Patents
Literature
80 results about "Operative therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adjuvant immune therapy in the treatment of solid tumors through modulation of signaling pathways following engagement of humoral and cell mediated responses
InactiveUS20020187130A1Evaluating efficacyAccurate assessmentBiocideSaccharide peptide ingredientsAdjuvantWhite blood cell
Owner:KINDNESS GEORGE +2
Anti-cancer sustained-released injection containing epothilone derivate
InactiveCN101396342AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryPoly dl lactidePolyethylene glycol
The invention relates to an anti-cancer sustained release injection containing epothilone derivative, consisting of sustained microspheres and menstruum. The sustained microspheres comprise anti-cancer drugs selected from taxane, alkylating agent and / or plant alkaloid and the like, the epothilone derivative and sustained release auxiliary material. The menstruum is a special menstruum containing suspending agent. The epothilone derivative is selected from epothilone B, epothilone D, iso-epothilone D, BMS-247550, azaepothilone B, furan epothilone D or BMS-310705. The sustained release auxiliary material is selected from poly-dl-lactide, the glycolic acid copolymer of the poly-dl-lactide, polyethyleneglycol, the polylactide copolymer of the polyethyleneglycol, carboxyl terminated polylactide copolymer, fatty acid and decanedioic acid copolymer, etc. The suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The sustained release microsphere can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can release drug at partial position for 40 days approximately, therefore, the sustained release injection improves the local drug concentration selectively and enhances the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy and the like at the same time.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer sustained-released injection containing epothilone derivate
InactiveCN101396340AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryPoly dl lactidePolyethylene glycol
The invention relates to an anti-cancer sustained release injection containing epothilone derivative, consisting of sustained microspheres and menstruum. The sustained microspheres comprise anti-cancer drugs selected from taxane, alkylating agent and / or plant alkaloid and the like, the epothilone derivative and sustained release auxiliary material. The menstruum is a special menstruum containing suspending agent. The epothilone derivative is selected from epothilone B, epothilone D, iso-epothilone D, BMS-247550, azaepothilone B, furan epothilone D or BMS-310705. The sustained release auxiliary material is selected from poly-dl-lactide, the glycolic acid copolymer of the poly-dl-lactide, polyethyleneglycol, the polylactide copolymer of the polyethyleneglycol, carboxyl terminated polylactide copolymer, fatty acid and decanedioic acid copolymer, etc. The suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The sustained release microsphere can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can release drug at partial position for 40 days approximately, therefore, the sustained release injection improves the local drug concentration selectively and enhances the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy and the like at the same time.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer composition containing bendamustine
An anti-cancer composition comprises anti-cancer effective component selected from tyrosine kinase inhibitor and / or bendamustine and slow releasing adjuvant, and can be prepared into slow releasing injection and slow releasing implantable agent. The slow releasing injection also comprises special dissolvant containing suspending agent. The Suspending agent has a viscocity of 100cp-3000cp (at 20deg.C-30deg.C) and is selected from sodium carboxymethylcellulose, and so on. the slow releasing adjuvant is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p(BHET-EOP / TC), p(BHDPT-EOP / TC), p( BHDPT-EOP / TC), p(CHDM-HOP) or p(CHDM-EOP.Slow releasing injection and implantable agent can keep high medicinal concentration in tumour part through slow releasing for over 60 days after being injected or implanted in or around tumour. The anticancer composition may also be prepared into sustained-release implant. It can reduce systemic toxic reaction of anticancer drugs, and also selectively improve the therapeutic effect of non-operative therapy such as chemotherapy.
Owner:JINAN KANGQUAN PHARMA TECH
Neratinib sustained-release implant for treating solid tumor
InactiveCN101185633AOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerTherapeutic effect
A sustained release implant includes 0.1%-50% (w / w) nilotinib, 50-99% sustained release excipients and 0-15% sustained release moderator. Sustained release excipients are mainly one or combination of poly (L-lactide-co-ethyl phosphate), poly (L-lactide-co- phosphoric acid propyl), polylactic acid, the copolymer of polylactic acid and hydroxyacetic acid and polifeprosan; sustained release moderator is one or combination of mannitol, sorbic alcohol and chondroitin; sustained release implant applied in local tumor can slowly release nilotinib onto local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction; the invention not only reduces overall toxic reaction of nilotinib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy. The implant can be used for treating solid tumors including lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, pancreatic cancer, bladder carcinoma, cerebroma, and colorectal cancer.
Owner:SHANDONG LANJIN PHARMA +1
Sustained-release injection and preparation method thereof
InactiveCN101380291APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectSuspending Agents
A slow release injection contains bioactive components, slow release adjuvants, a suspending agent and a dissolvant, wherein, the dissolvant is a common dissolvant or a special dissolvant containing the suspending agent. The slow release adjuvants are chosen from copolymer of polylactic acid, polyglycolic acid and hydroxyacetic acid, copolymer of ethylene vinyl acetate, polifeprosan, etc. The injection can be slowly released to the local focuses of tumors, inflammation and tuberculosis during the decomposition and absorption of the slow release adjuvants, therefore, the slow release adjuvants can not only extremely reduce general toxic reactions thereof but also keep the effective drug concentration at the chronic local focuses of tumors, etc. The suspending agent is chosen from sodium carboxymethyl cellulose, mannitol, and the like, and is used for suspending the active components or suspending the slow release particles or microspheres containing anticancer active components. The slow release injection has the advantages of good injectivity, seldom blockage, strong stability, infrequent demixing, and little general toxic reaction of local injection; furthermore, the slow release injection can selectively increase the drug concentration in local focuses, and enhance the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like.
