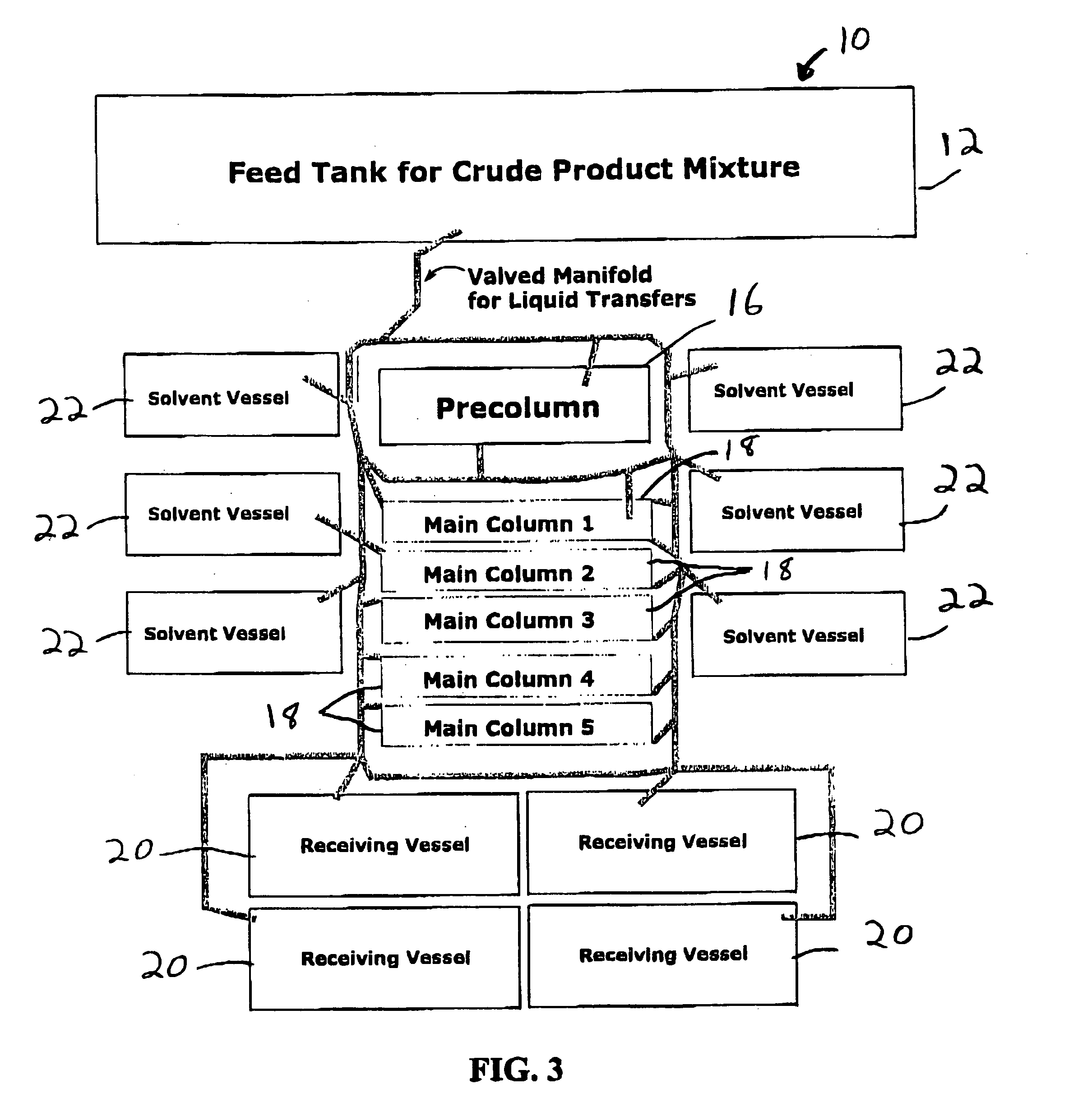
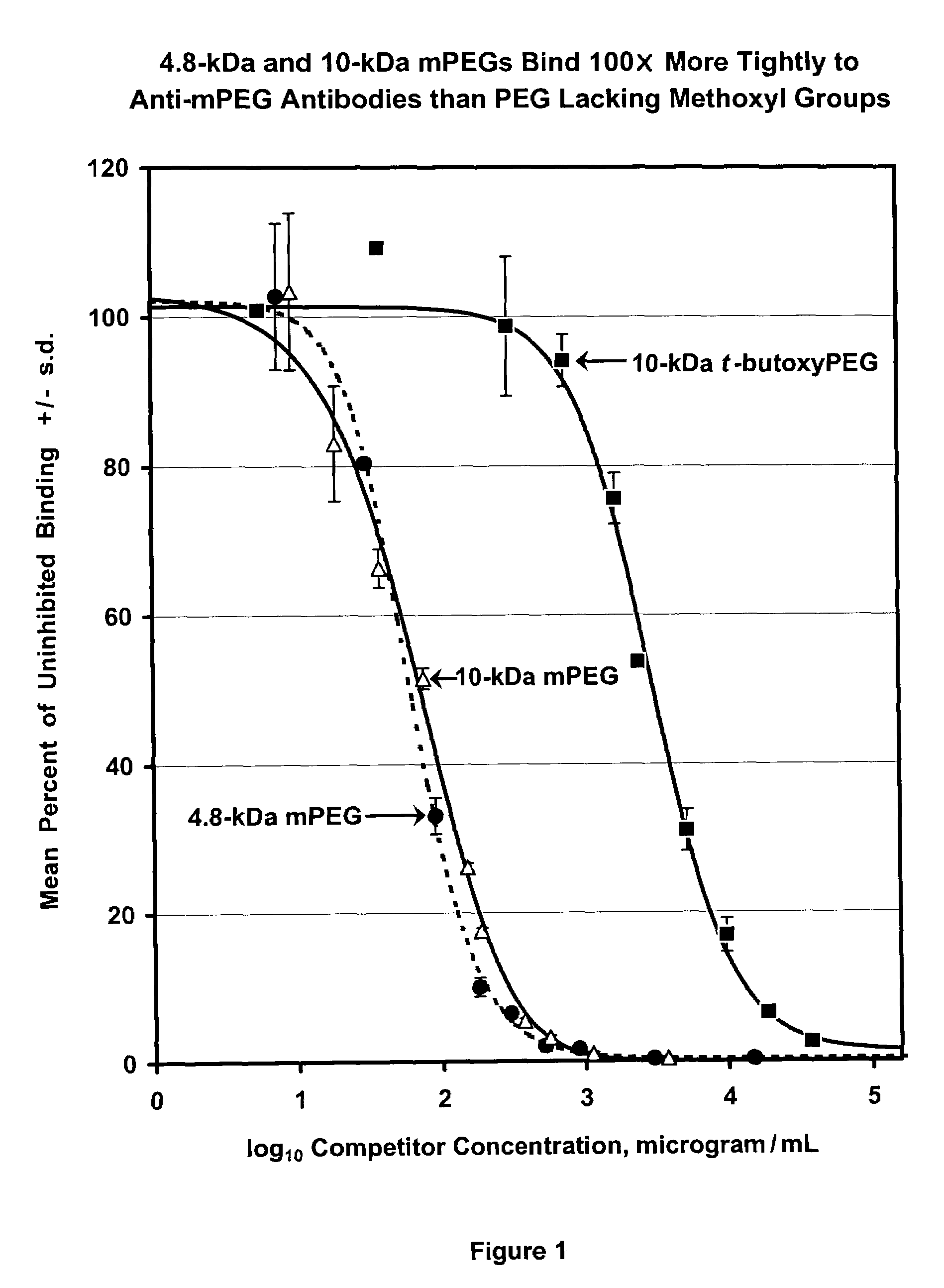
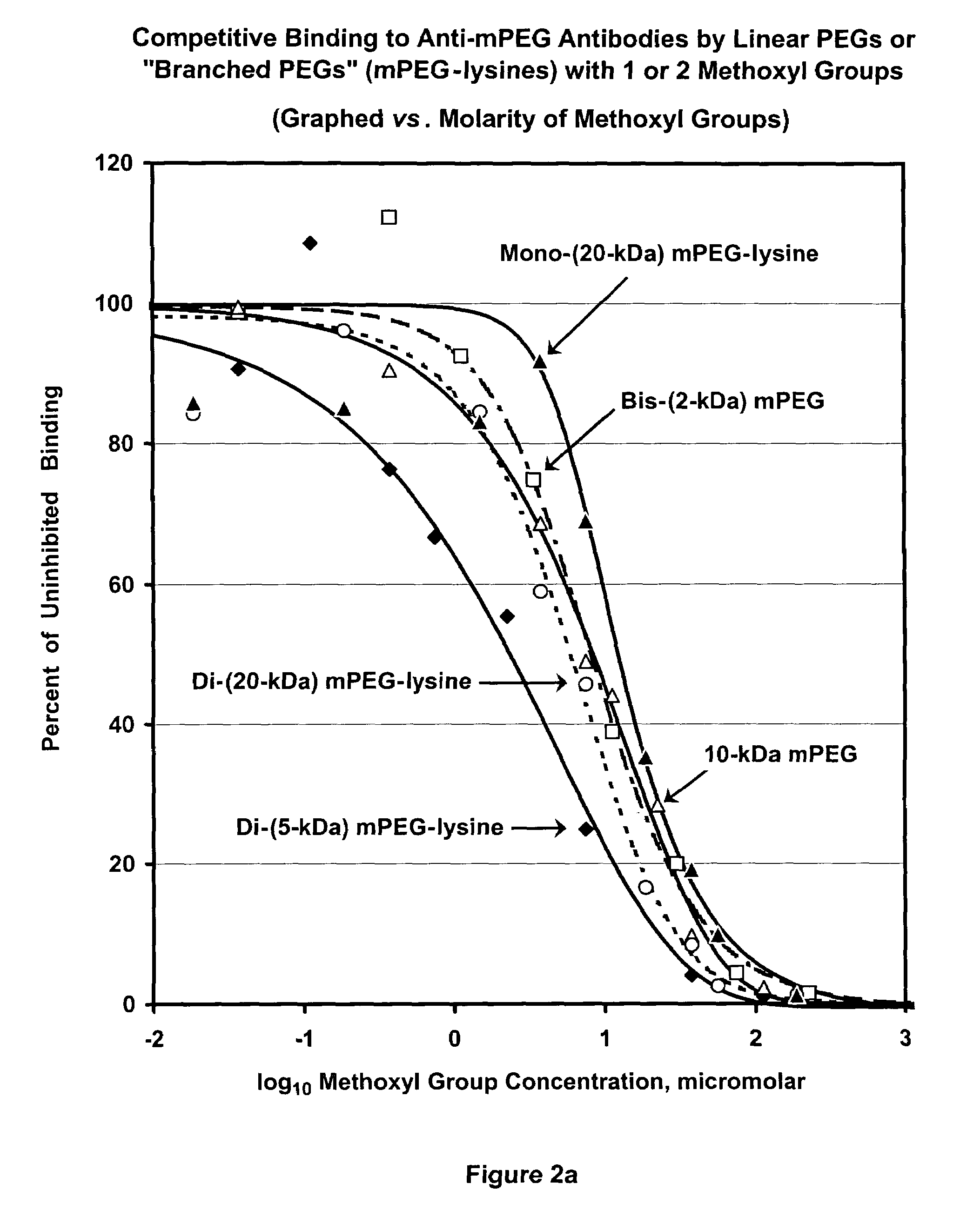
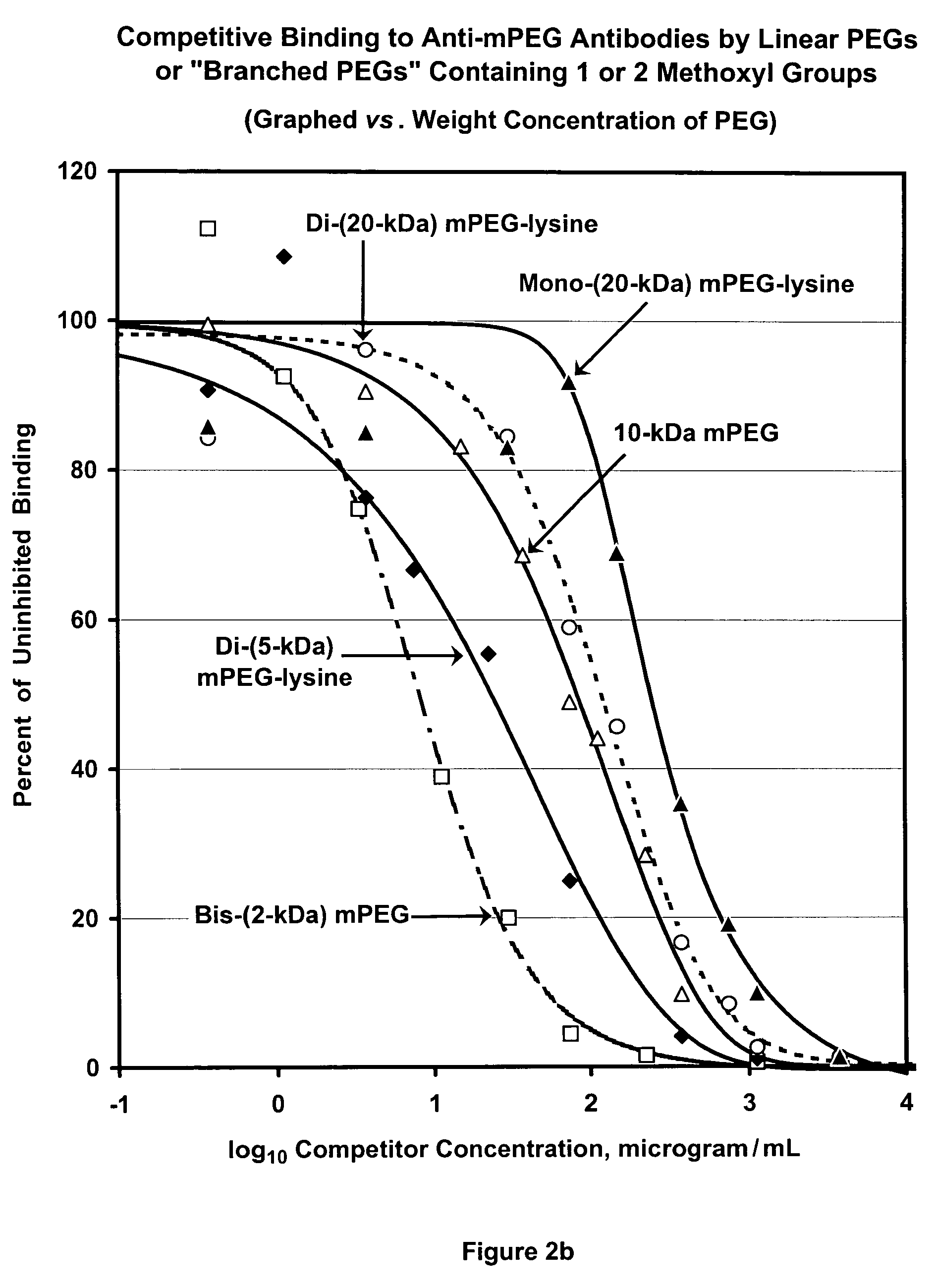
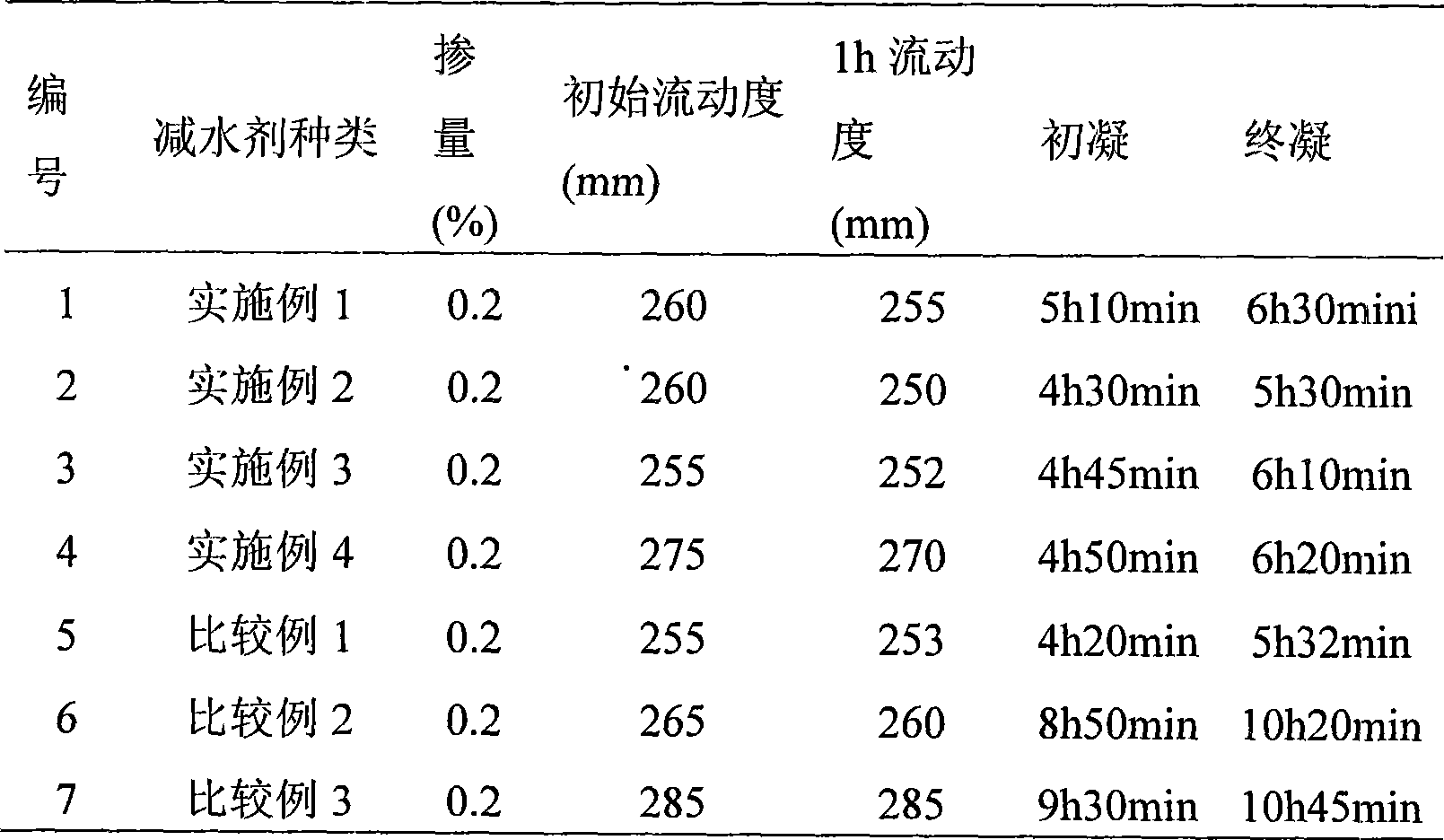
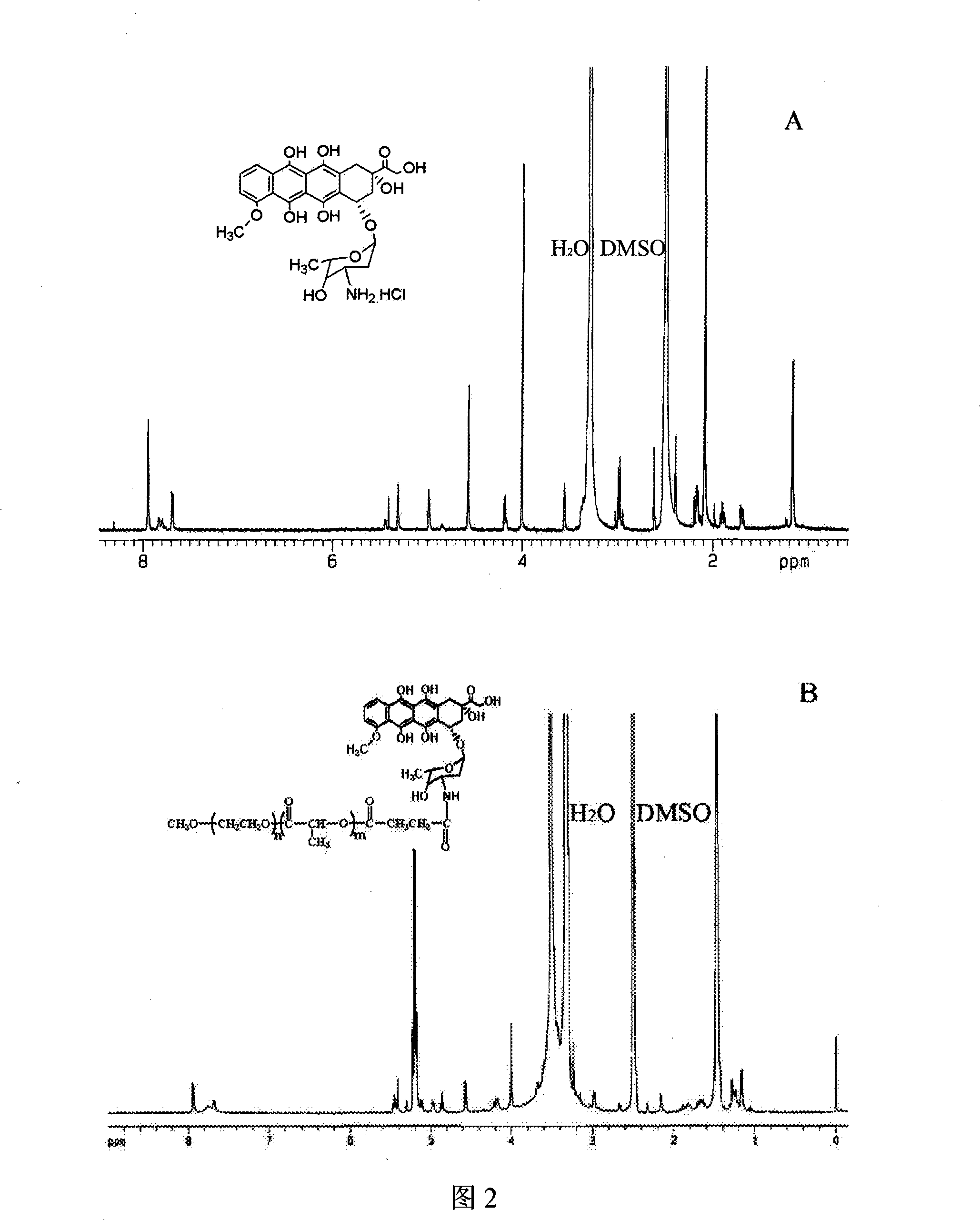
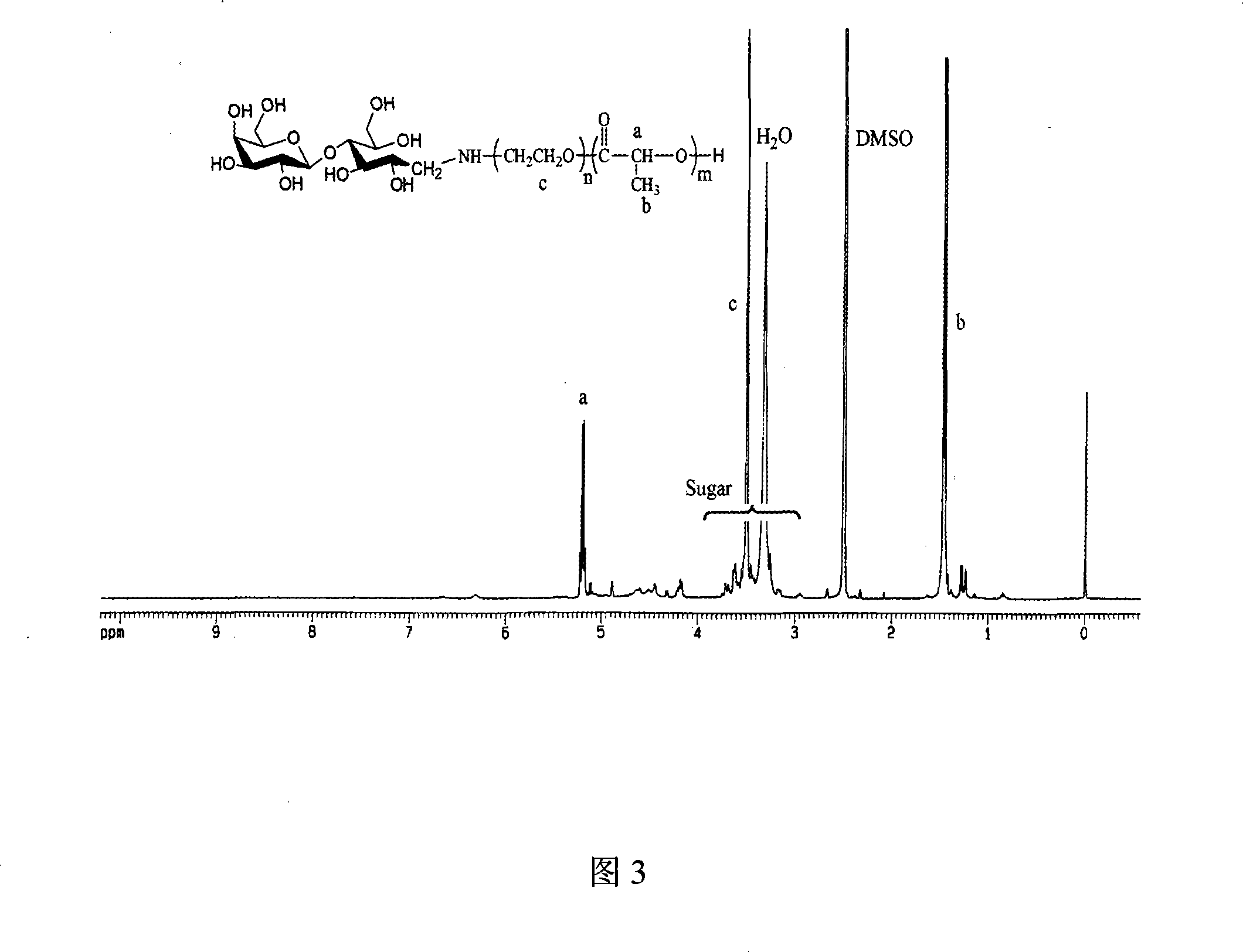
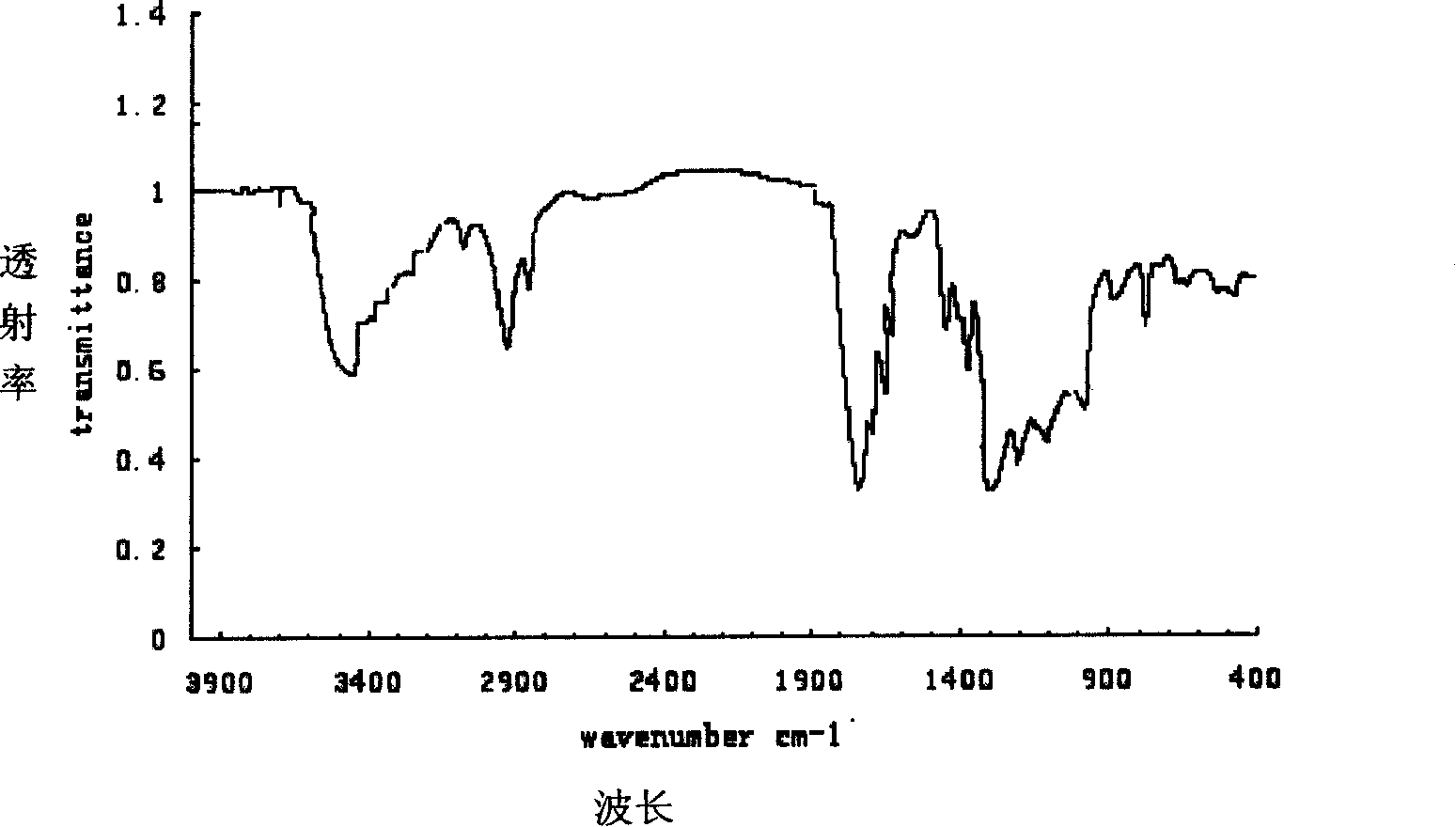
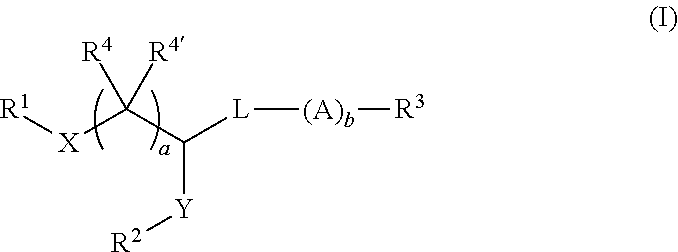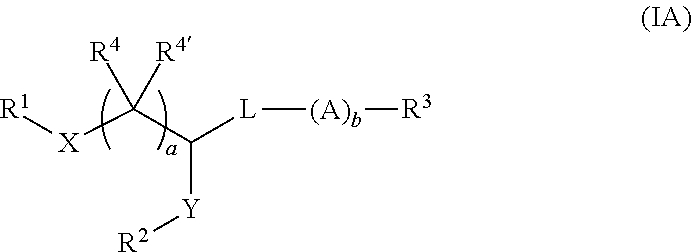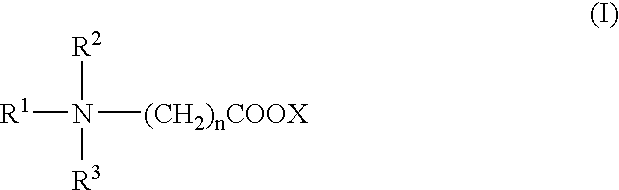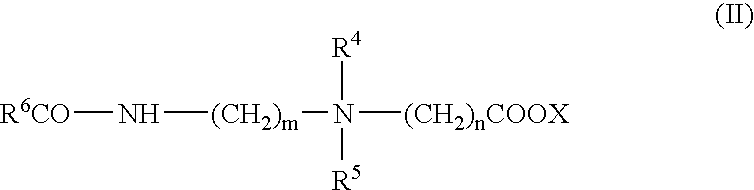