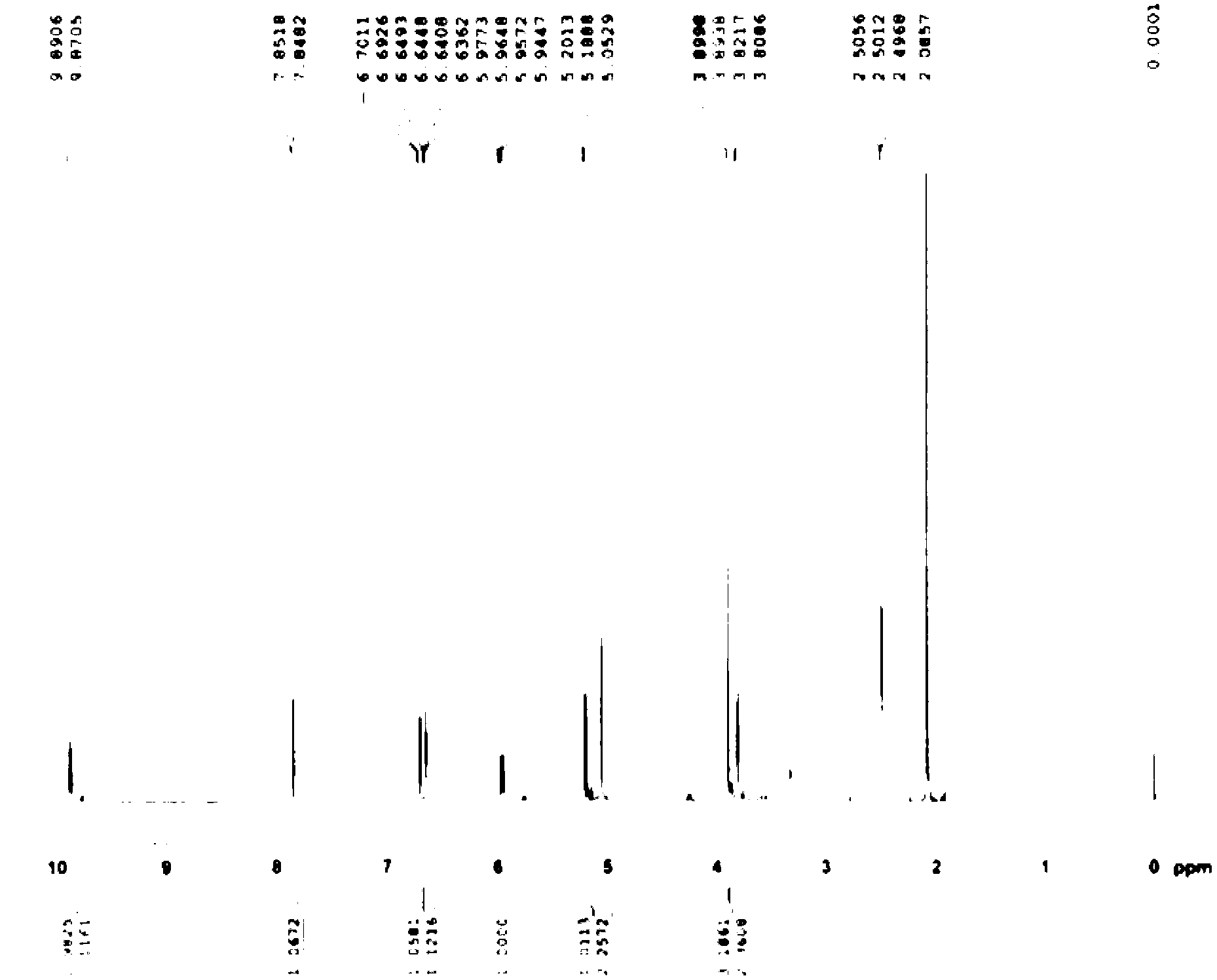
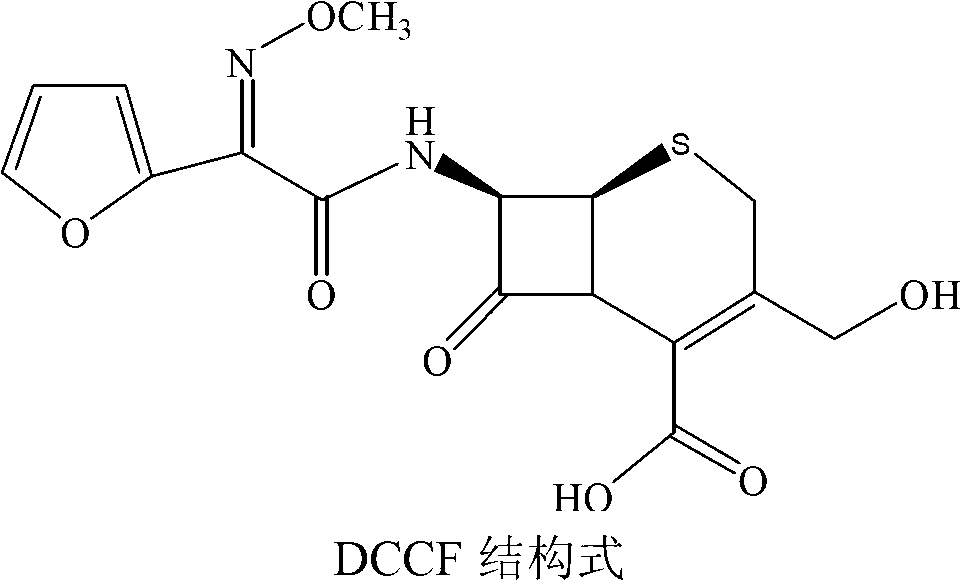
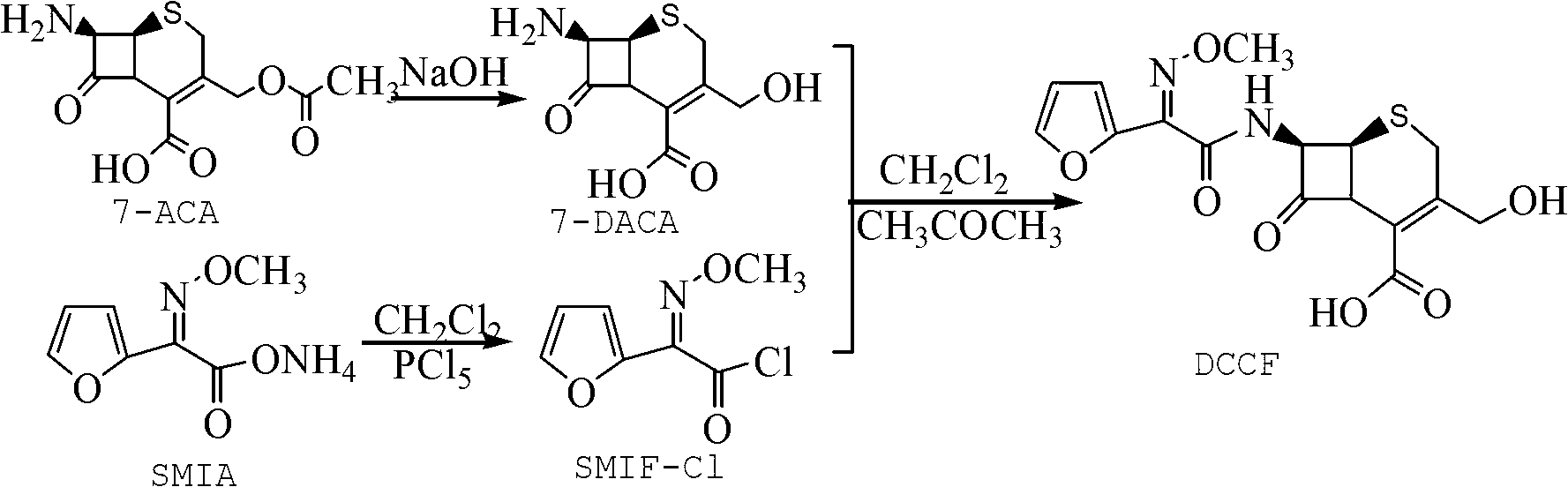
Method for preparing 3-descarbamoyl-cefuroxime acid
A technology of cefuroxime acid and aminocephalosporanic acid, which is applied in the field of drug synthesis, can solve the problems of large solvent residue, long cycle, and unsuitability for large-scale production, and achieve the effect of reducing synthesis steps and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention is achieved through the following technical solutions:
[0025] A kind of preparation method of 3-decarbamoyl-cefuroxetin acid, comprises the steps:
[0026] (1) Dissolve 7-aminocephalosporanic acid (7-ACA) in water or methanol solution at -5-5°C to prepare a 7-ACA solution with a concentration of 0.1-0.3g / ml, and add NaOH solution dropwise Adjust the pH value to 8-11, and keep the hydrolysis reaction for 80-120 minutes to obtain 3-deacetyl-7-amino-cephalosporanic acid (7-DACA) solution;
[0027] (2) Dissolve the acyl chloride reagent in the solvent, stir at room temperature for 20-40 minutes, cool down to -20--10°C, add co-solvent, then add ammonium furan (SMIA), react for 90-120 minutes, filter, and take the filtrate, Add water or rotary evaporation to remove excess acid chloride reagent, then vacuum rotary evaporation to remove solvent, add homogeneous reagent to dissolve, and obtain SMIF-Cl solution;
[0028] Wherein, the molar ratio of the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com