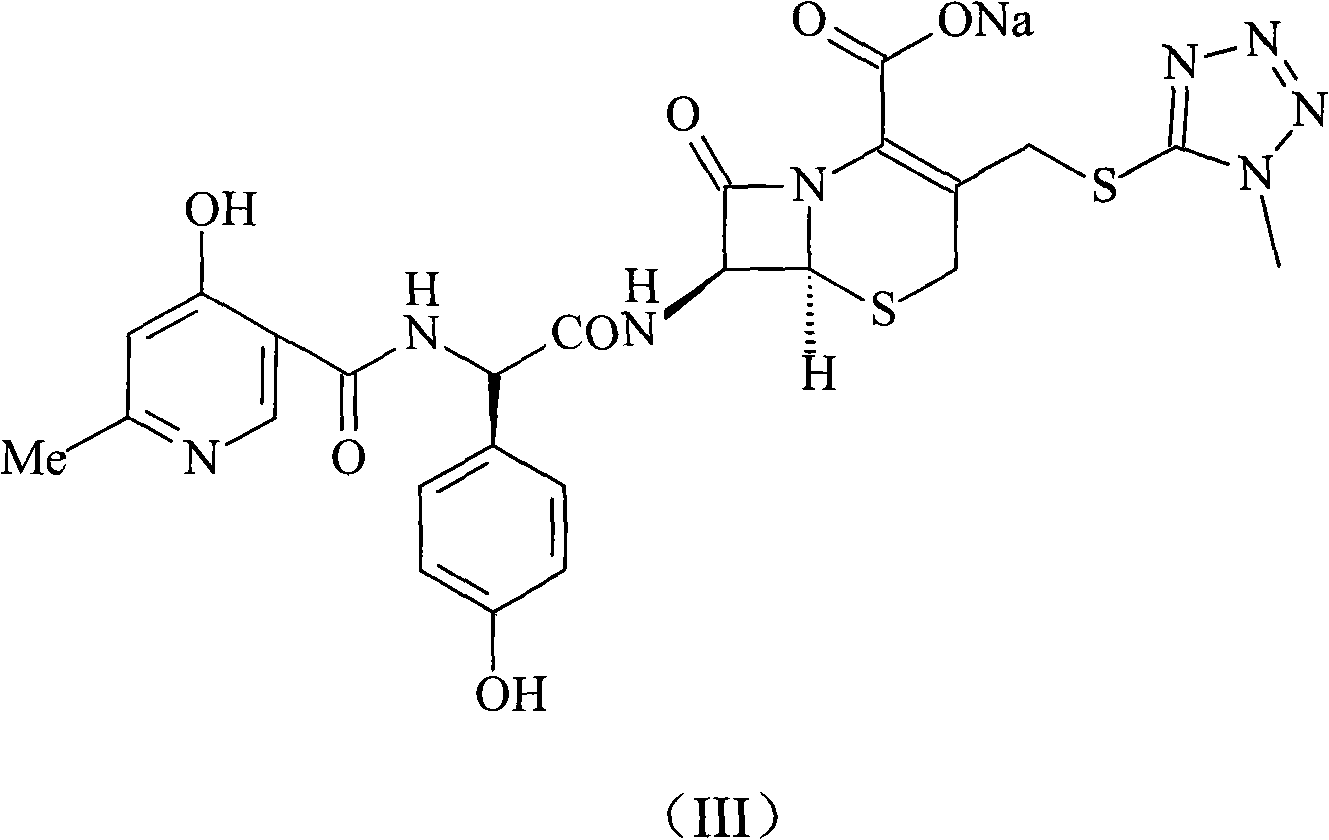
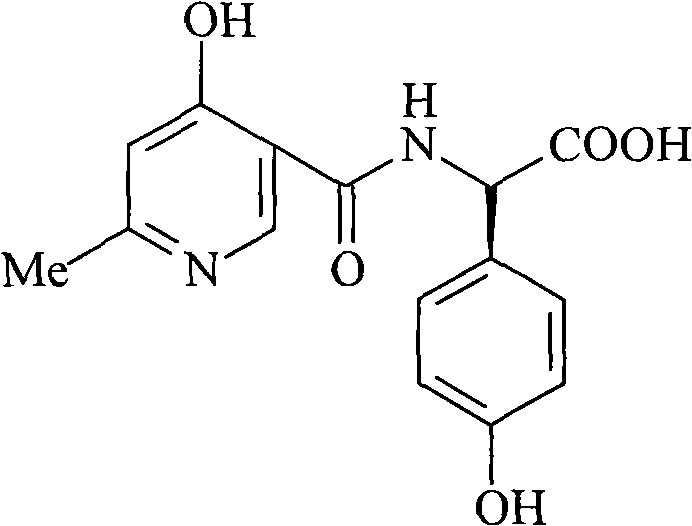
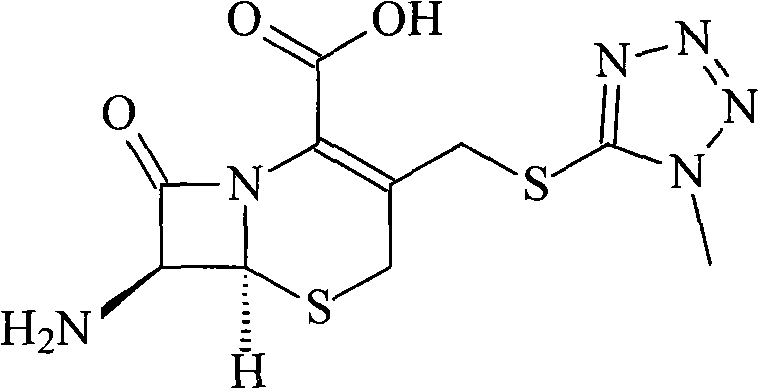
Cefpiramide sodium compound of new way
A technology of cefpiramide sodium and its compounds, which is applied in the field of drug synthesis, can solve the problems of uneven crystal form, poor stability, and low purity, and achieve the effects of improved purity and yield, simple equipment, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 cefpiramide sodium
[0027] Dissolve 600 grams of triphosgene in 1 liter of dichloromethane, and add dropwise to 302 grams of D-α-(6-methyl-4-hydroxynicotinamide)-p-hydroxyphenylacetic acid and 556 grams of triphenyl In the system of phosphine and 2 liters of dichloromethane, react at -5°C for 4 hours, then add dropwise to 1 liter of water containing 328 grams of 7-TMCA, adjust pH=7.5 with sodium hydroxide in the reaction, The reaction temperature was controlled at 0°C. After the dropwise addition, reacted for 2 hours, distilled off part of the organic solvent under reduced pressure, added 3 liters of acetone, stirred, precipitated crystals, filtered, and vacuum-dried at 40°C to obtain 584 grams of the product, with a yield of 92 %.
Embodiment 2
[0028] The synthesis of embodiment 2 cefpiramide sodium
