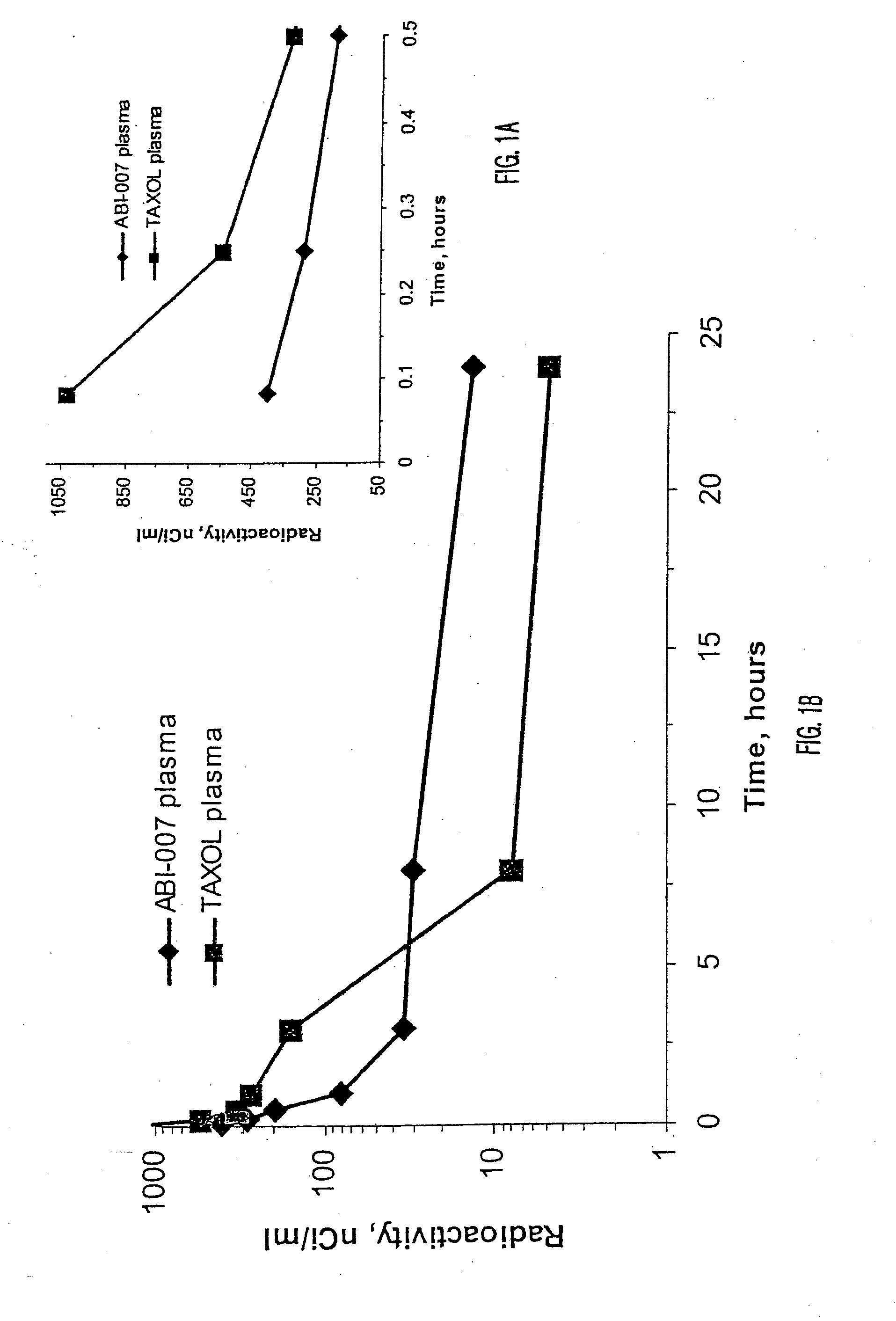
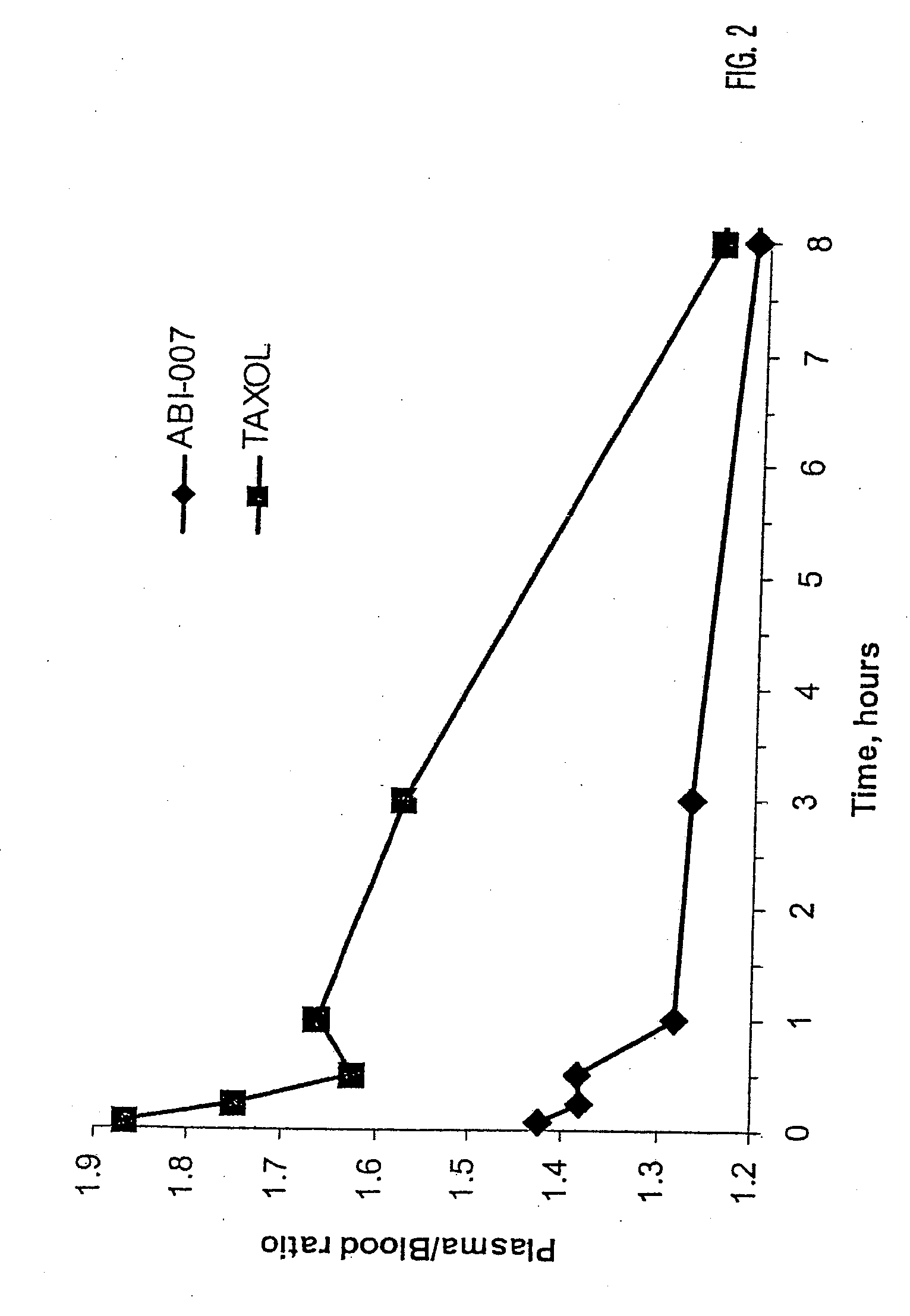
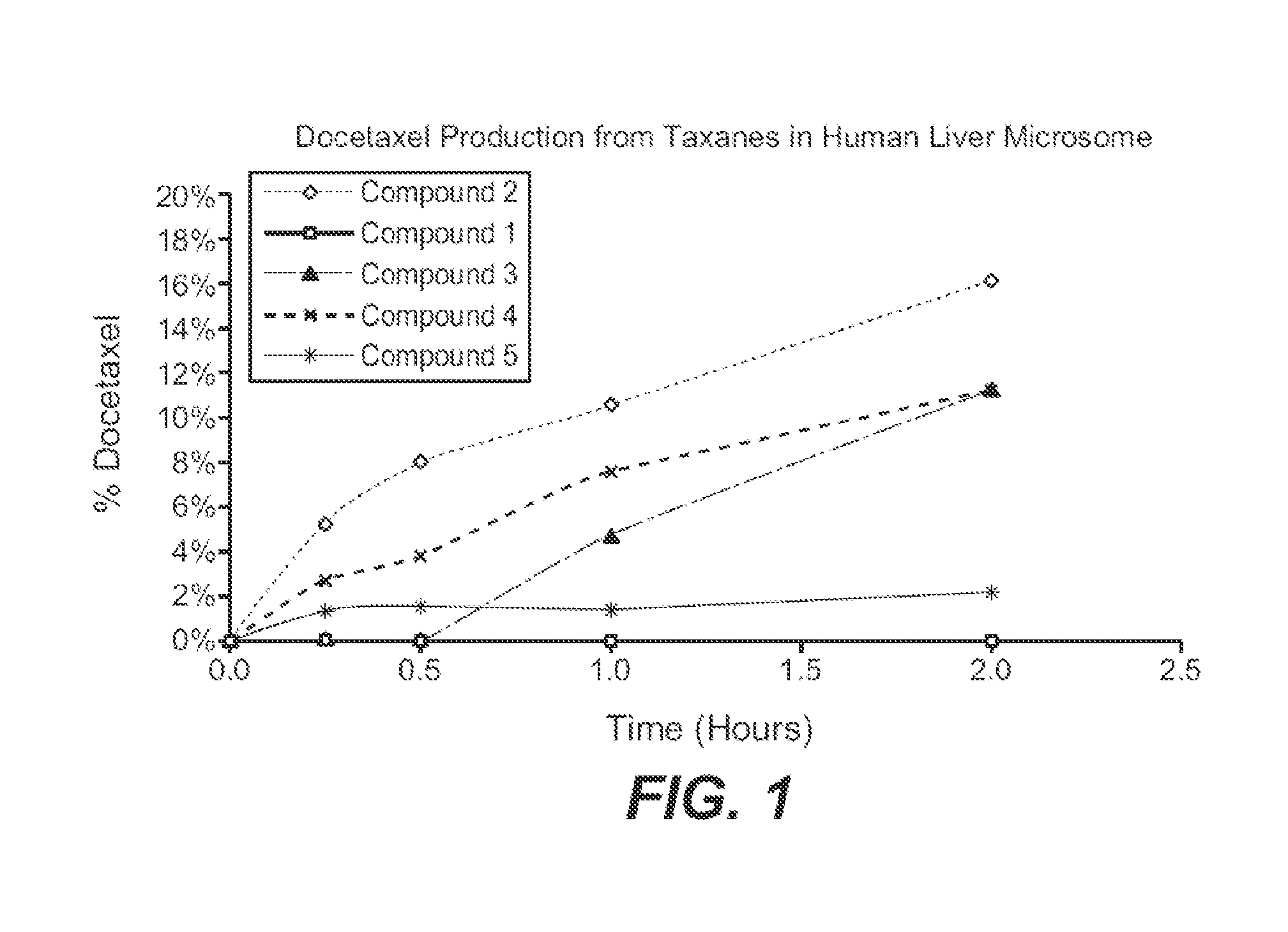
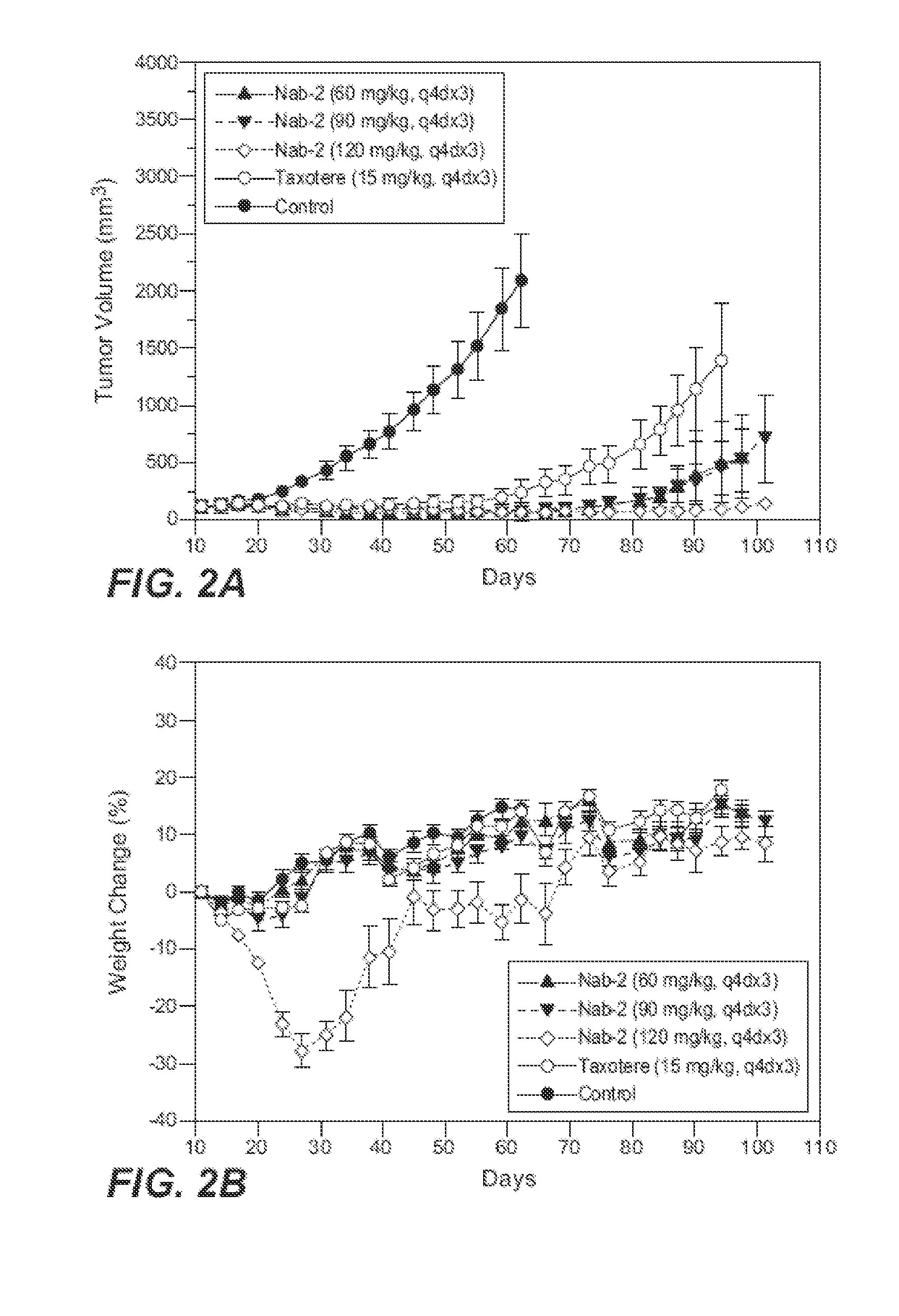
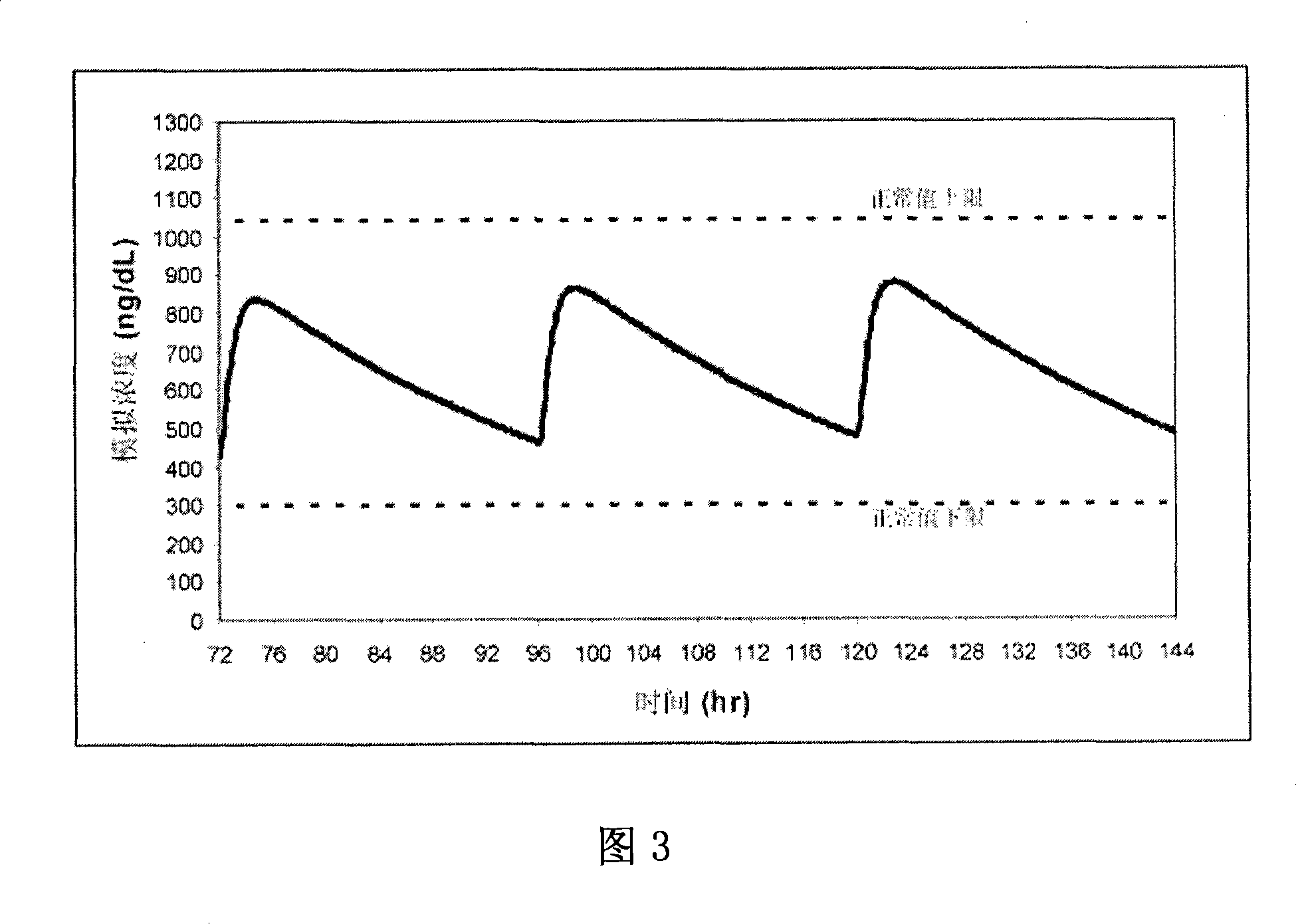
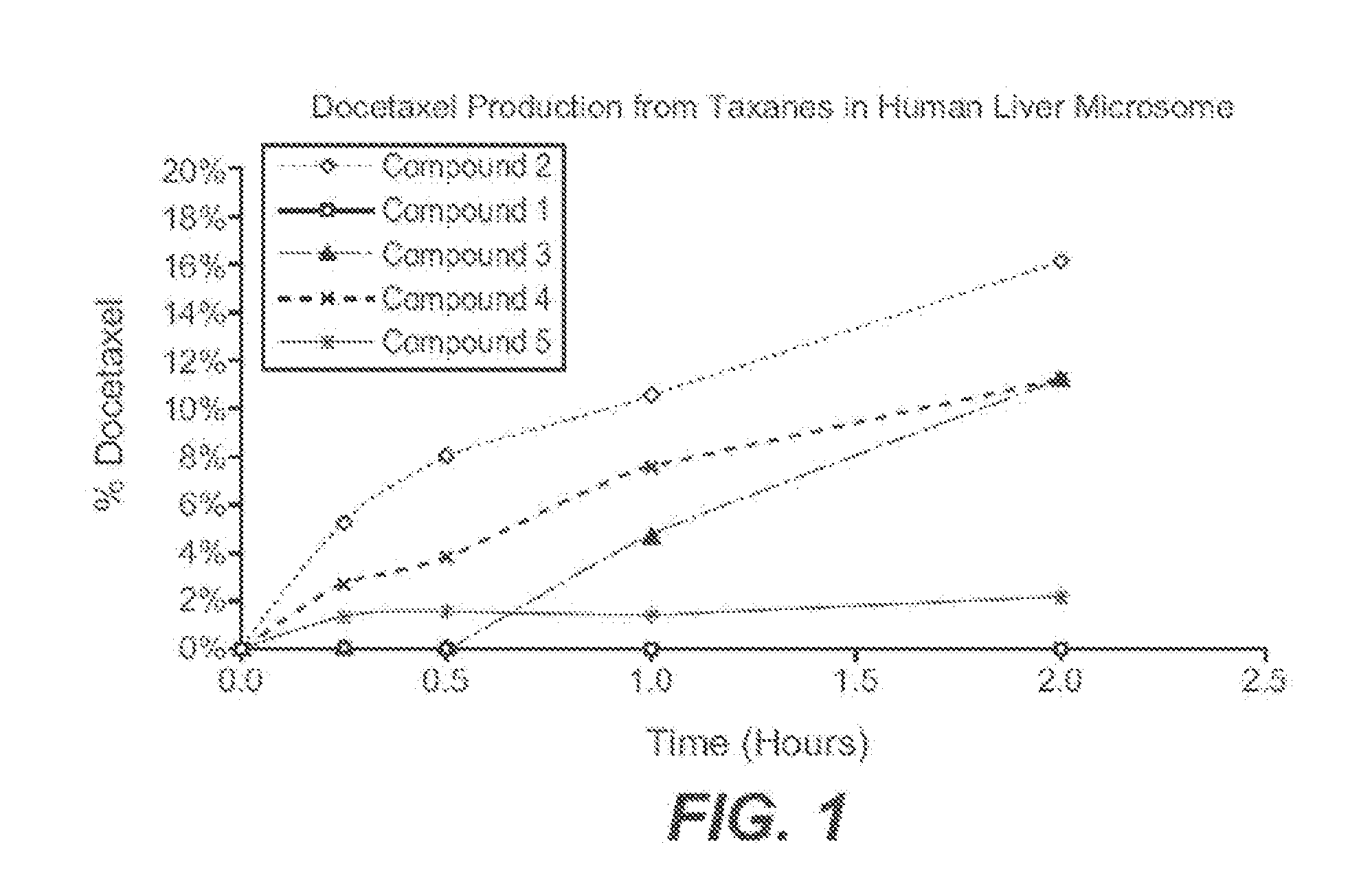
