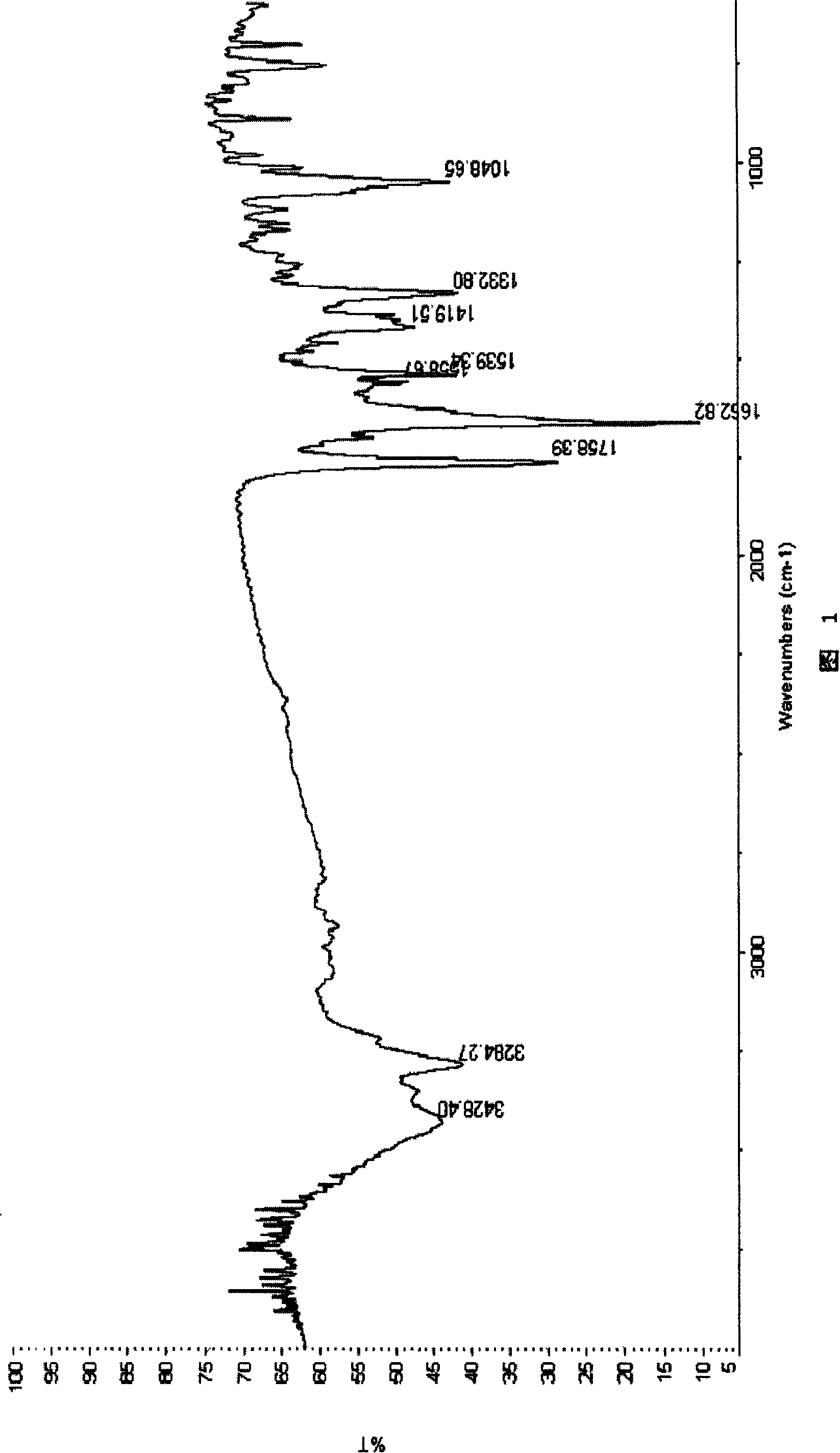
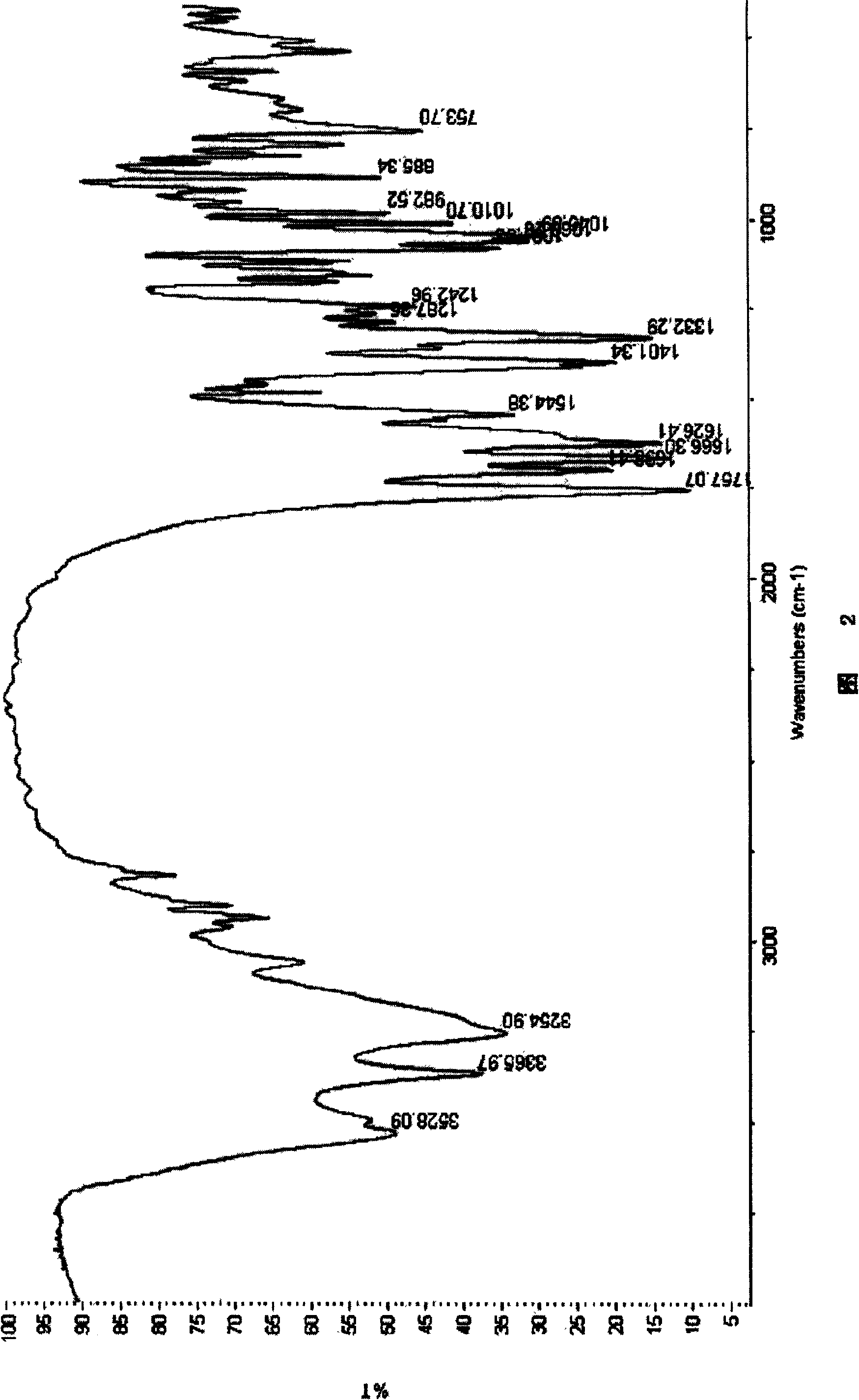
Patents
Literature
72 results about "Cefuroxime" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
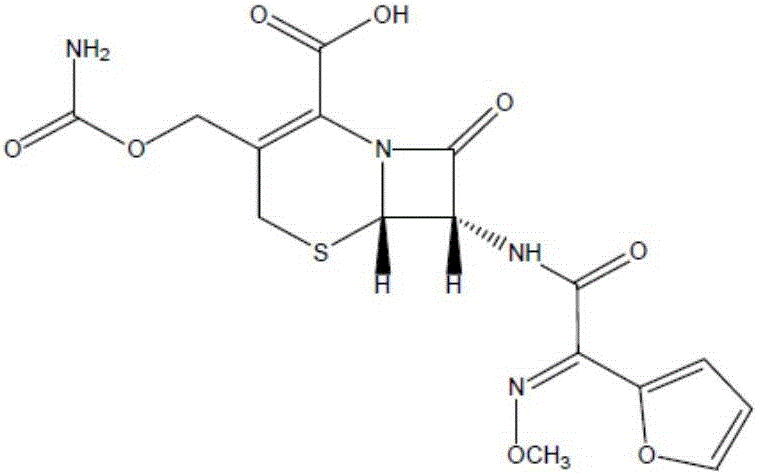
Cefuroxime, sold under the brand name Zinacef among others, is an antibiotic used to treat and prevent a number of bacterial infections. These include pneumonia, meningitis, otitis media, sepsis, urinary tract infections, and Lyme disease. It is used by mouth or by injection into a vein or muscle.
Anti beta-bactamase antibiotic compound prepn.
InactiveCN1513457ABroad spectrum antibacterialImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsCefuroximeSulbactam
A compound anti-beta-lactamase antibiotic is prepared from cefuroxime or its salt, sulbactam or its salt, and tazobactan or its salt. Its advantages are broad spectrum and high antibacterial effect.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Method of synthesizing cefixime
InactiveCN101016305AEliminate the dissolution processReduce usageOrganic chemistryCefuroximeCefixime
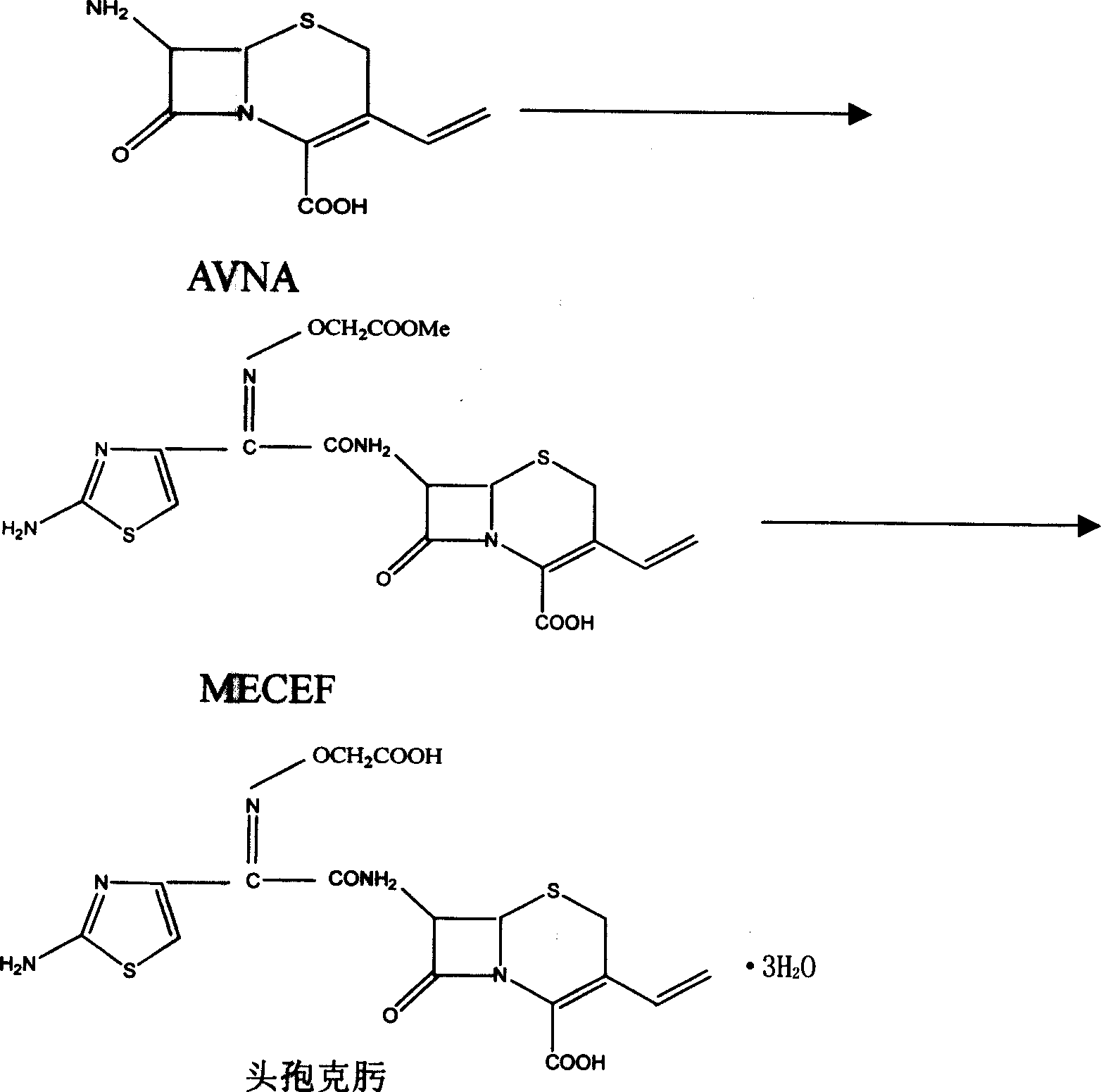
The invention discloses a synthesizing method of cefuroxime, which is characterized by the following: crystallizing cefuroxime intermediate MECEF; synthesizing cefuroxime through water-phased extract of MECEF without dissolving MECEF; saving cost to improve receiving rate effectively; shortening manufacturing period.
