Method for synthesizing cefuroxime acid
A technology of cefuroxime acid and mixed solution, applied in the direction of organic chemistry, etc., can solve the problems of increasing the cost of cefuroxime acid, harming human health, high processing cost, etc., and achieves low solvent recovery cost, reduced production cost, and shortened operation period Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
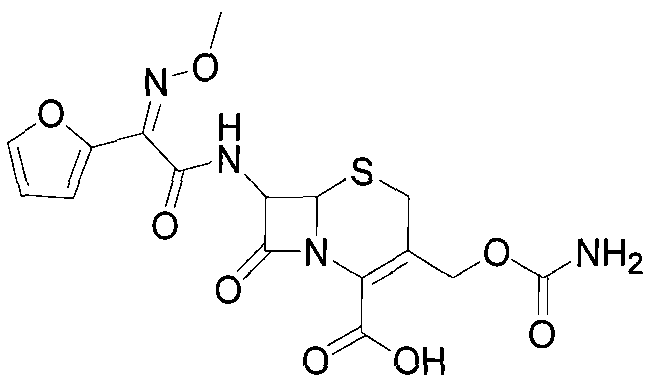
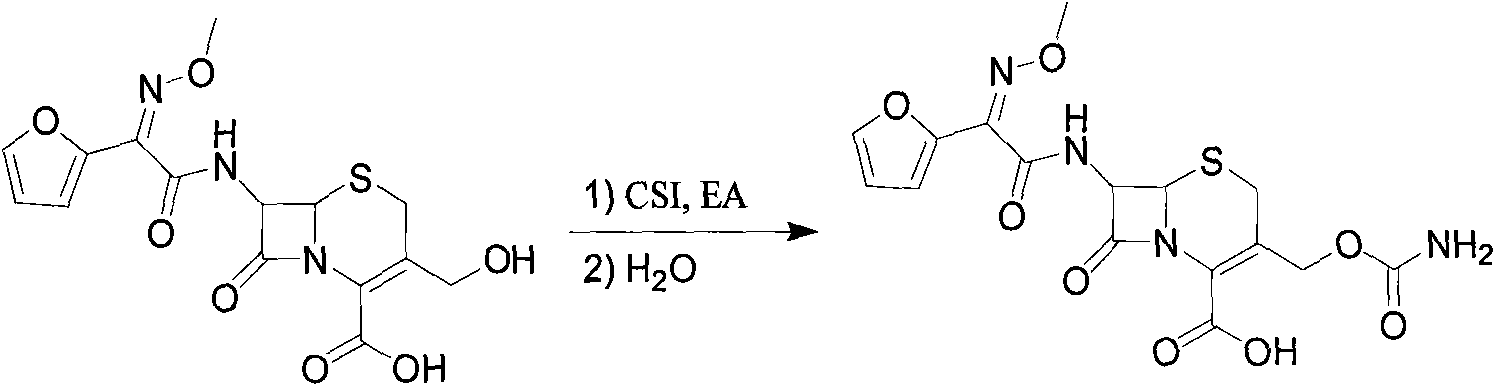
[0033] Add ethyl acetate (500ml), DDC (50g, 117.82mmol) to a 1000ml three-necked flask in sequence, then control the temperature T1 = 5-10°C, and drop 15mL of CSI in 10 minutes. Control temperature T2=15-20°C, add 2.5g of activated carbon, stir and react for 30min, then filter. The filtrate was cooled to about 5°C, then 70mL of purified water was added dropwise for 5min, followed by NaHCO3 solution (53gNaHCO 3 + 284mL water), dripped in 30min. The liquid was separated, the ethyl acetate layer was discarded, and 30% HCl was added dropwise to adjust the pH value, and the temperature was lowered to T3=0-5° C. to grow crystals for 1 h. Suction filtration, wash the filter cake twice with water, 50 mL each time, drain it, and dry it in vacuum at 50°C for 3 hours. The reaction products are shown in Table 1:
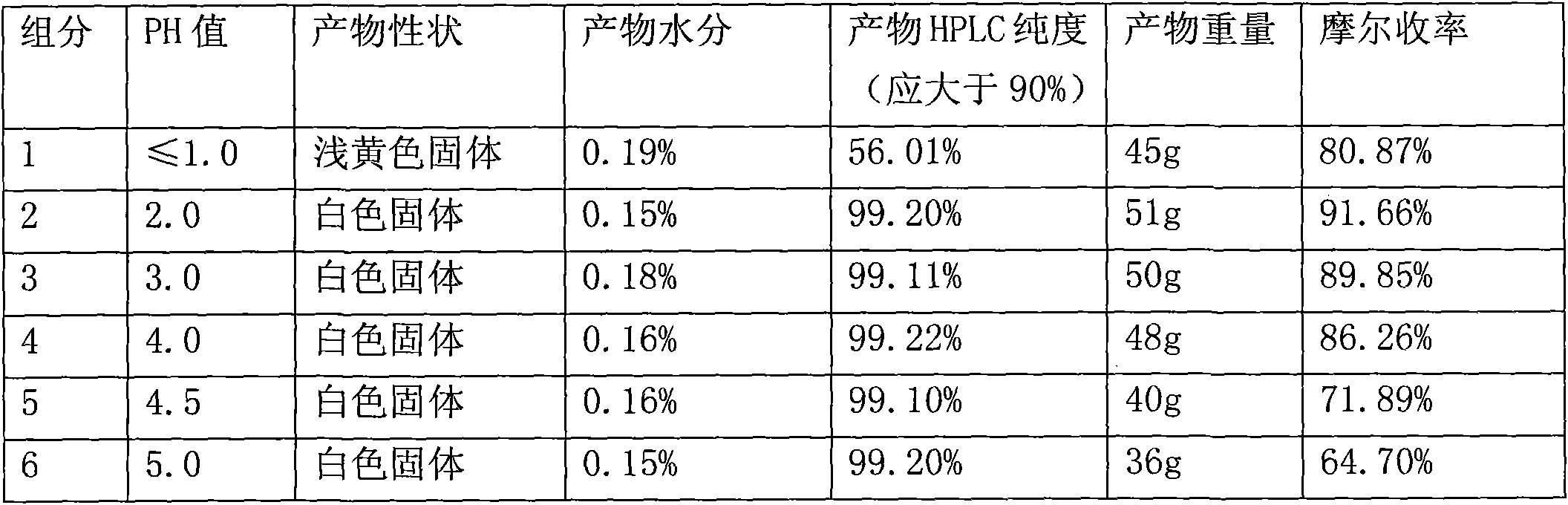
[0034] Table 1. The influence of different pH values on the reaction products
[0035]
[0036] Conclusion: From Table 1, it can be found that when the pH value is 1-4, ...
Embodiment 2
