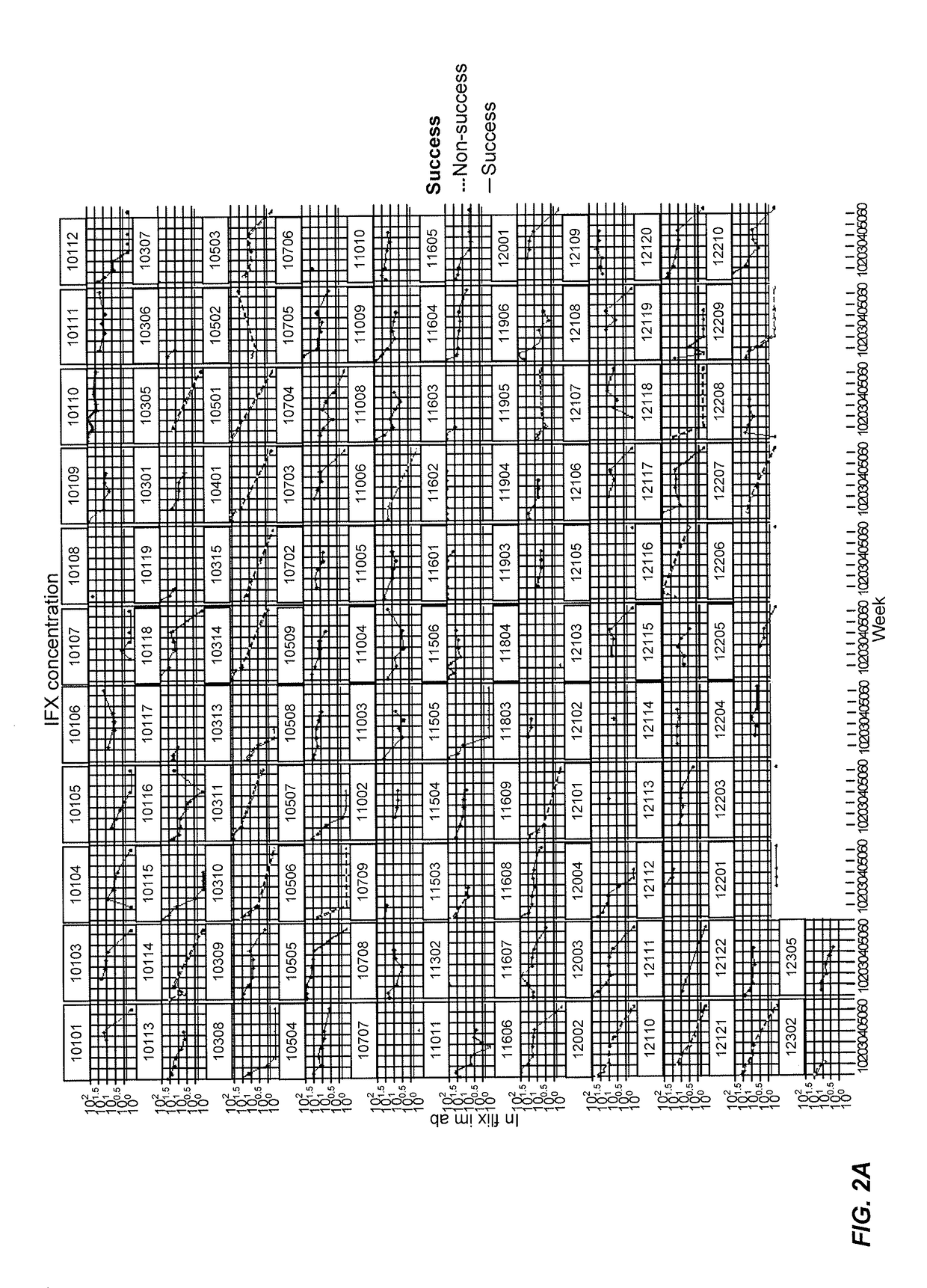
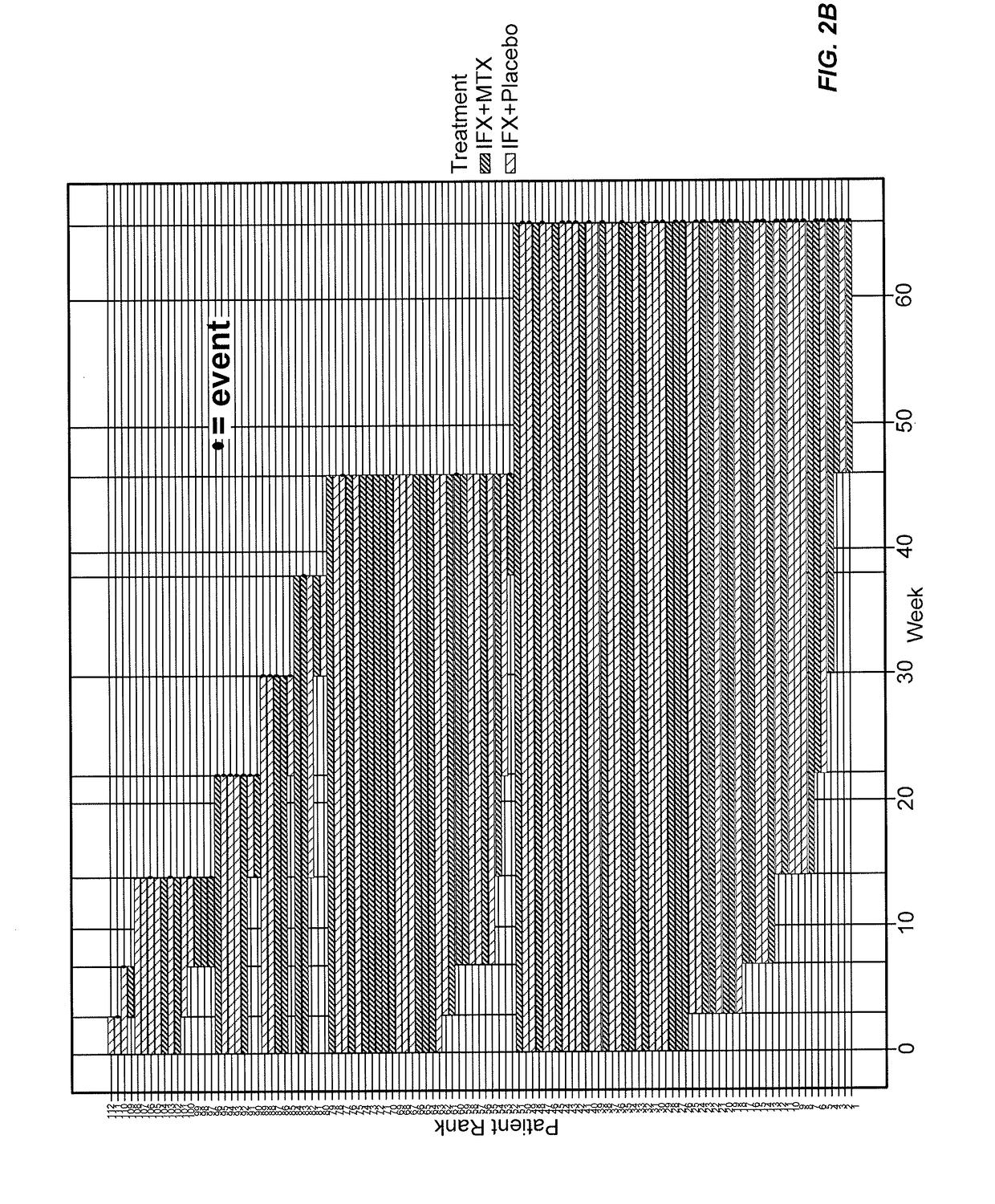
Patents
Literature
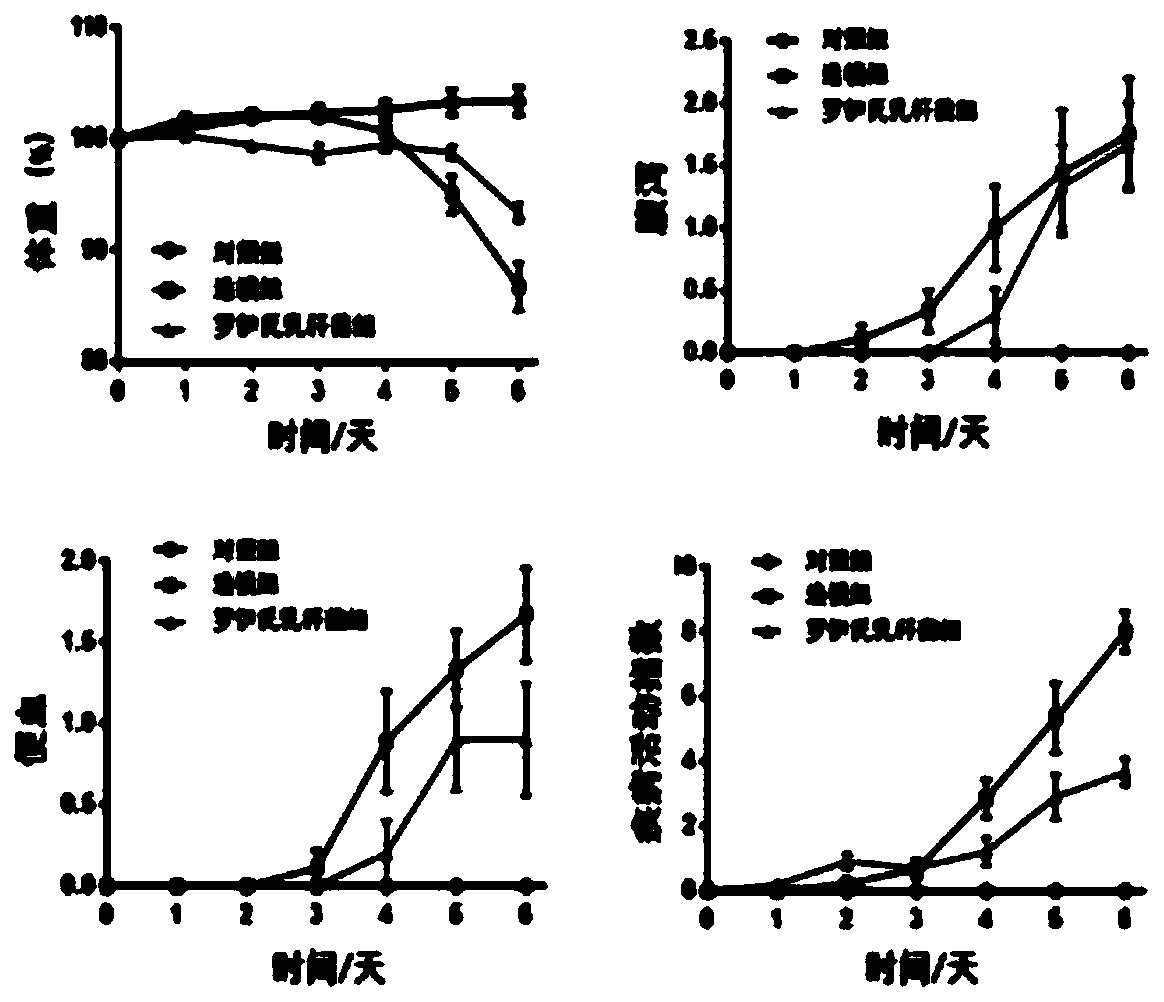
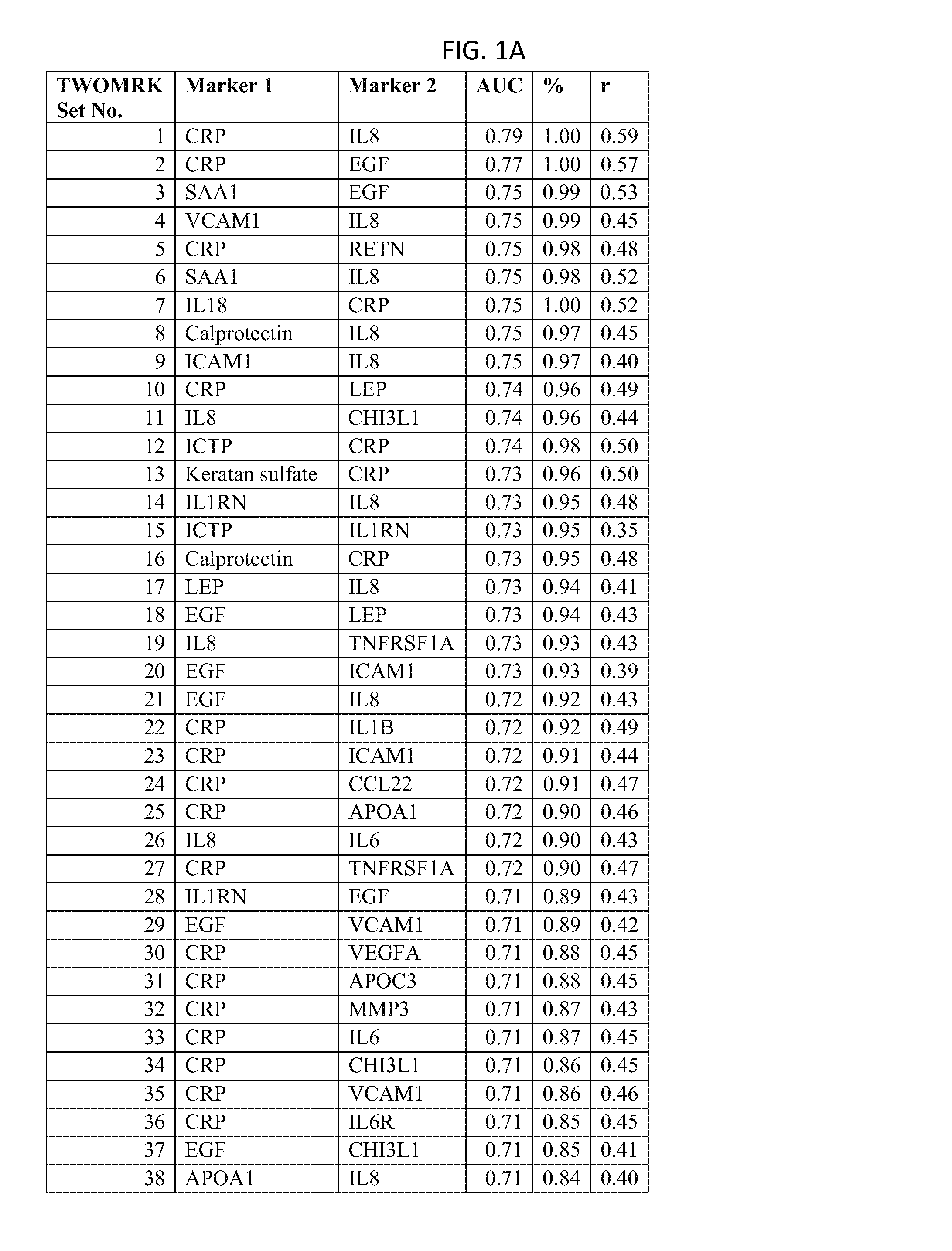
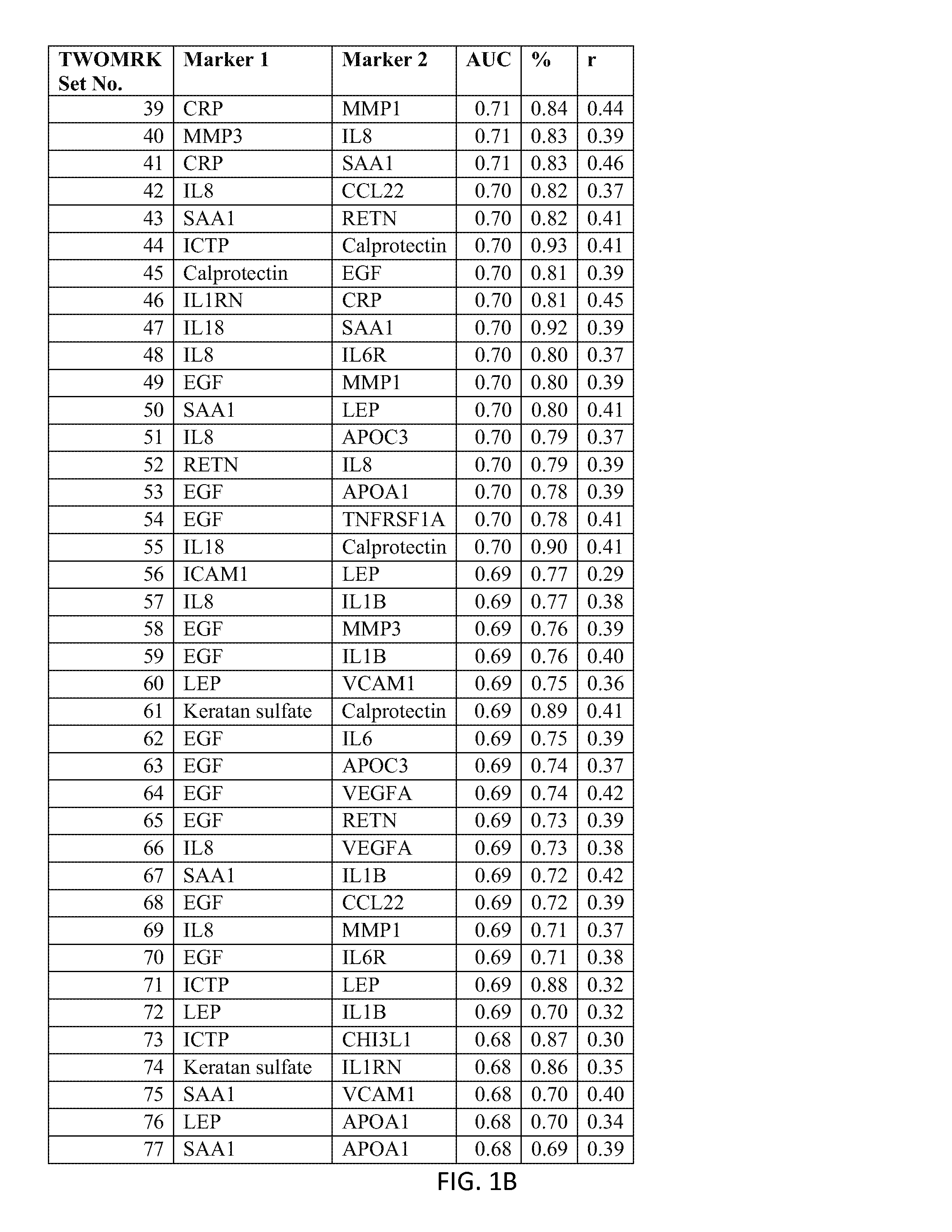
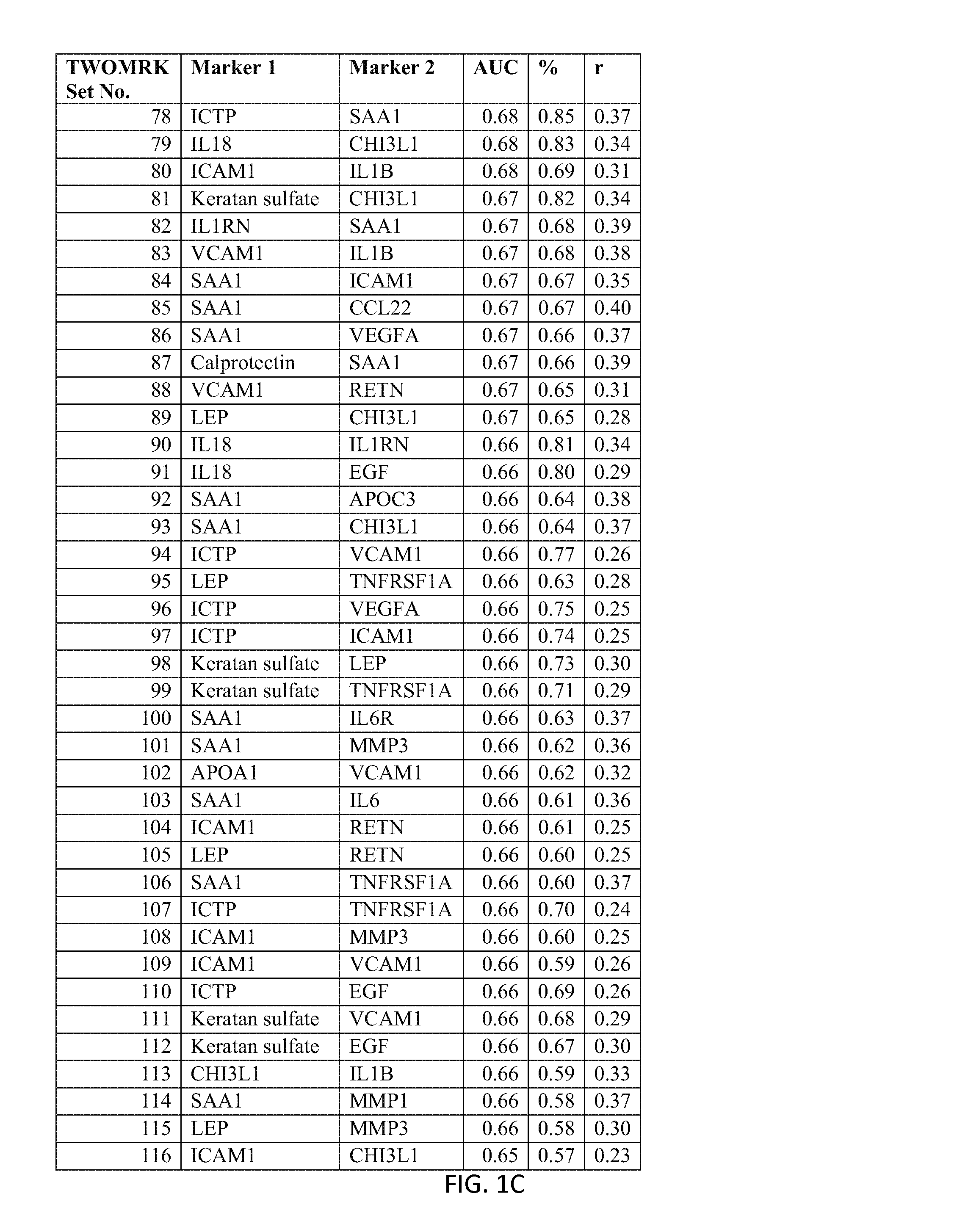
176 results about "Disease activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The concept of disease activity is useful for characterizing the current degree of severity and the progression of the disease. Disease activity has to be differentiated from disease severity, which is a concept encompassing much broader aspects of the disease process and its conse- quences.