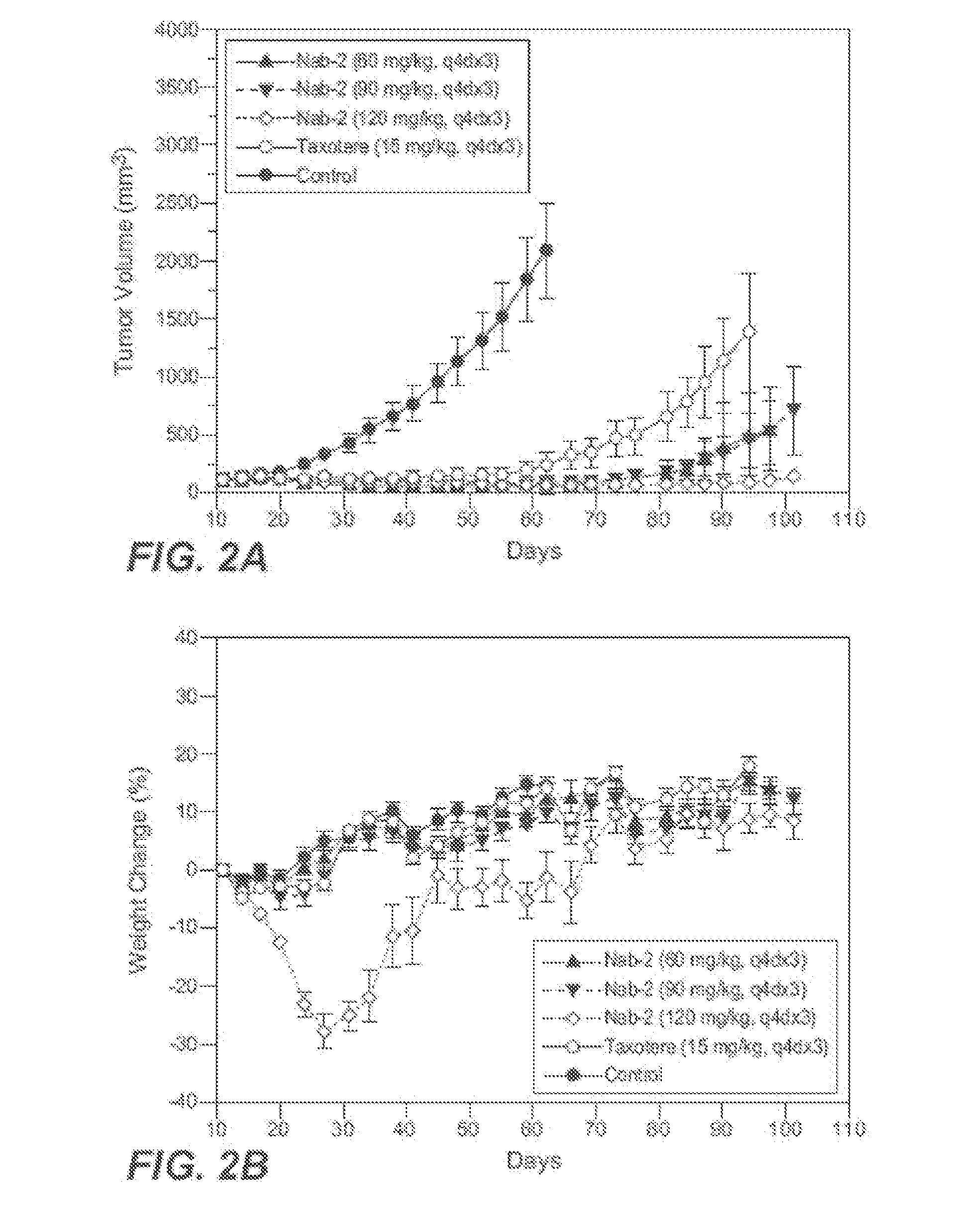
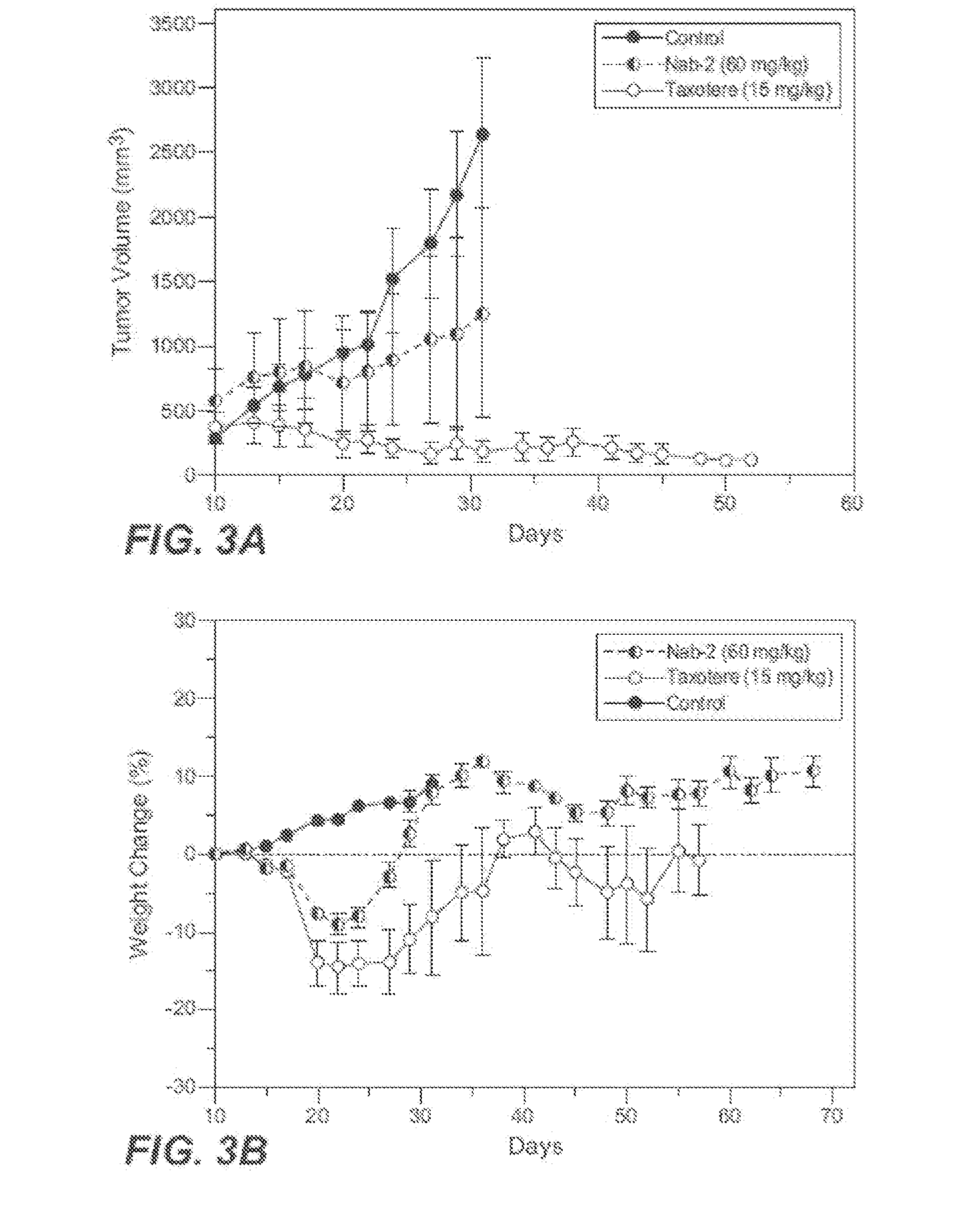
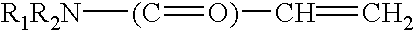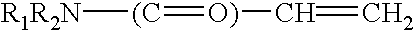Patents
Literature
720 results about "Hydrophobic drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrophilic drugs or lipophobic drugs are are drugs that love waters. So hydro means water, philic means love. Now any drug that loves water is going to hate oil. So lipophobic and hydrophilic are the same thing.