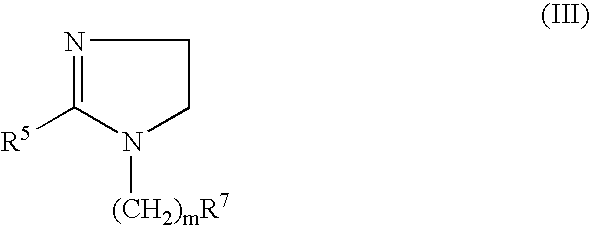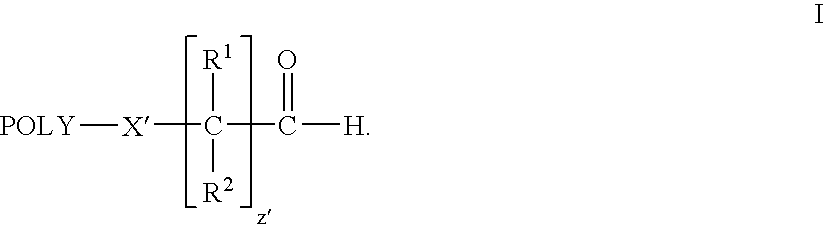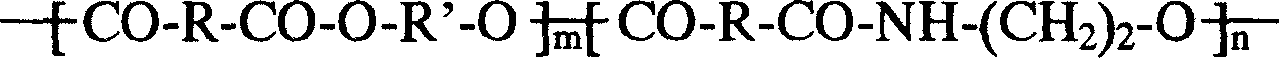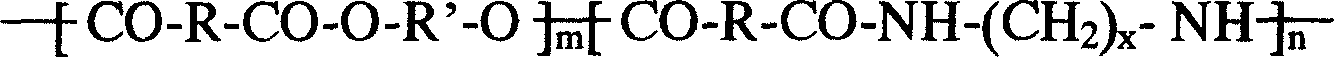Patents
Literature
2341 results about "Polythylene glycol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyethylene glycol 3350 is used to treat occasional constipation. Polyethylene glycol 3350 is in a class of medications called osmotic laxatives. It works by causing water to be retained with the stool.