Owner:孔庆忠
Anti-cancer sustained-released agent
InactiveCN101396341AInhibition formationSpeed up entryPeptide/protein ingredientsSolution deliveryDepressantSuspending Agents
The invention relates to an anti-cancer sustained release injection, consisting of sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise sustained release auxiliary material, angiogenesis inhibitor and / or proteolytic enzyme; and the menstruum contains suspending agent. The angiogenesis inhibitor is selected from gefitinib, erlotinib, lapatinib, vatalanib, pelitinib, endostatin, imatinib, semaxanib, dasatinib, avastin, sorafenib, sunitinib, telcyta or panitumumab; the proteolytic enzyme is selected from one or the combination of collagenase, hyaluronidase, relaxin and plasmase; the sustained release auxiliary material is selected from polifeprosan, decanedioic acid copolymer, EVAc, polylactic acid, the mixture or the copolymer thereof, and the like; and the suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The injection can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can improve the local drug concentration selectively, reduce the general reaction of the drug, inhibit the growth of tumour cell and blood vessel and enhance the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Orthopedic therapy system and device and a method of use
InactiveUS8348811B2Maximize the benefitsEasy to usePerson identificationSensorsPhysical medicine and rehabilitationOrthopedic department
Owner:KAMINS PAUL
Temperature controlled sustained-release injection containing steroids anti-cancer drugs
InactiveCN101273963APharmaceutical delivery mechanismPharmaceutical non-active ingredientsGoserelinTherapeutic effect
The invention relates to a temperature-controlled sustained-release injection containing a hormone anti-cancer drug, which comprises the anti-cancer drug, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained hormone anti-cancer drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the viscosity of the temperature-controlled sustained-release injection is 10cp to 3000cp ( at 5 DEG C to 30 DEG C ), and the gelatinization temperature is 35 DEG C to 37 DEG C. The sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, selectively strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be triptorelin, goserelin, leuprorelin, anastrozole, idoxifene, tamoxifen and other hormone anti-cancer drugs.
Owner:SHANDONG LANJIN PHARMA +1
Sustained-release injection containing methotrexate and synergist thereof
InactiveCN101396338AOrganic active ingredientsPharmaceutical delivery mechanismTreatment effectWhole body
The invention relates to a sustained release injection containing methotrexate, consisting of sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise effective anti-cancer component and sustained release auxiliary material; and the menstruum is ordinary menstruum or special menstruum containing suspending agent. The effective anti-cancer component is the combination of the methotrexate and the methotrexate synergist selected from platinum compound, topoisomerase inhibitor and / or tetrazine compound. The sustained release auxiliary material is selected from one or the combination of the copolymer of polylactic acid, polyglycolic acid and glycolic acid, ethylene vinyl acetate copolymer and the copolymer of polifeprosan, FAD and decanedioic acid. The suspending agent is selected from carboxymethyl cellulose, etc. The suspending agent is used for suspending the effective anti-cancer component or sustained release particle or microsphere containing the effective anti-cancer component, so as to be made into the sustained release injection. The sustained release injection is injected into tumor, which can not only reduce the general toxic reaction of the drug, but also improve the partial drug concentration at the tumour selectively and enhance the treatment effect of non-operative treatments, such as chemotherapeutic drug, radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer composition loading both platinum compound and synergist
InactiveCN101011351APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinMitozolomide
Disclosed is a slow release injection agent of anticancer composition containing platinum-group compounds and synergistic agent, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the platinum-group compounds are selected from cisplatin, Carboplatin, Nedaplatin or Oxaliplatin, the synergistic agent can be selected from tetrazine drugs such as Mitozolomide or Temozolomide, and / or anticancer antibiotics such as Adriamycin, Aclarubicin, Epirubicin, mitomycin or pidorubicin, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer medicine sustained-released injection loaded with platinum compound and synergist thereof
InactiveCN101380303AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismEptaplatinPicoplatin
A anticarcinogenic slow release injection carrying platinum compounds and a synergist thereof is composed of slow release microspheres and a dissolvant, wherein, the slow release microspheres comprise anticancer active components and a slow release adjuvant, and the dissolvant is a special dissolvant containing a suspending agent. The anticancer active component comprises platinum compounds such as sunpla, dicycloplatin, eptaplatin, (cis-amminedichloro(2-methylpyridine) platinum, camphoramine chloroacetic platinum or picoplatin, and the like, and a cytotoxic drug selected from a phosphoinositide 3-kinase inhibitor, pyrimidine analogue and / or a DNA repair enzyme inhibitor; the slow release adjuvant is biocompatible macromolecules such as polylactic acid and copolymer thereof, polyethylene glycol, carboxyl end polylactic acid copolymer, copolymer of dienoic fatty acid and sebacic acid, poly (erucic acid dimer-sebacic acid), poly (fumaric acid-sebacic acid), polifeprosan, polylactic acid, EVAc, and the like, and the suspending agent has the viscosity of 100cp-3,000cp (at the temperature of 20-30 DEG C) and is selected from sodium carboxymethyl cellulose, and the like. The slow release microspheres can also be made into a slow release implant. The slow release injection is injected or placed in tumors or around the tumors, which can improve the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like.