Polymeric drug delivery system for hydrophobic drugs
InactiveUS20050249799A1Low oral bioavailabilityStable against aggregationAntibacterial agentsPowder deliveryHydrophobic polymerImmediate release
An oral delivery system for Class II drugs that have low oral bioavailability due to their insolubility in water and slow dissolution kinetics and method for making such a drug delivery system are disclosed herein. The formulation may be a controlled release or immediate release formulation. The immediate release formulation contains a Class II drug, together with a hydrophobic polymer, preferably a bioadhesive polymer. In one embodiment, the drug and polymer are co-dissolved in a common solvent. The solution is formed into small solid particles by any convenient method, particularly by spray drying. The resulting particles contain drug dispersed as small particles in a polymeric matrix. The particles are stable against aggregation, and can be put into capsules or tableted for administration. The controlled release formulations contain a BCS Class II drug and a bioadhesive polymer. The controlled release formulations may be in the form of a tablet, capsules, mini-tab, microparticulate, or osmotic pump. Enhancement of oral uptake of the drug from use of bioadhesive polymers occurs through (1) increased dissolution kinetics due to stable micronization of the drug, (2) rapid release of the drug from the polymer in the GI tract; and (3) prolonged GI transit due to bioadhesive properties of the polymers. The combination of these effects allows the preparation of a compact, stable dosage form suitable for oral administration of many class II drugs.
Owner:SPHERICS
Pharmaceutical delivery systems for hydrophobic drugs and compositions comprising same
ActiveUS8241664B2Preventing and alleviating symptomInhibition releaseBiocideOintment deliveryOral medicationMedicine
A drug delivery system for oral administration of hydrophobic drugs with enhanced and extended absorption and improved pharmacokinetics is provided. In one embodiment, formulations comprising testosterone and testosterone esters, e.g., testosterone palmitate, are disclosed. Methods of treating a hormone deficiency or effecting male contraception with the inventive formulations are also provided.
Owner:TOLMAR INC
Pharmaceutical Delivery Systems for Hydrophobic Drugs and Compositions Compositions Comprising Same
ActiveUS20080317844A1Preventing and alleviating symptomInhibition releaseBiocideOintment deliveryOral medicationDelivery system
A drug delivery system for oral administration of hydrophobic drugs with enhanced and extended absorption and improved pharmacokinetics is provided. In one embodiment, formulations comprising testosterone and testosterone esters, e.g., testosterone palmitate, are disclosed. Methods of treating a hormone deficiency or effecting male contraception with the inventive formulations are also provided.
Owner:TOLMAR INC
Polymeric systems for drug delivery and uses thereof
InactiveUS20050042293A1Precise moldingMore solid formPowder deliveryKetone active ingredientsWater insolubleIn vivo
Biodegradable polymeric implants can provide a safe and efficient means to deliver drugs in the treatment of various diseases. Although a polymeric drug delivery system can be implanted as a solid device within a subject, it is also possible to administer such a system as an injectable liquid which solidifies in vivo. An improved formulation of a polymeric drug delivery system comprises a water insoluble copolymer that is a solid or wax at 37° C., a water soluble polymer that is a liquid at 25° C., and a hydrophobic drug. These drug delivery systems can be administered by injection, and do not require the use of a toxic curing agent or inconvenient temperature manipulations.
Owner:ANGIOTECH INT AG (CH)
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs
InactiveUS20100137271A1Improve bioavailabilitySuture equipmentsOrganic active ingredientsActive agentBioavailability
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs are provided. The pharmaceutical compositions include a therapeutically effective amount of a hydrophobic drug, preferably a steroid; a solubilizer, and a surfactant. The synergistic effect between the hydrophobic drug and the solubilizer results in a pharmaceutical formulation with improved dispersion of both the active agent and the solubilizer. As a result of the improved dispersion, the pharmaceutical composition has improved bioavailability upon administration. Methods of improving the bioavailability of hydrophobic drugs administered to a patient are also provided.
Owner:LIPOCINE
Lipid particles and suspensions and uses thereof
The present invention relates to formulations and methods for the mucosal and parenteral administration of lipid particles an suspensions. The formulations of this invention are stable lipid particles useful for oral delivery of water-insoluble therapeutic agents, vaccines and diagnostics. The compositions of this invention promote the mucosal absorption of biologically active molecules across mucosal epithelial barriers. Stabilization of lipid particles is achieved by coating the hydrophobic central core with a polymer shell. The polymer shell can include bioadhesive agents, ligands, and absorption promoting agents. This invention relates to oral drug delivery systems for hydrophobic drugs, and in particular is concerned with improving the bioavailability of hydrophobic drugs from such systems. Using this system, anticancer drugs such as taxanes are orally effective.
Owner:CONSTANTINIDES PANAYIOTISP +2
Micelle composition of polymer and passenger drug
InactiveUS20060251710A1Improving micelle encapsulation efficiencyBiocidePowder deliverySolubilitySide effect
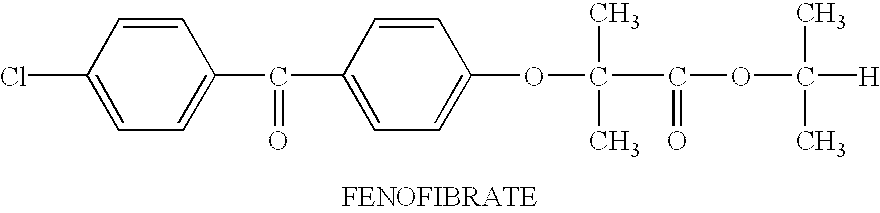
Hydrophobic drugs become more practical for treatments by being encapsulated in micelle compositions for increasing solubility. Micelle compositions may include an excipient tocopherol and / or prodrug formulations of the drug. Micelles extend the time period the drug remains in the micelles to improve drug circulation time and thereby drug delivery. Hydrophobic drugs for micelle encapsulation may include rapamycin, geldanamycin, and paclitaxel. Administration of these micelle compositions does not require Cremophor EL or Tween 80, avoiding serious side effects associated with these products which would previously accompany such drug administration.
Owner:WISCONSIN ALUMNI RES FOUND
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs, particularly fenofibrate, are provided. The compositions comprise a therapeutically effective amount of an active agent and a solubilizer. The solubilizer is selected to effectively solubilize active agent in the composition. The solubilizers employed as part of the invention include: a vitamin E substance; monohydric alcohol esters such as trialkyl citrates, lactones and lower alcohol fatty acid esters; nitrogen-containing solvents; phospholipids; glycerol acetates such as acetin, diacetin and triacetin; glycerol fatty acid esters such as mono-, di- and triglycerides and acetylated mono- and diglycerides; propylene glycol esters; ethylene glycol esters; and combinations thereof. The pharmaceutical dosage forms contain the compositions in a suitable dosage form unit such as a capsule. Methods of treating patients comprising administering the compositions are also provided.
Owner:LIPOCINE
Tunable sustained release of a sparingly soluble hydrophobic therapeutic agent from a hydrogel matrix
ActiveUS20100291191A1Improve solubilityGood effectAntibacterial agentsBiocideSolubilityMethyl cellulose
The incorporation of polymeric excipients into an injectable hydrogel matrix, for example, methyl cellulose in the case of a hydrogel matrix comprising hyaluronan and methylcellulose (HAMC) has been found to increase the solubility of sparingly soluble hydrophobic drugs and tune their rate of release. The hydrogel matrix may also include other sparingly soluble hydrophobic food or cosmetic agents.
Owner:THE GOVERNINIG COUNCIL OF THE UNIV OF TORANTO
Osmotic delivery of therapeutic compounds by solubility enhancement
ActiveUS20050053653A1Low water solubilityImprove solubilityMetabolism disorderPill deliveryChemical compoundAqueous solubility
The present invention is directed to the oral osmotic delivery of therapeutic compounds that have limited solubility in an aqueous environment due to inherent hydrophobicity or to saturation limitations in the core of the osmotic system. The present invention is suitable for the osmotic delivery of glipizide and other hydrophobic drugs, but runs the spectrum to other therapeutic agents with higher aqueous solubilities, yet having a solubility limitation in an osmotic dosage unit due to high drug load.
Owner:SUPERNUS PHARM INC
Osmotic delivery of therapeutic compounds by solubility enhancement
ActiveUS7611728B2Low water solubilityImprove solubilityMetabolism disorderPill deliveryChemical compoundAqueous solubility
The present invention is directed to the oral osmotic delivery of therapeutic compounds that have limited solubility in an aqueous environment due to inherent hydrophobicity or to saturation limitations in the core of the osmotic system. The present invention is suitable for the osmotic delivery of glipizide and other hydrophobic drugs, but runs the spectrum to other therapeutic agents with higher aqueous solubilities, yet having a solubility limitation in an osmotic dosage unit due to high drug load.
Owner:SUPERNUS PHARM INC
Sustained release and long residing ophthalmic formulation and the process of preparing the same
The present invention relates to sustained release and long residing opthalmic formulation having thermosensitivity, mucoadhesiveness, hydro gel properties and small particle size. The said formulation comprises micelle solution of random block co-polymer having a hydrophobic component and a hydrophillic component of general formula -(X+Y+Z-)m, and at least one hydrophobic drug with the block co-polymer solution. The invention also provides a process of preparing said formulation.
Owner:UNIVERSITY OF DELHI
Hydrophobic drug compositions containing reconstitution enhancer
Disclosed are compositions comprising a hydrophobic active agent, a polymer and a reconstitution enhancing agent. Reconstitution of the lyophilized form of the compositions takes less time than in the absence of the enhancing agent.
Owner:NOVARTIS AG
Nanoparticle formulations and uses thereof
InactiveUS20090263483A1Improve bindingGood curative effectBiocideOrganic active ingredientsDiseaseNanoparticle
The present invention provides compositions comprising nanoparticles comprising: 1) a drug, such as a hydrophobic drug derivative; and 2) a carrier protein. Also provided are methods of treating diseases (such as cancer) using the compositions, as well as kits and unit dosages.
Owner:ABRAXIS BIOSCI LLC
Sustained release and long residing ophthalmic formulation and the process of preparing the same
The present invent relates to sustained release and long residing ophthalmic formulation having thermosensitivity, mucoadhesiveness, hydro gel properties and small particle size. The said formulation comprises of micelle solution of random block co-polymer having a hydrophobic component and a hydrophilic component of general formula -(X+Y+Z-)m, wherein m is an integer greater than 2 X is a monomer which will provide hydrogel formation properties of the co-polymer to reduce the irritability of the eye and is selected from vinyl group of compounds Y is a monomer which will provide thermosensitivity properties of the co-polymer having a general formula R1-R2N-(C=O)-CH=CH2, R1=a proton or CnH2n+1 in which n may have the value from 3 to 6 and R2=alkyl group having chain length of C3 to C6 Z is a monomer which will provide mucoadhesiveness and pH-sensitivity properties to the co-polymer and is selected from acrylate based monomers at least one hydrophobic drug with the said block co-polymer solution; The invention also provides a process of preparing said formulation.
