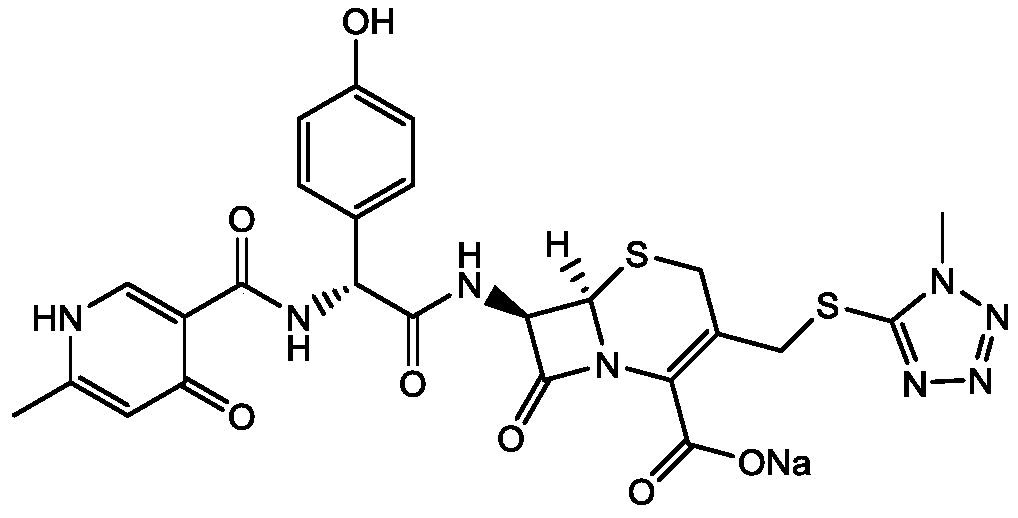
A kind of preparation method of cefpiramide sodium
A technology of cefpiramide sodium and cefpiramic acid, which is applied in the direction of organic chemistry, can solve the problems of difficult drying of products, long process time and high residual solvents, and achieve the effects of shortening drying time, high purity and low residual solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A preparation method of cefpiramide sodium comprises the steps of preparing cefpiramide salt with cefpiramide as a raw material, forming salt and crystallizing, drying, and purging with atomized water for injection driven by gas before drying.
[0031] The preparation method comprises the following steps:
[0032] A. Add cefpiramin to the solvent Ⅰ which is 3 to 6 times the weight of cefpiramin, and stir at 20 to 30°C to obtain a cefpiramin solution;
[0033] In step A, solvent I is any one or a combination of ethanol, acetone, ethyl acetate, isopropanol or water;
[0034] B. Add an organic base to the cefpiramide solution, stir, and control the pH to be 7.5 to 8.0, then add 5 to 10% of the mass of cefpiramide with activated carbon or white clay, decolorize for 0.5 to 1 hour, and filter to obtain cefpiramide Amine amine salt solution;
[0035] The organic base is any one of diethylamine, triethylamine or isopropylamine;
[0036] C. Add sodium isooctanoate to solvent ...
Embodiment 1
[0043] A preparation method of cefpiramide sodium comprises the steps of preparing cefpiramide salt with cefpiramide as a raw material, forming salt and crystallizing, drying, and purging with atomized water for injection driven by gas before drying.
[0044] The preparation method comprises the following steps:
[0045] A. Add 20kg of cefpiramic acid to the mixed solution of 5L purified water-35L acetone-20L ethyl acetate-60L ethanol, and stir at 20°C to obtain a cefpiramic acid solution;
[0046] B. drip triethylamine in the cefpiramide acid dissolving liquid, stir, control pH is 7.5, then add 2.0kg gac, stir and decolorize 1h, filter, obtain cefpiramide salt solution;
[0047]C. Add 7.05kg of sodium isooctanoate into 600L of acetone, stir at 30°C, filter to obtain sodium isooctanoate solution, add cefpiramide salt solution to the sodium isooctanoate solution within 30 minutes under stirring, and stir at 10°C Crystal growth 1h;
[0048] D. The crystals are filtered, washed...