[0038] Add ethyl acetate (500ml) and DDC (50g, 117.82mmol) to a 1000ml three-necked flask in sequence, then control the temperature T1 = 0-5°C, and drop 15mL of CSI in 10 minutes. Control temperature T2=15-20°C, add 2.5g of activated carbon, stir and react for 30min, then filter. Cool the filtrate to about 5°C, then add 70 mL of purified water dropwise for 5 minutes, then add NaHCO dropwise 3 Solution (53gNaHCO 3 + 284mL water), dripped in 30min. The liquid was separated, the ethyl acetate layer was discarded, 30% HCl was added dropwise, the pH was adjusted to 2.0, and the temperature was lowered to T3=0-5°C to grow crystals for 1 h. After suction filtration, the filter cake was washed twice with 50 mL of water each time, sucked dry, and dried in vacuum at 50° C. for 3 hours to obtain 50.8 g of a white solid product with a moisture content of 0.18%, an HPLC purity of 99.25%, and a molar yield of 91.30%.
Embodiment 3
[0040] Add ethyl acetate (500ml) and DDC (50g, 117.82mmol) to a 1000ml three-necked flask in sequence, then control the temperature T1 = 10-15°C, and drop 15mL of CSI in 10 minutes. Control temperature T2=15-20°C, add 2.5g of activated carbon, stir and react for 30min, then filter. Cool the filtrate to about 5°C, then add 70 mL of purified water dropwise for 5 minutes, then add NaHCO dropwise 3 Solution (53gNaHCO 3+ 284mL water), dripped in 30min. The liquid was separated, the ethyl acetate layer was discarded, 30% HCl was added dropwise, the pH was adjusted to 2.0, and the temperature was lowered to T3=0-5°C to grow crystals for 1 h. After suction filtration, the filter cake was washed twice with 50 mL of water each time, sucked dry, and dried in vacuum at 50° C. for 3 h to obtain 49.5 g of a white solid product with a moisture content of 0.16%, an HPLC purity of 99.00%, and a molar yield of 88.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com