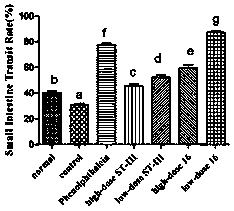
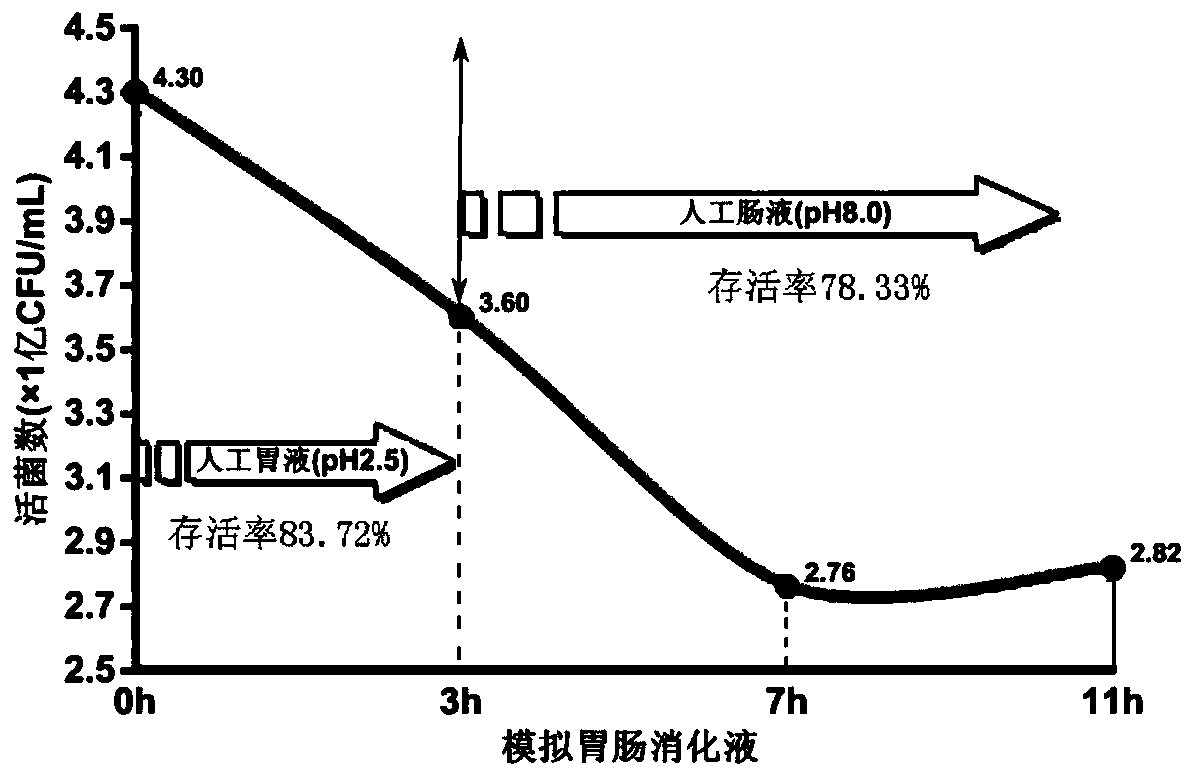
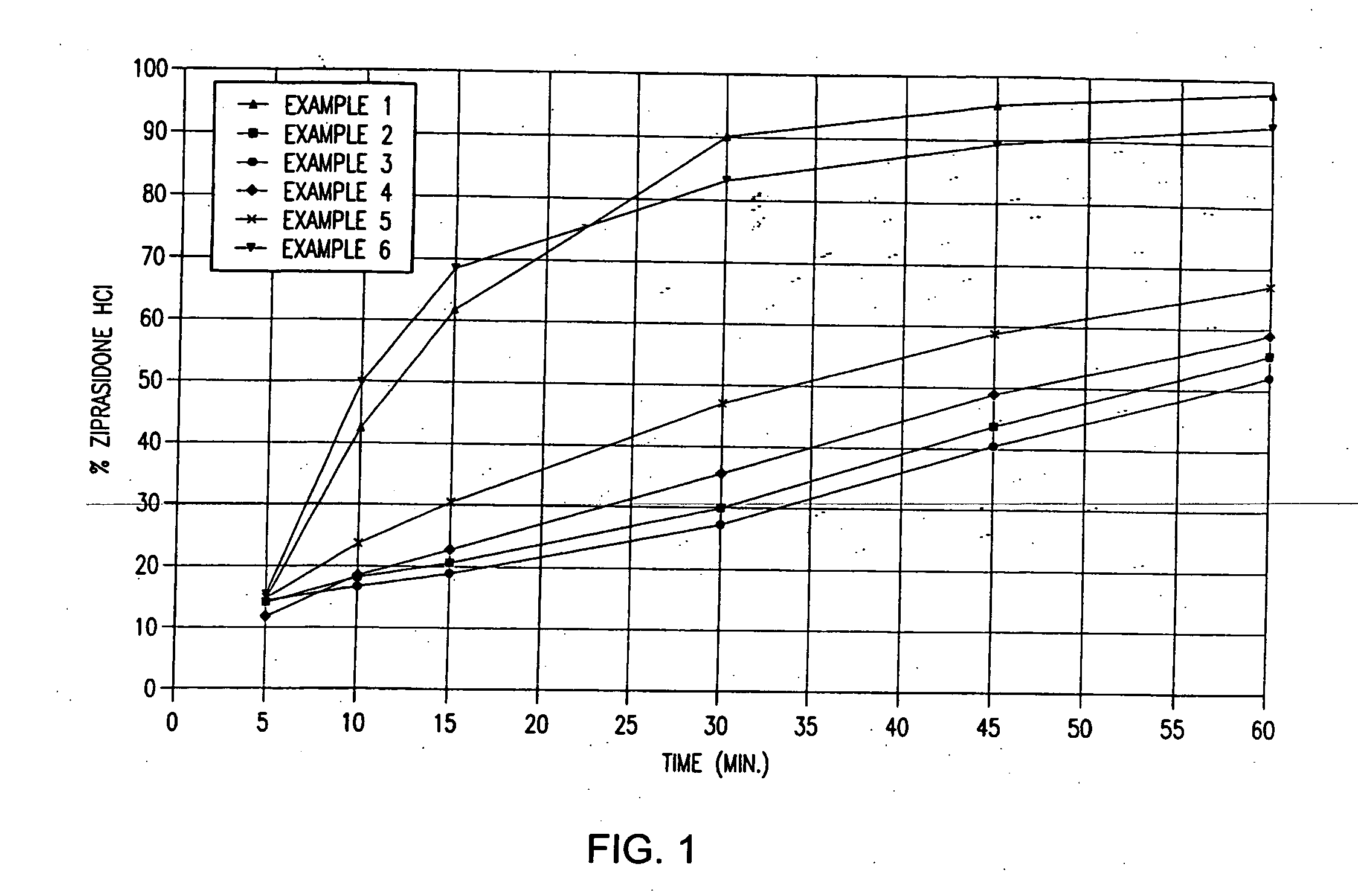
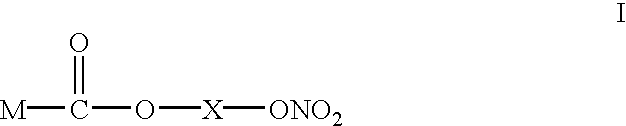Patents
Literature
151 results about "Gastrointestinal fluids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
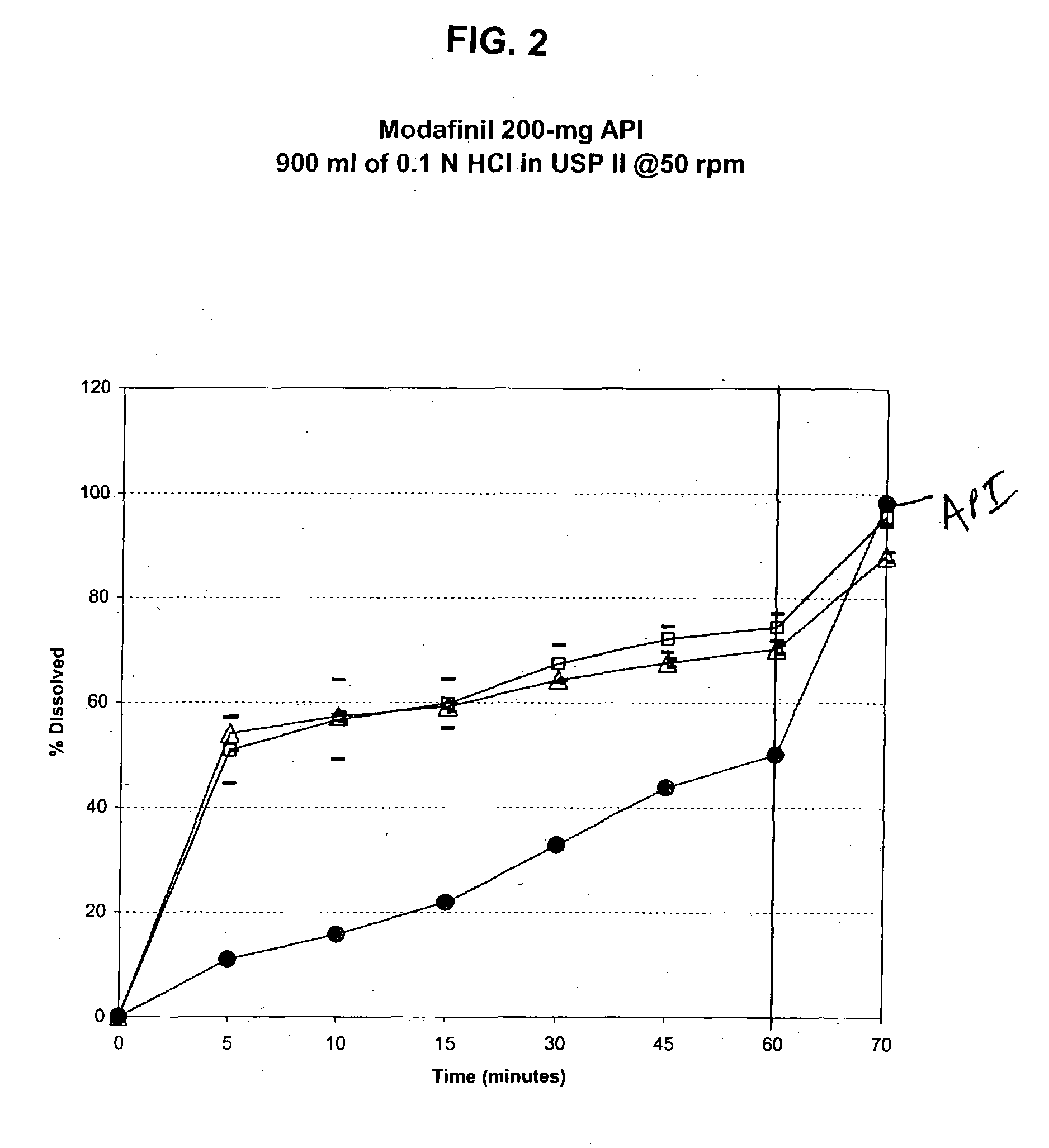
Controlled release sulfonylurea formulation
InactiveUS20030157166A1Effective controlBiocideSulfonylurea active ingredientsControl releaseSulfonylurea
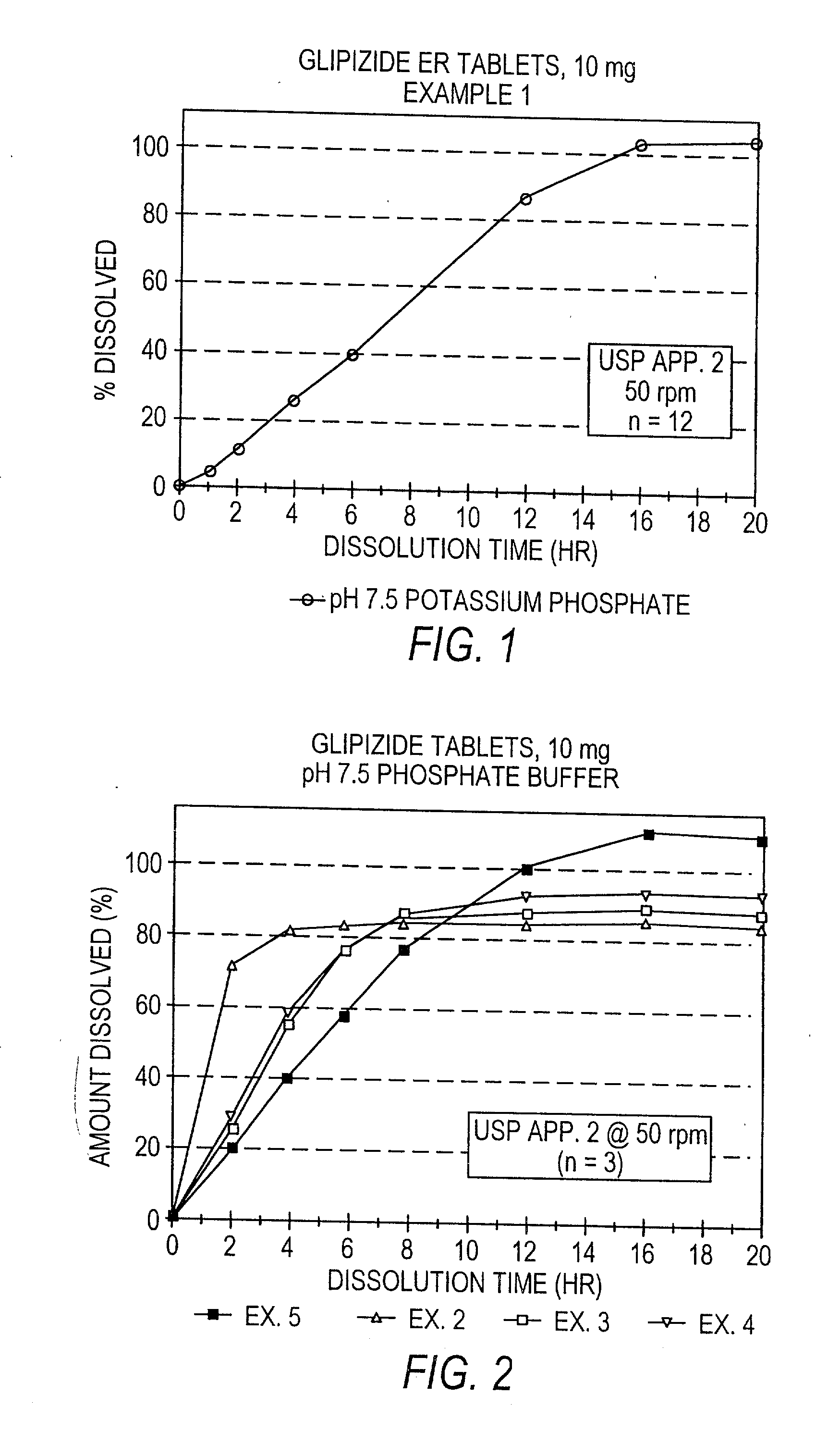
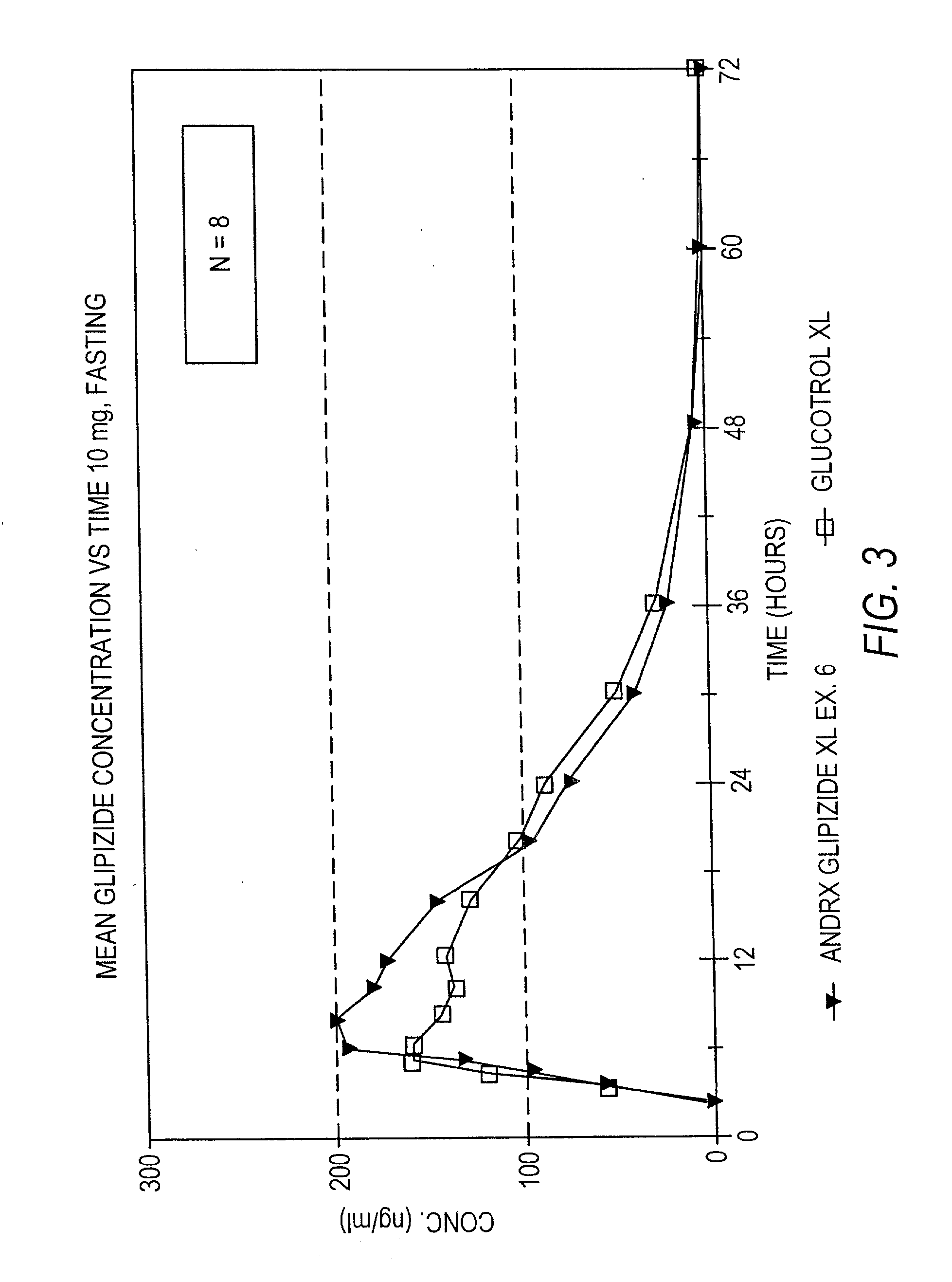
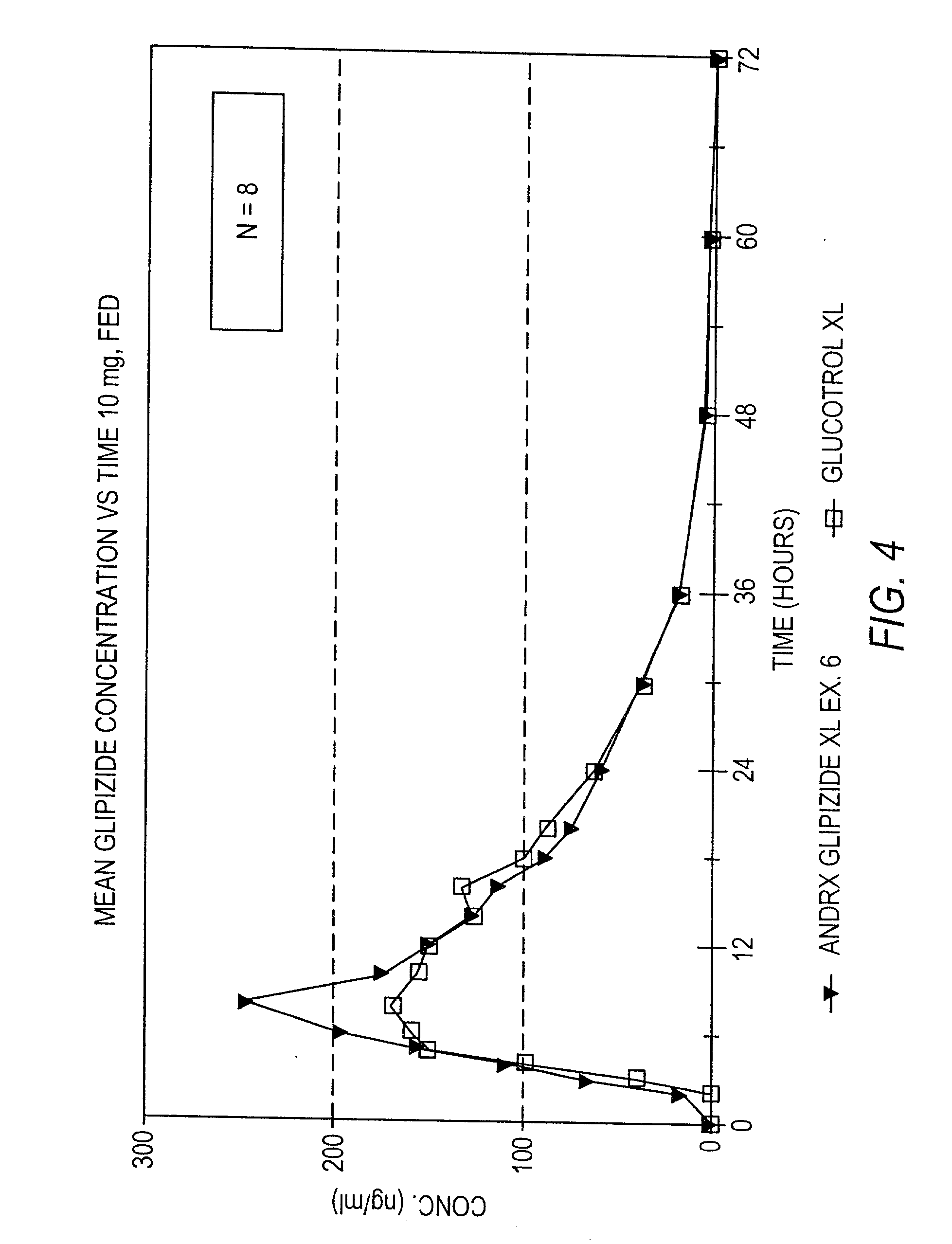
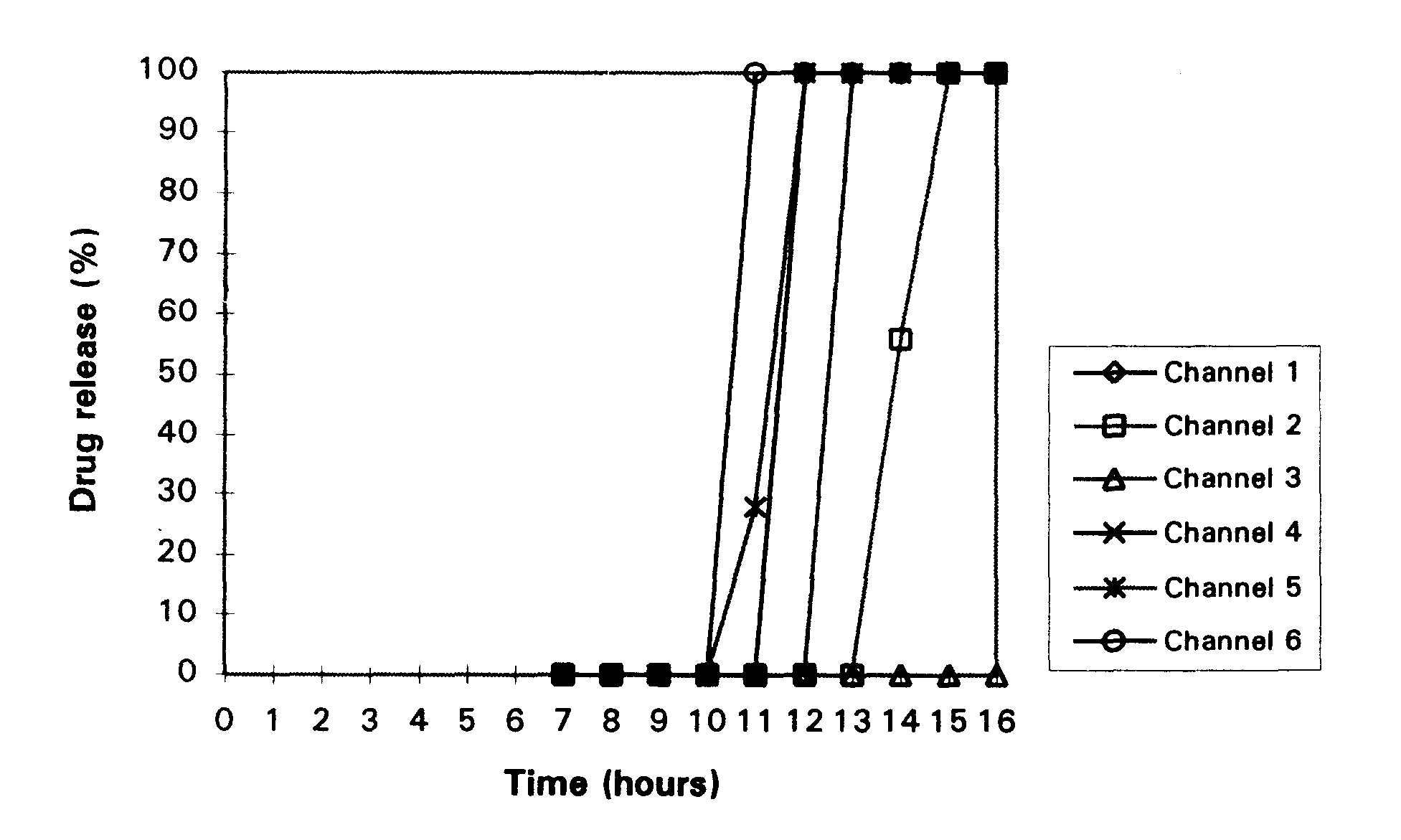
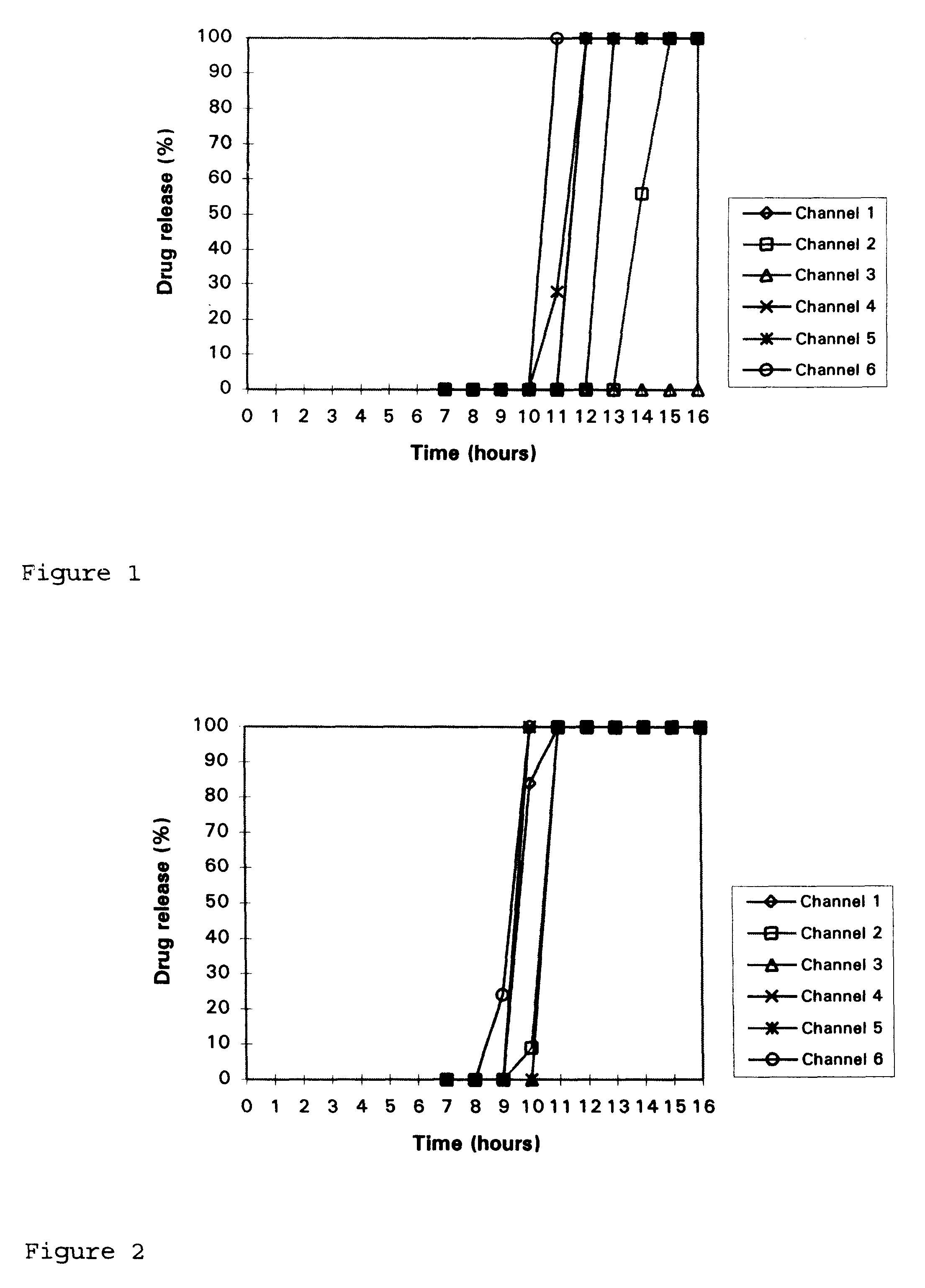
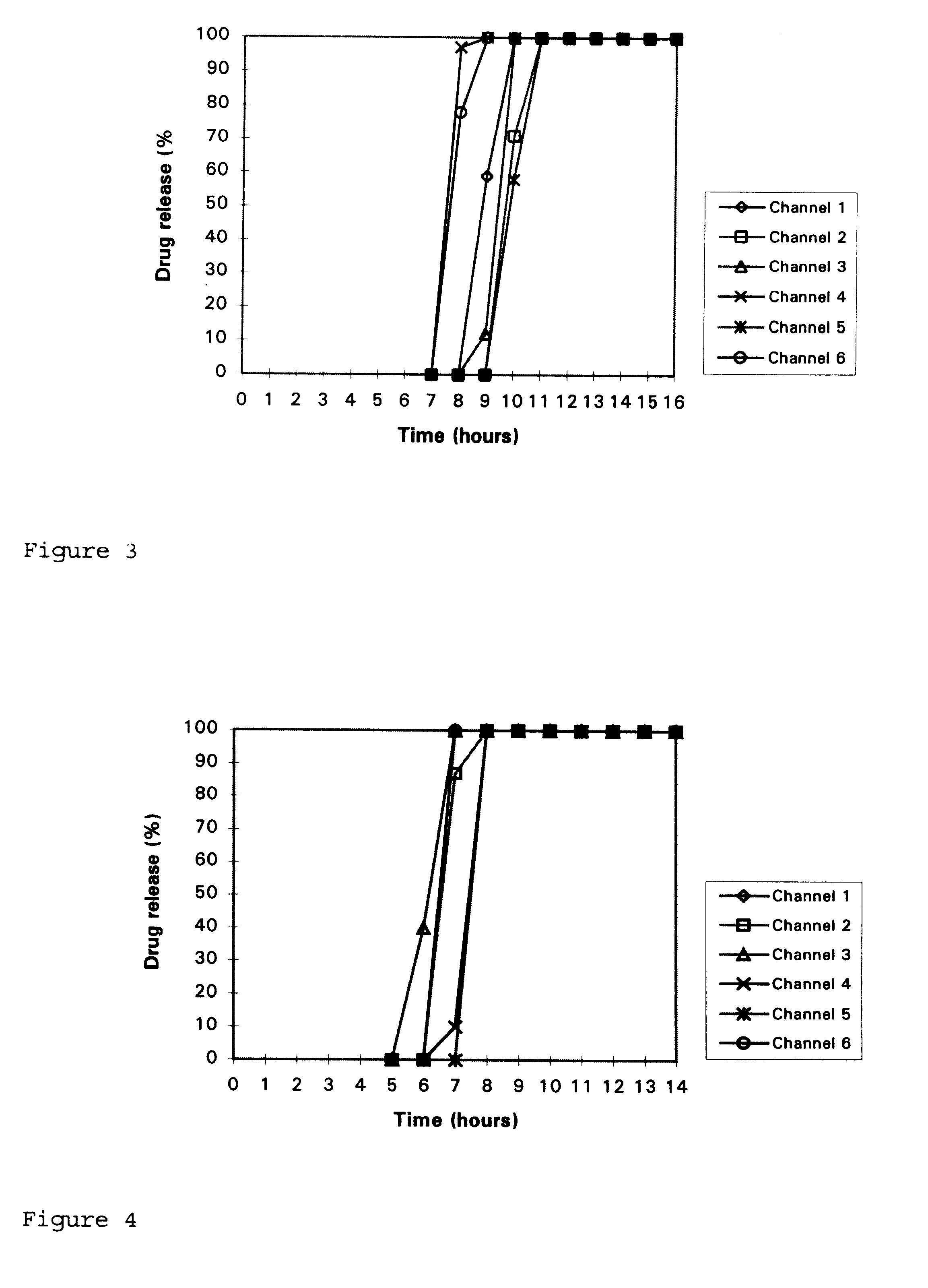
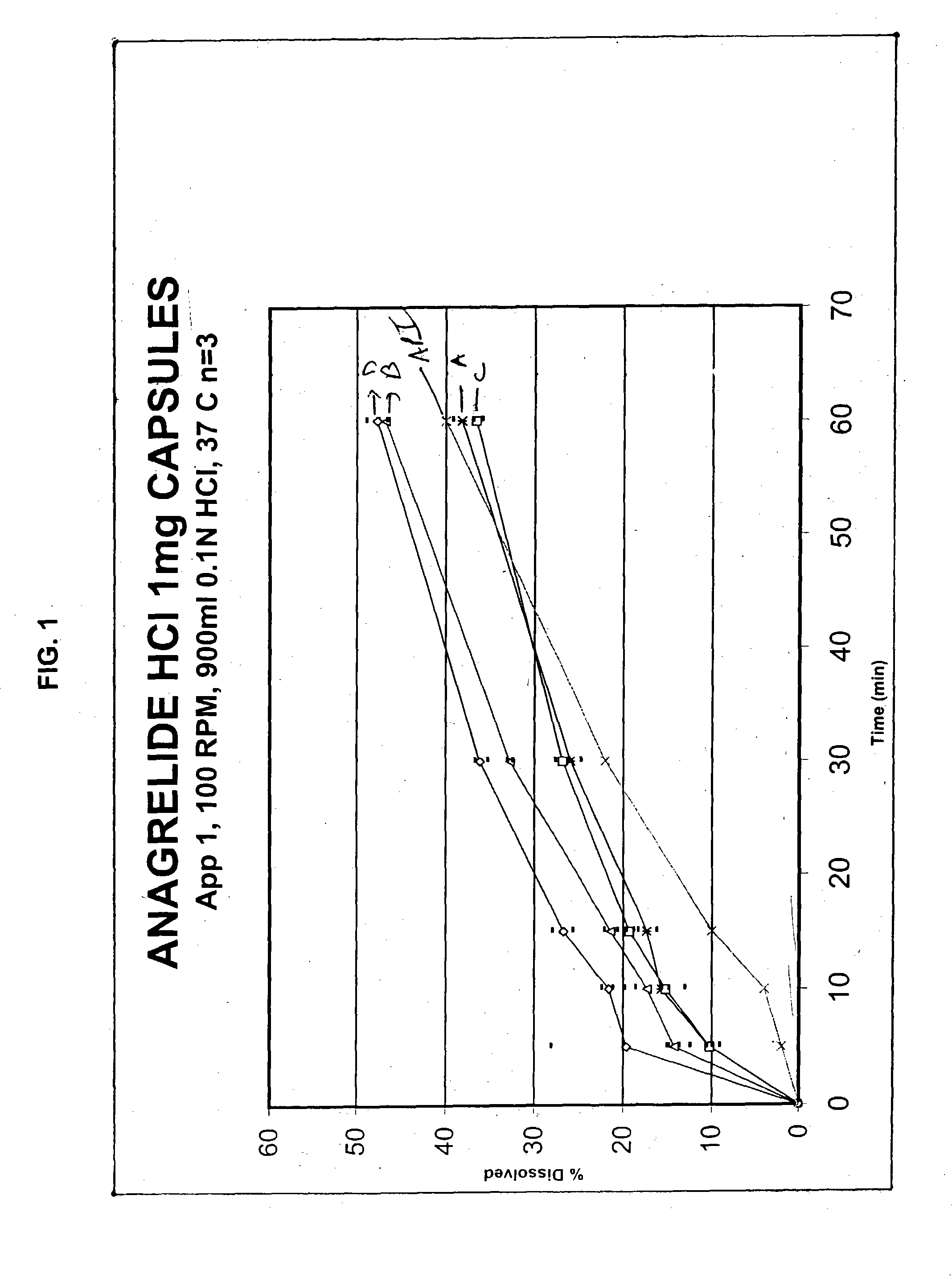
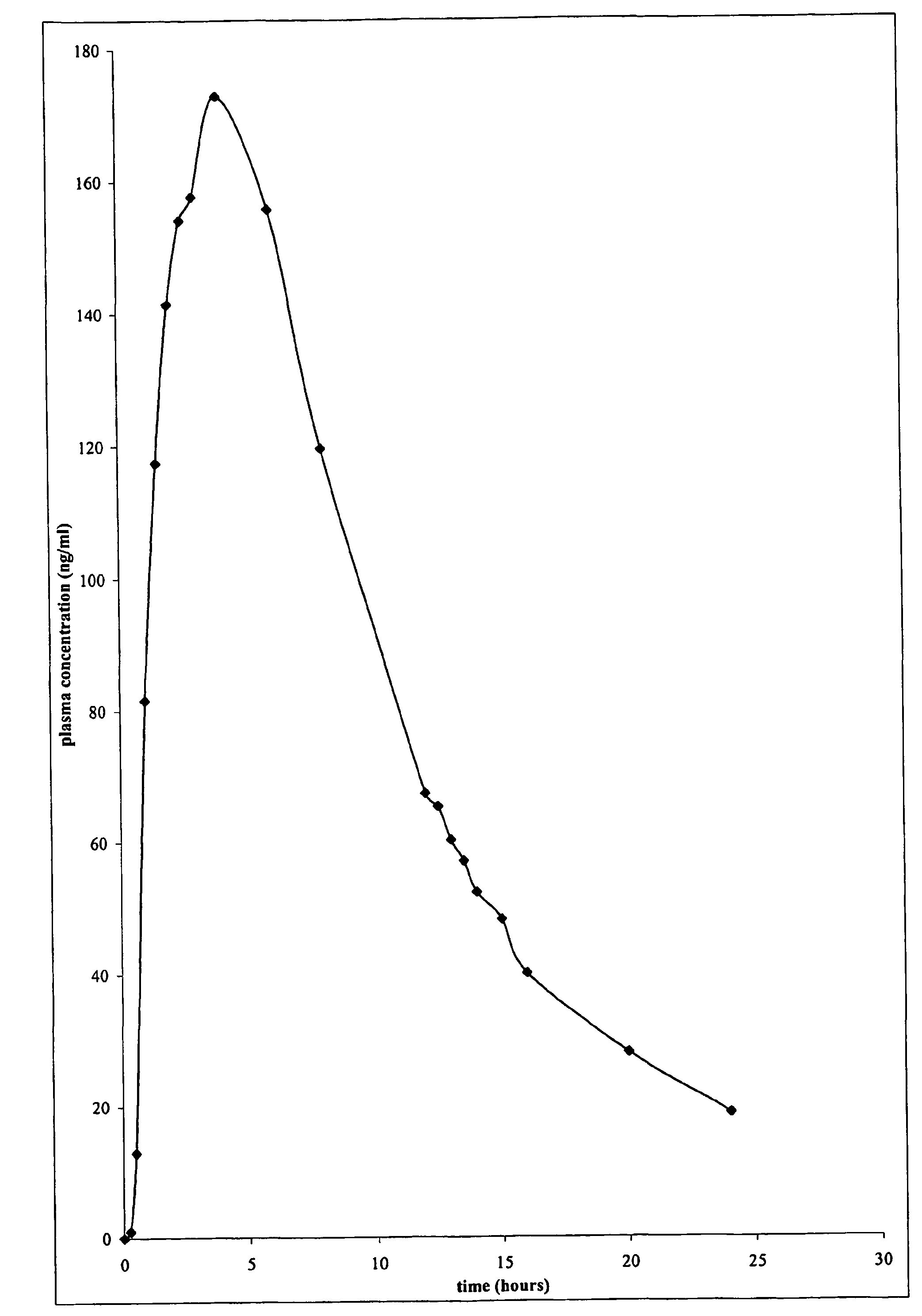
Disclosed in a controlled release sulfonylurea formulation. In certain embodiments, the invention comprises: (a) a core comprising: (i) a sulfonylurea or a pharmaceutically acceptable salt thereof; (ii) a pharmaceutically acceptable polymer; (b) a membrane surrounding the core which is permeable to the sulfonylurea and gastrointestinal fluid, wherein said dosage form provides a mean time to maximum plasma concentration (Tmax) of said sulfonylurea.
Owner:ANDRX PHARMA INC
Oral delayed immediate release formulation and method for preparing the same
InactiveUS6183780B1Simple and inexpensive formulation methodSimple and inexpensive methodNervous disorderPeptide/protein ingredientsAnti-emeticTranquilizing Agents