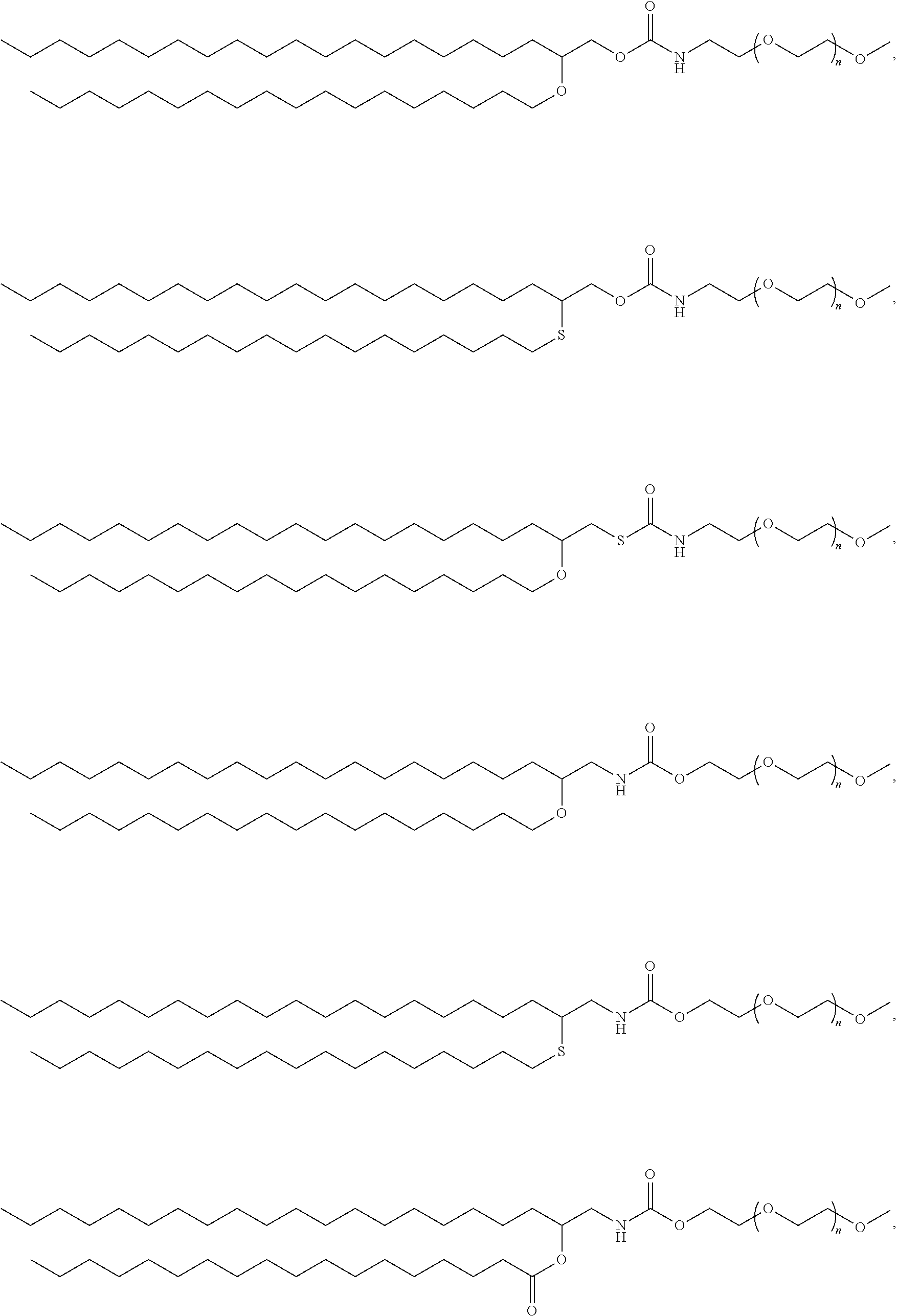
Pegylated lipids and their use for drug delivery
Owner:ALNYLAM PHARM INC
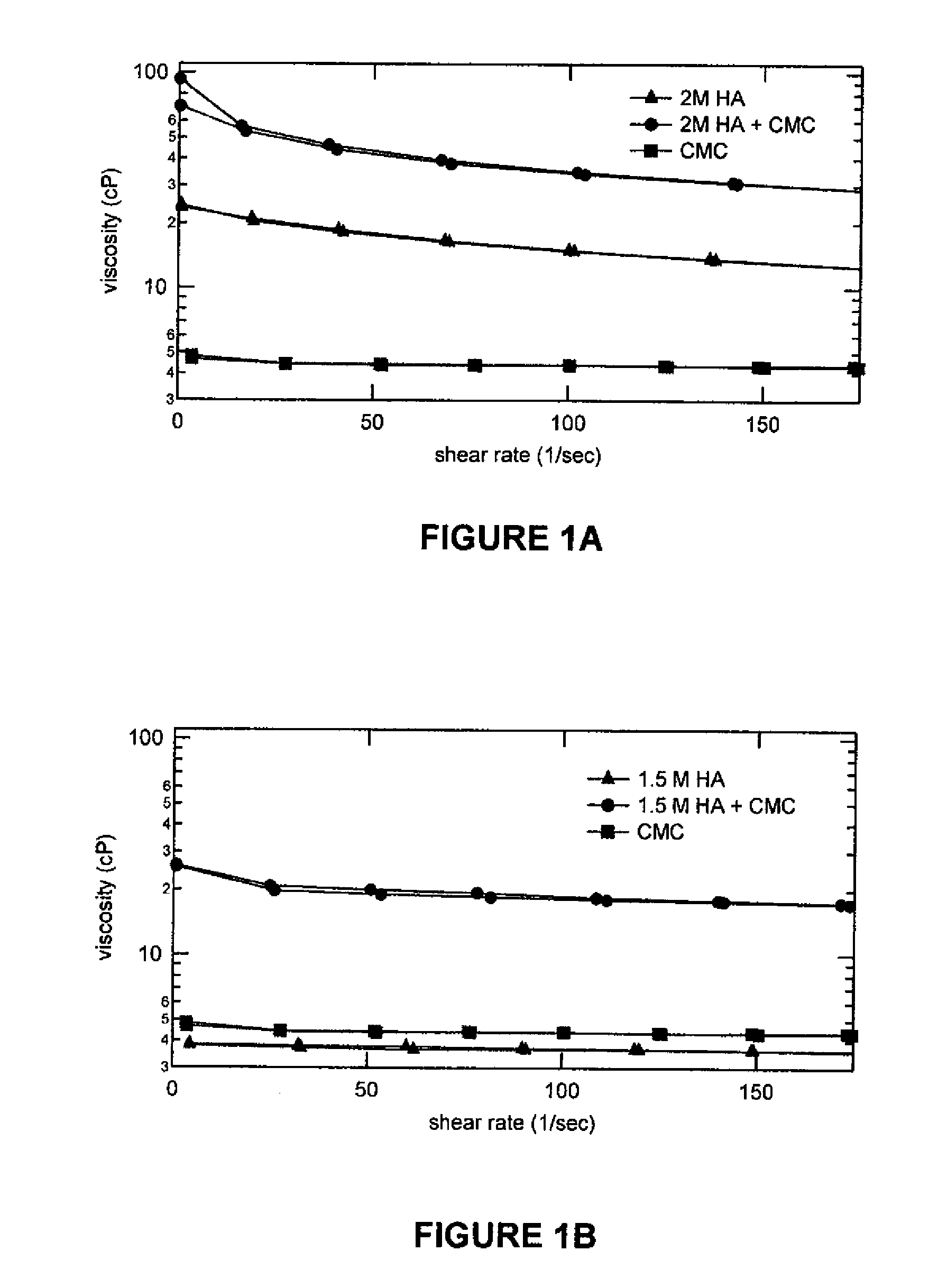
Stabilized Glycosaminoglycan Preparations and Related Methods
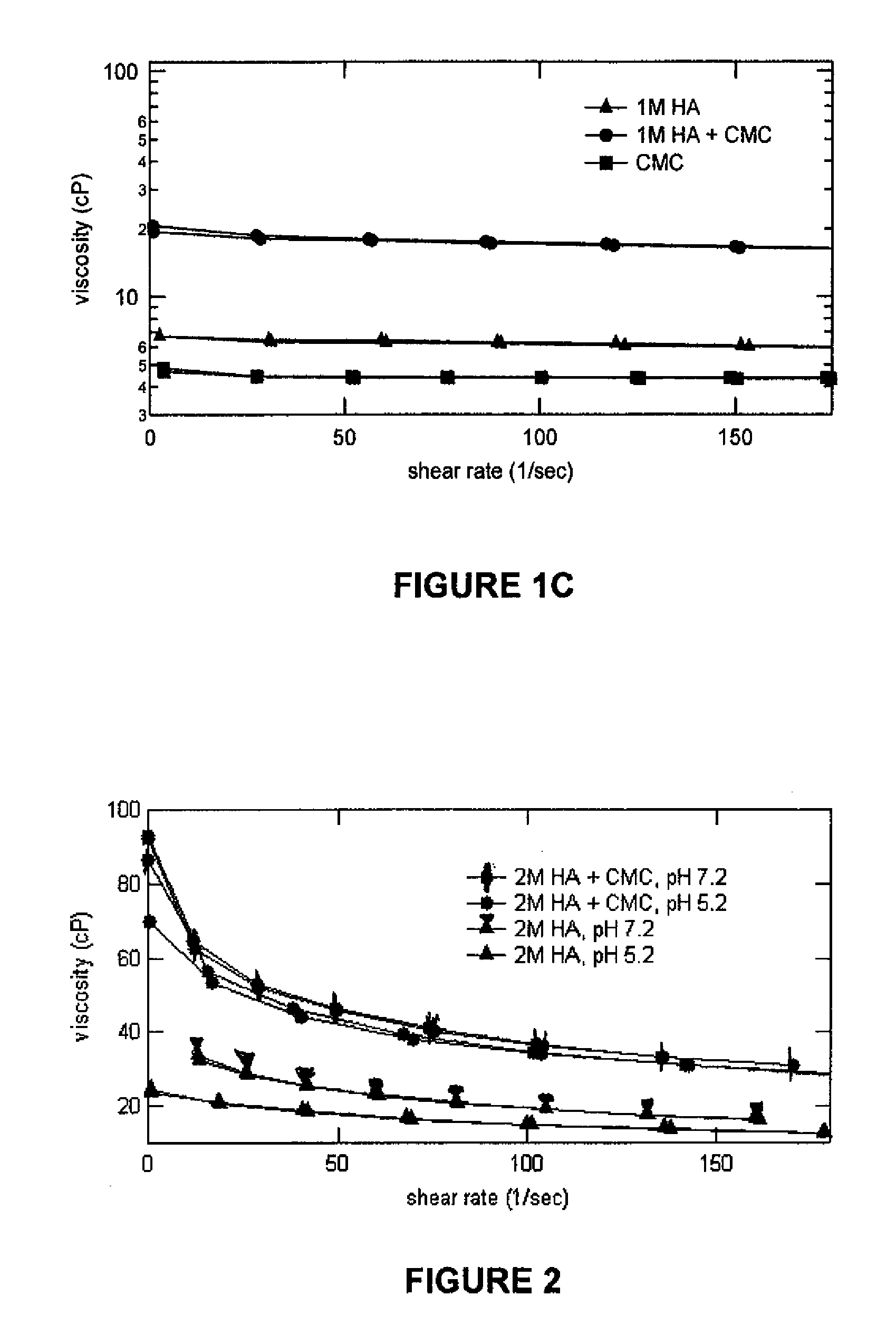
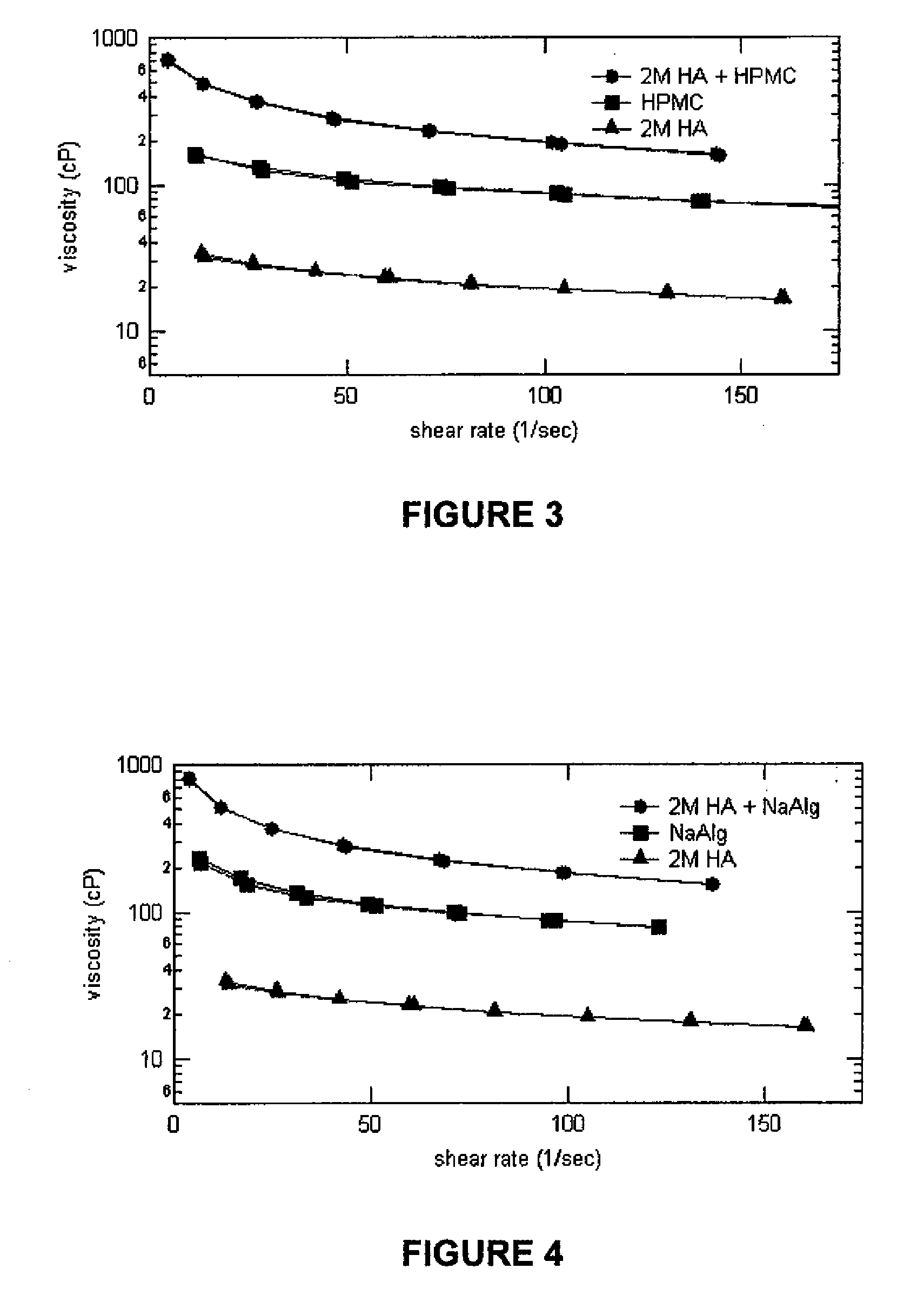
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
Thermoreversible pharmaceutical formulation for anti-microbial agents comprising poloxamer polymers and hydroxy fatty acid ester of polyethylene glycol
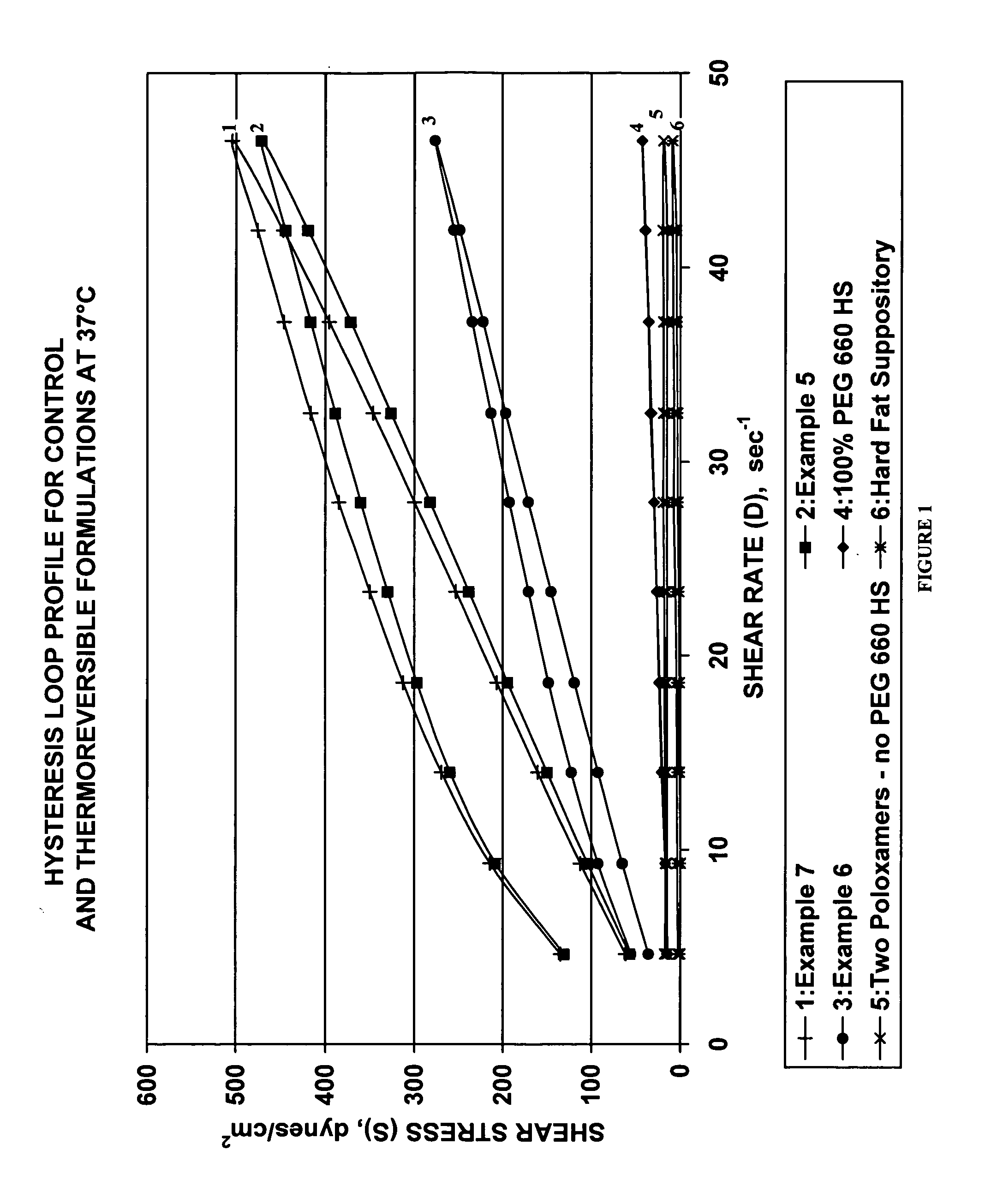
The present invention provides a pharmaceutical formulation having thermoreversible properties, comprising: a) an anti-microbial agent; b) a poloxamer mixture containing at least two poloxamer polymers; and c) a hydroxy fatty acid ester of polyethylene glycol, wherein the formulation is a solid at room temperature and is a liquid-gel at body temperature. The thermoreversible pharmaceutical formulation has a viscosity of about 8,500 cP to about 400,000 cP at room temperature, and a viscosity of about 1,000 cP to about 8,000 cP at body temperature and exhibits a hysteresis loop behavior. The present invention further provides a process of preparing as well as a method of treating a microbial infection in a mammal using the same.