Owner:SHANDONG LANJIN PHARMA +1
Axitinib sustained-release implplant treating for solid tumor
InactiveCN101204367AOrganic active ingredientsPharmaceutical delivery mechanismWhole bodyProstate cancer
The invention relates to an Axitinib sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of Axitinib and sustained-release excipients and a certain amount of sustained-release regulator. The solid tumors include the liver cancer, the lung cancer, the esophageal canner, the gastric canner, the breast canner, the ovarian canner, the prostate canner, the bladder canner and the rectum canner. The sustained-release excipients are mainly a copolymer of polylactic acid, glycollic acid and hydroxyacetic acid and one of the three materials, polifeprosan, poly-(L-lactide-co-ethyl phosphonate) and Poly(L-lactide-co-propyl phosphonate) or a combination of the three materials, in the degradation and absorption process of which the Axitinib is sustainedly released to part of the tumor, thus the entire toxicity of the Axitinib is significantly reduced while an effective medicine consistency is maintained on part of the tumor. That the sustained-release implant is implanted inside part of the tumor can not only reduce the entire toxicity of the Axitinib, but also enhance the medicine consistency on part of the tumor, thereby increasing the curing effect of non-operative therapeutics such as chemotherapeutic drugs and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Bosutinib sustained release implant for treating solid tumors
InactiveCN101224185AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a bertschtinib sustained-release implant for treating solid tumors, which is characterized in the sustained-release implant contains anti-cancer effective dose of bertschtinib, sustained-released auxiliary materials and a certain amount of sustained-release regulator. Solid tumors comprise lung cancer, esophageal cancer, gastric cancer, liver cancer, mammary cancer, oophoroma, prostatic cancer, carcinoma of urinary bladder and colorectal cancer. The sustained-released auxiliary materials are mainly one or composition of the copolymer of glycolic acid and glycollic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co- propyl phosphate), which can slowly release bertschtinib to the tumor local during degeneration and absorption, thus keeping concentration of effective medicine in the part of the tumor while distinctively reducing overall toxic reaction. Putting the sustained-release implant in the tumor can not only reduce overall toxic reaction of bertschtinib, but also selectively improve drug concentration in the part of the tumor, thereby promoting treatment effect of non-operative treatment, such as chemotherapeutics, radiotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Nilotinib sustained-release implant for treating solid tumor
InactiveCN101185627AOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerTherapeutic effect
A sustained release implant includes 0.1%-50% (w / w) nilotinib, 50-99% sustained release excipients and 0-15% sustained release moderator. Sustained release excipients are one or the combination of poly (L-lactide-co-ethyl phosphate), poly (L-lactide-co- phosphoric acid propyl), glycolic acid and copolymer of glycolic acid and hydroxyacetic acid, and polifeprosan; sustained release moderator is one or combination of mannitol, sorbic alcohol and chondroitin; sustained release implant applied in local tumor can slowly release nilotinib onto local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction, thus the invention not only reduces overall toxic reaction of nilotinib, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy. The implant can be used for treating solid tumors including lung cancer, esophageal carcinoma, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic carcinoma, pancreatic cancer, bladder carcinoma, cerebroma, and colorectal cancer.
Owner:SHANDONG LANJIN PHARMA +1
A kind of controlled release injection of carried fluorouracil and synergis
InactiveCN1957923AEasy injectionIncrease drug concentrationSolution deliveryPharmaceutical non-active ingredientsTreatment effectMicrosphere
A slowly-release anticancer medicine in the form of injection or implant is disclosed. Said slowly-release injection is composed of a special solvent containing suspending aid and the slowly-release microballs consisting anticance medicine, iterstitial hydrolyte and slowly-releasing auxiliary. Said anticancer medicine is chosen from 5-FU, oxaliplatin, etc. Said interstitial hydrolyte is chosen from collagenase, relaxin, etc. Said slowly-releasing auxiliary is chosen from polylactic acid, FAD, polyethanediol, etc.
Owner:SHANDONG LANJIN PHARMA +1
Anticancer sustained-released formulation loaded with blood vessel inhibitor and synergist thereof
InactiveCN101380304AIncreased sensitivityGrowth inhibitionOrganic active ingredientsPharmaceutical delivery mechanismAngiostatinDepressant
An anticarcinogenic slow release injection carrying an angiogenesis inhibitor and a synergist thereof is made from slow release microspheres and a dissolvant. The slow release microspheres comprise anticancer active components and a slow release adjuvant, and the dissolvant is a special dissolvant containing a suspending agent. The anticancer active components are angiogenesis inhibitors such as gefitinib, erlotinib, lapatinib, vatalanib, pelitinib, thalidomide, ranolamine, angiostatin, endostatin, imatinib, thalidomide, ranolamine, simatinib, dasatinib, avastin, kanatini, sorafenib, sunitinib, telstar or panitoma, and the like, and / or cytotoxic drugs selected from a phosphoinositide-3-kinase inhibitor, pyrimidine analogue and / or a DNA repair enzyme inhibitor; the slow release adjuvant is biocompatible macromolecule; the viscosity of the suspending agent is 100cp-3,000cp (at the temperature of 20-30 DEG C), and the suspending agent is selected from sodium carboxymethyl cellulose, and the like. The slow release microspheres can be also made into a slow release implant, and the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like, can be improved when the slow release injection is injected or placed in tumors or around the tumors.
Owner:SHANDONG LANJIN PHARMA +1
Anticancer sustained release agent containing fluorouracil and synergist thereof
InactiveCN1969824AEasy injectionIncrease drug concentrationSolution deliveryPharmaceutical non-active ingredientsSodium carboxymethylcelluloseDoxorubicin
Disclosed is an anticancer slow release injection containing fluorouracil and synergistic agents, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The suspending agent is selected from sodium carboxymethylcellulose, mannitol, sorbitol, Tween-80 or their combination. The anticancer active constituents are the combination of 5-FU and Carboplatin, Carmofur, adriacin as 5-FU synergistic agent. The slow release auxiliary materials are selected from polylactic acid, ethylene-vinylacetate copolymer, Polifeprosan, FAD, sebacic acid copolymer, polyglycolic acid and glycolic acid copolymer or their combination. The viscosity of the injection is 50-1000cp (at 20-30 deg C), the slow release microspheres can also be prepared into slow release implanting agent, when in-tumor or around-tumor injected or placed, the slow release agent can not only lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, and improve the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation.