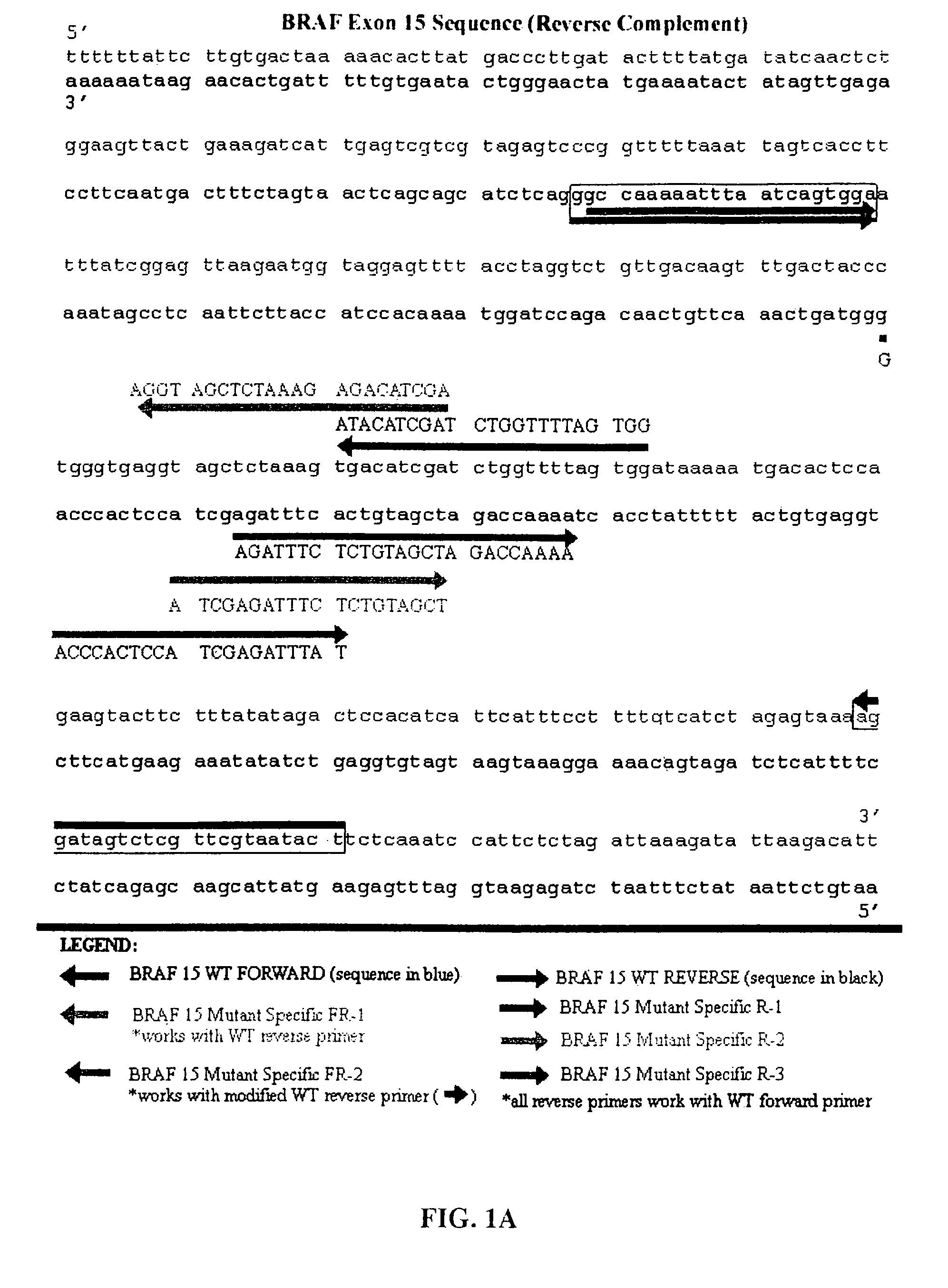
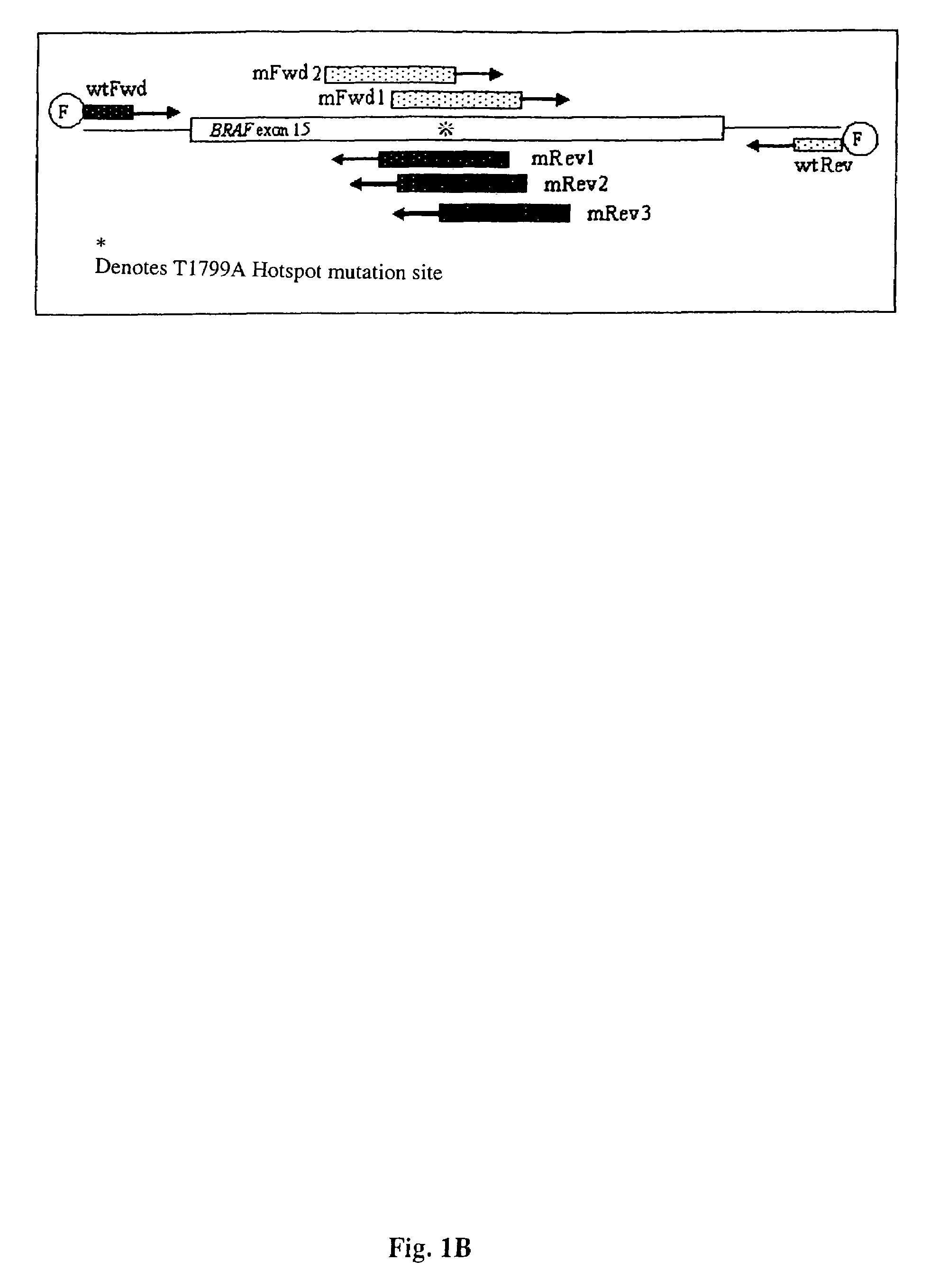
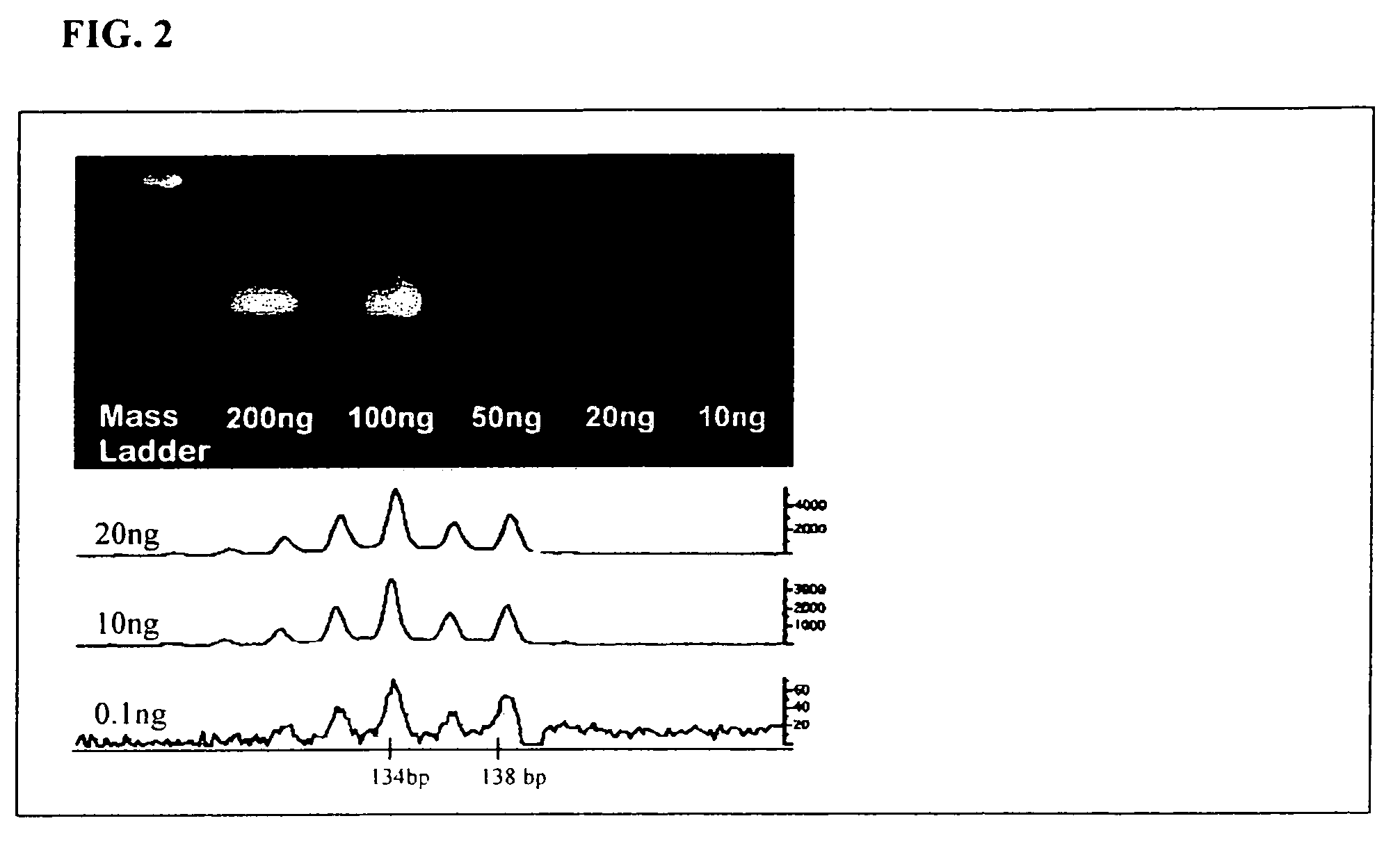
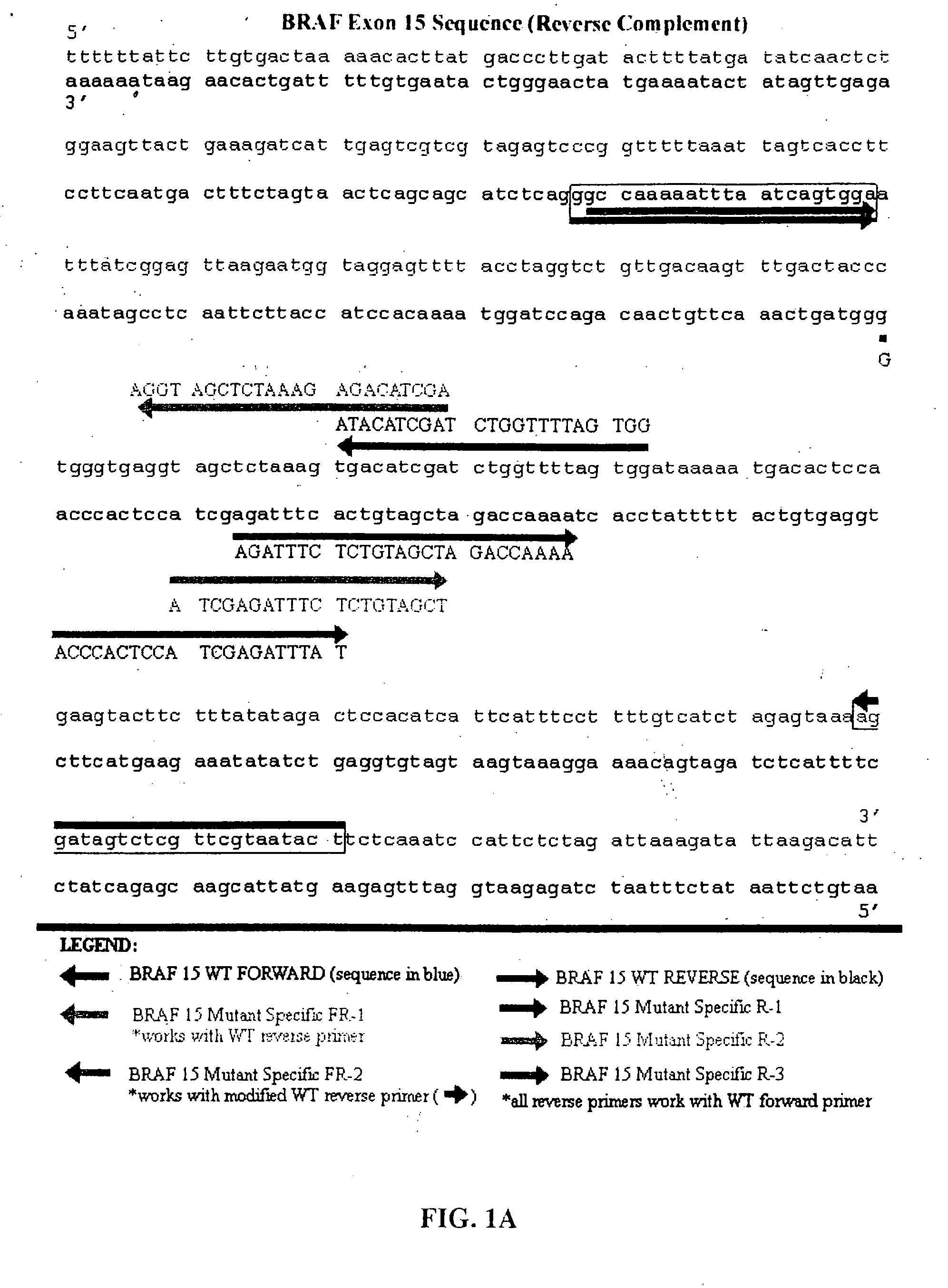
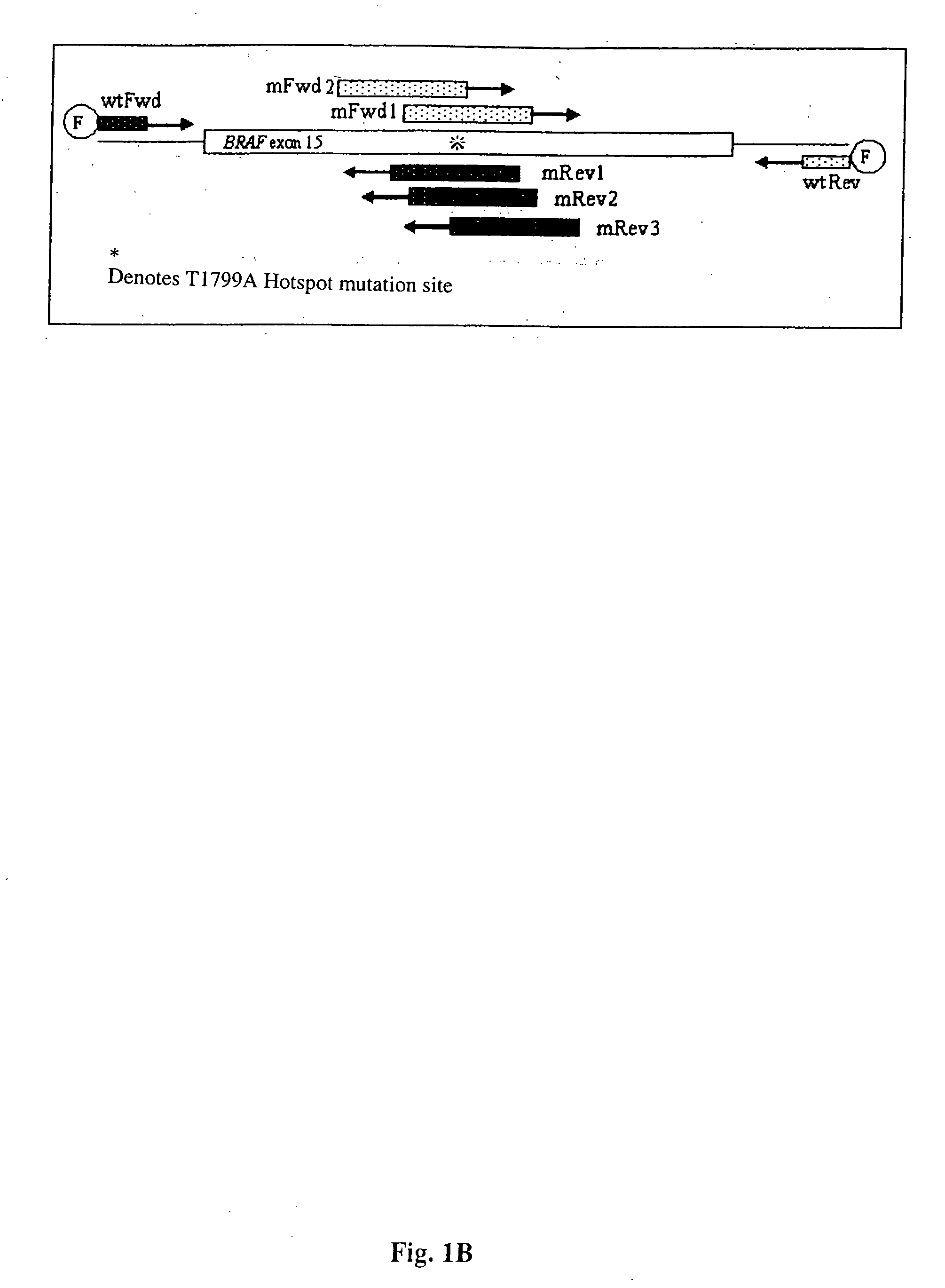
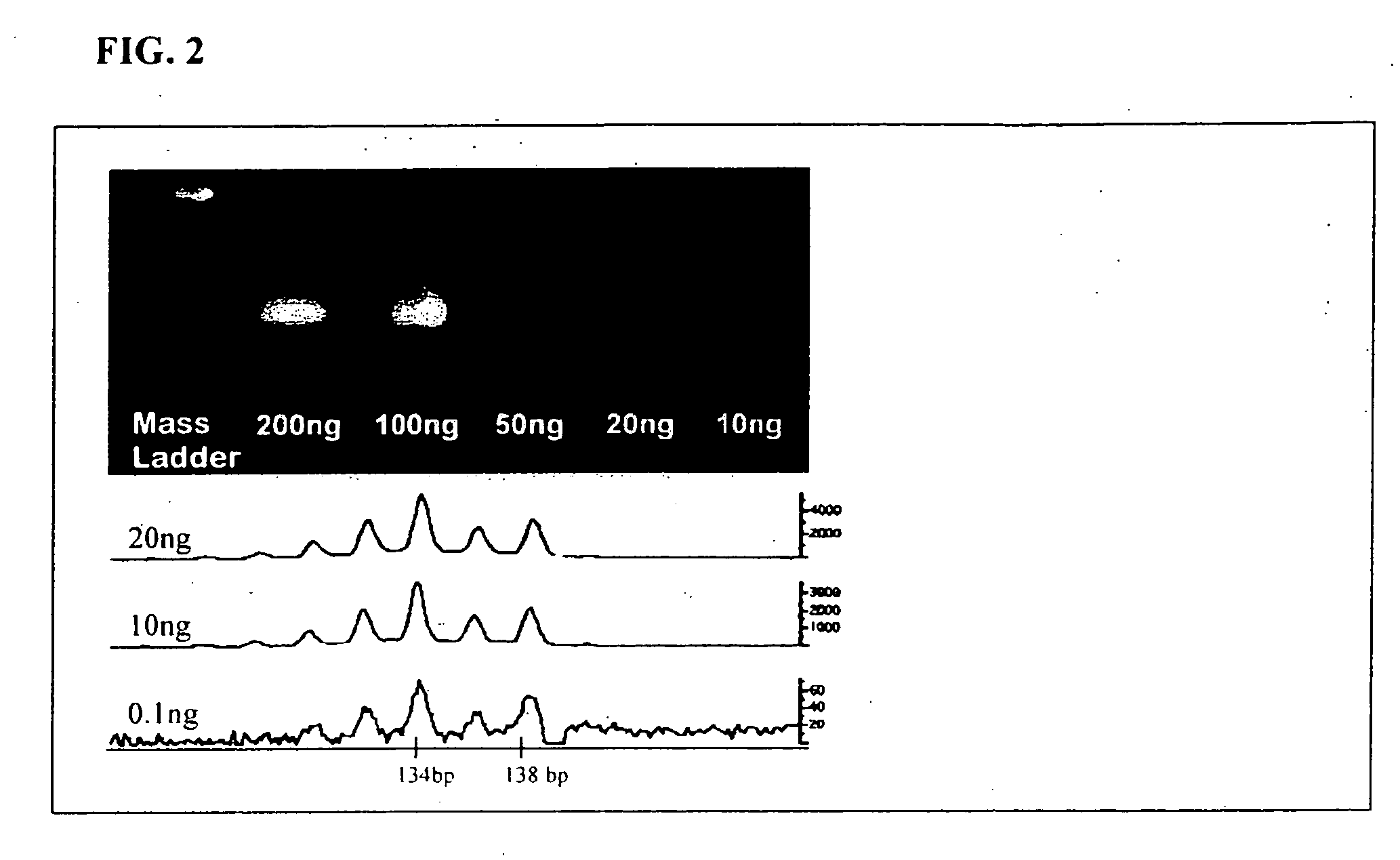
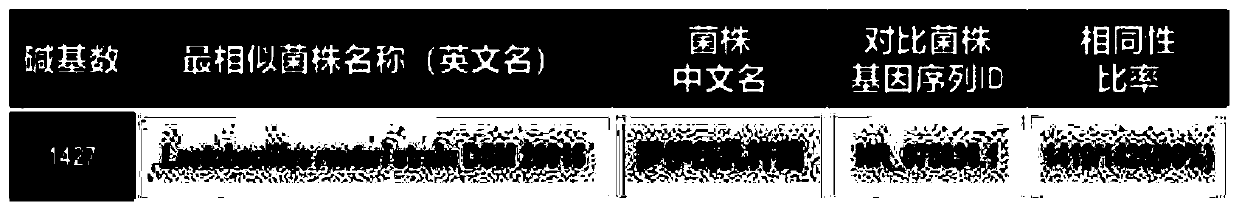
Methods for detecting circulating mutant BRAF DNA
ActiveUS7442507B2High sensitivityMicrobiological testing/measurementFermentationMelanomaNucleic acid sequencing
The present invention relates to a method for detecting the presence of circulating mutant BRAF DNA, which may be present in circulating melanoma cells or as DNA shed from tumor cells. Methods, compositions and kits which employ one or more sets of BRAF mutant specific primer pairs for detection of circulating mutant BRAF DNA are presented. Also provided are methods for diagnosing and / or determining stage / progression of a melanoma in a mammal based on detection of a BRAF mutant nucleic acid sequence. Such methods are also well suited to monitoring disease activity in patients with active disease or those in remission.