Owner:河源市制药工程技术研究开发中心
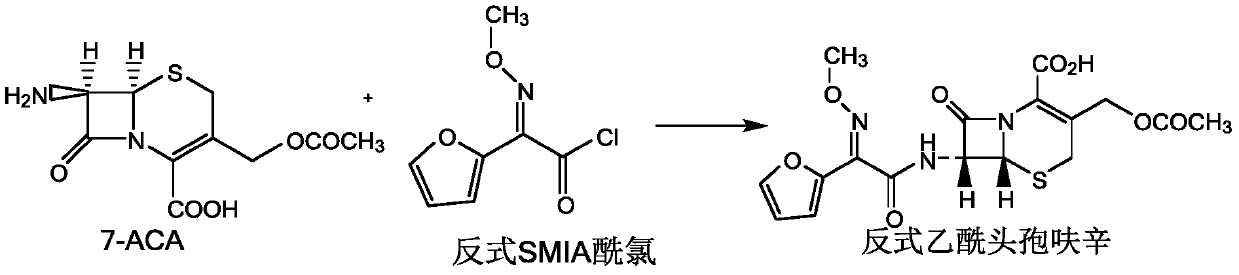
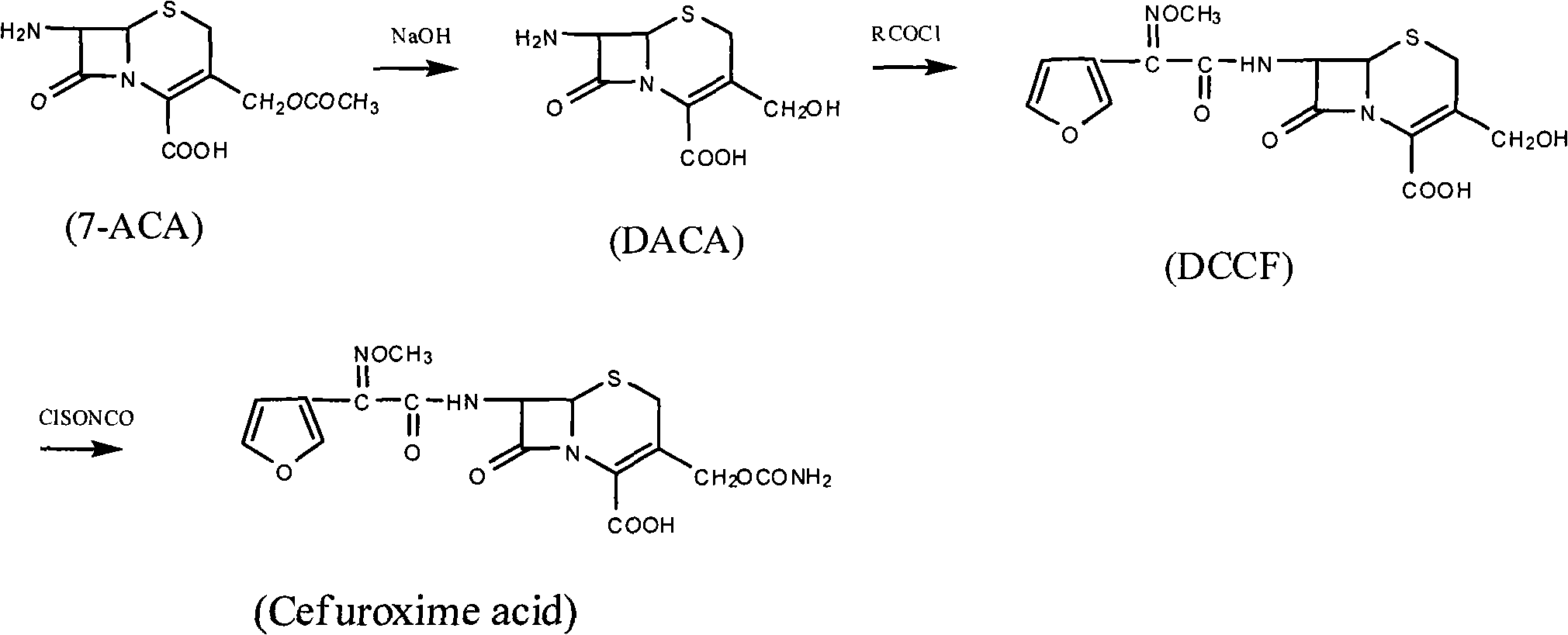
Method for preparing cefuroxime acid
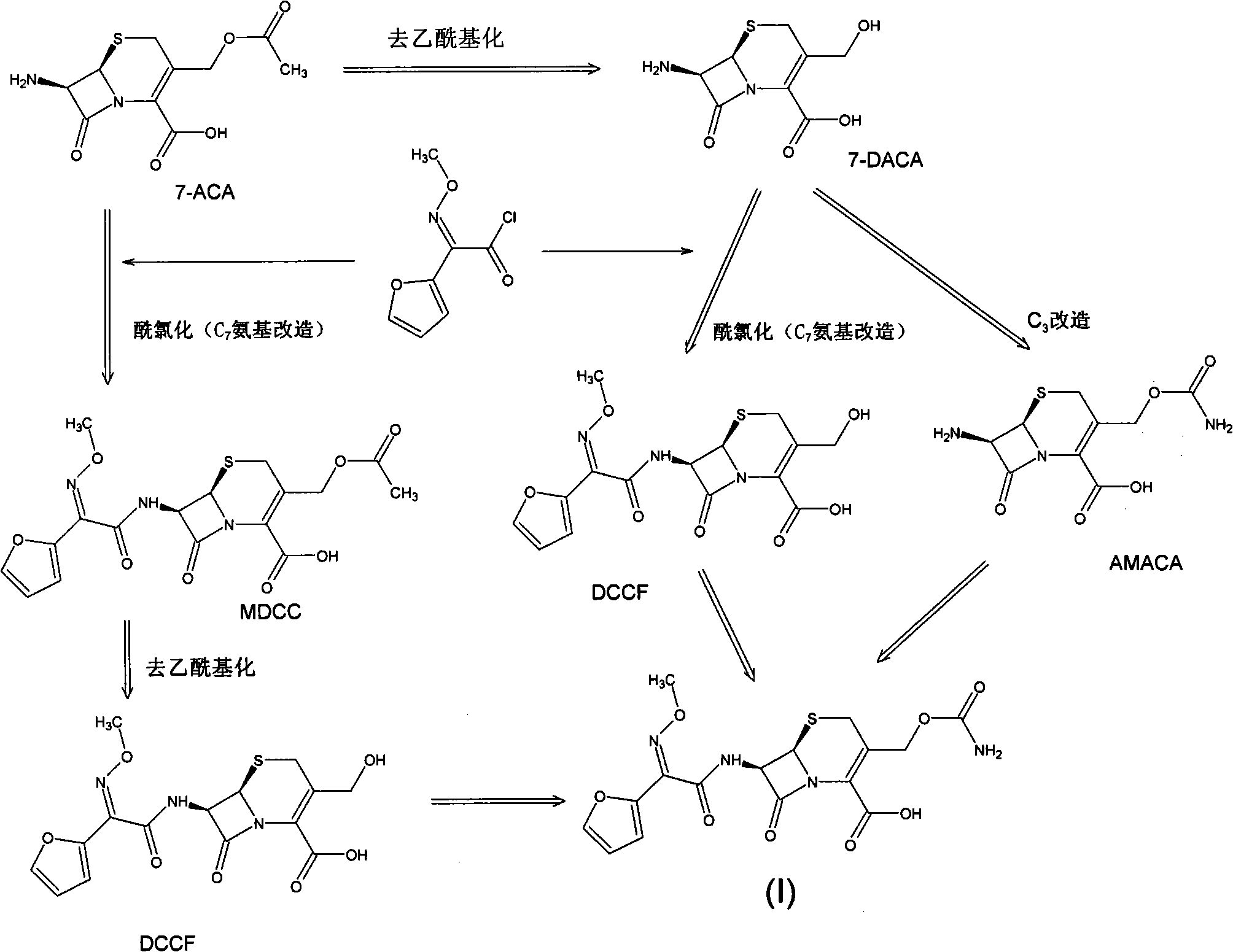
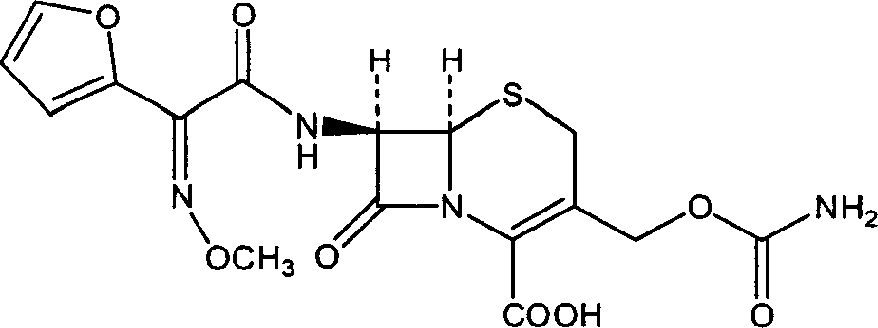
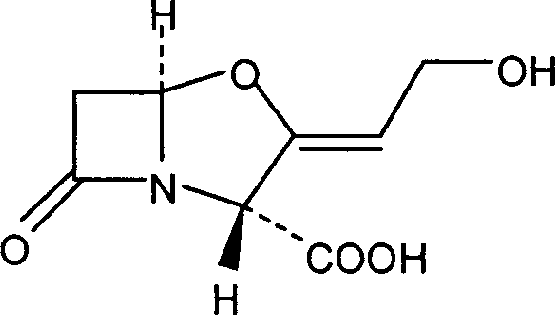
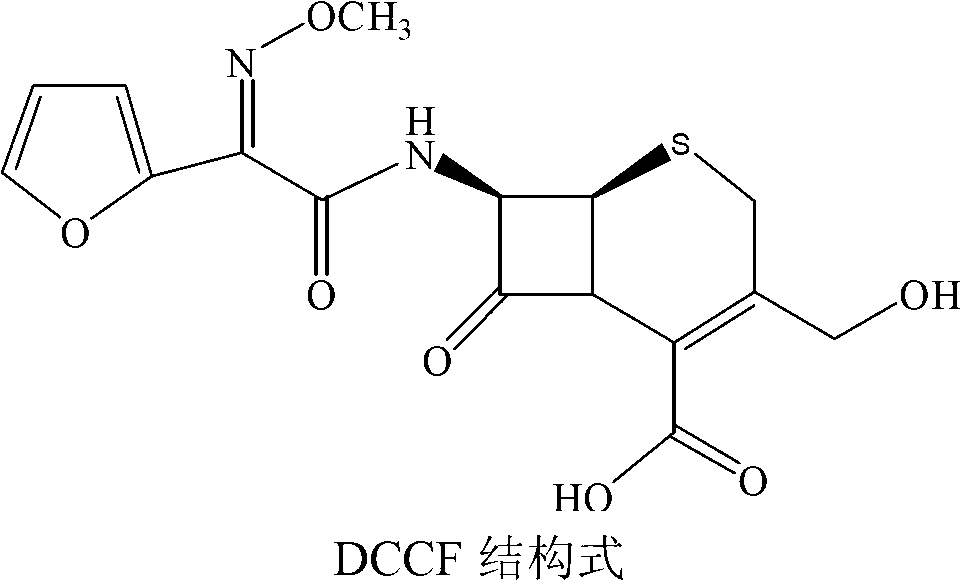
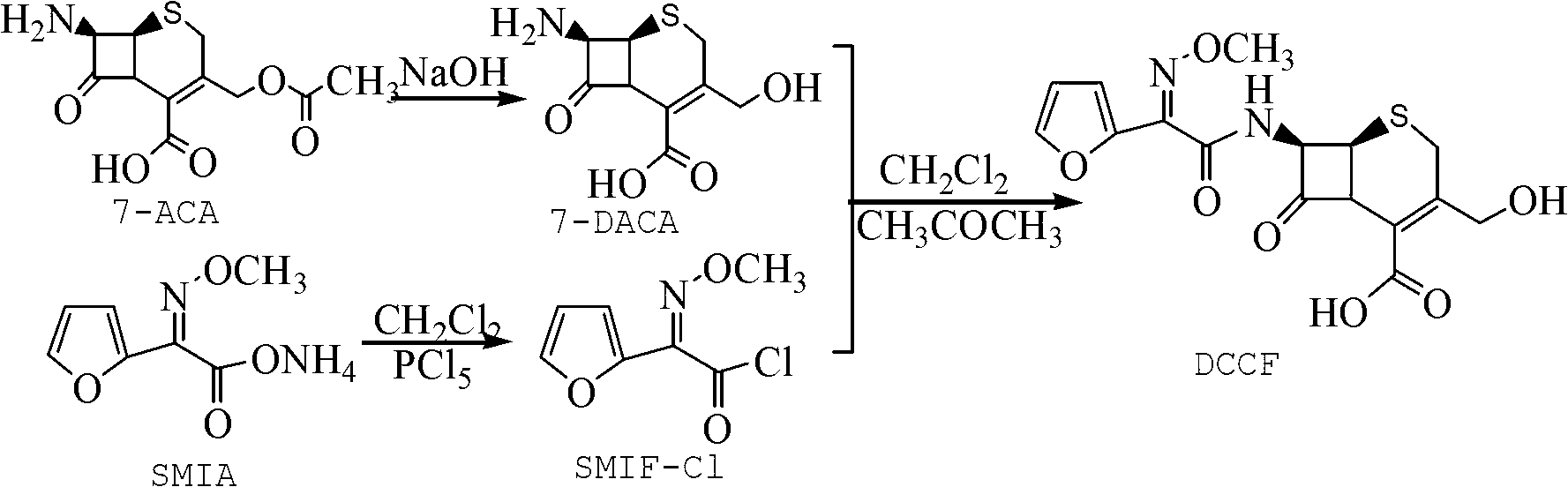
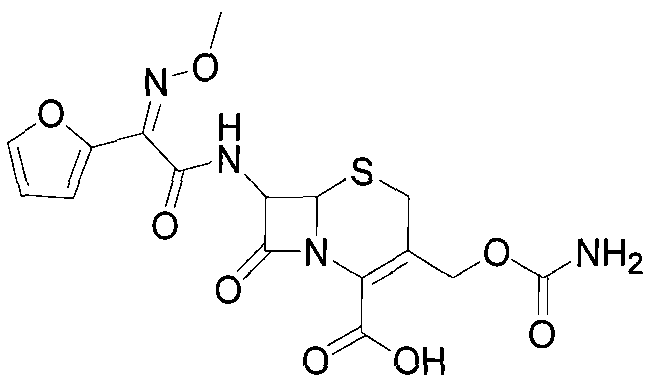
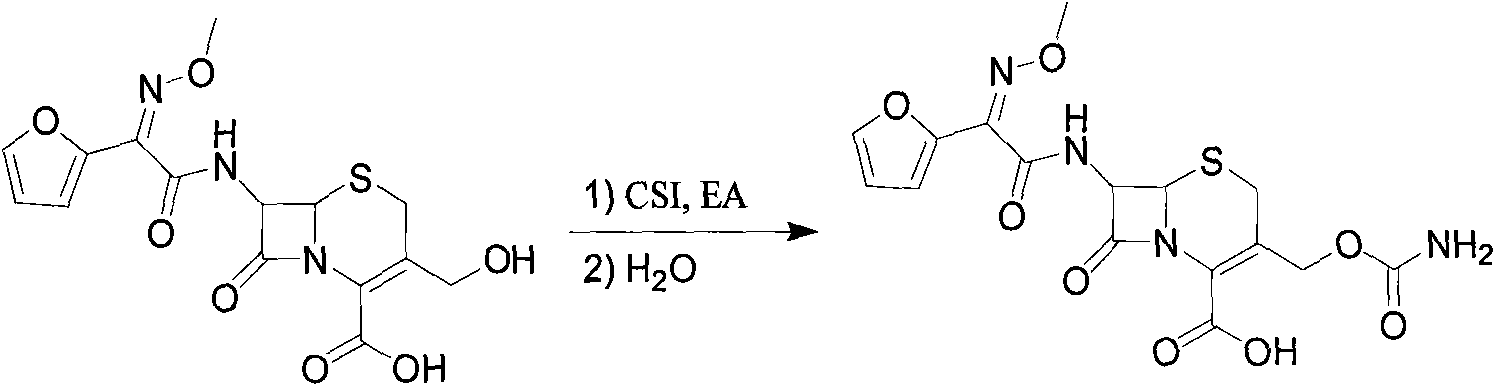
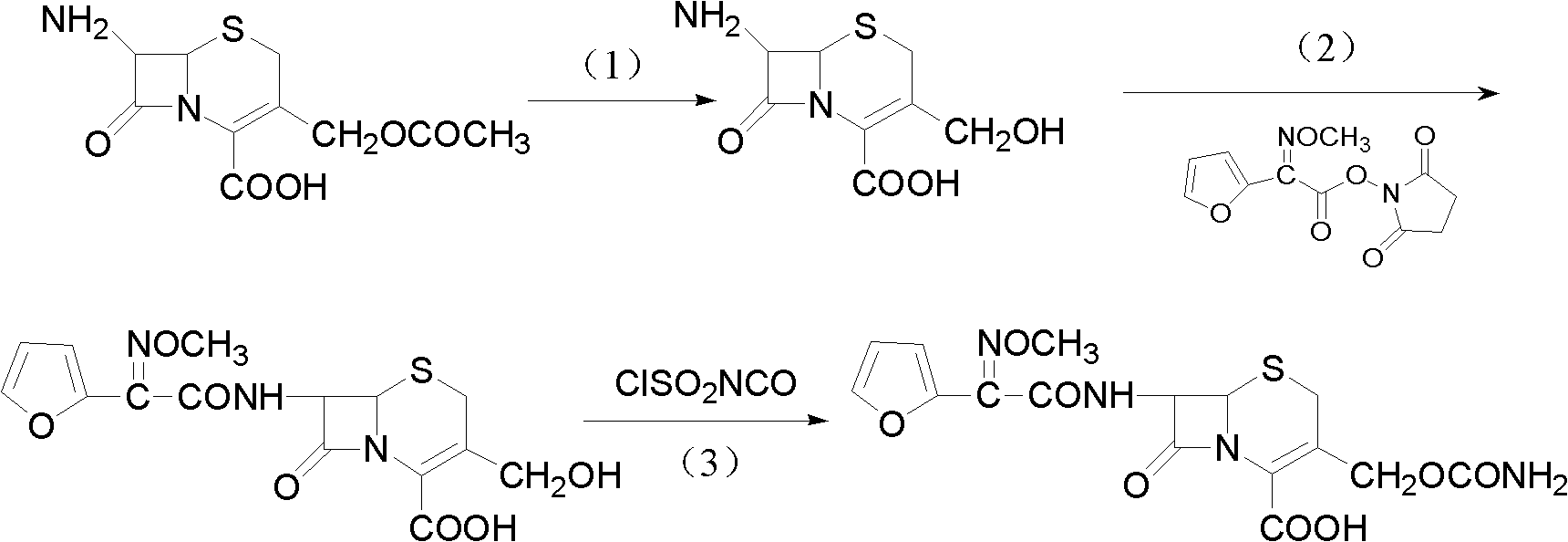
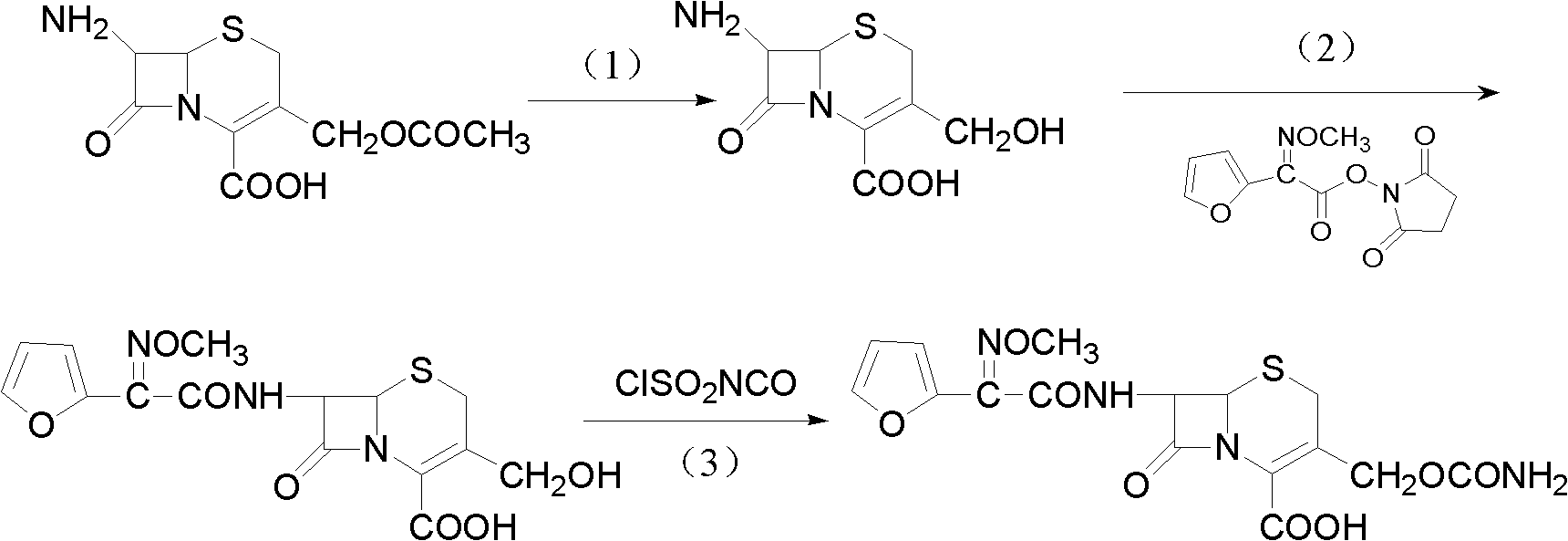
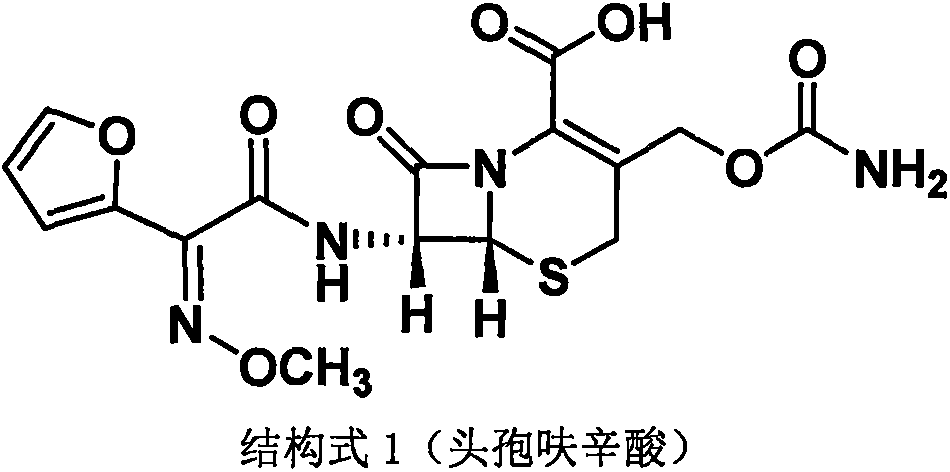
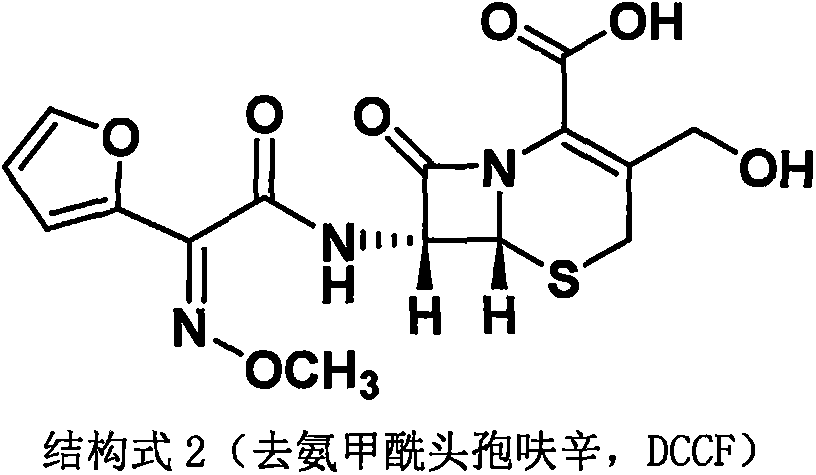
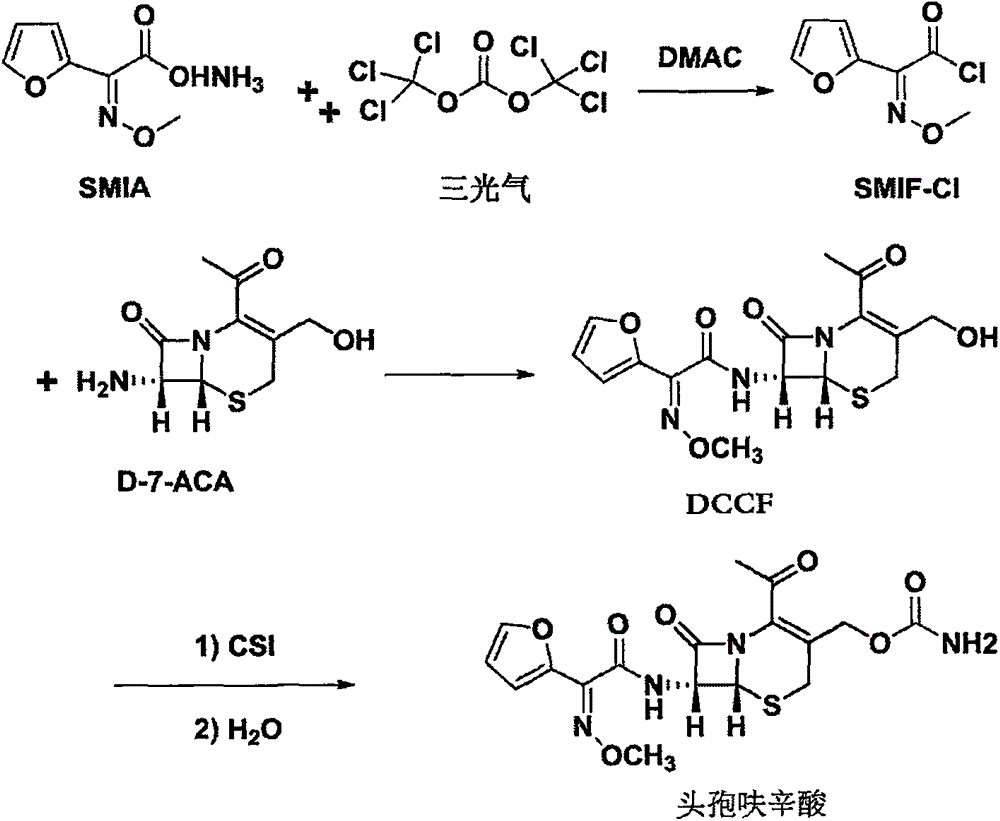
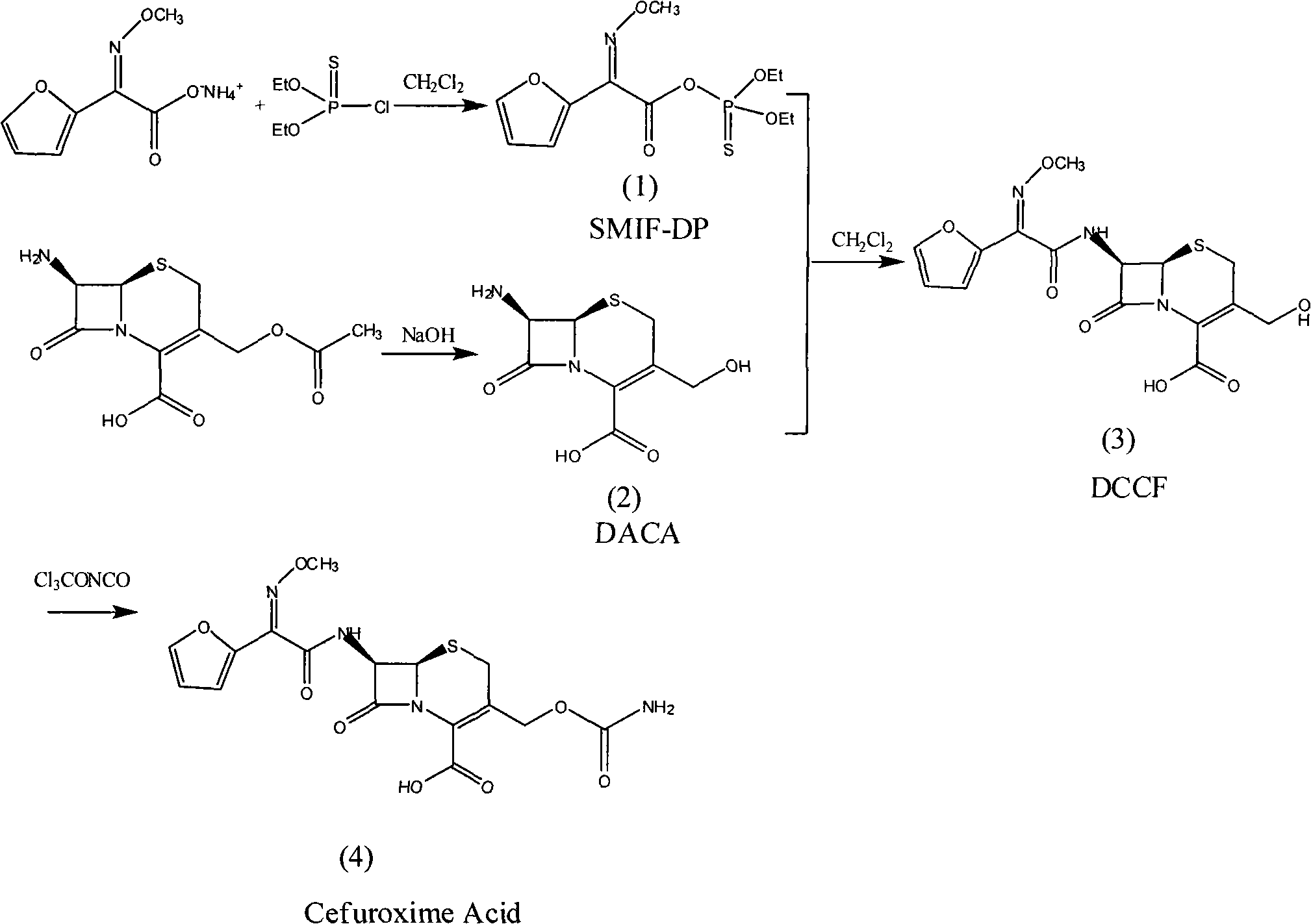
The invention discloses a method for preparing cefuroxime acid. The method comprises the following steps of: performing a chloride acylation reaction on 7-aminocephalosporanic acid (7-ACA) and methoxyiminofuran acetate serving as raw materials; performing deacetylation to synthesize DCCF; performing nucleophilic addition on the DCCF and chlorosulfonyl isocyanate (CSI) serving as a strong carbamoyl reagent to obtain chlorosulfonyl cefuroxime; and hydrolyzing the chlorosulfonyl cefuroxime to obtain cefuroxime acid. In the preparation method, the preparation process is simple, and the cefuroximeacid is crystallized by adopting aqueous solution, so that the loss of organic solvents is reduced; simultaneously, aids are added selectively in the reaction process to improve the quality of products, so that finished products with high purity and yield and good colors are obtained. The purity of the cefuroxime acid prepared by the method is more than 98.5 percent, and the weight yield is approximately 100 percent.
Owner:国药集团致君(苏州)制药有限公司
Cefuroxime, beta-lactamase inhibitor containing composition
