins antibiotics cefpiramide, D-alpha-(6-methyl-4-hydroxy nicotinamido)-p-hydroxyphenyl acetic acid. The method comprises the steps of: reacting carboxyl group protected D-p-hydroxyphenyl glycine and anhydride or acyl chloride of 4-hydroxy-6-methylnicotinic acid to obtain acylated product, and hydrolyzing to remove the protecting group to obtain the target product D-alpha-(6-methyl-4-hydroxy nicotinamido)-p-hydroxyphenyl acetic acid. The method has the advantages of easy and feasible operation, and applicability to large scale production.
Owner:QILU ANTIBIOTICS PHARMA
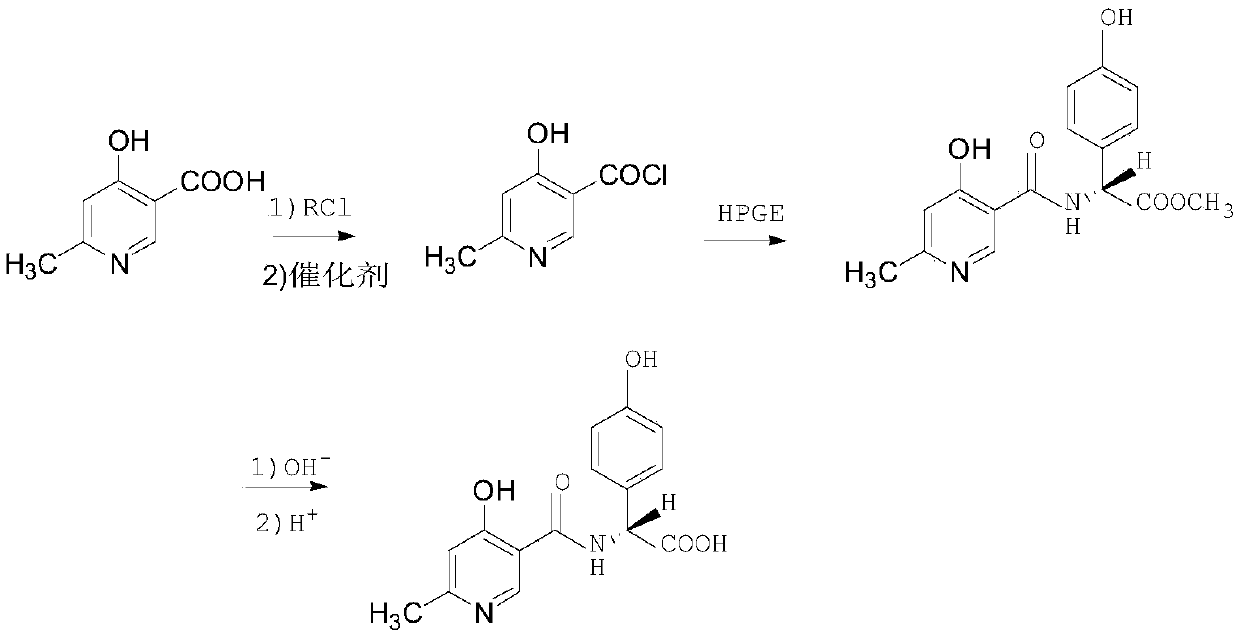
Synthesis method of cefpiramide side chain acid
InactiveCN111116463AEasy to operateRaw materials are easy to getOrganic chemistry methodsChemical synthesisSide chain
The invention belongs to the technical field of chemical synthesis, and particularly relates to a synthesis method of cefpiramide side chain acid. Pyridine-3-formic acid and methyl p-hydroxyphenylglycinate are used as raw materials, and the cefpiramide side chain acid is obtained through chlorination, acylation, hydrolysis and crystallization. The synthesis method of the cefpiramide side chain acid is simple to operate, easily available in raw materials and low in cost, adopts a dichloromethane and N, N-dimethylacetamide mixed solvent as a solvent, greatly improves the product yield and stability, has good economic benefits, and is convenient for large-scale industrial production.
