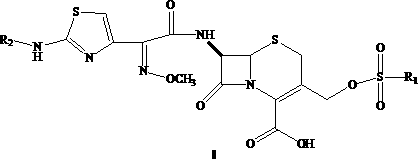
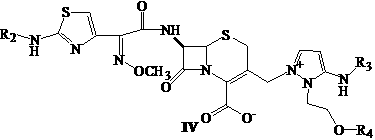
Cephalosporin nucleus derivative compound, cephaene onium salt compound prepared from same, and method for preparing cefpiramide sulfate from cephalosporin nucleus derivative compound and cephaene onium salt compound
A technology for cefepizole sulfate and compound, which is applied in the field of preparing cefepizole sulfate and cephem onium salt compounds, and achieves the effects of mild reaction conditions, three-waste treatment and easy three-waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
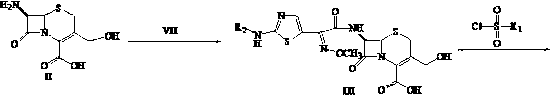
[0042] Embodiment 1, the first step: 7β-amino-3-hydroxymethyl-3-cephalosporin-4-carboxylic acid ( II ) synthesis: put 20 ml of methanol and 20 ml of water into a 100 ml three-neck flask, add 6 g of 7-ACA, cool to -40°C, add 1.76 g of sodium hydroxide in 5 ml of aqueous solution dropwise, after the addition, keep stirring After 30 minutes, adjust the pH to 1-2 with 15% hydrochloric acid, stir for 2 hours, filter with suction, wash the filter cake with water, and dry it in vacuum at 40 degrees to obtain 4.5 grams of white solid with a yield of 90% and a purity of 98.7%;
[0043] 1 H-NMR (DMSO-d 6 )d 3.45 (1H,d, J=18Hz), 3.55 (2H,d,J=18Hz),4.18 (1H, d, J=13.6Hz) 4.23 (1H,d, J=13.6Hz), 4.72 (1H ,d, J=4.8Hz), 4.92 (1H,d, J=4.8Hz),6.25 (2H,brs)
[0044] The second step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(methoxyiminoacetamide)]-3-hydroxymethyl-3-cephem-4-carboxylic acid Synthesis of (III): Put 40 ml of tetrahydrofuran and 20 ml of water into a 200 ml three-neck flask, add 4 g...
Embodiment 2
[0052] Embodiment 2, the first step is with embodiment 1;
[0053] The second step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(methoxyiminoacetamide)]-3-hydroxymethyl-3-cephem-4-carboxylic acid Synthesis of (III): Put 30 ml of acetonitrile and 20 ml of water into a 200 ml three-necked flask, add 4 g of the compound (II) synthesized in the first step, add dropwise saturated sodium bicarbonate solution to pH=7-8, and cool to 0-5°C, add 4.0 grams of active ester in batches, while adding, use saturated sodium bicarbonate solution to control pH = 7-8, after the addition, slowly heat up to about 40°C, stir for 6 hours, filter, add 30 ml of water to the filtrate , cooled to about 5°C, adjusted the pH to 1-2 with 10% hydrochloric acid, stirred for 8 hours after dripping, filtered with suction, washed with ice water, and dried in vacuum at 30°C to obtain 6.6 grams of light yellow solid with a yield of 87% and a purity of 95 %;
[0054] The third step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(m...
Embodiment 3
[0057] Embodiment 3, the first step is with embodiment 1;
[0058] The second step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(methoxyiminoacetamide)]-3-hydroxymethyl-3-cephem-4-carboxylic acid Synthesis of (III): Put 20 ml of N,N-dimethylformamide and 20 ml of water into a 200 ml three-necked flask, add 3 g of the compound (II) synthesized in the first step, and add a saturated sodium bicarbonate solution dropwise to pH=7-8, cooled to 0-5°C, added 4.0g of active ester in batches, controlled pH=7-8 with saturated sodium bicarbonate solution while adding, slowly raised the temperature to about 50°C after adding, stirred and reacted for 4h , filter, add 30 ml of water to the filtrate, cool to about 5°C, adjust the pH to 1-2 with 10% hydrochloric acid, stir for 8 hours after dripping, filter with suction, wash with ice water, and vacuum-dry at 30°C to obtain 6.5 g of light yellow solid , yield 86%, purity 95%;
[0059] The third step: 7β-[2-(2-formylaminothiazol-4-yl)-2-(methoxyimino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com