Owner:NEW YORK UNIV +1
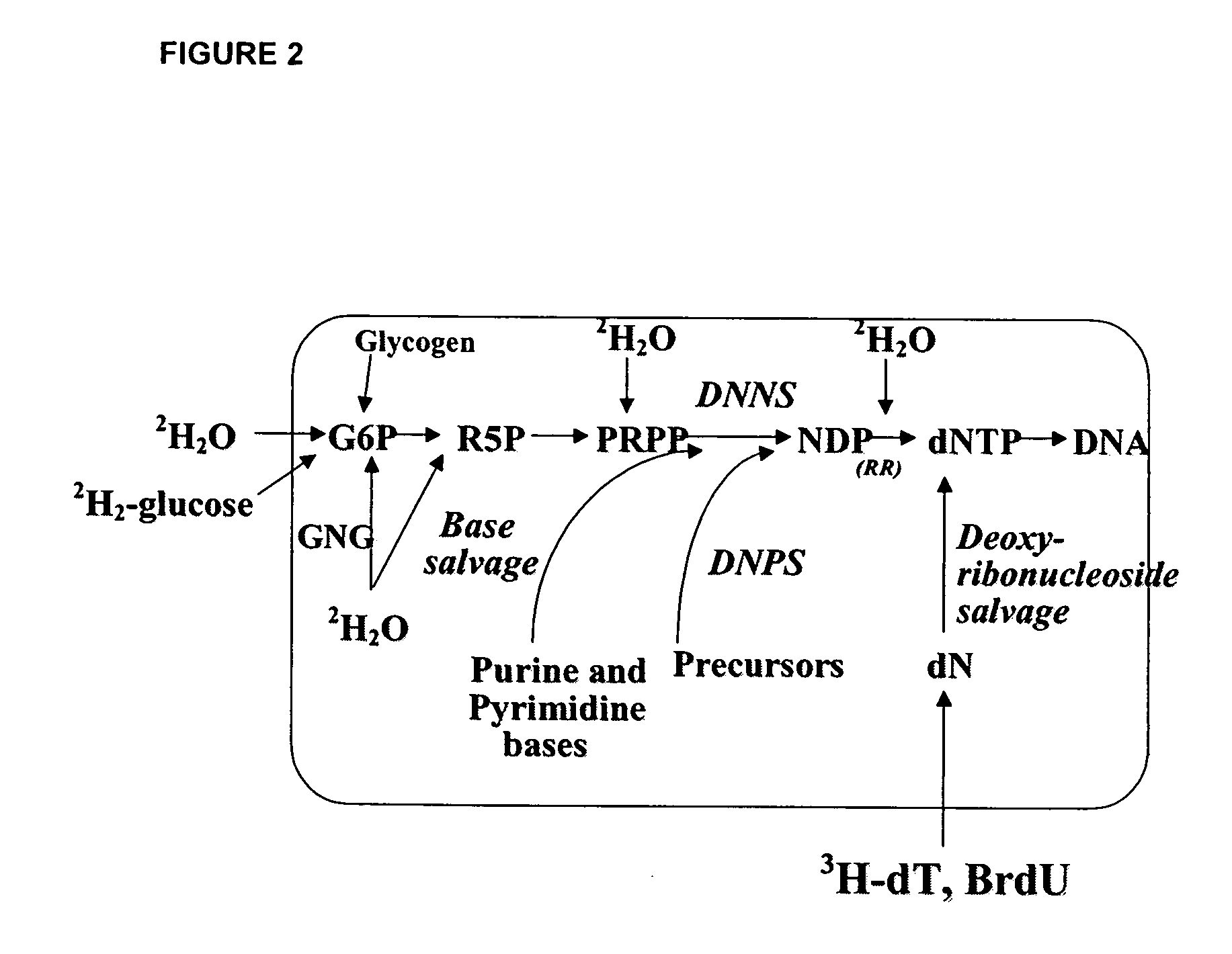
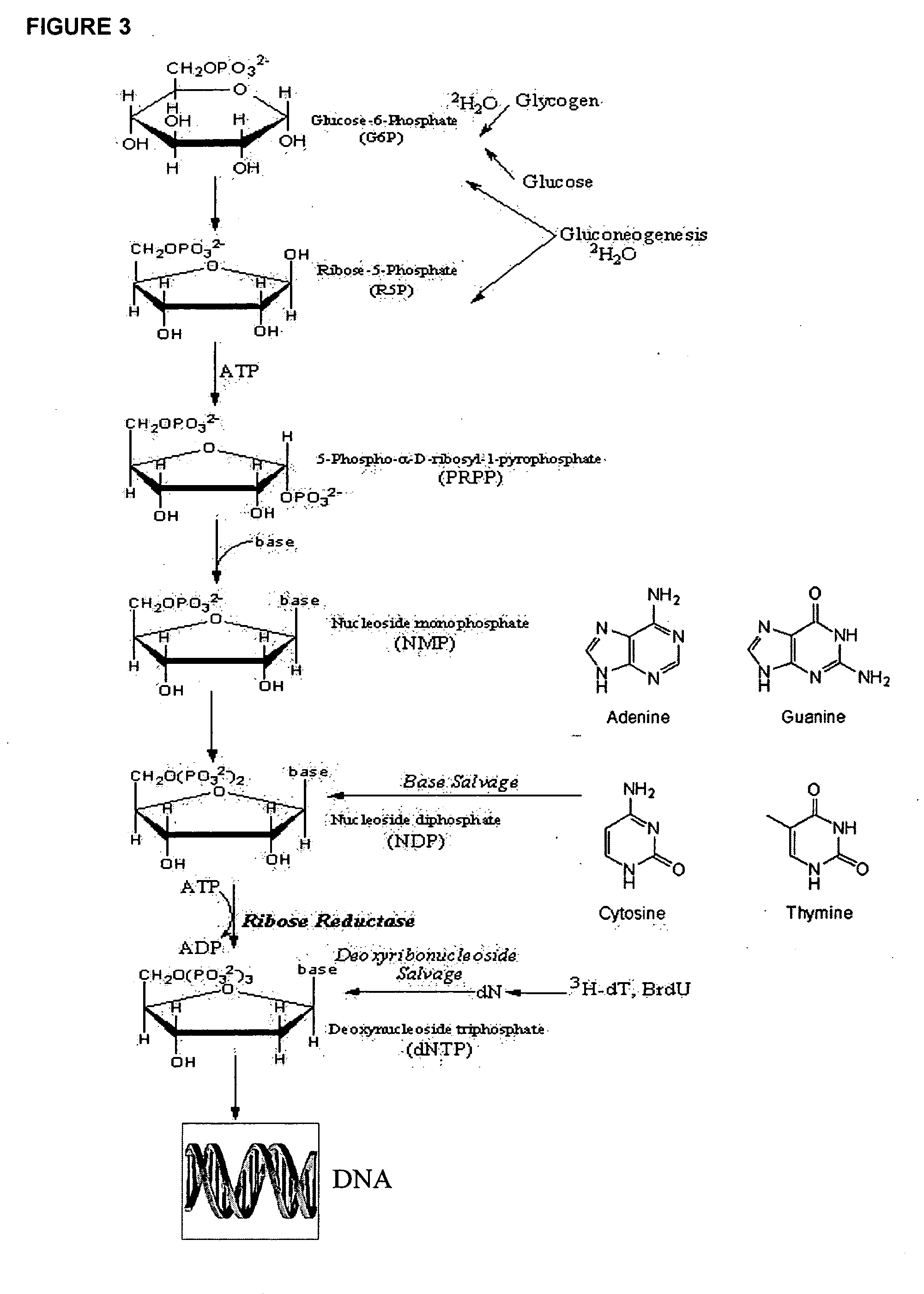
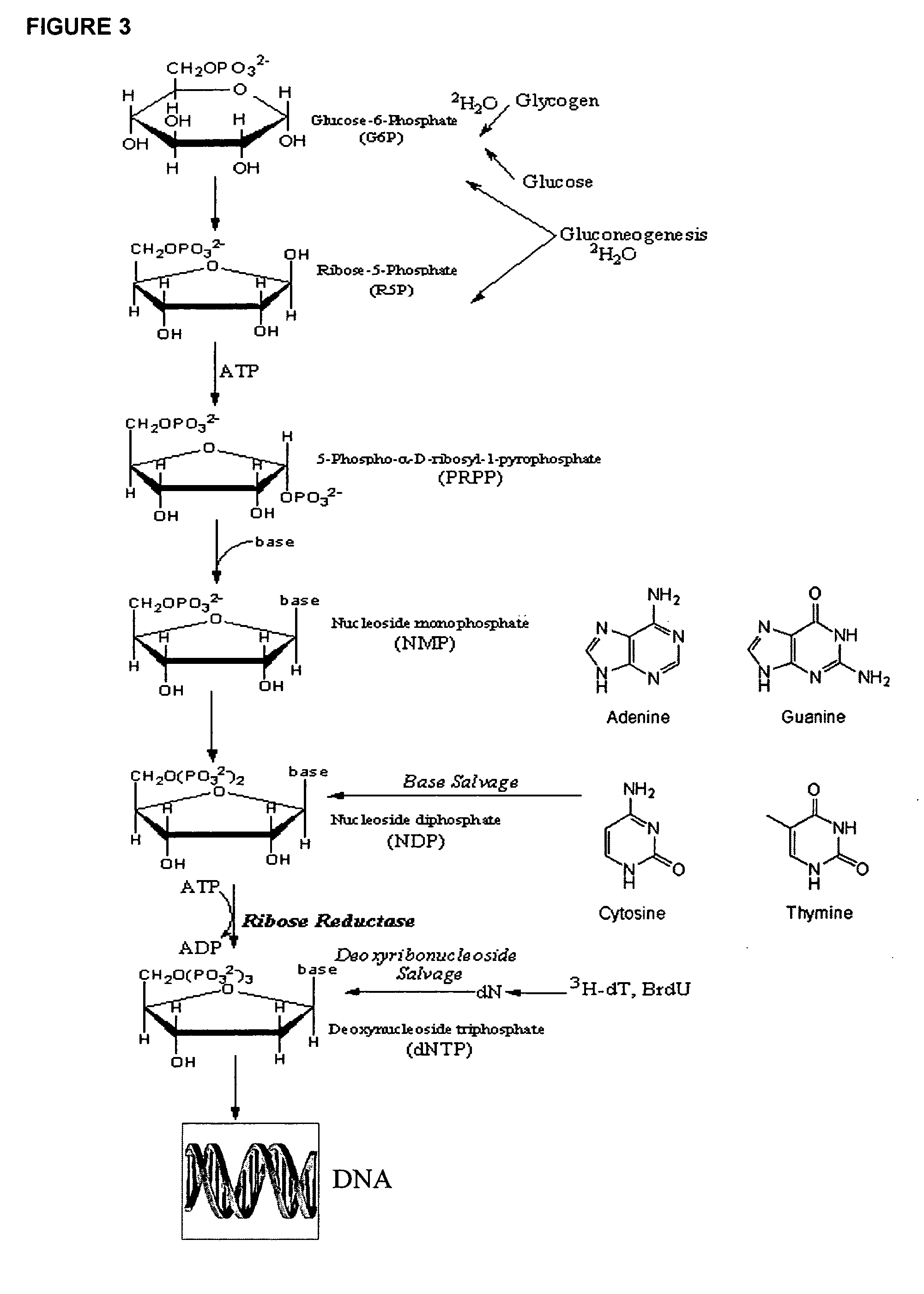
Molecular flux rates through critical pathways measured by stable isotope labeling in vivo, as biomarkers of drug action and disease activity
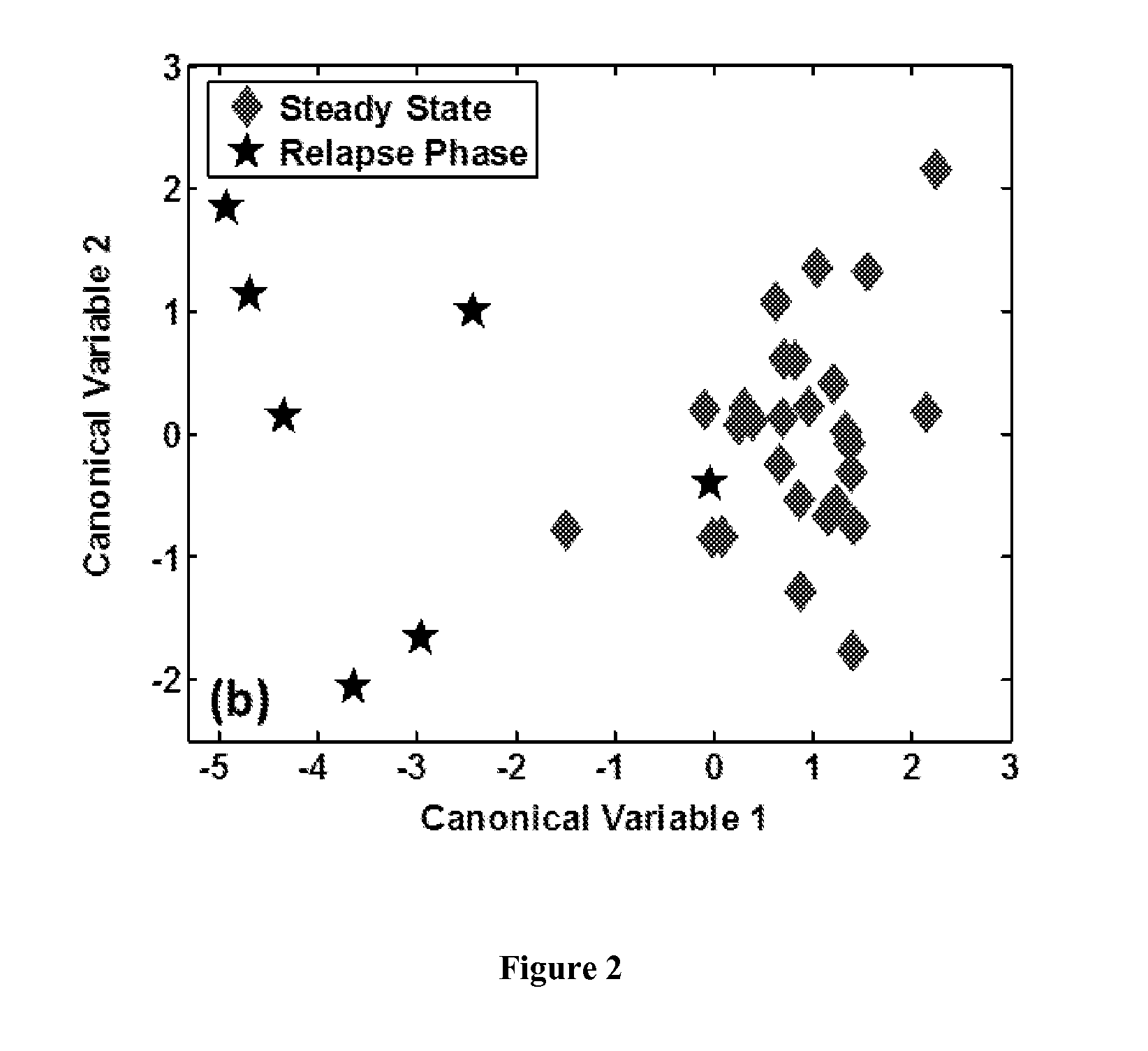
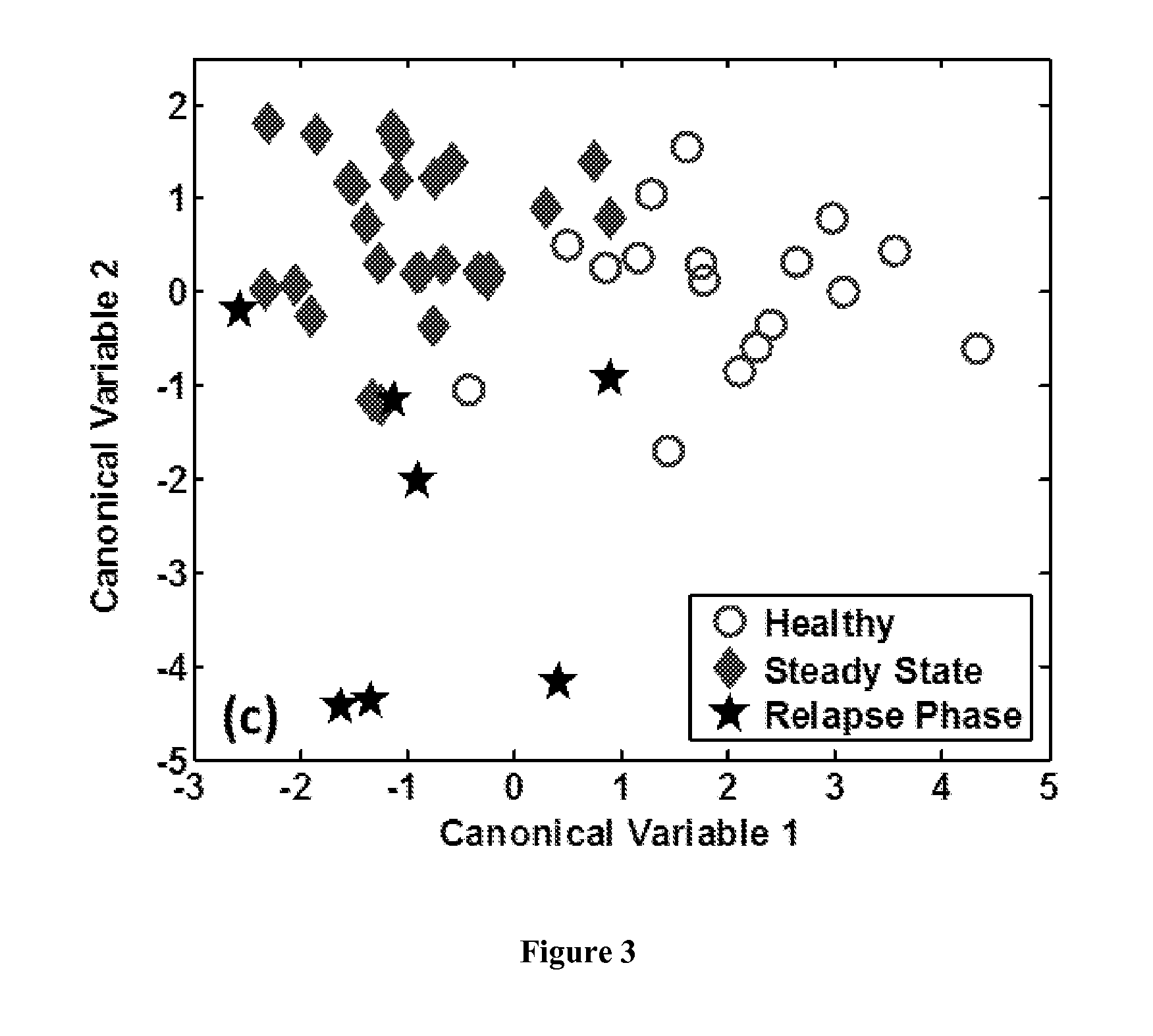
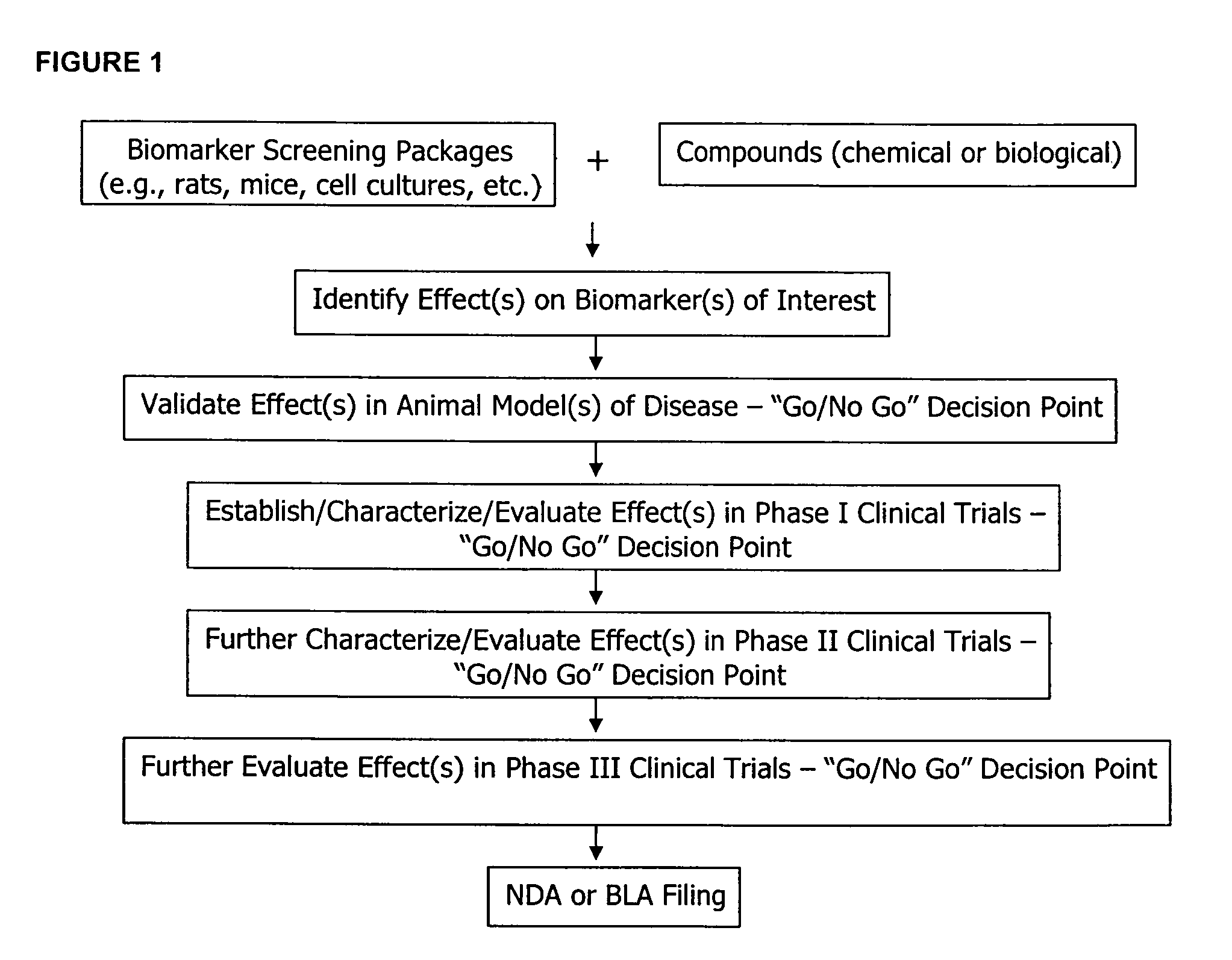
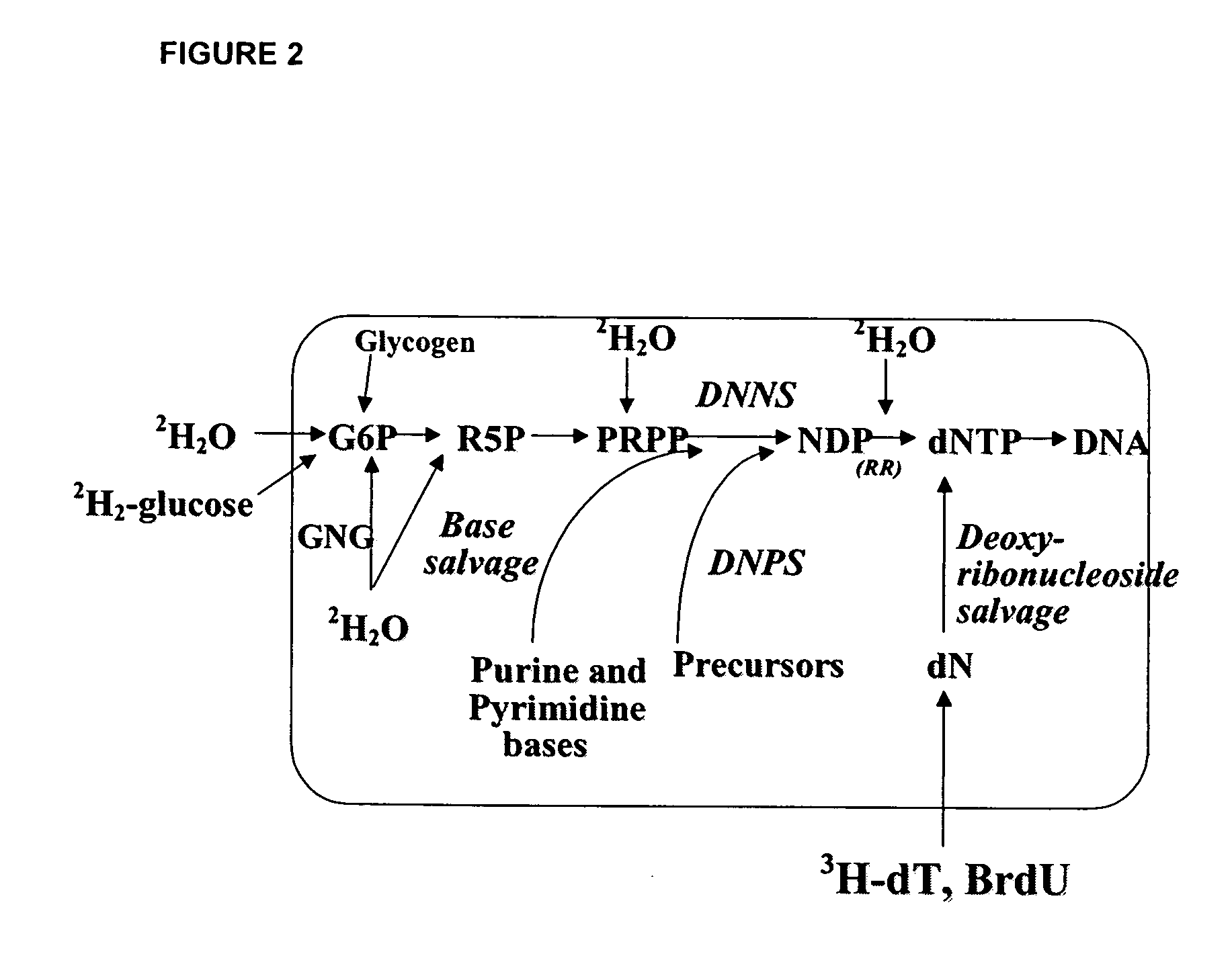
The methods described herein enable the evaluation of compounds on subjects to assess their therapeutic efficacy or toxic effects. The target of analysis is the underlying biochemical process or processes (i.e., metabolic process) thought to be involved in disease pathogenesis. Molecular flux rates within the one or more biochemical processes serve as biomarkers and are quantitated and compared with the molecular flux rates (i.e., biomarker) from control subjects (i.e., subjects not exposed to the compounds). Any change in the biomarker in the subject relative to the biomarker in the control subject provides the necessary information to evaluate therapeutic efficacy of an administered drug or a toxic effect and to develop the compound further if desired. In one aspect of the invention, stable isotope-labeled substrate molecules are administered to a subject and the label is incorporated into targeted molecules in a manner that reveals molecular flux rates through one or more metabolic pathways of interest. By this method, a comparison between subjects and control subjects reveals the effects of the chemical entity or entities on the biomarkers. This, in turn, allows for the identification of potential therapeutic uses or toxicities of the compound. Combinations of compounds can also be systematically evaluated for complementary, synergistic, or antagonistic actions on the metabolic pathways of interest, using the methods of the present invention as a strategy for identifying and confirming novel therapeutic or toxic combinations of compounds.
Owner:RGT UNIV OF CALIFORNIA
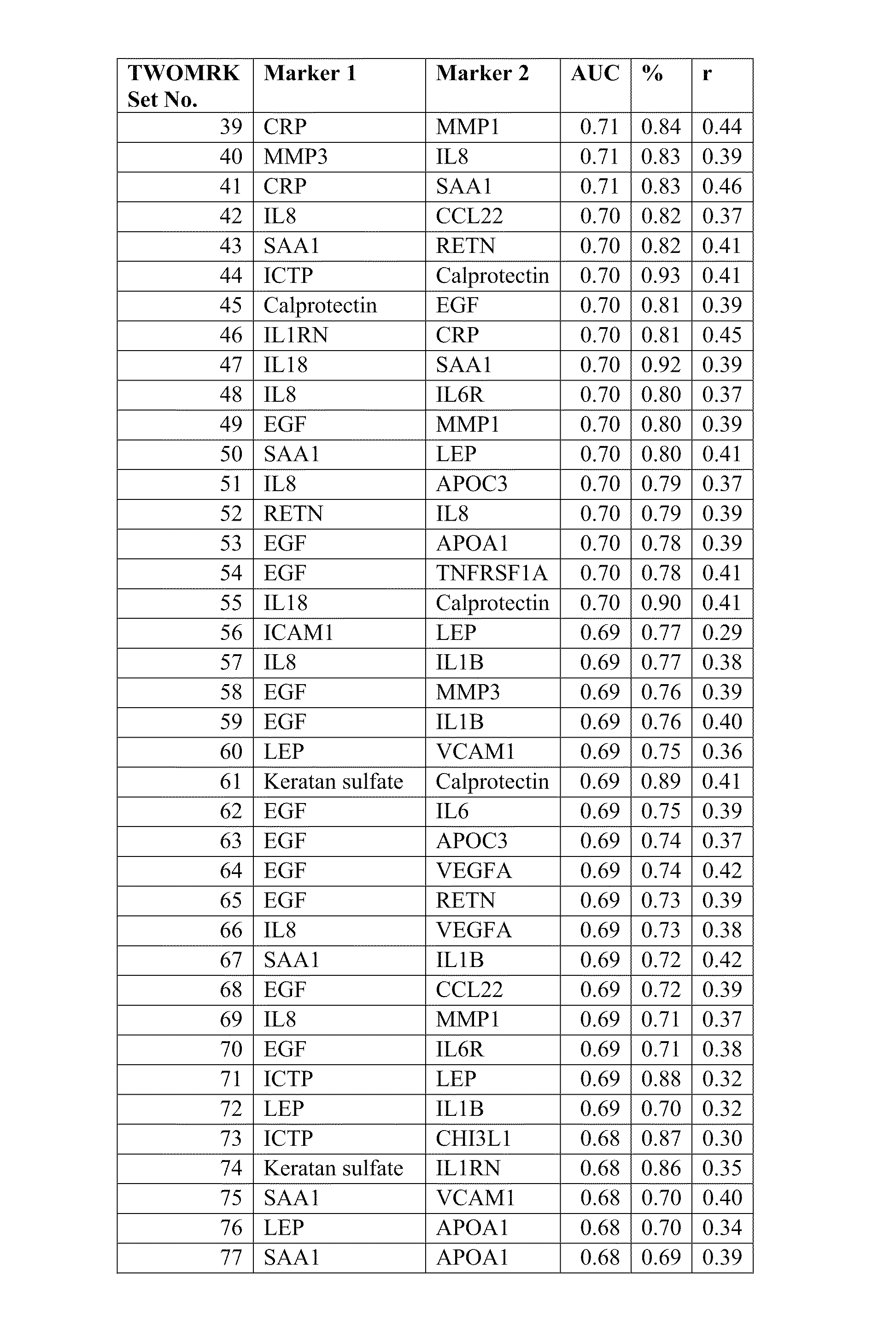
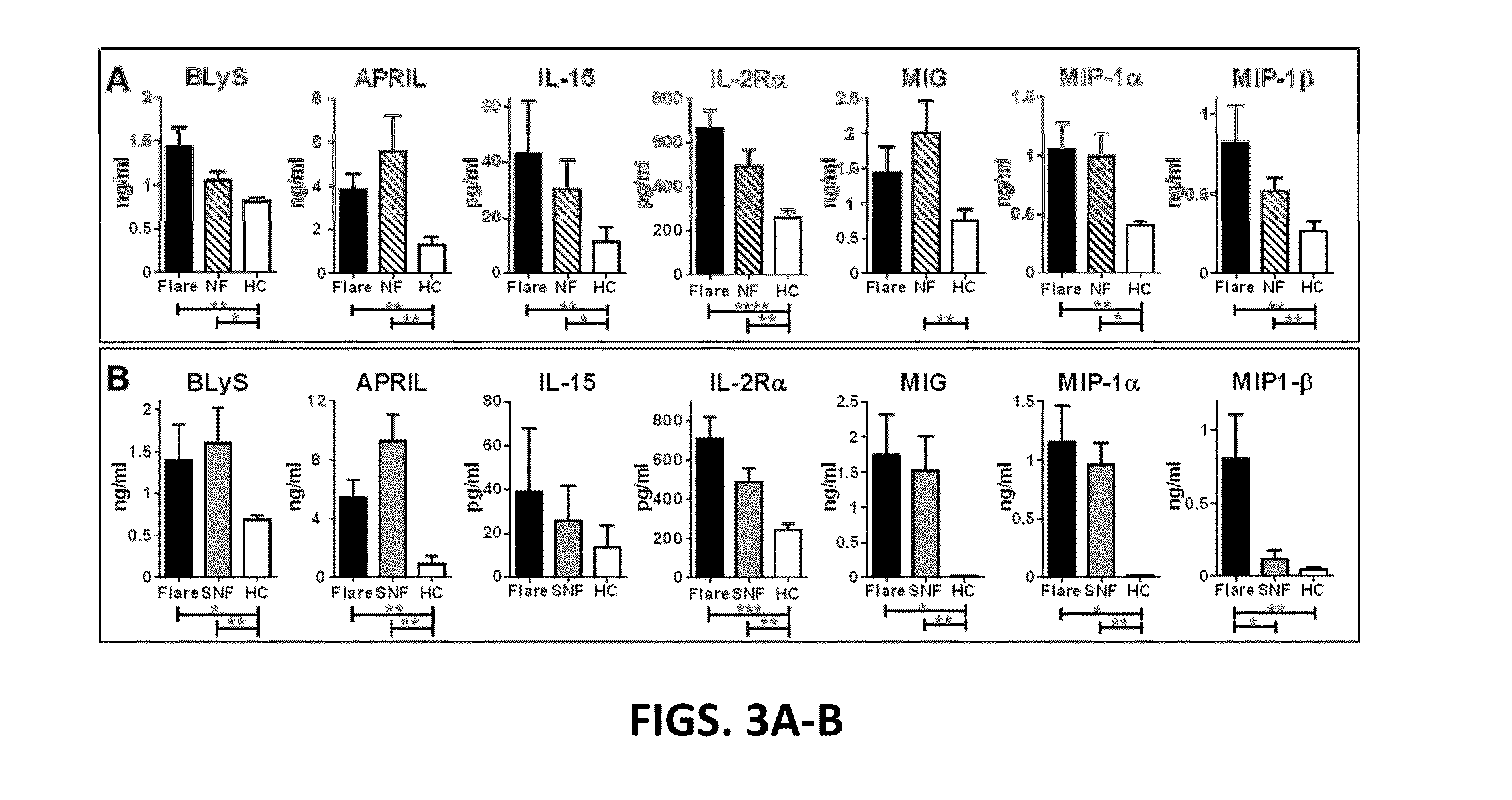
Biomarkers and methods for measuring and monitoring inflammatory disease activity
ActiveUS20110137851A1Microbiological testing/measurementSkeletal disorderComputerized systemDisease activity
Biomarkers useful for diagnosing and assessing inflammatory disease are provided, along with kits for measuring their expression. The invention also provides predictive models, based on the biomarkers, as well as computer systems, and software embodiments of the models for scoring and optionally classifying samples. The biomarkers include at least two biomarkers selected from the DAIMRK group and the score is a disease activity index (DAI).
