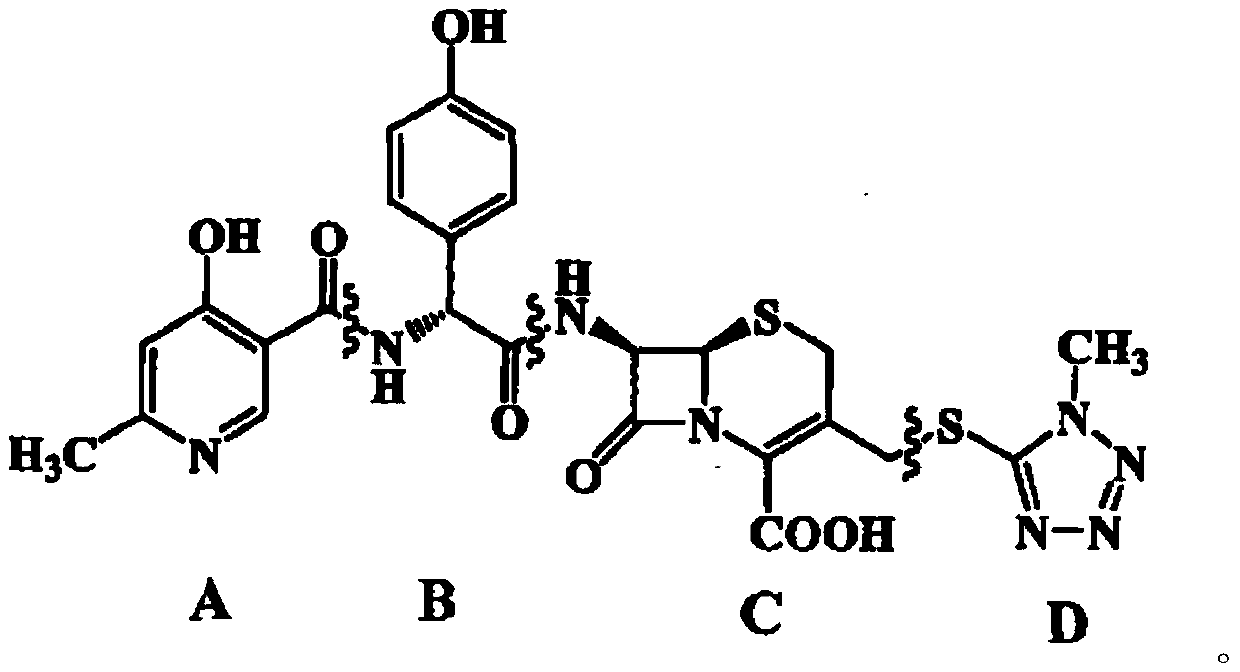
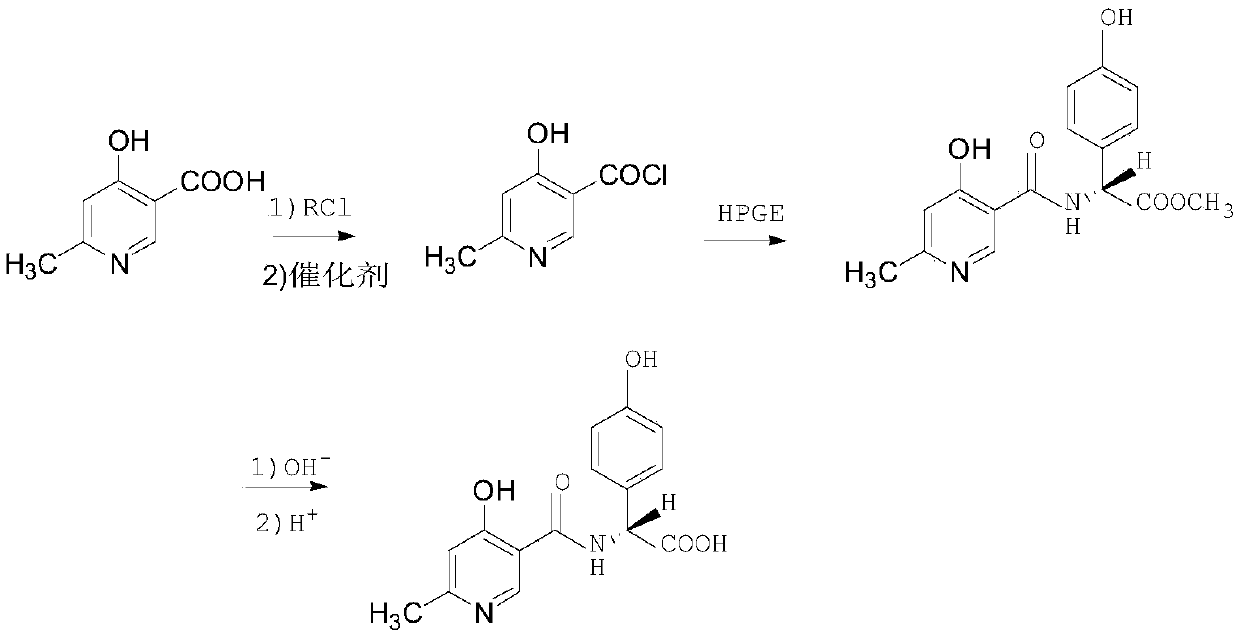
Synthesis method of cefpiramide side chain acid
The technology of a cefpiramide and a synthetic method is applied in the field of synthesis of cefpiramide side chain acid, which can solve the problems of poor product stability and low product yield, and achieve the effects of low cost, easy availability of raw materials, and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthetic method of the cefpiramide side chain acid described in present embodiment 1, the steps are as follows:
[0035] (1) Chlorination and acylation
[0036] Add 22g of pyridine-3-carboxylic acid and 25.4g of HPGE into a mixed solvent consisting of 480ml of dichloromethane and 80ml of DMAC, stir at normal pressure and cool down to -25°C, add 4.2ml of pyridine, and then slowly dropwise add 13.4ml of phosphorus oxychloride , Control the temperature during the dropwise addition to not exceed -15°C, after the dropwise addition, control the temperature at -15°C, and react for 5 hours.
[0037] (2) Hydrolysis and crystallization
[0038] After the reaction, the temperature was raised to 15°C, and then 30% sodium hydroxide solution was slowly added dropwise to the reaction solution to adjust the pH to 8.5, and the reaction was carried out at 15°C for 1 hour. After the reaction, add 240ml of water and 1.0g of sodium metabisulfite to the reaction bottle; slowly add 480...
Embodiment 2
[0040] The synthetic method of the cefpiramide side chain acid described in present embodiment 2, the steps are as follows:
[0041] (1) Chlorination and acylation