Owner:YIYUAN XINQUAN CHEM
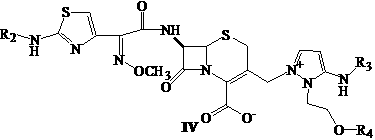
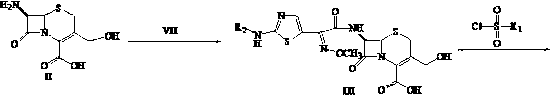
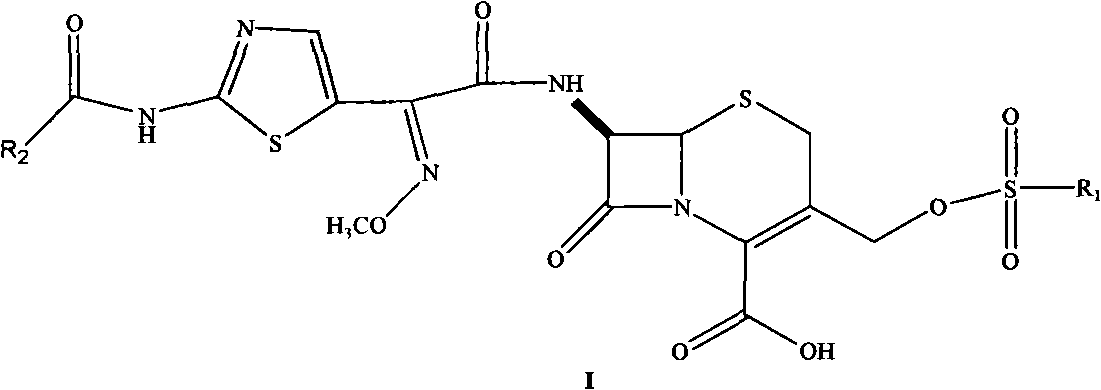
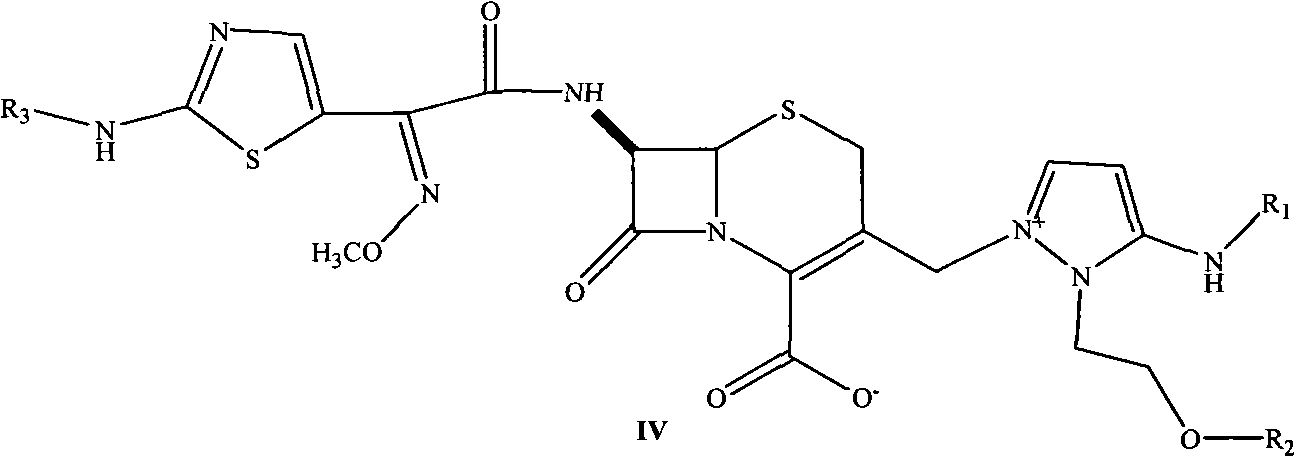
Cephalosporin nucleus derivative compound, cephaene onium salt compound prepared from same, and method for preparing cefpiramide sulfate from cephalosporin nucleus derivative compound and cephaene onium salt compound
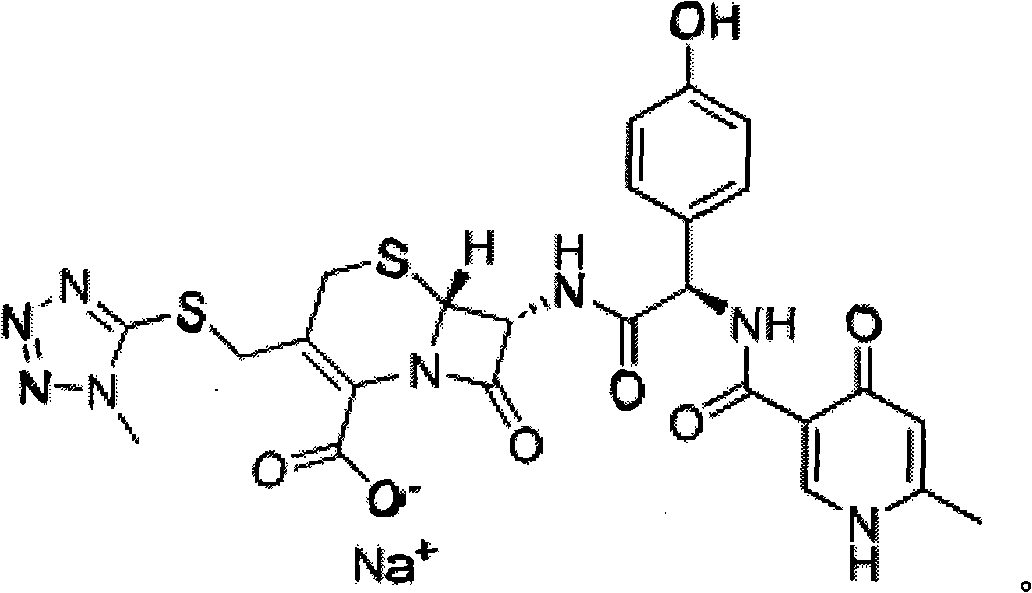
The invention discloses cephalosporin nucleus derivative compound and cephaene onium salt compound prepared from the cephalosporin nucleus derivative compound, which are respectively intermediates for synthesizing cefpiramide sulfate, and a method for preparing the cefpiramide sulfate from the two intermediates, namely preparing 7beta-[2-(2-alkanol aminothiazol-5-yl)-2-methoxyimino acetamid]]-3-[3-alkanol amino-2-(2-alkanol oxy ethyl)-1-pyrazol onium]methyl-3-cephem-4-carboxylate (IV) by coupling 7beta-[2(2-alkanol aminothiazol-5-yl)-2-methoxyimino acetamid]]-3-(hydrocarbon sulfonate)-3-cephem-4-carboxylic acid (I) serving as a raw material with 5-alkanol amino-2-(2-alkanol oxy ethyl)-1-pyrazol, and then converting to obtain the cefpiramide sulfate. The method has the advantages of mild reaction conditions, short steps, cheap raw materials, industrial production, no special reagents, simple process and easy amplification.
Owner:YANTAI BAOHUA BIO TECH +1
Cephalosporin nucleus derivative compound, cephaene onium salt compound prepared from same, and method for preparing cefpiramide sulfate from cephalosporin nucleus derivative compound and cephaene onium salt compound
Owner:YANTAI BAOHUA BIO TECH +1
Composite method for cefpiramide midbody D-alpha-(4-Hydroxy-6-methylnicotinamido) hydroxyphenylacetic acid
