Cefpiramide composition
A technology of cefpiramide and cefpiramide, which is applied in powder transportation, antibacterial drugs, organic active ingredients, etc., can solve the problems of content reduction, low temperature storage, poor stability of cefpiramide sodium, etc., and achieve high stability, Rapid dissolution and stable storage quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
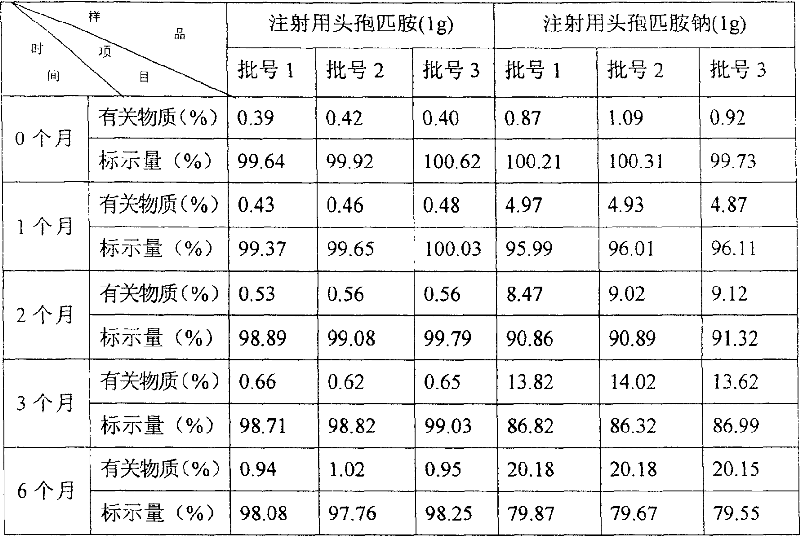
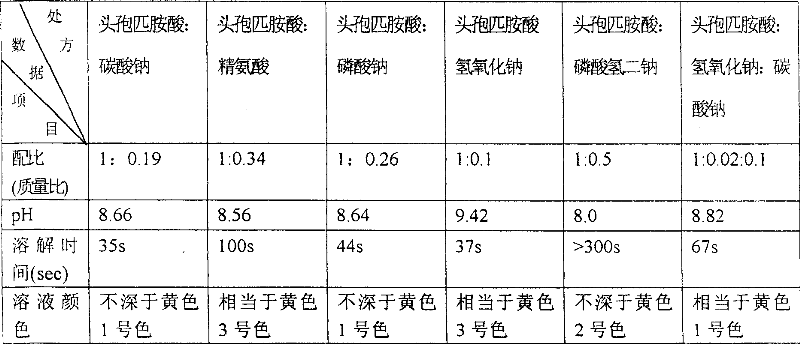
[0033] Co-solvents and best co-solvents
[0034]
[0035] It can be seen from the above table that sodium carbonate, sodium phosphate and sodium hydroxide have the shortest dissolution time. Clinically, the faster the drug dissolves, the shorter the dispensing time, which is convenient for clinical application. Therefore, analyze from the dissolution rate of product, the stability of drug and the safety of medication: sodium carbonate is the best cosolvent for preparing cefpiramide for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com