Owner:UNIVERSITY OF DELHI
Progesterone Solutions for Increased Bioavailability
ActiveUS20100255085A1Improve bioavailabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionProgesterones
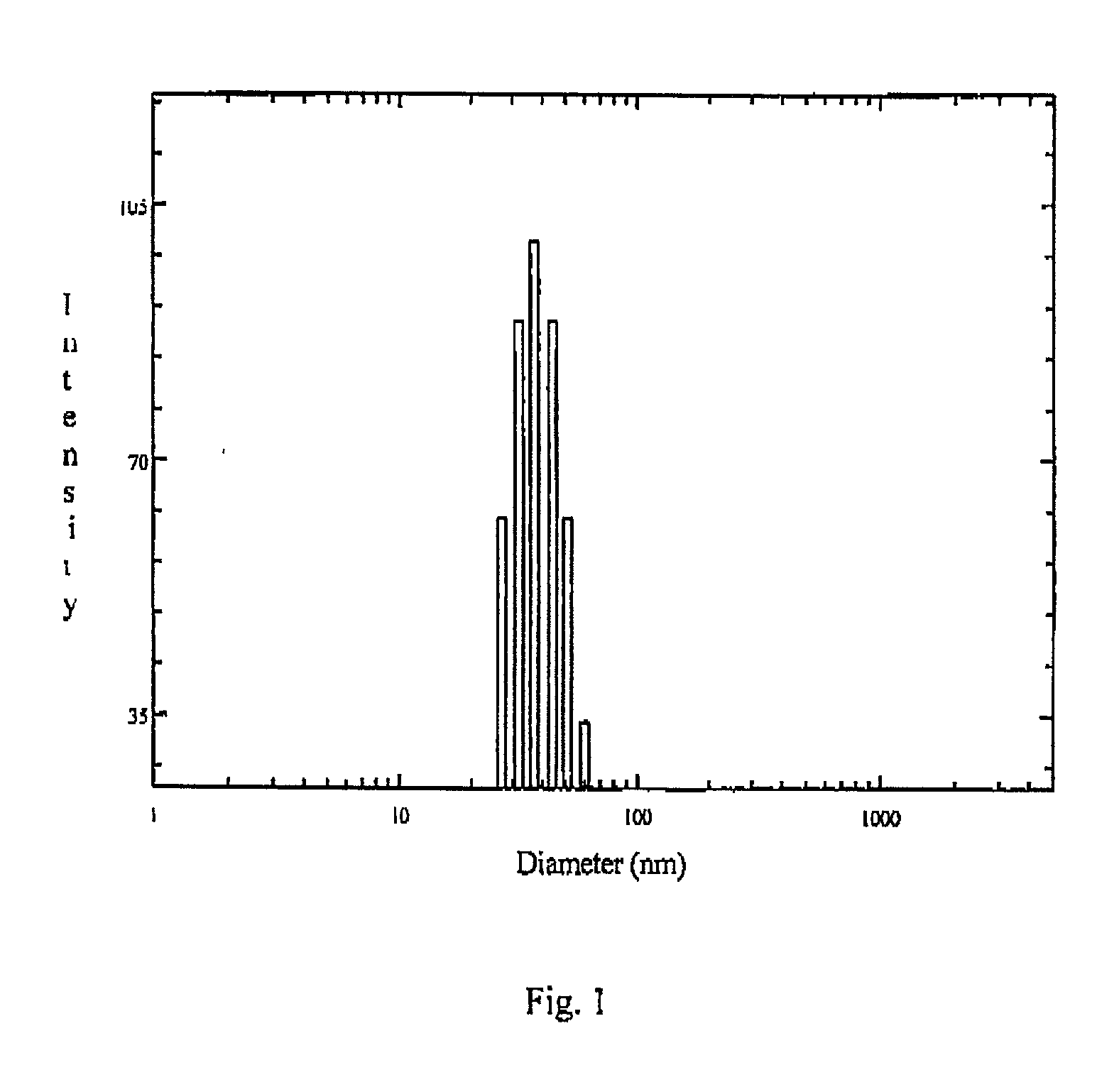
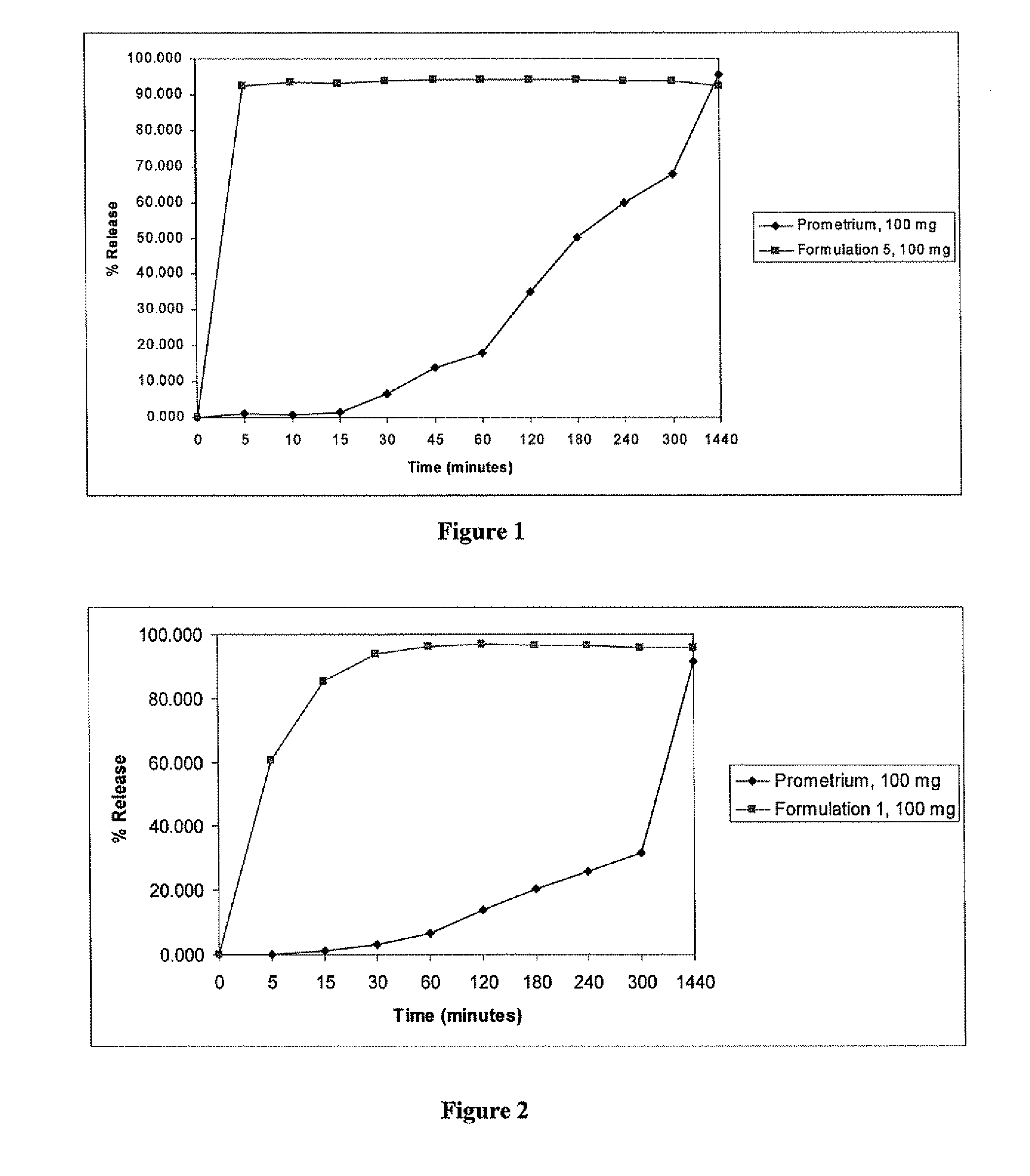
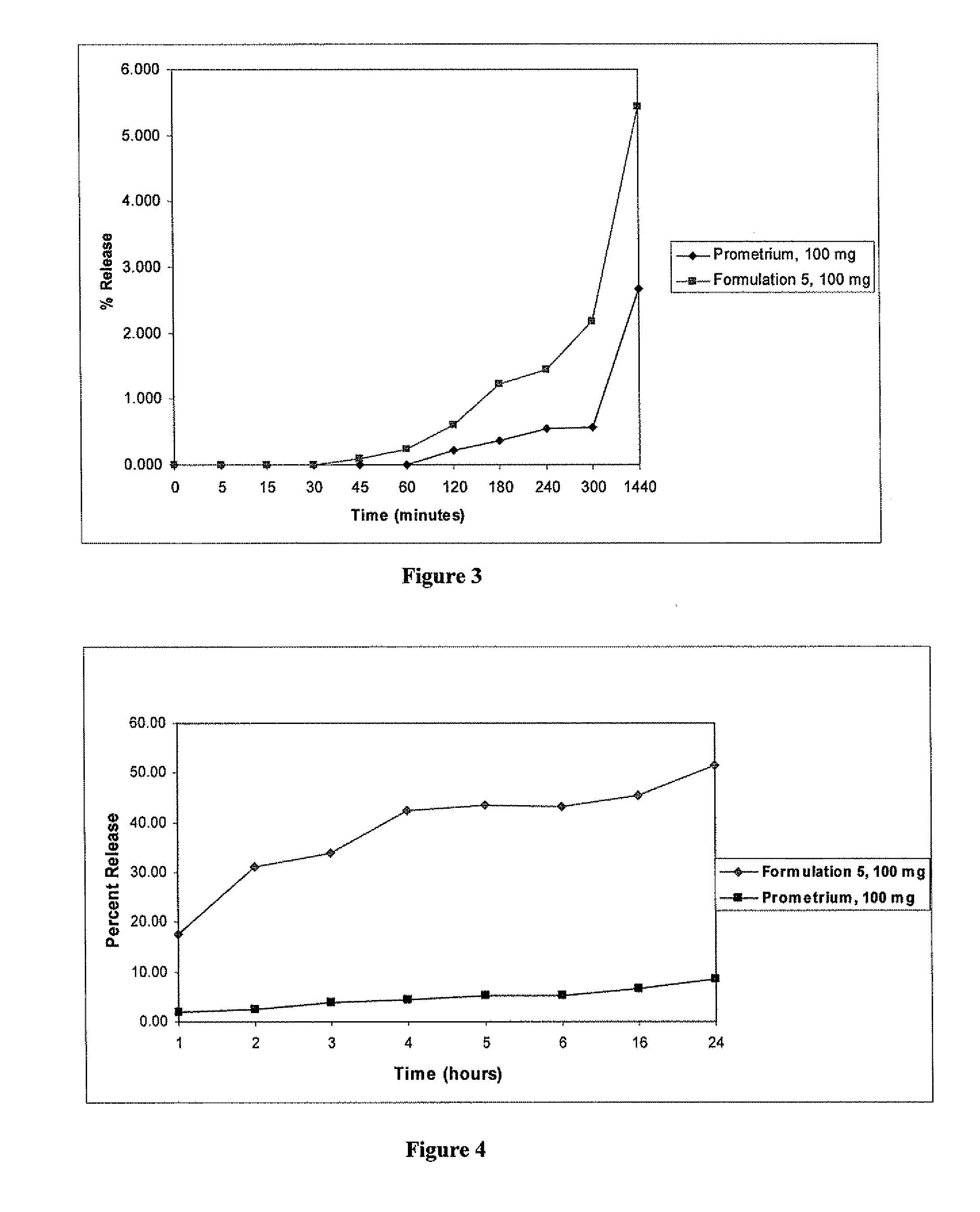
Fill materials for hydrophobic drugs, such as progesterone, and methods of making and using thereof are described herein. The fill material contains the hydrophobic drug dissolved in one or more fatty acids. The concentration of the hydrophobic drug is typically from about 7% to about 50% by weight of the fill material. The concentration of the one or more fatty acids is from about 60% to about 95% by weight of the carrier. The formulation also contains an organic acid and one or both of one or more pharmaceutically acceptable alcohols and one or more pharmaceutically acceptable mono-, di-, or triesters of medium or long chain fatty acids. The fill material can be encapsulated in a hard or soft capsule. The formulations described herein have a higher dissolution rate and faster onset of dissolution compared to micronized progesterone suspended in an oil and thus should have increased bioavailability in vivo.
