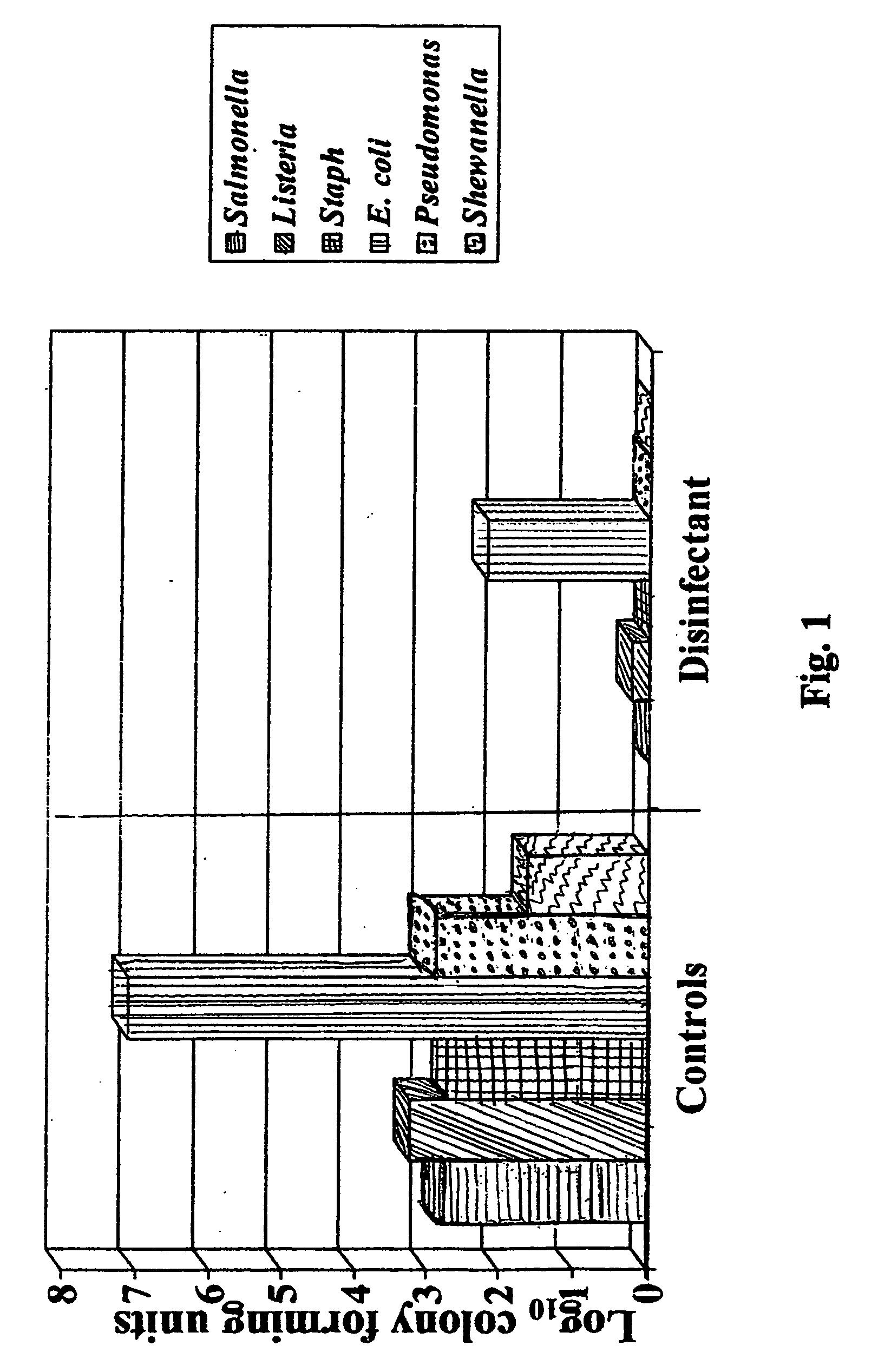
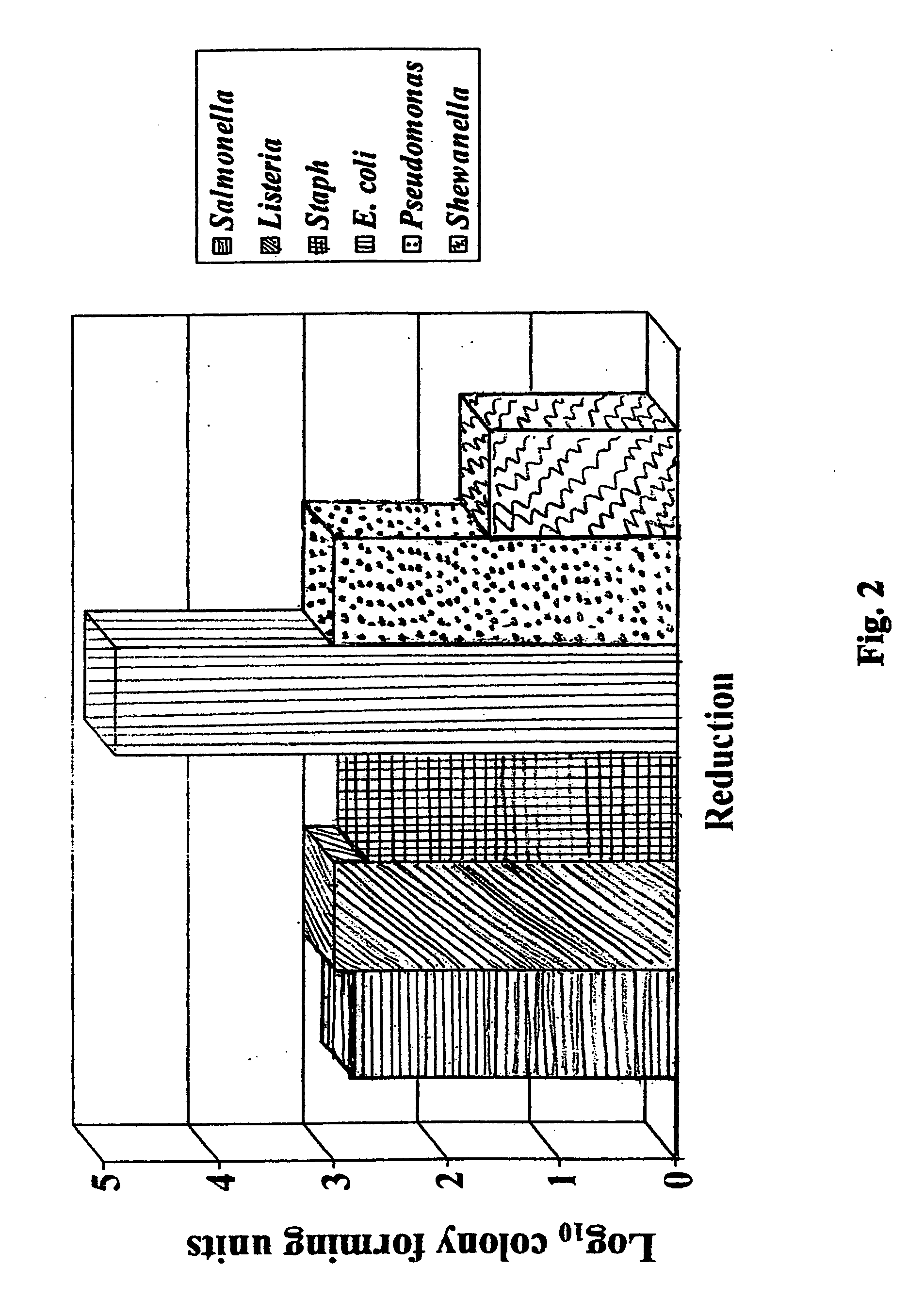
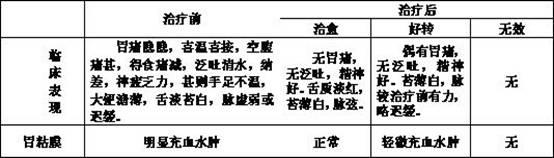
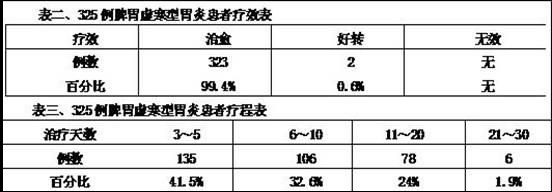
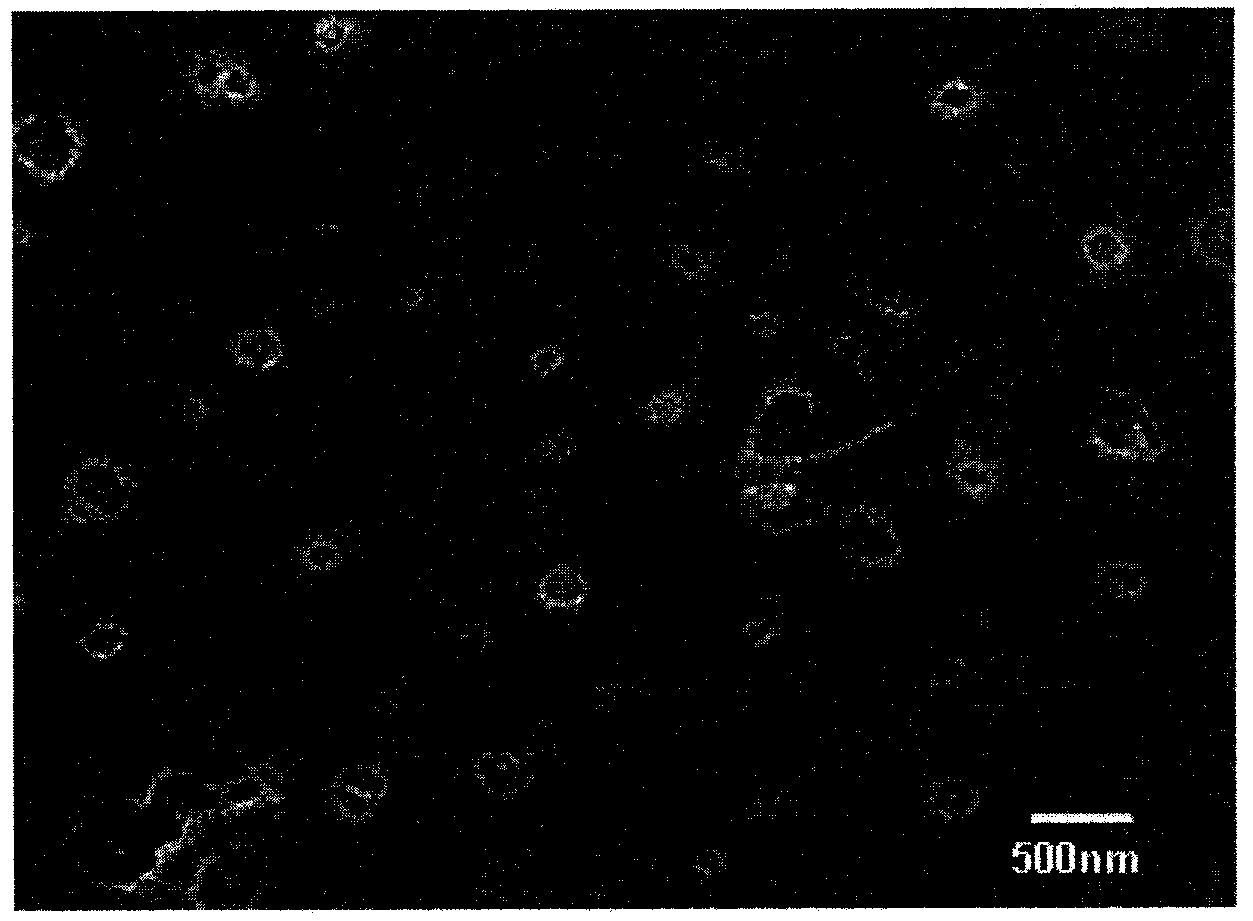
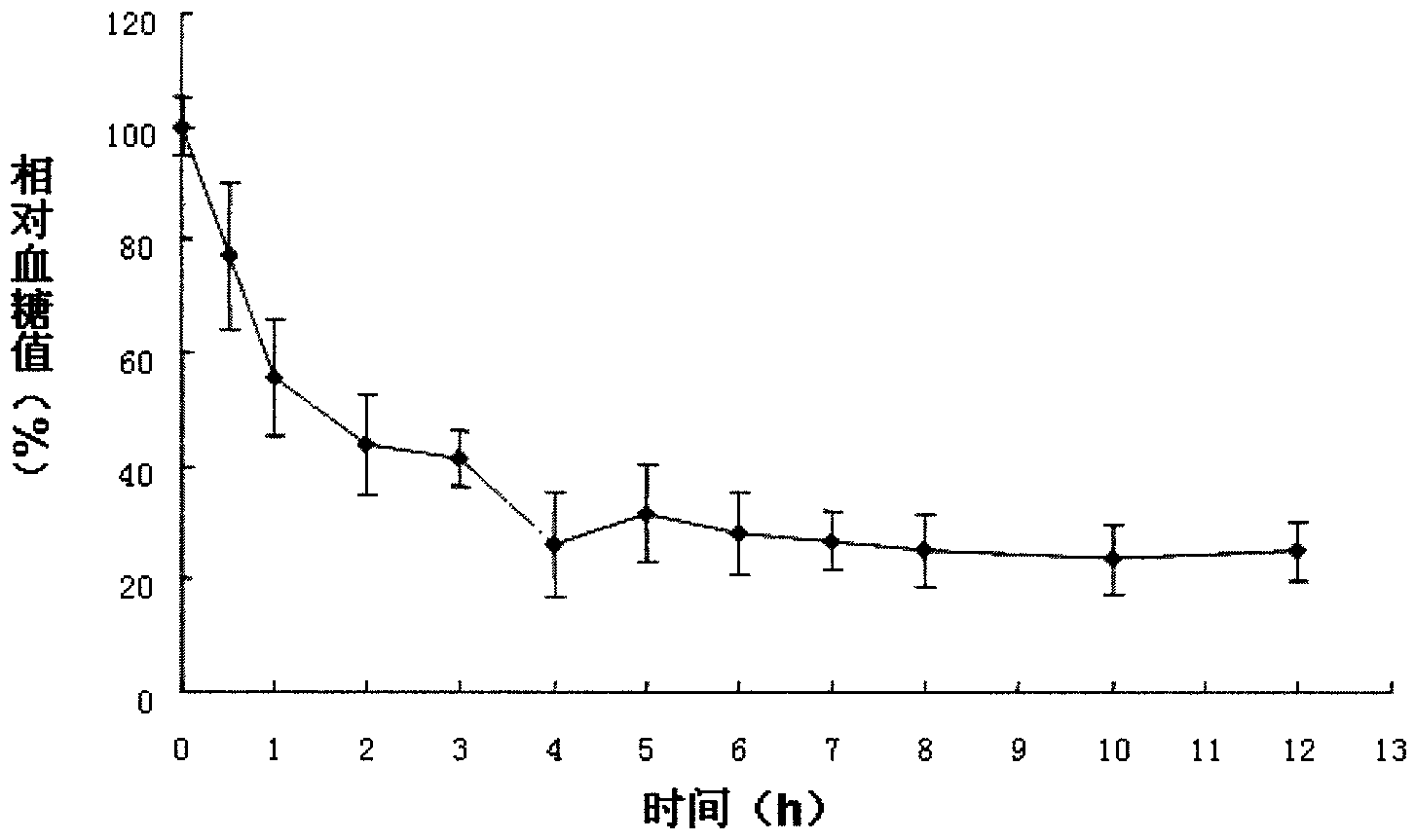
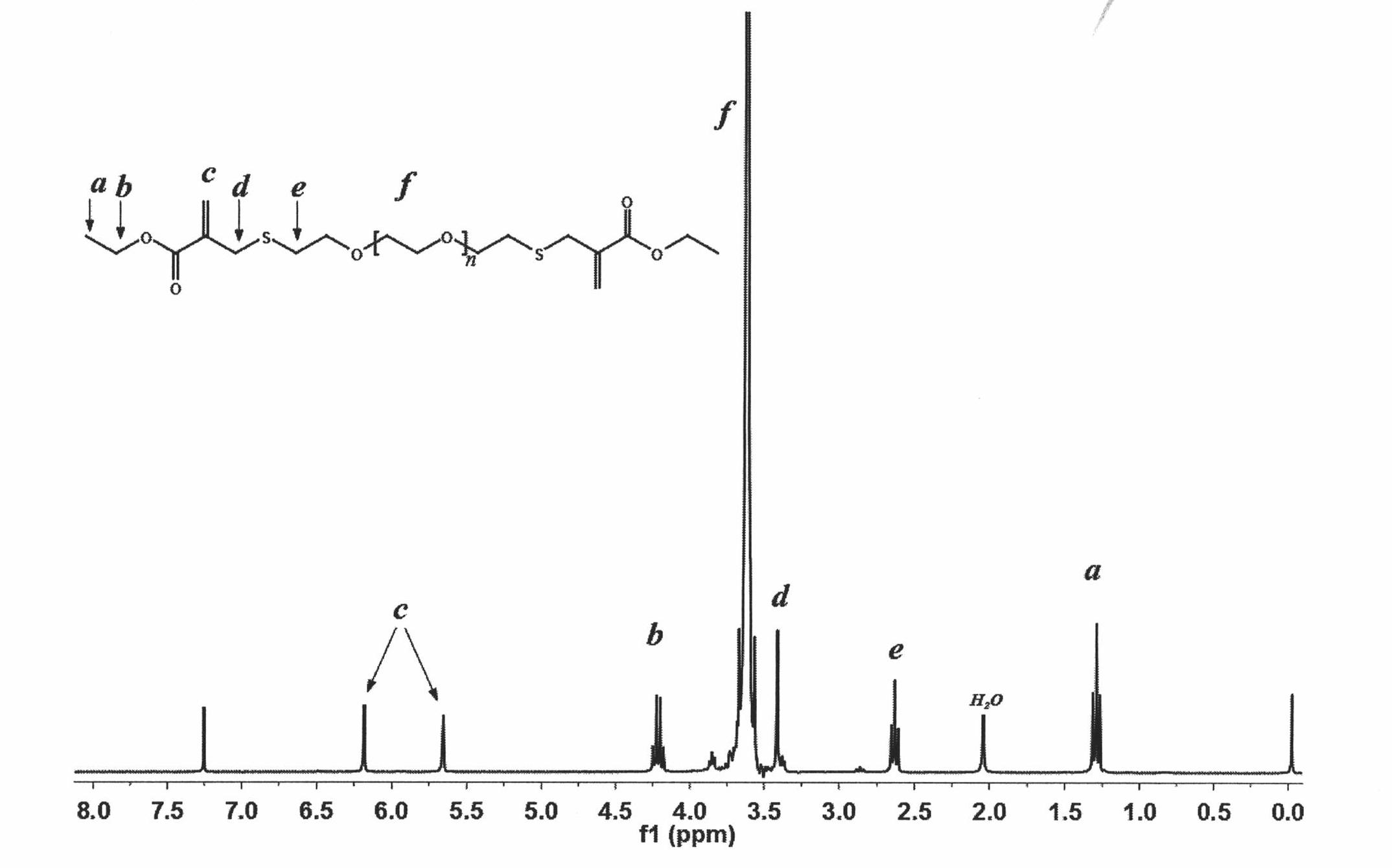
Patents
Literature
311 results about "Toxic reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Topics under Toxic Reactions Incl Drug and Substance Abuse. Alcoholism (34 drugs in 12 topics) Anticholinergic Syndrome (2 drugs) Barium Poisoning (2 drugs) Benzodiazepine Overdose (4 drugs in 2 topics) Digitalis Glycoside Toxicity (2 drugs) Drug Dependence (28 drugs in 6 topics) Extrapyramidal Reaction (50 drugs in 3 topics)
Method and form of a drug delivery device, such as encapsulating a toxic core within a non-toxic region in an oral dosage form
InactiveUS7276252B2Promote absorptionSmall crystal sizeBiocideAdditive manufacturing apparatusHazardous substanceBioavailability
A drug delivery device such as an oral dosage form (ODF) with a toxic or potent core encapsulated by a non-toxic region. The non-toxic region may be a region including multiple layers, coatings, shells, and combinations thereof, which provides protection to and isolation from the toxic or potent core. The drug in the toxic or potent core is incorporated into the dosage form via, for example, three-dimensional printing, as a solution, solubilization or suspension of solid particles in liquid, rather than by the more conventional handling and compressing of dry powder. This minimizes the likelihood of creating airborne particles of the toxic drug during manufacturing, hence controlling and minimizing the exposure of manufacturing personnel to the hazardous substance. Wet dispensing of the toxic or potent drug further provides greater bioavailability of the drug to the patient.
Owner:APRECIA PHARMA LLC +1
Hangover remedy and alcohol abatement composition
InactiveUS20050191395A1Easy to carryRelieve pressureHeavy metal active ingredientsFood preservationNausea sicknessFood additive
An anti-intoxication composition and method of making and using are provided for treating and preventing drinker's remorse and other toxic effects of excessive alcohol consumption by humans. The anti-intoxication composition is an acidic mixture containing sweeteners, flavoring agents, food additives and a processed mixture of metallic salts, sulfuric acid, ammonium sulfate and water. When the anti-intoxication composition is ingested, it promotes the beneficial metabolism of alcohol, primarily ethanol, in the body. The beneficial metabolism results in the conversion of alcohol to amino acids and eliminates the toxic reactions of a hangover, including, but not limited to, a pounding headache, nausea, dry mouth, shaking hands, hypersensitivity to bright lights and sounds.