Owner:TARO PHARMA INDS
Composition for sustained delivery of hydrophobic drugs and process for the preparation thereof
InactiveUS7153520B2Enhance pharmacological effectsPowder deliverySolution deliveryPolythylene glycolPharmaceutical Substances
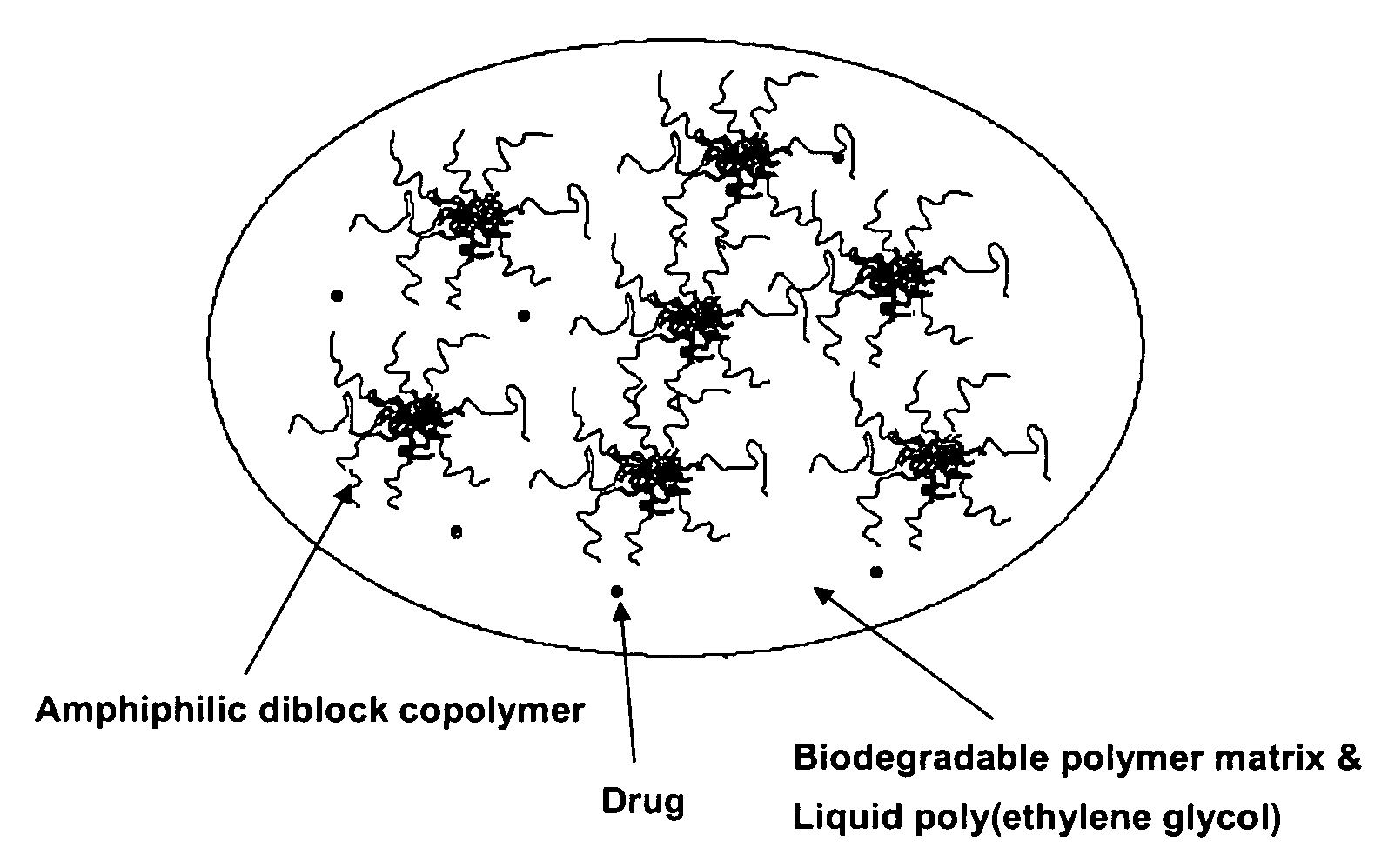
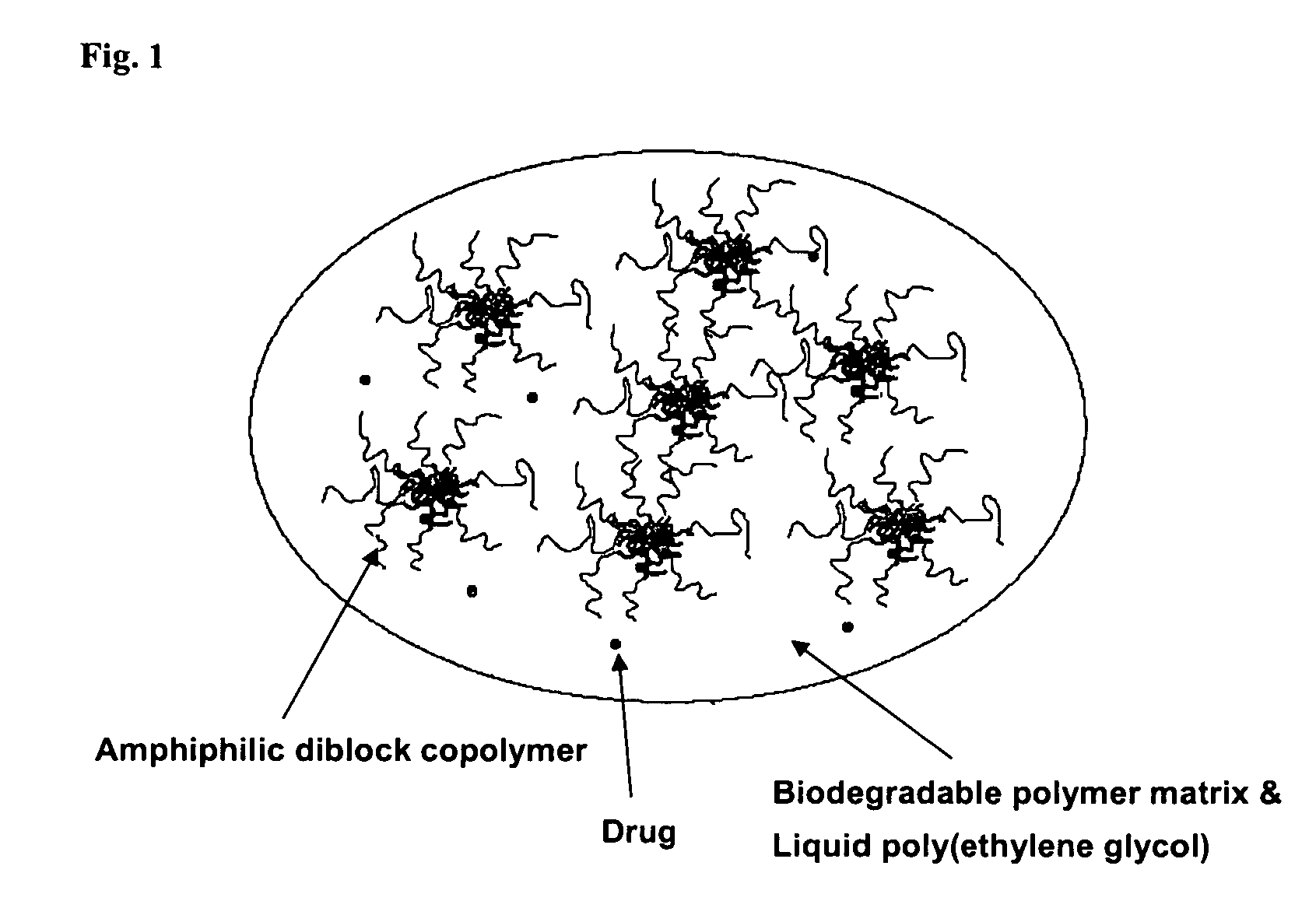
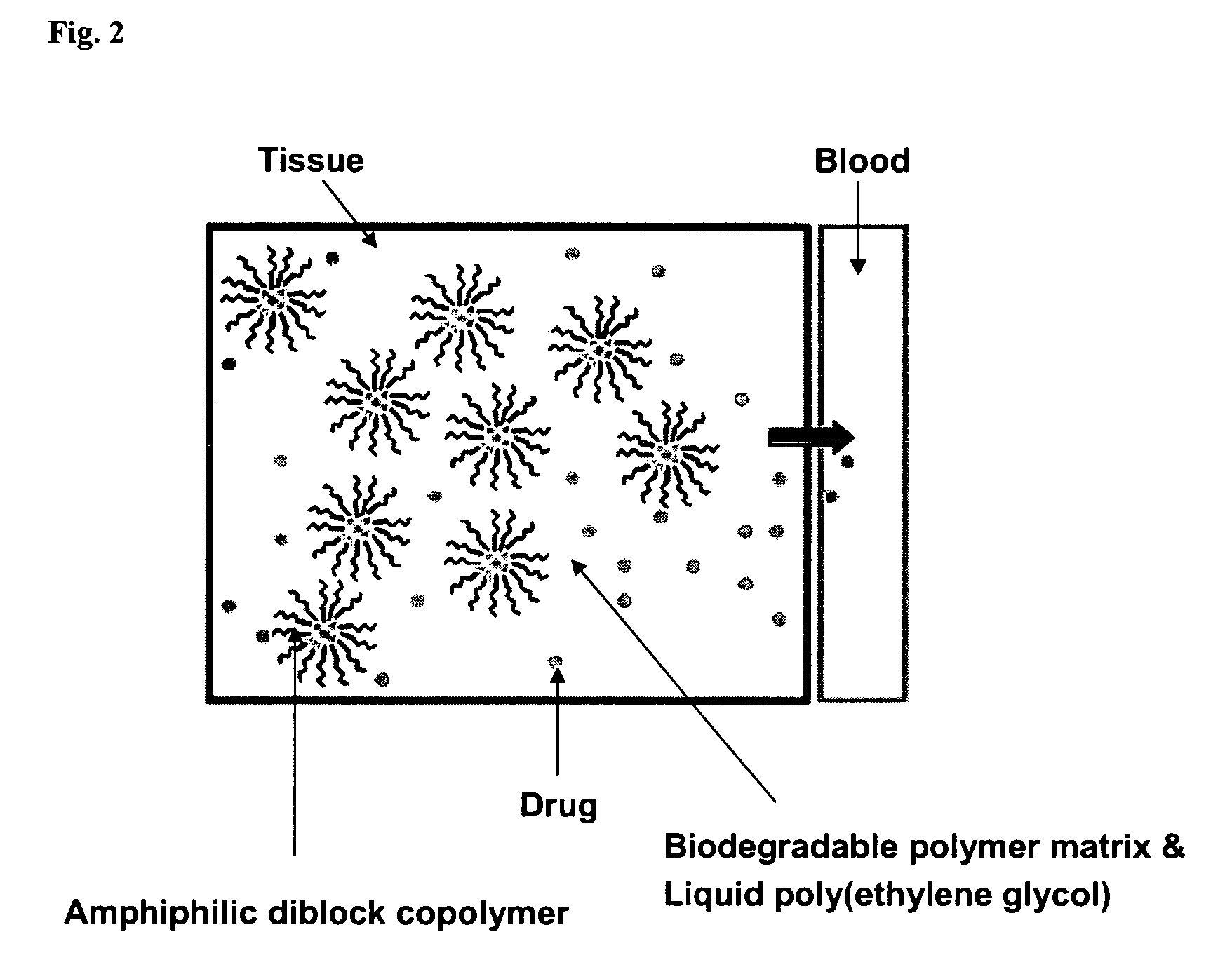
A composition for the sustained delivery of a drug comprising an amphiphilic diblock copolymer; a poorly water-soluble drug; a biodegradable polymer; and liquid poly(ethylene glycol) or functional derivatives thereof and a process for preparing the composition are disclosed. When administered into a particular body site, the composition forms an implant containing the drug and drug containing polymeric micelles, which are slowly released from the implant to maintain a constant drug concentration for an extended period of time.
Owner:SAMYANG HLDG CORP
Gel laundry detergent composition
InactiveUS7297674B2Improve clarityHigh transparencySoap detergents with organic compounding agentsNon-ionic surface-active compoundsActive agentPolypropylene glycol
The present invention provides a shear thinning, transparent gel laundry detergent composition, comprising a surfactant system containing surfactant material selected from an anionic surfactant, a nonionic surfactant or a mixture thereof, and from 0.1 to 10% by weight of a clarity improving agent being a glycol dialkyl ether selected froma mono-or polyethylene glycol dialkyl ether having the formula(CpH2p+1)O—(CH2CH2O)n—(CqH2q+1), (I)a mono- or polypropylene glycol dialkyl ether having the formula(CpH2p+1)O—(CH2CH (CH3)O)n—(CqH2q+1), (II) and mixtures thereof,wherein p and q independently are integers in the range of from 1 to 5, and n is an integer in the range of from 1 to 50, preferably 1 to 10. It has been found that this gel laundry composition is highly transparent, such that particles can be suspended therein for improving visual appearance.
Owner:HENKEL IP & HOLDING GMBH
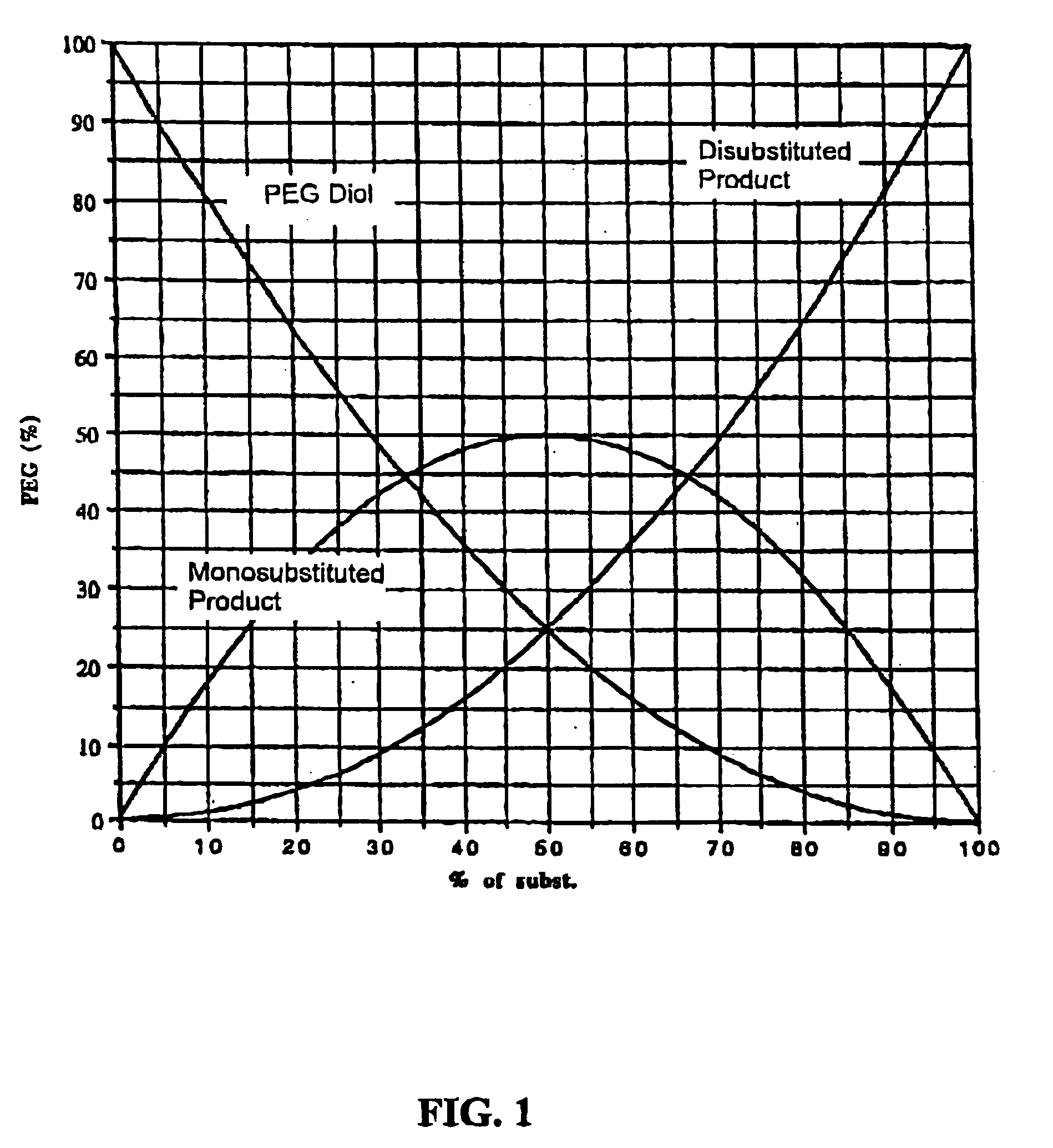
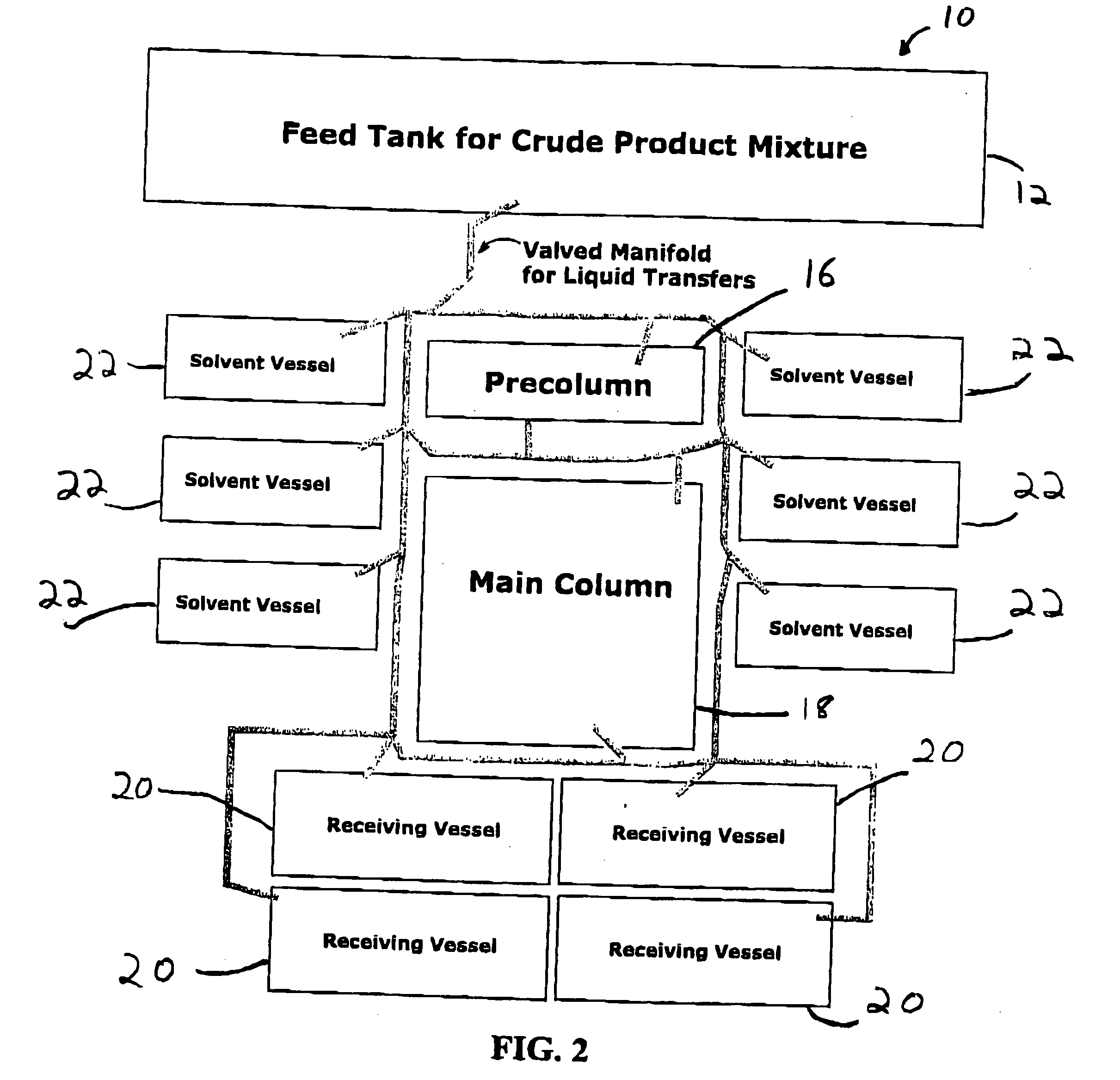
Method for preparing functionalized polymers from polymer alcohols
ActiveUS20050054816A1Solid sorbent liquid separationPharmaceutical non-active ingredientsPolyethylene glycolPolymer composition
Owner:NEKTAR THERAPEUTICS INC
Polymer conjugates with decreased antigenicity, methods of preparation and uses thereof
ActiveUS8129330B2Reduced and substantially reduced antigenicityReduced and substantially reduced and undetectable antigenicity and immunogenicityAntibacterial agentsNervous disorderWater solubleImmunogenicity
Methods are provided for the preparation of conjugates of a variety of bioactive components, especially proteins, with water-soluble polymers (e.g., poly(ethylene glycol) and derivatives thereof), which conjugates have reduced antigenicity and immunogenicity compared to similar conjugates prepared using poly(ethylene glycol) containing a methoxyl or another alkoxyl group. The invention also provides conjugates prepared by such methods, compositions comprising such conjugates, kits containing such conjugates or compositions and methods of use of the conjugates and compositions in diagnostic and therapeutic protocols.