InactiveCN1557321ABroad spectrum antibacterialImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsCefuroximePharmacology
The present invention is one kind of antiseptic medicine composition containing antibiotic cefuroxime and beta-lactamase inhibitor in the weight ratio of 0.5-20. The said medicine composition as new generation of antiseptic medicine composition has the advantages of wide antiseptic spectrum and strong antiseptic effect.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
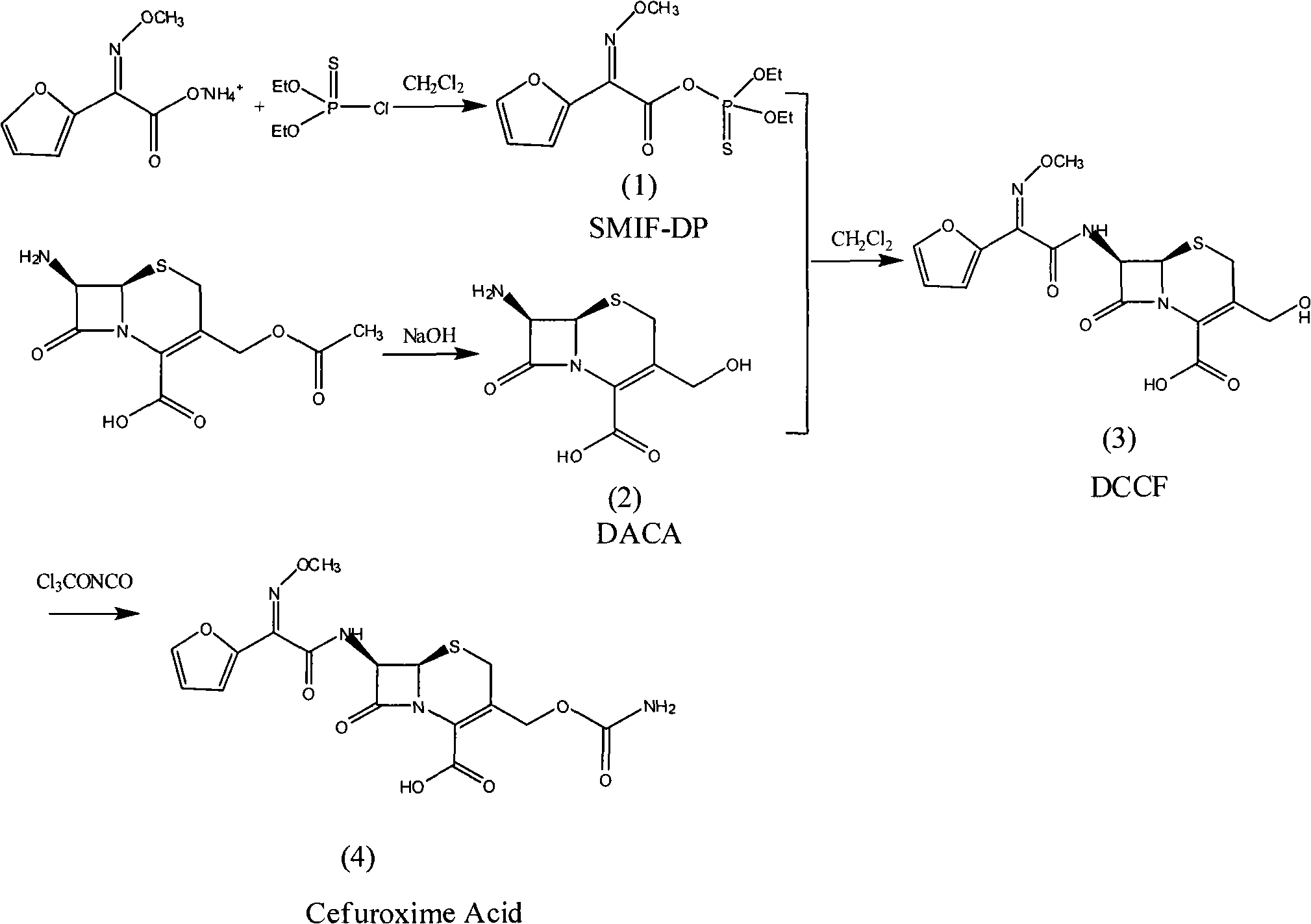
Method for preparing 3-descarbamoyl-cefuroxime acid
The invention relates to a method for preparing 3-descarbamoyl-cefuroxime acid. The method comprises the following steps of: (1) dissolving 7-aminocephalosporanic acid (7-ACA) in water or a methanol solution to prepare a 7-ACA solution, and performing hydrolysis reaction to obtain a 3-deacetyl-7-aminocephalosporanic acid (7-DACA) solution; (2) dissolving an acylchlorination reagent in a solvent, adding a cosolvent, adding (Z)-2-methoxyimino-2-(furyl-2-yl)acetic acid ammonium salt (SIMA), reacting, filtering, adding water or performing rotary evaporation to remove the excessive acylchlorination reagent, performing vacuum rotary evaporation, adding a homogenization reagent, and dissolving to obtain a (Z)-2-(furyl-2-yl)-2-(methoxyimino)-acetyl chloride (SMIF-Cl) solution; (3) adding the homogenization reagent into the 7-DACA solution, dripping the SMIF-Cl solution, regulating the pH value, preserving heat and reacting to obtain a reaction solution; and (4) decolorizing the reaction solution, regulating the pH value, adding purified water, growing a crystal, filtering, and performing vacuum drying. Reactants form a homogeneous system by adding the homogenization reagent, so that the contact area of the reactants is expanded, the reaction rate is increased, and reaction time is shortened.