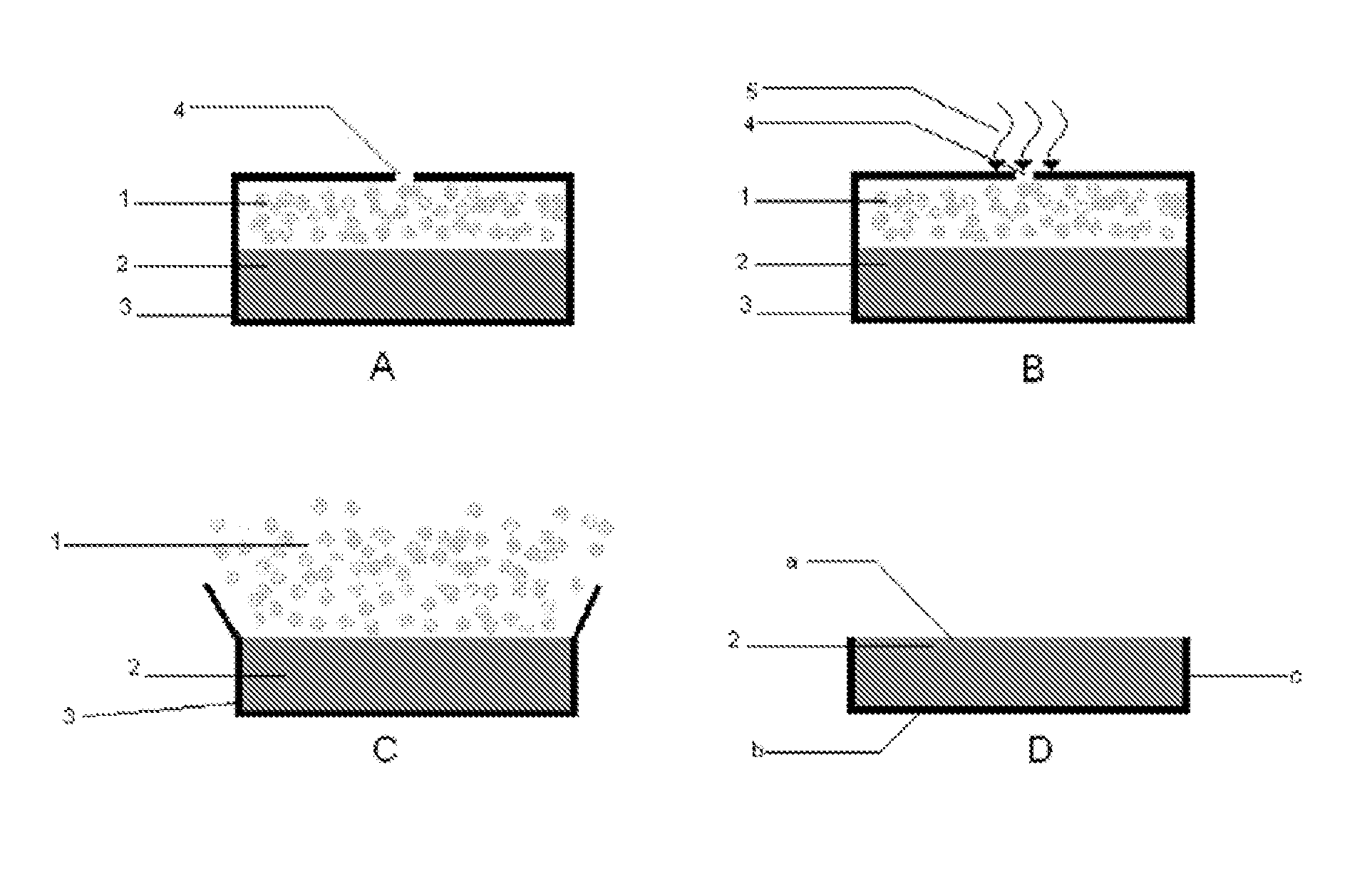
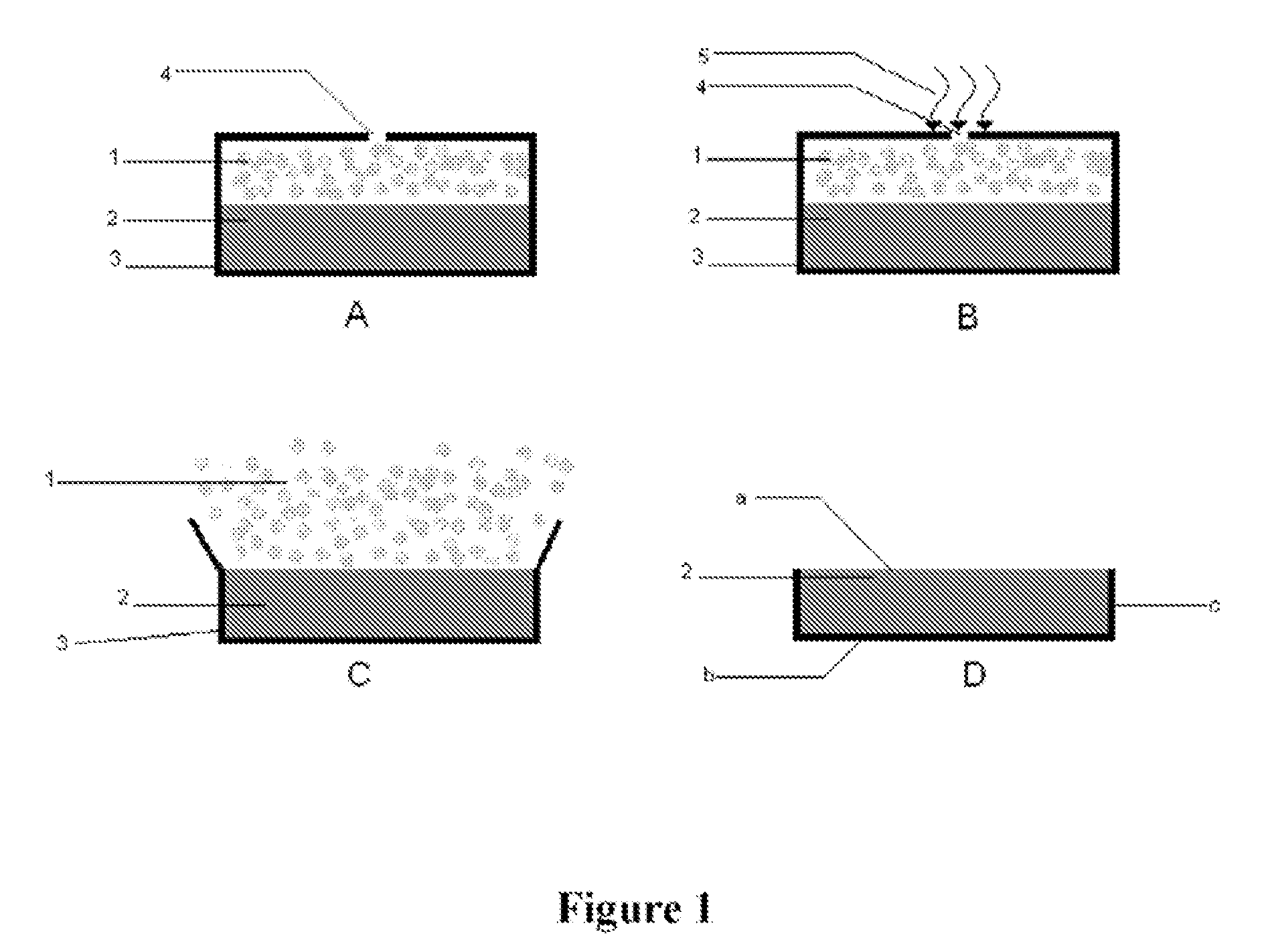
The invention relates to an Oral Delayed Immediate Release formulation comprising a compressed core containing one or more active substances surrounded with a coating, wherein release of active substance from the core is caused by rupture of the coating after a definite lag-time, said core comprising one or more immediate release carriers and having no substantial swelling properties upon exposure to gastrointestinal fluids. The invention further relates to formulations containing an Immediate Release formulation combined with one or more Oral Delayed Immediate Release formulations with different lag-times and to a method of preparing an Oral Delayed Immediate Release formulation.The Oral Delayed Immediate Release formulation may be used for the application of active substances whenever a certain lag-time before release is advantageous, such as in be the case of anti-asthmatics, anti-emetics, cardiotonics, vasodilators, anti-vertigo and anti-meniere compounds, anti-hypertensives, sedatives, anti-depressants, anti-anxiety compounds, cortico-steroids, general anti-inflammatory compounds, anti-inflammatory compounds for gastrointestinal use, anti-ulceratives, analgetics, anti-aritmics, anti-rheumatics, anti-arthritic compounds and anti-angina compounds.The Oral Delayed Immediate Release formulation may also be used for the application of biological active compounds such as proteins, peptides, enzymes, vaccines and oligonucleotides.
