Method for preparing powder injection using superfine communication technique and prepared products
A kind of superfine pulverization technology and technology, which is applied in the field of preparation of powder injections and products prepared by superfine pulverization technology, which can solve the stability problem that has not been completely solved, the freeze-dried powder injection process is complicated, the application of superfine pulverization technology, etc. problem, to achieve the effect of facilitating packaging, good clarity and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Invert Sugar Sterile Powder for Injection
[0033] prescription
[0034] Fructose 6250g
[0035] Glucose 6250g
[0036] Preparation steps:
[0037] 1. Aseptic pretreatment of chemical drugs fructose and glucose
[0038] a. In the 10,000-class clean area, put fructose: ethanol which is 1:8 by weight to volume ratio; glucose: 80% ethanol which is 1:4 by weight to volume ratio, and put into two reaction tanks respectively, and slowly heat up to 70 ℃, stir separately to dissolve, add medicinal charcoal with a dosing volume of 0.2-0.5% (g / ml), stir for 30 minutes, filter to remove charcoal while it is hot, then fine filter through a 0.22μm filter membrane, and cool to 25±5 ℃, add appropriate amount of fructose and glucose seed crystals respectively, stir and crystallize for 10-30 hours (preferably 12-18 hours);
[0039] b. Transfer the crystallized drug solution to a sterile room, filter, and wash with ethanol approximately equivalent to 0.15-0.25 t...
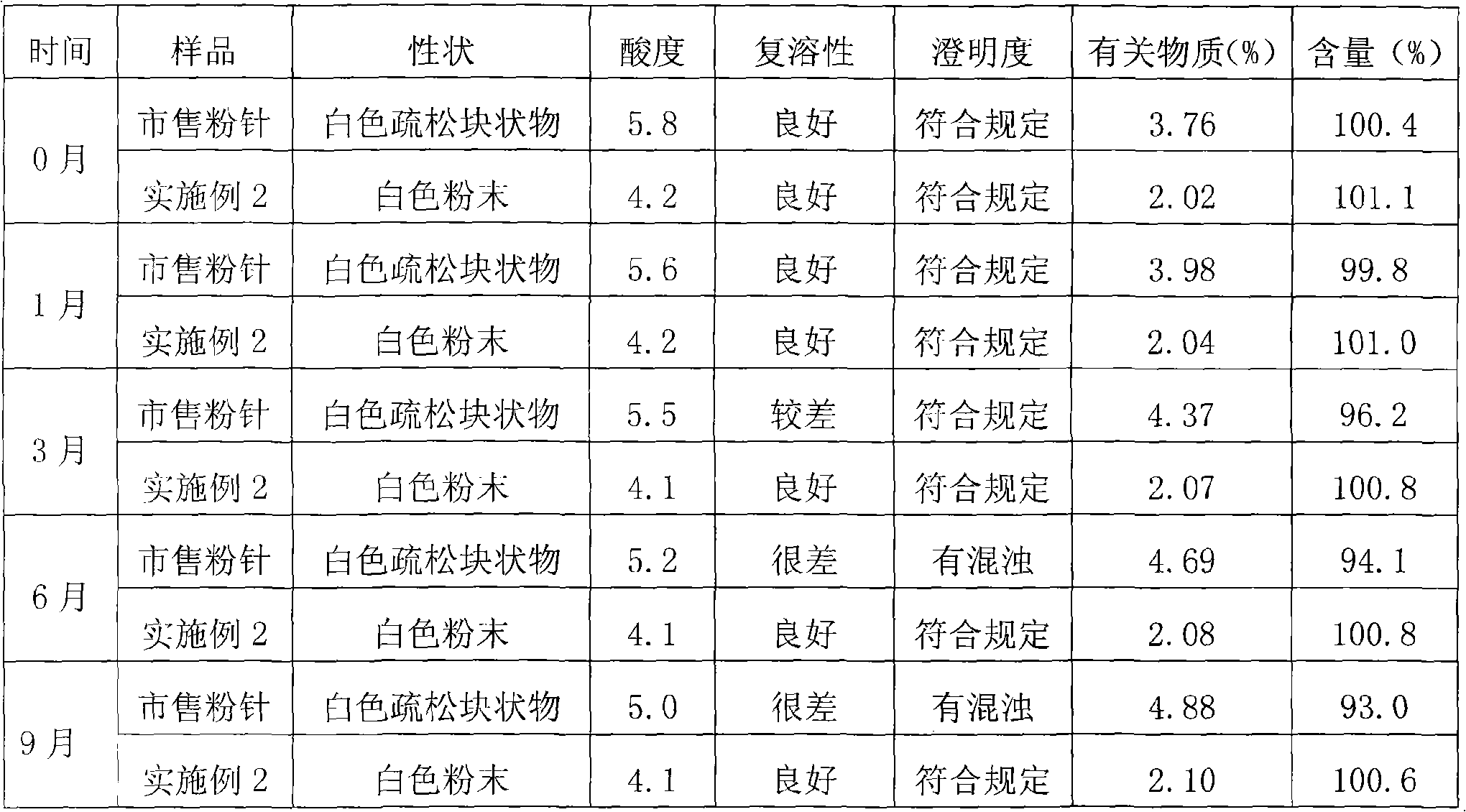
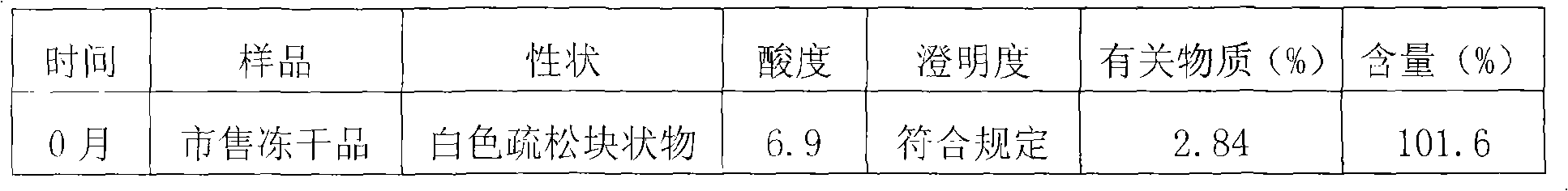
Embodiment 2
[0045] Preparation of Clindamycin Phosphate Sterile Powder for Injection
[0046] Preparation steps:
[0047] 1. Aseptic pretreatment of the chemical drug clindamycin phosphate