Owner:SHANDONG UNIV
Method for synthesizing cefuroxime acid
The invention relates to a preparation method for synthesizing cefuroxime acid, which comprises the following steps: A) dropwisely adding isocyanate chlorosulfonate into a mixed solution of ethyl acetate and 3-decarbamoyl-cefuroxime acid at 0-15 temperature; B) while controlling the temperature at 15-25 DEG C, continuing adding activated carbon into the reaction solution, stirring to react, and filtering; and C) taking the filtrate, cooling, adding water, continuously adding sodium bicarbonate, skimming, discarding the ethyl acetate layer, dropwisely adding 30% HCl to regulate the pH value, cooling to 0-10 below, growing the grain, carrying out vacuum filtration, and washing the filter cake with water.
Owner:珠海保税区丽珠合成制药有限公司
Cefuroxime dibenzyl ethylenediamine salt and preparation method and application thereof
ActiveCN101157697AImprove qualityAvoid Freezing RequirementsOrganic chemistryEthylenediamineCefuroxime
The invention provides a novel compound of cefuroxime dibenzyl ethylenediamine salt and a preparation method as well as the method for the preparation of cefuroxime dibenzyl ethylenediamine salt or purification of cefuroxime or cefuroxime salt.
Owner:SHANDONG SALUBRIS PHARMA +1
Method for preparing cefuroxime acid
ActiveCN102093390AEasy to operateReduce the discharge of three wastesOrganic chemistryAcetic acid7-ACA
The invention provides a method for preparing cefuroxime acid. The method comprises the following steps: (1) selectively hydrolyzing 7-aminocephalosporanic acid (7-ACA) with aqueous alkali so as to obtain 3-deacetylation-7-aminocephalosporanic acid (7-DACA); (2) condensing 2-(2-furyl)-2-(methoxyimino)acetic acid-(2,5-dioxo-pyrrolidyl)-1-ester and 7-DACA so as to obtain 3-decarbamyl-cefuroxime acid (DCCF); and (3) modifying 3-hydroxymethyl of DCCF with chlorosulfonyl isocyanate so as to obtain the cefuroxime acid. In the method, low-temperature selective hydrolysis is carried out by using inorganic base to remove the 3-ester group of 7-ACA so as to prepare 7-DACA; C7-amino modification is carried out by an active ester method so as to obtain DCCF; and the 3-hydroxymethyl of DCCF is modified into carbamoyl methoxyl so as to obtain the cefuroxime acid. The method has the advantages of less emission of three wastes and high yield, is simple and convenient to operate, and is suitable for industrial production.
Owner:蚌埠丰原涂山制药有限公司
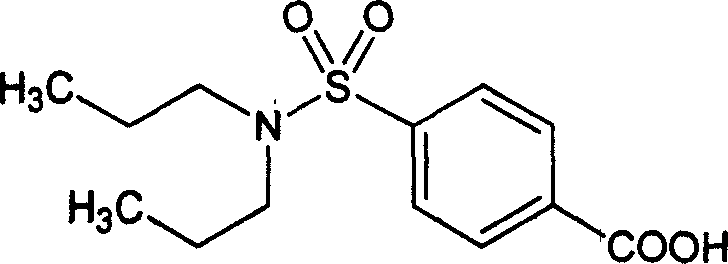
Compound injection with probenecid, potassium and beta-lactam antibiotic and its use
InactiveCN1853724AIncrease the total amount of drugReduce dosageAntibacterial agentsOrganic active ingredientsCefotaximePenicillin
A compound powder injection or freeze-dried powder injection is prepared from the sodium (or potassium) salt of probenecid and the beta-lactam kind of antibiotics including penicillin G, ampicillin, ancef, cefuroxime, cefotaxime and cefoxitin. It can elongate the half life of antibiotic, increase the AUC and plasma level and prevent the generation of drug-resistant bacteria.
Owner:吴晓辉
Method for preparing cefuroxime acid
InactiveCN106432267AThe synthetic method is green and environmentally friendlyHigh yieldOrganic chemistryCefuroximeOrganic layer
The invention provides a method for preparing cefuroxime acid. The method is characterized by comprising the following steps: (1) mixing deammonized formyl cefuroxime in liquid ester, adding a strong amino carbamylation reagent sulfonylisocynate, and performing a temperature-controlled reaction; (2) adding purified water after the reaction is completed, and performing hydrolysis; (3) adding the liquid ester after hydrolysis is completed, adjusting the pH value to be 1.9-2.0 by using hydrochloric acid, standing for layering, and performing vacuum concentration on an organic layer so as to obtain an organic layer concentrated solution; (4) adding purified water into the organic layer concentrated solution, performing crystallization, and filtering so as to obtain cefuroxime acid, wherein the liquid ester used in the step (1) and the step (3) is selected from methyl acetate, acetic ether, n-butyl acetate, methyl formate, ethyl formate and propyl formate. The method for preparing cefuroxime acid, which is provided by the invention, is green and environmental-friendly, low in cost, high in yield and good in quality.
Owner:珠海保税区丽珠合成制药有限公司
Preparation method of descarbamoyl cefuroxime lactone
The invention discloses a preparation method of descarbamoyl cefuroxime lactone. The method includes the steps of: taking descarbamoyl cefuroxime as a raw material, dissolving it in a solvent, and then adding a catalyst to perform an esterification reaction, thus obtaining the descarbamoyl cefuroxime lactone. The preparation method provided in the invention has the advantages of simplicity, easy operation and high yield, and the prepared descarbamoyl cefuroxime lactone has purity not less than 99.5%, thus being convenient for structural analysis and pharmacological study on the descarbamoyl cefuroxime lactone.
Owner:GUANGDONG LIGUO PHARMACY
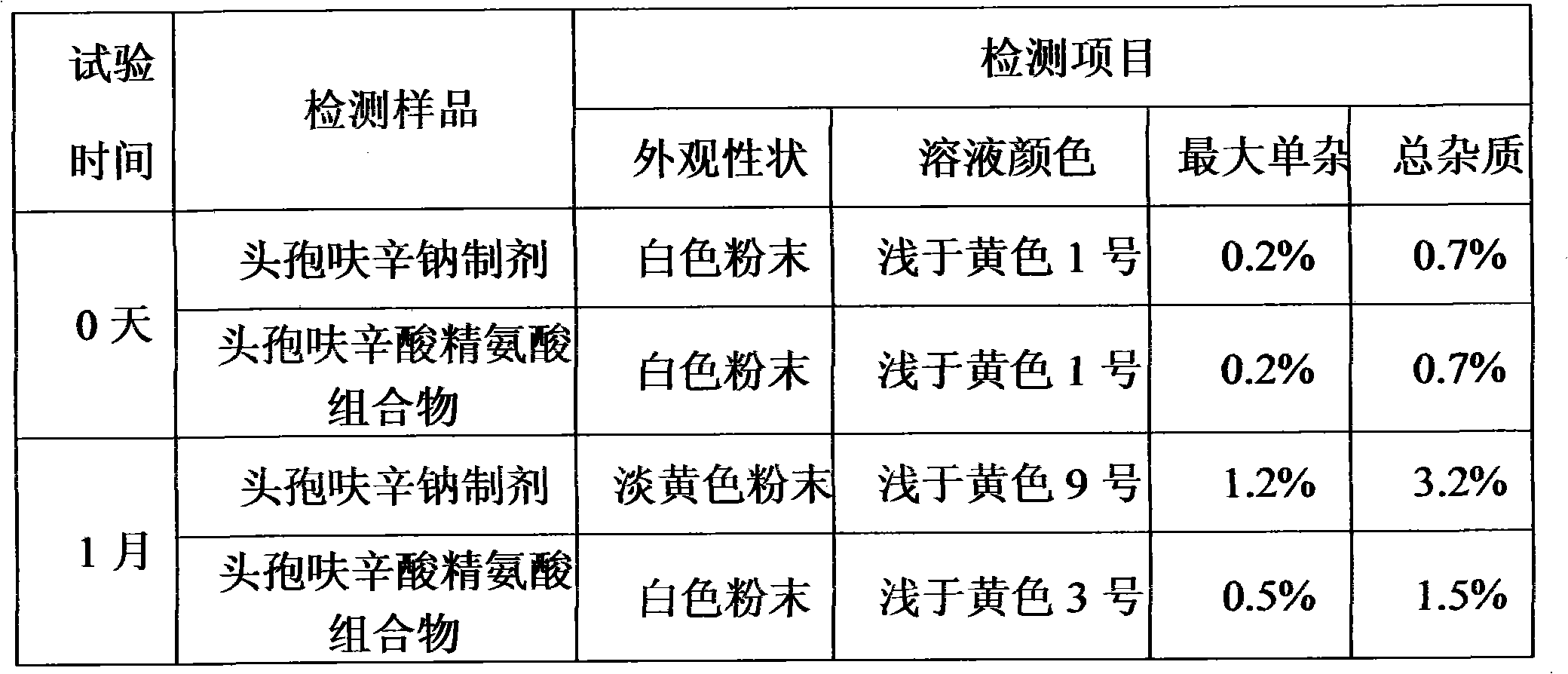
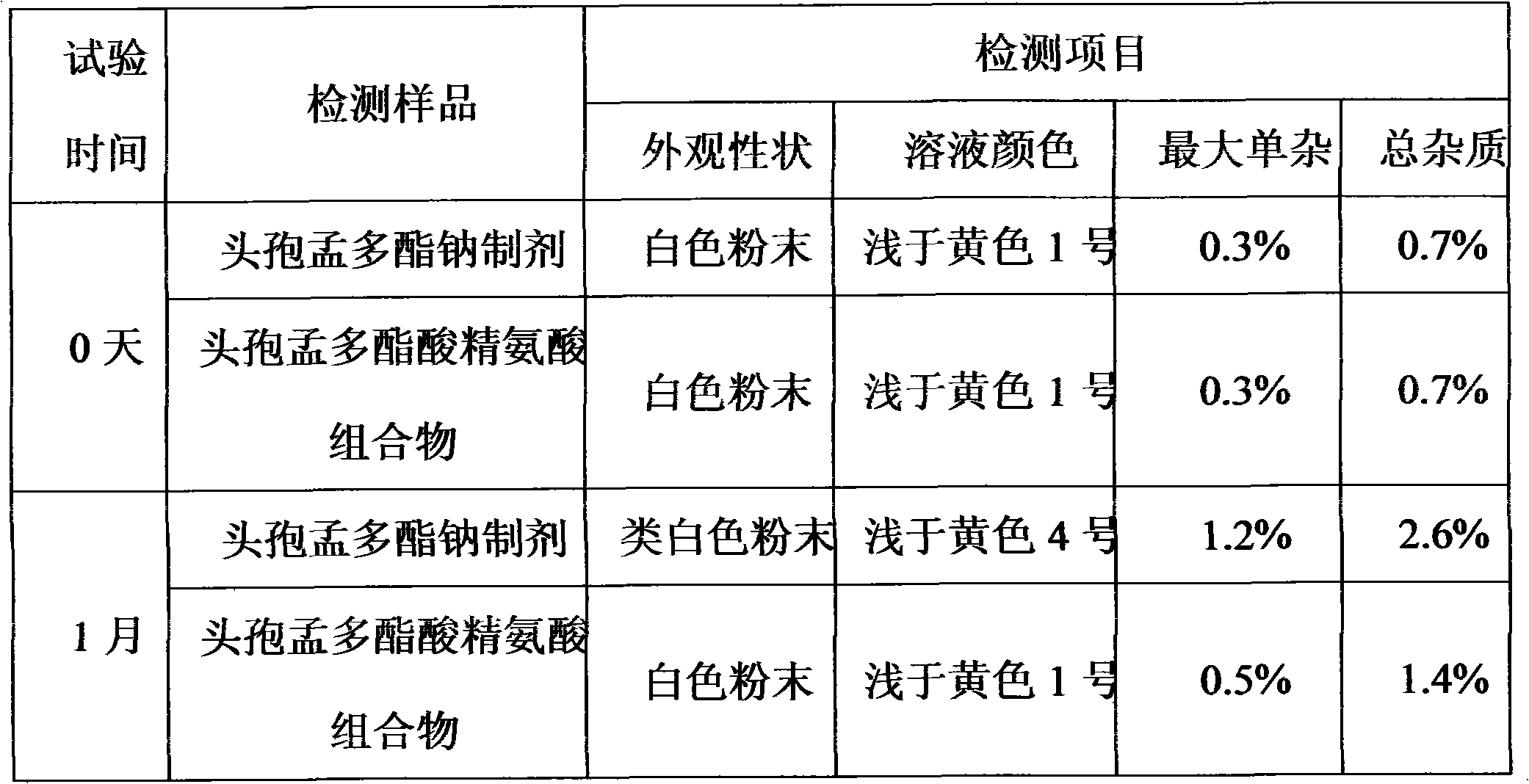
Cefuroxime lysine medicinal composition
ActiveCN103142617ALow hygroscopicityHigh purityAntibacterial agentsOrganic active ingredientsSodium metabisulfiteMANNITOL/SORBITOL
The invention relates to cefuroxime lysine, and particularly relates to a cefuroxime lysine medicinal composition. The cefuroxime lysine medicinal composition comprises cefuroxime lysine and sodium chloride serving as active ingredients in a weight ratio of 10: (0.1-2), and at least one of mannitol, sorbitol, sodium bisulfite and sodium metabisulfite can be added to the active ingredients. The cefuroxime lysine medicinal composition has the advantages of low impurity content, good stability and high flowability and is very suitable for clinical application.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Beta-lactam compound antibiotic composition
ActiveCN102327270AImprove liquidityImprove product quality yieldAntibacterial agentsPowder deliveryActive componentCefuroxime
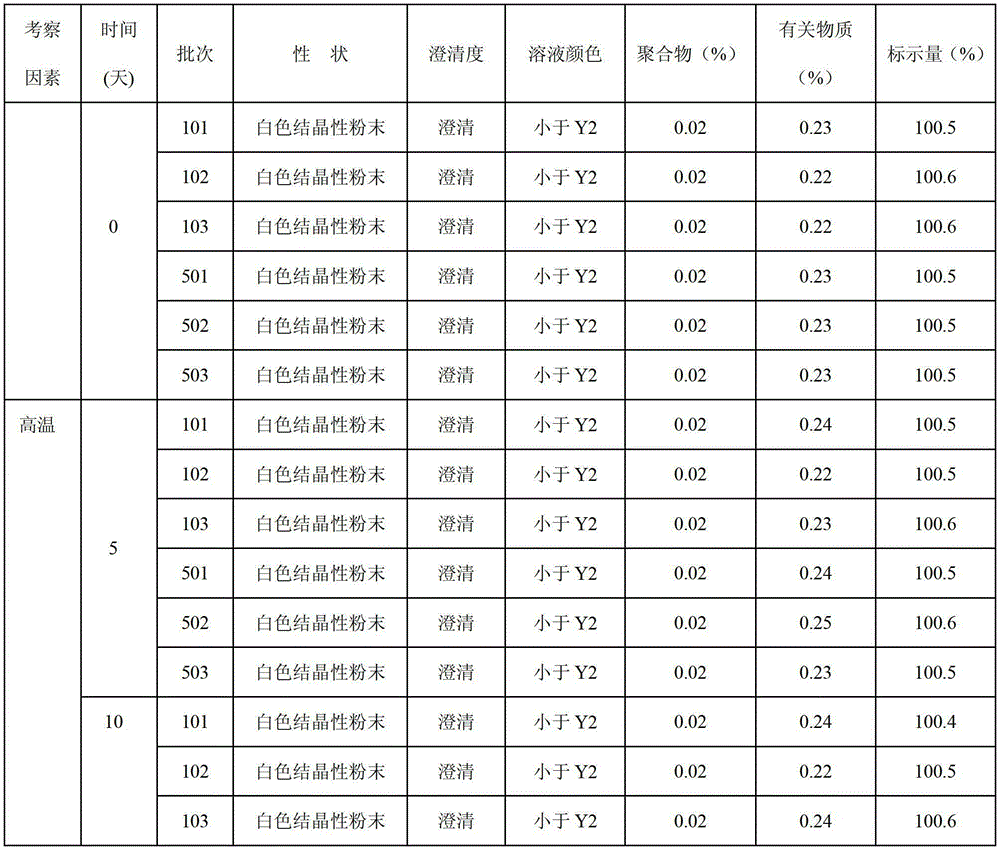
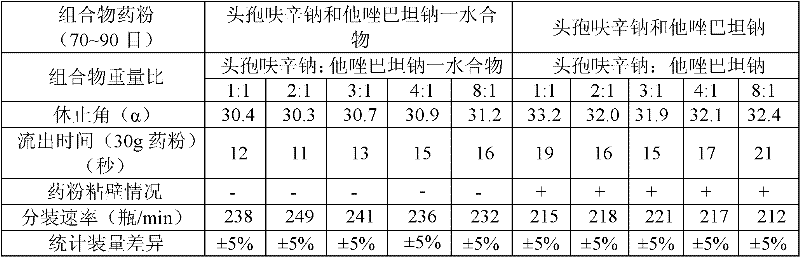
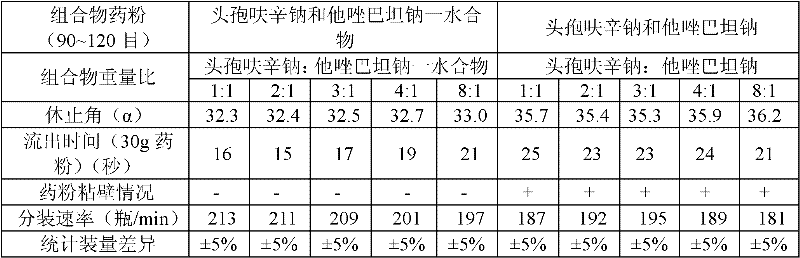
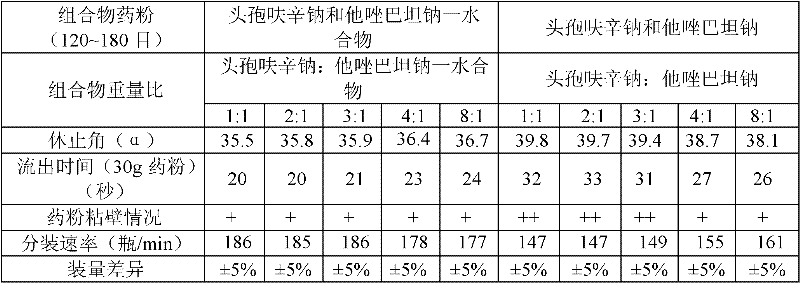