Owner:SHANDONG LANJIN PHARMA +1
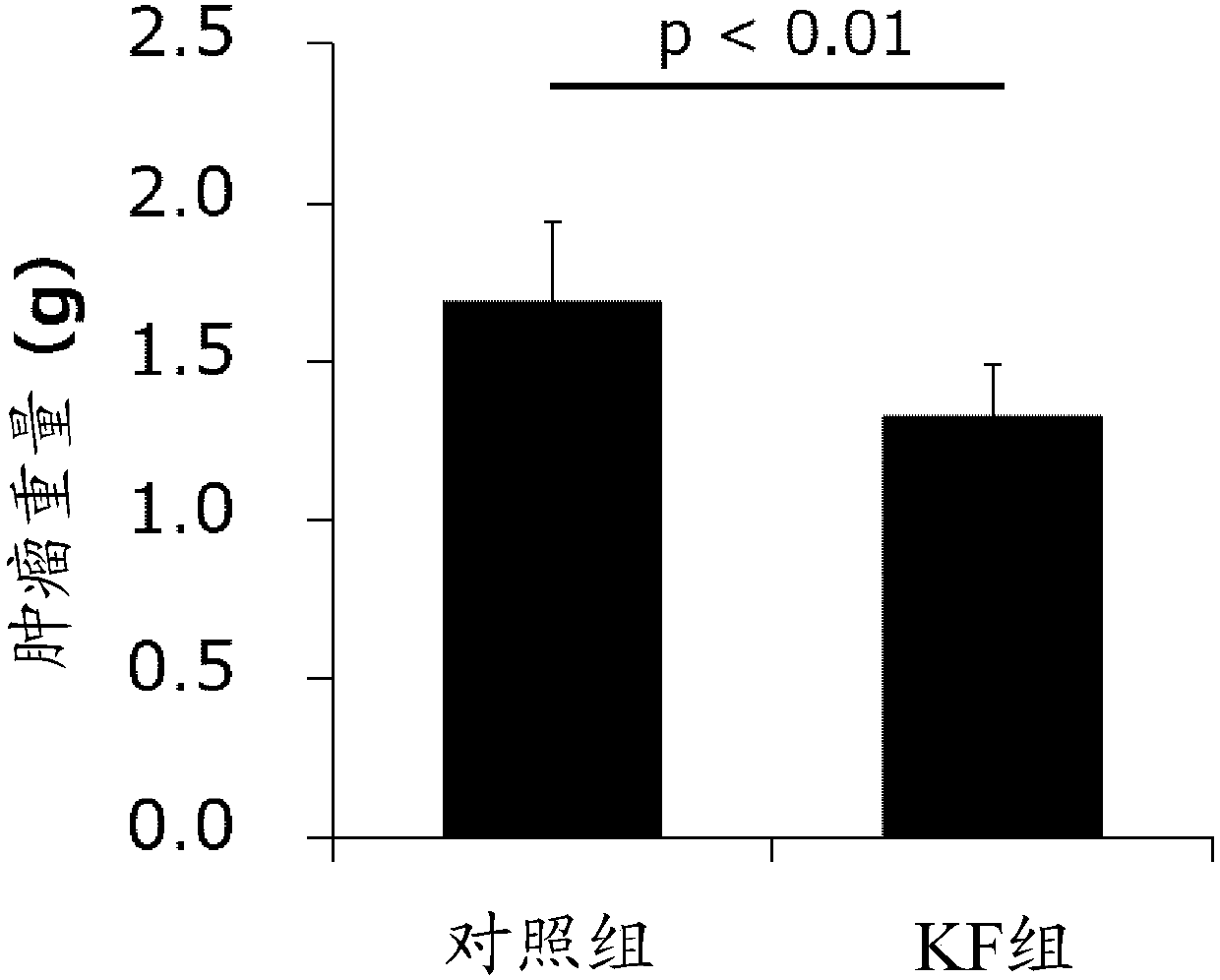
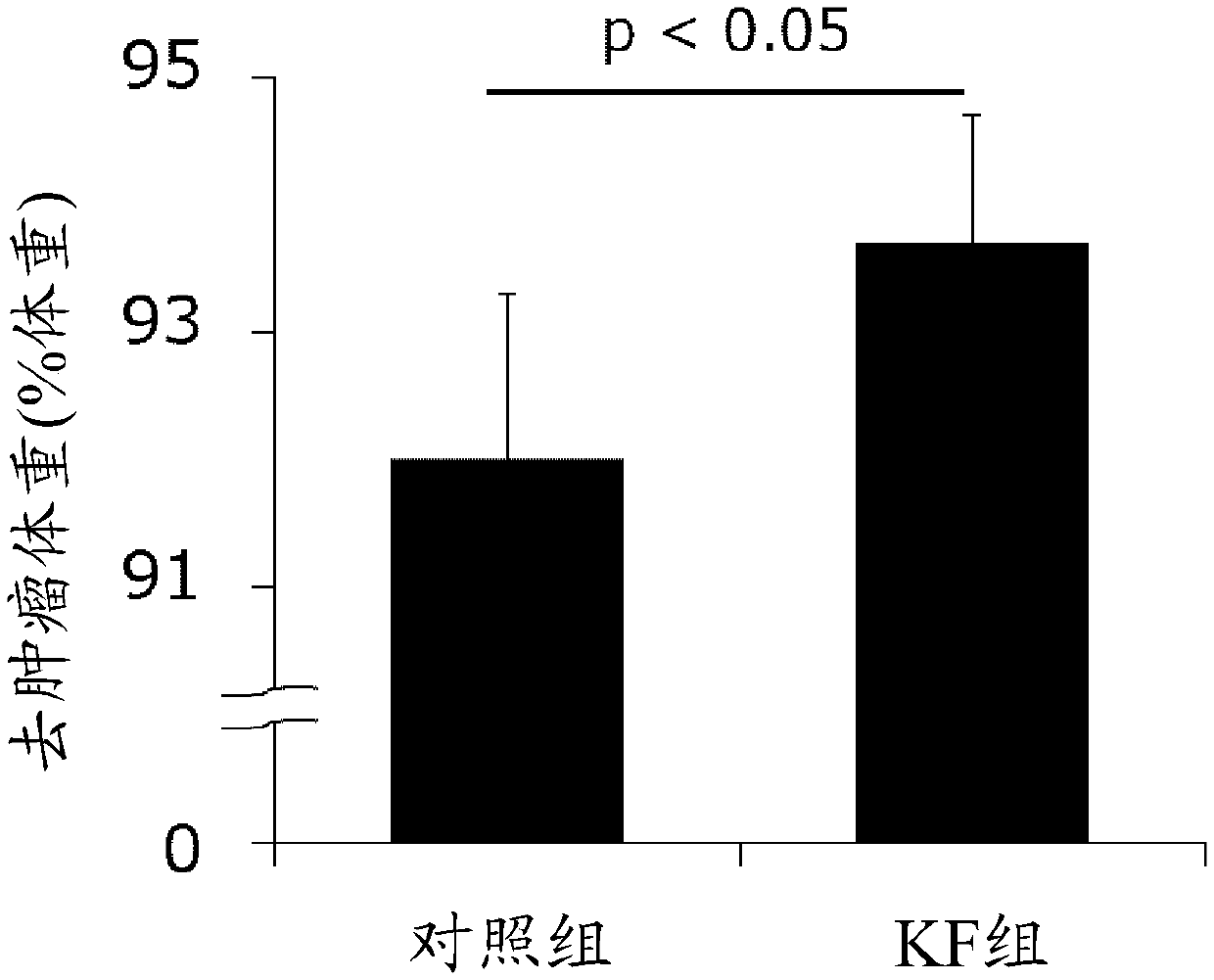
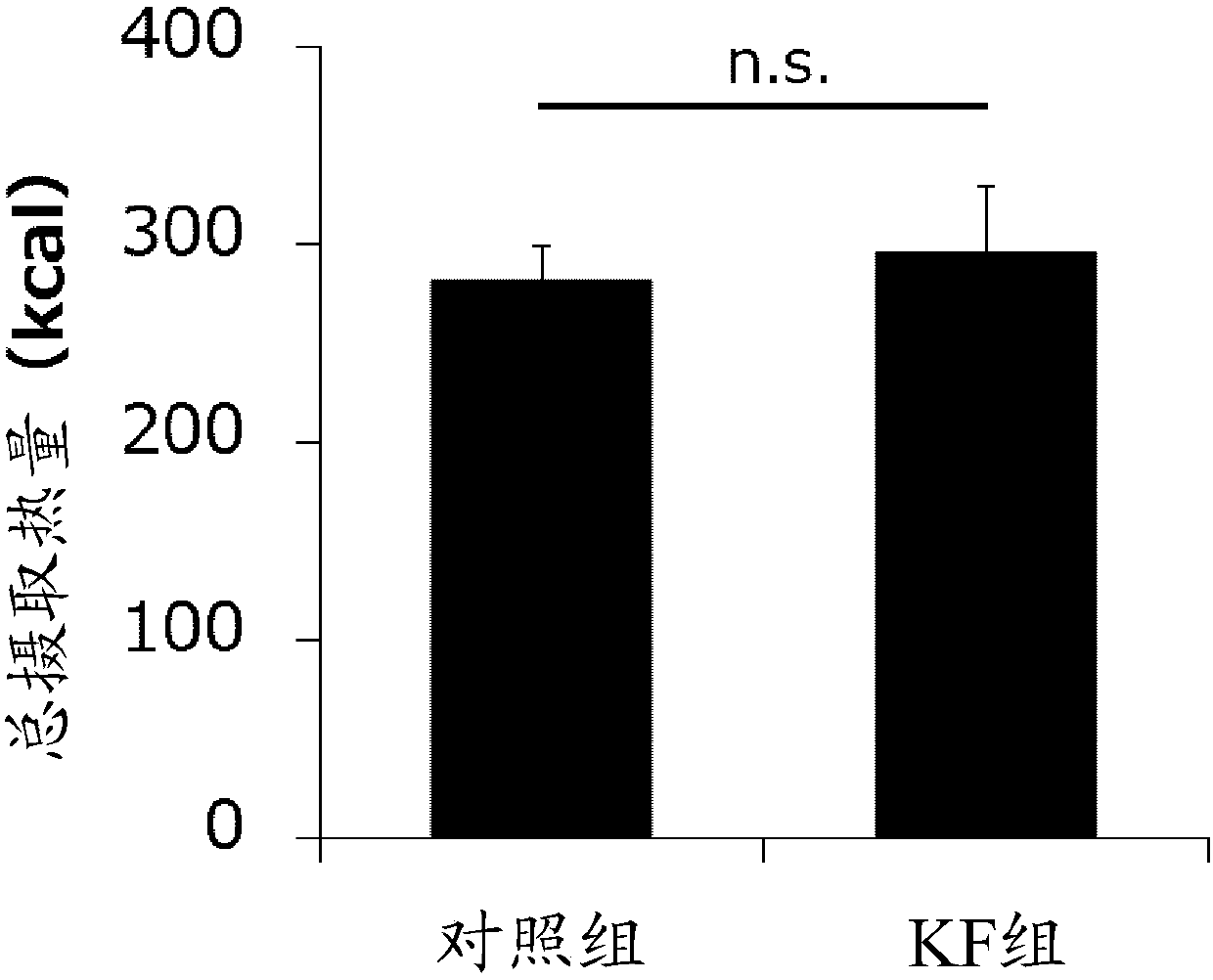
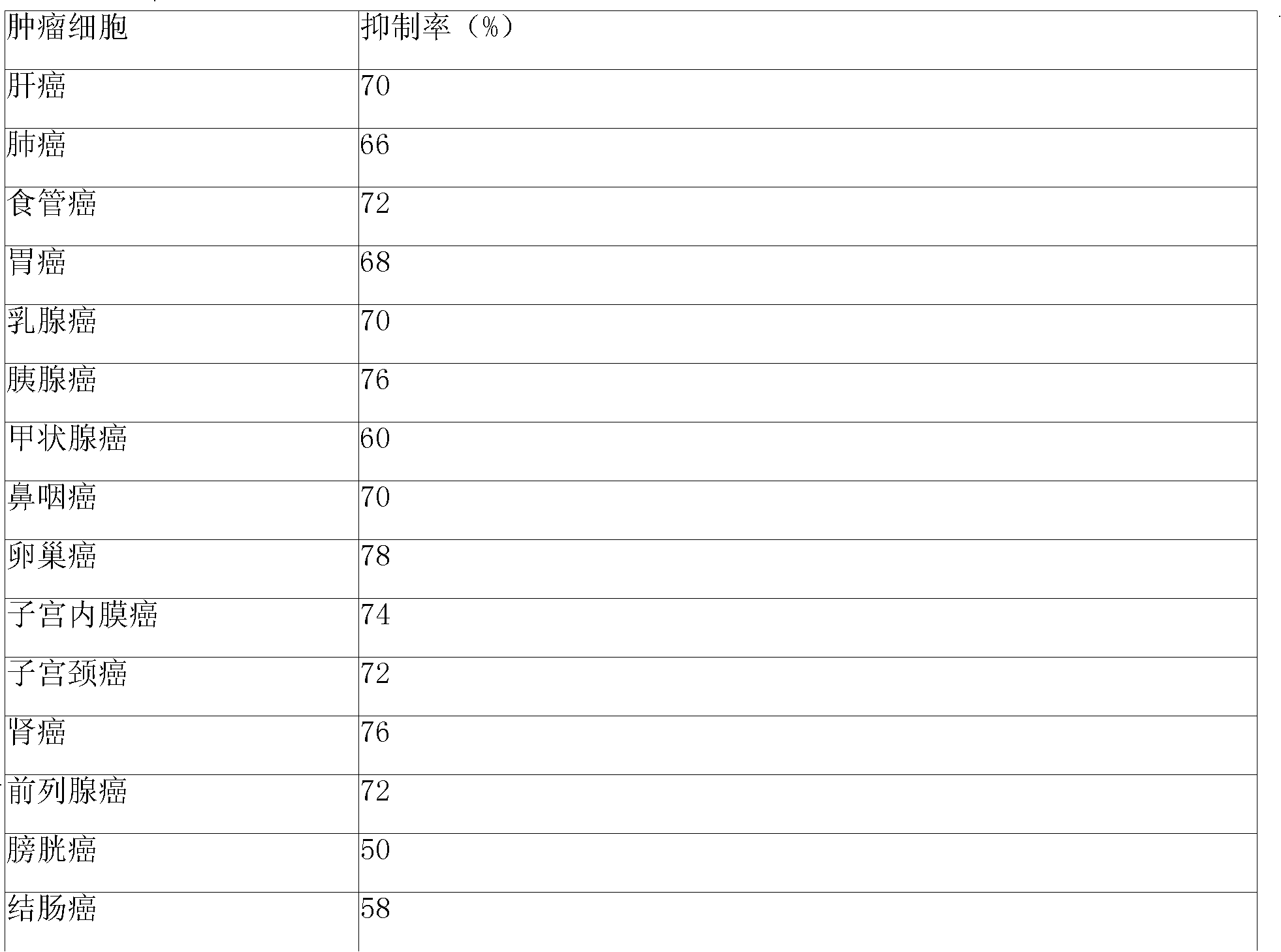
Development of dietary therapy in cancer
InactiveCN107921133AReduce the number of dosesProlong lifeEster active ingredientsAntineoplastic agentsFormularyRadical radiotherapy
The invention provides new compositions or combinations thereof for the treatment of cancer. More specifically, the invention provides compositions or combinations thereof for cancer treatment including a high-fat diet. Specifically, the high-fat diet is characterized by having approximately 120 g / day or more, or approximately 70% or more of the total daily energy, from fat, based on a real weightof 50 kg. The diet is preferably a carbohydrate-restricted high-fat diet, and more preferably provided by a ketone formula and / or MCT oil. The dietary therapy by a high-fat diet of the present invention is provided along with surgical treatment, chemotherapy or radiation therapy, or combinations thereof.
Owner:OSAKA UNIV +1
Mitoxantrone sustained-release implantation agent for curing entity tumour
InactiveCN101176710AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a mitoxantrone sustained-release implant capability of curing solid tumors, such as lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer, colon cancer and rectum cancer. The invention is characterized in that: the sustained-release implant comprises mitoxantrone, sustained-release excipient and a certain amount of sustained-release regulator; the amount of the mitoxantrone and sustained-release excipient is sufficient to control cancer; the sustained-release excipient is mainly one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate); the mitoxantrone can be released slowly into part of the tumor during the degradation and adsorption, significantly reducing the systemic toxicity and sustaining the effective medicine concentration simultaneously. The invention has the advantages that: the systemic toxicity of mitoxantrone can be significantly reduced; the effective medicine concentration can be improved selectively at part of the tumor, and the therapeutic effects of non-operative treatments such as chemotherapeutic drugs and radiotherapy can be reinforced.