Owner:ABBOTT PROD OPERATION AG
Lactobacillus reuteri and its application
ActiveCN107523526AImprove the level ofImprove triglyceridesMilk preparationNervous disorderDiseaseGut flora
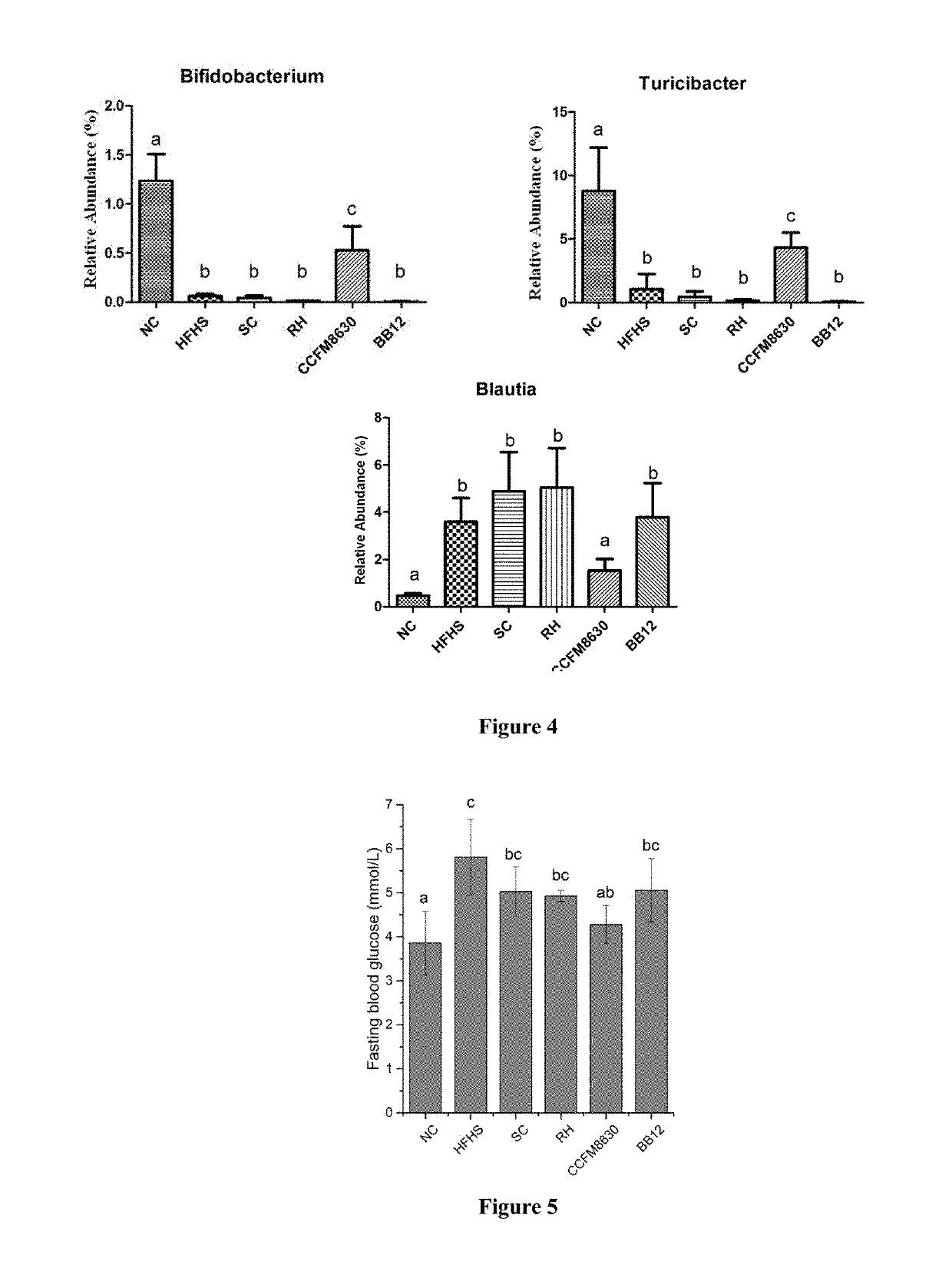
The invention relates to the technical field of microorganism, and discloses a Lactobacillus reuteri and its application. The preservation number of the Lactobacillus reuteri CCFM8631 is CGMCC No.14394; the level of mice peripheral neurotransmitter 5-hydroxytryptamine can be significantlyimproved; the rise of the mice peripheral blood testonsterone level and the abundant abnormity of Blautia, Turicibacter, Oscillospira and Bifidobacterium in the intestinal flora by high glucose and high fatty diets are recovered; the tolerated simulative gastrointestinal fluid is rapidly planted in an intestinal tract, so as to significantly improve the pathological injury of metabolic syndrome mice liver and duodenum and rise of triglyceride and total cholesterol content in the serum by high glucose and high fatty diets are significantly improved; the Lactobacillus reuteri can be used for preventing, delaying or treating metabolic disorder such as metabolic syndrome, irritable bowel syndrome, and anxiety, depression and other metal diseases related to irritable bowel syndrome.