The invention provides a beta-lactam compound antibiotic composition, which contains cefuroxime and a tazobactam sodium monohydrate, or cefuroxime salts and the tazobactam sodium monohydrate, wherein the weight ratio of the cefuroxime salts counted according to the cefuroxime to the tazobactam sodium monohydrate counted according to the tazobactam is (1-8):1. The beta-lactam compound antibiotic composition disclosed by the invention is used for being prepared into a clinically-acceptable medicinal preparation with the cefuroxime and salts thereof as well as the tazobactam sodium monohydrate as active components. According to the beta-lactam compound antibiotic composition, the tazobactam sodium monohydrate is mixed with the cefuroxime and salts thereof so as to improve the flowability of composition powder, enable the uniformity of the medicament to be better in the production process and improve the yield of the product; and the beta-lactam compound antibiotic composition has saved production cost and is especially suitable for popularization and application.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
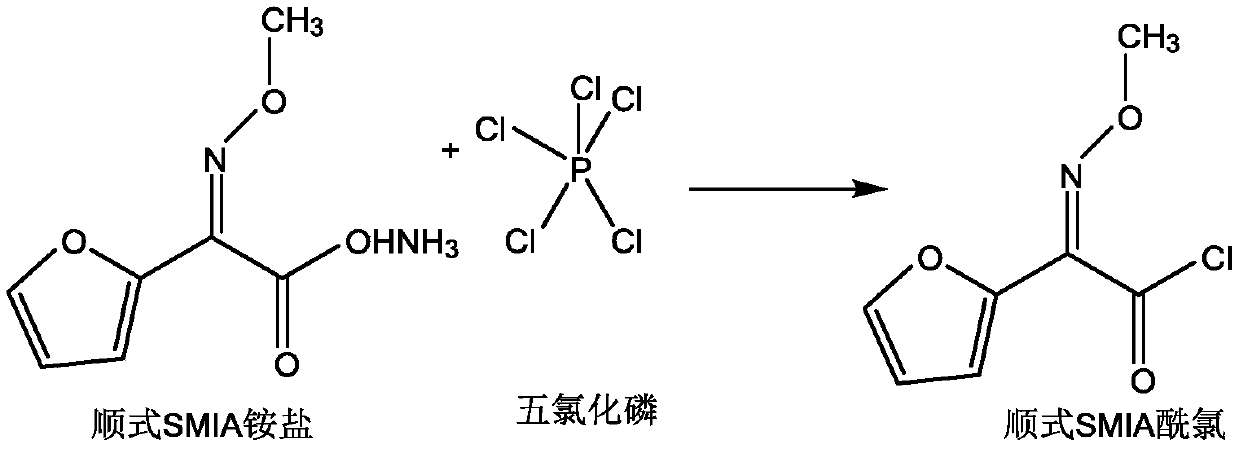
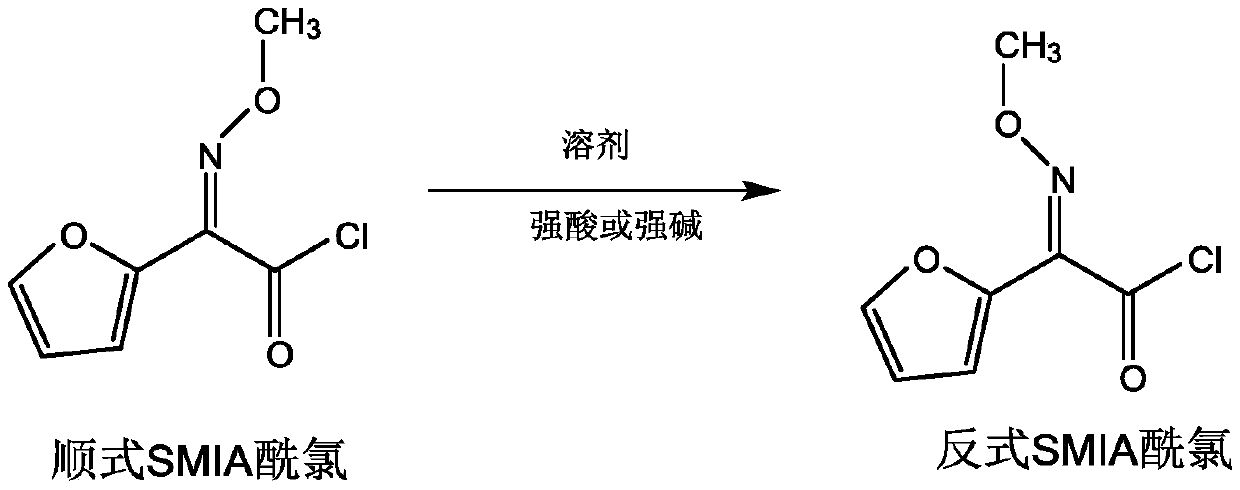
Preparation method of anti-form cefuroxime derivative
The invention relates to a preparation method of an anti-form cefuroxime derivative. The preparation method comprises the steps that A, phosphorus pentachloride is dissolved in a solvent, in the presence of an acidamide reagent, cis-form methoxyiminofurylacetic acid amonium salt is added, and through a reaction, a cis-form methoxy-imino furan acetyl chloride solution is obtained; B, a solvent is added to the cis-form methoxy-imino furan acetyl chloride solution, then strong acid or strong base is added, stirring is conducted, and the cis-form methoxy-imino furan acetyl chloride solution is converted into anti-form methoxy-imino furan acetyl chloride. According to the preparation method of the anti-form cefuroxime derivative, on the basis of original preparation of the anti-form methoxy-imino furan acetyl chloride, an innovative method is provided, by adding a proper quantity of solvent, in the presence of the strong acid or strong base, cis-trans isomerism conversion is conducted, andaccordingly the high-purity anti-form cefuroxime derivative can be efficiently prepared through a synthesis method.
Owner:GUANGDONG LIGUO PHARMACY
Care medicine composition used before operative anesthesia and preparation method thereof
InactiveCN105535489AImprove immunityReduce tensionAntibacterial agentsNervous disorderAdditive ingredientCefuroxime
The invention discloses a care medicine composition used before operative anesthesia and a preparation method thereof. The medicine composition is composed of Chinese herbal medicinal ingredients and Western medicinal ingredients. The Chinese herbal medicinal ingredients mainly comprise fruits of Celastrus rosthornianus Loes, sow thistle flower and seeds, rhapis excelsa, the root of Laxleaf Beautifulflower Millettia, all-grass of auriculate dichrocephala, pine among the Indian Bread, fructus terminaliae billericae, aizoon stonecrop herb, valerian, sandalwood extract and crowndaisy chrysanthemum extract. The Western medicinal ingredients mainly comprise aminomethylbenzoic acid, diaminocaproic acid, taurine and cefuroxime. The care medicine composition is scientifically matched according to different medicine effects and characteristics of Chinese and western medicine, is applied to according to indications, has the effects of soothing nerves and calming heart, relieving pain and removing swelling, resisting bacteria and diminishing inflammation, stopping bleeding, enhancing human body immunocompetence and resisting virus infection, and effectively eases preoperative tension, anxiety, fear and other psychic reactions of a patient. The care medicine composition used before operative anesthesia is simple in preparation technology, remarkable in treatment effect and gentle in medicine effect and has the good medical value.
Owner:张宝英
Preparation method of descarbamoyl cefuroxime
InactiveCN103450223AShorten the production cycleReduce energy consumptionOrganic chemistryFuranCefuroxime
The invention discloses a preparation method of descarbamoyl cefuroxime, which comprises the following steps: performing condensation reaction on 3-deacetyl-7-aminocephalosporanic acid used as a parent nucleus for synthesis of the descarbamoyl cefuroxime and a 2-methoxyimino-2-furanammoniumacetate acyl chloride solution, standing, crystallizing with ethanol / hydrochloric acid, washing, performing vacuum filtration, and drying to obtain the product descarbamoyl cefuroxime. The method uses 3-deacetyl-7-aminocephalosporanic acid as the raw material, uses ethanol for crystallization, shortens the production cycle, and is safe and low in toxicity; and meanwhile, the method is mild in reaction conditions and convenient to realize industrial production.
Owner:GUANGDONG LIGUO PHARMACY
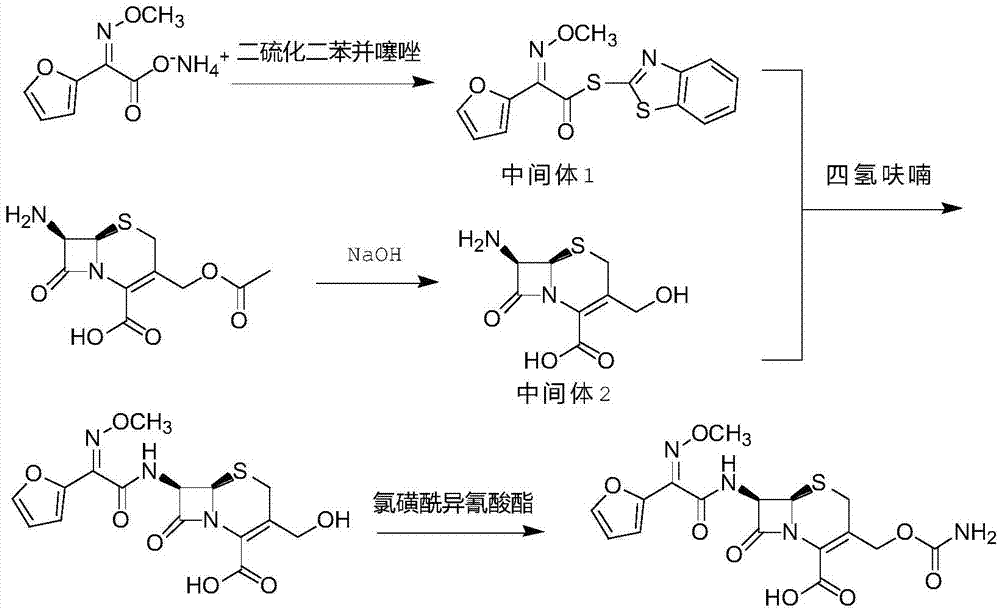
Original quality cefuroxime acid and drug preparation thereof
ActiveCN105440055AReduce contentIncrease fat solubilityAntibacterial agentsOrganic active ingredientsTriethylphosphiteCefuroxime
The invention discloses original quality cefuroxime acid and a drug preparation thereof. A preparation method of original quality cefuroxime acid comprises the following steps: (1), adding SMIA, dibenzothiazyl disulfide, phenylamine, dichloromethane, acetonitrile and triethylamine into a reaction bottle, stirring and increasing the temperature, dropwise adding triethyl phosphate, carrying out thermal reaction, cooling for crystallization, carrying out suction filtration, and drying to obtain an intermediate 1; (2), after hydrolyzing, crystallizing 7-ACA, filtering, and drying to obtain an intermediate 2; (3), in a tetrahydrofuran solvent, enabling the intermediate 1 and the intermediate 2 to be subjected to N-acylation reaction, after reaction, dropwise adding chlorosulfonyl isocyanate, and hydrolyzing to obtain cefuroxime acid reaction liquid; (4), purifying the cefuroxime acid reaction liquid to obtain cefuroxime acid products. The preparation method can effectively reduce the content of isomers and solve the residue problem of an accelerator M, is easy to operate, reliable in quality, and suitable for large-scale industrial production.