Owner:LAB OF AMERICA HLDG +1
Bifidobacterium longum and application thereof
ActiveCN108220206AGood ability to tolerate simulated gastrointestinal fluidNo side effectsMilk preparationBacteriaHuman bodyBiotechnology
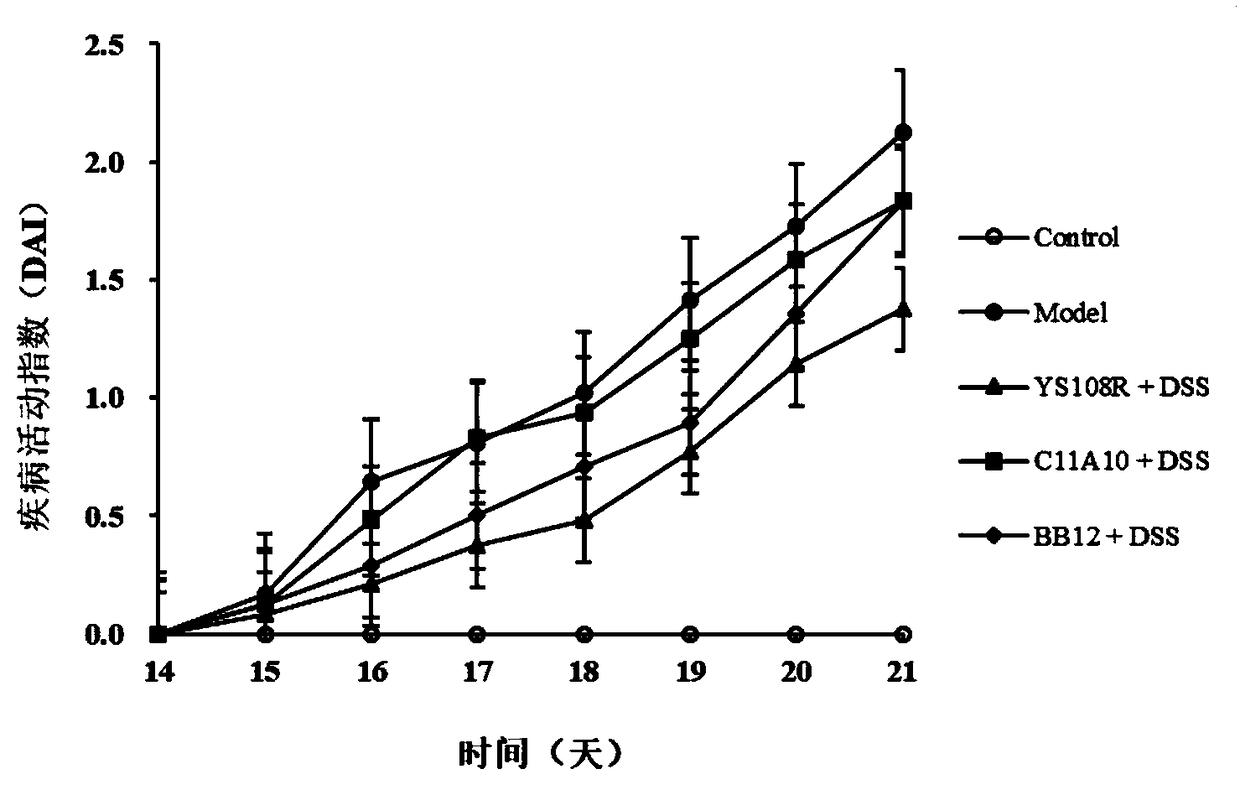
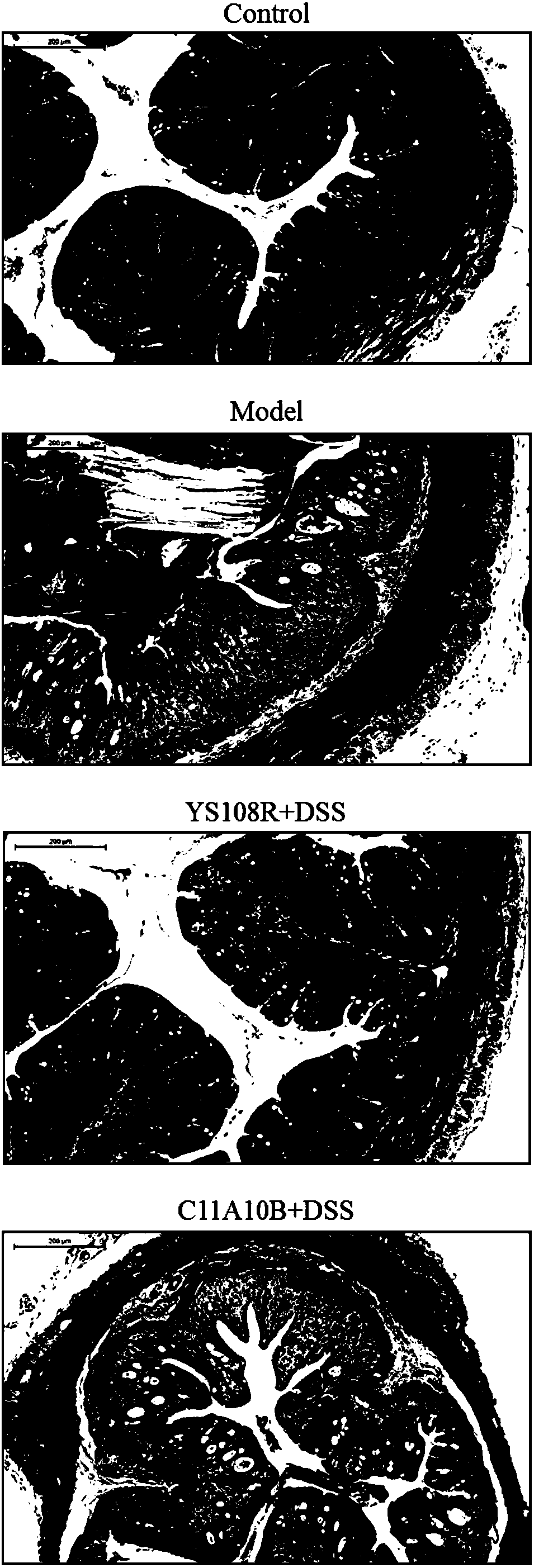
The invention discloses bifidobacterium longum and the application thereof and belongs to the technical field of biologics. The invention provides a bifidobacterium longum YS108R strain which has thecharacteristic of generating viscous exopolysaccharide, and has a remarkable improvement function on DSS (Dextran Sulfate Sodium) induced mouse colitis models. The strain has a relatively good capability of enduring simulated gastrointestinal fluids, is capable of remarkably reducing disease activity indexes of mice in the DSS induction period, and has an effective protection function on colon tissue. In addition, the bifidobacterium longum YS108R disclosed by the invention is separated from intestinal florae of healthy people, is free of toxic and / or side effects on human bodies, and has certain advantages when being compared with conventional medicine treatment. The strain can be used for preparing probiotic powder, fermented milk, and the like, and has wide market prospects.
Owner:无锡特殊食品与营养健康研究院有限公司
Antiproliferative factor
InactiveUS20020016443A1Sugar derivativesPeptide/protein ingredientsBladder cancerInterstitial cystitis
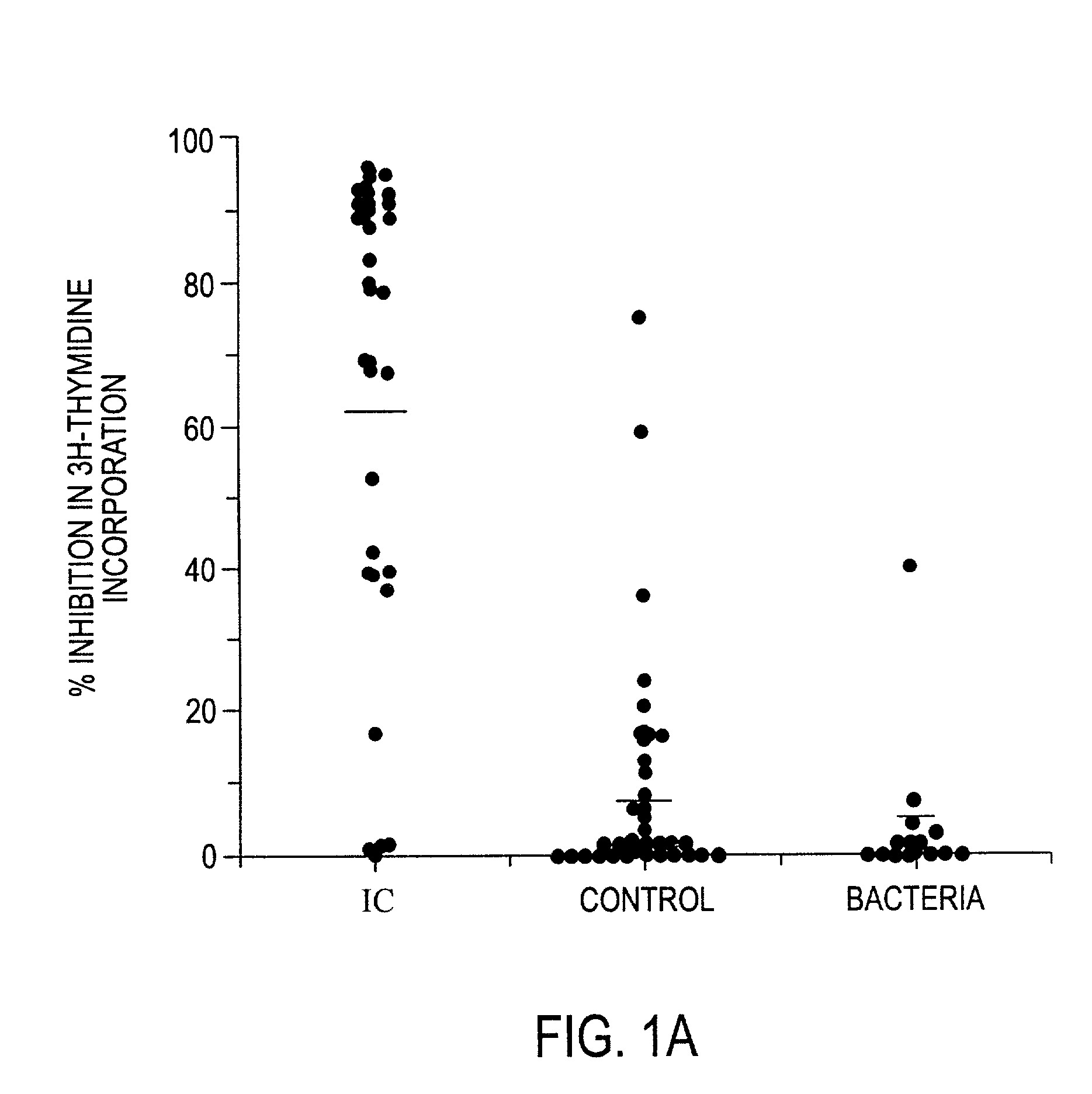
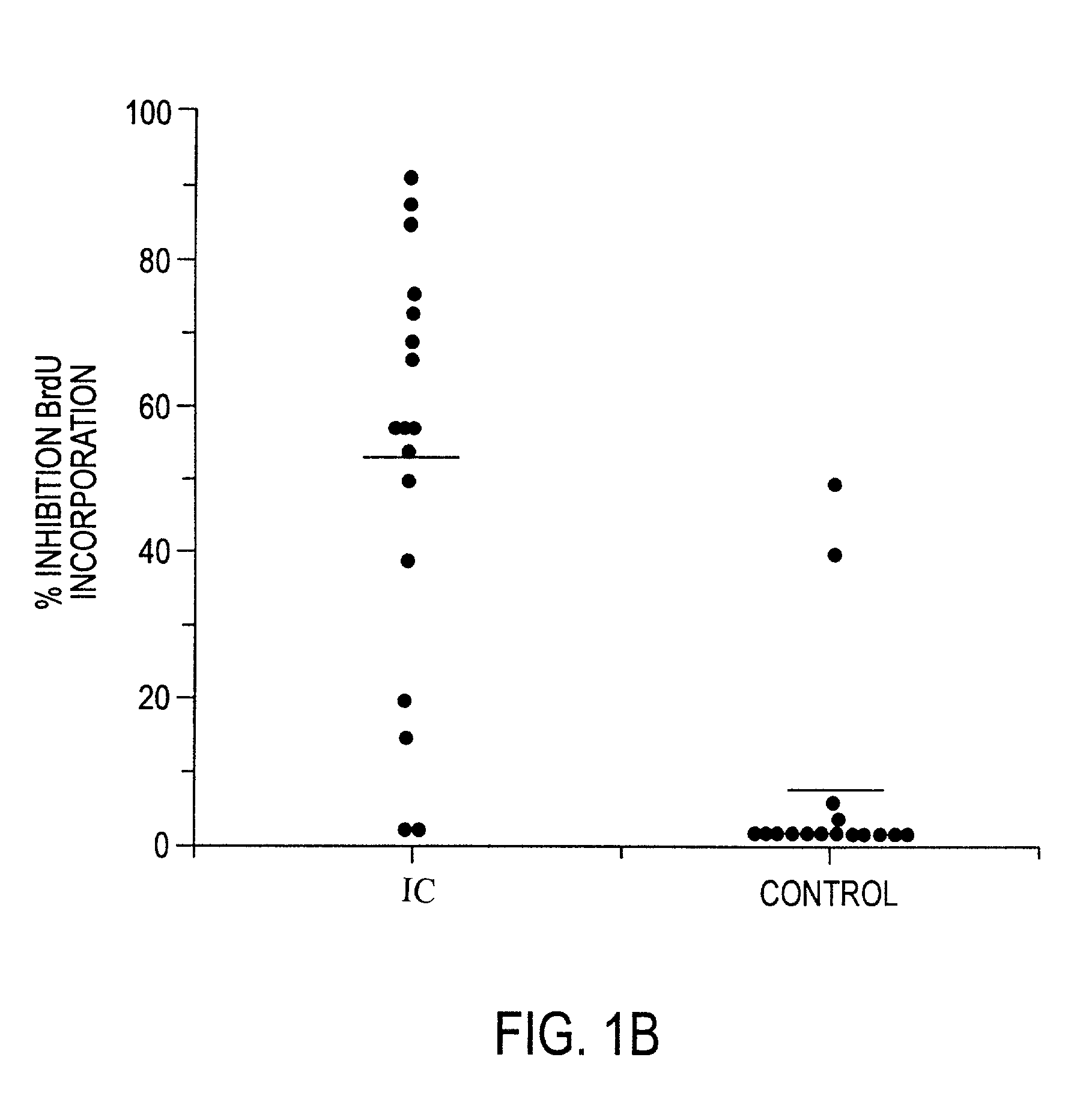
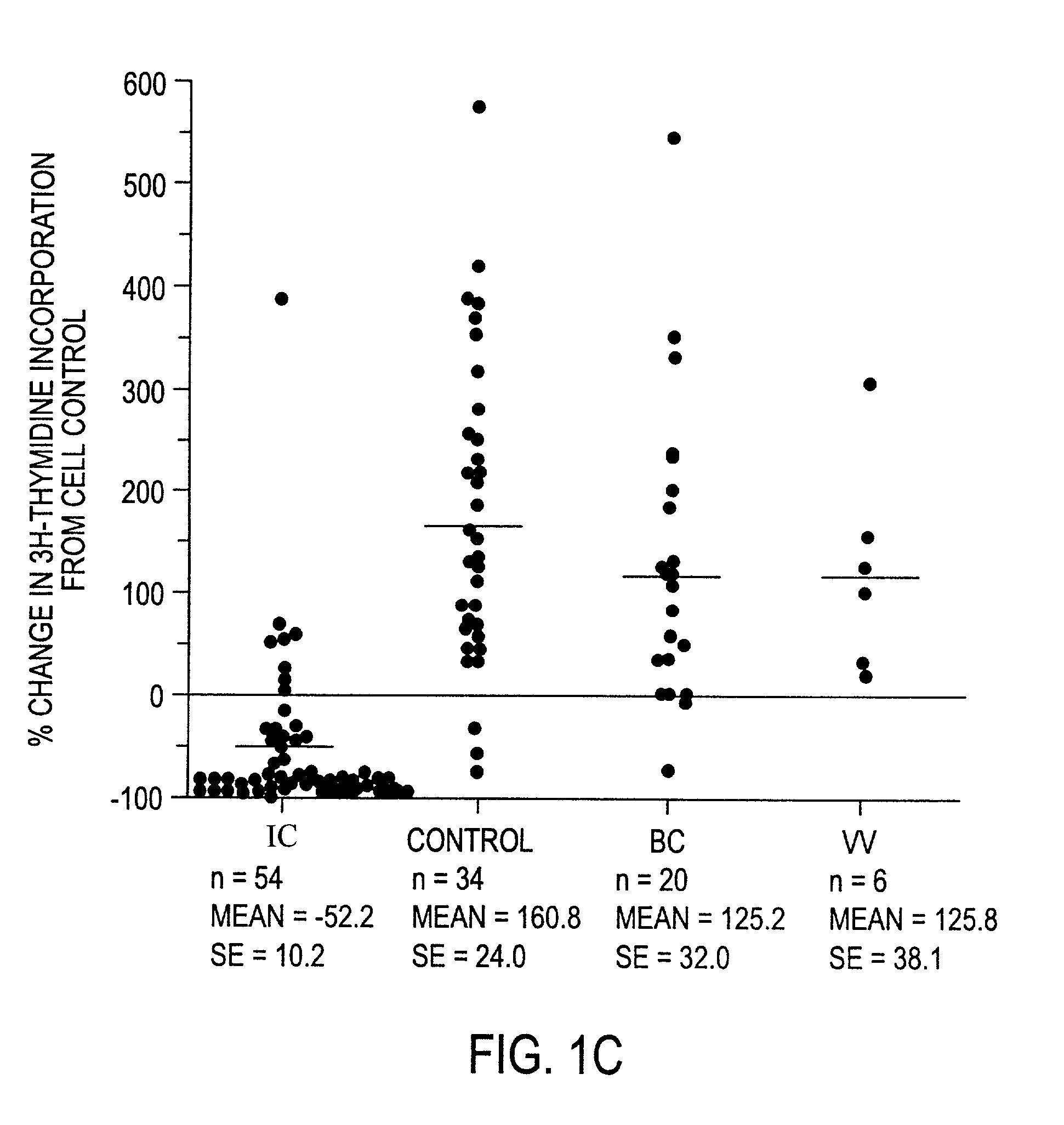
The invention relates to a novel antiproliferative factor (APF) present in urine of patients with interstitial cystitis (IC). APF is useful as a marker for disease activity and its antagonists are useful as therapeutic medicaments for IC and other conditions associated with elevated APF. APF and its agonists are useful in the treatment of diseases associated with cell proliferation, such as bladder cancer.
Owner:KEAY SUSAN K +3
Diagnosing, prognosing and monitoring multiple sclerosis
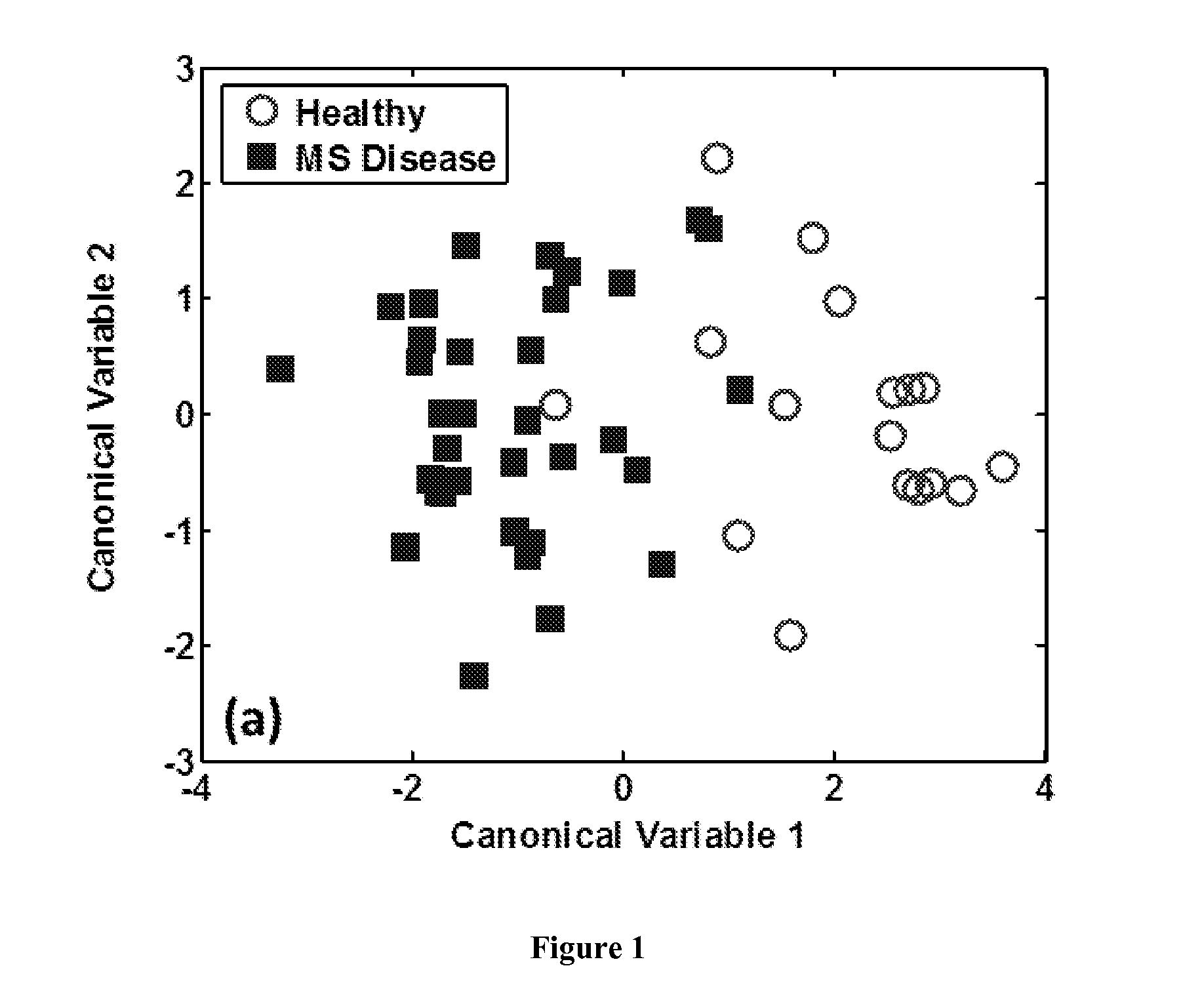
The present invention provides a system and method for diagnosing, monitoring or prognosing Multiple Sclerosis at different stages as well as affording the prediction of disease activity and response to a treatment regimen, using at least one sensor comprising carbon nanotubes or metal nanoparticles, each coated with various organic coatings in conjunction with a pattern recognition algorithm.
Owner:TECHNION RES & DEV FOUND LTD +2
Molecular flux rates through critical pathways measured by stable isotope labeling in vivo, as biomarkers of drug action and disease activity
The methods described herein enable the evaluation of compounds on subjects to assess their therapeutic efficacy or toxic effects. The target of analysis is the underlying biochemical process or processes (i.e., metabolic process) thought to be involved in disease pathogenesis. Molecular flux rates within the one or more biochemical processes serve as biomarkers and are quantitated and compared with the molecular flux rates (i.e., biomarker) from control subjects (i.e., subjects not exposed to the compounds). Any change in the biomarker in the subject relative to the biomarker in the control subject provides information to evaluate therapeutic efficacy of an administered drug or a toxic effect and to develop the compound further if desired. In one aspect of the invention, stable isotope-labeled substrate molecules are administered to a subject and the label is incorporated into targeted molecules in a manner that reveals molecular flux rates through metabolic pathways of interest.
Owner:RGT UNIV OF CALIFORNIA
Steroid responsive nucleic acid expression and prediction of disease activity
InactiveUS20070248978A1Microbiological testing/measurementBiological testingSteroid responsiveTransplant rejection
The invention relates to methods useful for diagnosing and monitoring the steroid responsiveness of a subject by detecting expression of steroid modulated genes and for predicting transplant rejection and non-rejection.
Owner:XDX
Methods for detecting circulating mutant BRAF DNA
ActiveUS20060246476A1High sensitivityMicrobiological testing/measurementFermentationMelanomaNucleic acid sequencing
The present invention relates to a method for detecting the presence of circulating mutant BRAF DNA, which may be present in circulating melanoma cells or as DNA shed from tumor cells. Methods, compositions and kits which employ one or more sets of BRAF mutant specific primer pairs for detection of circulating mutant BRAF DNA are presented. Also provided are methods for diagnosing and / or determining stage / progression of a melanoma in a mammal based on detection of a BRAF mutant nucleic acid sequence. Such methods are also well suited to monitoring disease activity in patients with active disease or those in remission.
Owner:NEW YORK UNIV +1
Method for diagnosis and monitoring of disease activity and response to treatment in systemic lupus erythematosus (SLE) and other autoimmune diseases
InactiveUS20100233752A1Sensitive methodMicrobiological testing/measurementDisease diagnosisAutoimmune thyroid diseaseWhite blood cell
The present invention provides methods of diagnosing and monitoring systemic lupus erythematosus and drug-induced lupus erythematosus by measuring cell-based complement activation products in a subject's blood. In particular, the invention describes a diagnostic method employing the measurement of multiple complement activation products, such as C3d and C4d, on the surfaces of red blood cells, white blood cells, and platelets. Kits and automated systems for performing the methods of the invention are also disclosed.