[0029] Dissolve 800 grams of triphosgene in 2 liters of xylene and add dropwise to 604 grams of D-α-(6-methyl-4-hydroxynicotinamide)-p-hydroxyphenylacetic acid and 988 grams of triphenyl In the system of oxonphos and 3.5 liters of xylene, react at -3°C for 5 hours, then add dropwise to 3 liters of water containing 656 grams of 7-TMCA, adjust pH=7.8 with sodium hydroxide in the reaction, and the reaction temperature Controlled at 2°C, after dropwise addition, reacted for 3 hours, distilled off part of the organic solvent under reduced pressure, added 3 liters of acetone, stirred, precipitated crystals, filtered, and vacuum dried at 45°C to obtain 1182 grams of product, yield 93.1%.
Embodiment 3
[0030] The synthesis of embodiment 3 cefpiramide sodium
[0031] Dissolve 300 grams of triphosgene in 1 liter of 1,2-dichloroethane, and add dropwise to 151 grams of D-α-(6-methyl-4-hydroxynicotinamide)-p-hydroxyphenylacetic acid and In the system of 282 grams of triphenylphosphine and 2 liters of 1,2-ethylene dichloride, react at 0°C for 6 hours, then add dropwise to 1 liter of water containing 164 grams of 7-TMCA, in the reaction Use sodium hydroxide to adjust PH=7.3, and control the reaction temperature at 5°C. After the dropwise addition, react for 4 hours, remove part of the organic solvent by distillation under reduced pressure, add 2 liters of acetone, stir, precipitate crystals, filter, and vacuum at 40°C After drying, 291 grams of the product were obtained, with a yield of 91.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com