Owner:INFINITUS (CHINA) CO LTD
Bifidobacterium bifidum and application thereof
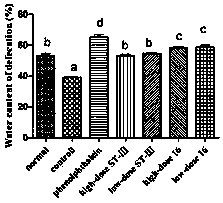
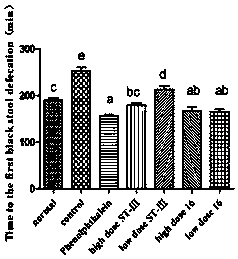
ActiveCN106834187AImprove toleranceIncrease the water content of fecesBacteriaDigestive systemSide effectFeces
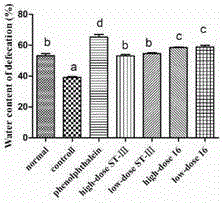
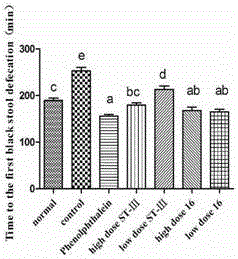
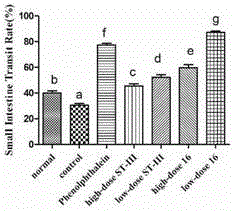
The invention discloses bifidobacterium bifidum capable of remarkably relieving constipation and application thereof and belongs to the technical field of microorganisms. The bifidobacterium bifidum CGMCC NO.13632 can well tolerate simulative gastrointestinal fluid, can be well attached to colon cancer cells HT-29, and can remarkably increase the water content of excrement of constipation mice and shorten the first tarry stool time. On the aspect of increasing the small intestine driving rate, the bifidobacterium bifidum CGMCC NO.13632 is superior to cathartic phenolphthalein, a best effect is shown, secretion of gastrointestinal regulatory peptide relevant to constipation in serum is also adjusted while constipation pathological factors are remarkably improved, and the bifidobacterium bifidum does not have toxic and side effects of medicine for treating constipation, and is a first choice for preventing and treating constipation. The bifidobacterium bifidum CGMCC NO.13632 is used for preparing pharmaceutical compositions and fermented food for relieving constipation, and has very wide application prospects.
Owner:JIANGNAN UNIV
Bifidobacterium longum and application thereof
ActiveCN108220206AGood ability to tolerate simulated gastrointestinal fluidNo side effectsMilk preparationBacteriaHuman bodyBiotechnology
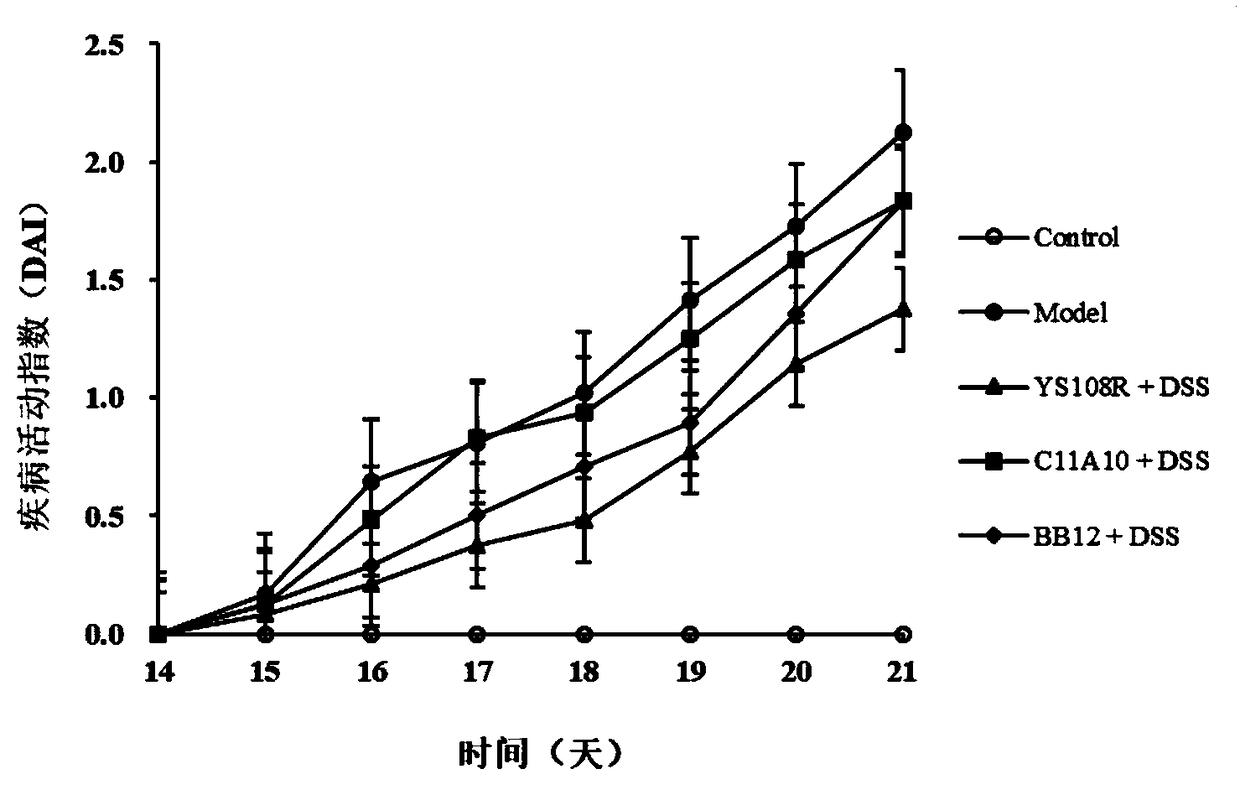
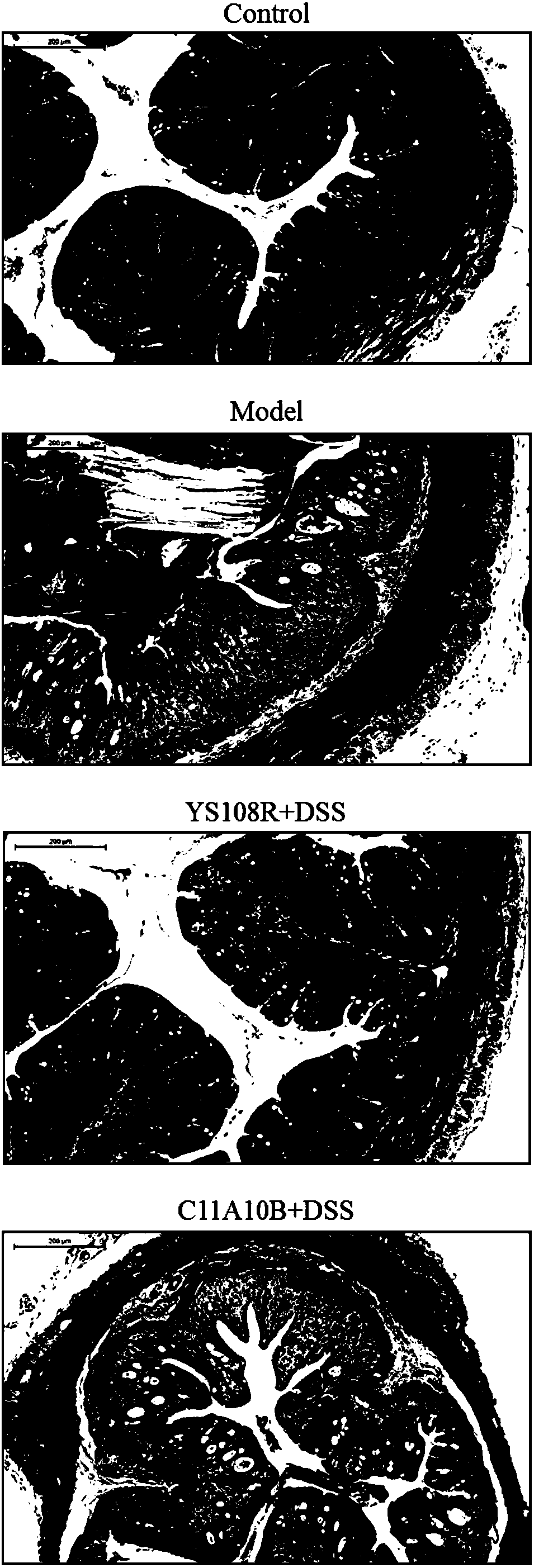
The invention discloses bifidobacterium longum and the application thereof and belongs to the technical field of biologics. The invention provides a bifidobacterium longum YS108R strain which has thecharacteristic of generating viscous exopolysaccharide, and has a remarkable improvement function on DSS (Dextran Sulfate Sodium) induced mouse colitis models. The strain has a relatively good capability of enduring simulated gastrointestinal fluids, is capable of remarkably reducing disease activity indexes of mice in the DSS induction period, and has an effective protection function on colon tissue. In addition, the bifidobacterium longum YS108R disclosed by the invention is separated from intestinal florae of healthy people, is free of toxic and / or side effects on human bodies, and has certain advantages when being compared with conventional medicine treatment. The strain can be used for preparing probiotic powder, fermented milk, and the like, and has wide market prospects.
Owner:无锡特殊食品与营养健康研究院有限公司
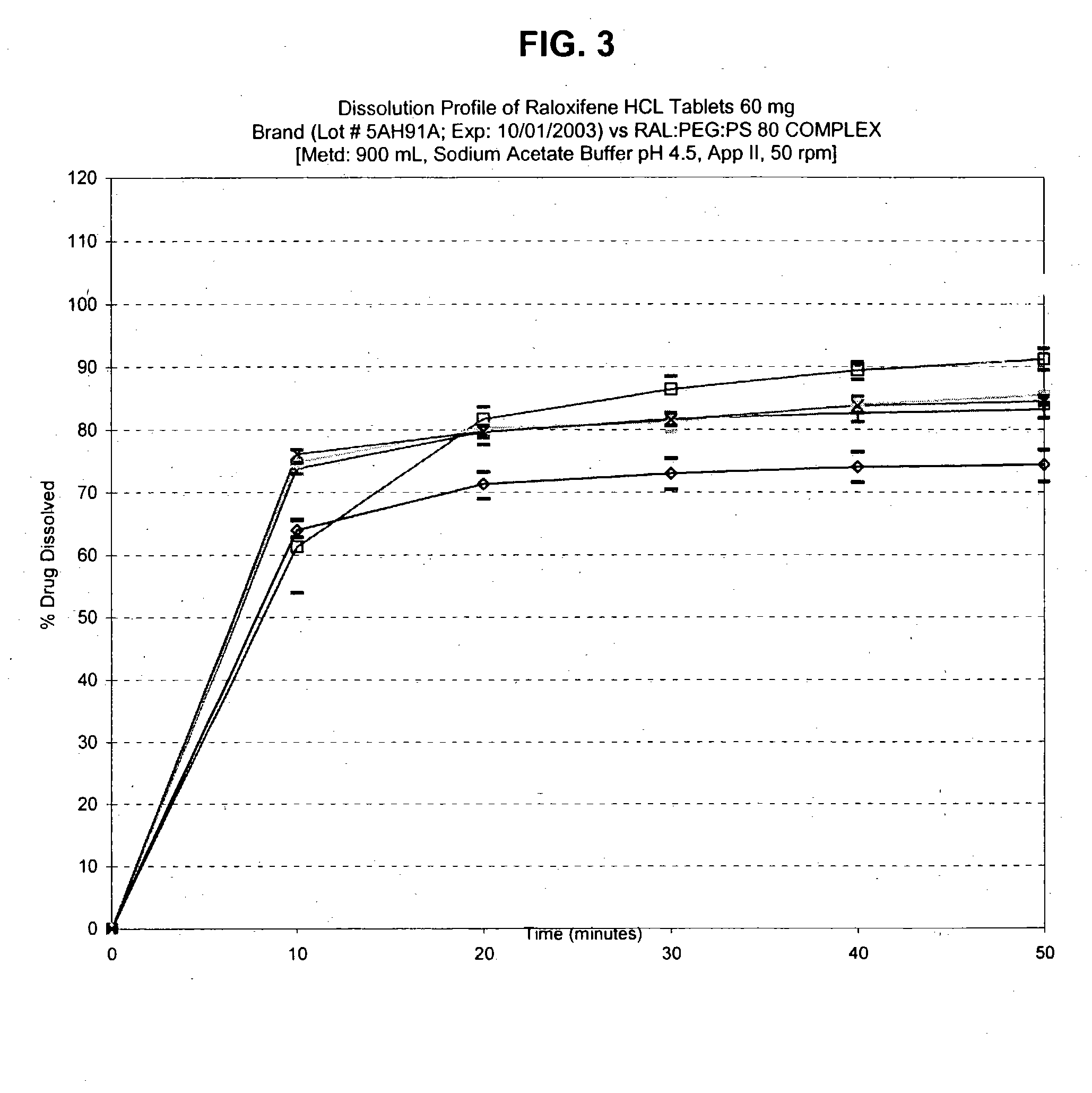
Pharmaceutical composition for solubility enhancement of hydrophobic drugs
InactiveUS20050008704A1Improve solubilityDissolve fastBiocidePowder deliverySolubilityPolyethylene glycol
The present invention provides a pharmaceutical composition having enhanced solubility comprising a drug and polyethylene glycol, wherein the ratio of polyethylene glycol to drug by weight is from about 0.2:1 to about 10:1, and the polyethylene glycol has a melting point of at least 37° C. The pharmaceutical compositions exhibit rapid dissolution upon contact with physiological solvents, such as water, saliva or gastrointestinal fluids.
Owner:RAY ANUP KUMAR +4
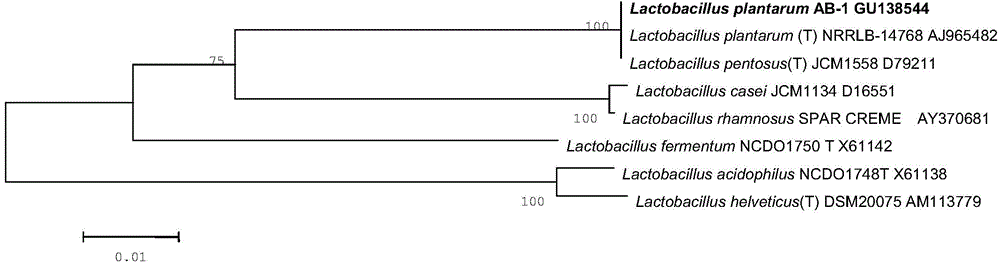
Lactobacillus plantarum AB-1 with broad-spectrum bacteriostasis and application thereof
ActiveCN104830731AGrowth inhibitionImprove survival rateMilk preparationBiocideFood flavorHigh survival rate
The invention discloses lactobacillus plantarum with broad-spectrum antifungal properties. The lactobacillus plantarum is named lactobacillus plantarum AB-1 and is preserved in China General Microbiological Culture Collection Center of China Committee for Culture Collection of Microorganisms with a preservation number of CGMCC No.9653 on September 15th, 2014. The lactobacillus plantarum shows relatively strong broad-spectrum antifungal properties in whole-milk culture mediums and participant compounded and fermented yoghourt; when the lactobacillus plantarum participates in the fermentation of the yoghourt, the flavor features of the fermented yoghourt can be effectively enhanced, and supernatant of the compounded and fermented yoghourt can still show relatively good broad-spectrum bacteriostasis after being treated by pepsin and trypsin. The relatively high survival rate of the lactobacillus plantarum in gastrointestinal fluid further proves that the lactobacillus plantarum has excellent probiotic properties and relatively strong survival ability in human bodies. Besides, the lactobacillus plantarum has relatively good bacteriostasis for fusariwn oxysporum and phytophthora drechsleri.
