Cefpiramide composite
A technology of cefpiramide and its composition, which is applied in the field of pharmaceutical manufacturing, can solve the problems of high energy consumption, difficult quality assurance, unfavorable production and application, etc., and achieve the effects of easy-to-obtain raw materials, good drug stability, and simple operation by nurses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
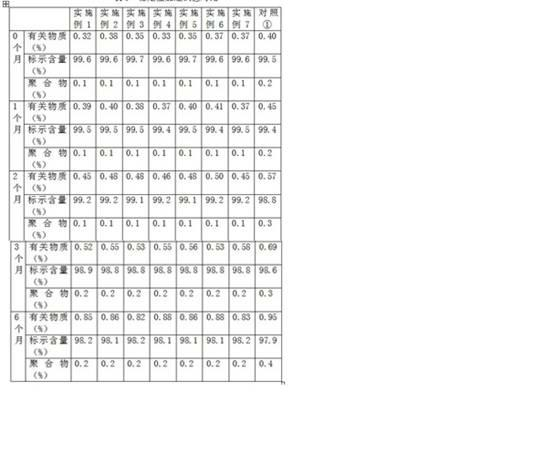
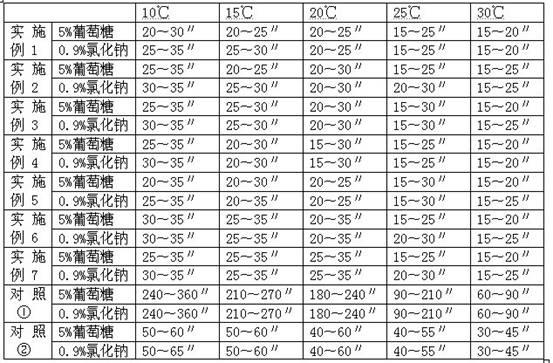
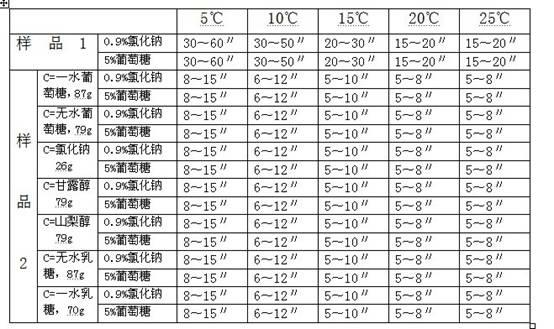
[0017] Example 1 Investigation of the dissolution rate of the composition of glucose, etc. and sodium carbonate
[0018] The following two sets of samples are used to investigate the dissolution rate of pure sodium carbonate (sample 1) and a mixture of sodium carbonate and (c) (sample 2) using clinically commonly used infusions at different temperatures:
[0019] Sample 1: Weigh 50g of sodium carbonate and pack it in 0.50g / bottle.
[0020] Sample 2: Weigh 50g of sodium carbonate, (c) an appropriate amount of component (see Table 1), mix well, and pack at 0.50g (containing sodium carbonate) / bottle.
[0021] Dissolution method: use common clinical infusion 0.9% sodium chloride injection and 5% glucose injection 10ml to dissolve according to routine operation. The results are shown in Table 1.
[0022] Table 1 Comparison of the dissolution rate of pure sodium carbonate and the mixture of sodium carbonate and (c) at different temperatures
[0023]
[0024] It can be seen from Table 1 tha...
Embodiment 2
[0026] Weigh 190g of sodium carbonate and 500g of dextrose monohydrate, add 1000g of cefpiramide after mixing uniformly, and then mix uniformly, and then divide into cefpiramide powder injection for injection according to the conventional production process of powder injection.
Embodiment 3
[0028] Weigh 190g of sodium carbonate and 50g of sodium chloride, mix well, add 1000g of cefpiramide, mix well, and then divide into cefpiramide powder for injection according to the conventional production process of powder injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com