Owner:EXAGEN DIAGNOSTICS +1
Biomarkers for disease activity and clinical manifestations systemic lupus erythematosus
ActiveUS20140135225A1Microbiological testing/measurementLibrary screeningProper treatmentClinical manifestation
This invention related to methods and assays for screening for, identifying, and predicting the severity and clinical manifestations of systemic lupus erythematosus (SLE). Specifically, this invention provides various biomarkers for the prediction of flares of the disease both in number and severity, as well as clinical manifestations of the disease, and methods of using these biomarkers to correctly subclassify patients with this disease, and prescribe appropriate treatment. The invention also provides for biomarkers of lupus disease activity, i.e., flares, as well as biomarkers for the prediction of future flares, and methods of using these biomarkers. The invention also provides, in these biomarkers, targets and methods for drug development and basic research for SLE.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
Adjuvant immune therapy in the treatment of solid tumors through modulation of signaling pathways following engagement of humoral and cell mediated responses
InactiveUS20020187130A1Evaluating efficacyAccurate assessmentBiocideSaccharide peptide ingredientsAdjuvantWhite blood cell
Owner:KINDNESS GEORGE +2
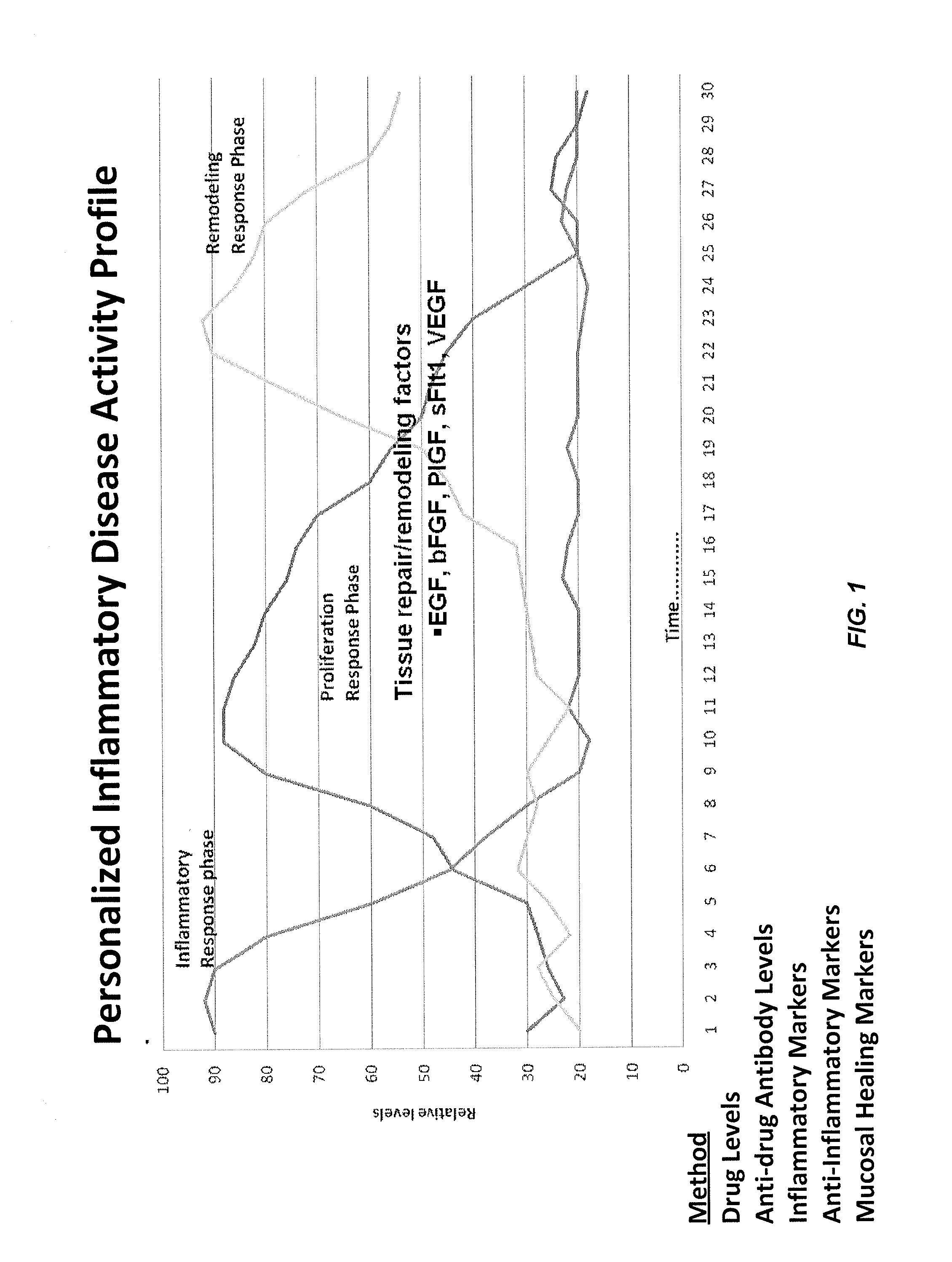
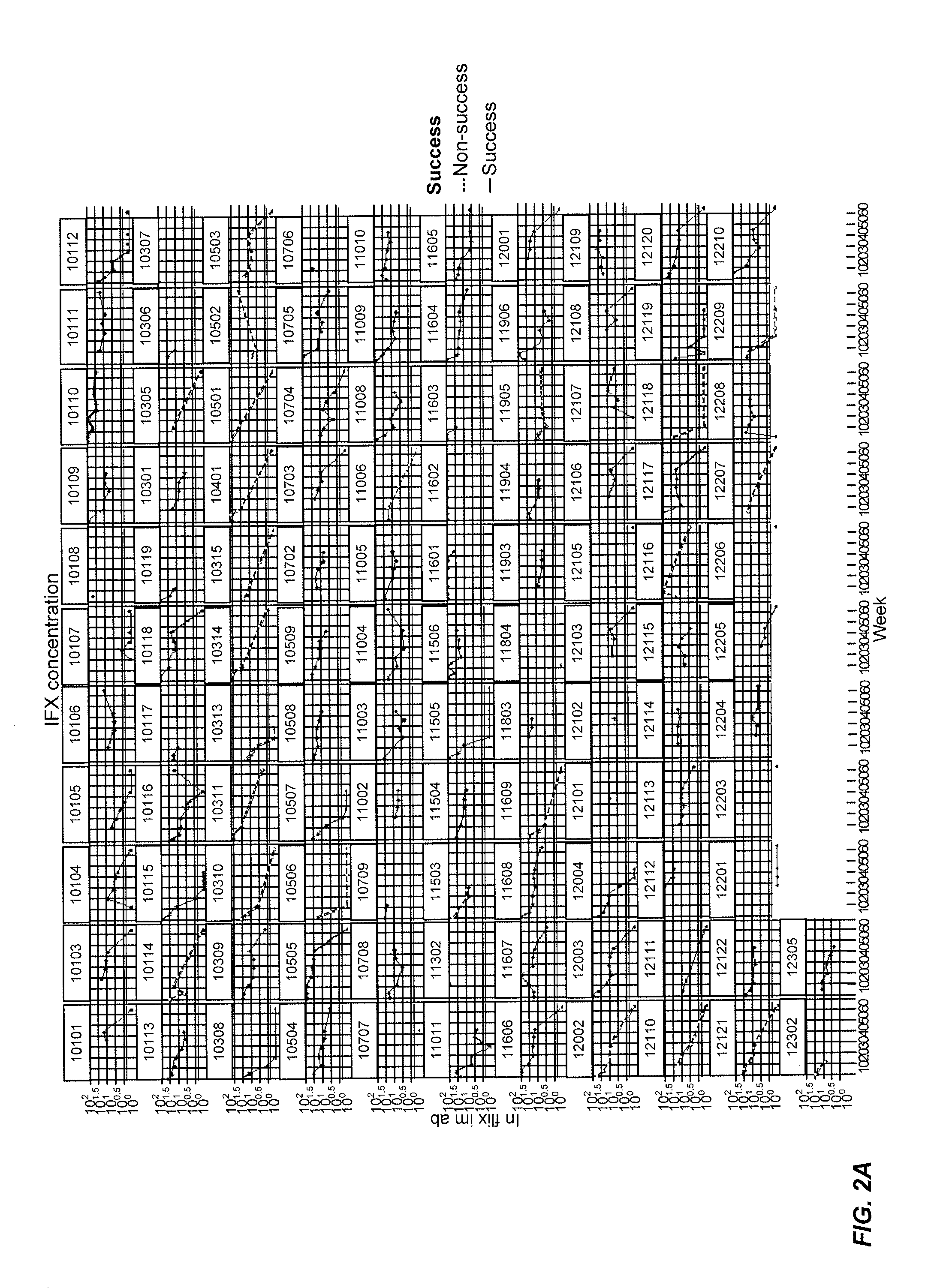
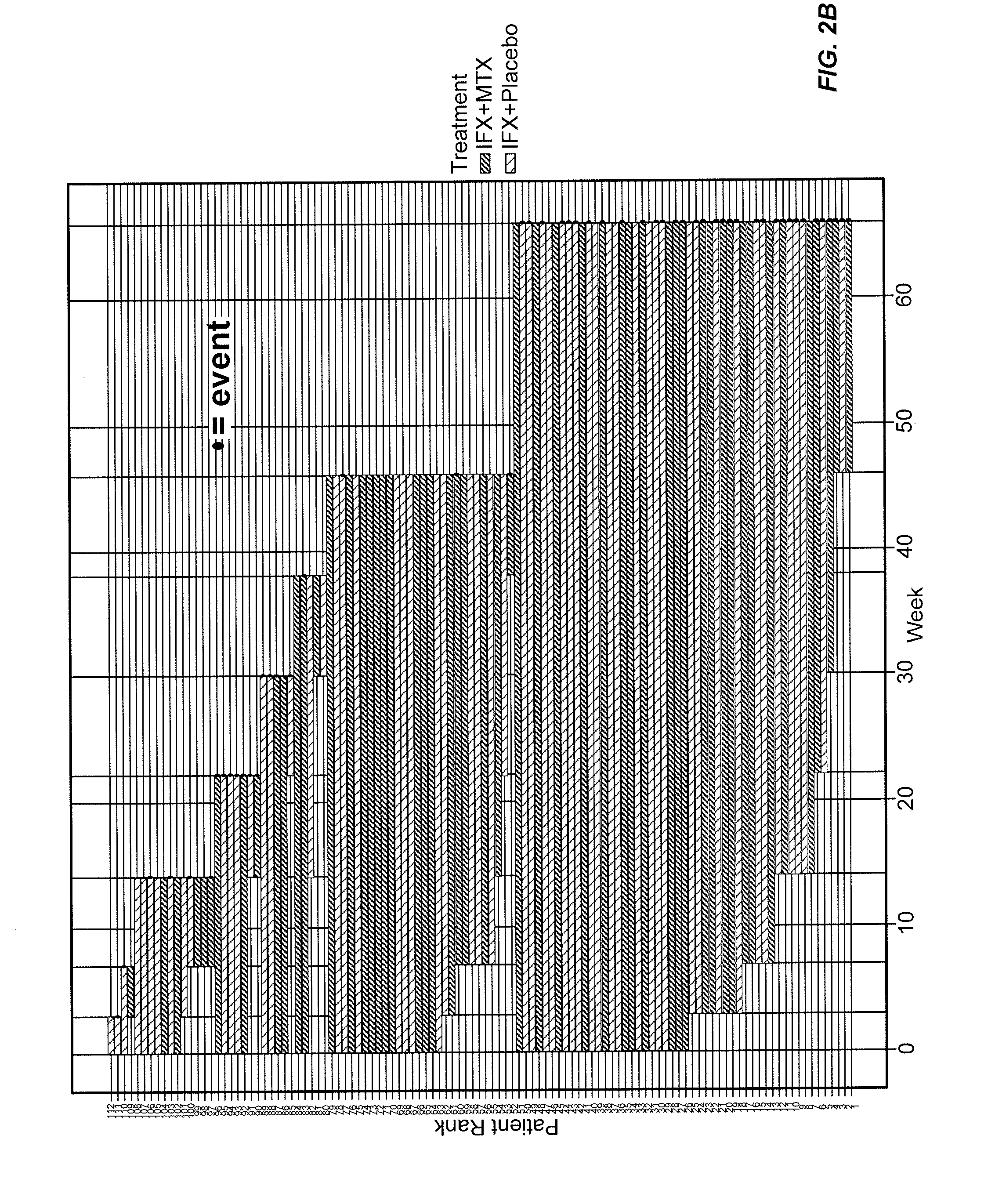
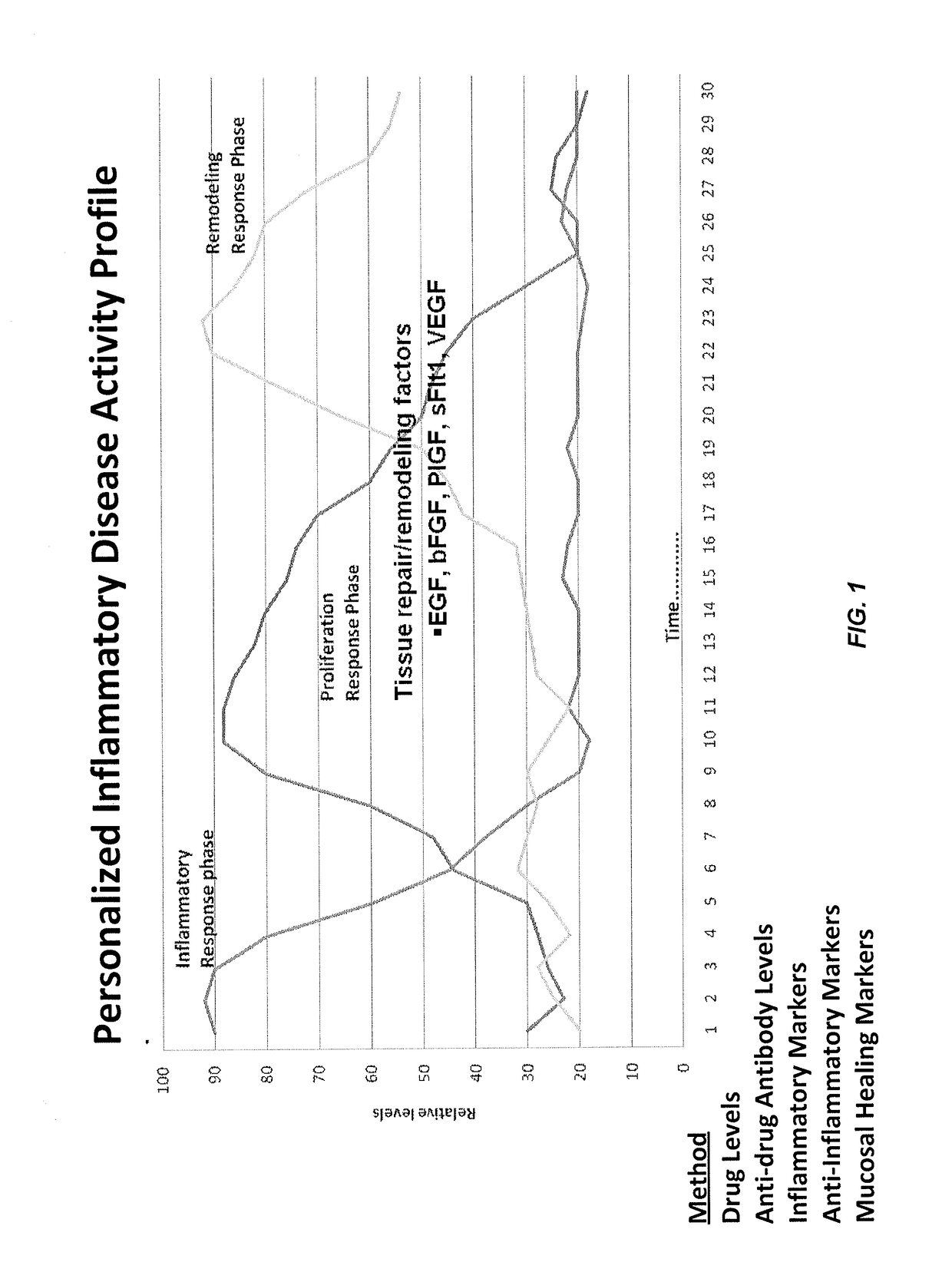
Methods of disease activity profiling for personalized therapy management
ActiveUS20140141983A1Prediction of responsivenessImprove therapeutic managementMicrobiological testing/measurementLibrary screeningTreatment effectDisease activity
The present invention provides methods for personalized therapeutic management of a disease in order to optimize therapy and / or monitor therapeutic efficacy. In particular, the present invention comprises measuring an array of one or a plurality of biomarkers at a plurality of time points over the course of therapy with a therapeutic agent to determine a mucosal healing index for selecting therapy, optimizing therapy, reducing toxicity, and / or monitoring the efficacy of therapeutic treatment. In certain instances, the therapeutic agent is a TNFα inhibitor for the treatment of a TNFα-mediated disease or disorder.
Owner:PROMETHEUS LAB
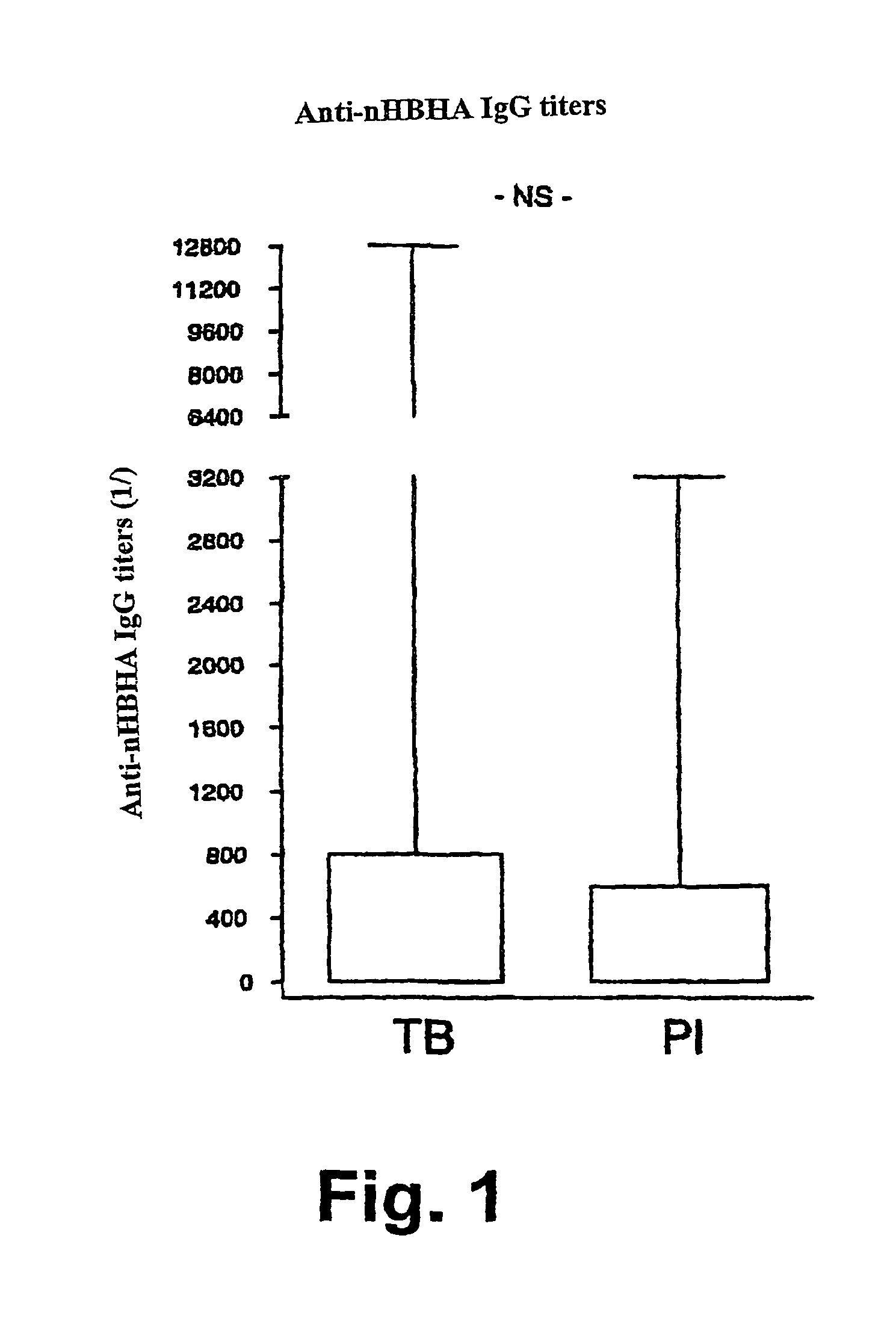
Detection of tuberculosis and infection by Mycobacterium tuberculosis using HBHA
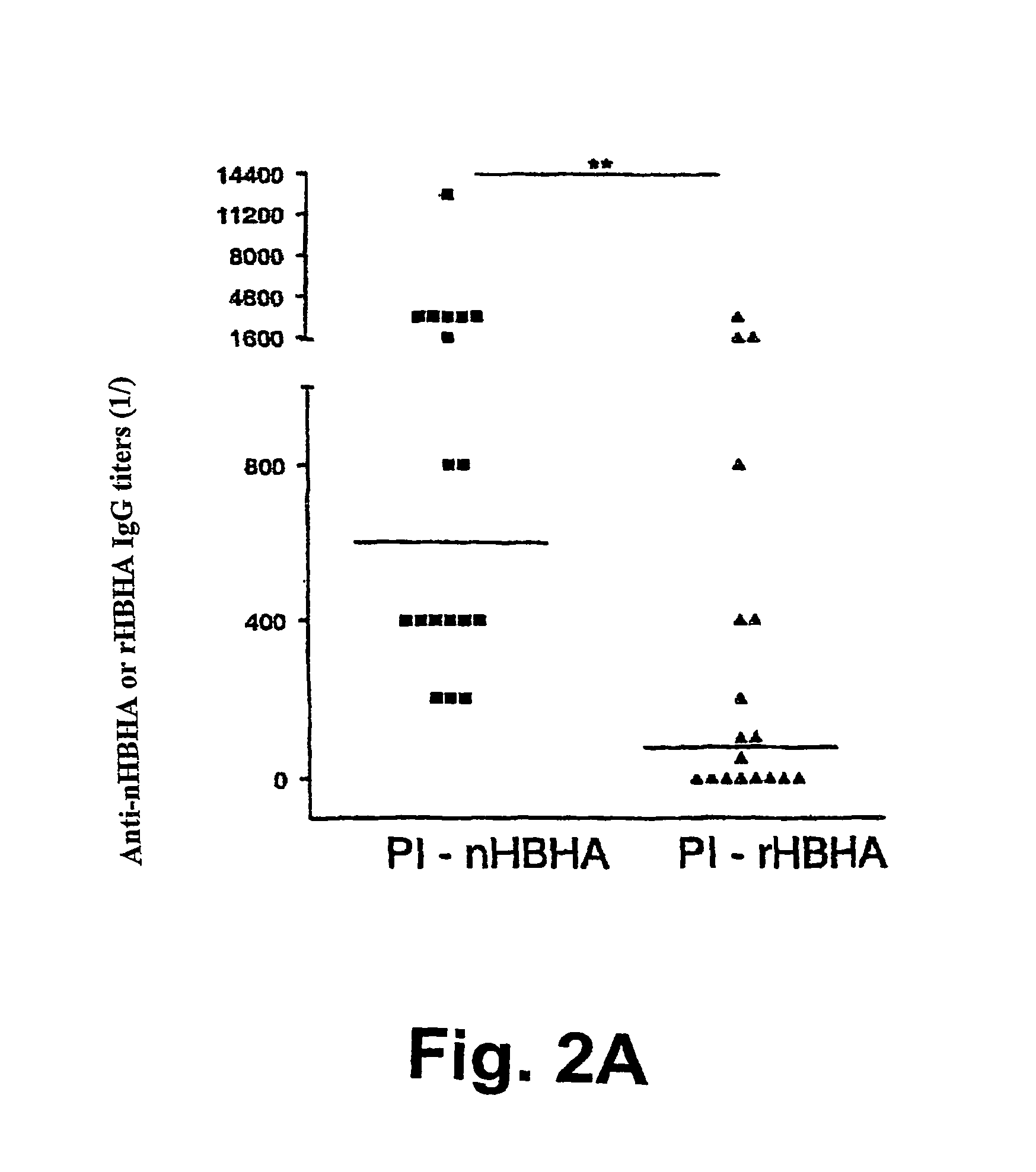
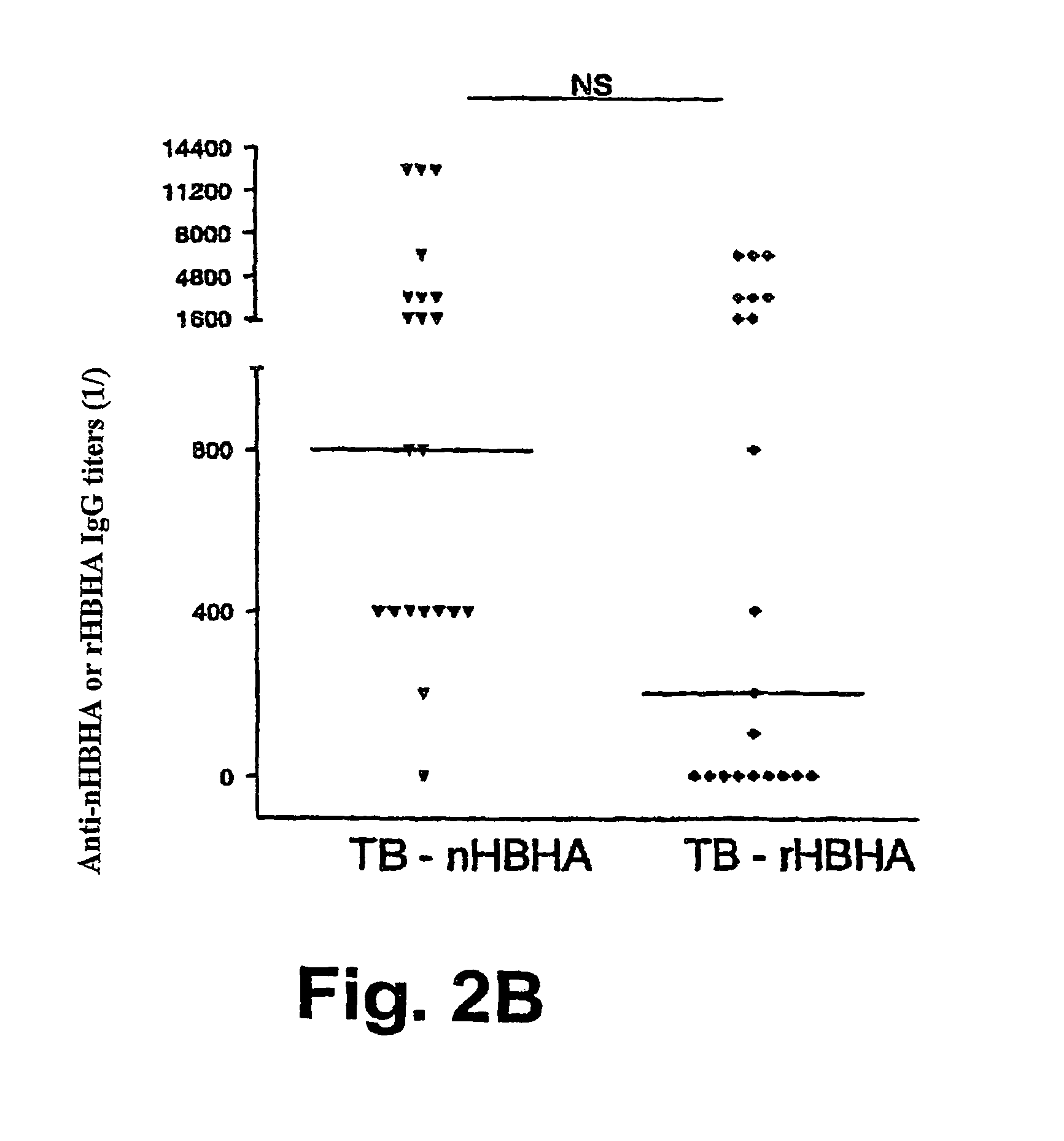
The present invention concerns methods for in vitro detection of an infection by Mycobacterium tuberculosis in mammals, and methods for in vitro distinction between mammals infected with Mycobacterium tuberculosis in which the disease is declared (active form) and mammals which are infected but asymptomatic for tuberculosis (latent form), and a method for in vitro distinction between mammals presenting an active form of tuberculosis and mammals not infected by M. tuberculosis or presenting a latent form of tuberculosis. The present invention also pertains to kits for detection and distinction between infected mammals presenting tuberculosis symptoms and infected mammals with no disease development, and a kit for distinguishing between mammals presenting an active form of tuberculosis and mammals not infected by M. tuberculosis or presenting a latent form of tuberculosis.