Owner:TASKER PRODS IP HLDG CORP
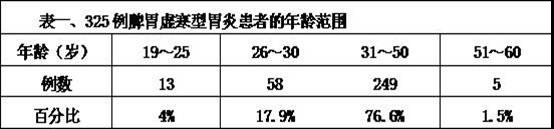
Preparation method of traditional Chinese medicine used for treating gastritis caused by insufficiency of spleen-yang
InactiveCN102366615ASmall side effectsEasy to makeAnthropod material medical ingredientsDigestive systemPolygonum fagopyrumSide effect
The invention belongs to the technical field of traditional Chinese medicine preparation method, and provides a preparation method of a traditional Chinese medicine used for treating gastritis caused by insufficiency of spleen-yang. According to prior arts, gastritis caused by insufficiency of spleen-yang is commonly treated by using omeprazole, which causes adverse reactions. The technical scheme of the invention is that: traditional Chinese medicines of ginseng, human milk, common jujube, common yam rhizome, wheat, different leaves pseudostellaria root tuber, balck-bone silky fowl, wild cabbage, dried longan pulp, largehead atractylodes rhizome, radix paeoniae alba, American ginseng, ligusticum, lotus seed, pilose asiabell root, sharpleaf galangal fruit, mongolian milkvetch root, Siberian solomonseal rhizome, nonglutinous rice, edible bird nest, dried ginger, bayberry, garden radish seed, carica papaya, roof iris rhizome, carrot, buckwheat, medicated leaven, cydonia oblonga mill, cerealose, honey, and licorice are soaked in water, and are decocted by using a mild fire; the obtained solution is filtered, a slag is removed, and the obtained medicine liquid is the traditional Chinese medicine used for treating gastritis caused by insufficiency of spleen-yang. The method is advantaged in that: the preparation method is simple; toxic and side-effects of the traditional Chinese medicine liquid are low; a treatment course is short; and a recovery rate is high. According to the invention, a good effect can be obtained with the cooperation of monarch and minister medicines. A traditional Chinese medicine preparation is adopted, such that adverse reactions, allergic reactions and toxic reactions caused by western medicines are avoided.
Owner:孟德芹
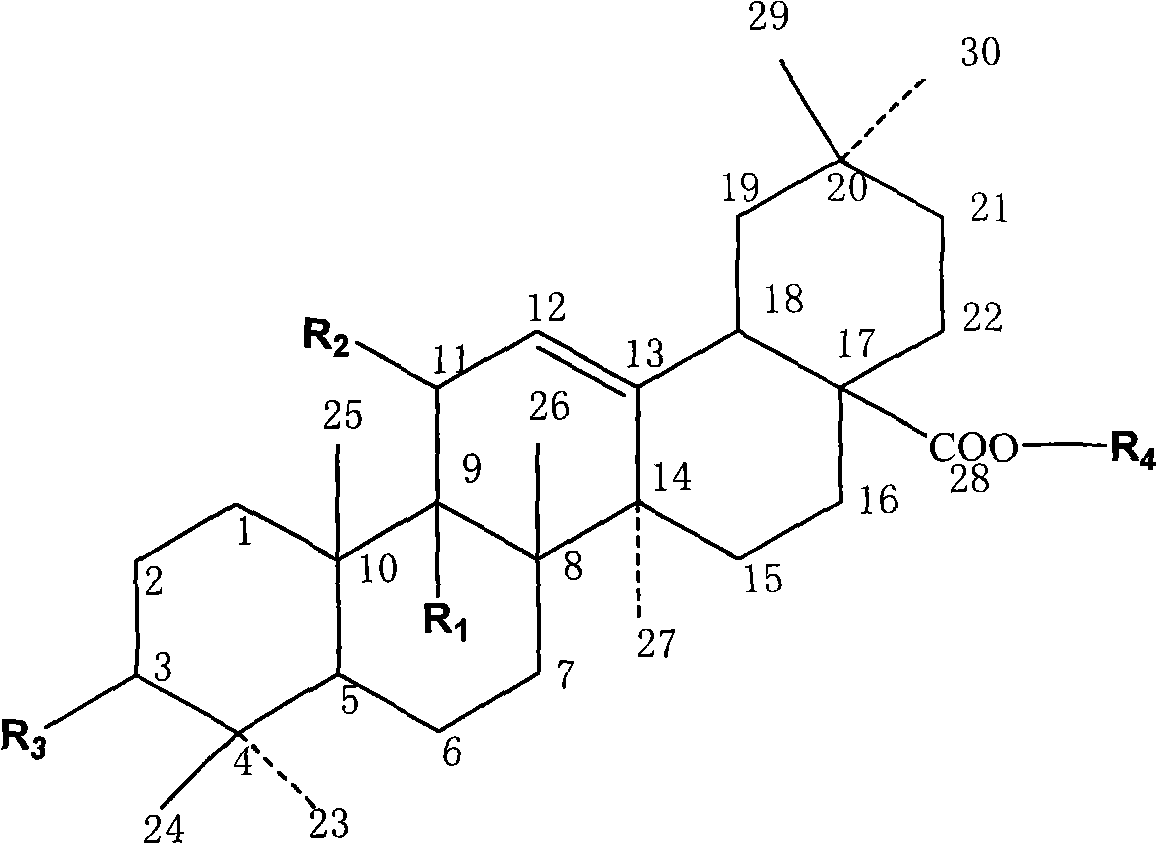
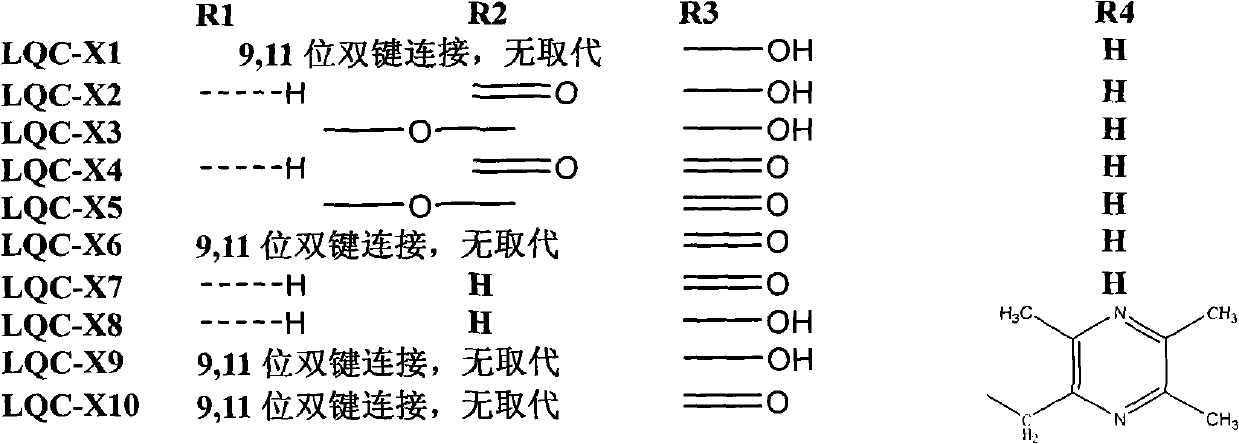
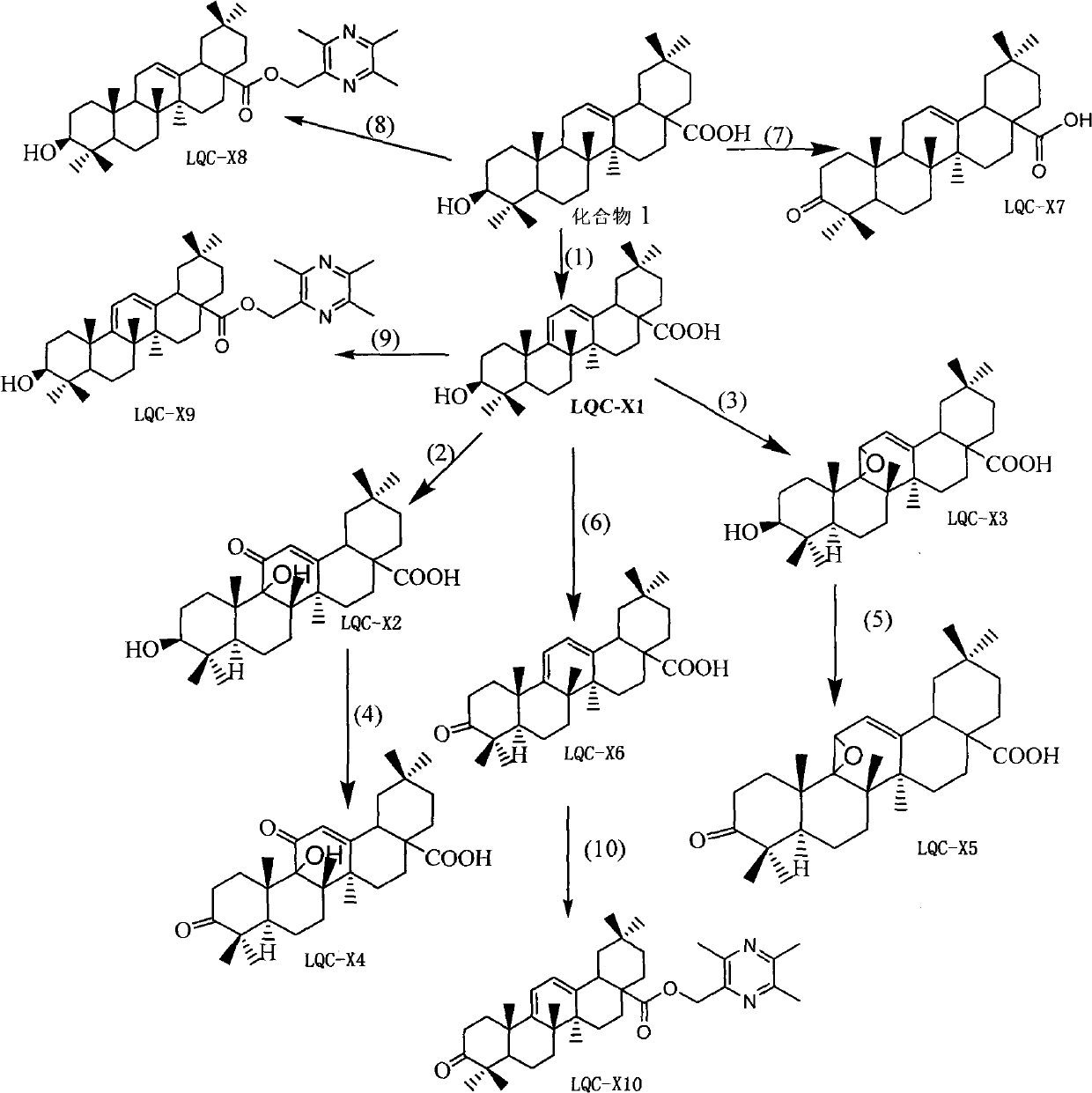
Synthesis of anti-hepatitis B medicine LQC-X and application thereof
The invention relates to the field of chemical and biological sciences, in particular to LQC-X structural formula I, and synthesis and application of intermediate of LQC-X, wherein a maximum dosage of LQC-X6 given to an mouse is 2000 mg / kg once in one day; the mouse is continuously observed for 7 days; no toxic reaction appears, and the medicine is proven to have quite high safety; and it is proved through pharmacodynamics experiments that LQC-X compounds have obvious anti-hepatitis B virus action and liver protecting and transaminase lowering action. The compounds can be used for preparing medicines for preventing and treating diseases of hepatitis B, chronic liver cirrhosis and the like. The structural formula I of LQC-X is shown in the description.