Owner:广东金城金素制药有限公司 +1
Preparation process for cefuroxime acid crystallization
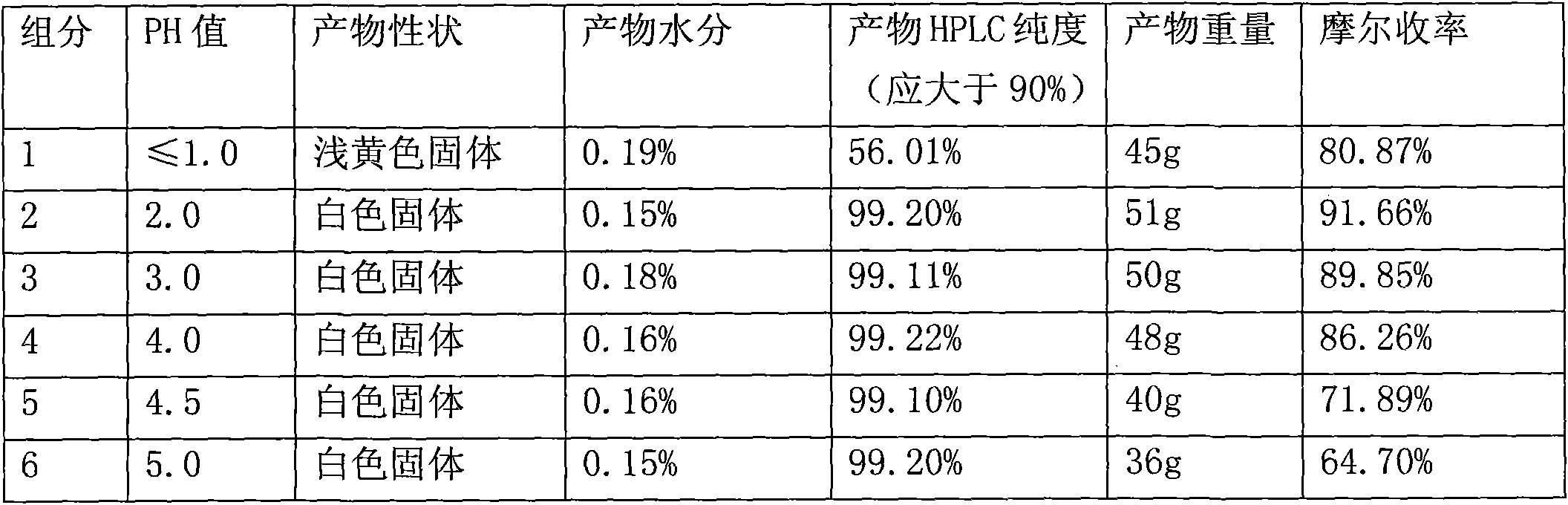
The invention belongs to the technical field of crystallization of pharmaceutical intermediates, and discloses a preparation process for cefuroxime acid crystallization. The process provided by the invention comprises the following concrete steps: adding a cefuroxime acid material liquid prepared in an upstream process into a crystallizer, adding a proper amount of a crystal modifier, adjusting a certain temperature and stirring rate; dropwise adding hydrochloric acid and adjusting the pH value of the mixed solution to a certain value, adding a proper amount of seed crystals, and allowing crystals to grow; adjusting the stirring rate and the hydrochloric acid dropwise adding rate, continuously dropwise adding the hydrochloric acid, and after the completion of dropwise adding, allowing the crystals to grow again; and carrying out filtering, washing and vacuum drying so as to obtain a cefuroxime acid crystallization product. The cefuroxime acid crystal obtained by using the crystallization process provided by the invention has high purity, complete crystal habits and proper particle sizes and particle-size distribution, solves common problems like small product particle size, large crystal slurry viscosity, difficult filtration and drying in the production process of cefuroxime acid in the prior art, shortens the production cycle of products, and reduces production cost.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
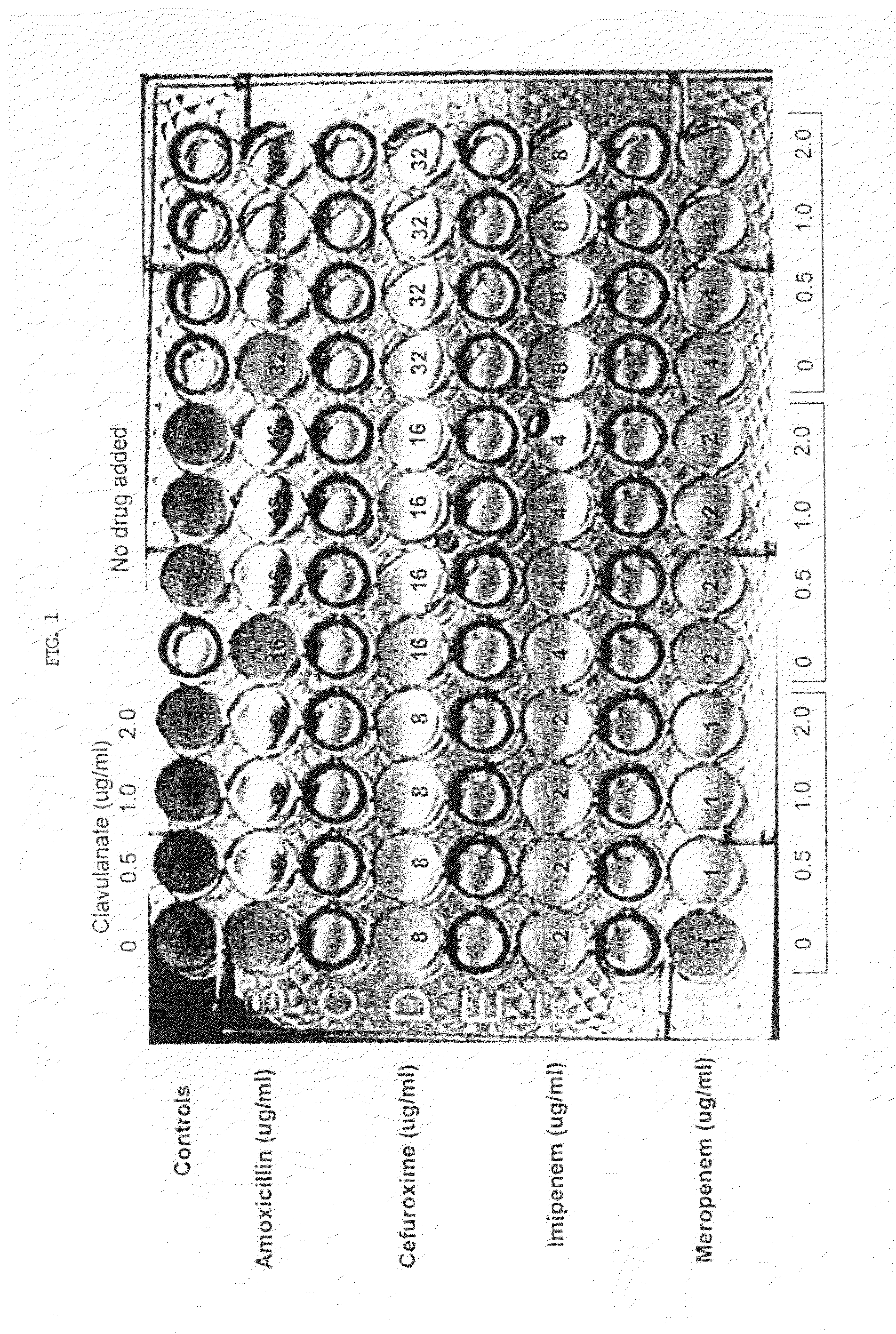
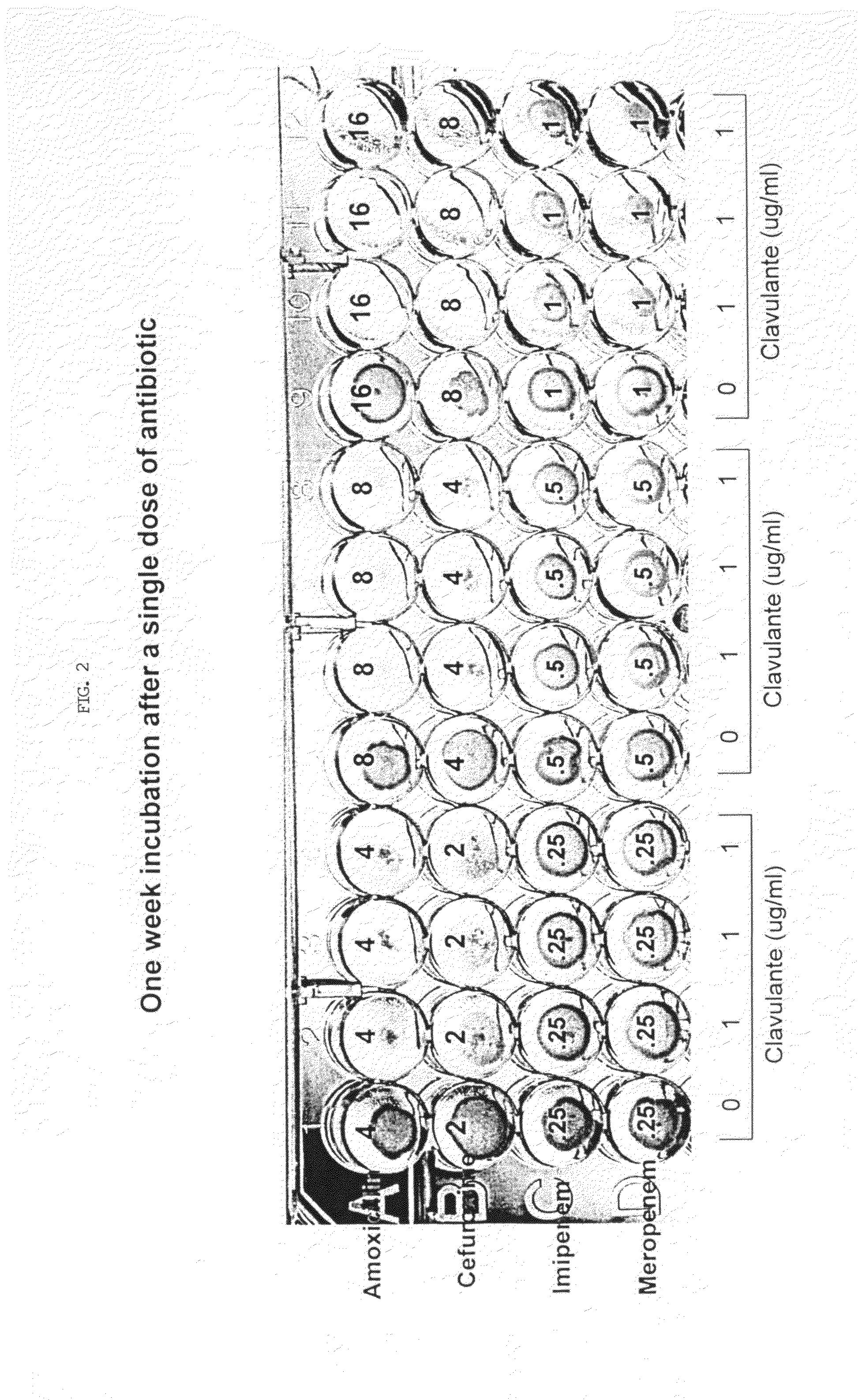
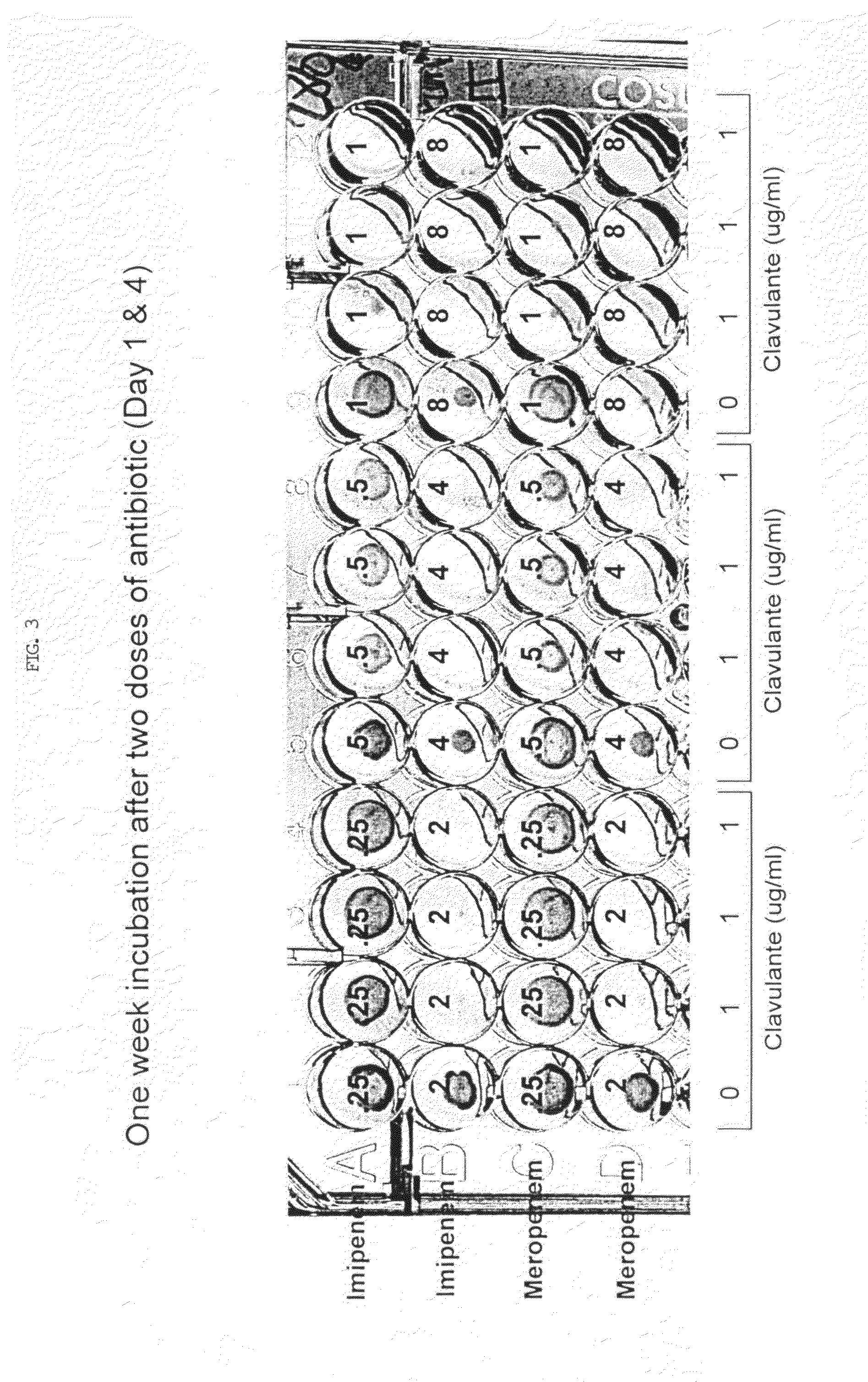
Method for treating tuberculosis
The present invention generally relates to methods for treating tuberculosis in a subject comprising administering to the subject an antibiotic in conjunction with clavulanic acid or salt thereof. The antibiotic can be carbapenem (e.g., meropenem or imipenem) or cefuroxime. The present invention also relates to related pharmaceutical compositions and methods for manufacturing said pharmaceutical compositions.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Amino butanetriol salt of cephalosporin compounds and preparing method
InactiveCN101012235AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefuroximeCefazolin
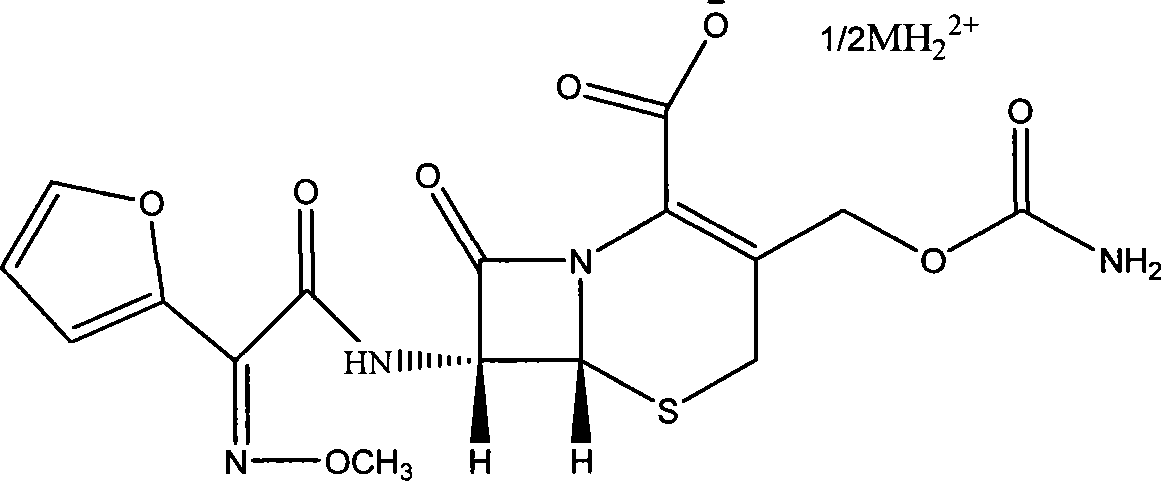
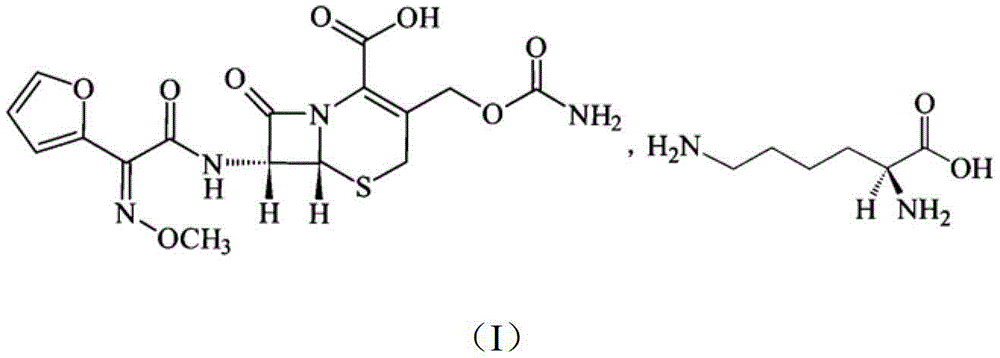
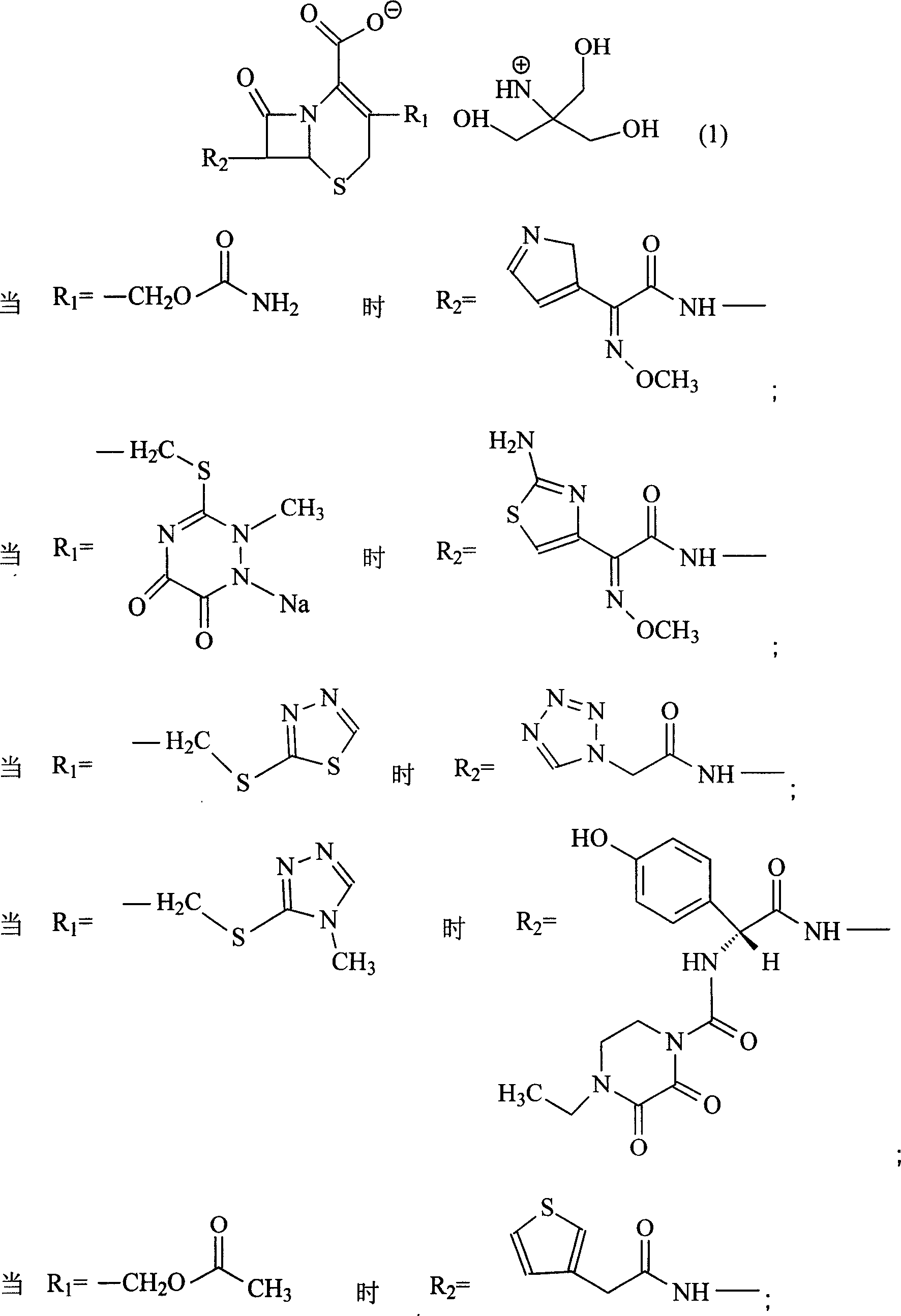
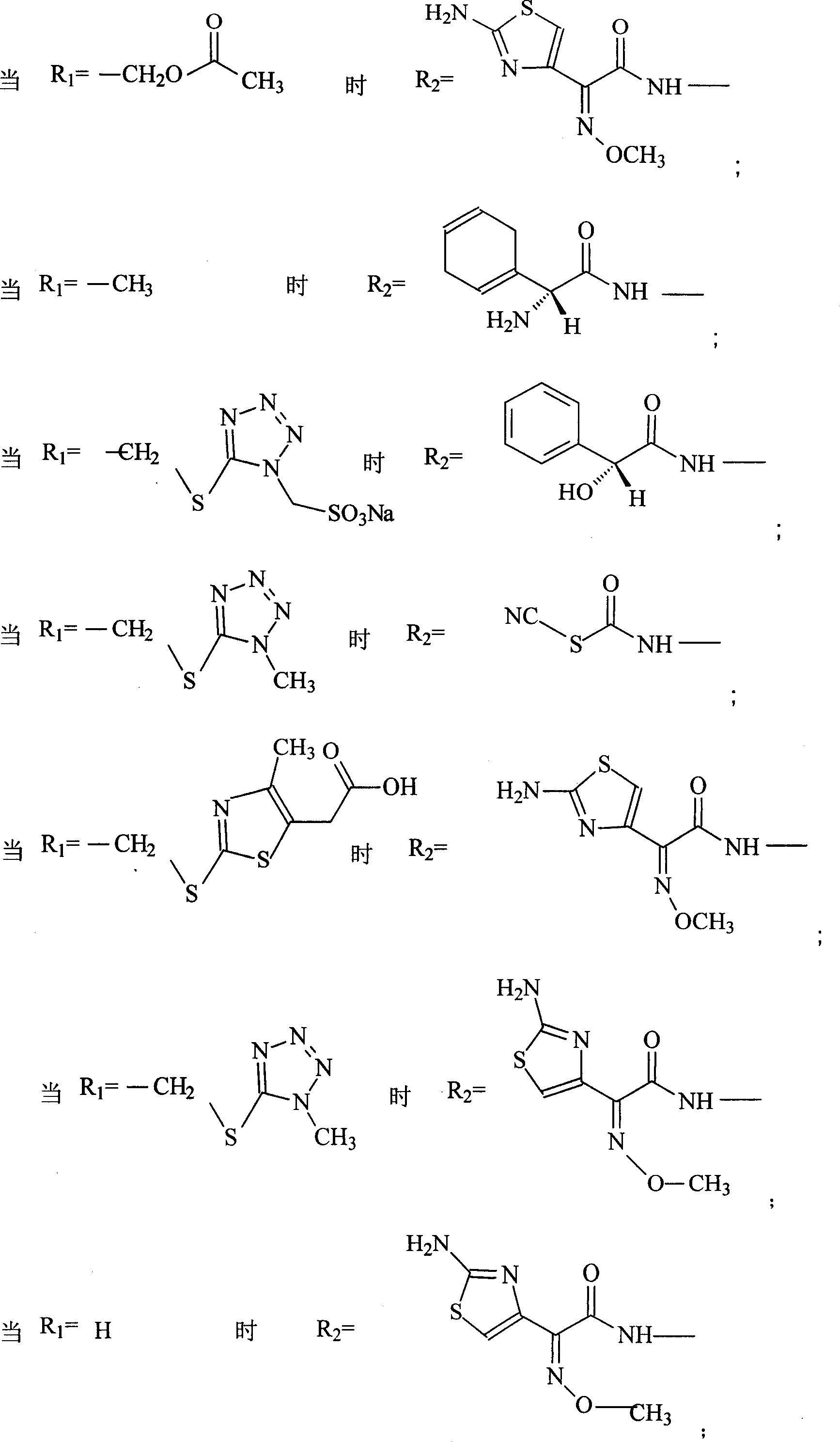
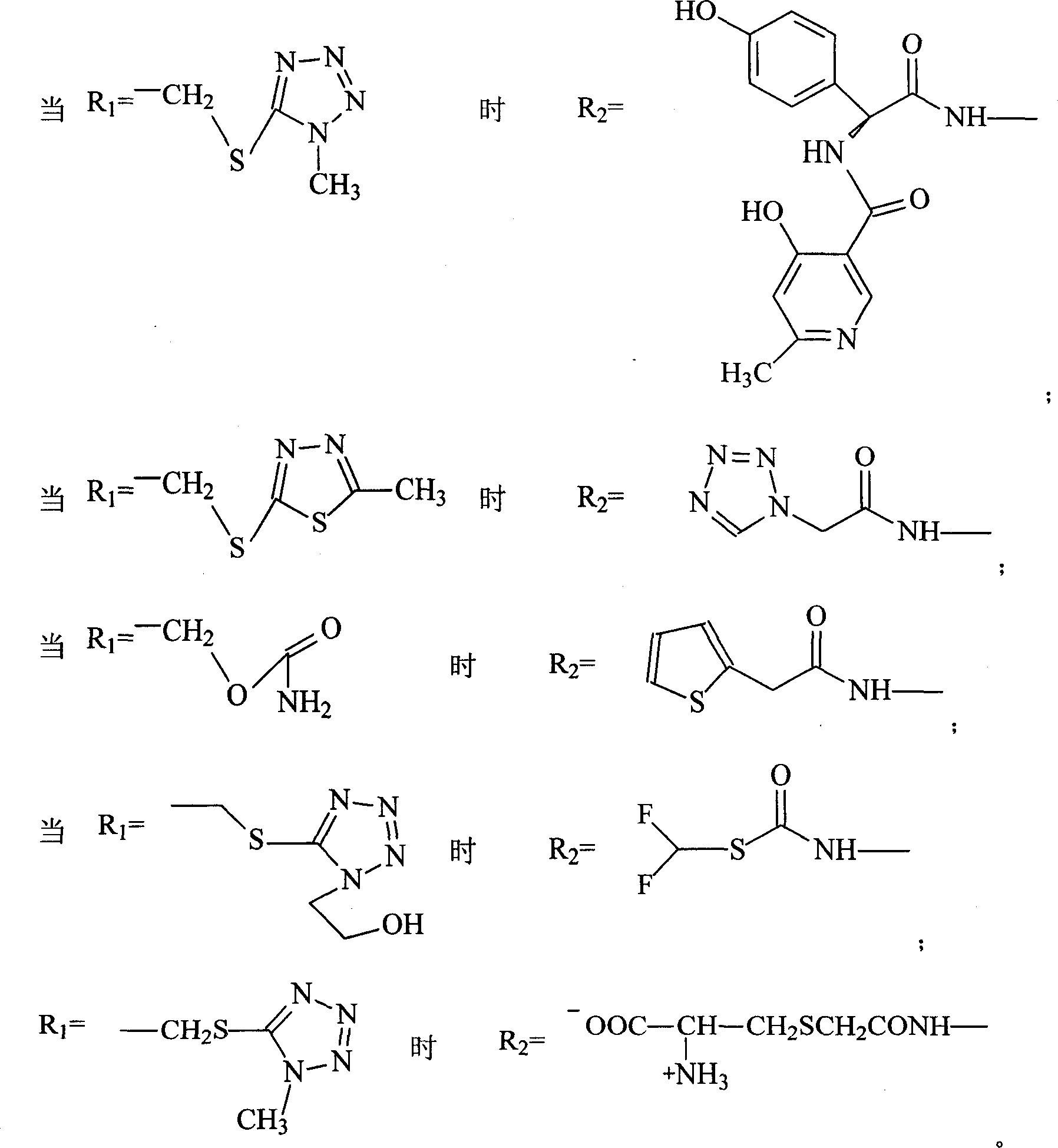
The invention discloses a pehanorm salt or hydrate with chemical formula as picture (1) and drug composition and application to treat bacterial infection, which comprises the following parts: cefuroxime oxtatromethane, cepham qusong tromethane, cepham thiotepa tromethane, cefoperazone tromethane, cephalothin tromethane, cefotaxime tromethane, cefolading tromethane, cefonixin tromethane, cefameizin tromethane, cefadizine tromethane, cefuroxime tromethane, cefazolin tromethane, cefapamine tromethane, cefazoline tromethane, cefaadid tromethane, cefaoxofluoride tromethane, cefaminol tromethane and their hydrate.
Owner:GUANGDONG ZHONGKE DRUG R&D
Cefuroxime safety delivery system