Owner:INSTITUT PASTEUR DE LILLE +3
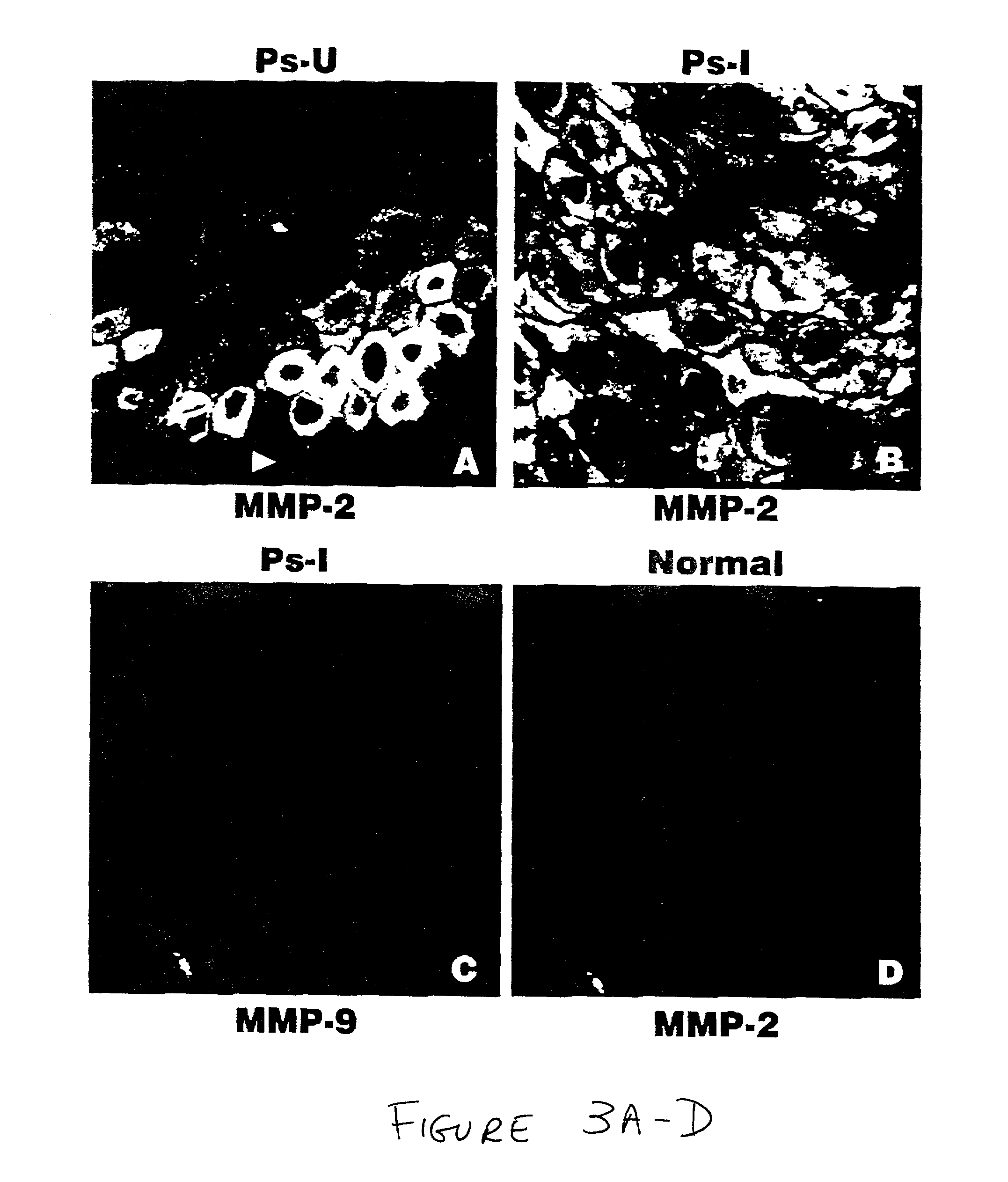
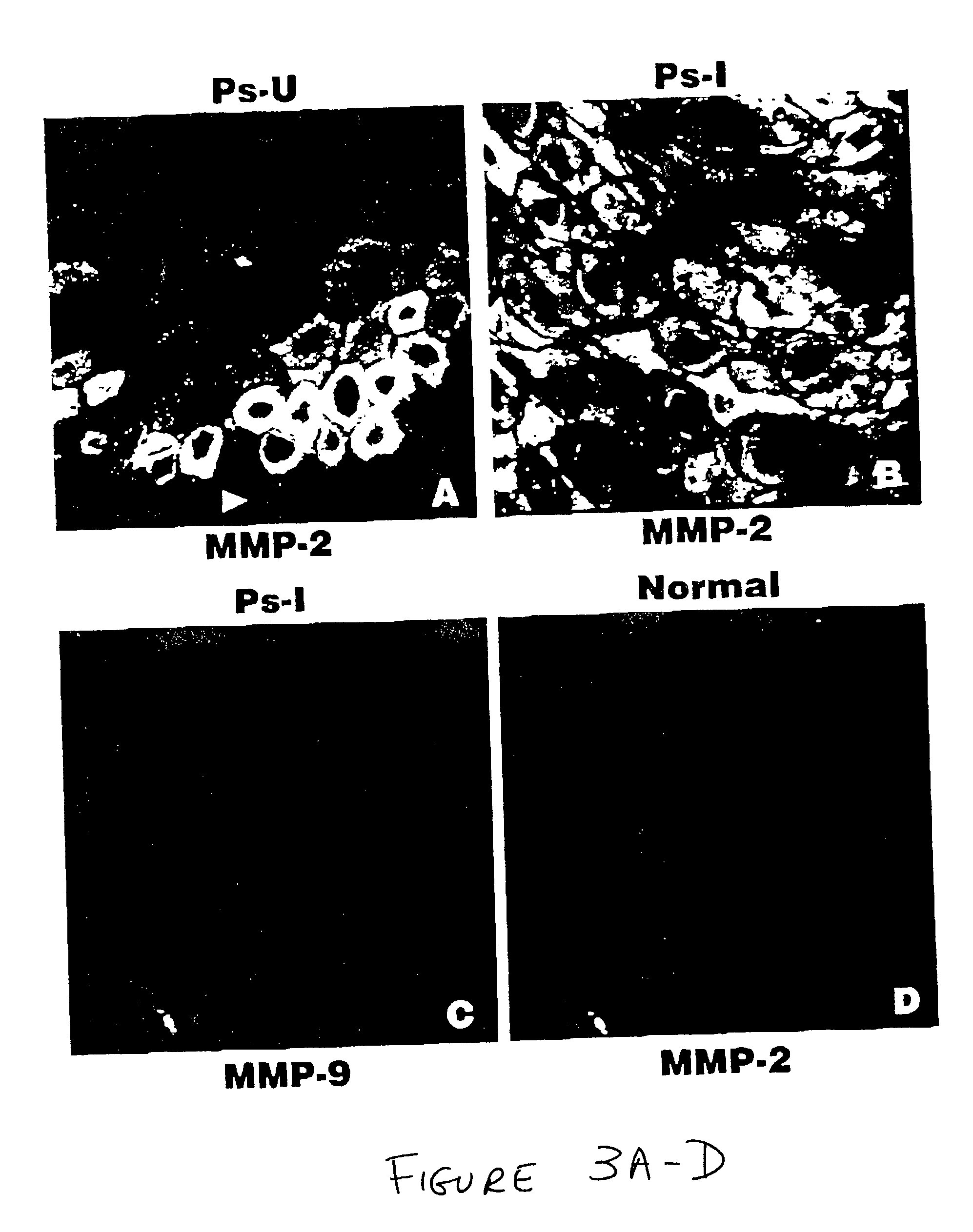
Treatment of psoriasis with matrix metalloproteinase inhibitors
The present invention relates to methods of treating psoriasis by inhibiting one or more matrix metalloproteinase enzymes ("MMPs"). It is based, at least in part, on the discovery that the expression patterns of certain MMPs and related molecules are altered in patients suffering from psoriasis, relative to normal subjects. Certain expression patterns are altered even in unaffected skin of psoriasis-afflicted patients, although aberrancies are more pronounced in psoriatic lesions. In various non-limiting embodiments, the present invention provides for methods of treating psoriasis, including preventing the development of new psoriatic lesions, comprising administering, to subjects in need of such treatment, effective concentrations of compounds which inhibit the enzymatic activity of one or more MMP. Suitable inhibitors include tetracycline and its derivatives and various hydroxymate, carboxylic acid, and phosphonic acid derivatives. Therapy may comprise systemic and / or local administration of inhibitor. In additional embodiments, the present invention provides for methods of diagnosing MMP inhibitor responsive skin lesions, for evaluating the level of disease activity in a subject, and for transgenic animal and tissue culture models of psoriasis.
Owner:FLEISCHMAJER RAUL
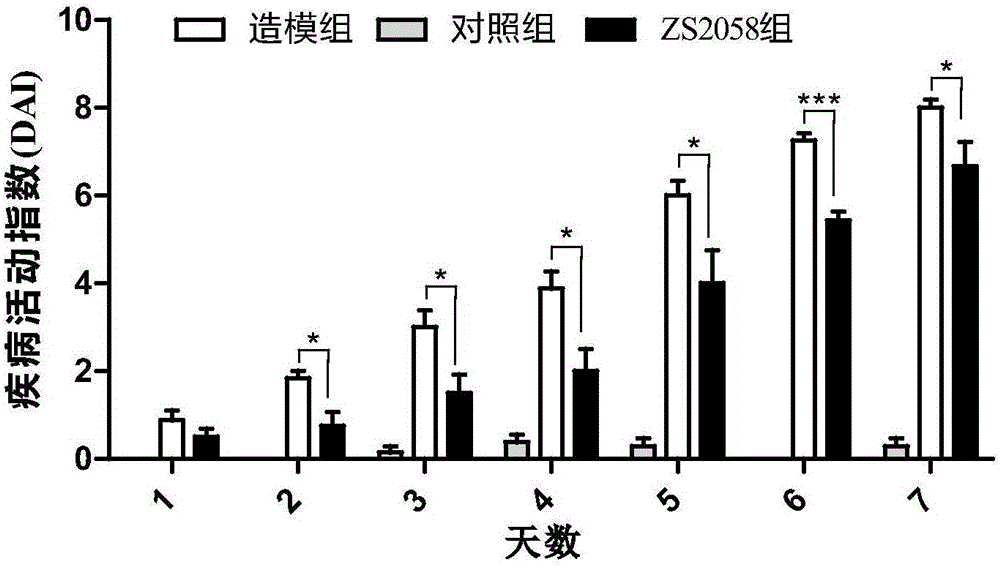
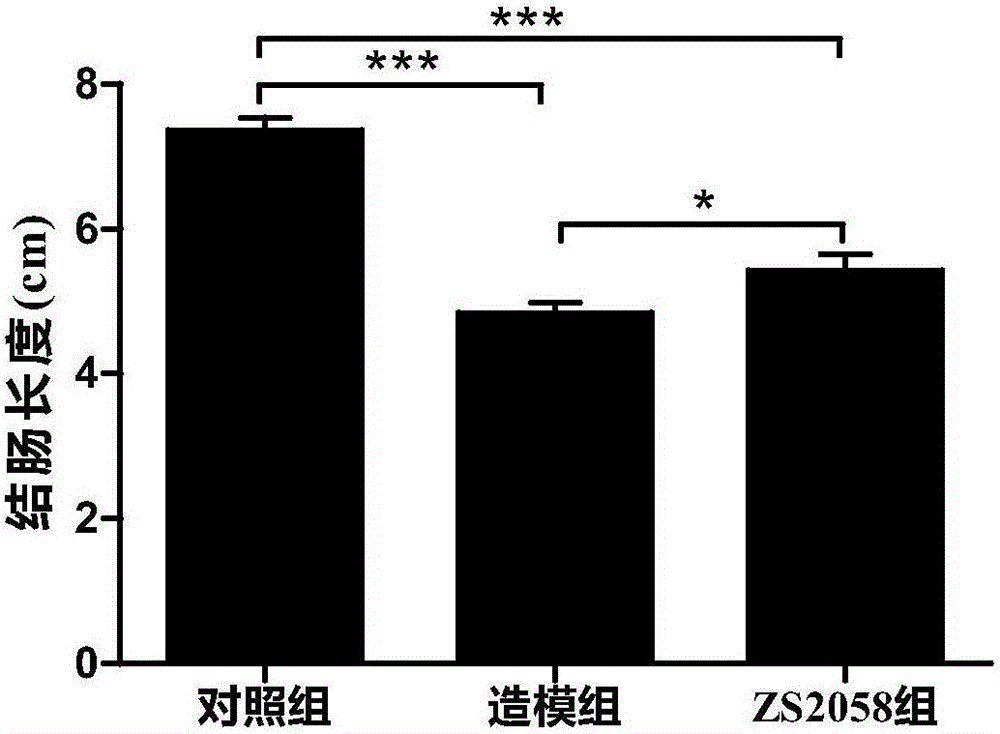
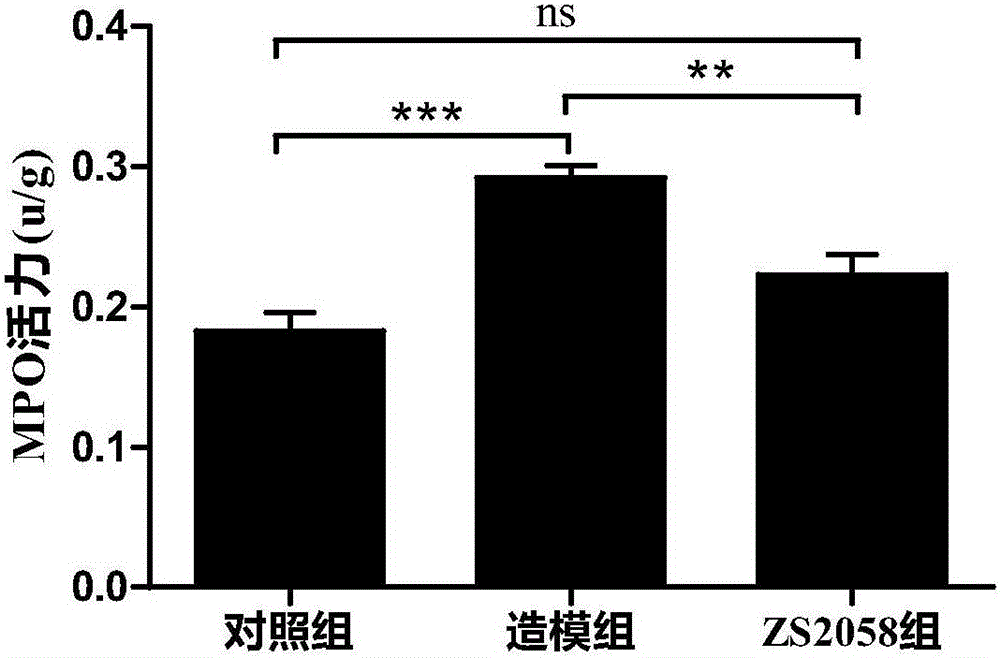
Lactobacillus plantarum ZS2058 and application thereof
InactiveCN105106246AImprove diarrheaImprove blood in stoolDigestive systemUnknown materialsStool occult bloodGenetics
The invention relates to lactobacillus plantarum ZS2058 and application thereof. The lactobacillus plantarum ZS2058 and the application have the advantages that colitis symptoms of diarrhea, stool occult blood, hematochezia and the like of mice can be effectively improved by the lactobacillus plantarum ZS2058, increase of disease activity indexes (DAI) can be suppressed, change of the lengths of colons of the mice can be improved, the activity of myeloperoxidase (MPO) can be obviously reduced, and the lactobacillus plantarum ZS2058 can be used for preparing medicine for preventing and treating intestinal inflammation or producing food beneficial to the intestinal health.
Owner:JIANGNAN UNIV
Individual assessment and classification of complex diseases by a data-based clinical disease profile
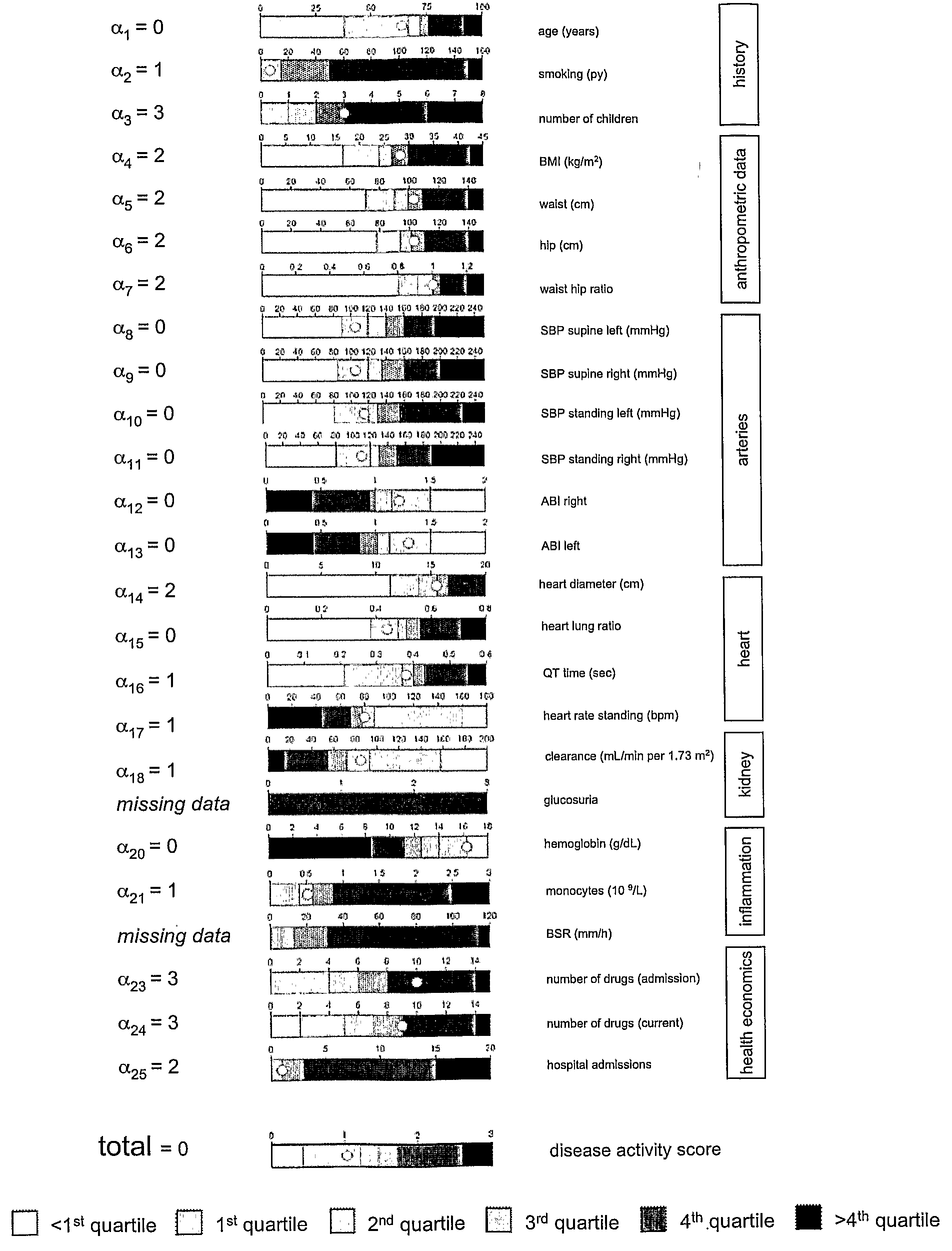
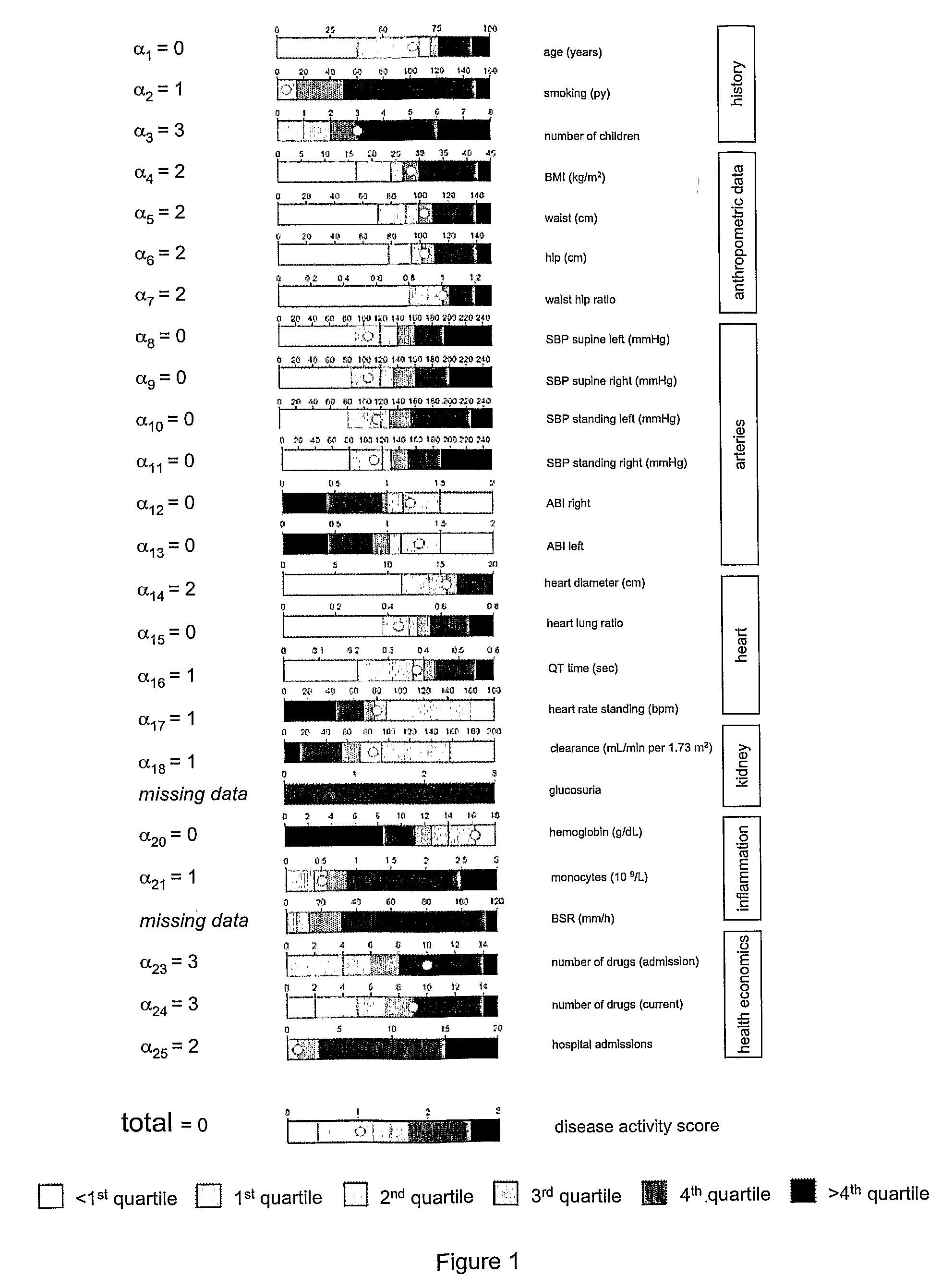
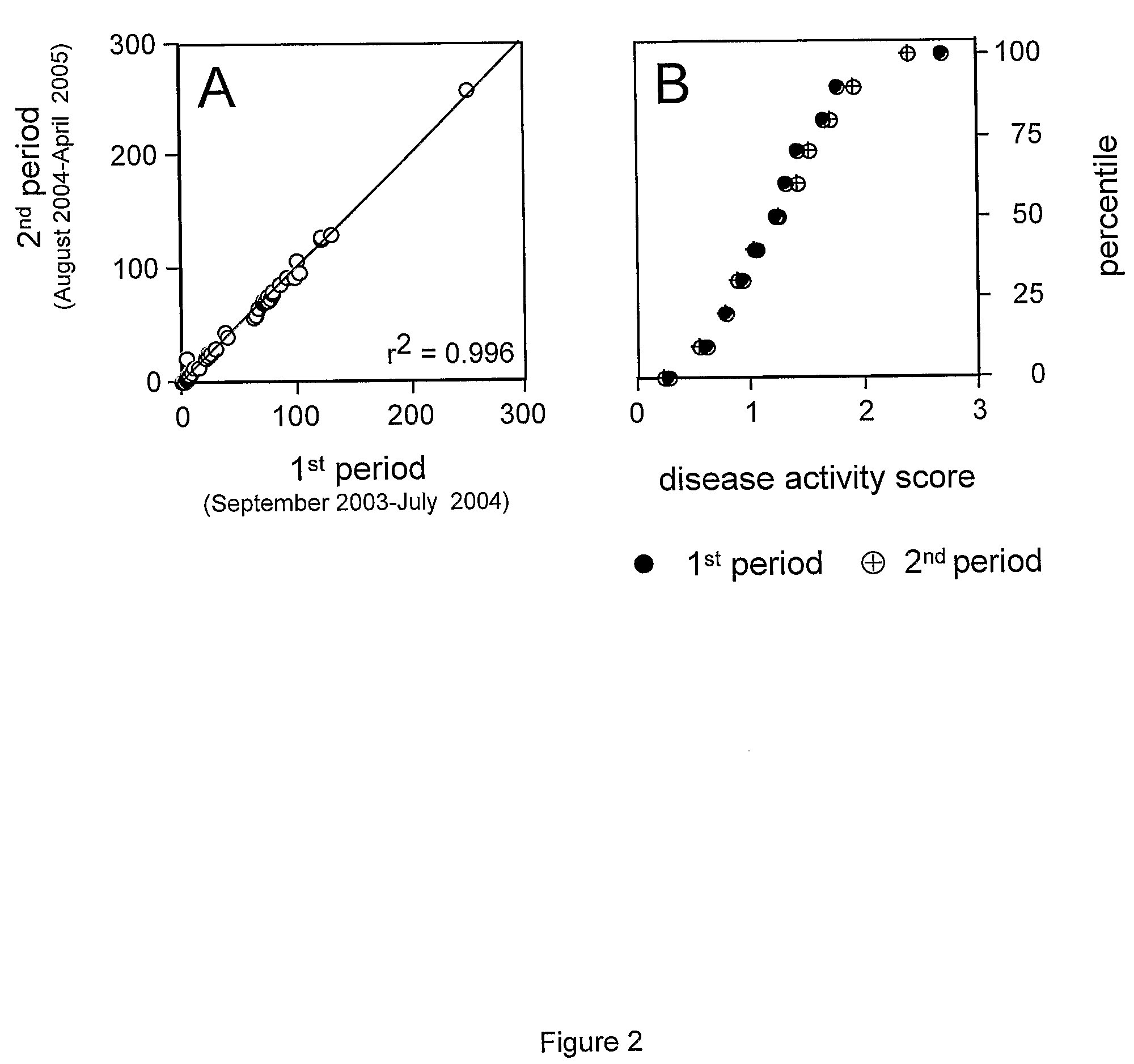
An tool and method is disclosed to assess disease activity and to classify complex diseases using basic clinical data. The tools and methods allow identifying and consulting affected individuals based on comprehensive bedside examinations and thus provide a basis for the personalized management of complex diseases.