[0042] Add 22g of pyridine-3-carboxylic acid and 28g of HPGE into a mixed solvent consisting of 468ml of dichloromethane and 55ml of DMAC, stir at normal pressure and cool down to -25°C, add 4.2ml of triethylamine, then slowly dropwise add 13.4ml of phosphorus oxychloride, During the dropwise addition, the temperature was controlled not to exceed -15°C. After the dropwise addition was completed, the temperature was controlled at -15°C, and the reaction was carried out for 5 hours.
[0043] (2) Hydrolysis and crystallization
[0044] After the reaction, the temperature was raised to 15°C, and then 30% sodium hydroxide solution was slowly added dropwise to the reaction solution to adjust the pH to 8.5, and the reaction was carried out at 15°C for 1 hour. After the reaction, add 240ml of water and 1.0g of sodium me...
Embodiment 3
[0046] The synthetic method of the cefpiramide side chain acid described in present embodiment 3, the steps are as follows:
[0047] (1) Chlorination and acylation
[0048] Add 22g of pyridine-3-carboxylic acid and 33g of HPGE to a mixed solvent consisting of 472ml of dichloromethane and 59ml of DMAC, stir at normal pressure and cool down to -25°C, add 4.2ml of pyridine, then slowly add 10.4ml of thionyl chloride dropwise, drop During the addition process, the temperature was controlled not to exceed -15°C. After the dropwise addition was completed, the temperature was controlled at -15°C, and the reaction was carried out for 5 hours.
[0049] (2) Hydrolysis and crystallization
[0050] After the reaction, the temperature was raised to 15°C, and then 30% sodium hydroxide solution was slowly added dropwise to the reaction solution to adjust the pH to 8.5, and the reaction was carried out at 15°C for 1 hour. After the reaction, add 240ml of water and 1.0g of sodium metabisulfi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com