Owner:PATHEON SOFTGELS INC
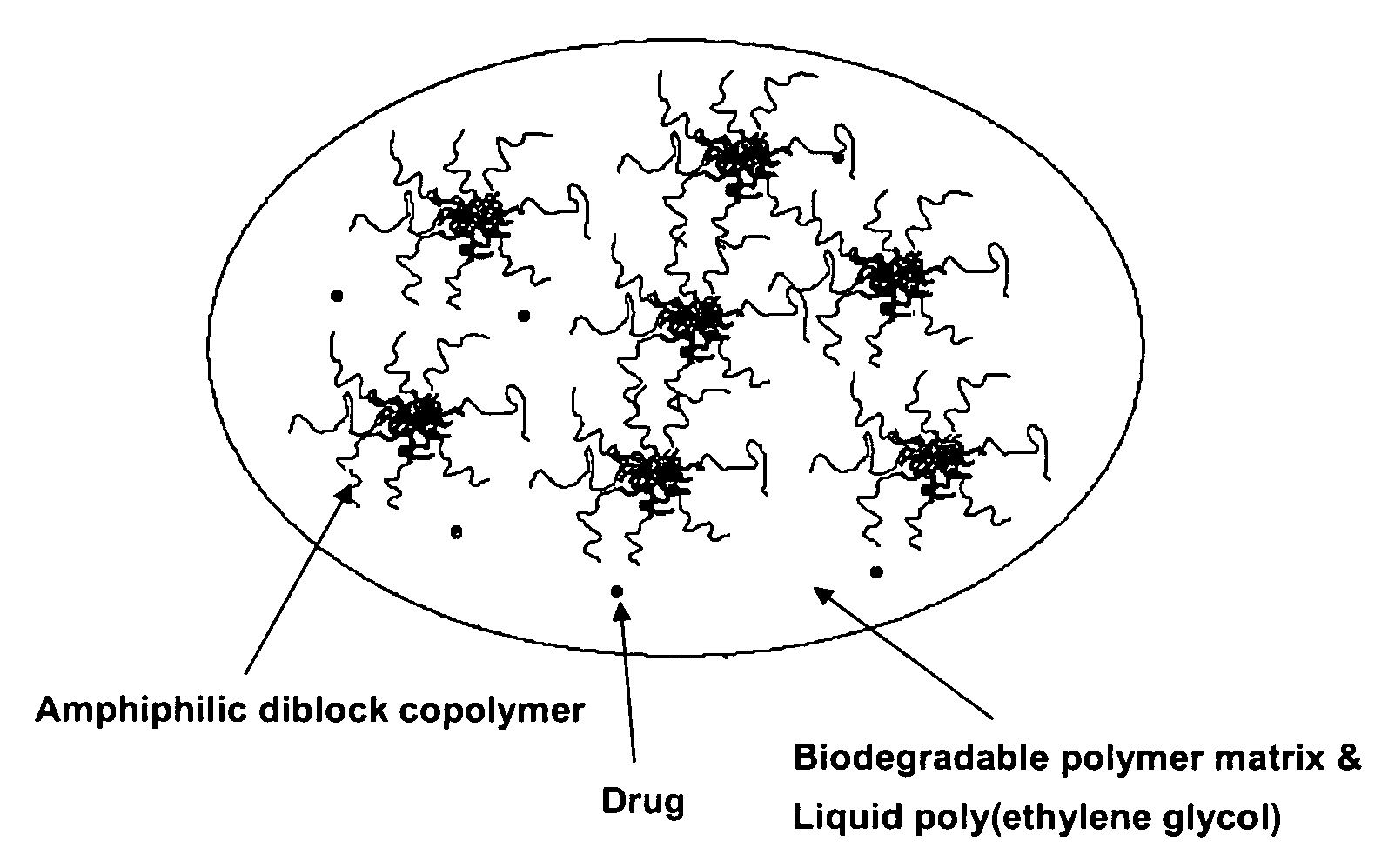
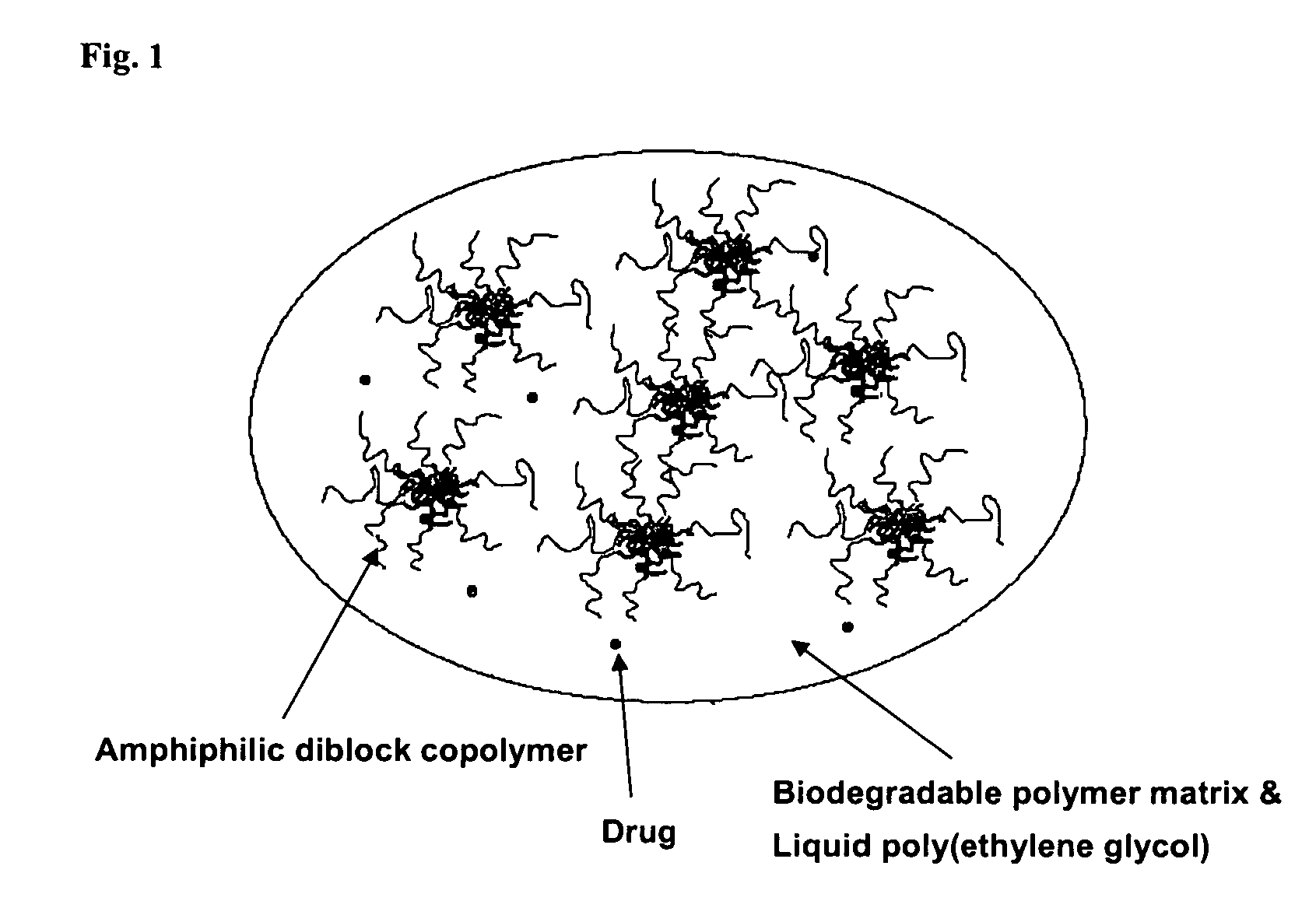
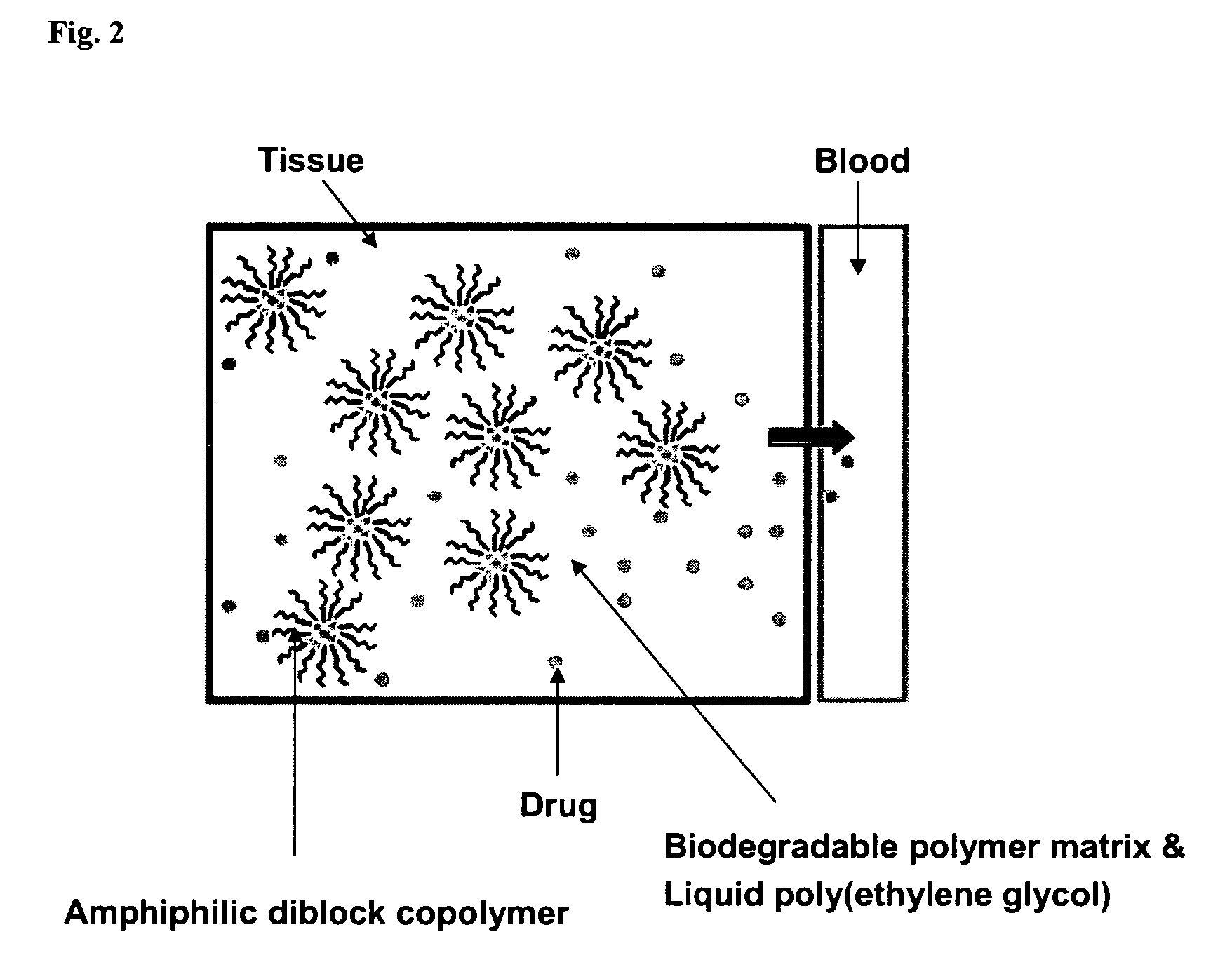
Composition for sustained delivery of hydrophobic drugs and process for the preparation thereof
InactiveUS7153520B2Enhance pharmacological effectsPowder deliverySolution deliveryPolythylene glycolPharmaceutical Substances
A composition for the sustained delivery of a drug comprising an amphiphilic diblock copolymer; a poorly water-soluble drug; a biodegradable polymer; and liquid poly(ethylene glycol) or functional derivatives thereof and a process for preparing the composition are disclosed. When administered into a particular body site, the composition forms an implant containing the drug and drug containing polymeric micelles, which are slowly released from the implant to maintain a constant drug concentration for an extended period of time.
Owner:SAMYANG HLDG CORP
Drug formulations for coating medical devices
The present invention relates to oil-based formulations of hydrophobic drugs for the uniform coating of medical devices such as vascular stents and balloons. Another aspect of the present invention is an intravascular medical device having an oil-based coating suitable for delivering a water-insoluble drug, comprising one or more of an anti-oxidant, an anti-inflammatory and an anti-restenotic agent.
Owner:REVA MEDICAL LLC
Biodegradable polymeric materials providing controlled release of hydrophobic drugs from implantable devices
InactiveUS20090110713A1Good miscibilityImprove breathabilityBiocideCosmetic preparationsBiological propertyControl release
The present invention is directed to polymeric materials (e.g., coatings) comprising biodegradable copolymers and implantable devices (e.g., drug-delivery stents) formed of such materials. The biodegradable copolymers are derived from at least two relatively polar monomers and at least one relatively nonpolar monomer. Incorporation of at least one relatively nonpolar monomer into the copolymer improves controlled release of a hydrophobic drug from the polymeric material by increasing the copolymer's miscibility with and permeability to the hydrophobic drug. The polymeric materials can also contain at least one biocompatible moiety, at least one non-fouling moiety, at least one biobeneficial material, at least one bioactive agent, or a combination thereof. The polymeric materials are designed to improve the mechanical, physical and biological properties of implantable devices formed thereof.
Owner:ABBOTT CARDIOVASCULAR
Methods and formulations for the delivery of pharmacologically active agents
InactiveUS20110052708A1Short timeEliminate side effectsPowder deliveryOrganic active ingredientsSide effectActive agent
In accordance with the present invention, novel formulations have been developed which are much more effective for the delivery of hydrophobic drugs to patients in need thereof than are prior art formulations. Invention formulations are capable of delivering more drug in shorter periods of time, with reduced side effects caused by the pharmaceutical carrier employed for delivery.
Owner:ABRAXIS BIOSCI LLC
Nanoparticle formulations and uses thereof