Owner:北京鸿测科技发展有限公司
Anti-cancer medicine composition
InactiveCN1616099AAntineoplastic agentsPharmaceutical active ingredientsWhole bodyTherapeutic effect
The present invention relates to an anticancer medicine composition and belongs to the field of medicine technology. The anticancer medicine composition contains topomerase inhibitor and medicinal supplementary material. The topomerase inhibitorcan inhibit intracellular DNA repair function and reduce the tolerance one tumor cell on anticancer nitrosourea medicine; and the medicine supplementary material is mainly degradable biocompatible polymer. During the degrading and absorption process of the supplementary material, the anticancer medicine is released slowly to the tumor part, and this can lower the systemic toxic reaction while maintaining local effective medicine concentration. The anticancer medicine composition contains also anticancer nitrosourea medicine, and is set to the tumor part locally to enhance the treatment effect.
Owner:孔庆忠
Insulin nano transdermal patch and preparation method thereof
InactiveCN103705494AHigh drug loadingHigh local concentrationPeptide/protein ingredientsMetabolism disorderTransdermal patchTreatment effect
The invention relates to an insulin nano transdermal delivery preparation and a preparation method thereof. The insulin nano transdermal delivery preparation takes recombinant human insulin as an active pharmaceutical ingredient and is a filled closed type transdermal preparation consisting of an anti-sticking layer, a drug-loaded layer and a back lining layer. The drug-loaded layer consists of nano recombinant human insulin, a carrier, a penetration enhancer and a plasticizer. The nano recombinant human insulin is obtained by dissolving insulin in a solvent, adding water-soluble auxiliary agents including liposome, chitosan, HPMC (hydroxy propyl methyl cellulose) and the like and an aqueous solution of a freeze-drying protective additive, performing low-temperature high-speed stirring and then performing freeze-drying. The patch disclosed by the invention realizes transdermal noninvasive administration through nano-scale combination of insulin and the carrier to ensure that the bioavailability of the insulin is greatly improved; the patch has the advantages of high transdermal absorption rate, fast response, good treatment effect and good product stability, does not have anaphylactic and toxic reactions to skins, and has the characteristics of good safety, small side effect and convenience in use. The preparation method of the insulin nano transdermal patch disclosed by the invention is simple, low in production cost, and easy to magnify.
Owner:HUBEI UNIVERSITY OF MEDICINE
Application of in-situ crosslinking hydrogel capable of intraocular injection in preparing artificial vitreous bodies
The invention discloses an application of in-situ crosslinking hydrogel in preparing artificial vitreous bodies, which is characterized in that the in-situ crosslinking hydrogel is prepared by in-situ crosslinking after mixing a four-arm-end mercapto polyethylene glycol solution shown in the formula (I) and a polymer solution shown in the formula (II); wherein m in the formula (I) is an integer larger than 1, and n in the formula (II) is an integer larger than 1; and the two solutions are filled in eyes without vitreous bodies after mixing and can effectively form gel. A cell experiment and an animal in vivo experiment prove that the in-situ crosslinking hydrogel does not cause obvious inflammatory reaction and intraocular organ toxic reaction and can be used as the artificial vitreous bodies.
Owner:PEOPLES HOSPITAL PEKING UNIV
Atropine sulfate in situ forming eye gel
InactiveCN101327216AVarious dosage formsEliminate side effectsSenses disorderPharmaceutical delivery mechanismSide effectAtropine sulfate
The invention discloses an instant atropine sulphate eye gel and a preparation method thereof. According to the invention, 1000ml of the instant atropine sulphate eye gel contains 1g to 20g of atropine sulphate, 0.05g to 1g of preservative, 1.0g to 7.5g of osmotic pressure adjusting agent, and 30g to 150g of gel stroma, and the rest includes acid-alkali buffer and water for injection. The invention, through the optimization of accessories and the improvement of the technique, enriches the pharmaceutical dosage form of the atropine sulphate, greatly prolongs the time for which the medicine stays in the eyes, and not only improves the curative effect and shortens the time for reaching the function peak, but also reduces the amount of the medicine flowing into and absorbed by dacryocysts and relieves the side effect and toxic reaction of the atropine sulphate.
Owner:肖正连
Artificial liver tissue and preparation method thereof
ActiveCN106581761AAccurate Pathological ResearchAccurate Drug Toxicity Test ScreeningHepatocytesArtificial cell constructsArtificial liverCell-Extracellular Matrix
The invention provides an artificial liver tissue and a preparation method thereof. The artificial liver tissue comprises a lower chip, a first microporous membrane, a middle chip, a second microporous membrane and an upper chip from the top to the bottom in sequence in a laminated manner. Three layers of cavities of a lower cavity, a middle cavity and an upper cavity are formed in the lower chip, middle chip and upper chip respectively. Three cavities are all grouted with culture solution containing growth factor, extracellular matrix containing hepatic parenchymal cells and suspension liquid containing endothelial cells. Adjacent cavities are separated by the microporous membrane. The microporous membrane can separate the matrix flow outside of the cell, and cause no influence to the cell migration. The culture solution and is fed through an inlet and an outlet of the upper cavity and lower cavity. Under the guidance of the growth factor, endothelial cells pass through the extracellular matrix of the middle cavity to form a lumen, and form the artificial liver tissue containing capillaries. The artificial liver tissue can simulate the human body liver tissue in a good way, and simulate the intrahepatic capillary functions of mass transfer, selective permeability, toxic reaction and the like.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Solid tumor treating medicine composition
The present invention is solid tumor treating medicine composition and belongs to the field of medicine technology. The medicine composition contains melphalan in effective anticancer amount and medicinal supplementary material. The medicinal supplementary material is mainly biocompatible, degradable and absorbable polymer, and during the degradation and absorption of the polymer, melphalan is released slowly to local tumor part to lower the systemic toxic reaction and to maintain local medicine concentration. The present invention has enhanced treating effect. The solid tumor includes cerebral tumor, liver cancer, lung cancer, esophagus cancer, gastric cancer, etc.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Tumor tissue tumor infiltrating lymphocyte (TIL) cell preparation method and dedicated culture medium
ActiveCN107384867AImprove scalabilityIncrease the amount of amplificationCell dissociation methodsCulture processAdditive ingredientSodium phosphates
The invention relates to a tumor tissue tumor infiltrating lymphocyte (TIL) cell preparation method and a dedicated culture medium. The method comprises the following steps of tumor surrounding tissue obtaining, cell digestion, cell primary culture, cell subculture and cell collection, wherein a primary culture medium is based on a RPMI 1640 culture medium and prepared from the following concentration ingredients of 10% volume of human-derived serum, 20 to 45ng / ml of basic fibroblast growth factor (bFGF), 1 to 5mg / ml of riboflavin, 70 to 90ng / ml of cortisol, 10 to 25mg / ml of sodium dihydrogen phosphate monohydrate, 47 to 62ng / ml of recombinant human leukaemia inhibitory factor (LIF) and 500 to 800U / ml of IL-2; a subculture medium is based on the RPMI 1640 culture medium and prepared from the following concentration ingredients of 10% volume of the human-derived serum, 20 to 40mmol / L of HEPES, 1000 to 2000U / ml of the IL-2, 0.03 to 0.07mmol / L of beta-mercaptoethanol and 5 to 15ng / ml of sodium phosphate. According to the preparation method, an existing culture medium is improved, different culture mediums are utilized to culture the TIL cells in pertinence, TIL cell expansion capacity is improved, meanwhile a culture period is reduced, a culture complexity degree is reduced, a use amount of the IL-2 is reduced, and toxic reaction is reduced.
Owner:CENTURY BIOSTRENGTH BEIJING PTY LTD
Slow-released injection containing methotrexate and its synergist
The slow released anticancer injection containing methotrexate and its synergist consists of slow released microballoon and solvent. The slow released microballoon includes effective anticancer component and slow releasing supplementary material, and the solvent is common solvent or special solvent containing suspending agent. The effective anticancer component is methotrexate synergist or the composition of methotrexate and its synergist; the methotrexate synergist is selected from phosphorinositide 3-kinase (PI3K) inhibitor, pyrimidine analogue and DNA repair enzyme inhibitor; the slow releasing supplementary material is PLA, PLGA, EVAc, etc or their composition; and the suspending agent is sodium carboxymethyl cellulose, etc. The slow released microballoon may be also prepared into slow released implantation preparation. Implanting or injecting the slow released preparation to local tumor part can lower the systematic toxic reaction of the medicine and raise the medicine concentration of local tumor part selectively to raise the treating effect.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer medicine composition containing antimetabolite
InactiveCN1634584AAntineoplastic agentsHeterocyclic compound active ingredientsWhole bodyTherapeutic effect
An anticancer pharmaceutical composition composed of pharmaceutic adjuvant and anti-metabolism medicine is disclosed. Wherein, the anti-metabolism medicine can effectively destroy DNA and / or protein synthesis and repairing function inside the tumor cell so as to inhibit the tumor cell growth, while the pharmaceutic adjuvant can mainly be biological compatible, degradable and absorbable macromolecule polymer, which can make the anti-metabolism drug to release slowly in the local tumor region in the degradation and absorption process, therefore it can both decrease considerably the whole body toxic reaction and sustain the local tumor effective drug level.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
A medicament containing effective components of glabrous sarcandra herb, its preparation method and application
InactiveCN1879672AHigh purityGood curative effectAntibacterial agentsAntineoplastic agentsChemical synthesisAlcohol
The invention relates to a medicine with effective component of plant nodicorn, which is characterized in that: said effective components can be polyphenol and monomer caffeic acid 3, 4- dihydroxyphenylglycol; the monomer caffeic acid 3, 4- dihydroxyphenylglycol is extracted and purified from nodicorn and other plants; the preparation of polyphenol and the extraction of monomer caffeic acid 3, 4- dihydroxyphenylglycol comprise: refluxing and extracting alcohols in different densities, boiling, extracting butanol, macroreticular resinous adsorbent chromatography and positive and passive silicon gel chromatography. The invention can treat cancer, attenuate, and treat general bacteria infection.
Owner:连晓媛
Anti-cancer composition containing bendamustine
An anti-cancer composition comprises anti-cancer effective component selected from tyrosine kinase inhibitor and / or bendamustine and slow releasing adjuvant, and can be prepared into slow releasing injection and slow releasing implantable agent. The slow releasing injection also comprises special dissolvant containing suspending agent. The Suspending agent has a viscocity of 100cp-3000cp (at 20deg.C-30deg.C) and is selected from sodium carboxymethylcellulose, and so on. the slow releasing adjuvant is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p(BHET-EOP / TC), p(BHDPT-EOP / TC), p( BHDPT-EOP / TC), p(CHDM-HOP) or p(CHDM-EOP.Slow releasing injection and implantable agent can keep high medicinal concentration in tumour part through slow releasing for over 60 days after being injected or implanted in or around tumour. The anticancer composition may also be prepared into sustained-release implant. It can reduce systemic toxic reaction of anticancer drugs, and also selectively improve the therapeutic effect of non-operative therapy such as chemotherapy.
