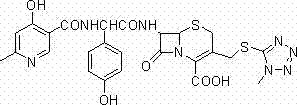
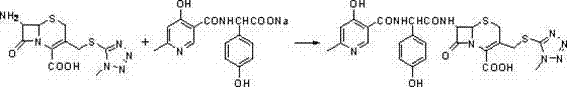
Method for preparing cefpiramide
A technology of cefpiramide and methylnicotinamide, applied in the field of medicine, can solve the problems of difficult control of conditions, easy destruction of beta-lactam, low practicability and the like, and achieves the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add D-a-(4-hydroxy-6-methylnicotinamide)-P-hydroxyphenylethylsodium 19.4g, N, N-dimethylacetamide (DMA) 160ml to a 1L three-necked flask, stir until dissolved, Cool down to 0°C and add 0.6 ml of pyridine dropwise, cool the solution to -25°C, control the temperature at -20°C, add 7.2g of pivaloyl chloride dropwise, stir at -20°C for 10 minutes, then add 80 mL of toluene was stirred for 20 minutes to obtain solution (a).
[0018] Add 7-amino-3-(1-methyl-1H-tetrazole-5-thiomethyl)-8-oxo-5-thia-1-azabicyclo[4.2. 0] Oct-2-ene-2-carboxylic acid (7-TMCA) 16.4g, dichloromethane 100ml, then add 8.8g hexamethyldisilazane (HMDS) and gradually increase the temperature and reflux for 1-2 hours until the solution is clear and transparent , (or blow nitrogen into the solution and stir at room temperature for 1 hour), and cool the solution to -20°C under the protection of nitrogen to obtain solution (b).
[0019] Slowly add solution (b) to solution (a), wash the wall of the bottle wi...
Embodiment 2
[0023] Add D-a-(4-hydroxy-6-methylnicotinamide)-P-hydroxyphenyl ethyl sodium 19.4g, N, N-dimethylacetamide (DMA) 160ml in sequence in a 1L three-necked flask, stir until dissolved, Cool down to 0°C, add 0.6ml of pyridine dropwise, cool to -25°C, add 7.2g of pivaloyl chloride dropwise at -20°C, stir for 10 minutes at -20°C, add 80ml of toluene dropwise at -20°C, stir After 20 minutes, solution (a) was obtained.
[0024] Add 7-amino-3-(1-methyl-1H-tetrazole-5-thiomethyl)-8-oxo-5-thia-1-azabicyclo[4.2. 0] Oct-2-ene-2-carboxylic acid (7-TMCA) 16.4g, acetonitrile 100ml, temperature control less than 25°C, add N, O-bis(trimethylsilyl)acetamide 12.7g, stir at 25°C for 30 Minutes until transparent and clear, cooled to -20°C to obtain solution (b).
[0025] Slowly add solution (b) to solution (a) dropwise, and drop 5.1 g of triethylamine into the reaction system under temperature control at -20°C, stir at -20°C for 15 hours, raise the temperature to 0°C for 2 hours, and then Pour th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com