InactiveUS20130195983A1Improve bindingGood curative effectPowder deliveryOrganic active ingredientsDiseaseNanoparticle
The present invention provides compositions comprising nanoparticles comprising: 1) a drug, such as a hydrophobic drug derivative; and 2) a carrier protein. Also provided are methods of treating diseases (such as cancer) using the compositions, as well as kits and unit dosages.
Owner:ABRAXIS BIOSCI LLC
pH-sensitive block copolymers for pharmaceutical compositions
The present invention relates to a pharmaceutical composition of a biologically active agent and a block copolymer composed of a poly(ethylene oxide) forming hydrophilic segment and a poly(butyl (alkyl)acrylate-co-(alkyl)acrylic acid) that is capable of forming supramolecular assemblies or micelles under favourable conditions. The supramolecular assemblies or micelles formed from said polymers associate or dissociate reversibly upon changes in the environmental pH. The pharmaceutical compositions of the present invention contain hydrophobic drugs, cations or polycationic compounds, which can be delivered to the body by oral route or other routes of administration.
Owner:PALADIN LABS INC
Nanoparticle formulations and uses thereof
The present invention provides compositions comprising nanoparticles comprising: 1) a drug, such as a hydrophobic drug derivative; and 2) a carrier protein. Also provided are methods of treating diseases (such as cancer) using the compositions, as well as kits and unit dosages.
Owner:ABRAXIS BIOSCI LLC
Pharmaceutical delivery systems for hydrophobic drugs and compositions comprising same
InactiveCN101217963APrevent or reduce deficiency symptomsOrganic active ingredientsOral medicationDelivery system
A drug delivery system for oral administration of hydrophobic drugs with enhanced and extended absorption and improved pharmacokinetics is provided. In one embodiment, formulations comprising testosterone and testosterone esters, e.g., testosterone palmitate, are disclosed. Methods of treating a hormone deficiency or effecting male contraception with the inventive formulations are also provided.
Owner:CLARUS THERAPEUTICS INC
Nanoparticle formulations and uses therof
InactiveUS20140302157A1Improve bindingGood curative effectBiocideOrganic active ingredientsDiseaseNanoparticle
The present invention provides compositions comprising nanoparticles comprising: 1) a drug, such as a hydrophobic drug derivative; and 2) a carrier protein. Also provided are methods of treating diseases (such as cancer) using the compositions, as well as kits and unit dosages.
Owner:ABRAXIS BIOSCI LLC
Pharmaceutical composition for solubility enhancement of hydrophobic drugs
InactiveUS20050008704A1Improve solubilityDissolve fastBiocidePowder deliverySolubilityPolyethylene glycol
The present invention provides a pharmaceutical composition having enhanced solubility comprising a drug and polyethylene glycol, wherein the ratio of polyethylene glycol to drug by weight is from about 0.2:1 to about 10:1, and the polyethylene glycol has a melting point of at least 37° C. The pharmaceutical compositions exhibit rapid dissolution upon contact with physiological solvents, such as water, saliva or gastrointestinal fluids.
