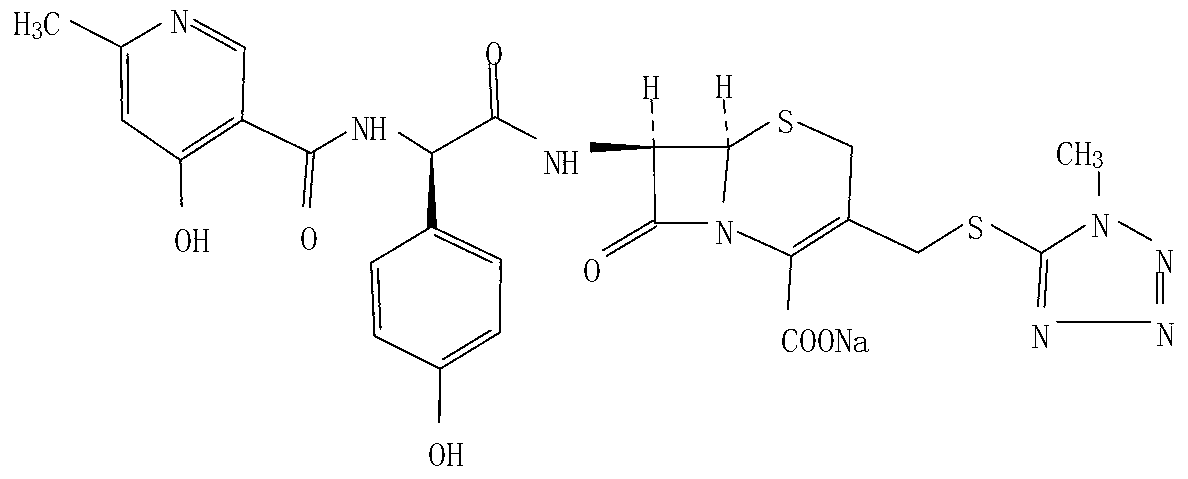
Method for crystallizing and producing cefpiramide sodium crystals
A technology of cefpiramide sodium and cefpiramic acid, which is applied in the field of medicine and chemical industry, can solve the problems of poor fluidity, difficulty in dispensing, and easy precipitation, and achieve the effects of good fluidity, easy dispensing, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Accurately measure 20g of cefpiramin, 60ml of methanol, 40ml of acetone, 20ml of acetonitrile, and 4.6ml of triethylamine into a four-necked bottle. The molar ratio of cefpiramin and triethylamine is 1:1. The ratio of the weight in grams to the volume in milliliters of the solvent I is 1:6. In a constant temperature reaction bath at 20°C, stir until completely dissolved to form a cefpiramide salt solution for later use. Accurately measure 1.5g of sodium hydroxide and 40ml of methanol into a conical flask. The amount of sodium hydroxide added is such that the molar ratio of sodium hydroxide to cefpiramin is 1.15:1. Stir until completely dissolved to form a hydroxide Sodium solution for later use. In a constant temperature reaction bath at 20°C, the sodium hydroxide solution was evenly added to the cefpiramide salt solution within 20 minutes, and then 0.03 g of cefpiramide sodium crystals were added as seed crystals, and the crystals were grown for 10 minutes. Then add 30...
Embodiment 2
[0041] Accurately measure 20g of cefpiramin, 60ml of methanol, 40ml of acetone, 20ml of acetonitrile, and 4.6ml of diisopropylamine into a four-necked bottle. The molar ratio of cefpiramin and diisopropylamine is 1:1. The ratio of the weight in grams to the volume in milliliters of solvent I is 1:6. Stir in a constant temperature reaction bath at 10°C until it is completely dissolved to form a cefpiramide salt solution for later use. Accurately measure 5.42g of sodium isooctanoate and 150ml of acetone into a conical flask. The amount of sodium isooctanoate is to make the molar ratio of sodium isooctanoate to cefpiramide 1:1, and stir until completely dissolved to form sodium isooctanoate The solution is ready for use. In a constant temperature reaction bath at 25°C, the sodium isooctanoate solution was evenly added to the cefpiramide salt solution within 25 minutes, and then 0.02 g of cefpiramide sodium crystals were added as seed crystals, and the crystals were grown for 25 m...
Embodiment 3
[0043] Accurately measure 20g of cefpiramin, 60ml of methanol, 40ml of acetone, 10ml of water, and 4.6ml of diisopropylamine into a four-neck bottle. The molar ratio of cefpiramin and diisopropylamine is 1:1. The ratio of the weight in grams to the volume in milliliters of solvent I is 1:5.5. Stir in a constant temperature reaction bath at 20°C until it is completely dissolved to form a cefpiramide salt solution for later use. Accurately measure 5.42g of sodium isooctanoate and 150ml of acetone into a conical flask. The amount of sodium isooctanoate is to make the molar ratio of sodium isooctanoate to cefpiramide 1:1, and stir until completely dissolved to form sodium isooctanoate The solution is ready for use. In a constant temperature reaction bath at 15°C, the sodium isooctanoate solution was evenly added to the cefpiramide salt solution in 30 minutes, and then 0.04 g of cefpiramide sodium crystals were added as seed crystals, and the crystals were grown for 15 minutes. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com