The present invention relates to safety delivery systems for intracameral administration of an appropriate dose of cefuroxime subsequent to cataract and other eye surgery. A preferred embodiment of an cefuroxime safety delivery system according to the invention comprises (a) a perforable sterile vial sterile-filled with a measured amount of cefuroxime, (b) a reconstitution syringe with a male luer fitting sterile-filled with 0.1 ml of isotonic salt solution per mg cefuroxime in the perforable vial and a vial adapter with a female luer fitting or one or more needles with female luer fitting, and (c) one or more sterilized delivery syringes with male luer fitting, each delivery syringe being capable of holding at least 0.1 ml of ejectable liquid and containing a marking indicating a fill volume of 0.1 ml of ejectable liquid.
Owner:CIS PHARMA
Composition of amino-cephalosporanic acid and arginine
The invention relates to a composition of amino-cephalosporanic acid and arginine, which is characterized in that amino-cephalosporanic acid in the composition comprises cefazolin acid, cefuroxime acid, cefamandole nanfate nitrosate, cefonicid acid and cefotetan acid, and one of the above amino-cephalosporanic acids is combined with arginine to obtain the composition. The invention comprises the following steps: in gnotobasis, scaling asepsis arginine and above amino-cephalosporanic acid which have suitable granularity and specific gravity according to ratio of recipe to serve as asepsis bulkdrugs; pouring the drugs into a mixer to operate according to the standard powder mixing operating instruction; after evenly mixed, discharging and slit charging. The composition of the invention solves the problems that sodium salt agent widely applied in cephems drugs specifically has poor crystal form, solution color is easily out of limits, no bacteria exist or pyrogen is not qualified, wateris hard to control, stability is poor and the like. The invention is predicted to have important clinic application value.
Owner:CHANGSHA KINGDAY BIO PHARMA TECH
Method for synthesizing cefuroxime acid
The invention provides a method for synthesizing cefuroxime acid. In the method, a phosphorus acyl active resin is subjected to hydrolysis reaction, the product of the hydrolysis reaction is subjected to aminoacylation, and a 3-decarba-moyl cefuroxime acid intermediate is synthesized by a one-pot method without separating the intermediate, the step of cefuroxime acid synthesis is saved, and the yield of the product is improved greatly.
Owner:SHANDONG JIANZHU UNIV +1
Preparation method of decarbamoyl cefuroxime
ActiveCN108440568AShorten the production cycleReduce energy consumptionOrganic chemistryCefuroximeFiltration
The invention belongs to the field of preparation of pharmaceutical intermediates, and particularly relates to a preparation method of decarbamoyl cefuroxime. The method comprises steps as follows: 7-aminocephalosporanic acid as a mother nucleus for synthesis of decarbamoyl cefuroxime is subjected to esterolysis, a product is then subjected to condensation reaction with an acyl chloride liquor of2-methoxyimino-2-furyl acetic acid ammonium, a reaction product is left to stand and subjected to crystallization by ethanol / hydrochloric acid, washing, suction filtration and drying are performed, and the product decarbamoyl cefuroxime is obtained. According to the method, 7-aminocephalosporanic acid is taken as the raw material and crystalized by ethanol and is safe and low in toxicity, and theproduction cycle is shortened; meanwhile, reaction conditions of the method are mild, and industrial production is facilitated.