Owner:KANTONSSPITAL BRUDERHOLZ
Excellent lactobacillus reuteri for preventing ulcerative colitis
InactiveCN110591945APrevent effectPromote infiltrationBacteriaDigestive systemUlcerative colitisDisease activity
The present invention provides lactobacillus reuteri for preventing ulcerative colitis. The strain is named as lactobacillus reuteri RAM0101 and deposited on May 27, 2019 at China General Microbiological Culture Collection Center and has a preservation number of CGMCC NO.17853. C57BL / 6N mice are used as test objects, dextran sodium sulfate (DSS) is used to induce the mice to form an ulcerative colitis model and the model is also used to investigate effectiveness of the strain in preventing the ulcerative colitis. The lactobacillus reuteri can relieve weight loss, diarrhea, hematochezia and disease activity index (DAI) score of the ulcerative colitis mice induced by the DSS, protects pathological damages of colon tissues of the mice induced by the DSS, can enhance colon tissue epithelial barrier completeness and is also free of toxin and harms.
Owner:JILIN UNIV
Biomarkers and methods for measuring and monitoring inflammatory disease activity
ActiveUS9200324B2Microbiological testing/measurementSkeletal disorderComputerized systemDisease activity
Biomarkers useful for diagnosing and assessing inflammatory disease are provided, along with kits for measuring their expression. The invention also provides predictive models, based on the biomarkers, as well as computer systems, and software embodiments of the models for scoring and optionally classifying samples. The biomarkers include at least two biomarkers selected from the DAIMRK group and the score is a disease activity index (DAI).
Owner:LAB OF AMERICA HLDG +1
Methylated marker IL-2RG in systemic lupus erythematosus (SLE) whole blood genome and application thereof
InactiveCN102140511AImprove diagnostic efficiencyReduce diagnostic costsMicrobiological testing/measurementCurative effectDisease activity
The invention discloses a methylated marker IL-2RG in a systemic lupus erythematosus (SLE) whole blood genome and application thereof. The sequence of the marker is shown as SEQ ID NO:1. The methylated marker IL-2RG in the SLE whole blood genome is applied to the preparation of a reagent for diagnosing SLE, in particular to a kit for diagnosing SLE. By detecting the methylation level of the methylated marker IL-2RG, a specific methylation locus with a great meaning and a remarkable difference is counted and taken as a basis for early diagnosis of SLE. By adopting the methylated marker, an accurate, cheap and quick clinical tool for early diagnosis of SLE, the disease activity, the curative effect and the prognostic evaluation is provided.
Owner:CENT SOUTH UNIV
Treatment of psoriasis with matrix metalloproteinase inhibitors
The present invention relates to methods of treating psoriasis by inhibiting one or more matrix metalloproteinase enzymes ("MMPs"). It is based, at least in part, on the discovery that the expression patterns of certain MMPs and related molecules are altered in patients suffering from psoriasis, relative to normal subjects. Certain expression patterns are altered even in unaffected skin of psoriasis-afflicted patients, although aberrancies are more pronounced in psoriatic lesions. In various non-limiting embodiments, the present invention provides for methods of treating psoriasis, including preventing the development of new psoriatic lesions, comprising administering, to subjects in need of such treatment, effective concentrations of compounds which inhibit the enzymatic activity of one or more MMP. Suitable inhibitors include tetracycline and its derivatives and various hydroxymate, carboxylic acid, and phosphonic acid derivatives. Therapy may comprise systemic and / or local administration of inhibitor. In additional embodiments, the present invention provides for methods of diagnosing MMP inhibitor responsive skin lesions, for evaluating the level of disease activity in a subject, and for transgenic animal and tissue culture models of psoriasis.
Owner:FLEISCHMAJER RAUL
Genotoxicity as a biomarker for inflammation
InactiveUS20100267037A1Efficacy of treatmentDecreased amount of presentMicrobiological testing/measurementMaterial analysis by electric/magnetic meansUveitisAutoimmune condition
The invention provides a method for detection of inflammatory disease in a subject that comprises assaying a test sample of peripheral blood from the subject for a marker of DNA damage. An elevated amount of marker present in the test sample compared to control sample is indicative of inflammatory disease activity, including sub-clinical inflammation. The method can be adapted for quantitatively monitoring the efficacy of treatment of inflammatory disease in a subject. Markers of DNA damage include single- and / or double-stranded breaks in leukocytes, oxidative DNA damage in leukocytes, or a marker of nitric oxide oxidative activity (protein nitrosylation in leukocytes). The inflammatory disease can be inflammatory bowel disease (ulcerative colitis or Crohn's disease). The invention may also be used for detection of other types of inflammatory disease, such as non-immune intestinal inflammatory disease (diverticulitis, pseudomembranous colitis), autoimmune diseases (rheumatoid arthritis, lupus, multiple sclerosis, psoriasis, uveitis, vasculitis), or non-immune lung diseases (asthma, chronic obstructive lung disease, and interstitial pneumonitis). This unexpected discovery of markers of genotoxicity present in circulating leukocytes enables detection of inflammation occurring at a localized site with a relatively simple and minimally invasive assay using peripheral blood.
Owner:RGT UNIV OF CALIFORNIA
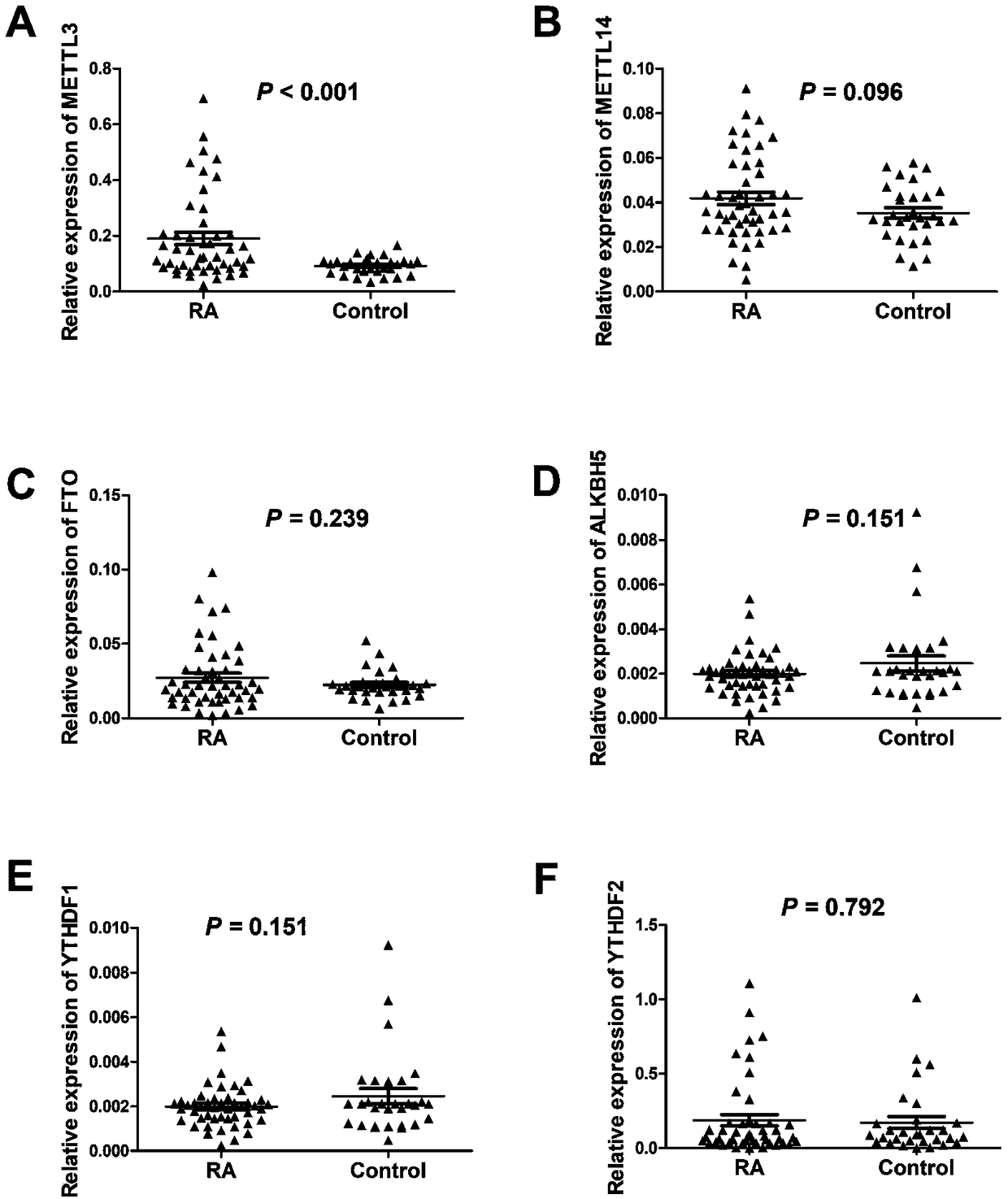
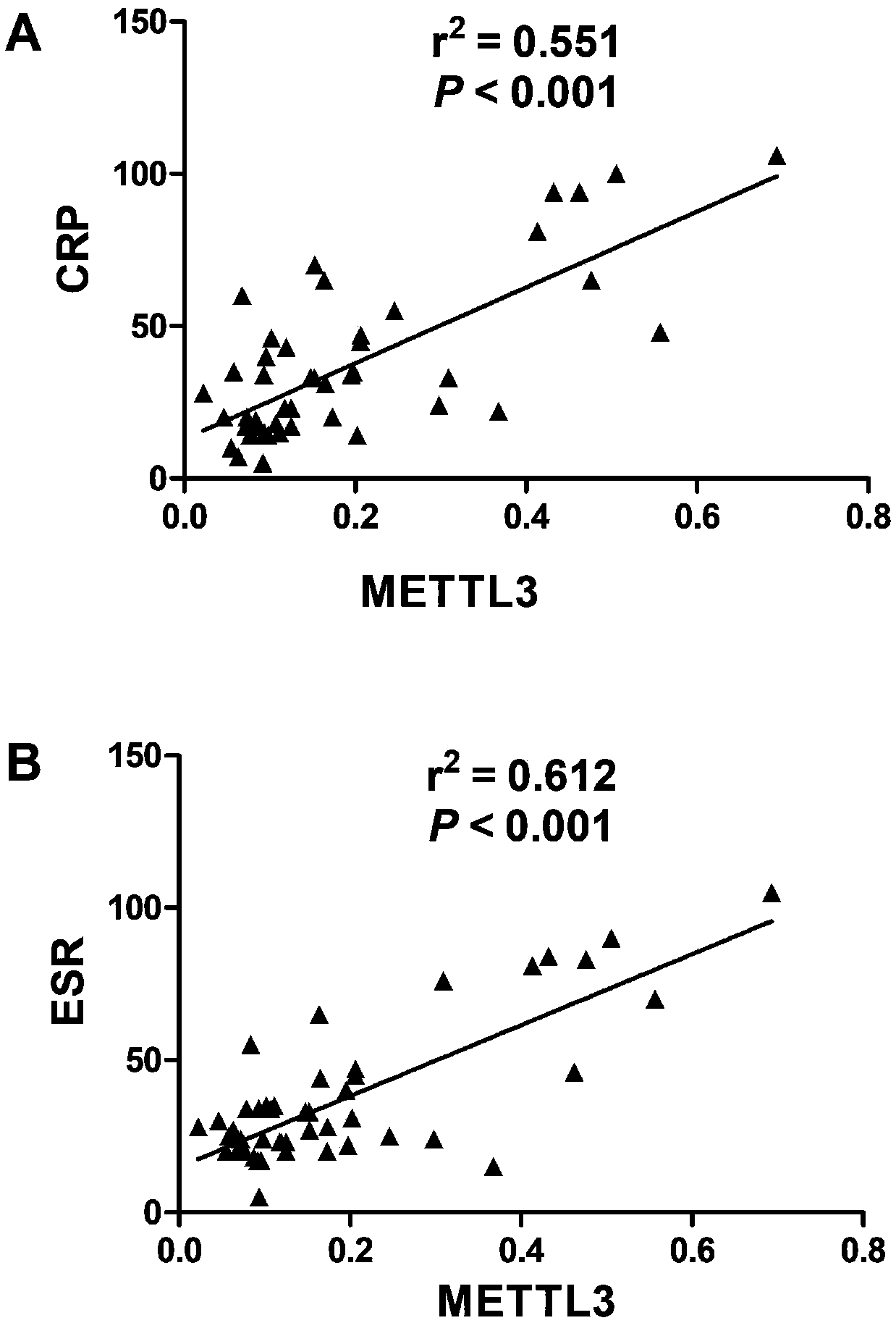
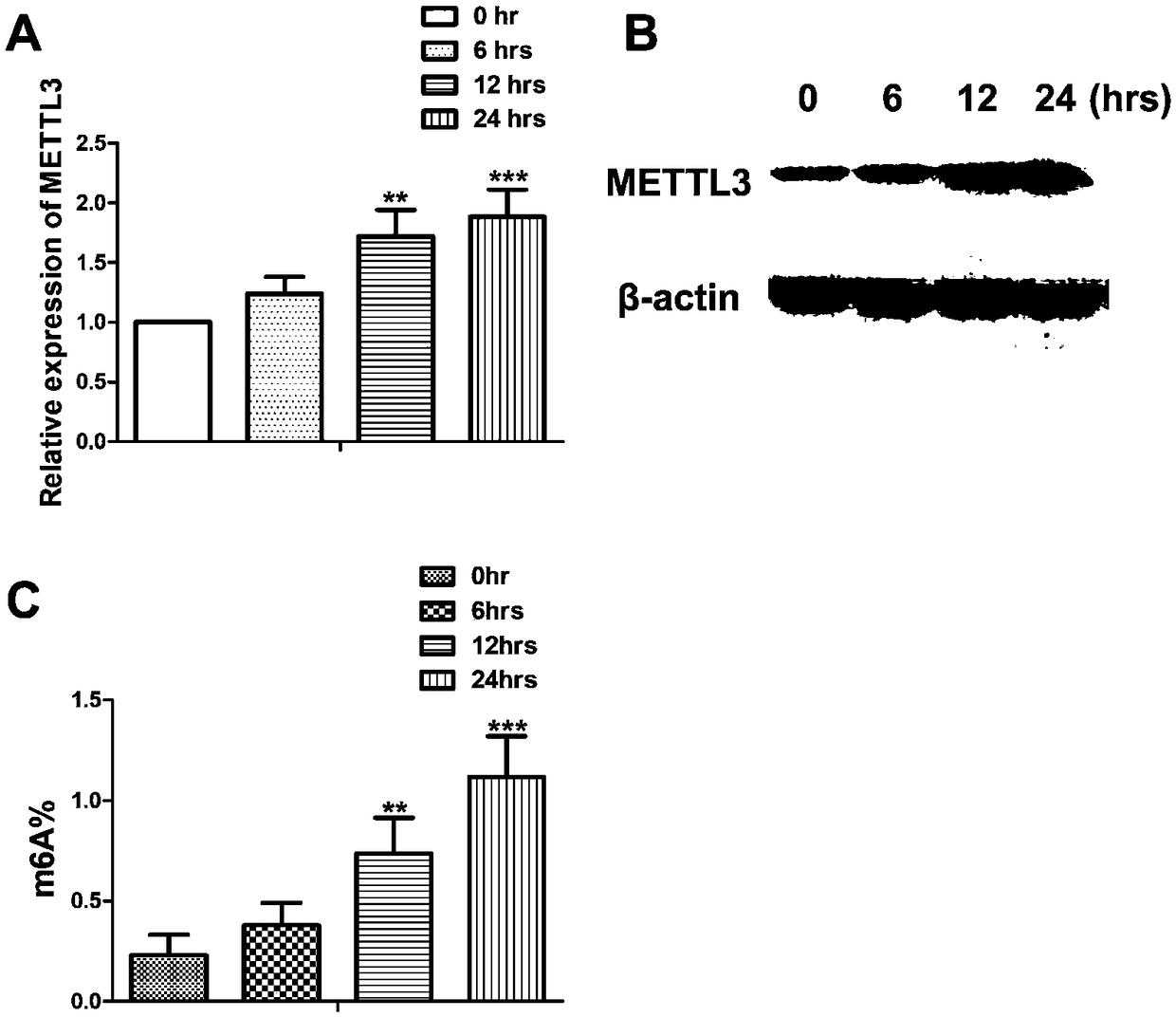
Application of METTL3 gene and detection method thereof
ActiveCN109457029AHigh expressionPrevent proliferationOrganic active ingredientsAntipyreticPeripheral blood mononuclear cellTotal rna
The invention relates to detection and application of a key gene (METTL3) in an m6A methylation modification process. A specific PCR primer is designed and synthetized according to an extracted totalRNA sequence; a real-time PCR technique is used for discovering that expression of METTL3 in a mononuclear cell of peripheral blood of rheumatoid arthritis patients is obviously improved and is obviously in a positive correlation relationship with CRP and ESR which are disease activity indexes of the rheumatoid arthritis, so that the gene METTL3 can be applied to preparation of preparations applied to auxiliary diagnosis and treatment or prognosis evaluation of the rheumatoid arthritis. Lv-METTL3 sequences with specific overexpression of the gene are simultaneously designed and synthetized; proliferation of macrophages and inflammatory response induced by LPS of the macrophages can be obviously inhibited by regulating and controlling an NF-kB signal path; the METTL3 can be used as a molecular targeted therapy tool of the rheumatoid arthritis.
Owner:潍坊医学院第一附属医院
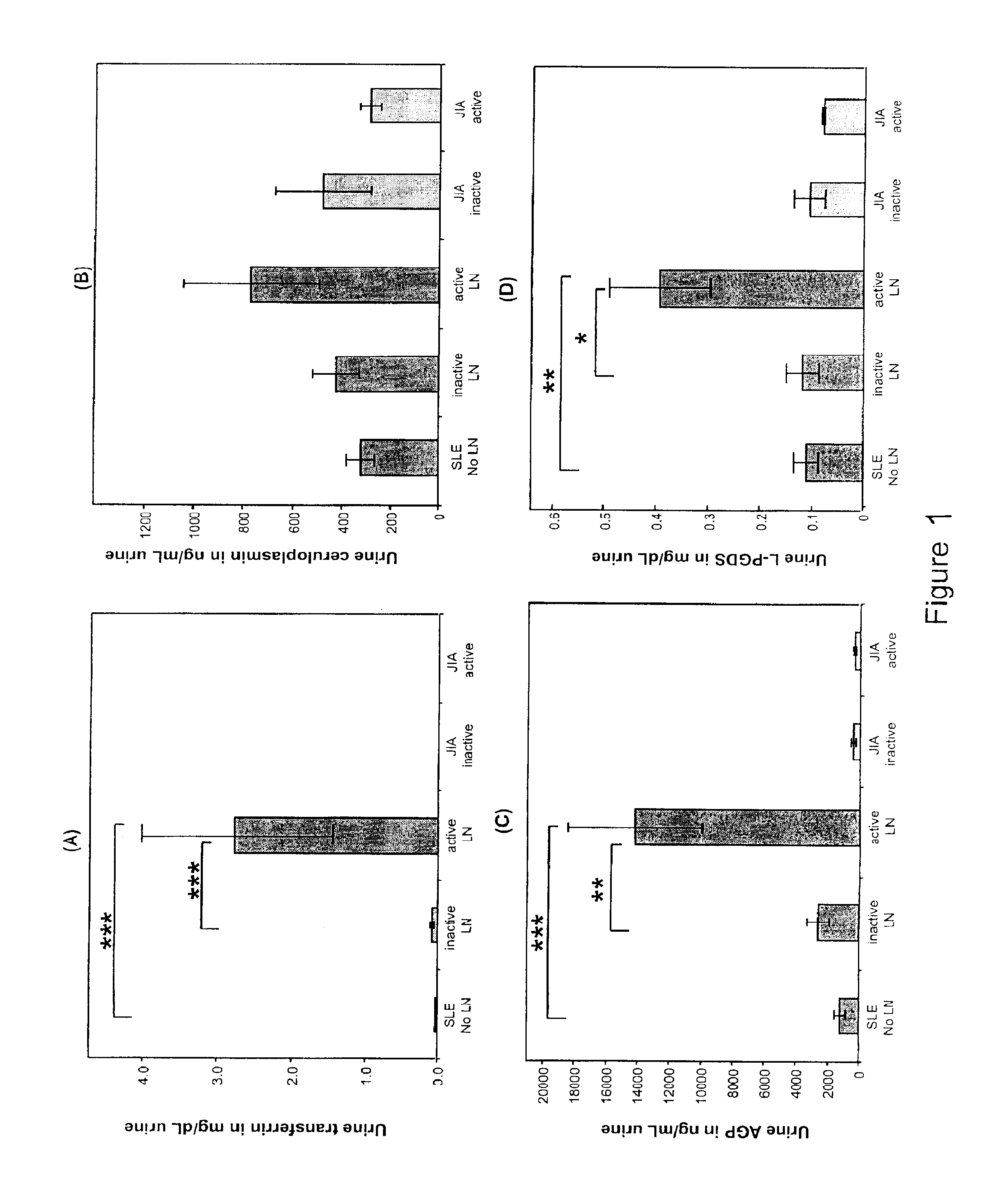
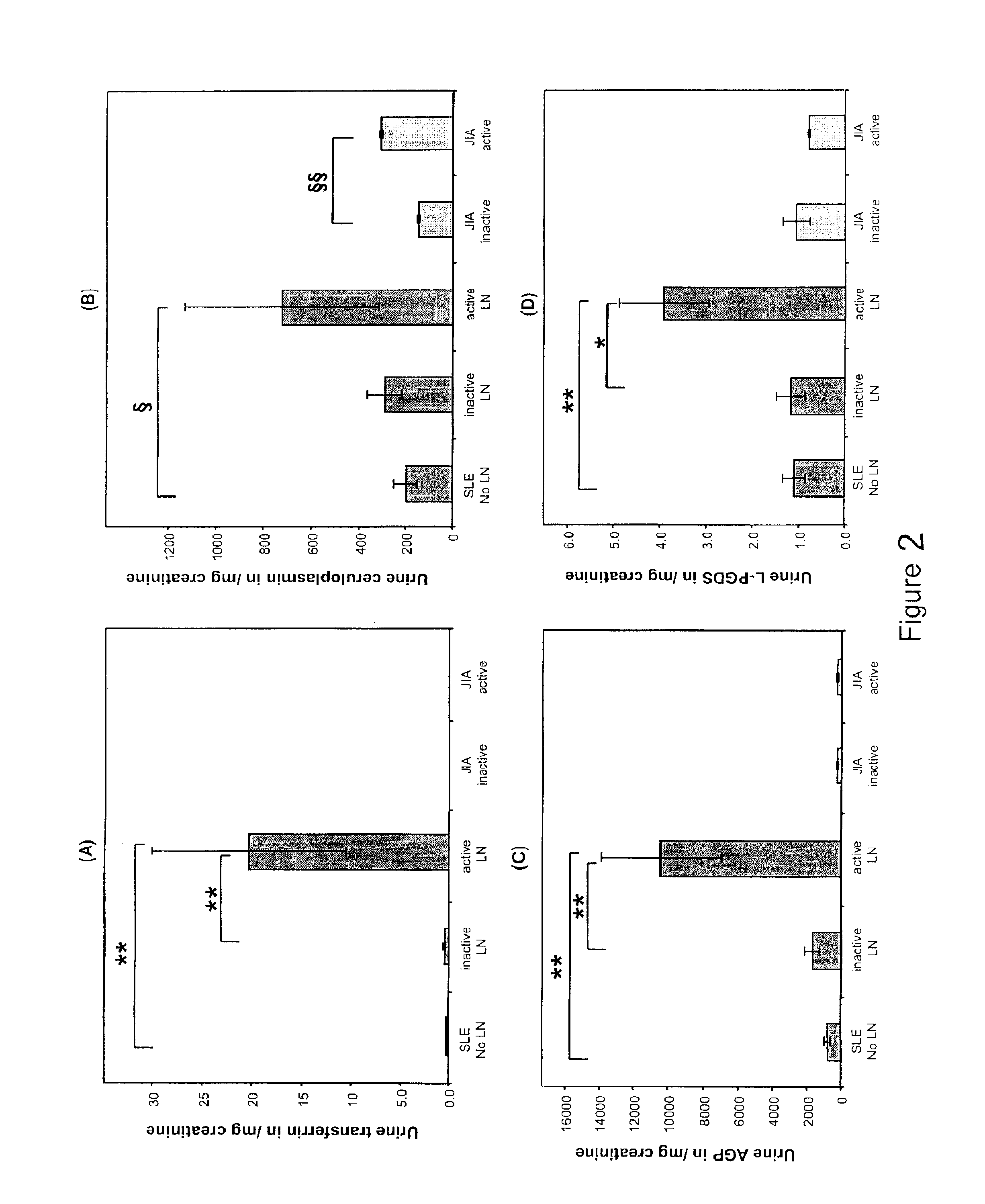
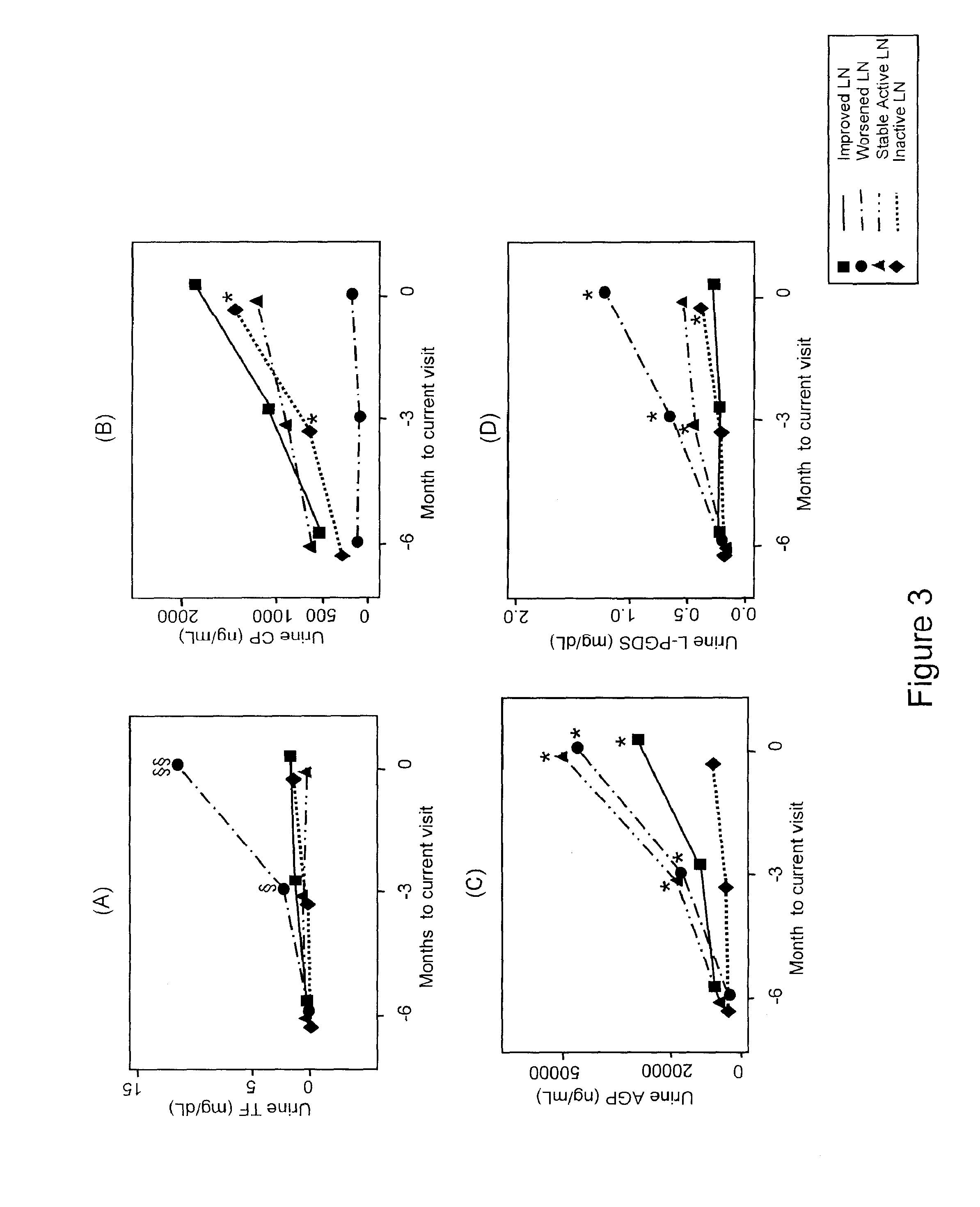
Detection of worsening renal disease in subjects with systemic lupus erythematosus
ActiveUS20100323911A1Worsening renal disease activityAccurate determinationLibrary screeningDisease diagnosisProstaglandins DDisease activity
Methods for the detection of active lupus nephritis (LN) and worsening renal disease activity and / or active LN in patients diagnosed with systemic lupus erythematosus, using a panel of biomarkers including transferrin (Tf), ceruloplasmin (Cp), alpha-1-acid glycoprotein (AGP1), lipocalin-like prostaglandin D synthetase (L-PGDS), and urinary neutrophil gelatinase associated lipocalin (UNGAL).