Owner:三主粮(和田)实业股份有限公司
Gastric retention controlled drug delivery system
ActiveUS7776345B2Fast flotationMaintain physical integrityOrganic active ingredientsNervous disorderControlled releaseExcipient
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Job's tears nut oil self-emusifying preparation and its making method
InactiveCN101028461AImprove stabilityPromote absorptionAntipyreticMetabolism disorderAdemetionineSoftgel
A self-emulsifying product of coix seed oil in the orally taken forms, such as softgel, liquid capsule, solid capsule, particle, etc, is proportionally prepared from coix seed, emulsifier, disperser, emulsifying aid, and solid adsorbent. It can be emulsified by itself in gastrointestinal tract for higher biologic utilization rate and higher curative effect. Its preparing process is also disclosed.
Owner:江苏圣典医药科技有限公司
Oral controlled release tablet
A method of reducing the risk of alcohol-induced dose-dumping of a therapeutically active ingredient comprising administering to human subjects who have ingested alcohol an oral controlled release tablet; said tablet comprising:a core comprisingan upper compressed layer comprising a swelling agent, anda lower compressed layer comprising at least one therapeutically active ingredient, and pharmaceutically acceptable excipient wherein at least one excipient is a release rate controlling excipient and wherein the percent by weight of excipients that are soluble in alcohol does not exceed 35% by weight of the layer and;a coating surrounding the said core, the coating comprising a polymer insoluble in an aqueous medium comprising from 0% v / v to 40% v / v of alcohol, whereby upon contact with aqueous gastrointestinal fluids, the upper compressed layer swells to cause removal of the coating from the upper surface of the upper compressed layer and then said upper layer disintegrates allowing the release of the active ingredient from the defined surface area of the upper surface of said lower compressed layer with the coating covering its bottom and side surfaces.
Owner:SUN PHARMA INDS
Drug composition containing abiraterone acetate and preparation method and application of drug composition
PendingCN110538150AUniform textureDecreased pre- and post-meal differencesOrganic active ingredientsUrinary disorderEthylic acidOil phase
The invention relates to the technical field of drug preparations, in particular to a drug composition containing abiraterone acetate, and a preparation method and application of the drug composition.The drug composition is prepared from an active ingredient, namely the abiraterone acetate and accessories including one or more oil phases, one or more emulsifiers and one or more co-emulsifiers. After being orally taken, the drug composition meets gastrointestinal fluid to be spontaneously dispersed under gastrointestinal peristalsis to form O / W-type nanoemulsion. The formed nanoemulsion is small in particle size, the permeability of intestinal epithelial cells is improved, and the bioavailability of drugs can be significantly improved. Compared with microemulsion, a self-emulsified solution has higher stability, and the requirement of long-term preservation can be met. The drug composition is stable in content. Compared with an original drug Zytiga, the difference before and after mealis significantly reduced; and a capsule can be further prepared from the drug composition, and the capsule is stable in property.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
Bifidobacterium adolescentis and use thereof
InactiveUS20190112674A1Improve their levelRelieve hyperglycemiaMilk preparationBacteriaBifidobacterium adolescentisDisease
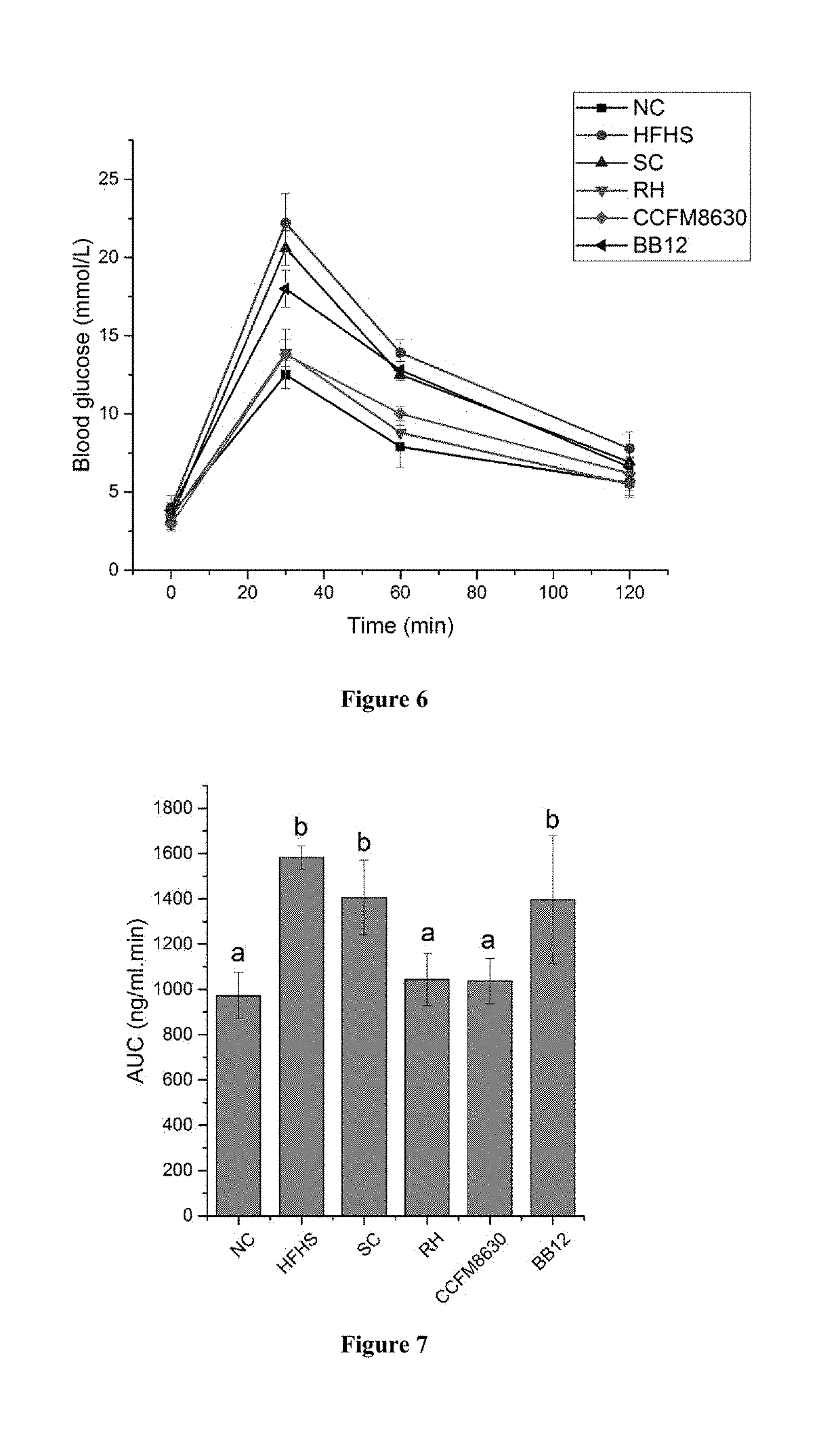
Provided is a strain CCFM8630 of Bifidobacterium adolescentis and use thereof. The strain CCFM8630 of Bifidobacterium adolescentis can significantly increase neurotransmitter 5-hydroxytryptamine level in peripheral blood of rat, recover the hormone levels, for example testosterone and so on in peripheral blood of rat, normalize abnormal abundances of Bifidobacterium genus, Blautia genus and Turicibacter genus in intestinal flora of rat affected by high-fat high-starch diet, show pretty good tolerance to simulated gastrointestinal fluid and quickly colonize in intestinal, significantly improve pathological damages of tissues such as liver, duodenum and so on, and increase triglyceride and total cholesterol levels in serum and oral glucose tolerance of rat with metabolic syndrome caused by high-fat high-starch diet. The strain CCFM8630 of Bifidobacterium adolescentis can be used for preventing, relieving or treating metabolic disorder, such as metabolic syndrome, irritable bowel syndrome and mental diseases related to metabolic syndrome such as anxiety, depression and so on.
Owner:INFINITUS (CHINA) CO LTD
Self emulsifying drug delivery system
The present invention claims and discloses a pharmaceutical composition suitable for oral administration, in form of an emulsion pre-concentrate, comprising (i) one or more NO-releasing NSAID(s); (ii) one or more surfactants; (iii) optionally an additional oil or semi-solid fat; said composition forming an in-situ oil-in-water emulsion upon contact with gastrointestinal fluids. The composition may optionally also comprise one or more short-chain alcohols. Also within the scope of the invention is a combination with a proton pump inhibitor. The pharmaceutical composition is useful in the treatment of pain and inflammation. Further within the scope of the invention is kit comprising a pharmaceutical composition according to the invention in a unit dosage form, in combination with a proton pump inhibitor, and said proton pump inhibitor is enteric coated
Owner:NICOX SA
Novel insulin-phospholipid-chitosan self-assembled microparticle carrier and preparation thereof
ActiveCN105617362APrevent leakageHigh encapsulation efficiencyPowder deliveryPeptide/protein ingredientsMass ratioMicroparticle
The invention discloses a novel insulin-phospholipid-chitosan self-assembled microparticle carrier and a drug delivery system thereof, wherein the mass ratio of insulin to phospholipid is 1 to (3-100); and the mass ratio of chitosan to the phospholipid is 1 to (5-50). The self-assembled microparticle carrier disclosed by the invention is prepared by preparing an insulin-phospholipid compound from the insulin and a proper amount of the phospholipid material in a special environment, injecting a non-aqueous solvent organic phase of the insulin-phospholipid compound to an aqueous-phase solution of the chitosan, and self-assembling in a warm stirring condition, so that the insulin-phospholipid-chitosan microparticle carrier is formed. The insulin-phospholipid-chitosan microparticle carrier disclosed by the invention is free from the addition of a cross-linking agent, is represented in a circular or elliptic form and has a multilayer capsule structure; the grain size distribution of the microparticle carrier is 50-5000nm and a drug entrapment rate reaches 70% or above; the microparticle carrier is good in quality stability in a gastrointestinal fluid environment and low in burst release; the microparticle carrier can break through the limitation of an enzyme barrier and a membrane barrier; and the microparticle carrier is used for preparing insulin non-injection drug delivery systems such as oral drug delivery, mucosal drug delivery, percutaneous drug delivery and the like.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
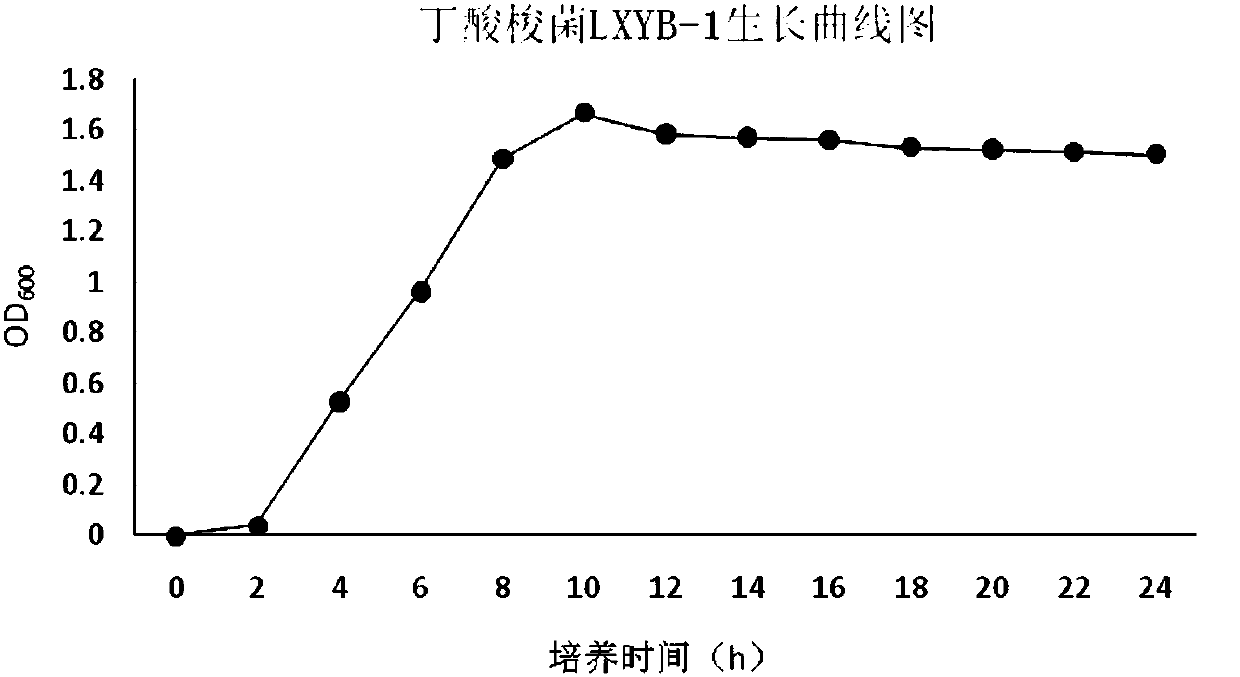
Clostridium butyricum agent with strong butyric acid resistance and stress resistance and application thereof
ActiveCN107937305AImprove survival ratePromote growthBacteriaAnimal feeding stuffPathogenPoultry product
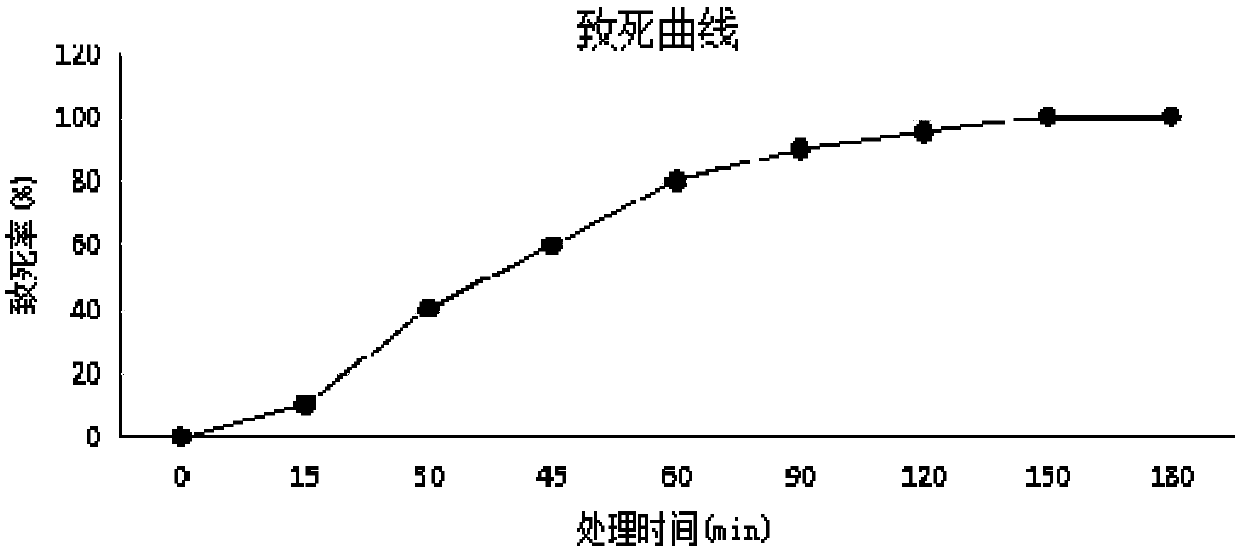
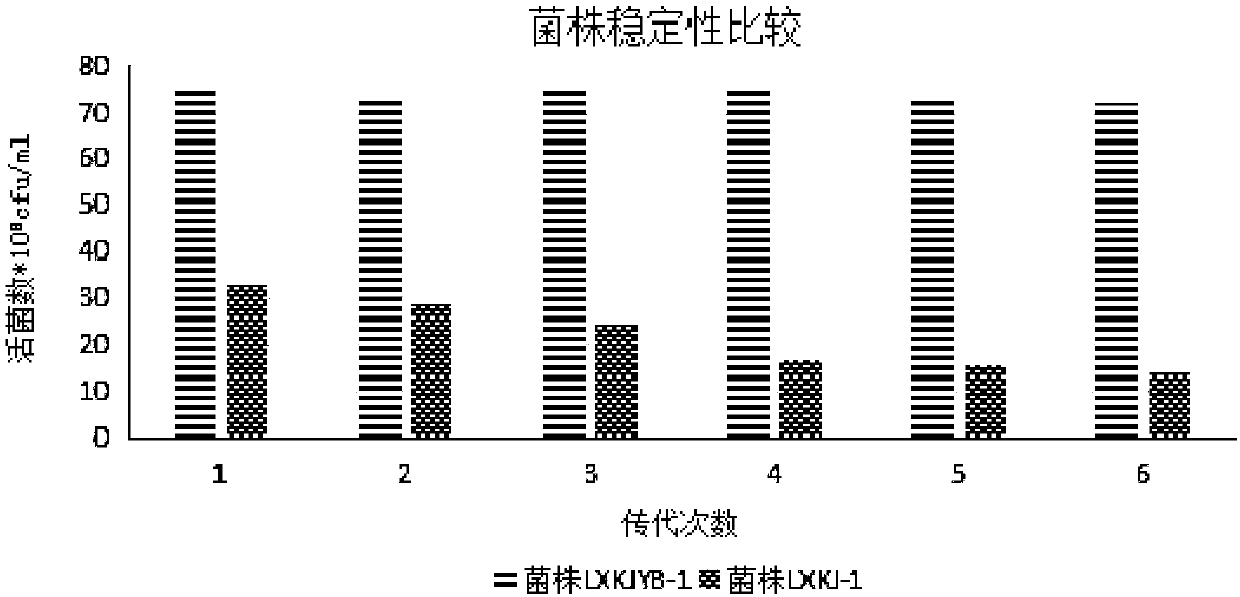
The invention belongs to the technical field of microbial fermentation, and in particular, relates to a clostridium butyricum inoculum with strong butyric acid resistance and stress resistance and anapplication thereof. Clostridium butyricum is specifically clostridium butyricum LXKJYB-1, and has the preservation number of CCTCC NO:M 2017485. The strain LXKJYB-1 has the characteristics of high fermentation level, high organic acid yield, inhibition of a variety of intestinal pathogens, and high tolerance capacity to gastrointestinal fluid, cholate and damp heat. The strain and the inoculum prepared therefrom are suitably applied for livestock and poultry breeding, increases the efficiency of breeding, and produces no-resistance livestock and poultry products.
