Pharmaceutical delivery systems for hydrophobic drugs and compositions comprising same
A composition and drug technology, applied in the field of hydrophobic drug delivery system and its composition, can solve the problem of inability to maintain blood drug concentration for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
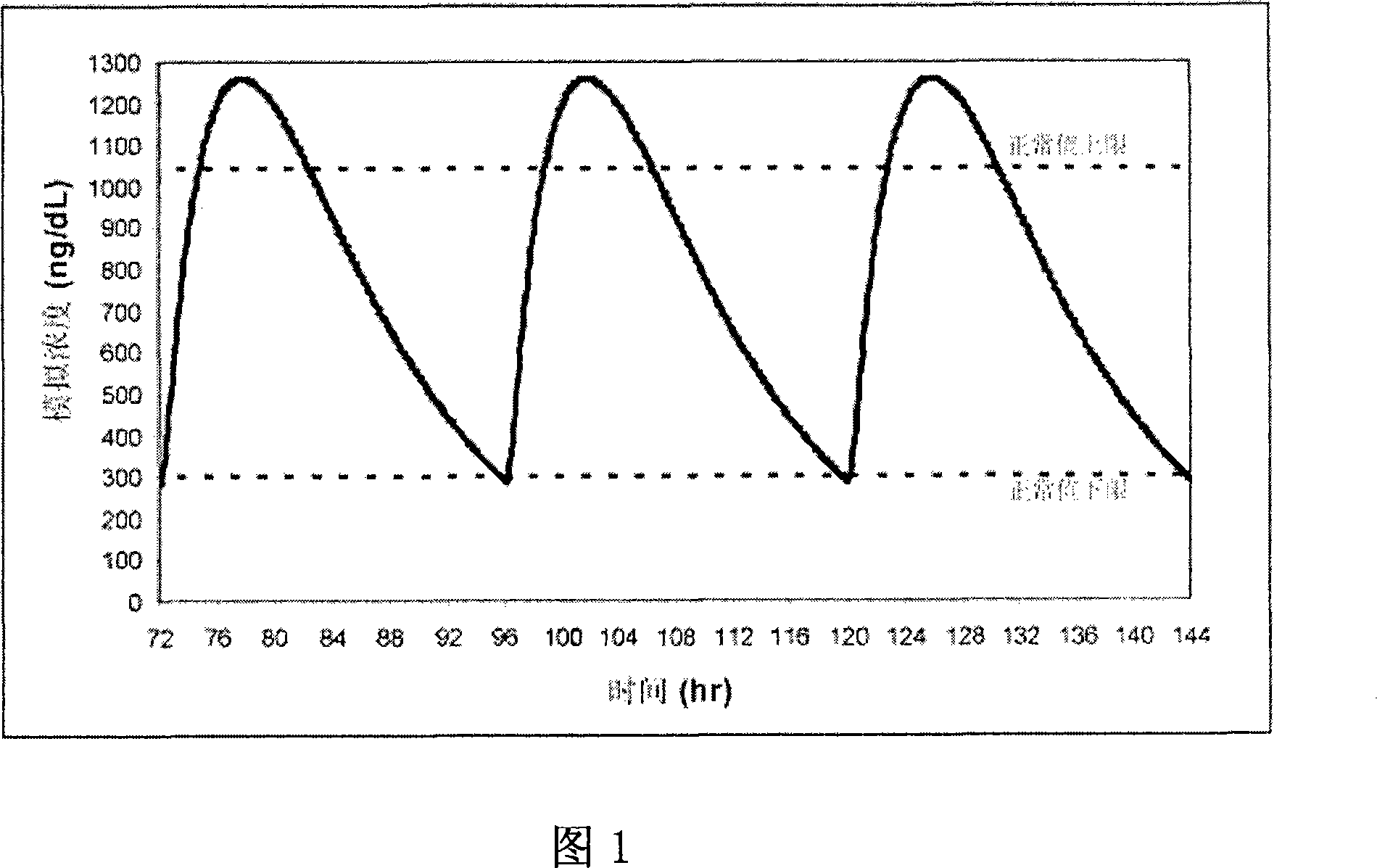
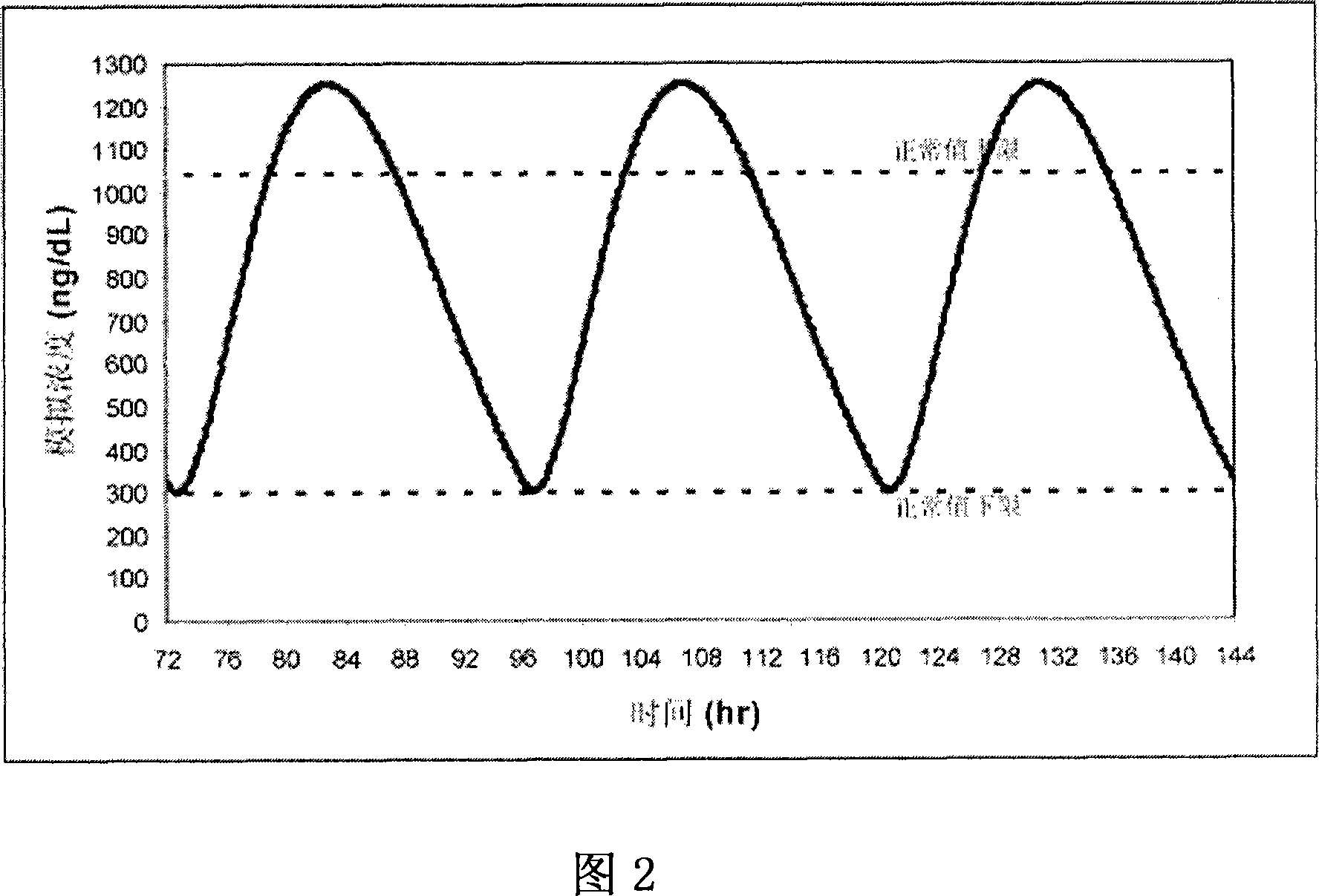
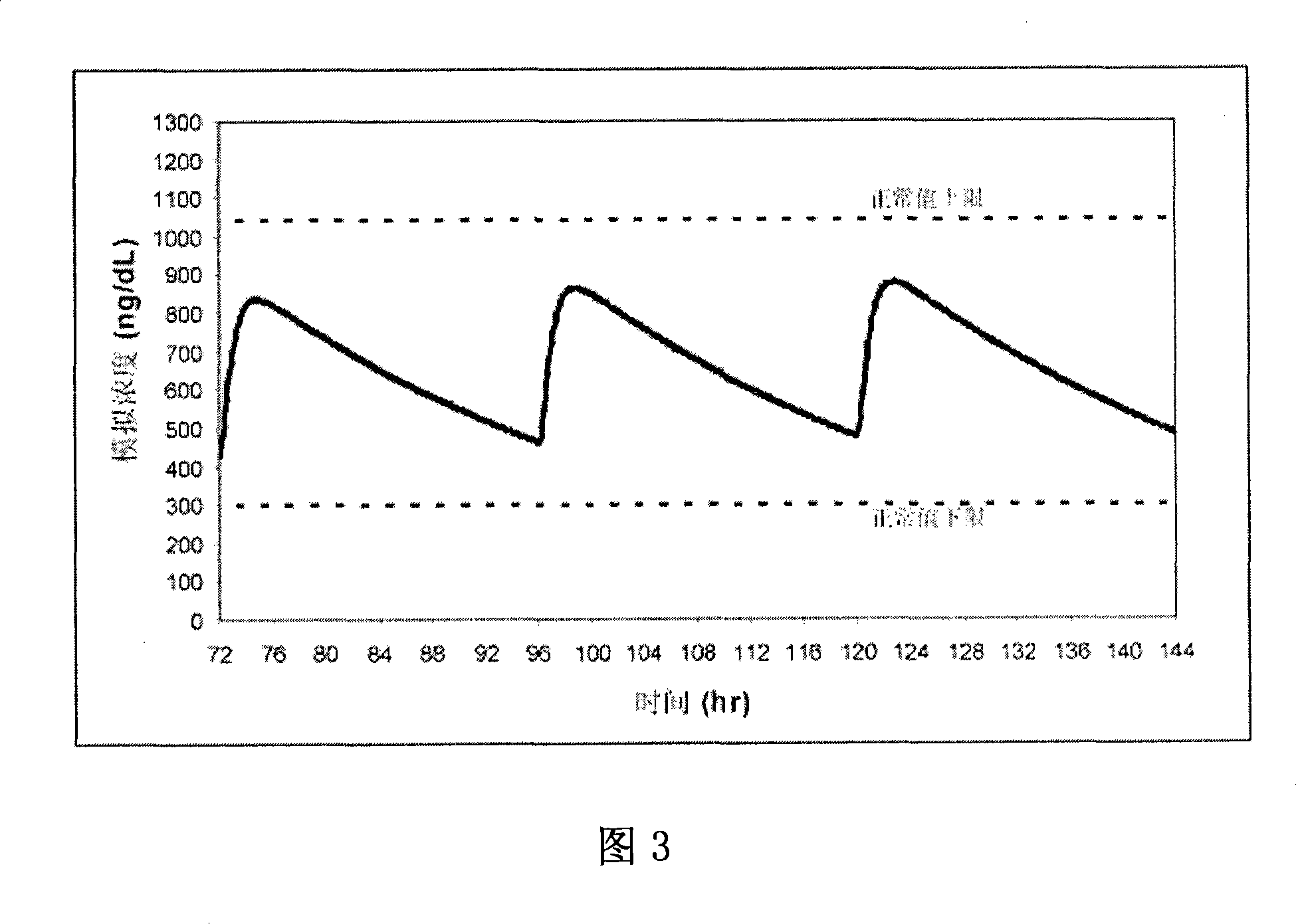
[0136] Formulations 50 and 54 were administered to 6 patients: Formulation 50 was administered once daily ("QD") in two capsules (each containing the equivalent of 100 mg of T). Formulation 54 was administered as a once-daily or twice-daily ("BID") regimen of three capsules (each containing the equivalent of 66 mg of T). After 7 days of treatment according to the above three administration methods, the average drug-time curves of patients in each group are shown in Fig. 11 . The average drug-time curve of the preparation 54BID administration group was relatively stable within 24 hours, and its lowest value was about 70% of the peak value. Additional kinetic data for Formulation 54 dosed groups included:
[0137] The mean T concentration in the serum increased by 275 ng / dL from the baseline level
[0138] The mean T concentration in the serum is below the bottom limit of the normal range (ie, about 325 ng / dL)
[0139] Relatively fast release (Tmax about 1 hour)
[0140] The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com