Owner:JINAN KANGQUAN PHARMA TECH
Neratinib sustained-release implant for treating solid tumor
InactiveCN101185633AOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerTherapeutic effect
A sustained release implant includes 0.1%-50% (w / w) nilotinib, 50-99% sustained release excipients and 0-15% sustained release moderator. Sustained release excipients are mainly one or combination of poly (L-lactide-co-ethyl phosphate), poly (L-lactide-co- phosphoric acid propyl), polylactic acid, the copolymer of polylactic acid and hydroxyacetic acid and polifeprosan; sustained release moderator is one or combination of mannitol, sorbic alcohol and chondroitin; sustained release implant applied in local tumor can slowly release nilotinib onto local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction; the invention not only reduces overall toxic reaction of nilotinib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy. The implant can be used for treating solid tumors including lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, pancreatic cancer, bladder carcinoma, cerebroma, and colorectal cancer.
Owner:SHANDONG LANJIN PHARMA +1
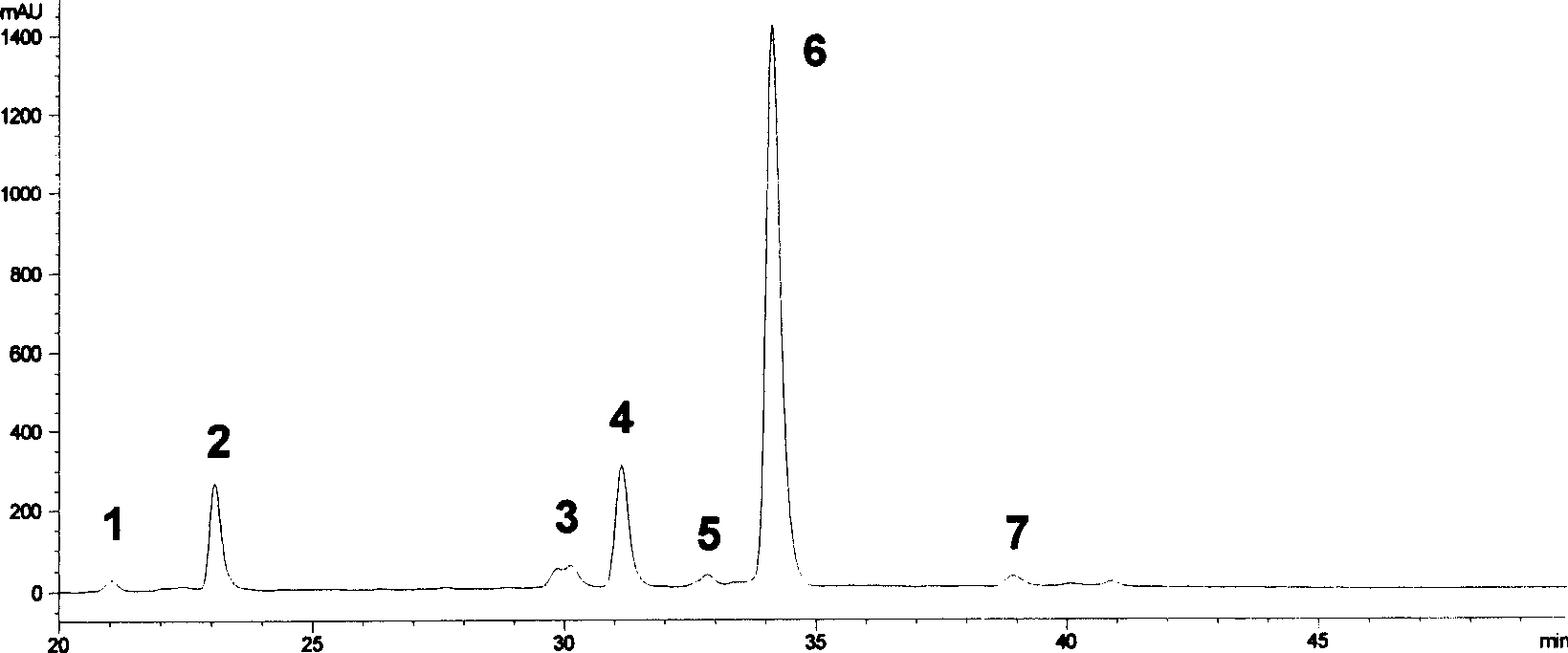
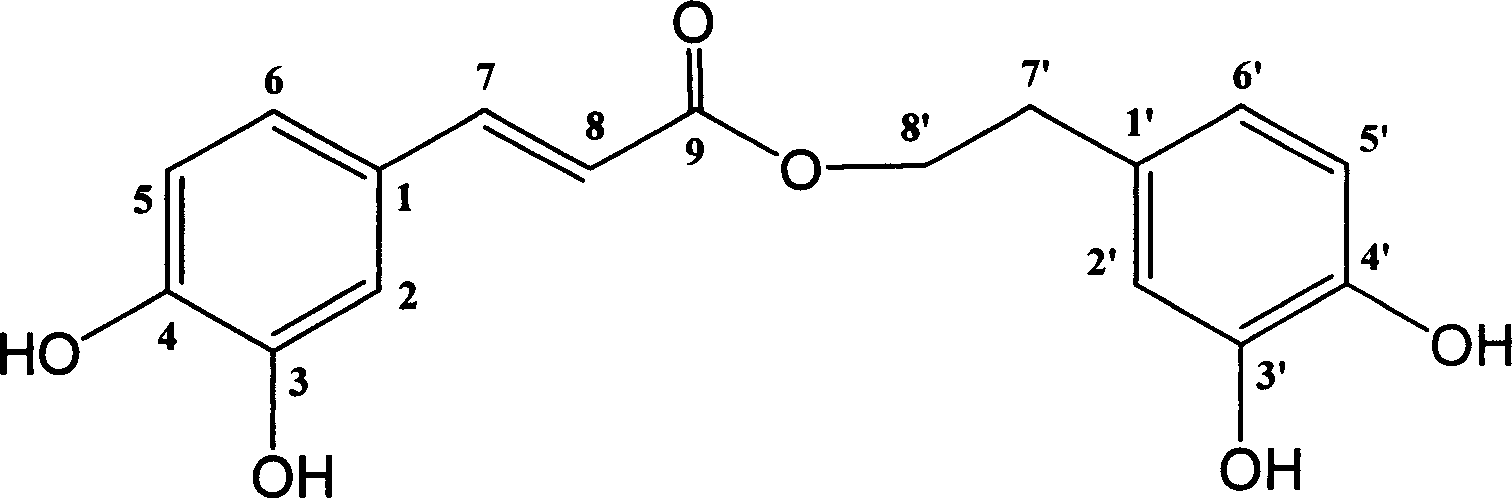
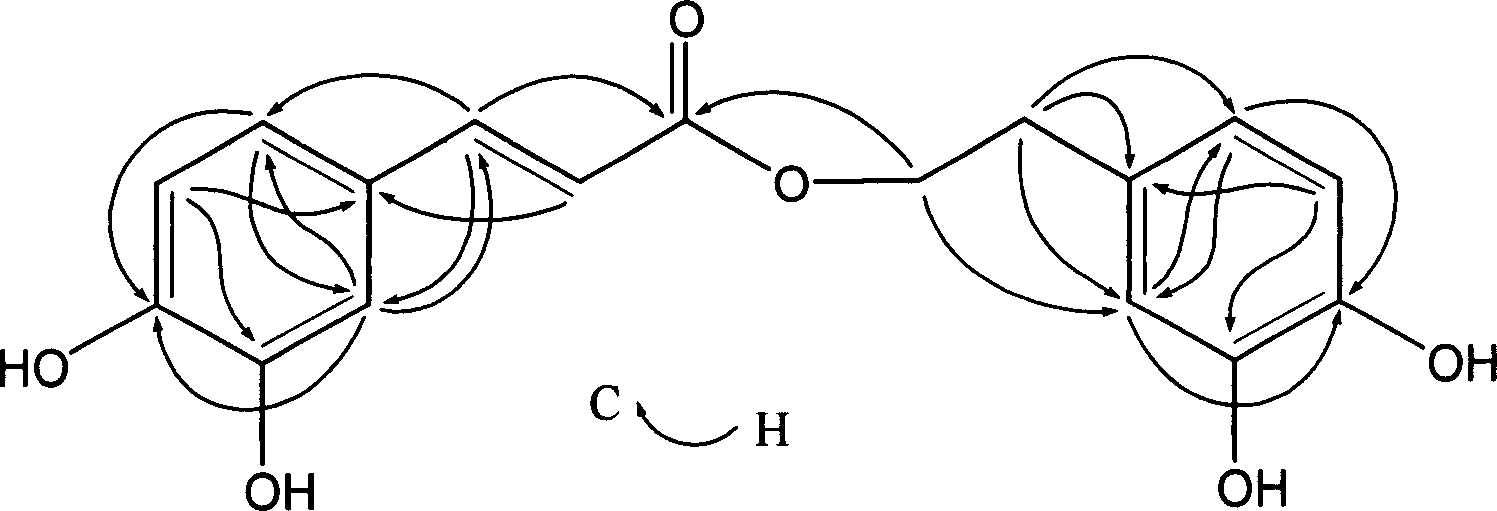
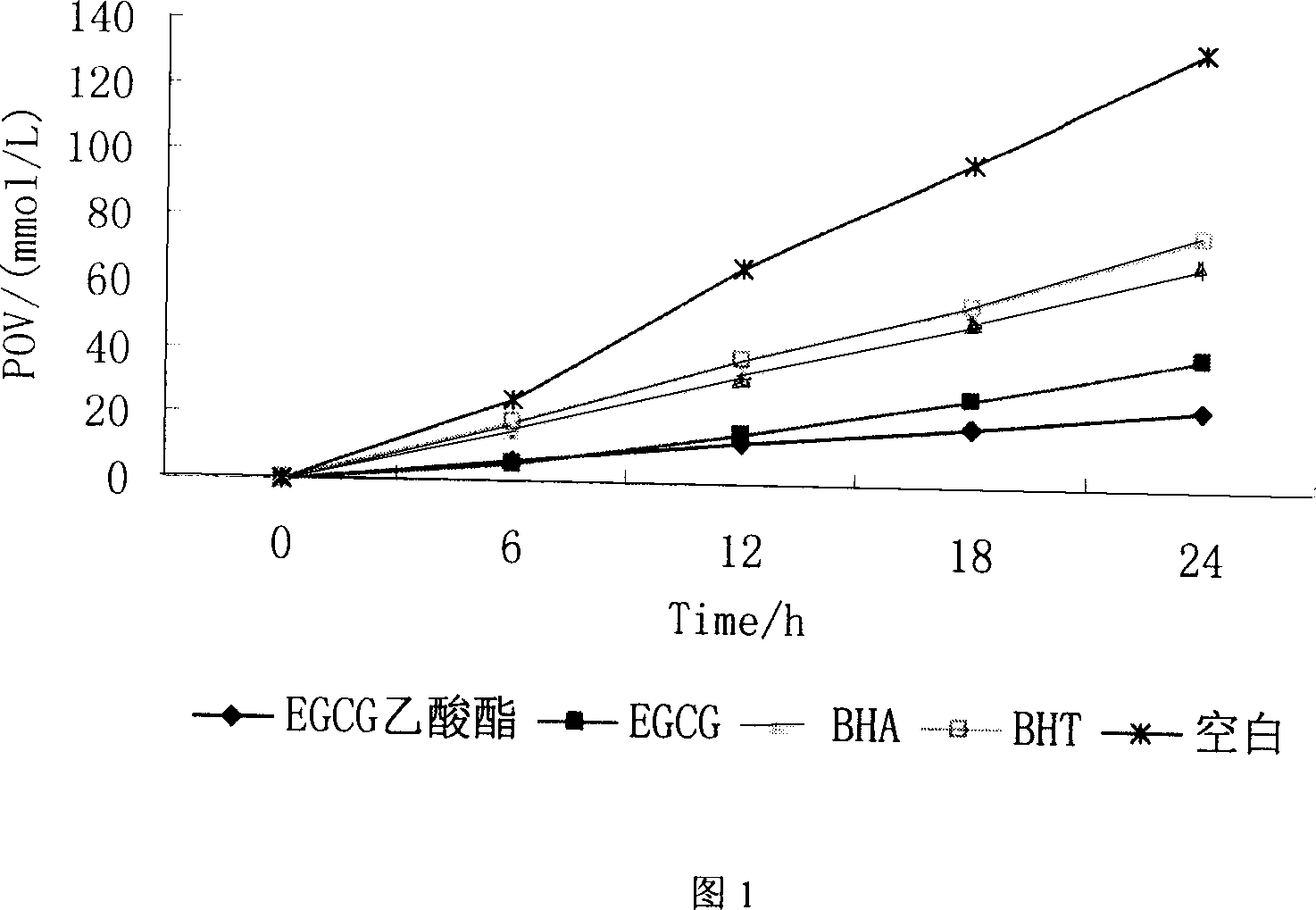
Method for synthesizing EGCG fatty acid ester catalyzed by immobilized enzyme
InactiveCN101086000AEasy to useHigh reaction conversion rateMicroorganism based processesFermentationOrganic solventVinyl ester
The invention relates to a method for preparing EGCG fatty acid ester by using immobilized lipase, belonging to enzyme catalysis field. It comprises following steps: taking catechin EGCG monomer and aliphatic acid or fatty acid vinyl ester containing 2- 18 carbon atoms as raw material, mixing with sifted organic solvent and immobilized lipase according to molar ratio of 1: 1- 10, the enzyme consumption amount is 0.5- 3 times of that of EGCG, reacting for 36- 200 hours at 30 -50 Deg. C, taking out catalyst, filtering raction liquid, decompressing and condensing, removing solvent and drying and getting EGCG fat lipase. The invention is characterized by good selectivity, high conversion rate, non toxic reaction solvent, definite EGCG lipase component, high content of monosubstituted product, good anti- oxidizing performance and stable structure.