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
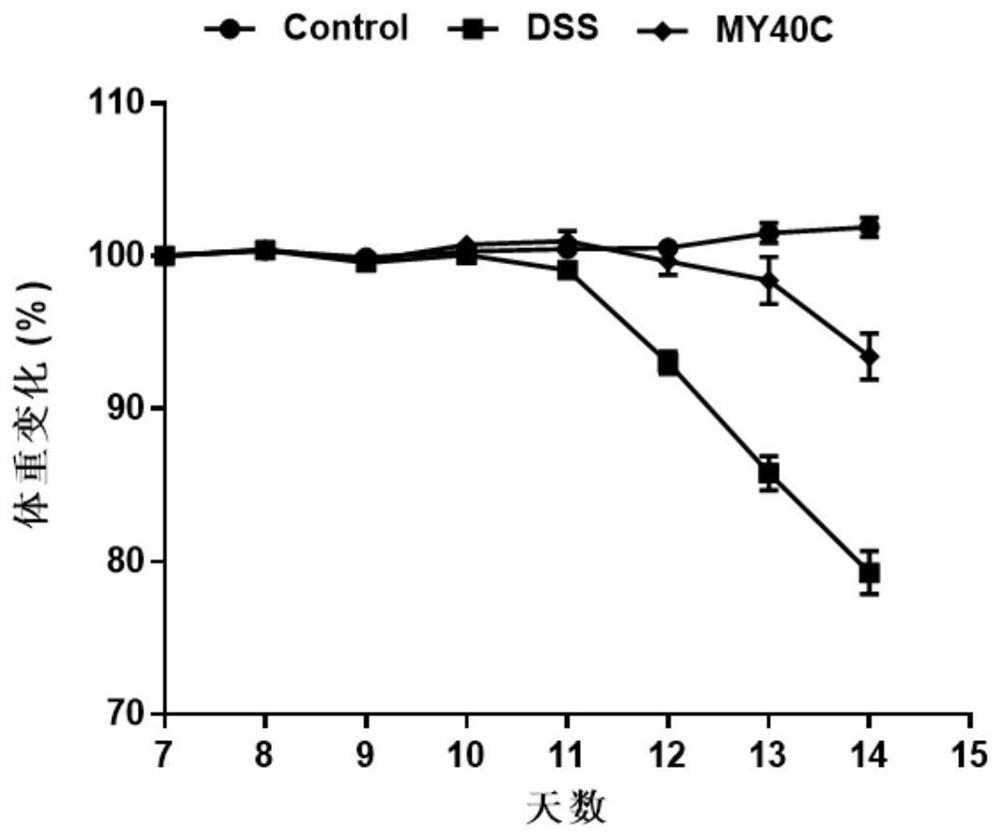
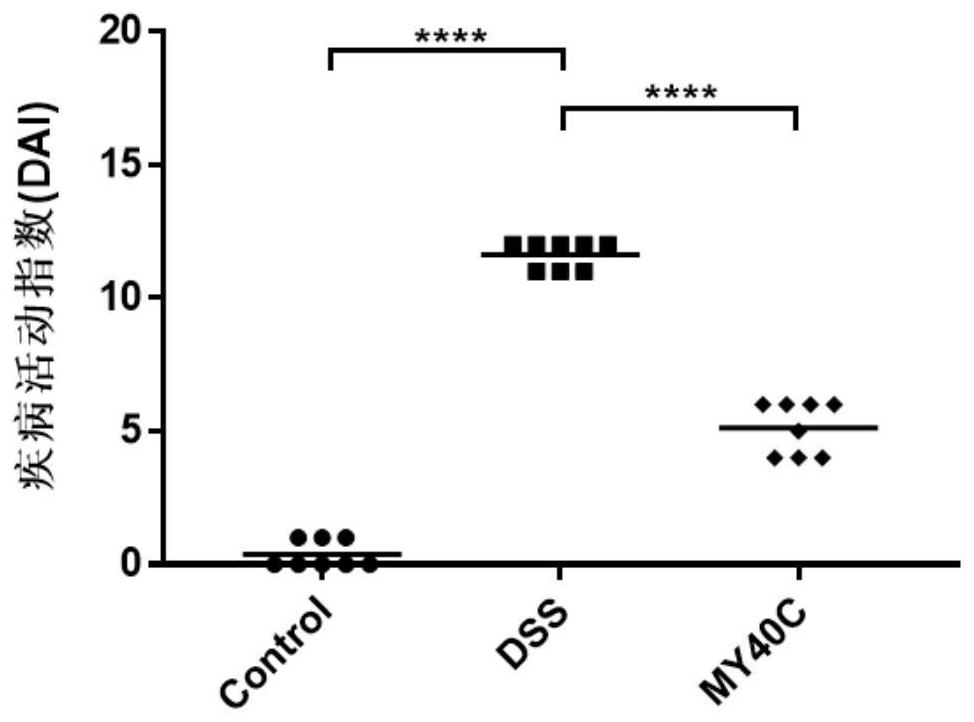
Bifidobacterium pseudocatenulatum capable of relieving colitis and application thereof
ActiveCN112111422ANo side effectsLower disease activity indexBacteriaDigestive systemBiotechnologyDisease activity
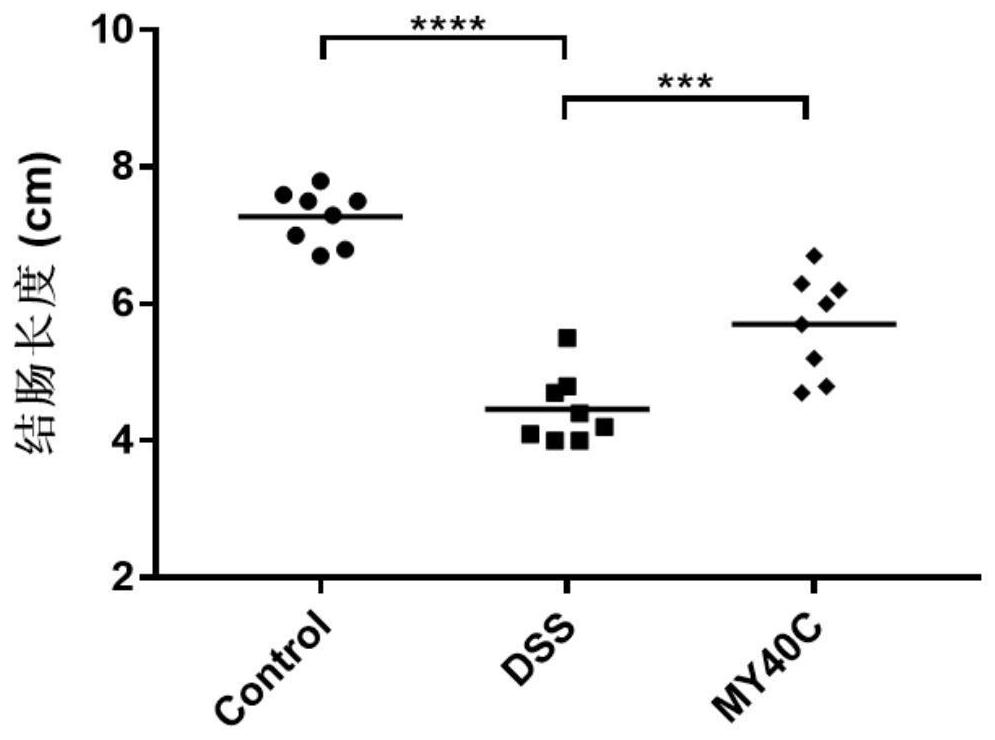
The invention discloses bifidobacterium pseudocatenulatum capable of relieving colitis and application thereof, and belongs to the technical field of biology. The invention provides bifidobacterium pseudocatenulatum MY40C which has immunoregulation capacity and can improve an intestinal tract barrier and relieve colitis. By adopting the bifidobacterium pseudocatenulatum provided by the invention,the disease activity index of a mouse during DSS induced colitis can be remarkably reduced, and the weight loss and the colonic shortening can be relieved. Compared with a mouse in a model group, themouse in a bifidobacterium pseudocatenulatum MY40C intervention group has the advantages that the levels of MPO and COX-2 in colonic tissues are obviously reduced, and the tissue injury is obviously relieved.
Owner:JIANGNAN UNIV
Methods for diagnosis and treatment of chronic immune diseases
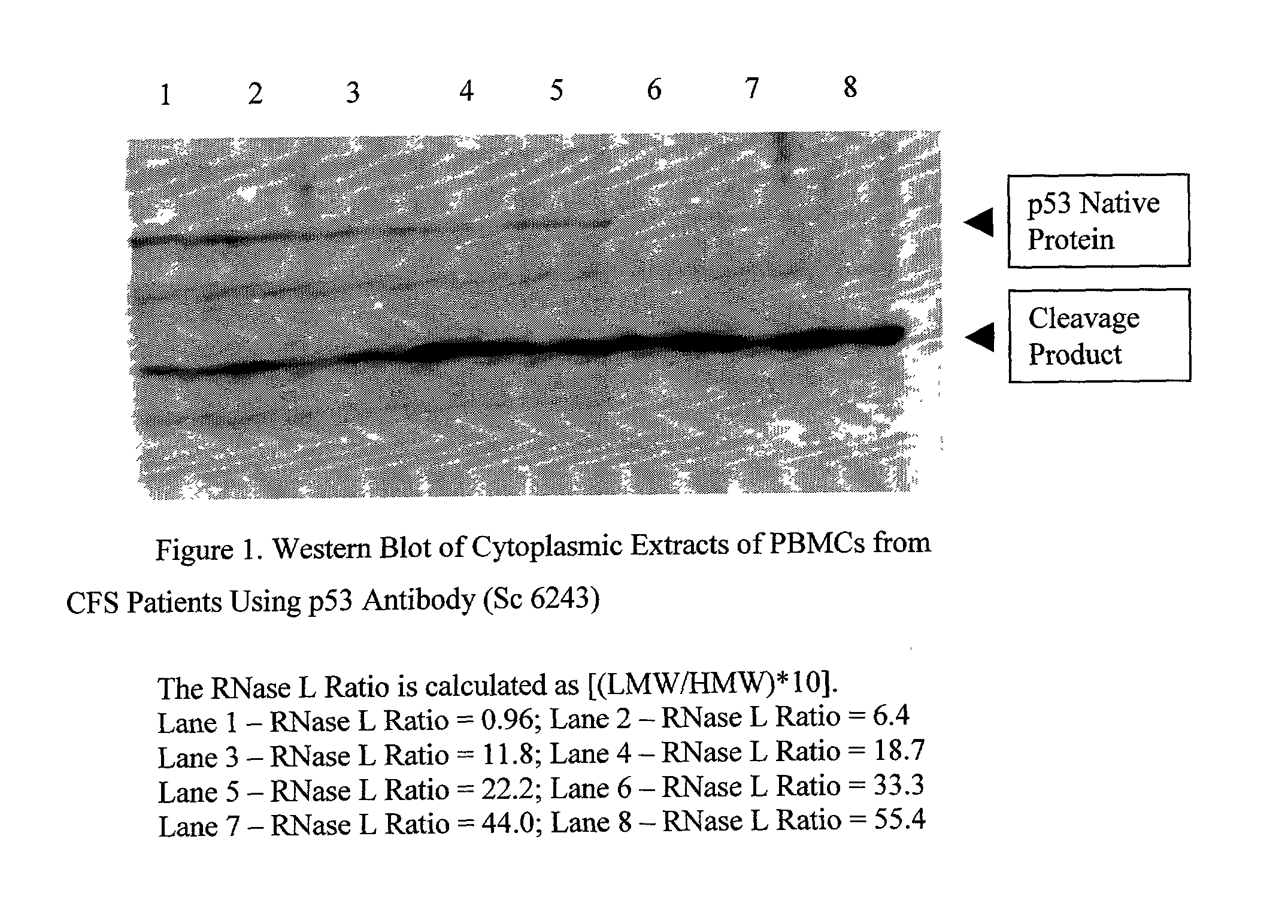
InactiveUS20030017492A1Microbiological testing/measurementBiological testingProper treatmentP53 protein
Methods are provided for diagnosing / characterizing chronic immune disease activity in a subject. In the subject methods, a sample is obtained from a subject suspected of having or known to have a chronic immune disease. The sample is then assayed for the presence and amount of intact (i.e., native) p53 protein and / or fragments thereof. The assay results are used to diagnose the presence of chronic immune disease and / or characterize chronic immune disease activity in a subject, and / or to determine appropriate treatments protocols. Also provided by the subject invention are methods of treating chronic immune disease conditions by enhancing p53 activity. Also provided by the subject invention are kits for practicing the methods.
Owner:PROTEA BIOPHARMA
Biomarkers for Systemic Lupus Erythematosus Disease Activity, and Intensity and Flare
InactiveUS20150098940A1Peptide librariesMicrobiological testing/measurementDisease activityBiomarker (petroleum)
The present invention involves the identification of biomarkers that are predictive of impeding systemic lupus erythematosus (SLE) disease flare. Methods for treating patients so identified are also provided.
Owner:OKLAHOMA MEDICAL RES FOUND
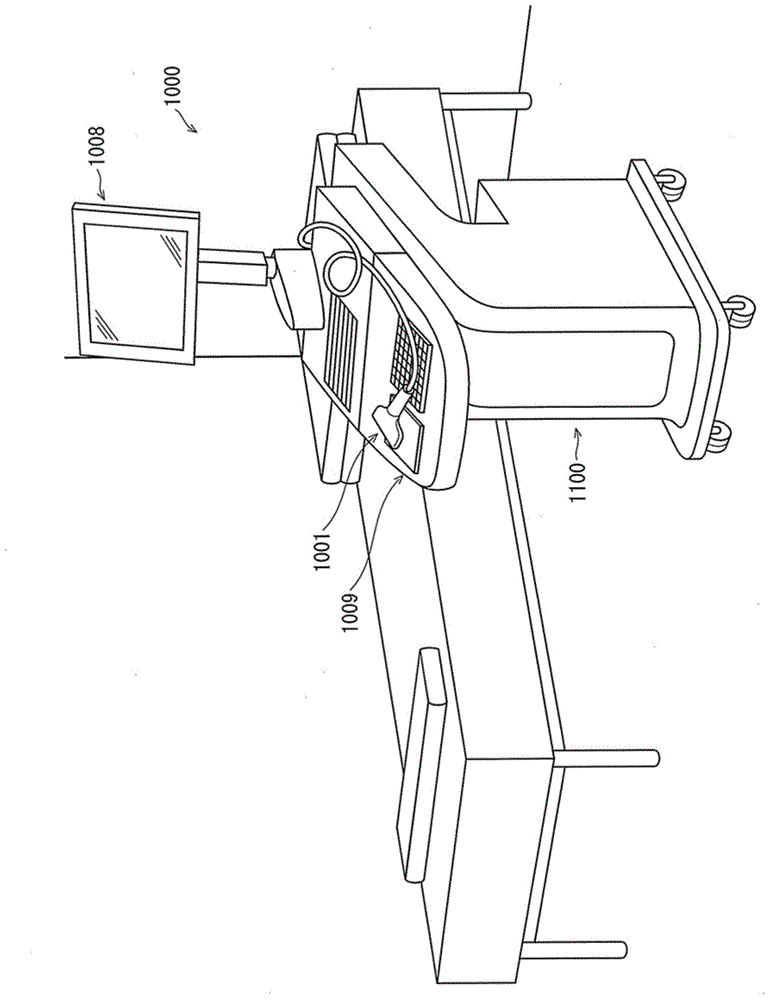
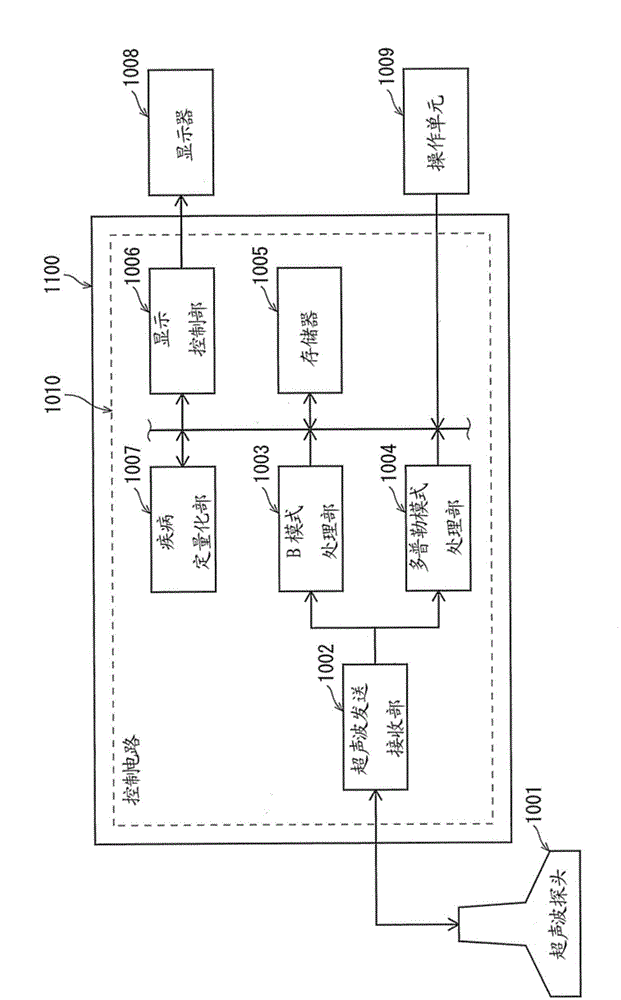
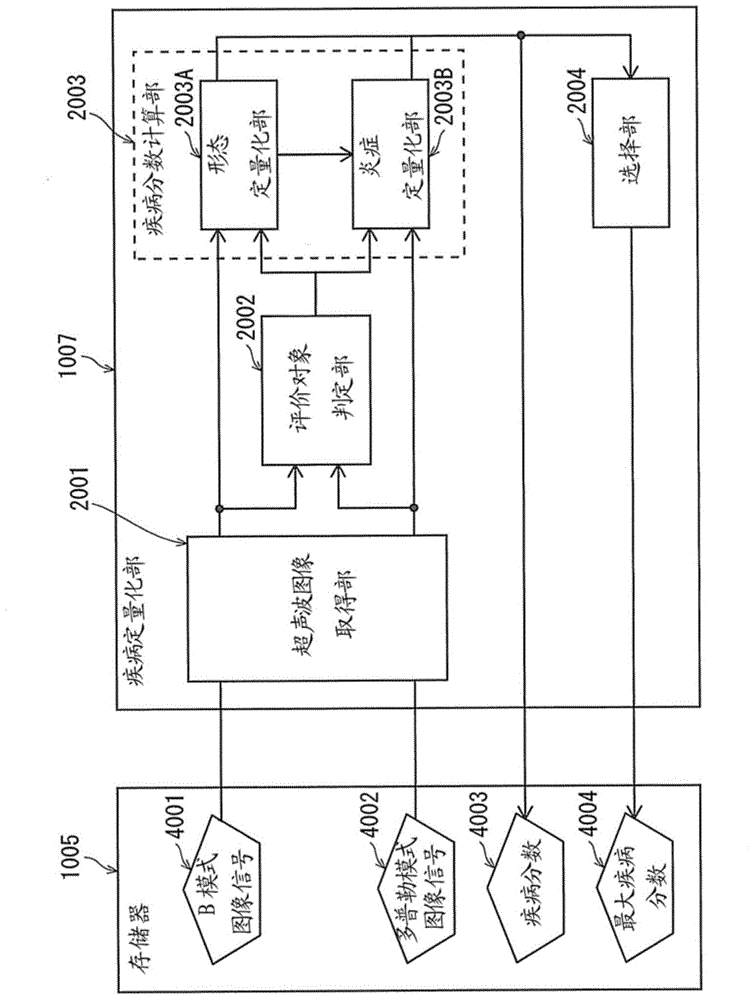
Ultrasound diagnostic apparatus and ultrasound image processing method
An ultrasound diagnostic apparatus includes an ultrasound image acquirer, an evaluation target determiner, a disease progression score calculator, a selector, and a display controller. The ultrasound image acquirer acquires ultrasound image signals of a plurality of frames. The evaluation target determiner analyzes the ultrasound image signal of each frame and determines the frame to be an evaluation target frame when the ultrasound image signal includes a target image section depicting a joint. The disease progression score calculator calculates, for each evaluation target frame, a disease progression score quantifying disease activity using an ultrasound image signal of the target image section included in the ultrasound image signal of the evaluation target frame. The selector selects at least one disease progression score in accordance with a predetermined numerical process. The display controller controls the display to display the selected disease progression score and an ultrasound image of a corresponding frame.
Owner:KONICA MINOLTA INC
Application of lactobacillus reuteri in preventing and relieving ulcerative colitis
ActiveCN112646744AImprove toleranceReduce abundanceMilk preparationBacteriaUlcerative colitisDisease activity
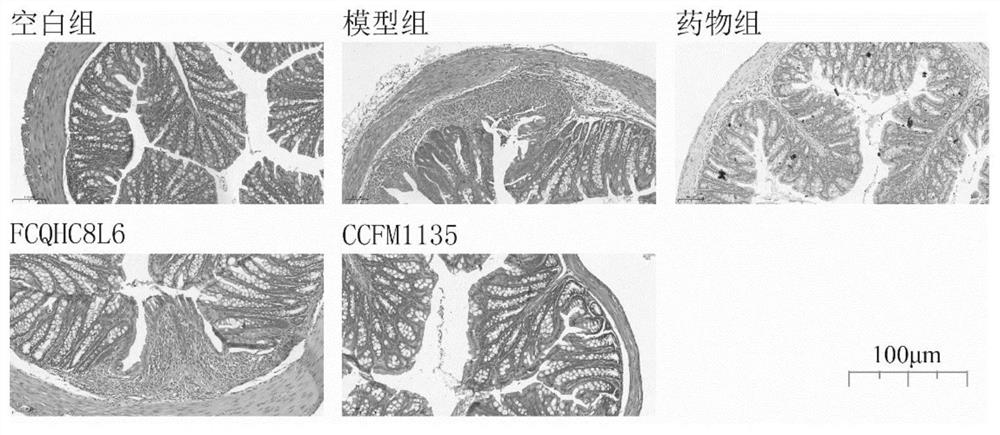
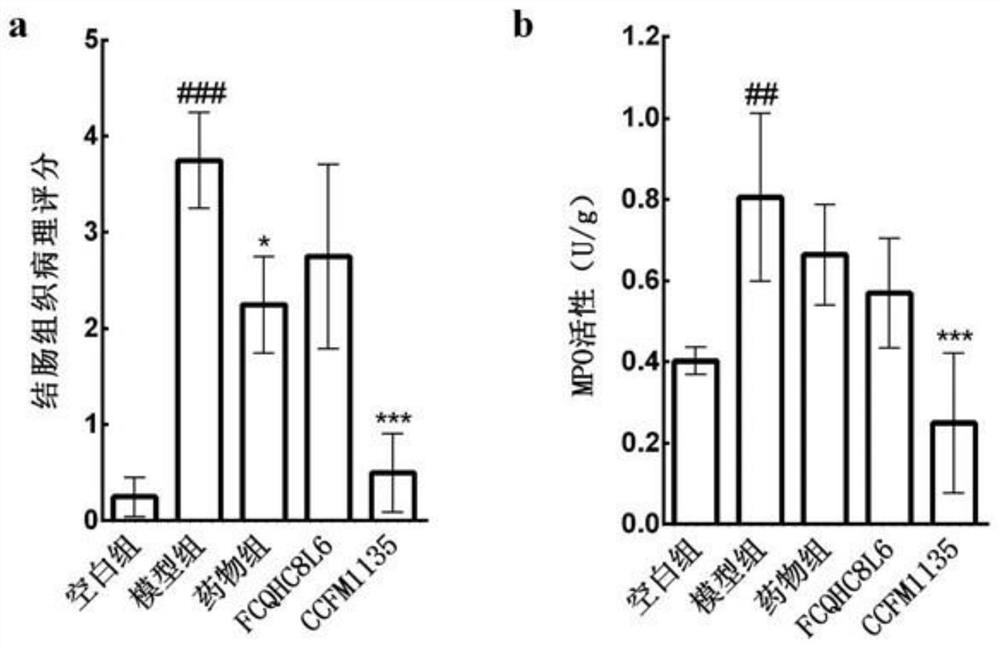
The invention discloses application of lactobacillus reuteri in preventing and relieving ulcerative colitis, and belongs to the technical field of functional microorganisms. Lactobacillus reuteri CCFM1135 can tolerate the gastrointestinal environment of a human body, significantly reduce the disease activity index during the ulcerative colitis, improve colon mucosal injury, reduce MPO activity, reduce the content of proinflammatory factors TNF-alpha, IL-6 and IFN-gamma in colon, up-regulate the transcription level of colon tight junction related proteins Claudin-3, ZO-1, ZO-2 and Occludin, up-regulate the transcription levels of colon antibacterial peptides Reg3g and Reg3b, improve the diversity of intestinal flora, and reduced the relative abundance of acinetobacter in excrement.
Owner:JIANGNAN UNIV
Methods of disease activity profiling for personalized therapy management
ActiveUS10086072B2Improve therapeutic managementReducing and minimizing riskMicrobiological testing/measurementAntibody ingredientsPersonalizationTherapeutic effect
The present invention provides methods for personalized therapeutic management of a disease in order to optimize therapy and / or monitor therapeutic efficacy. In particular, the present invention comprises measuring an array of one or a plurality of biomarkers at a plurality of time points over the course of therapy with a therapeutic agent to determine a mucosal healing index for selecting therapy, optimizing therapy, reducing toxicity, and / or monitoring the efficacy of therapeutic treatment. In certain instances, the therapeutic agent is a TNFα inhibitor for the treatment of a TNFα-mediated disease or disorder.
Owner:PROMETHEUS LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com