Embodiment 2~4
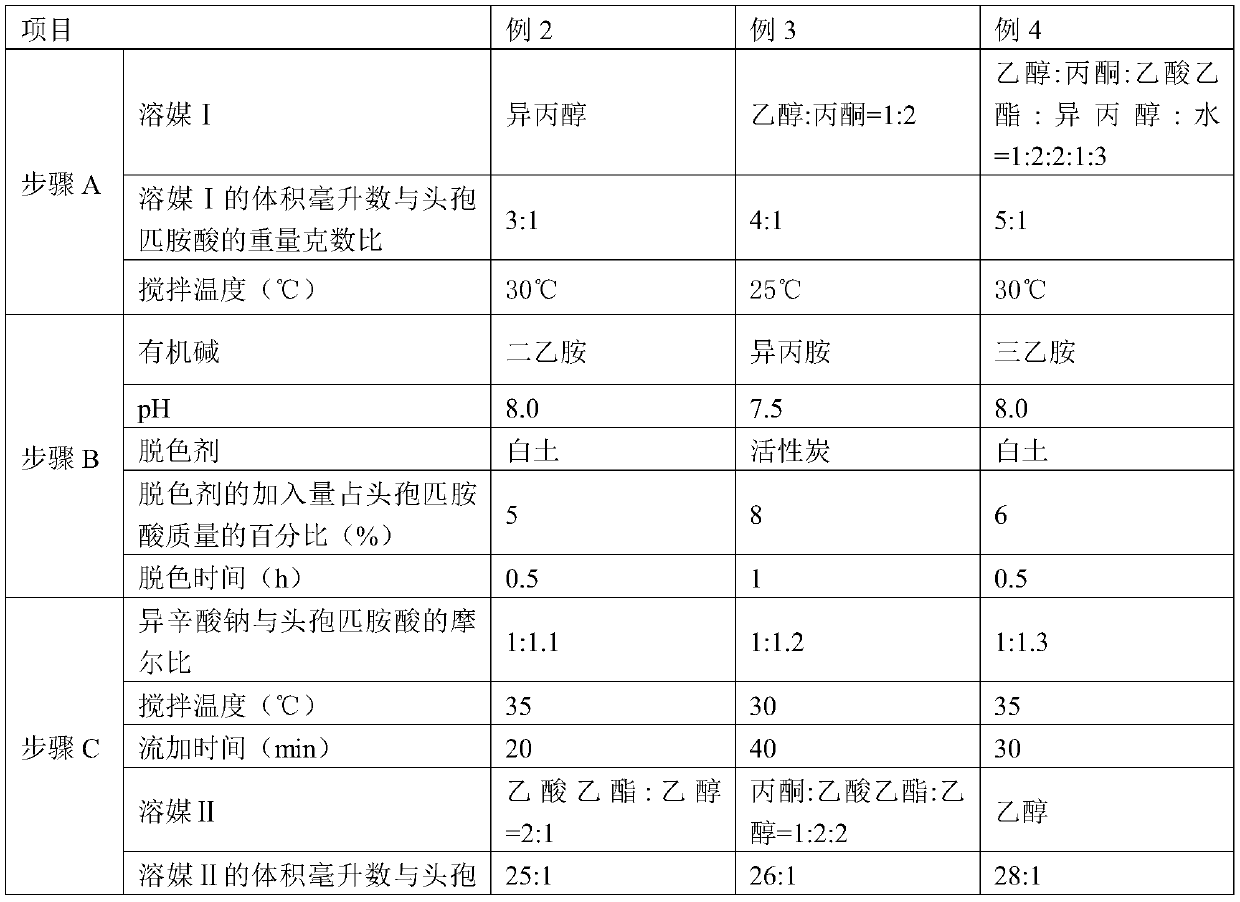
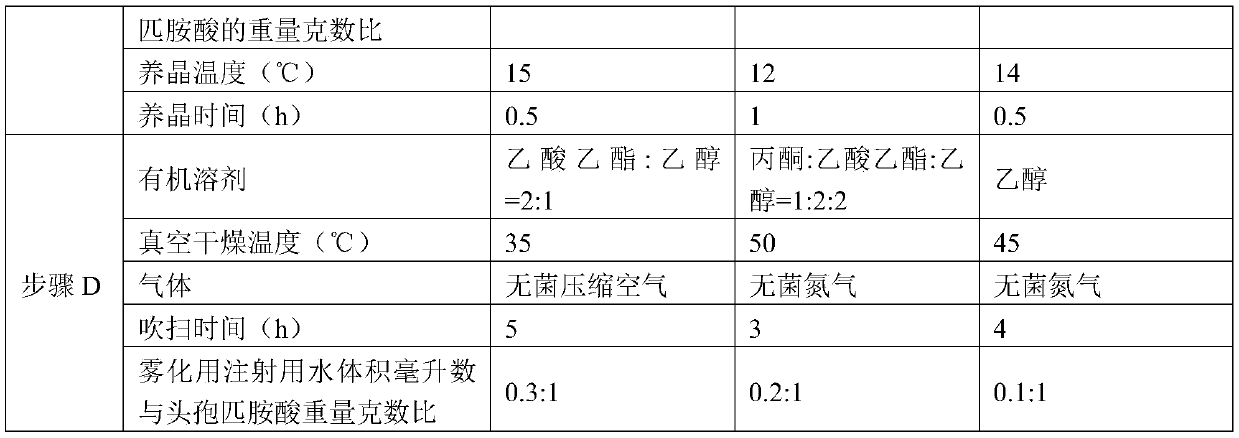
[0050] Embodiments 2-4 have the same production process steps as in Embodiment 1, the difference is the selection of process parameters, as shown in Table 1 below.
[0051] Table 1
[0052]
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com