Owner:BEIJING UNIV OF CHEM TECH
Composition for teriparatide injection, and preparation method and preparation thereof
InactiveCN103301058ANo degradationGuaranteed stabilityPowder deliveryPeptide/protein ingredientsPen InjectorTeriparatide
The invention relates to the technical field of medicaments, and particularly relates to a composition for teriparatide injection, and a preparation method and preparation thereof. The composition for teriparatide injection comprises teriparatide, a freezing and drying protecting agent and a pH regulator, wherein the mass ratio of the teriparatide to the freezing and drying protecting agent is 1:(10-100000). The composition for teriparatide injection is simple in formula; the freezing and drying protecting agent ensures that the teriparatide is not degraded in preparation and preservation processes; and the pH regulator is used for regulating the pH value in a preparation process and regulating the pH value after the composition for teriparatide injection is redissolved, so as to maintain the stability of the teriparatide. The used auxiliary materials are safe, and do not cause toxic reaction. Therefore, the composition for teriparatide injection provided by the invention is good in stability, simple and reasonable in formula, and good in redissolving performance, and can be prepared into powder-injection or injection and is needless to be prepared into a pen-type injector. Thus, the production technology is simple.
Owner:HYBIO PHARMA
Anti-cancer medicine composition
The present invention relates to an anticancer medicine composition and belongs to the field of medicine technology. The anticancer medicine composition contains tetrazine medicine and medicinal supplementary material. The tetrazine medicinecan inhibit intracellular DNA repair function and reduce the tolerance one tumor cell on anticancer nitrosourea medicine; and the medicine supplementary material is mainly degradable biocompatible polymer. During the degrading and absorption process of the supplementary material, the anticancer medicine is released slowly to the tumor part, and this can lower the systemic toxic reaction while maintaining local effective medicine concentration. The anticancer medicine composition contains also anticancer nitrosourea medicine, and is set to the tumor part locally to enhance the treatment effect.
Owner:广州多福医药科技有限公司
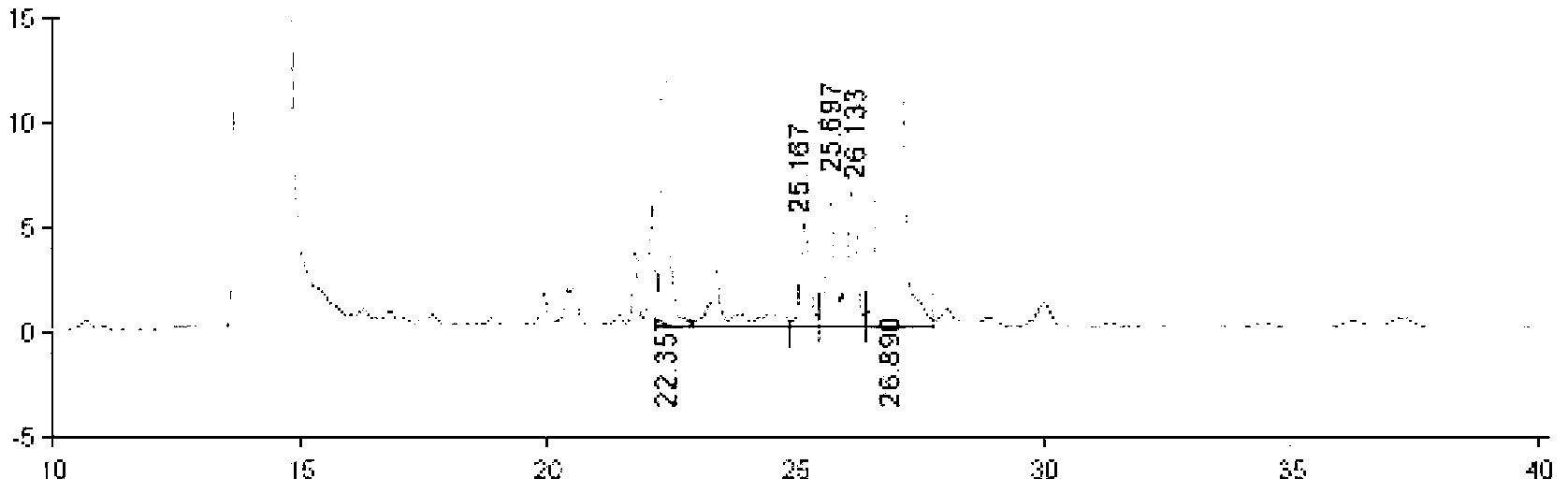
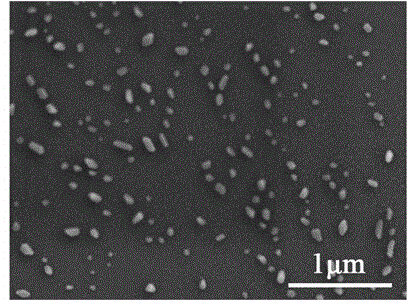
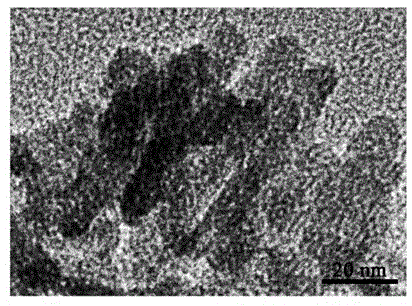
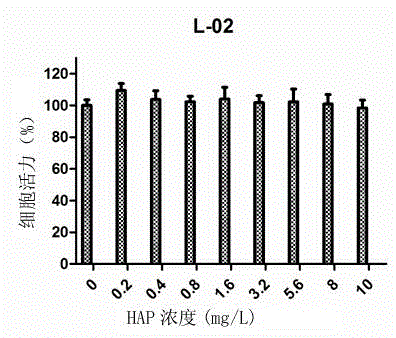
Method for quickly preparing polyethylene glycol regulated and controlled nano hydroxyapatite
InactiveCN104528676AGood dispersionLarge specific surface areaPhosphorus compoundsDispersityPhosphate
The invention discloses a method for quickly preparing polyethylene glycol regulated and controlled nano hydroxyapatite and belongs to the field of preparation of biomedical materials. The method comprises the following process steps: preparing a calcium salt solution and a phosphate solution respectively, and mixing the calcium salt solution and the phosphate solution with equal volumes; adjusting the pH value of a reaction system by using ammonia water while stirring; adding polyethylene glycol, and heating the reaction system; and performing centrifuging and washing after reaction, and collecting granular deposits to obtain nano hydroxyapatite granular powder. The method disclosed by the invention is simple in preparation process, strong in laboratory operability, low in production cost and high in product yield and is suitable for laboratory research application and industrial production; and moreover, the prepared rod-shaped nano hydroxyapatite has good dispersity, does not have toxic reaction to normal cells and cancer cells, and has a huge application potential in the fields of biomedicine and biological materials such as drug controlled-release vectors, gene therapy vectors and the like.
