Cefpiramide composite
A technology of cefpiramide and its composition, which is applied in the field of pharmaceutical manufacturing, can solve problems such as difficult quality assurance, high energy consumption, unfavorable production and application, and achieve the effects of simple operation by nurses, good drug stability, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
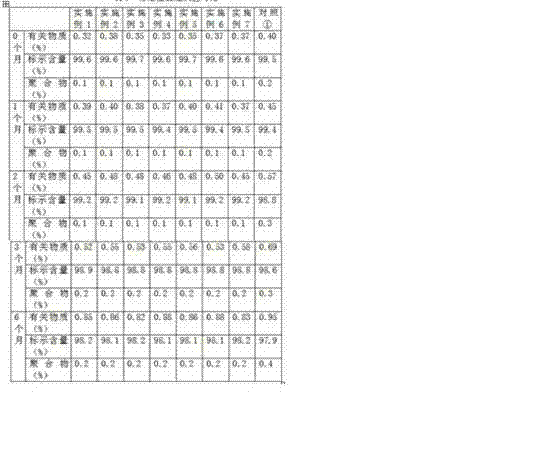
Embodiment 1
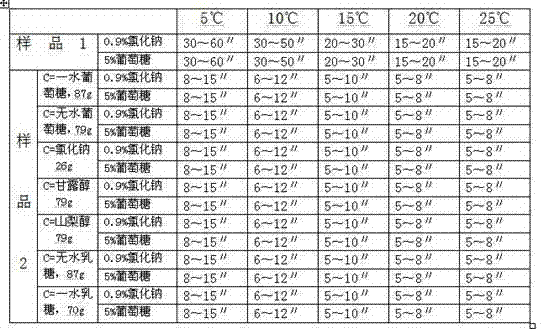
[0017] Example 1 Dissolution rate investigation of compositions such as glucose and sodium carbonate
[0018] The following two sets of samples were used to investigate the dissolution rate of pure sodium carbonate (sample 1) and the mixture of sodium carbonate and (c) (sample 2) at different temperatures using commonly used clinical infusions:
[0019] Sample 1: Weigh 50g of sodium carbonate and pack in 0.50g / bottle.
[0020] Sample 2: Weigh 50g of sodium carbonate, appropriate amount of component (c) (see Table 1), mix evenly, and pack in 0.50g (including sodium carbonate) / bottle.
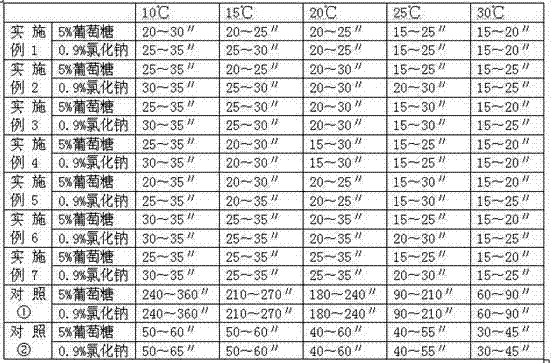
[0021] Dissolution method: According to the routine operation, 0.9% sodium chloride injection and 10ml of 5% glucose injection were used for dissolving. The results are shown in Table 1.
[0022] Table 1 Comparison of dissolution rates of pure sodium carbonate and mixtures of sodium carbonate and (c) at different temperatures
[0023]
[0024] It can be seen from Table 1 that the dissolu...
Embodiment 2
[0026] Take by weighing sodium carbonate 190g, glucose monohydrate 500g, add cefpiramide 1000g after mixing homogeneously, then mix uniformly, then pack by powder injection routine production process and make cefpiramide powder injection for injection.
Embodiment 3
[0028] Take by weighing sodium carbonate 190g, sodium chloride 50g, add cefpiramide 1000g after mixing, mix again, then pack by powder injection conventional production process and make injection cefpiramide powder injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com