Owner:湖北绿雪生物科技有限公司
Bifidobacterium bifidum and its use
ActiveCN106834187BIncrease moisture contentImprove the first black stool timeBacteriaDigestive systemBiotechnologySide effect
Owner:JIANGNAN UNIV
Lactobacillus rhamnosus M9 separated from breast milk and application thereof
ActiveCN110106119AIncrease diversityImprove stabilityMilk preparationBacteriaMicroorganismLactobacillus rhamnosus
The invention provides Lactobacillus rhamnosus M9 separated from breast milk. The microbial preservation number is CGMCC No.16002, and the Lactobacillus rhamnosus M9 has the excellent tolerance of gastrointestinal fluid and the good cholate tolerance, and has the basic condition as probiotics. The Lactobacillus rhamnosus M9 has the effect of enhancing immunity resistance, improving intestinal flora diversity and enhancing the intestinal flora stability, and the Lactobacillus rhamnosus M9 can be added into various common foods, healthcare products and the like as probiotics.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Solubility of hydrophobic drugs with a compound having a carboxylic acid moiety
A pharmaceutical composition having improved solubility comprising a hydrophobic drug or pharmaceutically acceptable salt thereof and a compound having at least one carboxylic acid moiety, wherein the molar ratio of the compound having at least one carboxylic acid moiety to the hydrophobic drug or pharmaceutically acceptable salt thereof is from about 0.1:1 to about 25:1. The pharmaceutical composition exhibits rapid dissolution upon contact with physiological solvents, such as water, saliva or gastrointestinal fluids.
Owner:SANDOZ AG
New self emulsifying drug delivery system
The present invention claims and discloses a pharmaceutical composition suitable for oral administration, in form of an emulsion pre-concentrate, comprising (i) a compound of formula (I); (ii) one or more surfactants; (iii) optionally an oil or semi-solid fat; said composition forming an in-situ oil-in-water emulsion upon contact with aqueous media such as gastrointestinal fluids. The composition may optionally also comprise one or more short-chain alcohols. The pharmaceutical composition is useful in the treatment of pain and inflammation.
Owner:NICOX SCI IRELAND
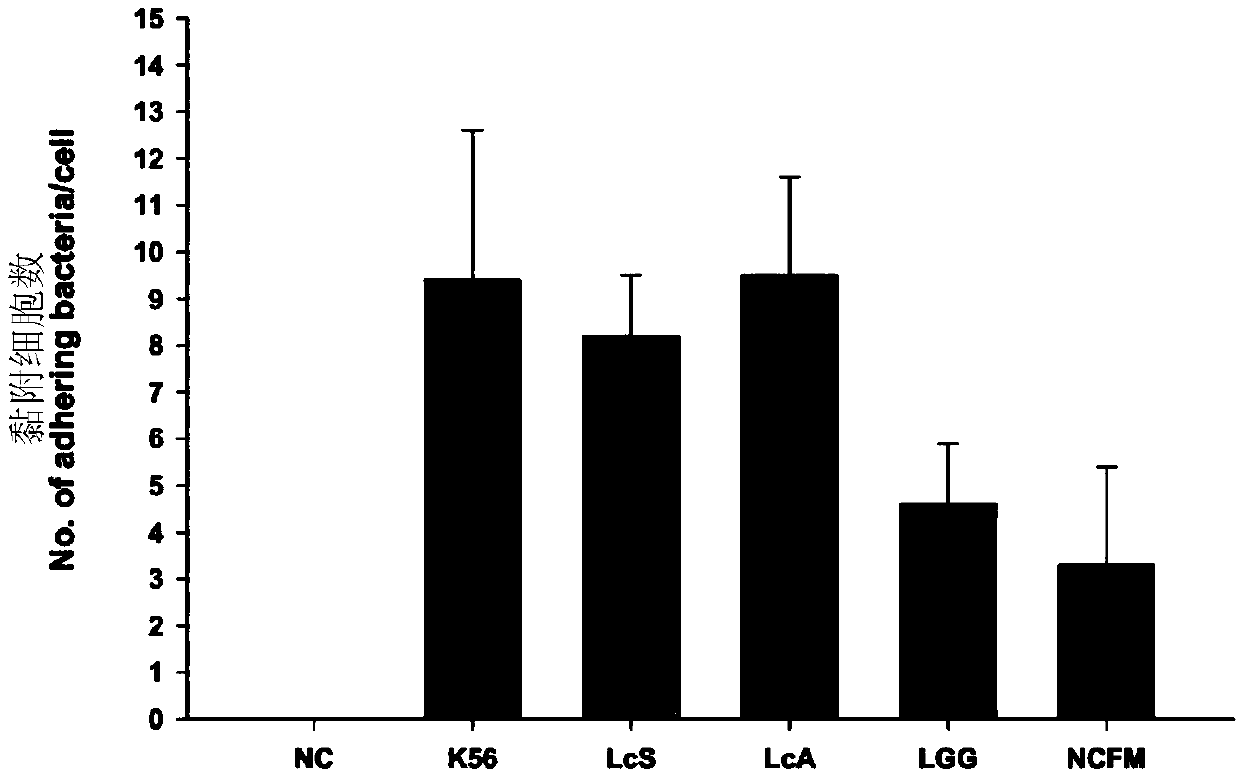
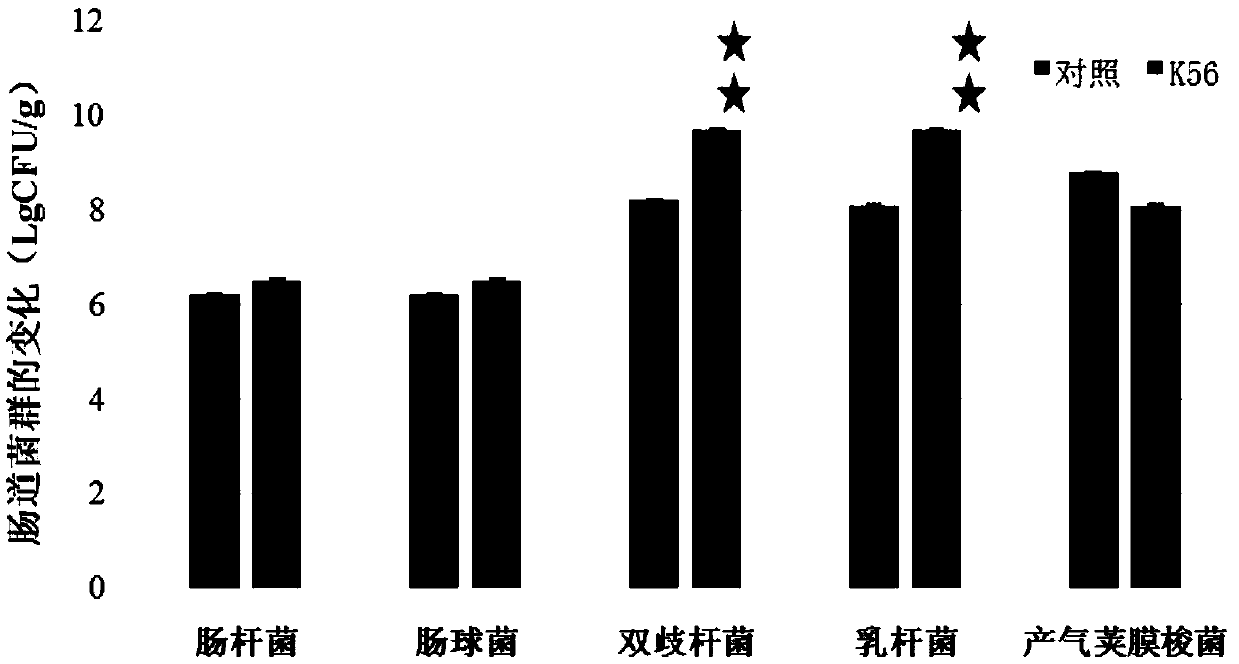
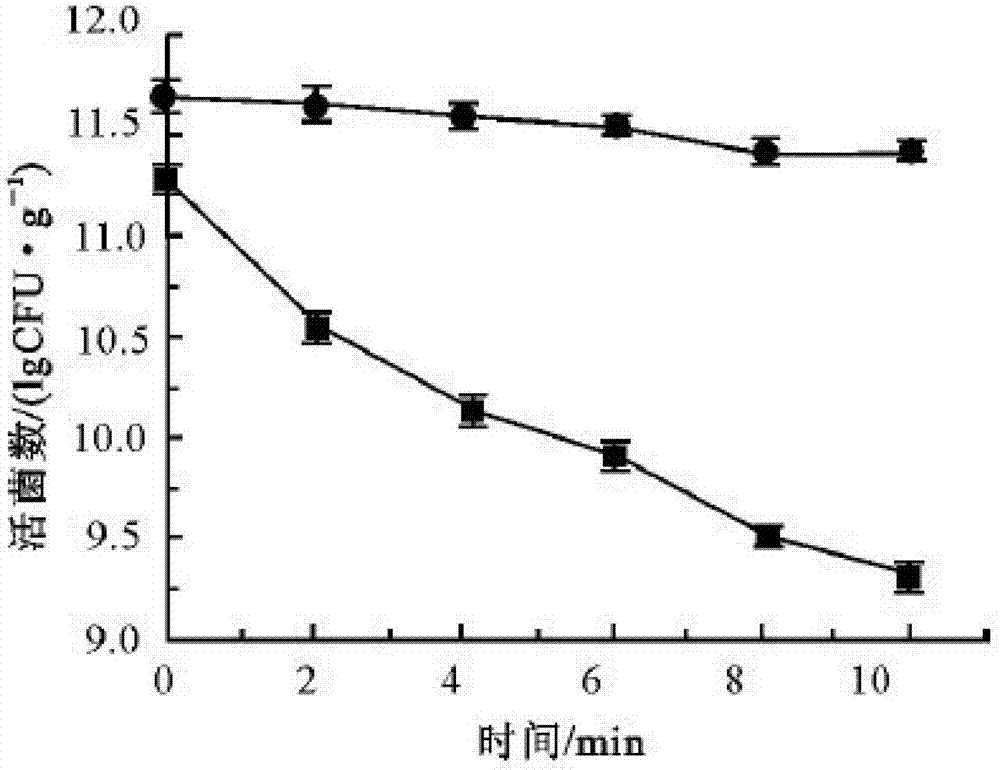
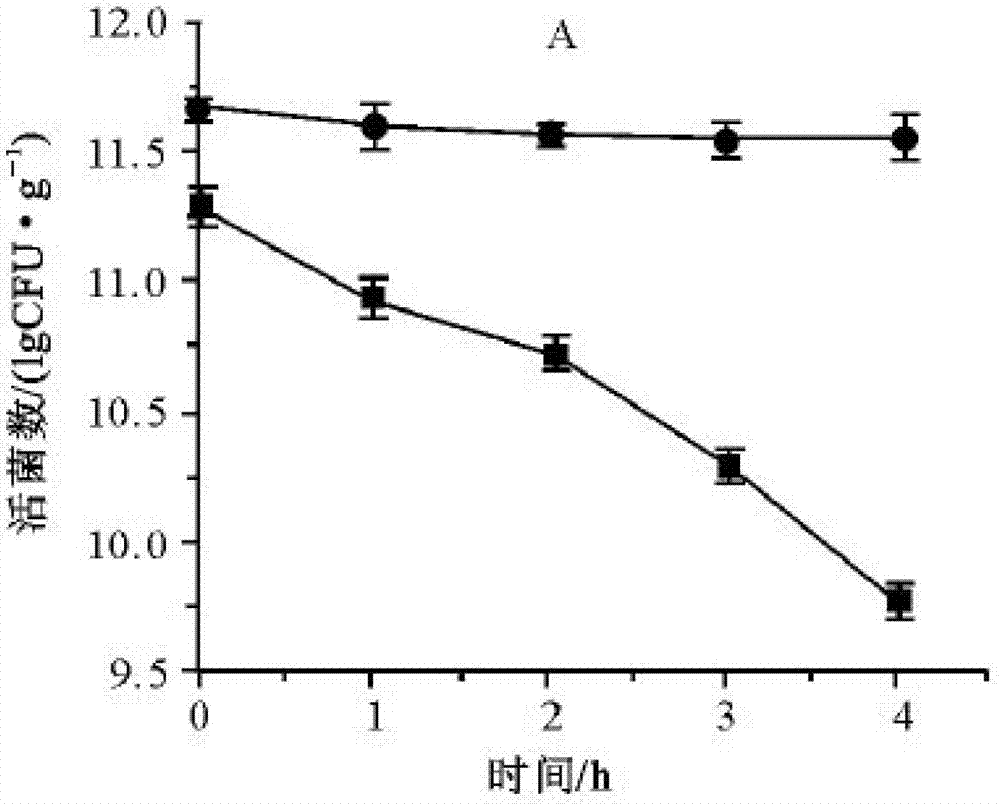
New application of lactobacillus paracausei K56 capable of adjusting gastrointestinal flora balance
The present invention provides a new application of lactobacillus paracausei K56 capable of adjusting gastrointestinal flora balance. The lactobacillus paracasei (lactobacillus paracausei subsp.paracasei) has a preservation number of DSM27447. The strain of the single bacteria has ability to significantly promote growth of intestinal bifidobacteria and lactic acid bacteria, can inhibit desulphovibrio and / or enterobacter, can inhibit helicobacter pylori and / or escherichia-shigella genera, and can tolerate stress environment of simulated gastrointestinal fluid in vitro. Mouse experiments show that the strain has no oral acute toxicity and no antibiotic resistance, is safe and can be used for food processing.
Owner:INNER MONGOLIA YILI INDUSTRIAL GROUP CO LTD
Microencapsulated Enterococcus faecium live bacterium preparation and its preparation method
InactiveCN103289983AImprove and enhance survivabilityImprove stabilityAnimal feeding stuffMicroorganism based processesBiotechnologySpoilage bacteria
The invention relates to a preparation method of a microencapsulated Enterococcus faecium live bacterium preparation and application of the preparation in breeding. The method employs a technological process of ''fermentation-coating-fermentation''. By adding 2g / L calcium chloride to a fermentation medium and conducting drying at 45DEG C, a microencapsulated Enterococcus faecium solid product with good sphericity and uniform size can be obtained, and its viable count can reach 11.57lgCFU / g. Compared with free Enterococcus faecium solid products, the microencapsulated Enterococcus faecium live bacterium preparation has stronger ability to tolerate a high temperature of 80DEG C and a simulated gastrointestinal fluid. After storage for 2 months under a normal temperature condition, the viable count basically has no change. The microencapsulated Enterococcus faecium live bacterium preparation has a simple pre-fermentation coating preparation process, good product appearance, strong stress resistance, high stability, and high encapsulation efficiency. Being use as a high activity microecological agent for feeding in actual production, the microencapsulated Enterococcus faecium live bacterium preparation can inhibit propagation of intestinal spoilage bacteria and pathogenic bacteria and intestinal infection, reduce the diarrhea rate, promote digestive absorption of nutrients, enhance immunity, improve feed intake and feed conversion rate, as well as lower feed-meat ratio.