Owner:ZHEJIANG SCI-TECH UNIV
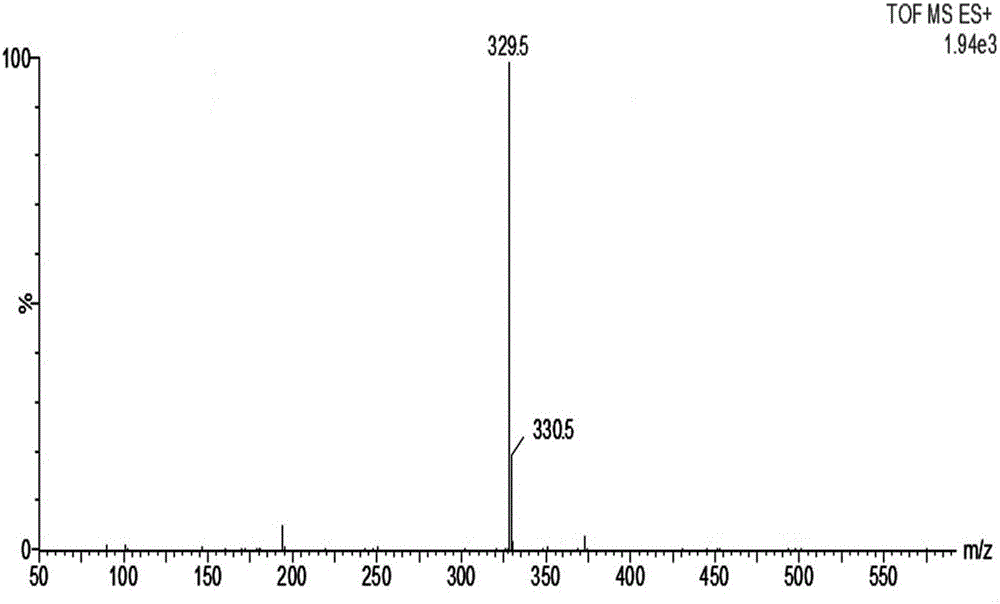
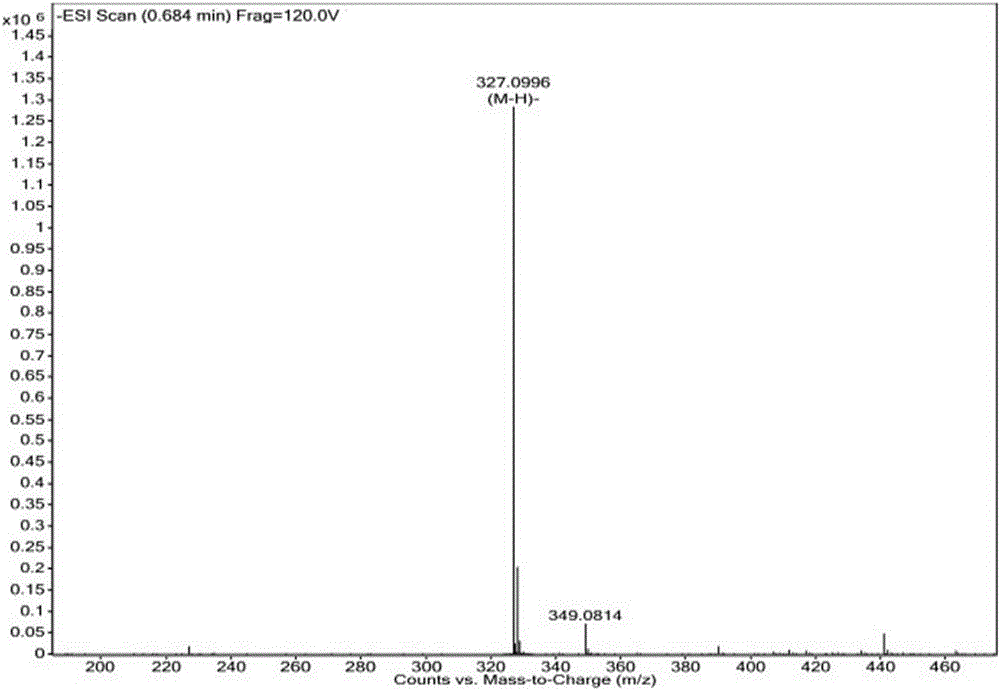
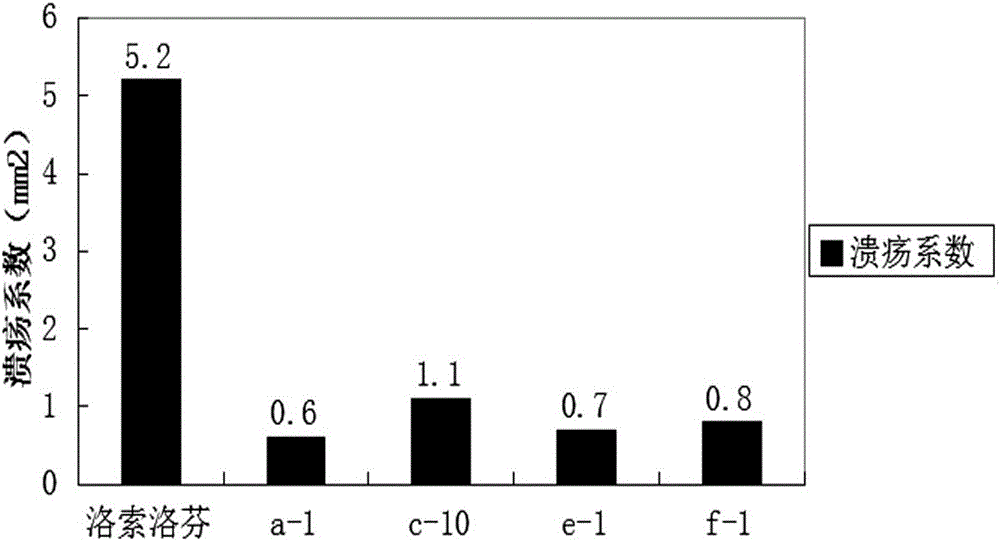
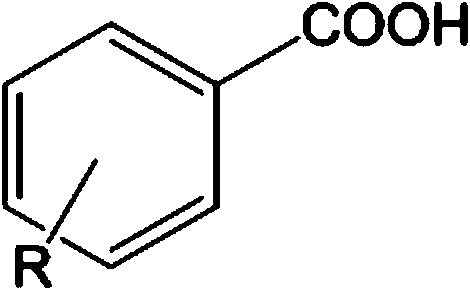
Aryl propionic acid derivative composition and pharmaceutical purpose
ActiveCN106661061ALess irritatingImprove medication complianceOrganic active ingredientsAntipyreticMetabolitePhosphate
The invention discloses an aryl propionic acid derivative composition and a pharmaceutical purpose. Alcohol metabolites of Loxoprofen having highest activity in metabolism is used as a nuclear parent to produce a series of derivatives of phosphate, sulphonate, carbonic ester, and amino-acid ester. The derivatives are used to enhance pesticide effect of anti-inflammatory and analgesic effects, and reduce toxic reaction, and have good pharmacokinetic property. And at the same time, the stimulation of the medicine on the gastrointestinal tract, and medication compliance of patients is improved, and therefore the aryl propionic acid derivative composition is the non-steroidal anti-inflammatory drugs having potentials.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
Chinese medicinal compound preparation for resisting virus and treating flu and cold
InactiveCN102526355ADefinite curative effectSmall side effectsAntiviralsPlant ingredientsAcute toxicity testingBaical Skullcap Root
The invention relates to a Chinese medicinal compound preparation for resisting virus and treating flu and cold. The preparation consists of the following raw materials by weight: 300 to 600g of honeysuckle, 200 to 400g of indigowoad leaf, 200 to 400g of weeping forsythia capsule, 200 to 400g of Chinese thorowax root, 200 to 400g of baical skullcap root, 200 to 400g of platycodon grandiflorum, and 1 to 10g of menthol. Pharmacodynamic tests prove that honeysuckle and Chinese thorowax root anti-flu capsules have the effects of reducing fever, diminishing inflammation and relieving cough, wherein the effect of reducing fever is more remarkable. Acute toxicity tests prove that: the safe dosage of the Chinese medicinal composition is equivalent to 371.2 times that of maximum clinical dosage of a human. Long-term toxicity test researching results prove that: remarkable toxic changes or delayed toxicity reactions caused by medicament are not found. The Chinese medicinal compound preparation has the functions of clearing heat and removing toxicity, coursing wind and dissipating heat, and ventilating lung and relieving cough, and has the advantages of convenient use, controllable quality, definite drug effect and safety and reliability.
Owner:武汉双龙药业有限公司
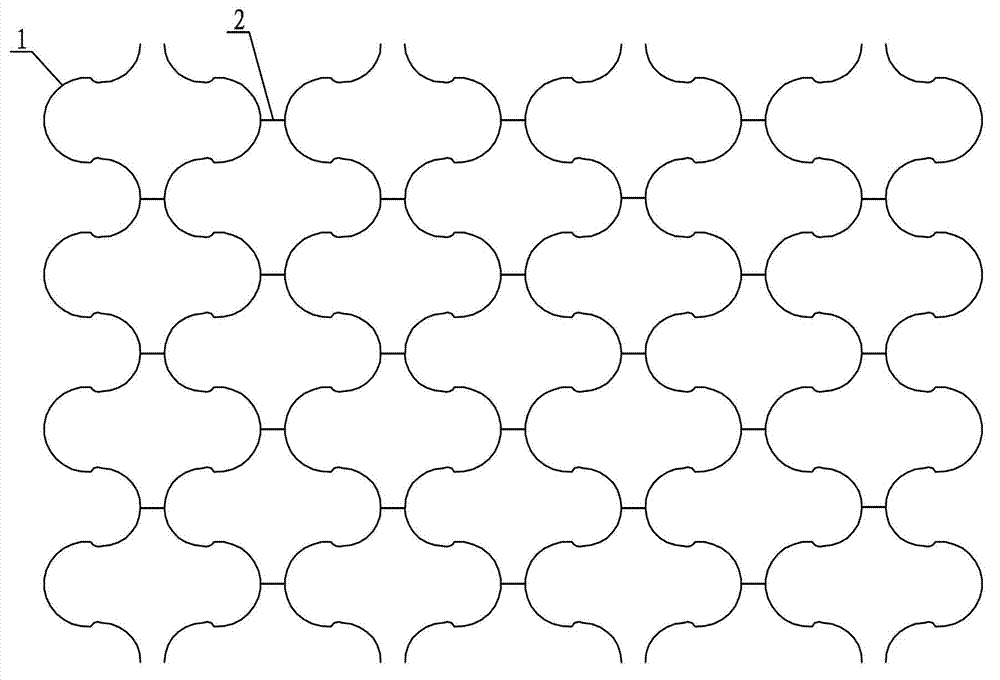
Magnesium alloy coronary support frame
ActiveCN103110465ADoes not cause toxic reactionsImprove the quality of lifeStentsSurgeryCoronary heart diseaseToxic reaction
The invention provides a magnesium alloy coronary support frame and relates to a metal coronary artery support frame. The problems that metal preparation which is not degradable of stainless steel, cobalt chrome alloy, platinum iridium alloy, chromel alloy, tantalum and the like are adopted by the prior support frame, toxic reaction is caused due to the fact that the support frame is implanted in a blood vessel as a foreign material are solved. The support frame is carved into a net shape round pipe structure by the magnesium alloy through a laser. The net shape round pipe structure is composed of a plurality of subject units and a plurality of straight line connecting bodies. Each subject unit is composed of a half circular ring, two quarters of round rings and two arcs. The two quarters of the round rings are arranged symmetrically at two ends of the half circular ring. The quarters of the round rings are connected with the half circular ring through the arcs. The plurality of the subject units are uniformly arranged along the axial direction and the circumference direction of a net pipe. Every adjacent two subject units are arranged symmetrically relative to a straight line connecting body in the plurality of the subject units arranged along the circumference direction. Arc top ends of every adjacent two half circular rings are connected with one straight line connecting body. The magnesium alloy coronary support frame is used for clinical curing coronary disease.