Owner:JINAN SHUAIHUA PHARMA TECH
Pirarubicin sustained-release implant treating for solid tumor
InactiveCN101204368AOrganic active ingredientsPharmaceutical delivery mechanismTreatment effectWhole body
The invention relates to a Pirarubicin sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of Pirarubicin and sustained-release excipients and a certain amount of sustained-release regulator. The solid tumors include the esophageal canner, the gastric canner, the breast canner, the ovarian canner, the prostate canner, the bladder canner, the liver canner, the lung canner, and the rectum canner. The sustained-release excipients are mainly a copolymer of glycollic acid and hydroxyacetic acid and one of the two materials, polifeprosan and poly-(L-lactide-co-ethyl phosphonate), or a combination of the two materials, in the process of degradation and absorption of which the Pirarubicin is sustainedly released to part of the tumor, thus the entire toxicity of the Pirarubicin is significantly reduced while an effective medicine consistency is maintained on part of the tumor. That the sustained-release implant is implanted inside part of the tumor can not only reduce the entire toxicity of the Pirarubicin but also enhance the medicine consistency on part of the tumor, thereby increasing the curing effect of non-operative therapeutics such as chemotherapeutic drugs and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Anticancer sustained-released formulation loaded with blood vessel inhibitor and synergist thereof
InactiveCN101380305AIncreased sensitivityGrowth inhibitionOrganic active ingredientsPharmaceutical delivery mechanismAngiostatinDepressant
An anticarcinogenic slow release injection carrying an angiogenesis inhibitor and a synergist thereof is made from slow release microspheres and a dissolvant. The slow release microspheres comprise anticancer active components and a slow release adjuvant, and the dissolvant is a special dissolvant containing a suspending agent. The anticancer active components are angiogenesis inhibitors such as gefitinib, erlotinib, lapatinib, vatalanib, pelitinib, thalidomide, ranolamine, angiostatin, endostatin, imatinib, thalidomide, ranolamine, simatinib, dasatinib, avastin, kanatini, sorafenib, sunitinib, telstar or panitoma, and the like, and / or cytotoxic drugs selected from a phosphoinositide-3-kinase inhibitor, pyrimidine analogue and / or a DNA repair enzyme inhibitor; the slow release adjuvant is biocompatible macromolecule; the viscosity of the suspending agent is 100cp-3,000cp (at the temperature of 20-30 DEG C), and the suspending agent is selected from sodium carboxymethyl cellulose, and the like. The slow release microspheres can be also made into a slow release implant, and the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like, can be improved when the slow release injection is injected or placed in tumors or around the tumors.
Owner:SHANDONG LANJIN PHARMA +1
Sustained-released injection including platinum compound and the alkylate agent
The invention relates to an anti-cancer compound as a slow release injection which contains platinum compound and / or alkyl agent, formed by slow release micro ball and solvent. The slow release micro ball comprises the anti-cancer effective components and slow release findings, selected from platinum compound of kpeitabing, peimeiquse, caplatinum, or jxitabing and / or alkyl agent, the solvent is a common solvent or a special solvent with suspending agent, while the viscosity of suspending agent is 100cp-3000cp (at 20-30Deg. C), selected from carboxymethyl cellulose, the anti-cancer effective component is phosphoinositide 3-kinase restrainer and / or the phosphoinositide 3-kinase restrainer booster selected from self-anti-cancer antibiotics and / or tetrazine drug, the slow release finding is selected from phosphate polyester as p (LAEG-EOP) or p (DAPG-EOP), or the polyester or mixture of phosphate, PLA, polyphenyl, PLGA, poly (erucic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid), and the alkyl agent is selected from ranimustine or the like. The anti-cancer compound can be made as slow release plant agent, to inject cancer or around cancer to hold the effective drug density for more than 60 days, while it can significantly reduce the general reaction of drug and selectively strengthen the effect of non-surgery treatments as chemotherapy or the like.
Owner:JINAN SHUAIHUA PHARMA TECH
Temozolomide sustained-release implant for treating solid tumor
InactiveCN101185630AIncreased sensitivityHigh clinical application valueOrganic active ingredientsPharmaceutical delivery mechanismPoly dl lactideTherapeutic effect
A temozolomide sustained release implant for treating solid tumors is characterized in that the sustained release implant includes effective dose of anticancer temozolomide, sustained release excipients and a certain quantity of sustained release moderator. Solid tumors include pancreatic cancer, lung cancer, liver cancer, breast cancer, cerebroma, ovarian cancer, prostatic carcinoma, esophageal carcinoma, lymphadenoma, osteosarcoma and colorectal cancer. Sustained release excipients mainly are one or combination of copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, and poly(L-lactide-co-ethyl phosphate); in the process of decompression, temozolomide can be slowly released to local tumor, thus maintaining effective drug concentration of local tumor as well as significantly reducing overall toxic reaction. Being placed in local tumor, the sustained release implant not only reduces overall toxic reaction of temozolomide, but also selectively improves drug concentration in local tumor, enhancing the therapeutic effects of non-operative therapy such as chemotherapy drugs and radiotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Meifaron sustained-release implantation agent for curing entity tumour
InactiveCN101176714AOrganic active ingredientsPharmaceutical delivery mechanismChitinPharmaceutical preservatives
The invention relates to a slow-release implant agent of Memphalan, which is characterized by effective amounts of Memphalan, slow-release excipients and releasing regulatory agents in the slow-release implant agent. The excipients comprise macromolecules which are biologically compatible and degradable, mainly PLA and PLGA. The releasing regulatory agents are selected from one or more items from mannitol, sorbitol, xylitol, oligosaccharide, chitin, potassium salt, sodium salt, hyaluronic acid, collagen, chondroitin, gelatin and albumin. The slow-release implant agent slowly releases Memphalanon the local tumor in the process of degradation and absorption, so the argent can evidently reduce the systemic toxicity and simultaneously apply to the effective drug concentration control on the local tumor. Therefore, the argent can be applied separately or combined with the non-surgical treatments such as chemotherapy drug and radiotherapy, which can be also widely used for tumor treatment of different phases, not only selectively improving the drug concentration on local tumor but also reinforcing the therapeutic effect of non-surgical treatments such as chemotherapy drug and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Sansheng medicine liquid capable of treating hemorrhoids
InactiveCN101507780ANo side effectsShort course of treatmentCardiovascular disorderPlant ingredientsVerbenaSide effect
The invention relates to a Sansheng hemorrhoid-relieving liquid, which is prepared from the following raw materials in portion by weight: 10 to 60 portions of Cimicifuga foetida, 50 to 60 portions of Astragalus membranaceus, 30 to 60 portions of Chinese scholartree flower, 30 to 60 portions of bupleurum, 60 to 70 portions of phellodendron bark and 60 to 70 portions of European verbena. The Sansheng hemorrhoid-relieving liquid overcomes the defects that the external application pharmacotherapy and the operative therapy cannot radically cure hemorrhoid, hemorrhoid is easy to relapse, and the cost is high. The Sansheng hemorrhoid-relieving liquid is prepared from pure Chinese herbal medicines, has the efficacies of invigorating vital energy and collecting lung Qi, ascending lucidity and descending turbidity, eliminating gloominess and releasing toxin, and activating blood circulation to dissipate blood stasis, has no toxic and side effect, has good treatment effect and high cure rate, and is economic and practical.
