Methods and formulations for the delivery of pharmacologically active agents
a technology formulations, applied in the field of formulations of pharmacologically active agents, can solve the problems of limiting the full potential of the very effective paclitaxel molecule, the association of significant side effects of the cremophor formulation, and the inability to deliver intravenously
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preclinical Studies Confirm the Modulation of Paclitaxel Release by the Protein Nanosphere and Increased Efficacy of Equi-dose of ABI-007 vs Taxol
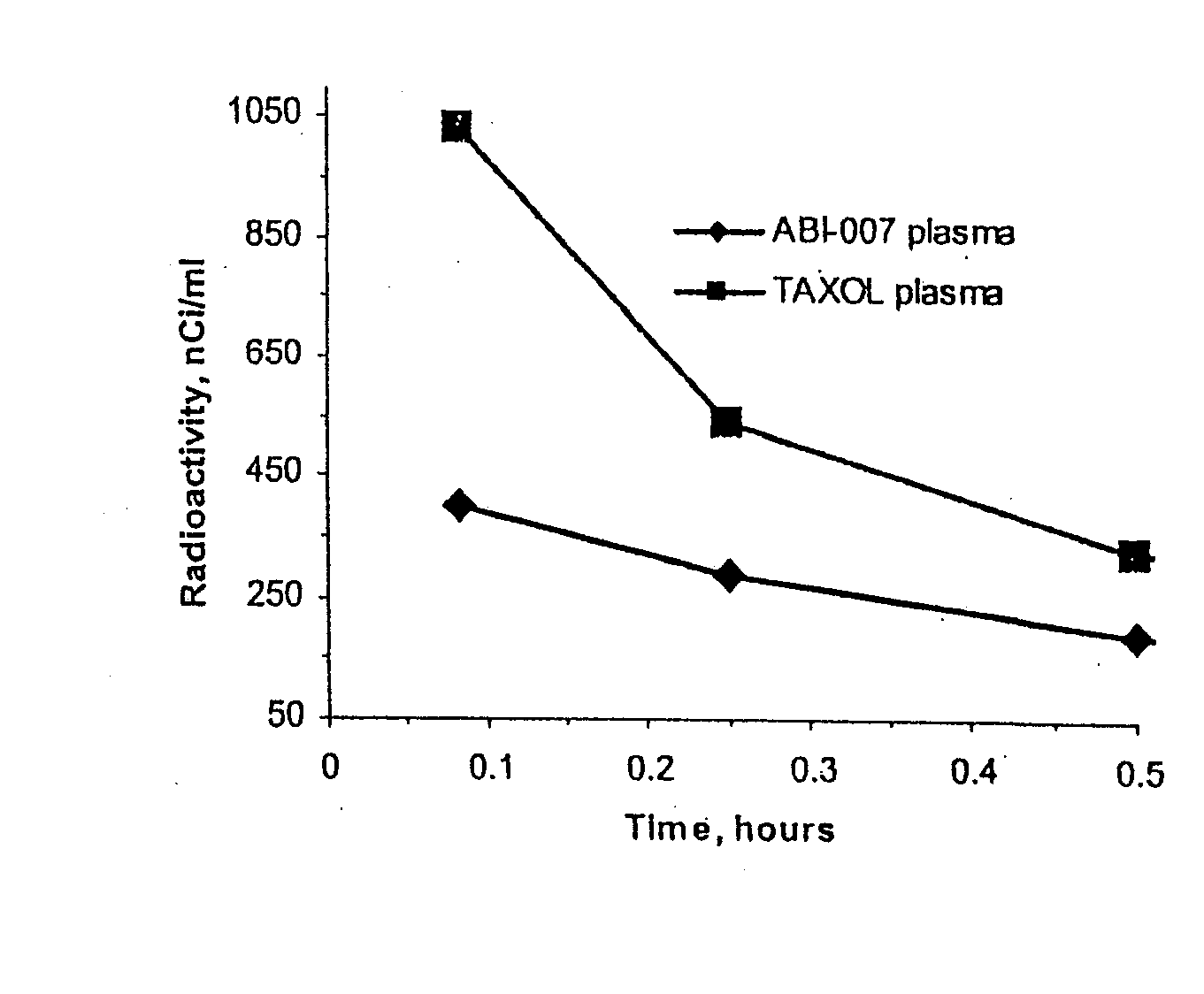
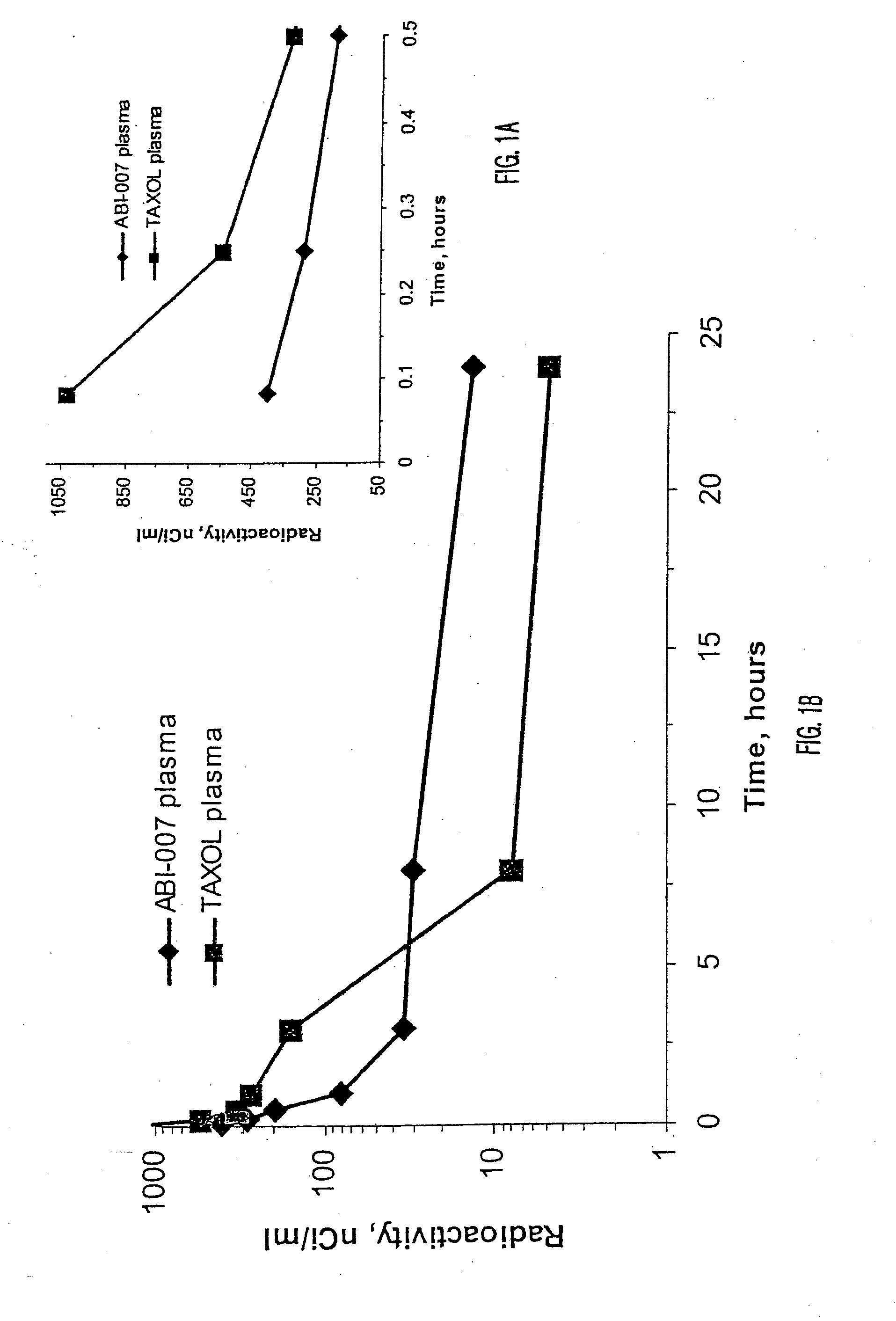
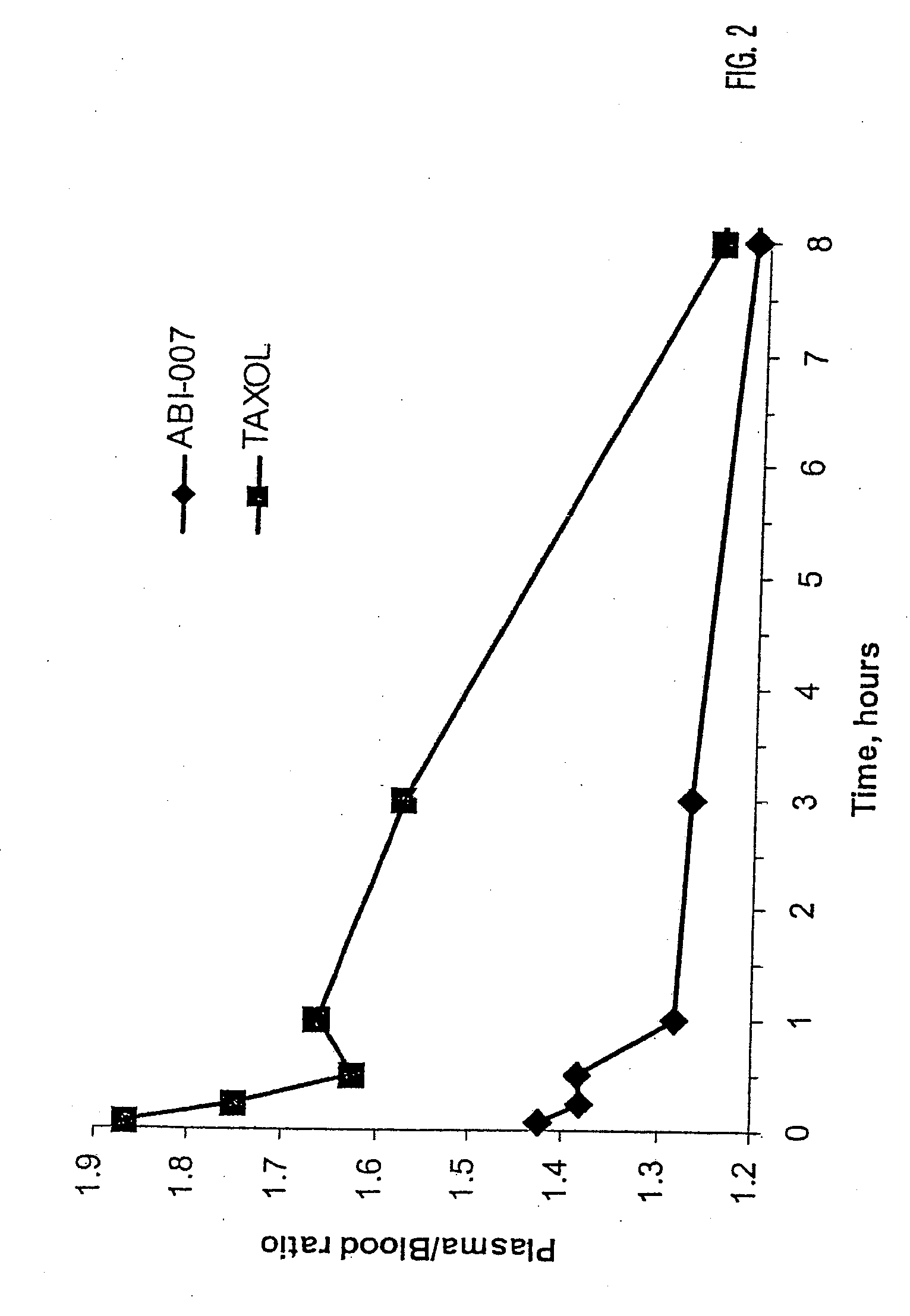
[0051]Using radio labeled paclitaxel, the enahanced intra-cellular availability of paclitaxel has been confirmed following injection of ABI-007. In addition, the entrapment of Cremophor-bound paclitaxel has also been confirmed. This difference in findings correlates with in-vivo studies