Owner:MOUNTAIN VIEW PHARMA
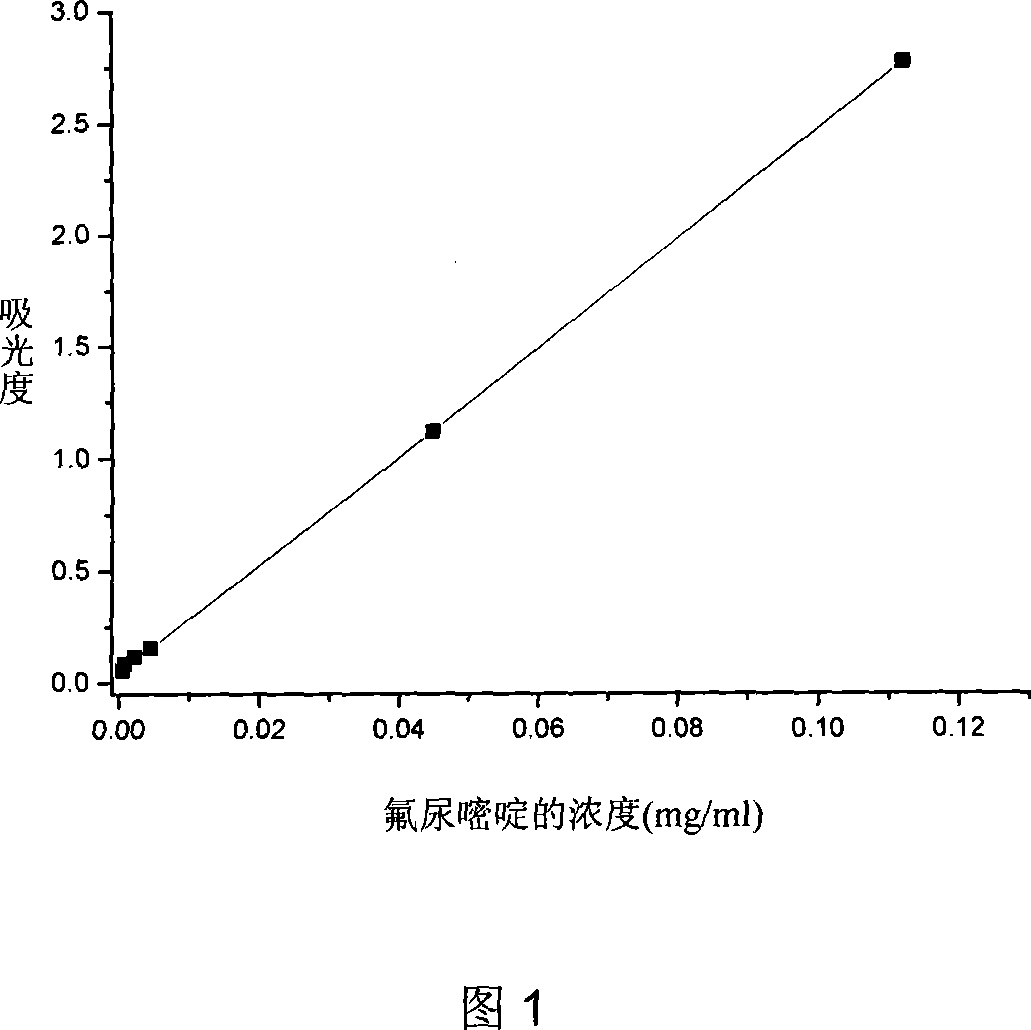
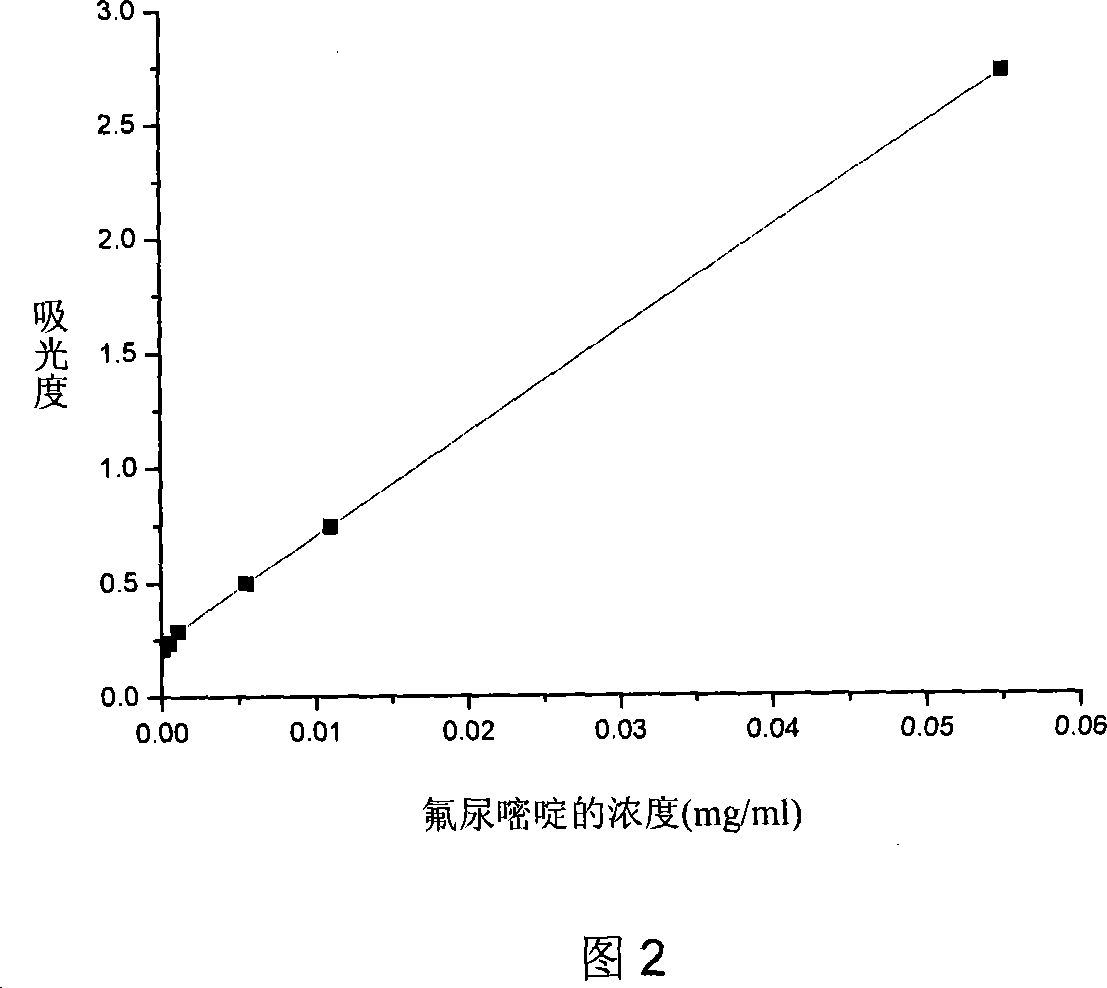
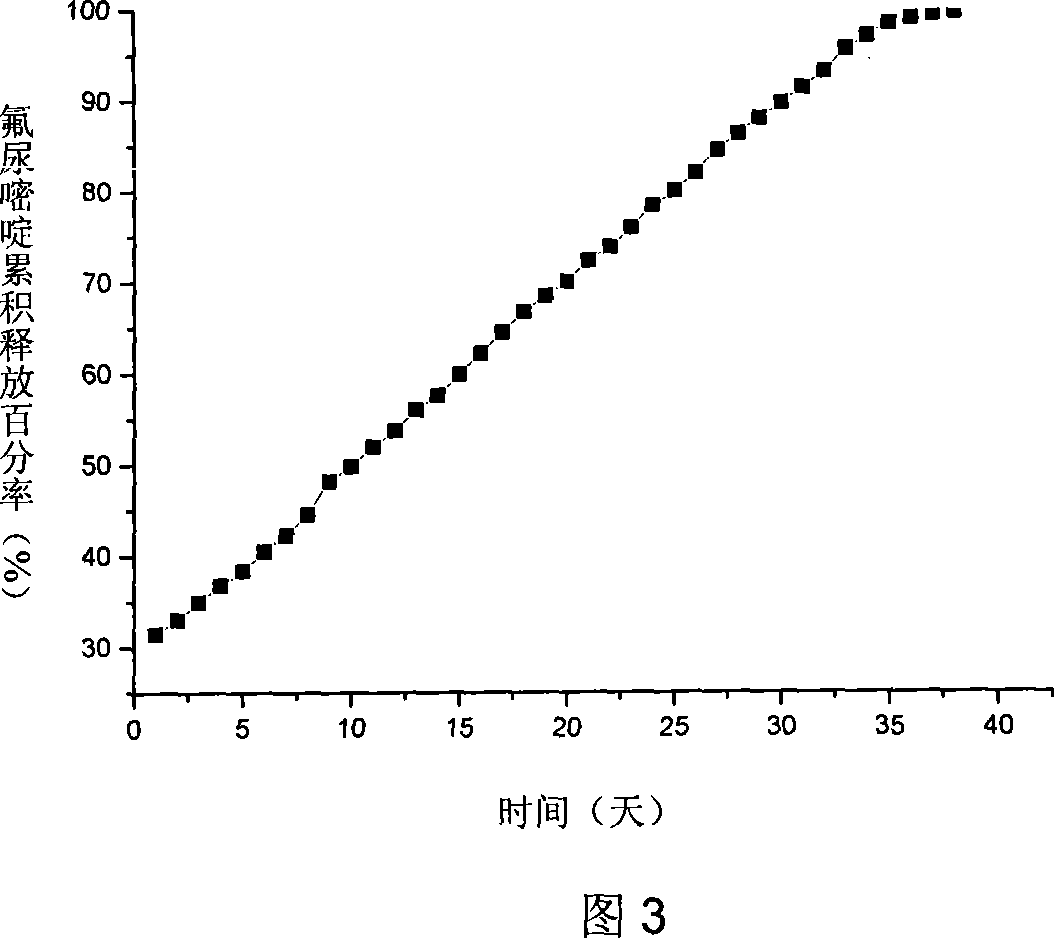
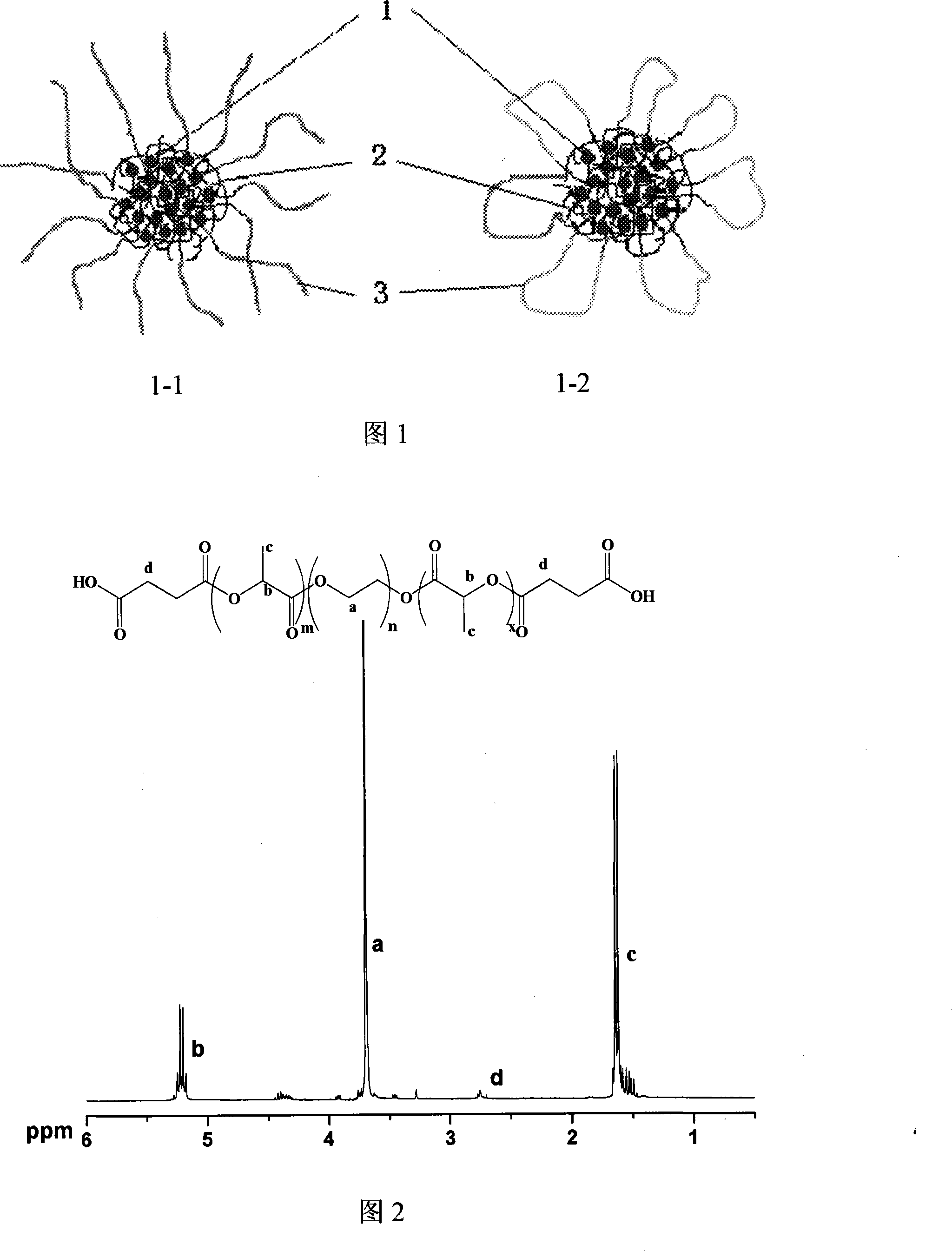
Biodegradable fluorourcacil polyester medicine-carried nanospheres and its preparation method
InactiveCN101053553AHigh drug loadingSmall particle sizeOrganic active ingredientsPowder deliveryPolyesterPolymer science
The invention relates to biodegradable fluorouracil(Fu) polyester drug-bearing manoparticles with a coating material of polylactic acid, polylactic acid-glycolic acid, polylactic acid-polyethylene glycol block copolymer or polylactic acid-glycolic acid-polyethylene glycol block copolymer and the producing method including: firstly, fully dissolving the copolymer in the dichloromethane, under the ultrasonic shock, injecting the fluorouracil NaOH solution in the dichloromethane solution, dispersing uniformly, forming W / O primary latex, and beating up the primary latex and injecting into the fluorouracil saturated water solution containing 5 wt% of polyvinylalcohol (PVA), and storing in the refrigeratory after freeze-dry. The drug-bearing manoparticle has a drug content which is 10-25% of the microparticle mass, and has a smooth surface, an even diameter distribution, a remarkable slow release function and not adhesive. The micropartical size is 100-1000nm.
Owner:JILIN UNIV +1
High molecule bonding adriamycin medicine, nano capsule and preparation thereof
InactiveCN101234204AReduce manufacturing costPlay a role in isolationOrganic active ingredientsPharmaceutical non-active ingredientsPolymer scienceEnd-group
The invention provides a macromolecule bonding adriamycin medicine, a nanometer capsule and a preparation method thereof. The bonding medicine is bonded by a block copolymer of poly ethylene glycol-polylactic acid and adriamycin. Firstly, the block copolymer of poly ethylene glycol-polylactic acid is acquired by ring-opening polymerization of aliphatic cyclic esters with poly ethylene glycol, solvent and catalyst being in existence; then a carboxyl end group is transformed by a hydroxyl-terminated; and then with a condensing agent being in existence, the carboxyl end group carries out amidation reaction with the adriamycin; thus acquiring the macromolecule adriamycin bonding medicine. The bonding medicine has amphiphilic property, thus being able to self assembly to prepare the nanometer capsule with a core-shell structure in the water solution. The adriamycin is wrapped inside the capsule to play a role of isolation and protection, which overcomes the deficiencies of short circulation time in vivo, large dosage and severe allergic reaction existing in the current adriamycin preparation. In addition, the nanometer particles are expected to congregate in the blood circulation through the so-called 'enhanced infiltration and retention effect', so as to improve the targeting action of the adriamycin on the tumor location.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Embolic occlusion of uterine arteries
InactiveUS20040202694A1Decreased blood flowSlow and stop flowSurgical adhesivesFemale contraceptivesWater basedUterine Disorder
A treatment procedure is disclosed which involves the short term, non-permanent occlusion of the patient's blood vessels by depositing a bioabsorbable embolic mass within the patient's blood vessel. The procedure is particularly suitable for treating uterine disorders by occluding a patient's uterine arteries. A therapeutically effective time period for occlusion of a uterine artery is from about 0.5 to about 48 hours, preferably about 1 to about 24 hours, with occlusion times of about 1 to about 8 hours being suitable in many instances. The embolic mass may bioabsorbable particulate with minimum transverse dimensions of about 100 to about 2000 micrometers, preferably about 300 to about 1000 micrometers. The particulate may be a polymeric material formed of polylactic acid, polyglycolic acid or copolymers thereof, or a swellable copolymer of lactic acid and polyethylene glycol. The embolic material may be delivered to an intracorporeal site as a biocompatible solution containing a solute which is relatively insoluble in a water based fluid and a solvent which is relatively soluble in the water based fluid, where the solute forms the embolic mass which occludes or partially occludes a body lumen or fills or partially fills a body cavity.
Owner:VASCULAR CONTROL SYST
PH-sensitive polymeric micelles for drug delivery
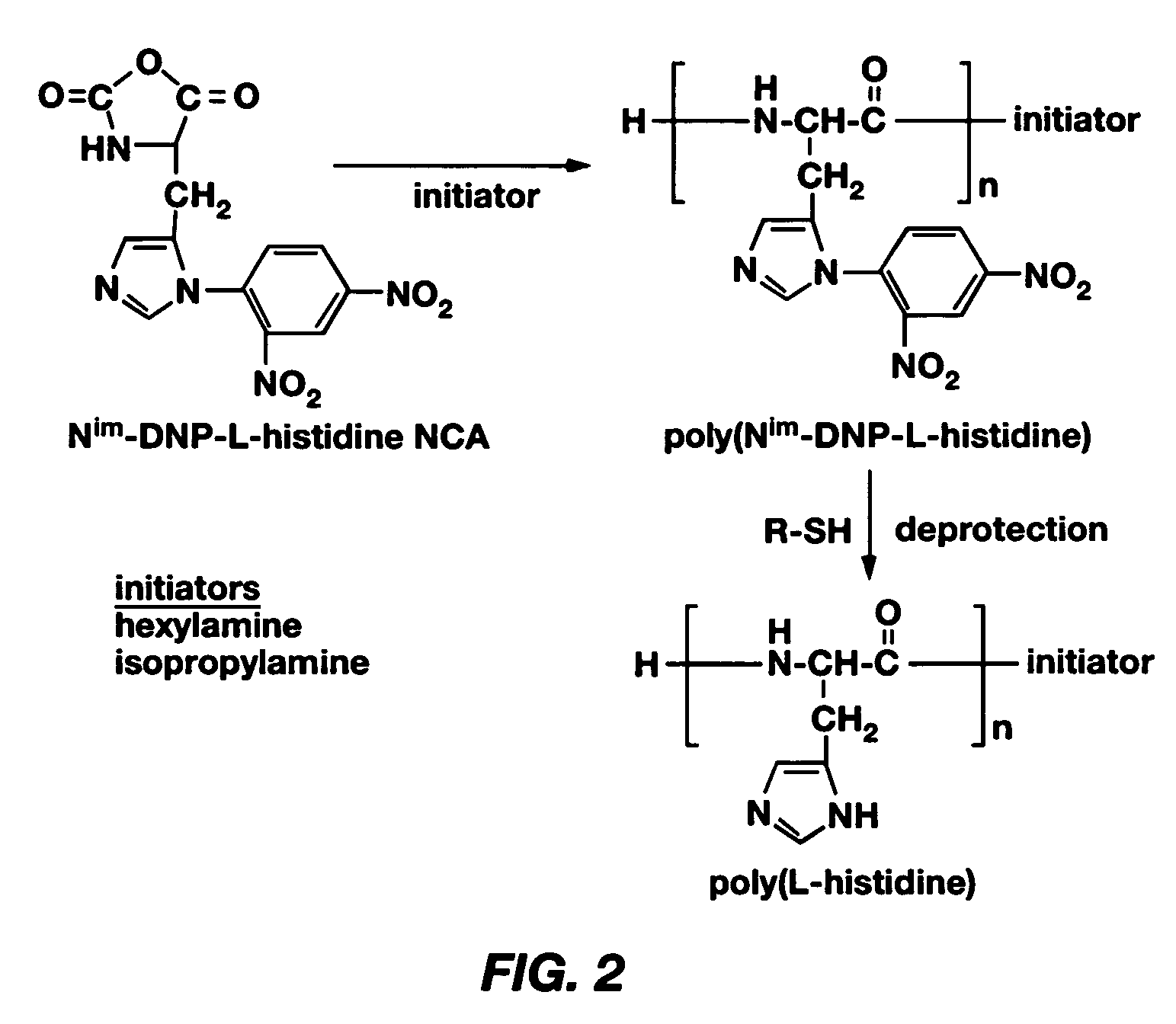
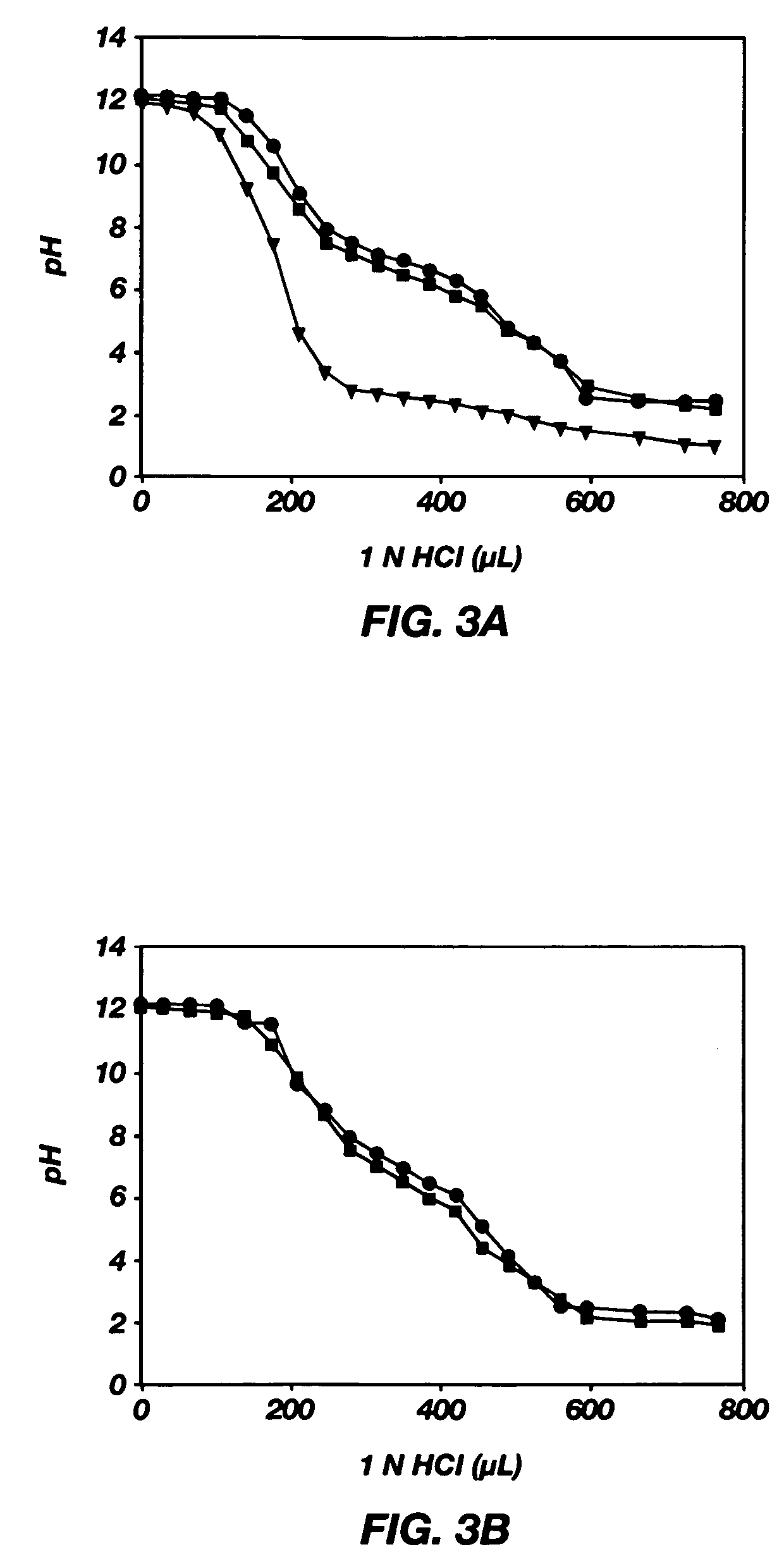
Mixed micelles containing poly(L-histidine)-poly(ethylene glycol) block copolymer and poly(L-lactic acid)-poly(ethylene glycol) block copolymer are a pH-sensitive drug carrier that release the drug in an acidic microenvironment, but not in the blood. Since the microenvironment of solid tumors is acidic, these mixed micelles are useful for treating cancer, including those cancers exhibiting multidrug resistance. Targeting ligands, such as folate, can also be attached to the mixed micelles for enhancing drug delivery into cells. Methods of making poly(L-histidine), synthetic intermediates, and block copolymers are also described.