[0048]a. In the 10,000-class clean area, put 900g of clindamycin phosphate and water for injection into the reaction tank at a weight / volume ratio of 1:1~2, slowly raise the temperature to 50°C, stir to dissolve, and add a dosing amount of 0.1~ 0.3% (g / ml) medicinal charcoal, stirred for 10 to 30 minutes, filtered while hot to remove charcoal, then finely filtered through a 0.22 μm filter membrane, cooled to room temperature, added 3 times the volume of the filtrate and mixed evenly, Then add an appropriate amount of clindamycin phosphate seed crystals, stir and crystallize for 8-20 hours (preferably 10-15 hours);
[0049] b. Transfer the crystallized medicinal solution to a sterile room, filter, and wash with an amount of absolute ethanol approximately equivalent to 0.1 to 0.3 times the volume ...
Embodiment 3
[0055] Preparation of sterile powder for injection of cefpiramide sodium
[0056] Preparation steps:
[0057] 1. Use 2000g of cefpiramide sodium aseptic raw material powder, purchased from Shandong Lukang Pharmaceutical Co., Ltd., without aseptic treatment.
[0058] 2. Pre-crushing the above-mentioned cefpiramide sodium sterile raw material powder through mortar grinding, and pulverizing into coarse particles that all pass through 80 meshes;
[0059] 3. Use QWJ-5 airflow vortex pulverizer to superfine the above coarse particles respectively, and pulverize them into superfine powders that all pass 1340 mesh;
[0060] Grinding conditions: the air temperature after freeze-drying is 0°C, the water content is 0.6%, the pressure is 1.5MP when injected into the ultrafine pulverizer, the working pressure of the ultrafine pulverizer is 1.5MP, the working temperature is 4°C, and the pulverization time is 60min .
[0061] 4. Dispense the above ultrafine powder into antibiotic glas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com