in mice bearing human breast cancer, with the finding that ABI-007 at equi-dose to Taxol, resulted in improved outcomes that these 130 nanometer size particles distributed throughout the body.
[0052]Thus, human MX-1 mammary tumor fragments were implanted subcutaneously in female athymic mice. Radiolabelled drug was administered when tumors reached about 500 mm3. Tritium-labelled ABI-007 or tritium-labelled Taxol were administered at a dose of 20 mg / kg. Both groups received about 7-10 μCi / mouse of tritium-labelled paclitaxel. Saline was used as the diluent fo...
example 2
Toxicity Studies
[0056]Toxicity was assessed for Taxol, cremophor and ABI-007. ABI-007 was found to be 50-fold less toxic than Taxol, and 30-fold less toxic than the cremophor vehicle alone, as illustrated in the following table:
AgentLD50, mg / kgTaxol9.4Cremophor13.7ABI-007448.5
example 3
In vivo Tumor Xenografts
[0057]Human tumor fragments were implanted subcutaneously in female athymic mice. Treatment was initiated when tumors reached about 150 mm3. The mice received either CONTROL (saline), ABI-007 (4 dose levels: 13.4, 20, 30 and 45 mg / kg) or TAXOL (3 dose levels: 13.4, 20, and 30 mg / kg) administered I.V. daily for 5 days. Saline was used as the diluent for both drugs.
[0058]Determination of Equitoxic dose or MTD: The Equitoxic dose or MTD for each drug was determined by satisfying one of the following criteria:[0059]a) Dose for each drug that resulted in similar body weight loss (≦20%) if no deaths were seen;[0060]b) If body weight loss could not be matched, the highest dose at which no deaths were seen;[0061]If neither a) nor b) could be satisfied, the lowest dose that resulted in similar death rate.
[0062]Tumor response to the drugs was compared at the Equitoxic dose or MTD established as above. Results for several different tumor types are presented in FIGS. 4-8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
| Area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com