Owner:UNIV OF UTAH RES FOUND
Crosslinked hydrogel copolymers
The present invention relates to crosslinked polymers, synthesized through ring-opening polymerization of ethylenically unsaturated epoxides, in combination with α-hydroxy acids using a hydrophilic macroinitiator, such as poly(ethylene glycol), to form substituted copolymers having ethylenically unsaturated functionality randomly distributed along the polyester polymer backbone. That copolymer is subsequently crosslinked to form a hydrogel network. More particularly, the present invention relates to the synthesis of biodegradable poly(α-hydroxy acid-co-glycidyl methacrylate)-block-poly(ethylene glycol)-block-poly(α-hydroxy acid-co-glycidyl methacrylate) copolymers, which are subsequently crosslinked to form hydrogel networks. The invention also relates to the use of these hydrogel networks in various applications, in particular, for the controlled release of drugs and proteins.
Owner:ALKERMES INC
Topical Compositions
InactiveUS20080317684A1Maintain good propertiesAntibacterial agentsBiocidePolyethylene glycolEthanol
Topical compositions are disclosed that are useful for delivering a therapeutic level of an NSAID to a target within a subject having a local inflammatory disorder. A composition of the present invention comprises a Drug and a solvent system, wherein the solvent system comprises at least two solvent alcohols and wherein the solvent system is present in an amount sufficient to solubilize the Drug, the solvent system is a low alkanol system, and the composition is a single phase composition. Exemplary solvent systems are those for which one of the at least two solvent alcohols is polyethylene glycol, glycerin, butylene glycol, dipropylene glycol, propylene glycol, ethanol, isopropanol, or a derivative thereof. Optionally the local inflammatory disorder is pseudofolliculitis barbae, dermatitis, psoriasis, wounds, or sunburn.
Owner:ISW GRP INC
Low-viscosity opacifiers free from anionic surface-active agents
The invention relates to wax-based opacifier preparations containing (a) at least one amphoteric surfactant, (b) at least one fatty acid partial glyceride, (c) at least one fatty acid polyglycol ester and optionally (d) a polyol, with the proviso that the ratio by weight of (a) to (b) is between 10:1 and 4:1 and the ratio by weight of (b) to (c) is between 1:1 and 5:1 and the preparations are free from anionic surfactants.
Owner:COGNIS DEUT GMBH & CO KG
Preparation of polycarboxylic acid water reducing agent for prefabricated part
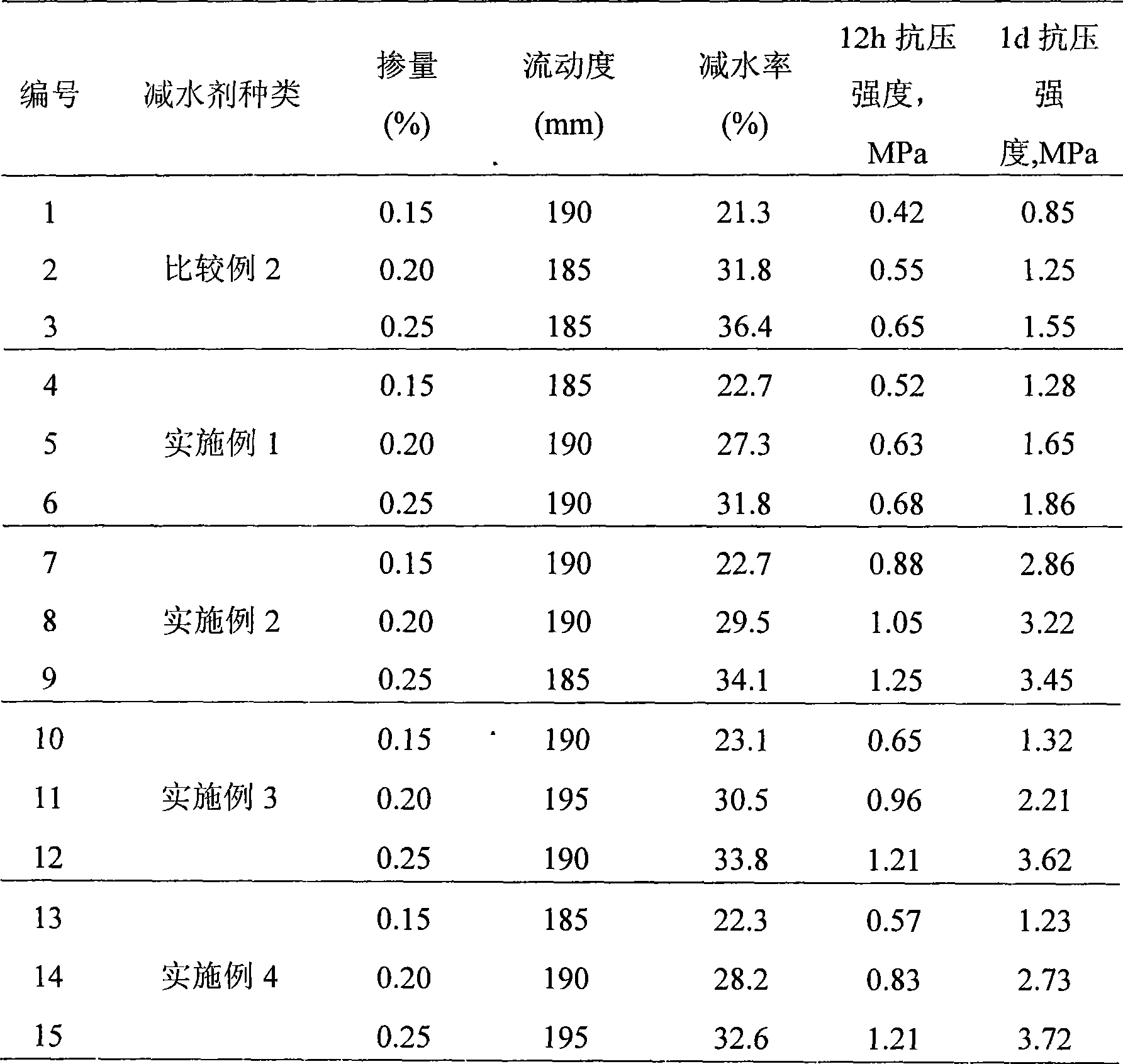
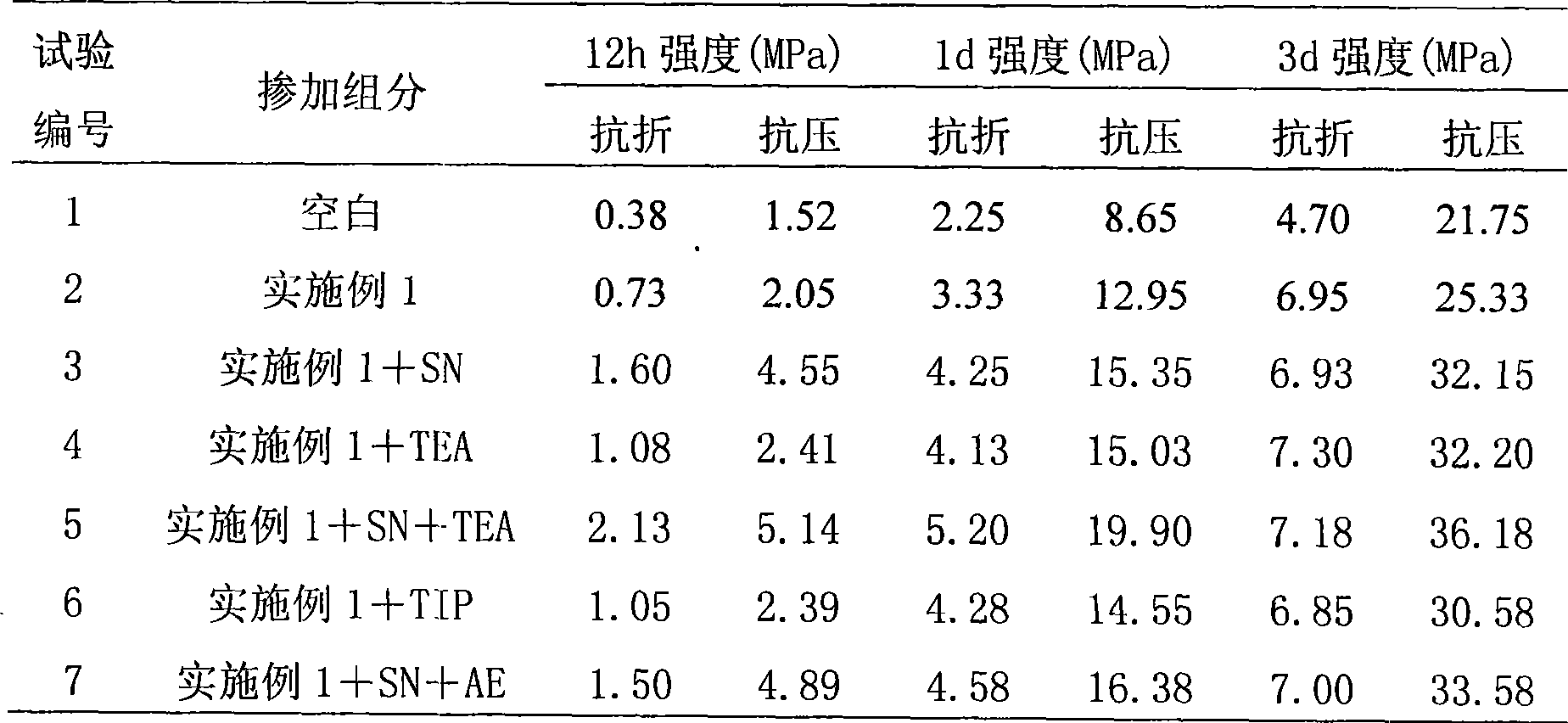
The invention discloses a preparation method for a polyocarboxy acid water reducing agent for precast units, which belongs to the field of water reducing agent. Under the action of a persulphate initiator, methoxypolyethylene glycol acrylate, acrylic acid, unsaturated sulfonic acid or a salt monomer b thereof, unsaturated amide or a salt monomer c thereof are copolymerized in an aqueous solution; a terminator is used to process the rest initiator of the reaction at a later polymerization stage; and finally alkaline solution is used for neutralization and the water reducing agent is obtained. The methoxypolyethylene glycol with high polymerization degree is introduced as a grafting side chain, thereby making the prepared water reducing agent effectively shorten the concrete coagulation time without reducing the concrete initial fluidity and improving the concrete compression strength. The early strength type polyocarboxy acid high performance water reducing agent can be widely applied to the construction of various concrete projects with a low temperature or early strength requirements, in particular to the low energy consumption production process of precast concrete units. The water reducing agent has wide generalization space.
Owner:HUIZHOU JIANKE IND
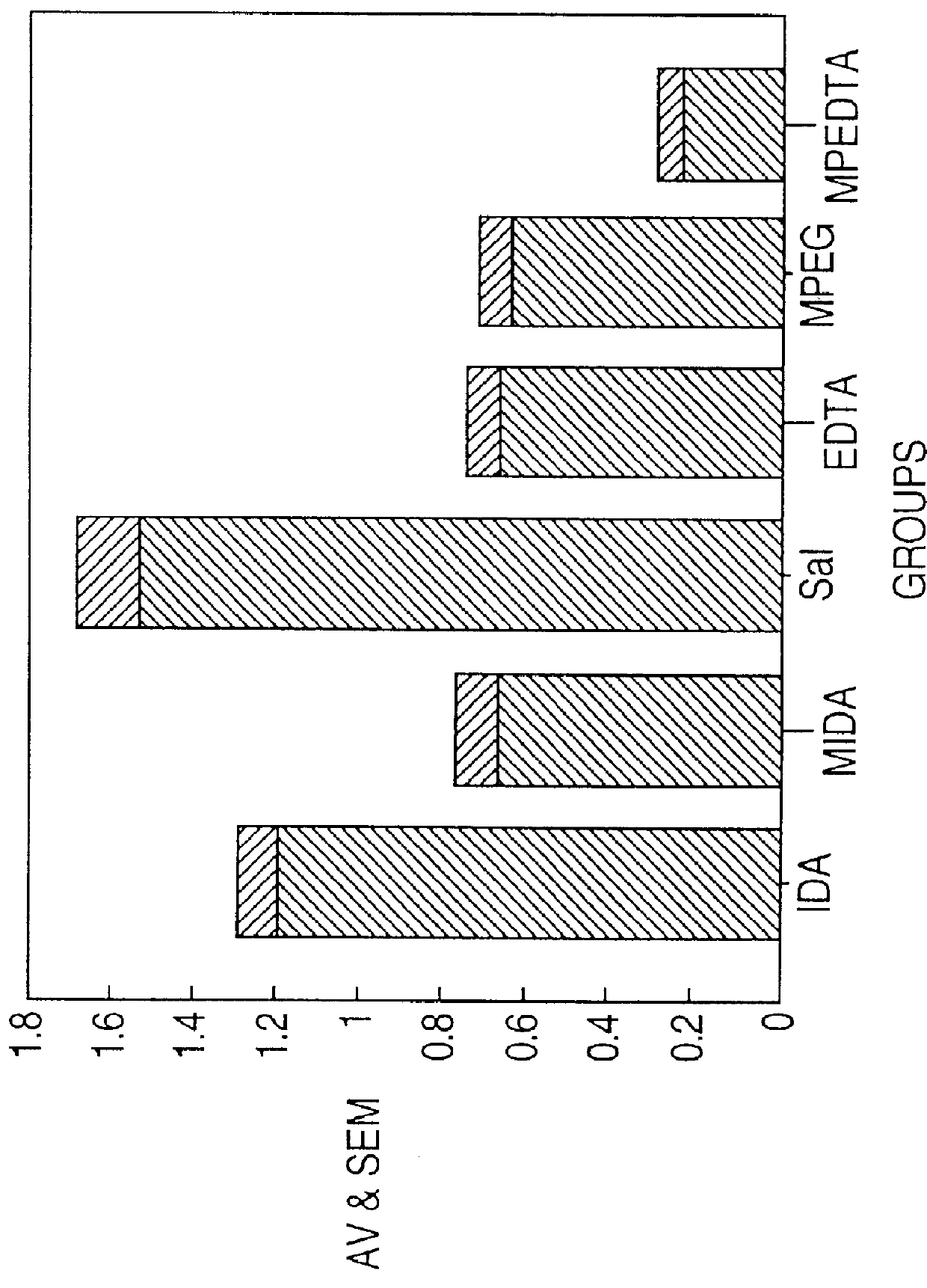
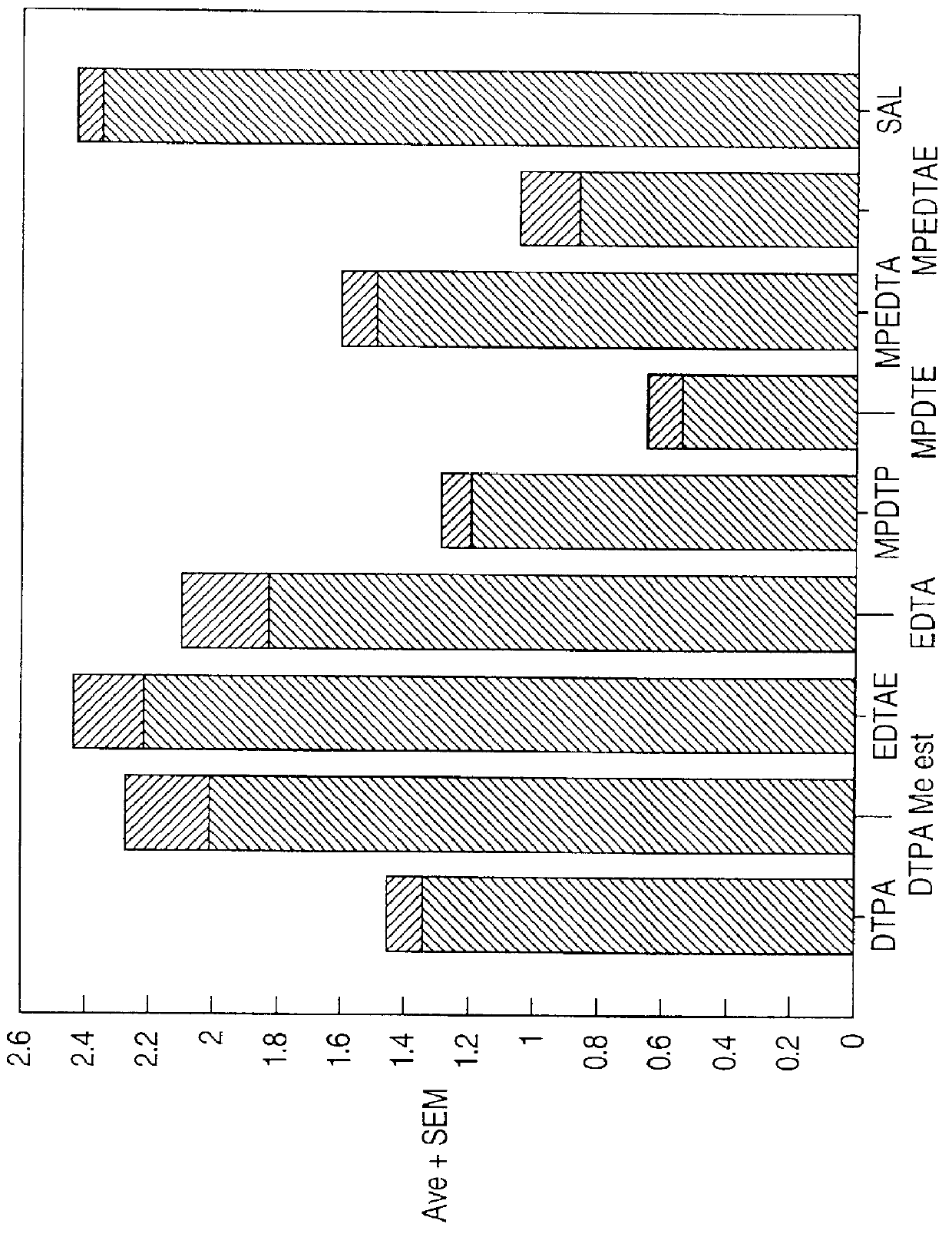
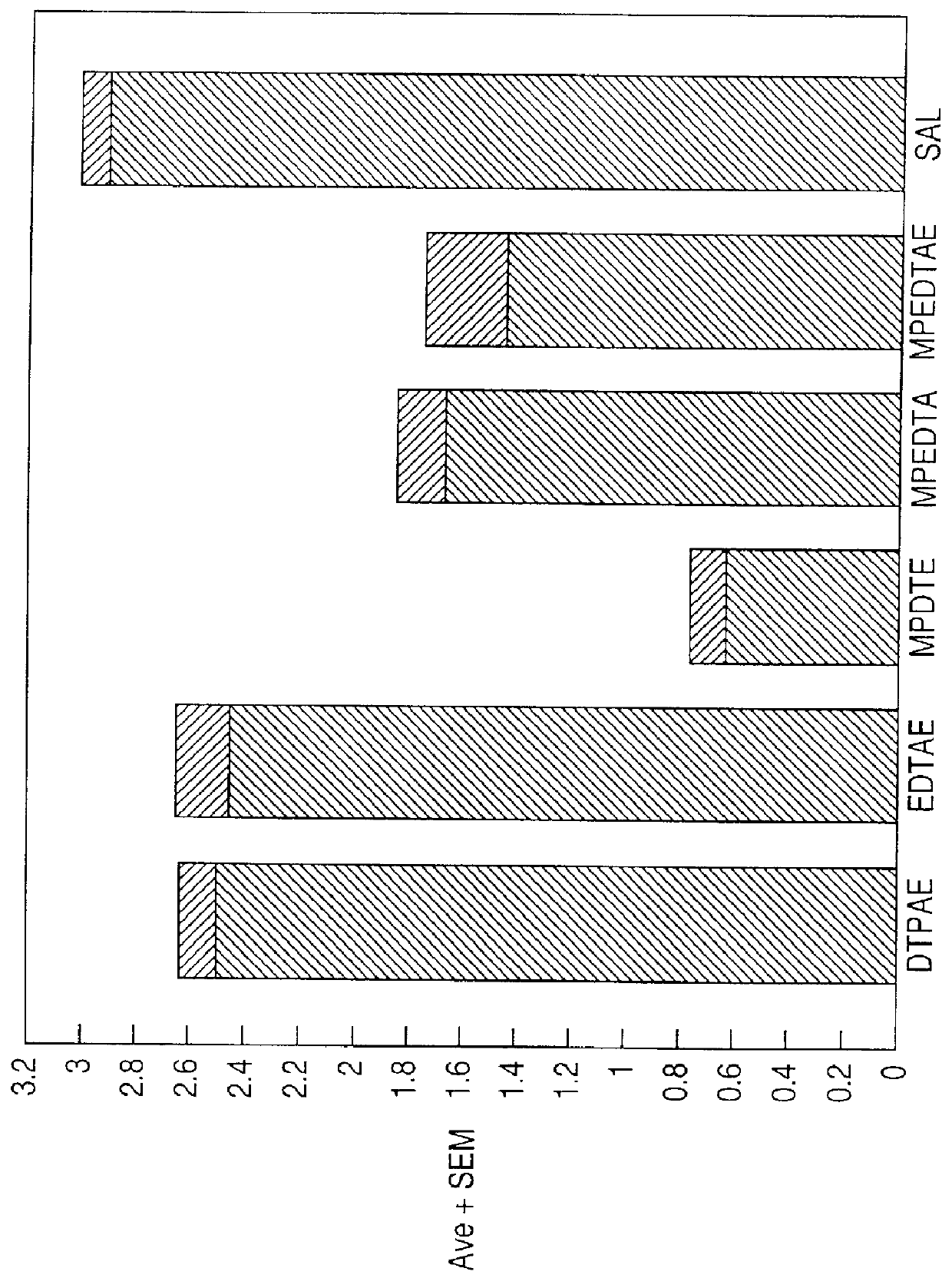
Chelate derivatives as protectors against tissue injury
Derivatives useful in the protection of living organisms against damage due to free radical reactions derived from methoxypolyethylene glycols (MPEG), which are modified by chemically attaching chelating groups in an amide or amine linkage to the nonmethyl end of the polymer. Such chelating groups include ethylene-diamine tetraacetic acid (EDTA), diethylene triamine pentaacetic acid (DTPA), and ethylene glycol aminoethyl ether tetraacetic acid (EGTA), and pharmacologically acceptable salts or esters thereof.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