Owner:GUANGZHOU GLAM BIOTECH
Lactobacillus plantarum GL-5 with oxidation resisting activity and application thereof
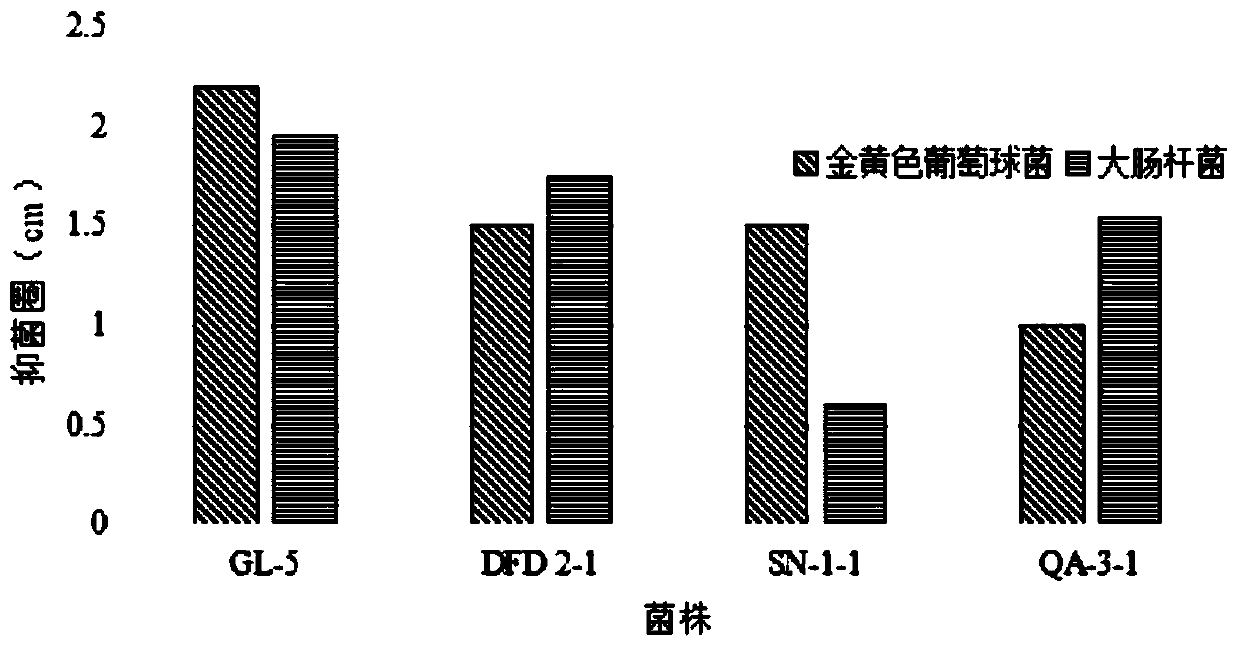
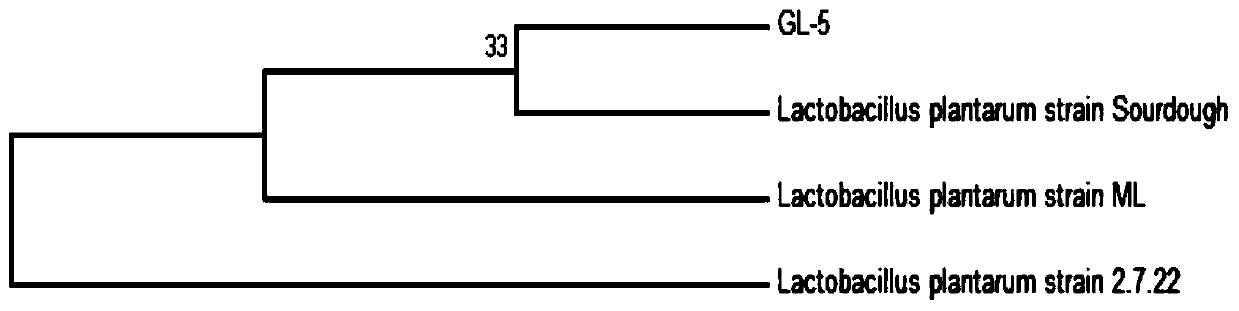
ActiveCN111304117AHigh antibacterial activityImproves antioxidant activityAntibacterial agentsBacteriaBiotechnologyEscherichia coli
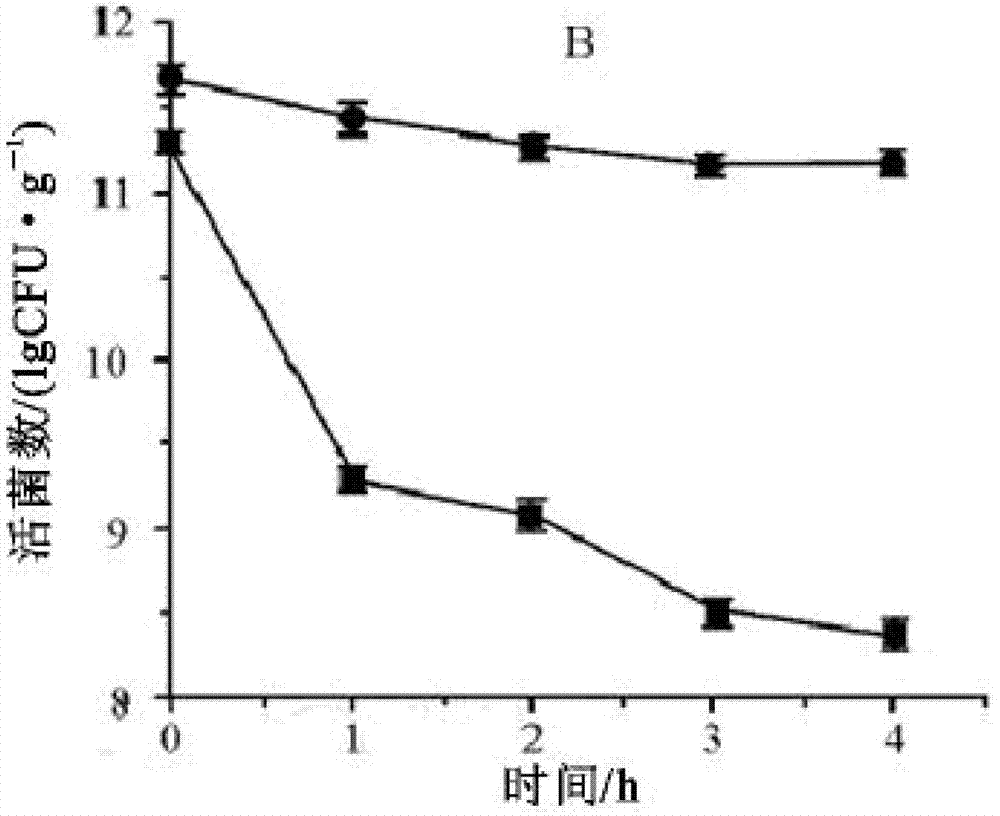
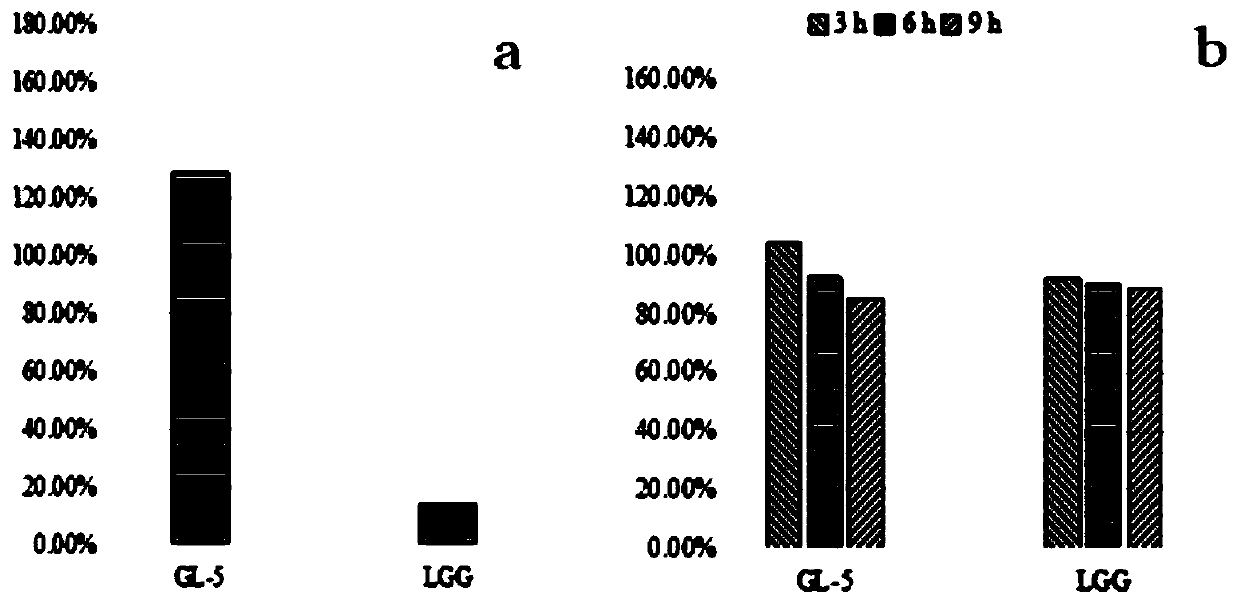
The invention belongs to the technical field of microbes and particularly relates to lactobacillus plantarum GL-5 with oxidation resisting activity and application thereof. The lactobacillus plantarumGL-5 with oxidation resisting activity disclosed by the invention is screened out from a home-made fermented dairy product of a herdsman's house of a Qinghai Guoluo region with an altitude of 4,000mor more, is collected in China General Microbiological Culture Collection Center of China Committee for Culture Collection of Microorganisms on November, 21, 2019 and has an accession number of CGMCCNo. 18988. The lactobacillus plantarum GL-5 has a 16S rRNA sequence shown in SEQ ID NO: 1. The lactobacillus plantarum GL-5 provided by the invention has relatively good bacteriostatic activity, and activity of enteropathogenic bacteria, i.e., staphylococcus aureus and escherichia coli can be remarkably inhibited; the lactobacillus plantarum GL-5 provided by the invention has relatively high oxidation resisting activity and can remarkably tolerate catalase; and the lactobacillus plantarum GL-5 provided by the invention has relatively high gastrointestinal fluid tolerance and cholate tolerance,and the survival rate of the lactobacillus plantarum GL-5 after culture in simulated gastric fluid and intestinal fluid is remarkably higher than that of lactobacillus rhamnosus LGG.
Owner:LANZHOU UNIVERSITY
Pharmaceutical composition for solubility enhancement of hydrophobic drugs
The present invention provides a pharmaceutical composition comprising a drug and polyethylene glycol, wherein the ratio of polyethylene glycol to drug by weight is from about 0.2:1 to about 10:1, and the polyethylene glycol has a melting point of at least 37oC. The pharmaceutical compositions of the invention exhibit rapid dissolution upon contact with physiological solvents, such as water, saliva or gastrointestinal fluids.
Owner:SANDOZ AG
Lactobacillus rhamnosus and application thereof
ActiveCN113604384AEffective colonizationExcellent simulated gastrointestinal fluid toleranceMilk preparationBacteriaDiseaseLactobacillus rhamnosus
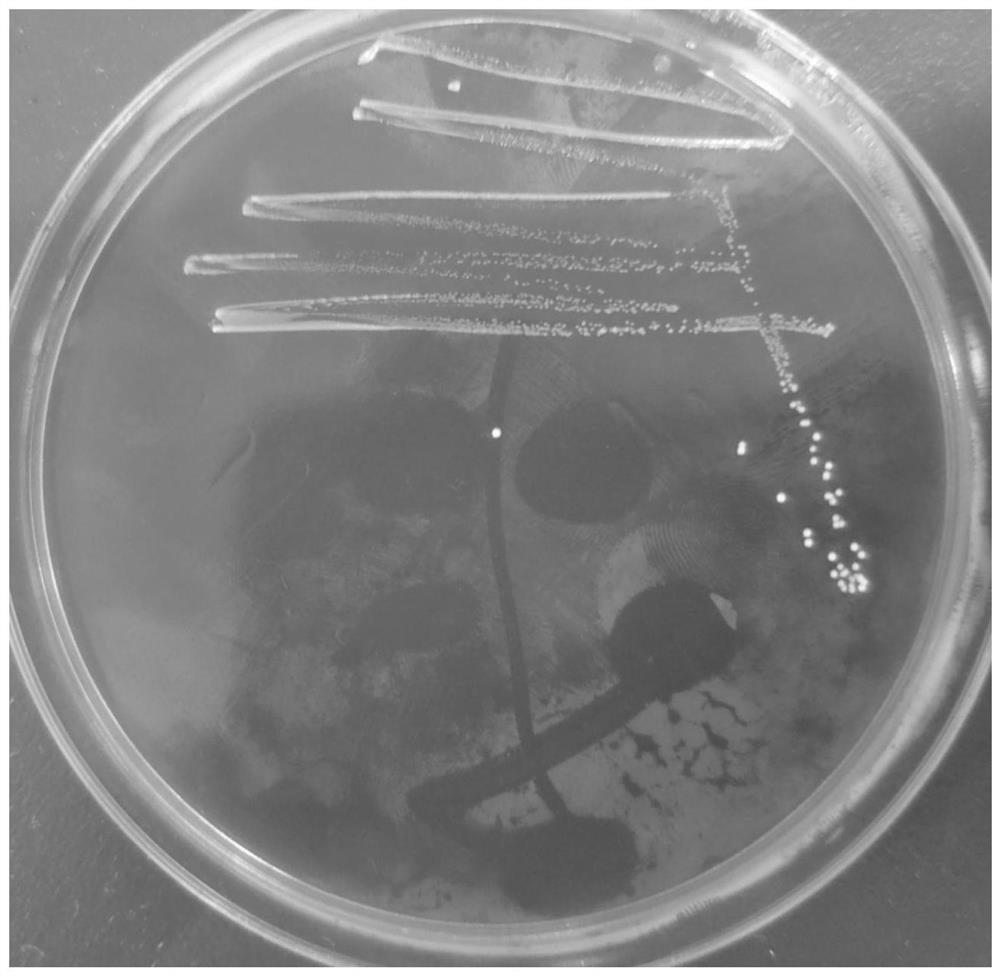
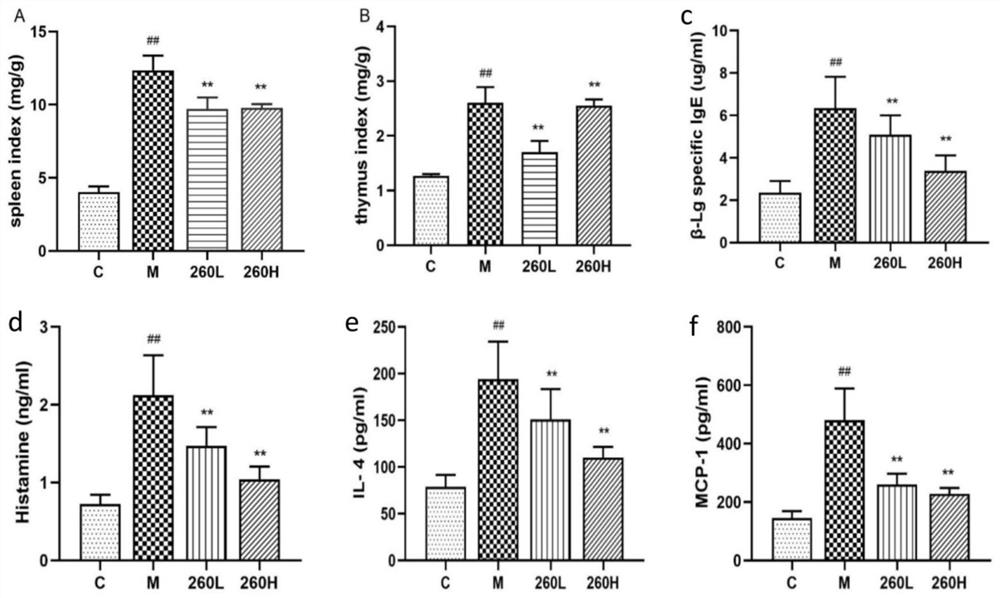
The invention discloses lactobacillus rhamnosus and an application thereof. The lactobacillus rhamnosus is named as lactobacillus rhamnosus AU9260 in taxonomy, and the preservation number of the lactobacillus rhamnosus is CGMCC No.21662. The strain has excellent in-vitro probiotic characteristics such as simulated gastrointestinal fluid tolerance, bacteriostatic activity, adhesion to Caco-2 cells and the like, and is preliminarily judged to be safe. In addition, the strain has a potential function of relieving and / or preventing allergic diseases of hosts, can significantly reduce histamine, specific IgE and cell factors in serum of mice induced and sensitized by beta-lactoglobulin, and can also protect damage to intestinal mucosa structures of the mice caused by allergy and regulate intestinal flora structures. The lactobacillus rhamnosus can be used as foods and / or health-care foods with a desensitization effect.
Owner:HUNAN AGRICULTURAL UNIV +1
Oral drug dosage forms compromising a fixed-dose of an adhd non-stimulant and an adhd stimulant
ActiveUS20210077410A1Small surface areaOrganic active ingredientsNervous disorderImmediate releaseNon stimulant
The present disclosure provides oral drug dosage forms comprising: (a) an erodible non-stimulant material admixed with an ADHD non-stimulant; and (b) an erodible stimulant material admixed with an ADHD stimulant, wherein the erodible non-stimulant material admixed with the ADHD non-stimulant is embedded in a substrate material, and wherein upon exposure to gastrointestinal fluid the ADHD non-stimulant is released according to a desired non-stimulant release profile and the ADHD stimulant is released according to a desired stimulant release profile. In some embodiment, the ADHD non-stimulant is released according to a sustained release profile. In some embodiments, the ADHD stimulant is released according to an immediate release profile. The oral drug dosage forms of the present disclosure are useful for the treatment of attention deficit hyperactivity disorder (ADHD). Also provided herein are methods of designing and manufacturing the oral drug dosage forms described herein.