Owner:JIANGYIN BIODEGRADE MEDICAL TECH CO LTD
Full composite human body antioxidant
InactiveCN102440386AProper balanceThe combination is reasonableCosmetic preparationsOrganic active ingredientsSide effectVitamin C
The invention relates to a full composite human body antioxidant, which firstly combines six key oxides of vitamin C, vitamin E, coenzyme Q10, lipoic acid, glutathione and superoxide dismutase (SOD) in the human body into the integral systematic, comprehensive and more effective antioxidant which has the function of eliminating free radicals in the human body. The full composite human body antioxidant can be produced into various preparation forms to be conveniently used, has a simple technology, uses raw materials which are abundant and easy to purchase, has no other toxic reaction side effect, has components with obvious respective functions and high perceived quality; and the full composite human body antioxidant can be used as functional food, medical assistant food, cosmetics, health food and even drugs to be popularized.
Owner:胥永贵
Sustained-release injection and preparation method thereof
InactiveCN101380291APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectSuspending Agents
A slow release injection contains bioactive components, slow release adjuvants, a suspending agent and a dissolvant, wherein, the dissolvant is a common dissolvant or a special dissolvant containing the suspending agent. The slow release adjuvants are chosen from copolymer of polylactic acid, polyglycolic acid and hydroxyacetic acid, copolymer of ethylene vinyl acetate, polifeprosan, etc. The injection can be slowly released to the local focuses of tumors, inflammation and tuberculosis during the decomposition and absorption of the slow release adjuvants, therefore, the slow release adjuvants can not only extremely reduce general toxic reactions thereof but also keep the effective drug concentration at the chronic local focuses of tumors, etc. The suspending agent is chosen from sodium carboxymethyl cellulose, mannitol, and the like, and is used for suspending the active components or suspending the slow release particles or microspheres containing anticancer active components. The slow release injection has the advantages of good injectivity, seldom blockage, strong stability, infrequent demixing, and little general toxic reaction of local injection; furthermore, the slow release injection can selectively increase the drug concentration in local focuses, and enhance the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like.
Owner:孔庆忠
Bone peptide injection preparation technology
InactiveCN101103999AGrowth inhibitionReduce toxicityPeptide/protein ingredientsSkeletal disorderUse medicationToxicant
Disclosed is a preparation process of ossotide injection (gutai), belonging to the medical technical field. To solve the defects of the existing ossotide injection (gunning), the novel ossotide injection (gutai) preparation process is produced by the change of the existing ossotide injection (gunning) process. In the invention, four bones of a pig is picked up, degreased, condensed, and then precipitated and condensed, and then after acid precipitation and alkaline precipitation, the heated liquid is cooled to be in the room temperature and then is placed in a refrigerator; the liquid is taken out from the refrigerator and acidic protein is removed from the liquid is filtrated and hyperfiltrated to become the ossotide stock solution which is mixed with water, filtrated, potted and sterilized, thus the ossotide injection (gutai) is prepared well. The invention removes sensitizer and toxicant, largely reduces toxic reaction for the ossotide injection (gutai), and makes medicine for intravenous injection possibly used.
Owner:石海
A traditional Chinese medicine compound preparation for anti-ulcer and alleviating toxic and side effects of radiotherapy and chemotherapy and its preparation method and use
ActiveCN102284049ARelieve gastrointestinal side effectsImprove the quality of lifeDigestive systemPlant ingredientsPolypillCompounded preparations
The invention relates to a traditional Chinese medicine compound preparation which is anti-ulcer and capable of alleviating toxic and side effects of radiotherapy and chemotherapy, and its preparation method and application. 3-10 parts of Scutellaria baicalensis, the above four herbs form the basic formula, supplemented with one or more of the following traditional Chinese medicines by weight: 5-15 parts of ginseng, 20-35 parts of astragalus, 3-15 parts of licorice, jujube 5 to 18 pieces. The present invention is a compound preparation obtained by screening prescriptions based on many years of clinical experience and optimizing the preparation process according to the theory of traditional Chinese medicine. This prescription has the effects of nourishing qi and harmonizing the stomach, combining cold and heat, dispelling mass and resolving stagnation, etc. It can be widely used in digestive system diseases such as ulcer disease, chronic gastritis, Helicobacter pylori gastropathy, enteritis, etc. The role of its definite curative effect, rapid effect, no toxic side effects.
Owner:SHENYANG PHARMA UNIVERSITY +1
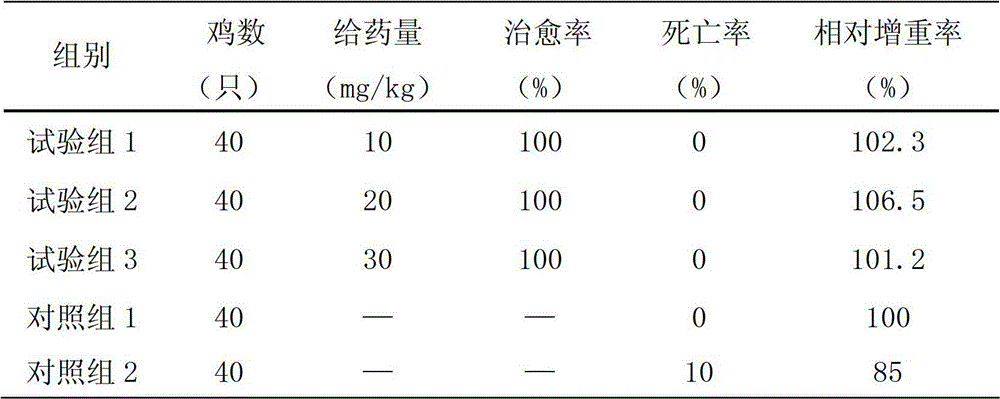
Toltrazuril injection and preparation method thereof
InactiveCN102743334AReduce systemic toxicityLittle systemic toxicityPharmaceutical delivery mechanismAntiparasitic agentsDiseaseTreatment effect
Owner:ZHENGZHOU HOUYI PHARMA
Ursolate microballoon preparation and its preparing process
InactiveCN1857273AMany years of clinical useStrong noveltyOrganic active ingredientsAntipyreticDiseaseVegetable oil
The present invention discloses a kind of ursolic acid lipoid microballoon preparation, and each 1000ml of the ursolic acid lipoid microballoon preparation contains ursolic acid 0.2-50.0g, vegetable oil for injection 50-300g, emulsifier 5-20g, isoosmotic regulator 20-60g and water for injection for the rest. Its preparation process includes dissolving, heating, mixing, emulsifying, cooling, filling and other steps. The present invention overcomes the demerit of water insolubility of ursolic acid, and the ursolic acid lipoid microballoon preparation has less toxic reaction, less stimulation, long medicinal acting period and targeting property. The ursolic acid lipoid microballoon preparation has both disease treating effect and energy providing function.
Owner:欧苏 +1
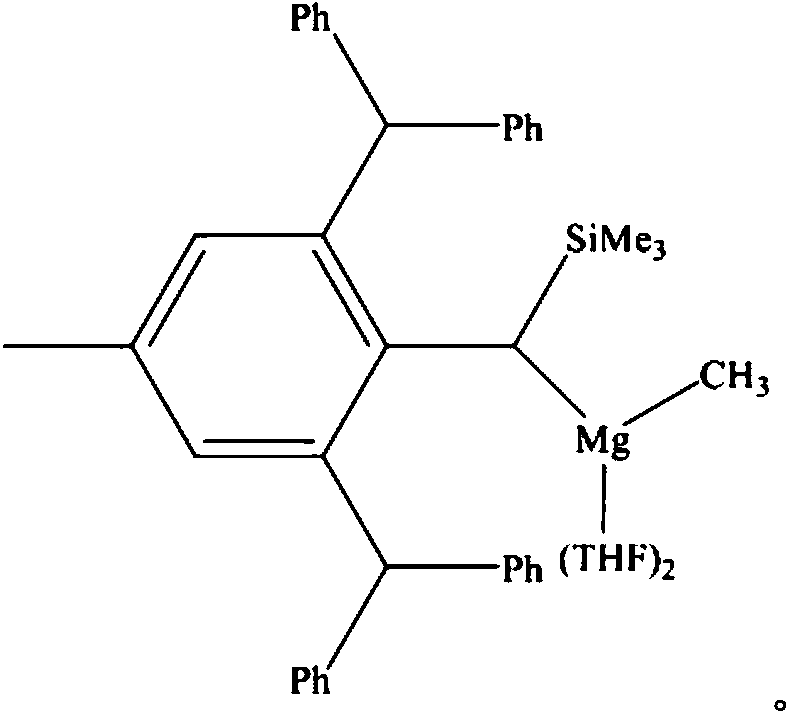
Carboxylic acid deoxidation hydroboration method
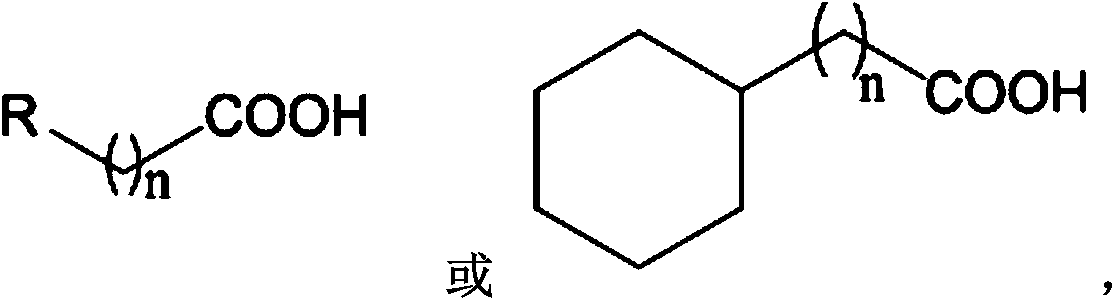
InactiveCN108948059AReduce dosageHigh catalytic activityGroup 3/13 element organic compoundsSolventToxic reaction
The invention discloses a carboxylic acid deoxidation hydroboration method. The method comprises the following steps: adding carboxylic acid into a Schlenk reaction bottle under the protection of nitrogen in an anhydrous oxygen-free and reaction solvent-free condition; adding pinacolborane, and uniformly mixing; adding a high steric hindrance amino magnesium alkyl compound, and reacting to obtaina hydroborated product. The preparation method adopts a magnesium alkyl compound with stable high steric hindrance amino ligand to catalyze the reaction for synthesizing borate compounds from carboxylic acid and borane, has high catalytic activity, no solvent and simple structure, is easy to synthesize, can provide a new scheme for borate compound synthesis from carboxylic acid and borane and canwiden the application range of the magnesium alkyl compound with stable high steric hindrance amino ligand. The method has the advantages of a non-toxic reaction system, no solvent, mild condition, low catalyst dosage, simple reaction step and the like, and highly accords with the concept of environment-friendly chemistry.
Owner:NANJING FORESTRY UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com