Owner:徐勋
Sustained-released injection including antimetabolite medicine and alkylate agent
The invention relates to a slow release injection which contains antimetabolite and / or alkyl agent, formed by slow release micro ball and solvent. The slow release micro ball comprises the anti-cancer effective components selected from platinum compound of kpeitabing, peimeiquse, caplatinum, or jxitabing and / or alkyl agent, the solvent is a common solvent or a special solvent with suspending agent, while the viscosity of suspending agent is 100cp-3000cp (at 20-30Deg. C), selected from carboxymethyl cellulose, the slow release finding is selected from phosphate polyester as p (LAEG-EOP) or p (DAPG-EOP), or the polyester or mixture of phosphate, PLA, polyphenyl, PLGA, poly (erucic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid), and the alkyl agent is selected from ranimustine or the like. The anti-cancer compound can be made as slow release plant agent, to inject cancer or around cancer to hold the effective drug density for more than 50 days, while it can significantly reduce the general reaction of drug and selectively strengthen the effect of non-surgery treatments as chemotherapy or the like.
Owner:JINAN SHUAIHUA PHARMA TECH
Solid tumor resisting release agent including nimustine and the intensifier
InactiveCN101040843AEasy injectionIncreased sensitivitySolution deliveryPharmaceutical non-active ingredientsTherapeutic effectPolymer
A nimustine slow release injection for treating solid cancer comprises slow release finding and nimustine, or the combination of nimustine and relative booster (pidorubicin, osaliplatinum, adriablastina, amethopterin or the like). The viscidity of slow-release injection is 10-650cp (20-30Deg. C). The anti-cancer effective component can be made into slow release plant agent. The slow release finding substantially comprises macromolecule polymer with biological soluble degradable and absorb property, which can slow release the anti-cancer effective component to cancer part in the degradation process, significantly reduce general reaction and hold effective drug density on cancer. The anti-cancer compound can be arranged part of cancer to reduce the general toxicity reaction, selectively improve the drug density locally, and strengthen the effect of non-surgery treatments as chemotherapy, and radiation therapy or the like. The solid cancer comprises glioma, lung carcinoma, intestinal cancer, breast cancer or the like.
Owner:JINAN KANGQUAN PHARMA TECH
Anticancer composition containing neovascularization inhibitor and bortezomib
InactiveCN101301470ABoron compound active ingredientsPharmaceutical delivery mechanismSodium carboxymethylcellulosePharmaceutical preservatives
The invention provides a sustained-release injection which is an anti-tumor composite containing agiogenesis inhibitors and bortezomib. The sustained-release injection comprises a sustained-release microsphere and a solution medium, wherein the sustained-release microsphere comprises effective anti-tumor ingredient and sustained-release excipient, the solution medium is a common solution medium or a special solution medium containing a suspending drug. The suspending drug has the viscosity between 100cp and 3000cp (under twenty centi degrees to thirty centi degrees) and is selected from sodium carboxymethylcellulose and others; the effective anti-tumor ingredient comprises the agiogenesis inhibitors and / or the combination thereof; the sustained-release excipient is selected from polyphosphonate copolymer like p(LAEG-EOP)or p(DAPG-EOP), PLA, polyphosphonate with PLA, polifeprosan, copolymer of dual fatty acid and sebacic acid, the polymer or blending polymer of ( erucic acid dipolymer-sebacic acid) or poly(boletic acid-sebacic acid); the anti-tumor composite can also be prepared to be a sustained-release implant which can maintain the effective concentration of drug over forty days for intratumor or tumor circumference injection or placement, can also reduce general reaction obviously and enhance the treatment effect of non-operative therapeutics such as chemotherapy and radiotherapy.
Owner:济南基福医药科技有限公司
Medicine for treating internal and external hemorrhoids and preparation method thereof
InactiveCN102552295AGood curative effectHas long-acting analgesic functionHydroxy compound active ingredientsAntipyreticSide effectExternal Hemorrhoid
The invention relates to a medicine for treating internal and external hemorrhoids and a preparation method thereof. The existing operative therapy can cure various hemorrhoids, but has many shortcomings such as great pain, much bleeding, sequela and easy relapse, and is hard for the old and infirm people to bear. The currently acknowledged (PPH) anastomat resection is only superior to the traditional operation in smaller wound and faster healing, and also has shortcomings such as pain and bleeding. The medicine provided by the invention is prepared by mixing carbolic acid, quinine, tannic acid and sterile water. The invention provides a medicine for treating internal and external hemorrhoids and a preparation method thereof, wherein the medicine does not hurt normal tissue or require long-term treatment, takes effect after once injection, avoids pain and relapse, and has wide raw material source, low cost, wide clinical application and little side effect.
Owner:秦克骏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com