Owner:TRIASTEK INC
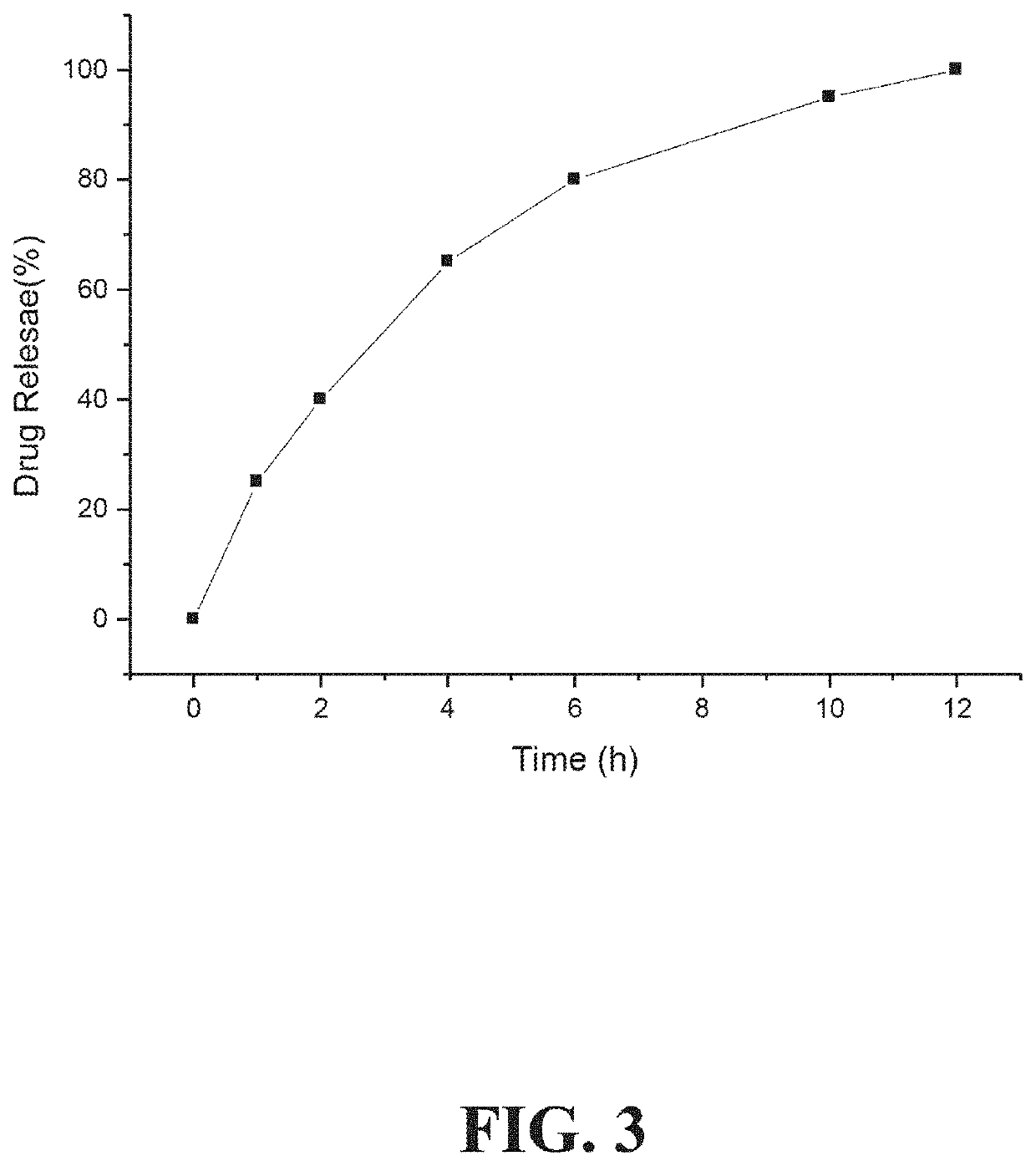
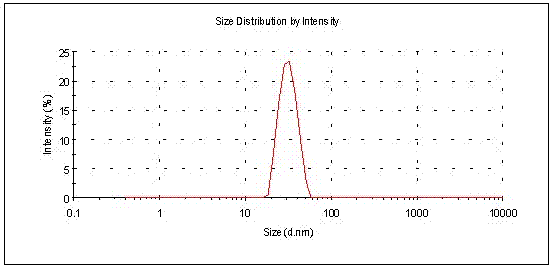
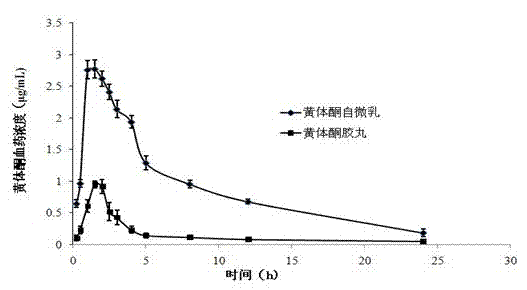
Oversaturated self-microemulsified progesterone composition and preparation method of same
ActiveCN102415995AImprove oral bioavailabilityOrganic active ingredientsEmulsion deliveryActive agentProgesterones
The invention provides an oversaturated self-microemulsified progesterone composition. The oversaturated self-microemulsified progesterone composition consists of progesterone, non-natural plant oil, emulsifier, assistant emulsifier, stabilizer and the like. A preparation method of the oversaturated self-microemulsified progesterone composition comprises the following steps of: uniformly mixing the non-natural plant oil and the assistant emulsifier; dissolving the progesterone in the mixed liquid after being subjected to ultrasonic treatment; and adding the emulsifier into the mixture, and adding to dissolve or suspend the stabilizer in the mixture to obtain the composition. In the invention, as the progesterone is solubilized in 10-90nm oil-in-water microemulsion, dissolubility and dissolution rate of the progesterone in gastrointestinal fluid are increased; the microemulsion which is formed after oral administration envelops the progesterone in the non-natural plant oil and a surfactant layer so as to prevent the progesterone from being degraded by acid and enzyme in a gastrointestinal tract; and the stabilizer can be used for inhibiting progesterone crystals from precipitation during the dilution of the microemulsion to form oversaturated progesterone solution, so that more progesterone can be absorbed. The oversaturated self-microemulsified progesterone composition has the characteristics of low viscosity, stability in quality, high bioavailability, low toxicity, less side effect, short preparation time, low energy consumption and the like.
Owner:GUANGDONG ZHONGSHENG PHARMA
Melt-processed polymeric cellular dosage form
InactiveUS20160184230A1Promotes quick releaseImprove uniformityPowder deliveryBiocideImmediate releaseSolid Dose Form
Presented herein are polymeric cellular dosage forms exhibiting improved immediate release properties, while maintaining high uniformity and satisfactory mechanical properties (e.g., to permit necessary handling). An exfoliating polymeric cellular dosage form is described herein that can be cost-effectively manufactured via batch or even non-batch (continuous or semi-continuous) melt processing. The solid dosage forms have a unique cellular microstructure featuring a number of open, interconnected cells. The cell walls contain the active ingredient(s) as well as an excipient that swells in the presence of a physiological fluid such as gastrointestinal fluid and / or saliva under physiological conditions.
Owner:BLAESI ARON H
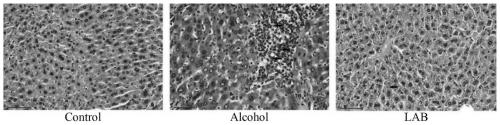
Lactic acid bacteria formula for preventing acute and chronic alcoholic liver injury and application thereof
ActiveCN111437294AGood simulated gastrointestinal fluid toleranceInhibit inflammationDigestive systemUnknown materialsLactobacillus acidophilusFatty acid
The invention discloses a lactic acid bacteria formula for preventing acute and chronic alcoholic liver injury and application thereof. Lactobacillus plantarum KLDS1.0344 and lactobacillus acidophilusKLDS1.0901 have good gastrointestinal fluid tolerance simulating capacity, have certain adhesion capacity to HT-29 cells and have basic probiotic characteristics; the lactobacillus plantarum KLDS1.0344 and the lactobacillus acidophilus KLDS1.0901 can prevent acute alcoholic liver injury; and the lactobacillus plantarum KLDS1.0344 and the lactobacillus acidophilus KLDS1.0901 can prevent chronic alcoholic liver injury. Specifically, the lactobacillus plantarum KLDS1.0344 and the lactobacillus acidophilus KLDS1.0901 have the functions of reducing the liver index, improving the intestinal barrier, improving the liver function, reducing the inflammation level in the liver, increasing the content of short-chain fatty acid in the intestinal tract, reducing protein expression of CYP2E1 in the liver and the like.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
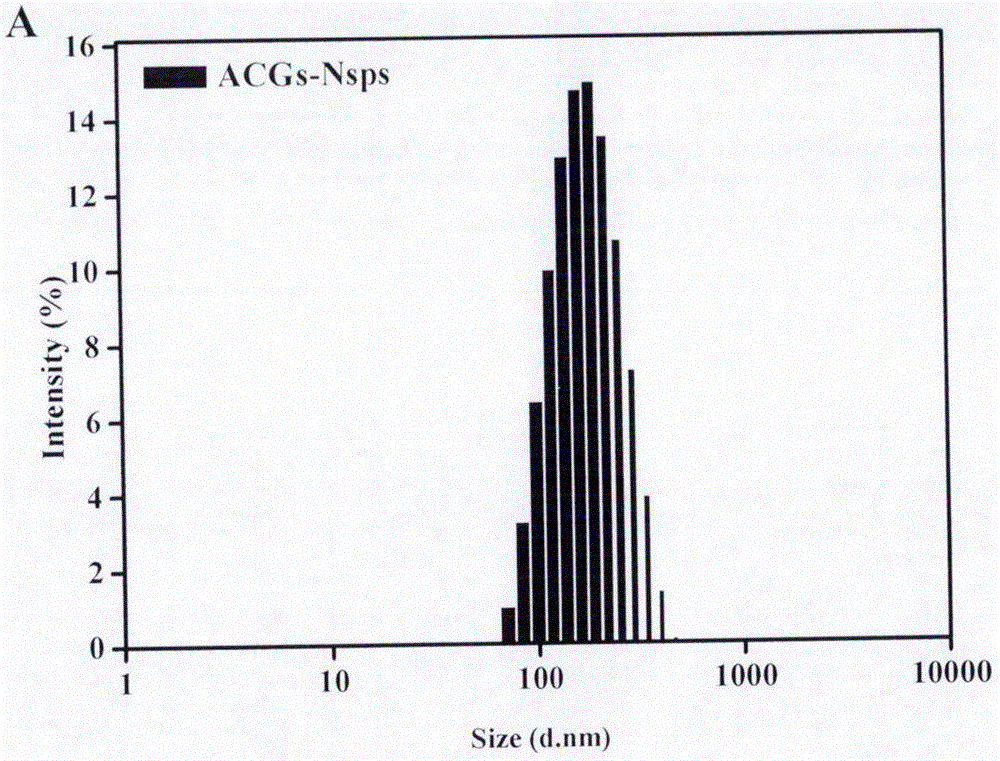
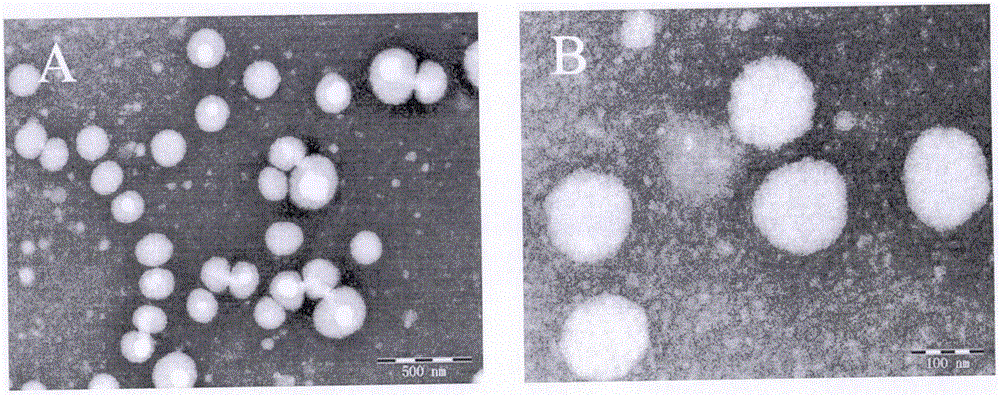
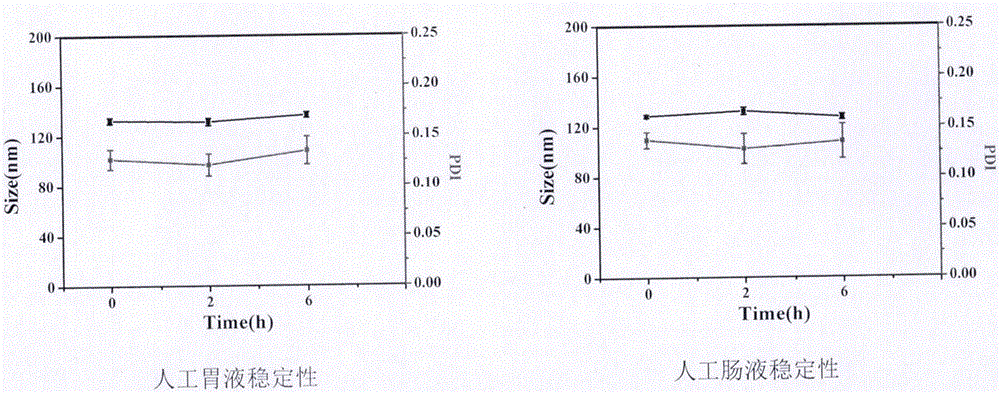
Nanosuspension of annonaceous acetogenin drugs and preparation method of nanosuspension
ActiveCN106420604AEasy to achieve passive targetingSimple prescriptionPowder deliverySolution deliverySide effectAnnonaceous Acetogenins
The invention belongs to the technical field of medicines, and in particular relates to a nanosuspension of annonaceous acetogenin drugs prepared from such amphiphilic stabilizers as mPEG PCL, mPEG PLA, mPEG PLGA, mPEG DSPE, mPEG Chol, SPC, Tween80, BSA, TPGS and the like, as well as a preparation method and an application of the nanosuspension. The annonaceous acetogenin total lactone nanosuspension is prepared by virtue of a solvent precipitation-ultrasonic injection method, and according to a prescription, the proportioning ratio of the annonaceous acetogenin drugs to the stabilizer is at 1 to (0.02-10) (in percentage by weight). The prepared annonaceous acetogenin nanosuspension can reach a load-loading capacity to 90% to the greatest extent and can reach a minimum grain size to 123.2nm, and the nanosuspension is good in polydispersity. The nanosuspension is stable in both gastrointestinal fluid and plasma, and the nanosuspension is applicable to oral administration and injection administration; the nanosuspension has a good in-vitro sustained-release effect and is free from burst release; the nanoparticle, in comparison with a crude drug solution, is more obvious in tumor cell inhibitory rate in vitro; the nanosuspension, in in-vivo tissue distribution, shows tumor passive targeting; therefore, the nanosuspension is beneficial for enhancing efficacy and reducing toxic and side effects. Meanwhile, an in-vivo efficacy experiment shows that the nanosuspension is outstanding in antineoplastic efficacy, and the nanosuspension has a broad development prospect.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Suspension composition containing lysozyme and florfenicol and preparation method thereof
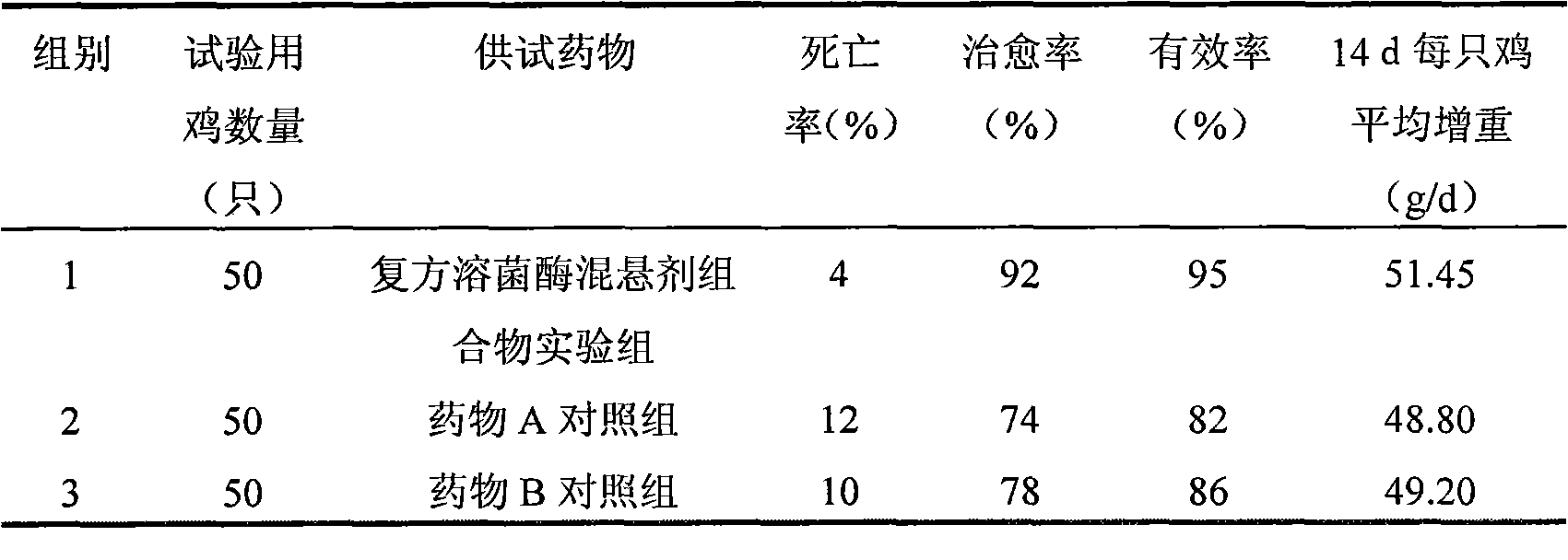
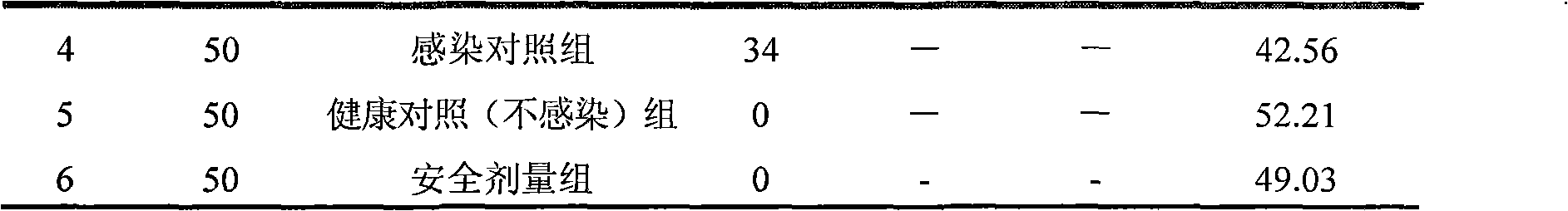
InactiveCN101874774ALong courseOrganic active ingredientsPeptide/protein ingredientsIn vivoBlood drug concentration
The invention relates to a suspension composition containing lysozyme and florfenicol, which is a microemulsion solution prepared from the lysozyme, the florfenicol, tween-80, sodium citrate, methylparaben, propylene glycol and water for injection. The composition adopts compatibility of the lysozyme and the florfenicol, not only enlarges the antimicrobial spectrum, but also has high efficiency, low toxicity, less residues and drug resistance prevention; and the prepared suspension not only can solve the problem that the florfenicol is hardly soluble in water, improves the biological utilization of the florfenicol, but also protects the activity of the lysozyme in gastrointestinal fluid, and plays a slow release effect on the lysozyme and the florfenicol to ensure that the medicament maintains long-term effective blood concentration in vivo, is integrated with instant effect, high efficiency and long action, and can be used for treating mycoplasma gallisepticum infections of livestock and poultry.
Owner:TIANJIN RINGPU BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com