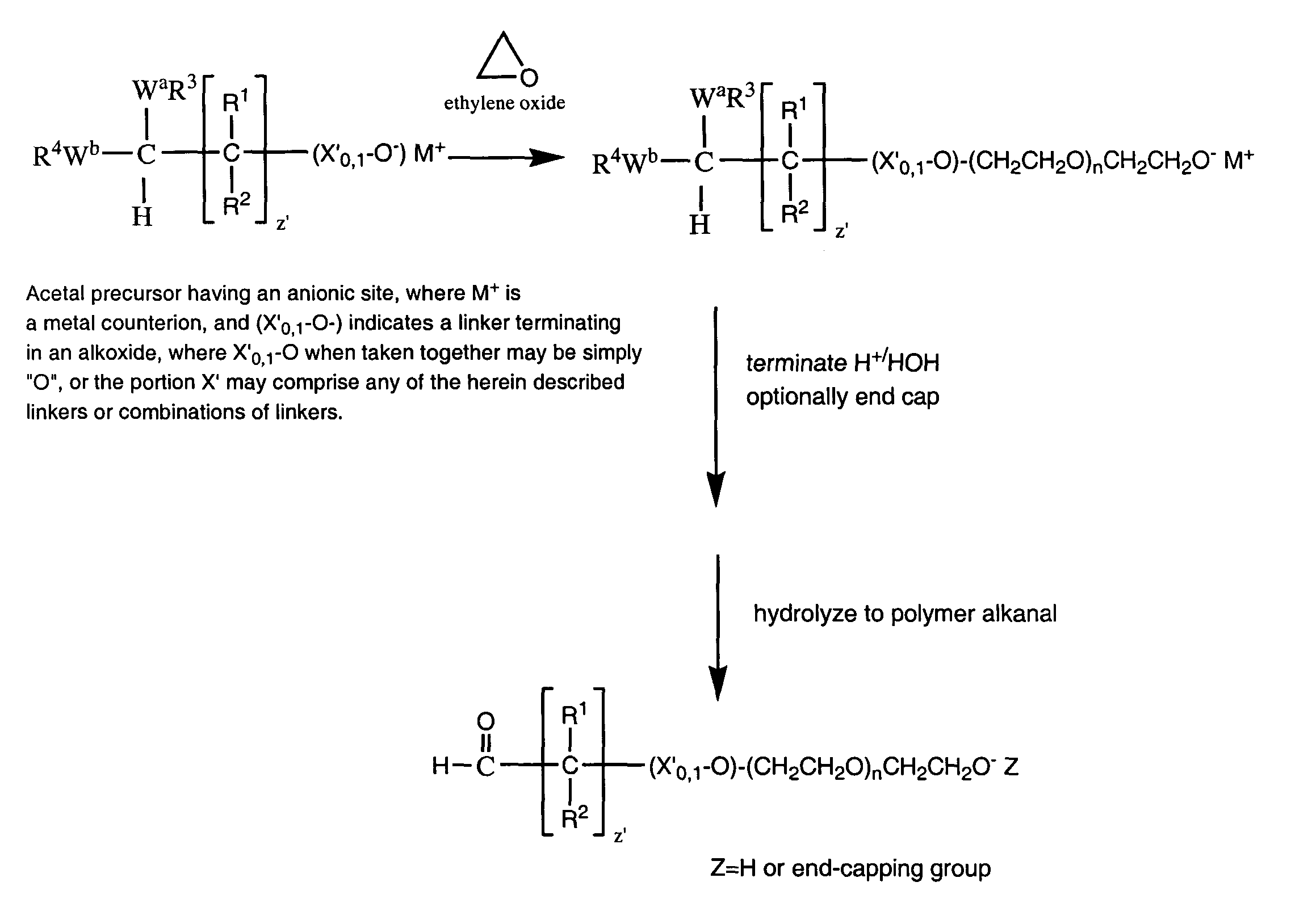
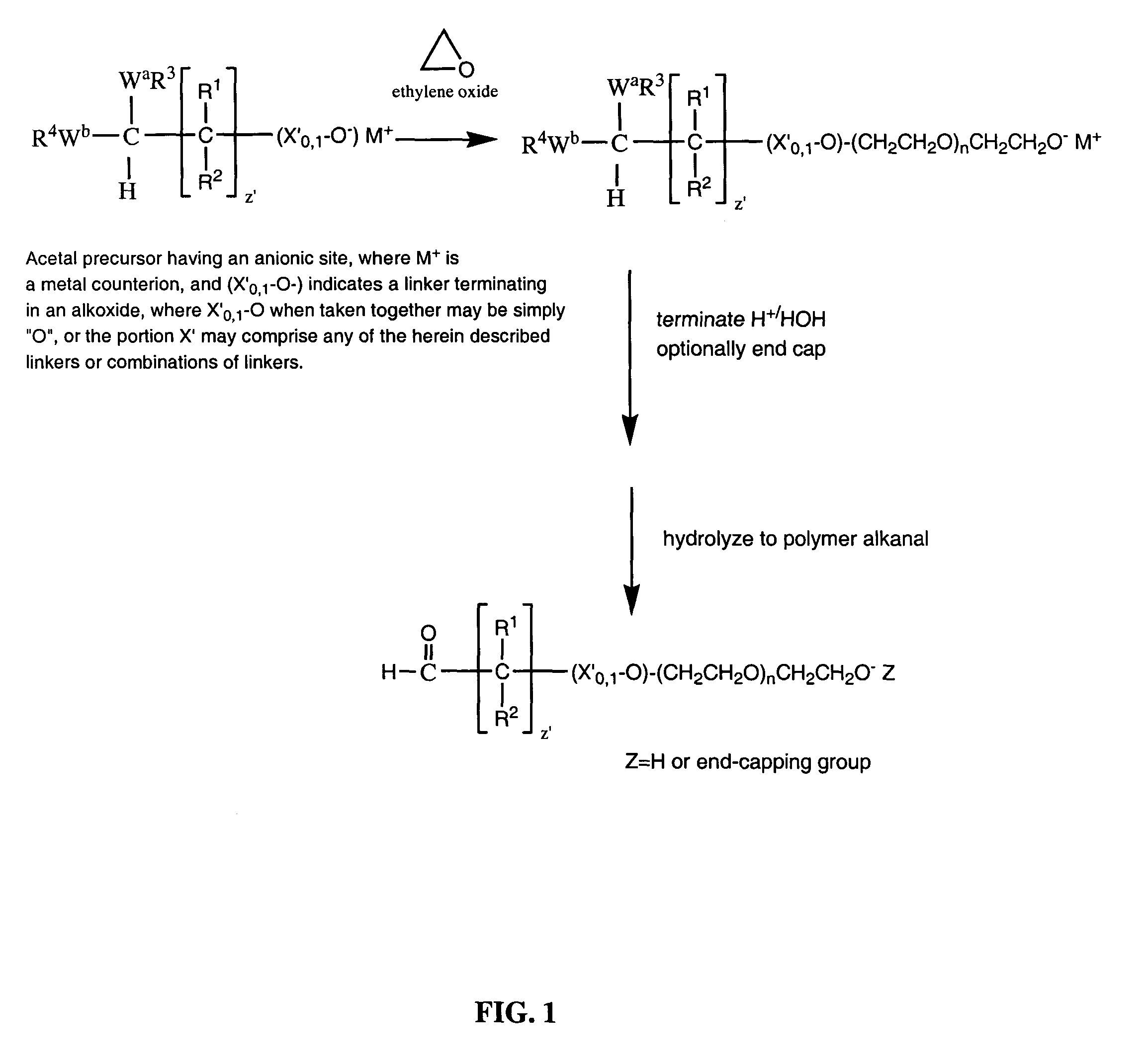
Water-soluble polymer alkanals
ActiveUS7157546B2Less reactiveHigh yieldPeptide/protein ingredientsOrganic compound preparationPolymer scienceWater soluble
The present invention is directed to alkanal derivatives of water-soluble polymers such as poly(ethylene glycol), their corresponding hydrates and acetals, and to methods for preparing and using such polymer alkanals. The polymer alkanals of the invention are prepared in high purity and exhibit storage stability.
Owner:NEKTAR THERAPEUTICS INC
Mono-functionalized polyethylene glycol with nitrogen atom branched center and its preparation method and biologically-relevant matter
ActiveCN104109235ARich varietyShort manufacturing processPharmaceutical non-active ingredientsOxidation-Reduction AgentNitrogen
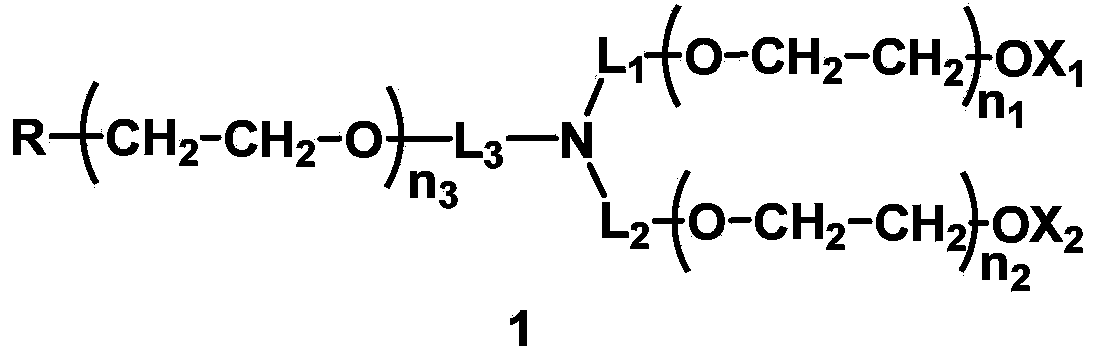
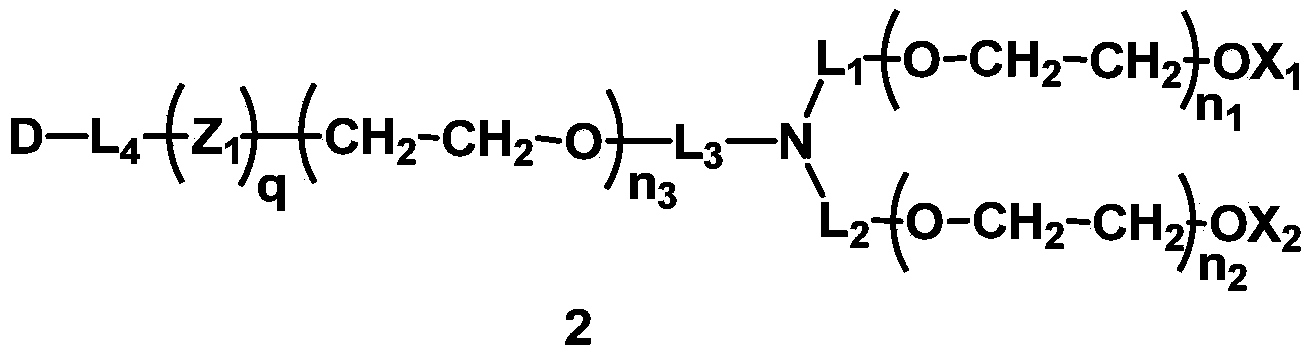
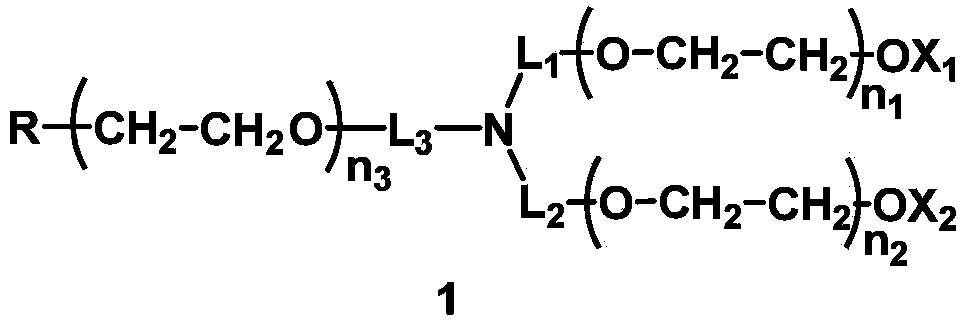
The invention discloses a mono-functionalized polyethylene glycol with a nitrogen atom branched center and its preparation method and biologically-relevant matter. The mono-functionalized polyethylene glycol with a nitrogen atom branched center is shown in the general formula (1). The polyethylene glycol-modified biologically-relevant matter is shown in the general formula (2). X1 and X2 represent alkyl containing 1-20 carbon atoms, n1 and n2 are integers of 2-2000, n3 is an integer of 1-2000, L1, L2 and L3 represent connection groups stably existing under the conditions of light, heat, enzyme, redox, acidic or alkaline condition, R represents a functional group or is in a protected form, D represents a biologically-relevant matter, Z1 represents a connection group, and L4 represents residue formed by a reaction of R and the biologically-relevant matter. The branched polyethylene glycol with the nitrogen atom center can interact with a substrate easily so that the substrate protection based on polyethylene glycol is promoted, the state of the substrate in the human body can be effectively improved and a prospect is wide.
Owner:XIAMEN SINOPEG BIOTECH
High molecule adriamycin bonding medicine nano capsule with targeting function and preparation thereof
InactiveCN101234205AReduce manufacturing costReduce doseOrganic active ingredientsPharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
The invention provides a nanometer capsule of a macromolecule adriamycin bonding medicine with a targeting function and a preparation method thereof. The nanometer capsule is mixed assembled with two block copolymers of poly ethylene glycol-polylactic acid, wherein the polylactic acid chain end of one block copolymer is connected with adriamycin, with a molar ratio of 98-70 percent in the nanometer capsule, while the poly ethylene glycol chain end of the other block copolymer is connected with lactose, with a molar ratio of 2-30 percent in the nanometer capsule. The adriamycin connected with the polylactic acid chain end is located in the capsule inner core, which is double protected by polylactic acid and poly ethylene glycol and has sustained release function; while the lactose connected with the poly ethylene glycol chain end is located in the outer layer of the capsule, which has targeting function and leads the nanometer capsule preferentially enter the cells carrying the lactose receptors.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Degradable unsaturated polyesteramide resin and synthesis method thereof
The invention adopts a melt polycondensation method. A few of monomers of C2-5 aliphatic dibasic alcohol, diethylene glycol, polyglycol, fumaric acid, maleic anhydride, lactic acid, glycolic acid, ethanolamine, C2 to C12 aliphatic diamine, glutamic acid, lysine, glycine, etc. are taken as basic materials to be synthesized to obtain non toxic unsaturated polyester-amide resin with adjustable degradation rate and lower cost; wherein, the partial fumaric acid or maleic anhydride can be replaced with phthalic anhydride, isophthalic acid, or adipic acid. The resin yearns for being used as matrix resin of medical bone internal fixation material, tissue engineering scaffold material, bone tissue temporary substitutes, environmental protection type bonding agent, environmental protection type fiberglass reinforced plastics, environmental protection type coating material, disposable tableware, packing material, shopping bags, disposable bags, drug coating or capsule, drug delivery (controlled-release) material, agricultural mulching films, etc., and can be recovered to be utilized.