Owner:GUANGDONG LIGUO PHARMACY
Preparation method of 3-deaminized formoxyl-cefuroxime acid crystal
InactiveCN106478667AUniform particle size distributionLow viscosityOrganic chemistryProduction lineMethylene Dichloride
The invention belongs to the technical field of a drug intermediate crystal, and particularly relates to a preparation method of 3-deaminized formoxyl-cefuroxime acid crystal. The raw material is 3- deaminized formoxyl-cefuroxime feed liquid prepared in an upstream synthesis process. The specific process includes steps of putting raw materials in a crystallizer according to matching ratio, removing methylene dichloride contained in the feed liquid, and maintaining constant temperature in the crystallizer; adjusting pH value by 10% of diluted hydrochloric acid by mass, and performing isoelectric point crystallization; before producing the crystal, adding the treated 3- deaminized formoxyl-cefuroxime crystal seed; after cultivating the crystal, adjusting pH value to the final point; performing secondary crystal cultivation, filtering, washing and drying, and obtaining a spherical 3-deaminized formoxyl-cefuroxime acid crystal product. The preparation process provided by the invention can effectively shorten the crystal time and avoid problems of too small product granularity and too wide granularity distribution in the production of the 3-deaminized formoxyl-cefuroxime acid; furthermore, the filtering and drying performances are improved, and the production capacity of the production line is promoted.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Cefuroxime lysine and preparation thereof
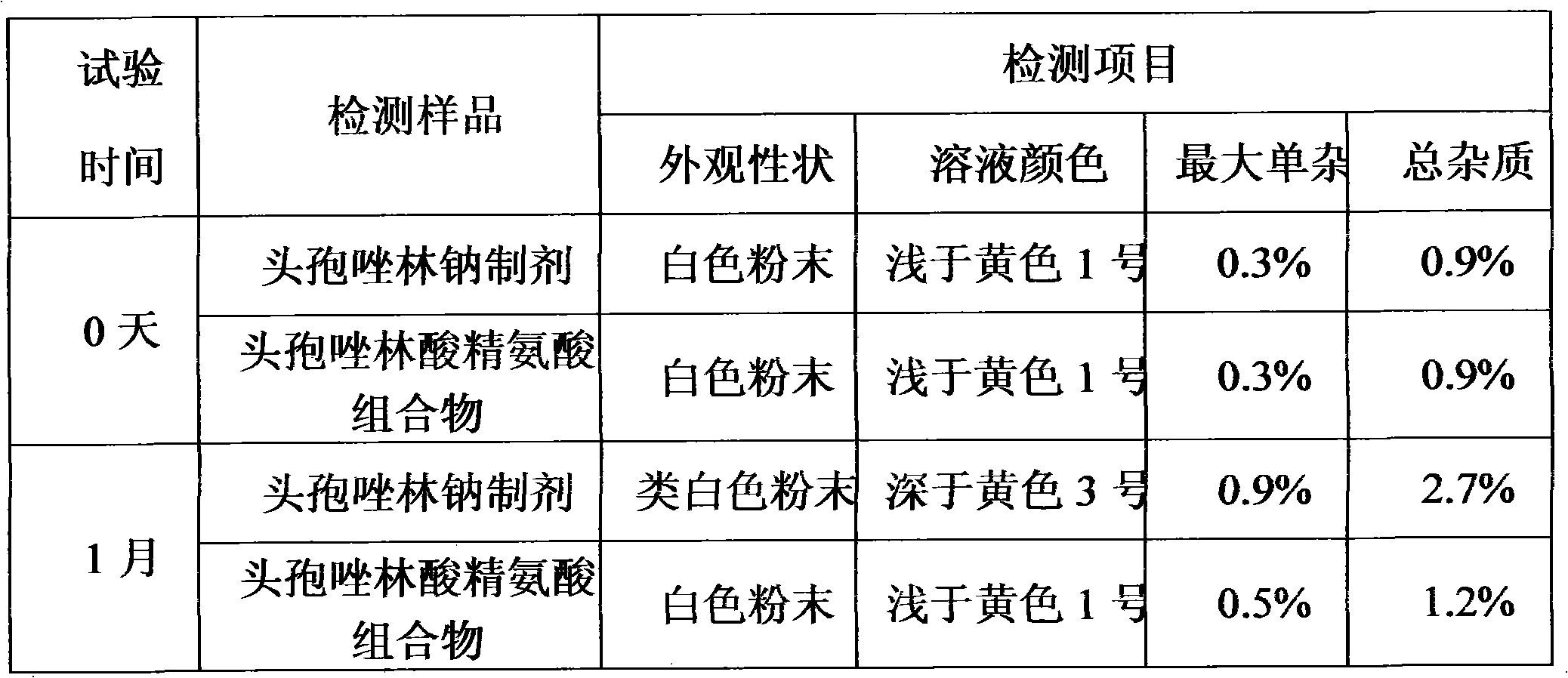
ActiveCN103159784AHigh purityStable clinical application effectAntibacterial agentsOrganic active ingredientsCefuroximeImpurity
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Cefuroxime lysine and preparation thereof
ActiveCN103130821AImprove stabilityLow impurity contentAntibacterial agentsOrganic active ingredientsCefuroximeCombinatorial chemistry
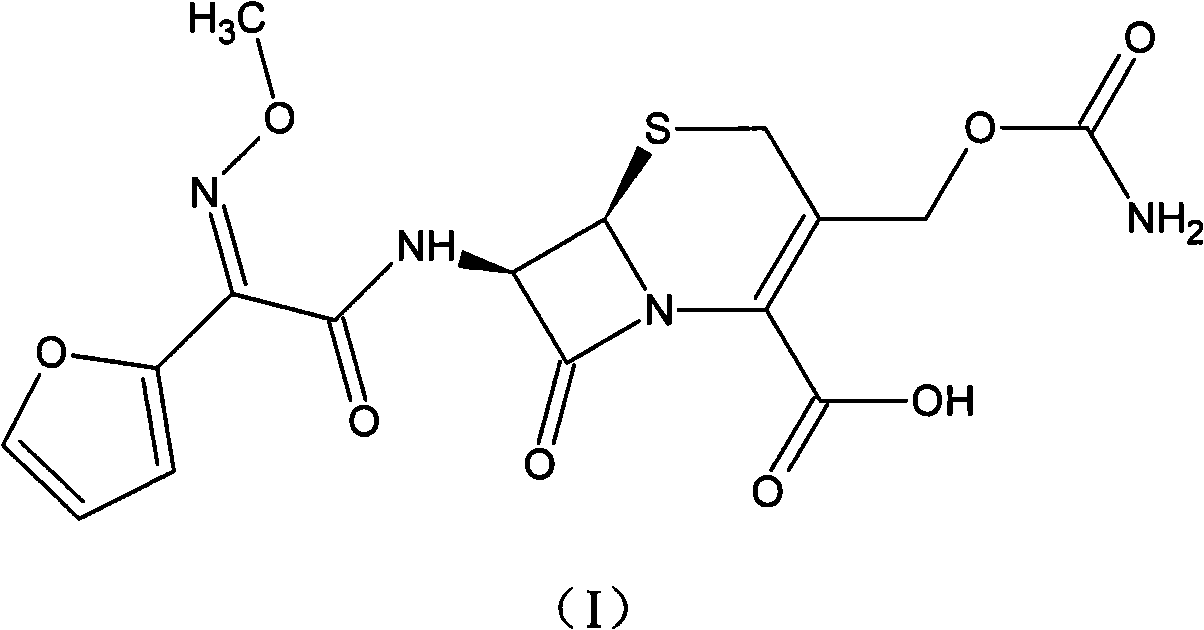
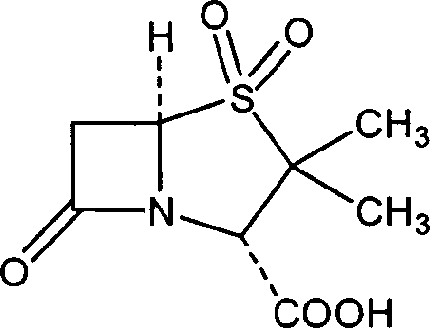
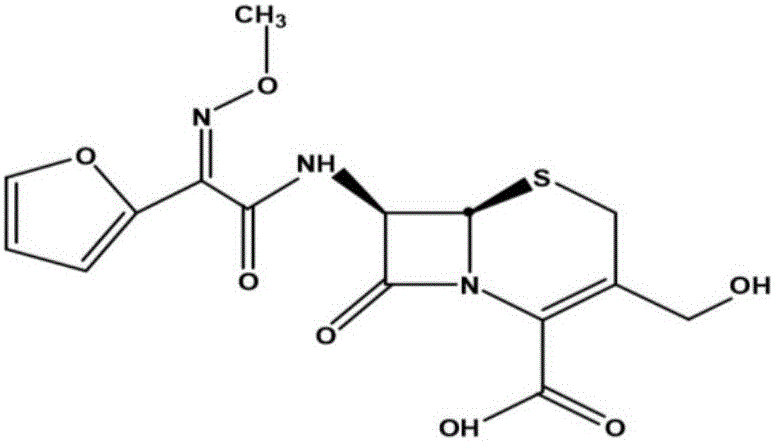
The invention relates to a cefuroxime lysine compound. The invention is characterized in that the cefuroxime lysine contains 98-99.99 wt% of cefuroxime lysine and 0.01-2 wt% of descarbamoyl cefuroxime, wherein the molecular formula of the descarbamoyl cefuroxime is disclosed as Formula I. The cefuroxime lysine provided by the invention has higher stability than the cefuroxime lysine in the prior art, and lower impurity content and polymer content than the cefuroxime lysine in the prior art, and is very suitable for clinical application.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Anti beta-bactamase antibiotic compound prepn.
InactiveCN1206995CBroad spectrum antibacterialImprove antibacterial propertiesAntibacterial agentsHeterocyclic compound active ingredientsCefuroximeSulbactam
The invention discloses an anti-beta-lactamase antibiotic compound preparation, which is specifically a compound preparation composed of cefuroxime and its salt, sulbactam and its salt, or tazobactam and its salt. The weight ratio range of cefuroxime and its salt and sulbactam and its salt or tazobactam and its salt in the compound preparation is 1:1 to 10:1; cefuroxime and its salt and sulbactam and its The preferred weight ratio range of its salt is 2:1 to 6:1, the optimum weight ratio is 4:1, the preferred weight ratio range of cefuroxime and its salt and tazobactam and its salt is 1:1 to 6 : 1, the optimum weight ratio is 2: 1. Compared with cefuroxime, the invention has wider antibacterial spectrum and stronger antibacterial effect.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
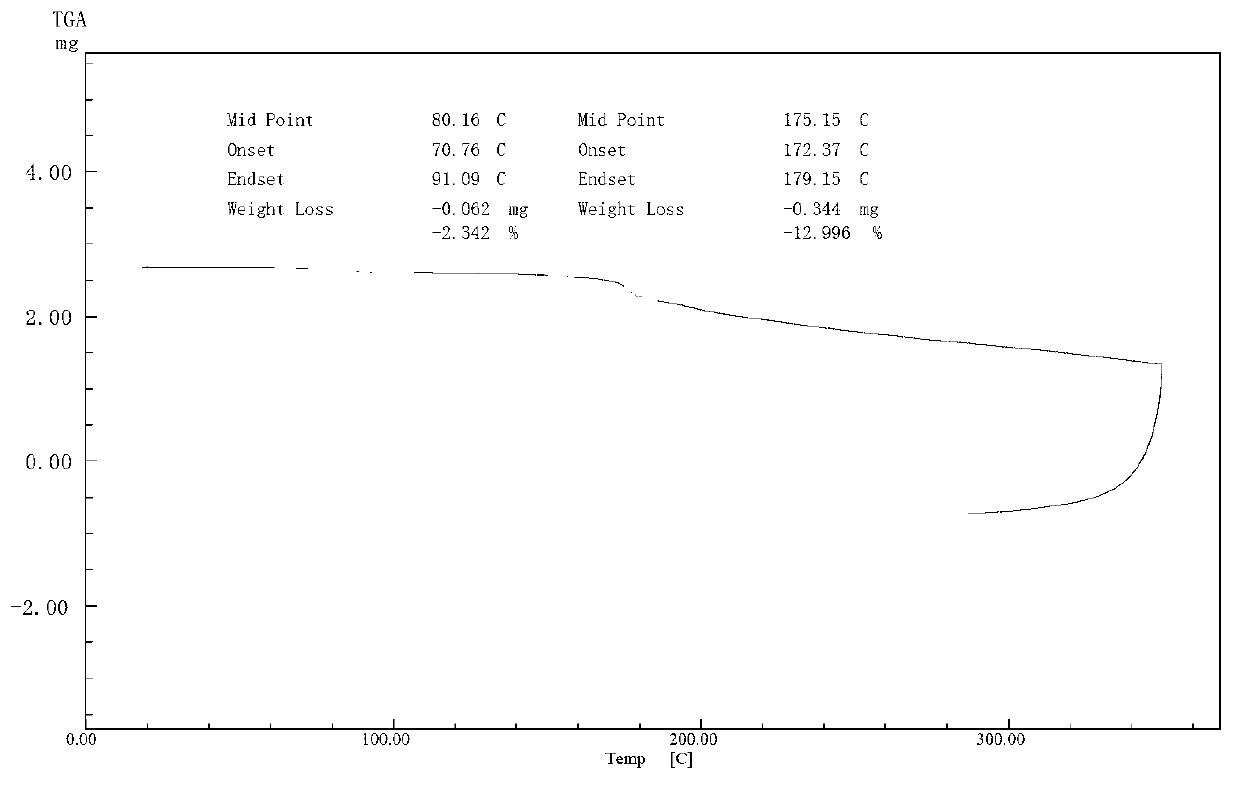
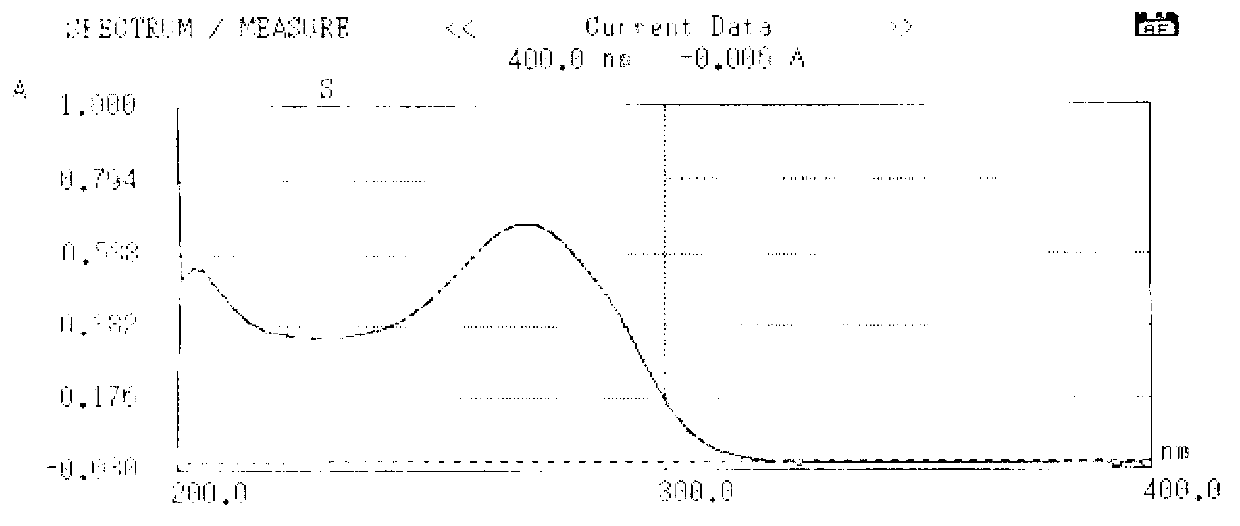
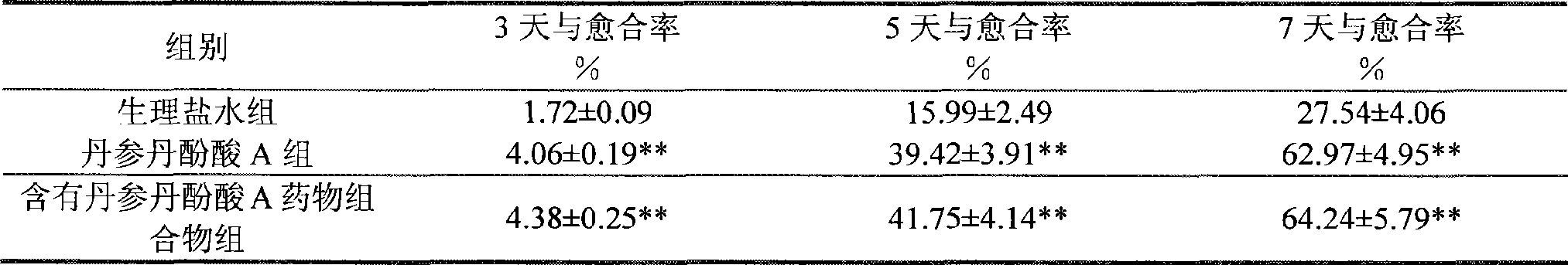
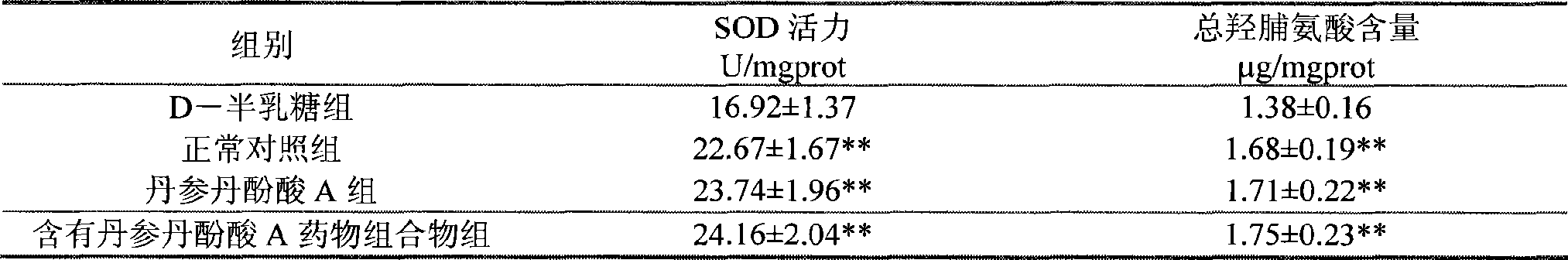
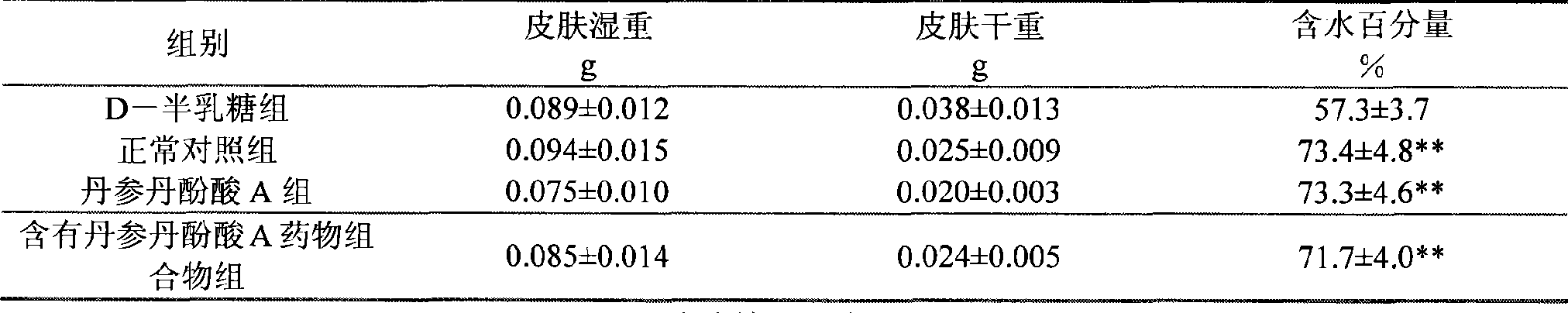
Use of salvianolic acid A and medicine composition containing salvianolic acid A from Salia miliiorrhiza Bge.
The present invention discloses salvianolic acid A and a novel use of pharmaceutical composition comprising salvianolic acid A. The invention is characterized by the application of salvianolic acid A in preparing the medicament for treating skin injury, and the application of pharmaceutical composition composed of salvianolic acid A, snow grass, pinellia tuber, phellodendron amurense, cefuroxime, cefalexin, ceftriaxone sodium, etc. in preparing the medicament for treating skin injury. The pharmaceutical and clinical researches show that the salvianolic acid A and pharmaceutical composition comprising salvianolic acid A have excellent treating function for skin injury.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Amidases, nucleic acids encoding them and methods for making and using them
The present invention provides amidases, polynucleotides encoding the amidases, methods of making and using these polynucleotides and polypeptides. In one aspect, the invention provides enzymes having secondary amidase activity, e.g., having activity in the hydrolysis of amides, including enzymes having peptidase, protease and / or hydantoinase activity. In alternative aspects, the enzymes of the invention can be used to used to increase flavor in food (e.g., enzyme ripened cheese), promote bacterial and fungal killing, modify and de-protect fine chemical intermediates, synthesize peptide bonds, carry out chiral resolutions, hydrolyze Cephalosporin C. The enzymes of the invention can be used to generate 7-aminocephalosporanic acid (7-ACA) and semi-synthetic cephalosporin antibiotics, including caphalothin, cephaloridine and cefuroxime. The enzymes of the invention can be used as antimicrobial agents, e.g., as cell wall hydrolytic agents. The invention also provides a fluorescent amidase substrate comprising 7-(epsilon-D-2-aminoadipoyladipoylamido)-4-methylcoumarin.
Owner:DIVERSA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com