The invention relates to a composite method for cefpiramide midbody D-alpha-(4-Hydroxy-6-methylnicotinamido) hydroxyphenylacetic acid, which includes the steps of using the acyl chloride of 4-hydroxy-6-methylnicotinic acid to directly acylate and esterify D-hydroxyphenylglycine, using water to remove the estersil of an acylation product and directly obtaining an objective product D-alpha-(4-Hydroxy-6-methylnicotinamido) hydroxyphenylacetic acid. Compared with the method of composing D-hydroxyphenylglycine into alkane ester or salt at first, the composite method for cefpiramide midbody D-alpha-(4-Hydroxy-6-methylnicotinamido) hydroxyphenylacetic acid simplifies the operation and is suitable for large-scale production.
Owner:山东众诚生物医药股份有限公司
Cefpiramide composite
ActiveCN101966152BEasy to operateRaw materials are easy to getAntibacterial agentsOrganic active ingredientsDextrose MonohydrateMannitol
The invention relates to a cefpiramide composite which is used for the cefpiramide powder-injection in the medicinal manufacture field. The invention discloses the following characteristic of the cefpiramide composite: the composite for the cefpiramide powder-injection which is prepare by the conventional production technology of powder-injection contains two or more of the following components: (a) sterile cefpiramide, (b) sterile sodium carbonate; and (c) sterile anhydrous glucose, dextrose monohydrate, sodium chloride, mannitol, sorbitol, anhydrous lactose or lactose monohydrate, wherein the weight ratio of the component (a) to the component (b) and the component (c) is 1:0.15-0.4:0.03-4.0. The invention has the advantages that the problem that the cefpiramide for injection on the market is difficult to dissolve, especially at a low temperature is solved; the operation of the nurse is easy; and the raw materials are accessible and the drug stability is good.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com