Owner:RAY ANUP KUMAR +4
Preparation method for nano-granular solid dispersion of hydrophobic drug by high-voltage electrostatic spraying
InactiveCN102218019AIncrease surface areaDry fastPharmaceutical product form changeOrganic solventPharmaceutical drug
The invention relates to a preparation method for a nano-granular solid dispersion of a hydrophobic drug by high-voltage electrostatic spraying, which comprises the following steps of: mixing a hydrophobic drug, a drug-carrying polymer and an organic solvent at a mass ratio of (1-10): (10-20): (80-94) to prepare a consolute electric spraying solution; and then carrying out high-voltage electrostatic spraying while controlling the flow rate of the consolute electric spraying solution at 0.5-2.5 mL / h, the distance between a receiving board and a wire spraying port at 15-30cm and the voltage at 5-30kV to obtain the nano-granular solid dispersion of the hydrophobic drug. The preparation method is simple to operate, is low in cost, is environmentally-friendly and is suitable for industrial production; and the prepared solid dispersion of the hydrophobic drug not only can highly disperse a composite of a polymer and the drug into an amorphous state and has a nano-structural characteristic.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Solubilising polysaccharides substituted with dydrophilic and hydrophobic groups
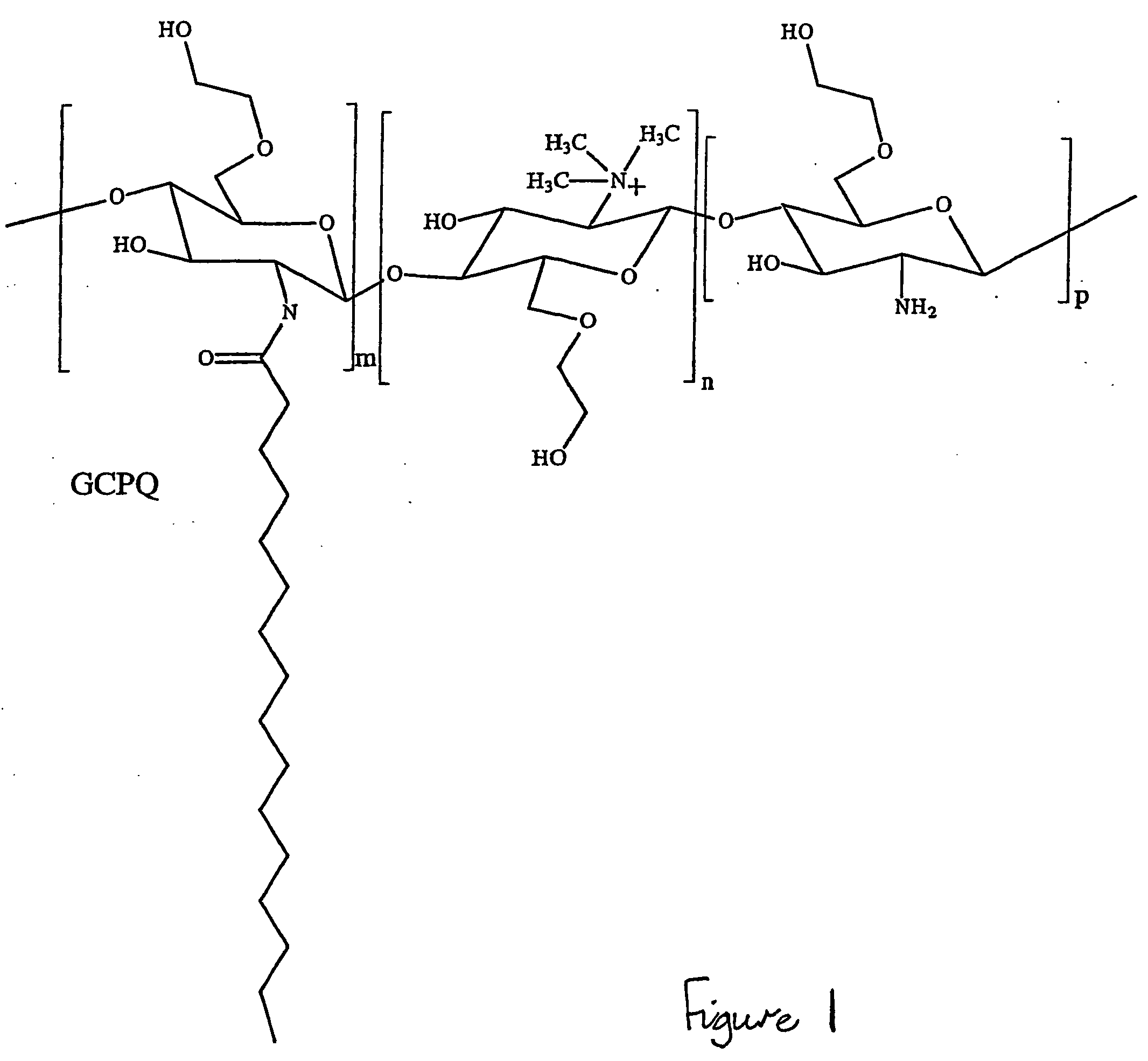
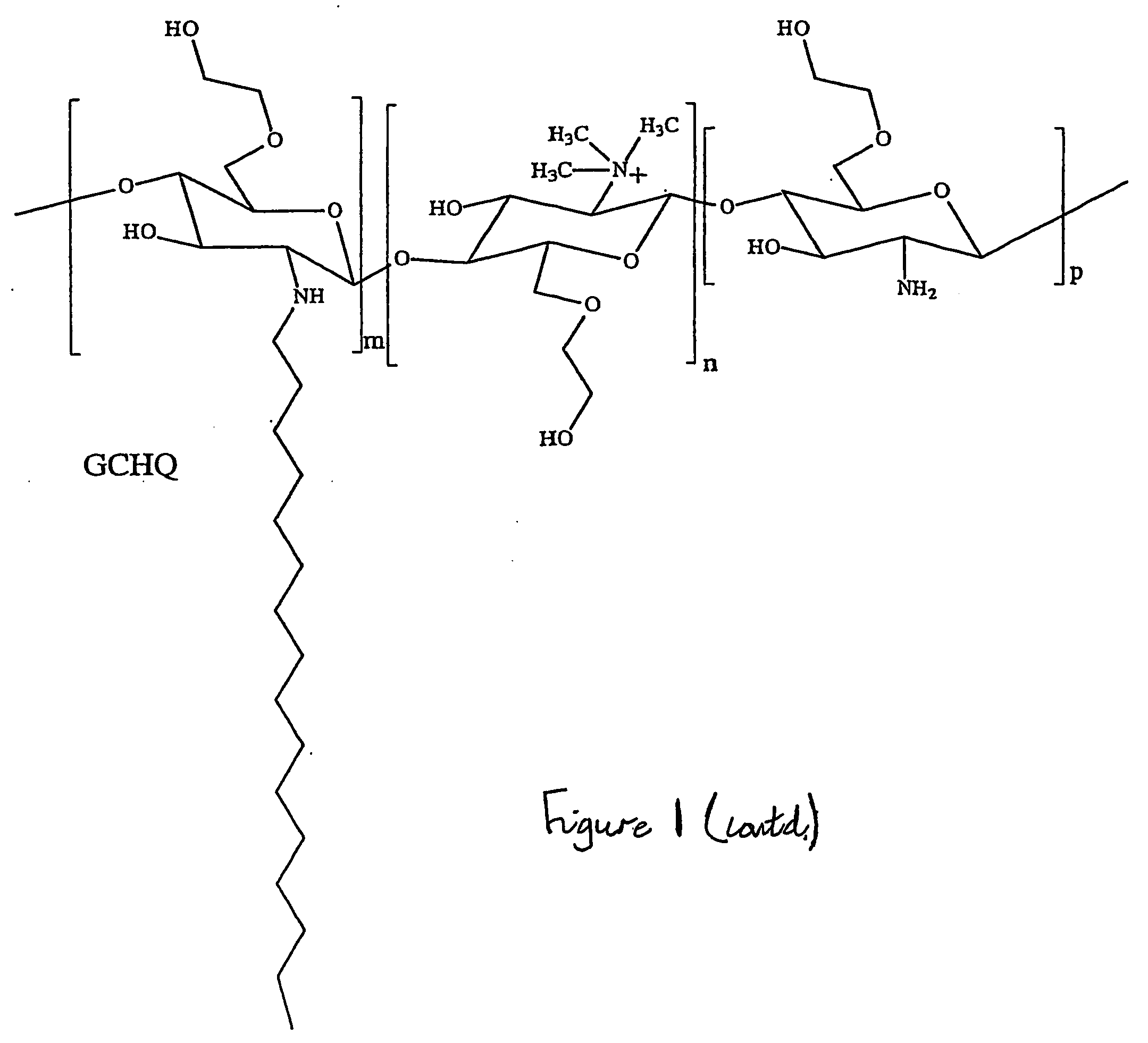
This invention relates to novel carbohydrate polymers with hydrophobic and hydrophilic side-groups suitable for solubilising, for example, hydrophobic drugs. The chain length of the carbohydrate polymeric backbone, and the type and number of the hydrophobic and hydrophilic side-groups are specifically chosen to improve the solubility properties of the carbohydrate polymers.
Owner:UNIV COLLEGE OF LONDON
Amphipathic three block copolymer and its preparation method and application
InactiveCN101265312AEvade captureAvoid the effect of adsorptionPharmaceutical non-active ingredientsPolyesterPolymer science
The invention relates to a polyethylene glycol / aliphatic polyester / cationic polymer amphiphilic triblock copolymer. The copolymer contains a hydrophilic non-ionic polymer block, a hydrophobic block and a pH-sensitive cationic polymer block. Potassium hydride is taken as an initiator, the polyethylene glycol and the aliphatic polyester monomers and the cationic monomer are sequentially added in a polymerization reactor for respective reaction, methanol is added for terminating the reaction; n-hexane is used for the precipitation and the purification of the triblock copolymer in tetrahydrofuran, the precipitation is repeated for 3 times, and a product undergoes the vacuum drying. The copolymer can form a micelle or a nanoparticle by self-assembly in a water medium, wherein, a core of a loading hydrophobic drug is formed by clustering the hydrophobic cationic polyester block, the polyethylene glycol block is assembled into a hydrophilic shell, thus having the functions of stabilizing the micelle and effectively avoiding the capture and protein absorption of a reticuloendothelial system of an organism; the cationic polymer block can be further acted with DNA, protein, peptide and other biological macromolecules, thus forming the biodegradable and pH-sensitive drug-loading polymer micelle or the nanoparticle which can be further dispersed,.
Owner:TIANJIN UNIV
Nanoparticle and preparation method thereof
InactiveCN101708162AGood biocompatibilityDimensional stabilityPharmaceutical non-active ingredientsGranular deliveryLactideHalf-life
The invention relates to a nanoparticle and a preparation method thereof. Poly(lactide-glycolide acid) (PLGA) is in the center to serve as a core, phospholipid surrounds the surface of the PLGA core in monolayer and distearoyl phosphatidylethanolamine-polyethyleneglycol-carboxyl (DSPE-PEG-COOH) penetrates the monolayer phospholipid to serve as the shell. The phospholipid, the DSPE-PEG-COOH and the PLGA have good biocompatibility, can entrap hydrophobic drugs and control the drugs to release slowly in the human bodies. The phospholipid surrounds the surface of the PLGA, thus ensuring that the particle can avoid immune system recognition so that the circulating half life of the particle is lengthened. The PEG shell ensures the particle to have spatial stability, static stability, long circulation and other characteristics and to be not easily agglomerated. Meanwhile, carboxyl is easily crosslinked with such ligands as antibodies, peptide, probes and the like, thus ensuring the particle to have targeting characteristic. The preparation method of the nanoparticle is simple, convenient practical and is easy to operate and popularize.
Owner:SHENZHEN INST OF ADVANCED TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com