Owner:HUNAN UNIV
Neutral blockage removing agent composition used for oil recovery formation in oilfield and preparation method thereof
ActiveCN104194759AImprove cleanlinessNo pollutionDrilling compositionActivated attapulgitePolythylene glycol
The invention relates to a neutral blockage removing agent composition used for an oil recovery formation in an oilfield. The neutral blockage removing agent composition is prepared from the following raw materials in parts by weight: 30-36 parts of disodium polyepoxysuccinicate, 18-25 parts of sodium gluconate, 2-3 parts of polyethylene glycol, 2-4 parts of triethanolamine, 9-12 parts of ethyl lactate, 7-10 parts of fatty alcohol polyoxyethylene ether sodium sulfate, 8-12 parts of amino acid sodium, 10-15 parts of activated attapulgite, 0.1-0.2 part of vanadium pentoxide, 5-7 parts of lipase, 5-8 parts of sorbitan fatty acid ester, 15-18 parts of P-hydroxy sodium sulfonate, 3-7 parts of tert-butyl hydroperoxide and 0.1-0.3 part of diethylene glycol monolaurate. The neutral blockage removing agent composition has high blockage removing speed, is neutral, is free of corrosion and can quickly dissolve material scales, asphalt sediment scales, carbonate scales, silicate scales and the like generated by an ASP flooding system; waste liquid for blockage removal can be degraded and does not need to be discharged onto the ground to be subjected to sewage treatment, the formation in the oilfield is cleaned to remove the blockage, no corrosion, dead angle, precipitation or secondary well blockage is generated, and the blockage removal time does not exceed 24 hours.
Owner:GANSU HEIMA PETROCHEM ENG
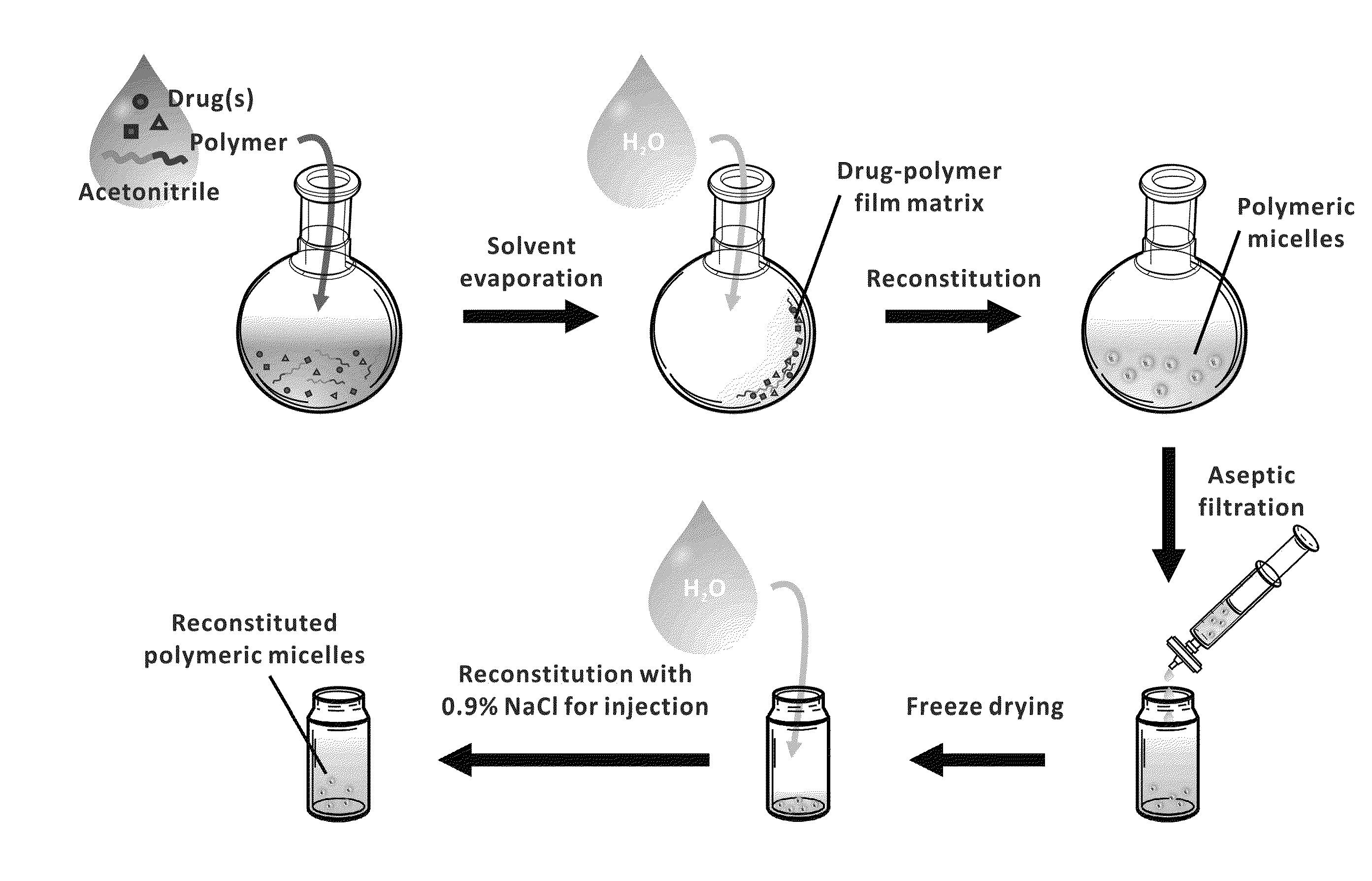
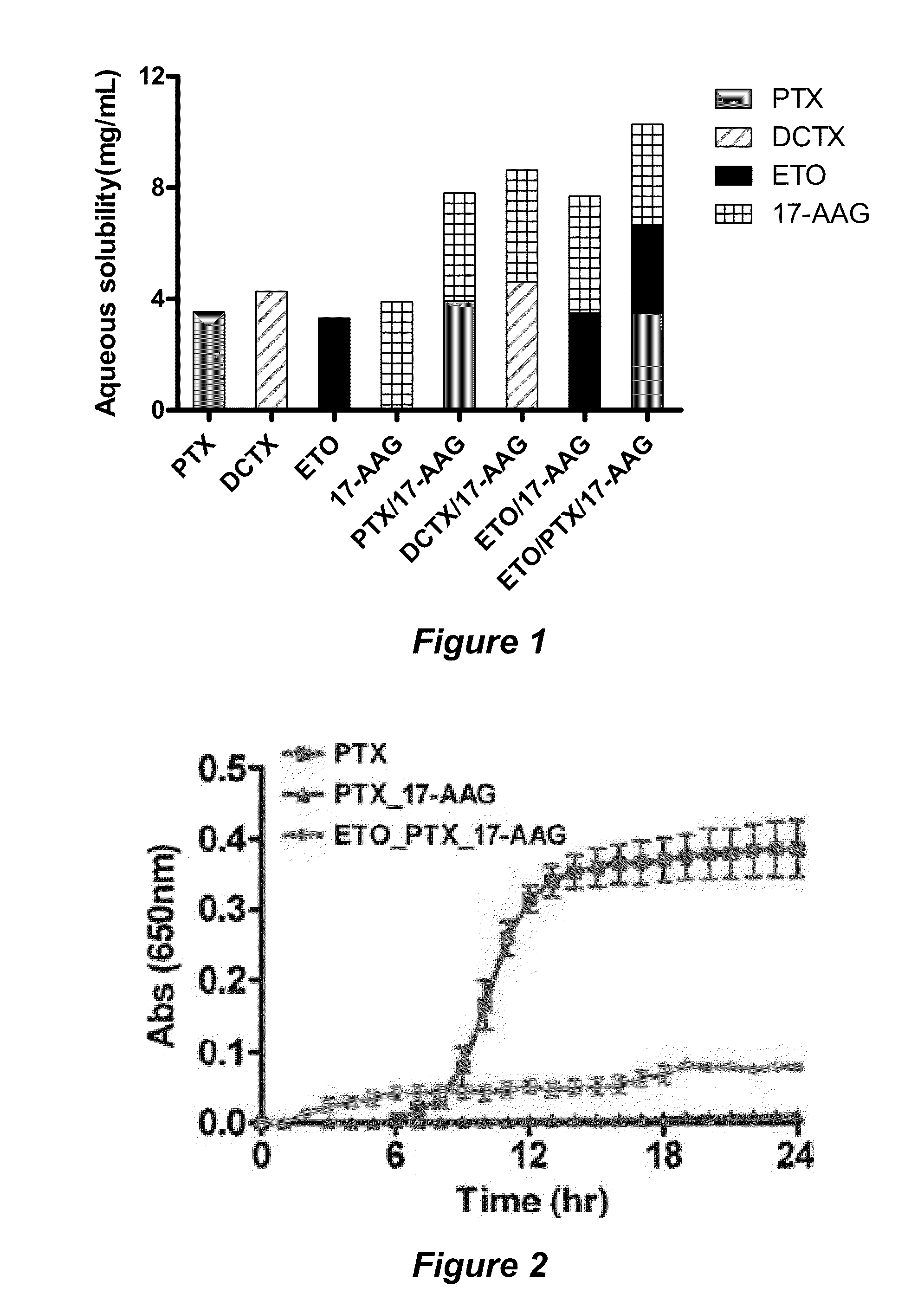
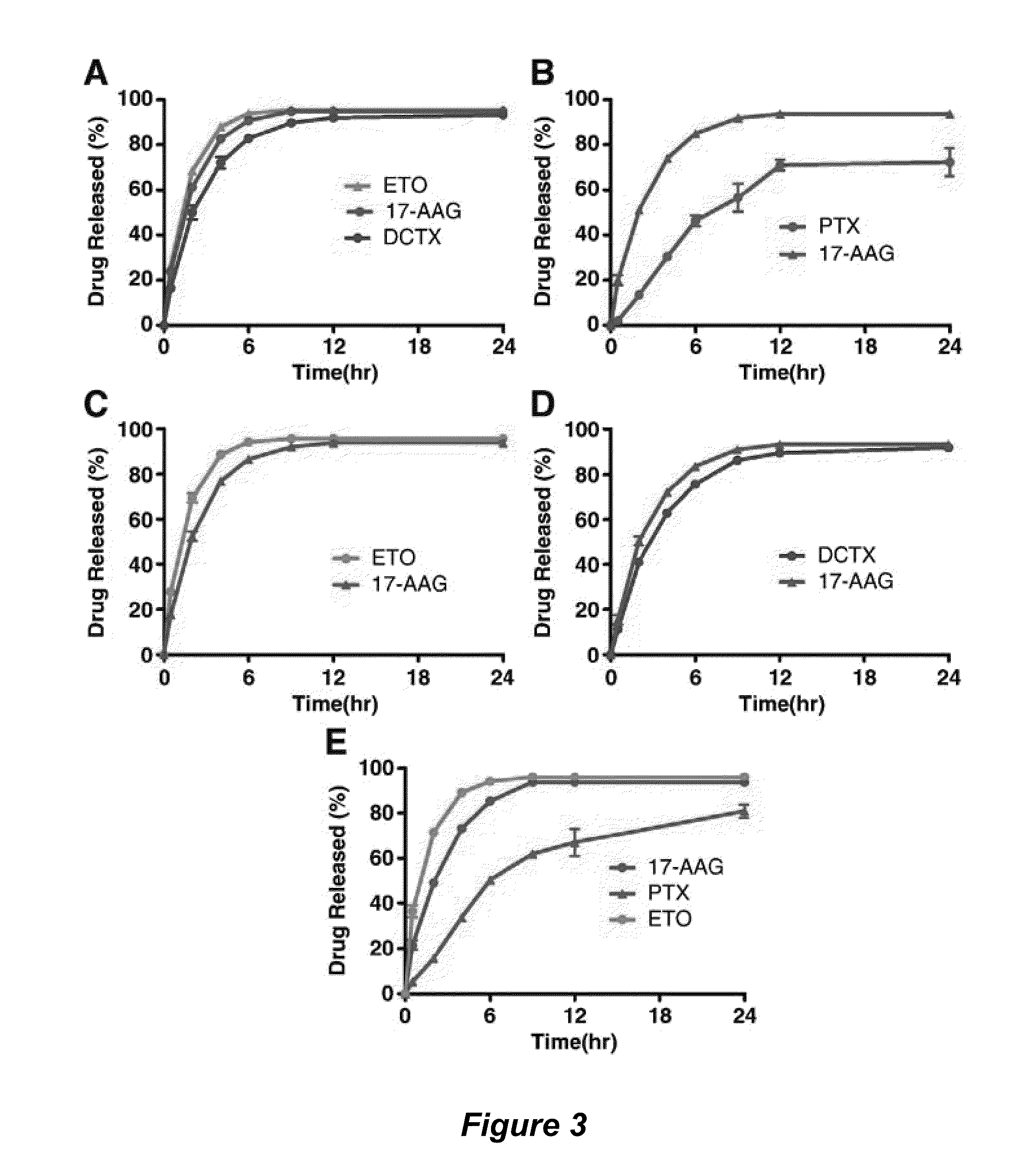
Micelle encapsulation of therapeutic agents
ActiveUS20110076308A1Effective dissolutionToxic reductionBiocideDispersion deliveryLarge doseActive agent
The invention provides active agents, such as paclitaxel, rapamycin, or 17-AAG, encapsulated by safe poly(ethylene glycol)-block-poly(lactic acid) (“PEG-b-PLA”) micelles. The compositions provide effective solubilization of drug combinations, such as paclitaxel, rapamycin, and 17-AAG, as well as others described herein. A significant advantage of PEG-b-PLA as a carrier is that it is less toxic than Cremophor® EL or DMSO, which are used in currently known compositions. Additionally, PEG-b-PLA micelles are easier to handle than DMSO and they do not possess a foul odor, which is a problem with formulations currently in clinical trials. Accordingly, the invention provides stable and biocompatible drug formulations that improve bioavailabilty without causing toxicity. It was also found that larger doses of individual drugs in micelle formulations can be administered compared to non-micelle formulations.
Owner:WISCONSIN ALUMNI RES FOUND
Preparation method of enhanced polyacrylonitrile hollow fiber membrane
InactiveCN102580577AImprove mechanical propertiesExtended service lifeSemi-permeable membranesMembranesHollow fibre membranePolymer science
The invention discloses a preparation method of an enhanced polyacrylonitrile hollow fiber membrane. The preparation method comprises the following steps of: (1) knitting a polyacrylonitrile fiber reinforcement body: knitting a polyacrylonitrile fiber hollow knitted tube by a two-dimensional knitting technology, wherein the polyacrylonitrile fiber hollow knitted tube is used as a reinforcement body of the hollow fiber membrane; (2) preparing polyacrylonitrile membrane casting liquid, wherein the polyacrylonitrile membrane casting liquid consists of the following components in percentage by mass: 3-25% of polyacrylonitrile resin, 50-95% of solvent and 2-30% of additive; (3) performing surface pretreatment of the reinforcement body: wetting the hollow knitted tube with low-polarity organic liquid for 1-60 seconds, wherein the low-polarity organic liquid is ethanol, glycerol, isopropyl alcohol or polyethylene glycol-600; and (4) preparing a hollow fiber membrane: co-extruding the hollow knitted tube and the membrane casting liquid through an annular spinning nozzle, and performing sufficient solidification in a coagulating bath to obtain the enhanced polyacrylonitrile hollow fiber membrane.
Owner:TIANJIN POLYTECHNIC UNIV
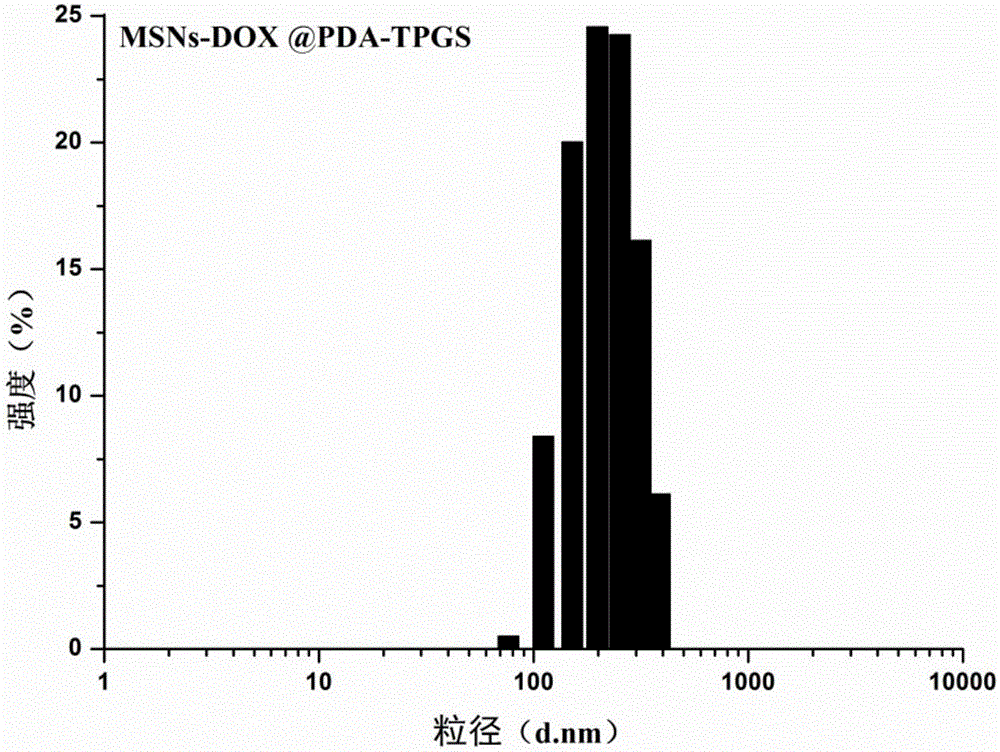
Polydopamine and polyethylene glycol vitamin E succinate-modified mesoporous silica nanoparticle as well as preparation method and application thereof
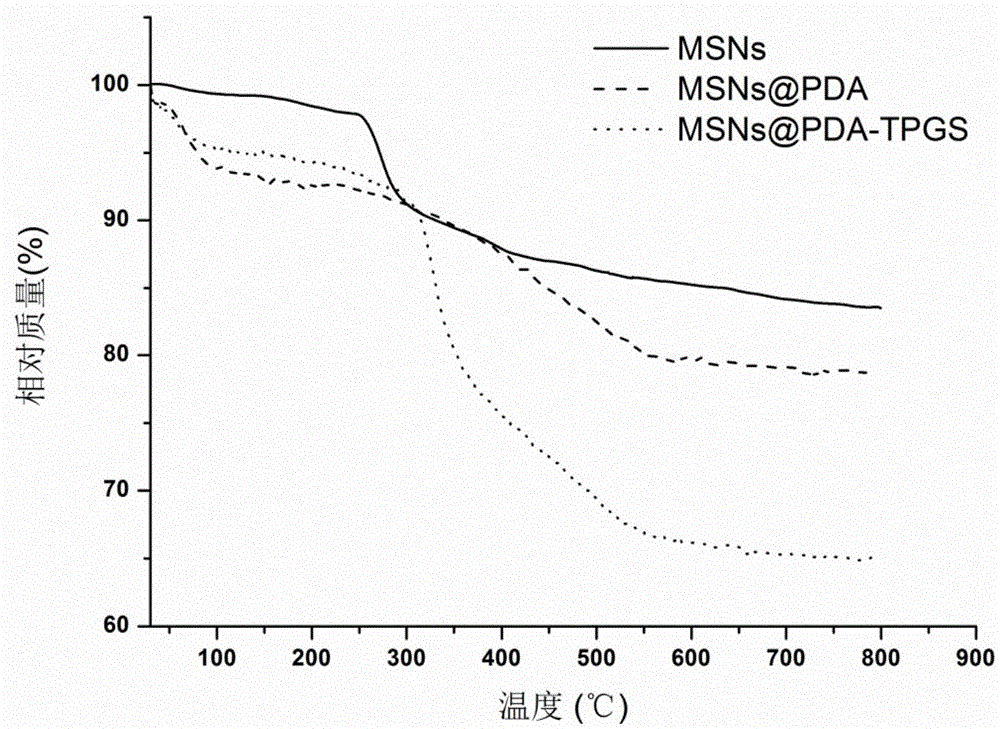
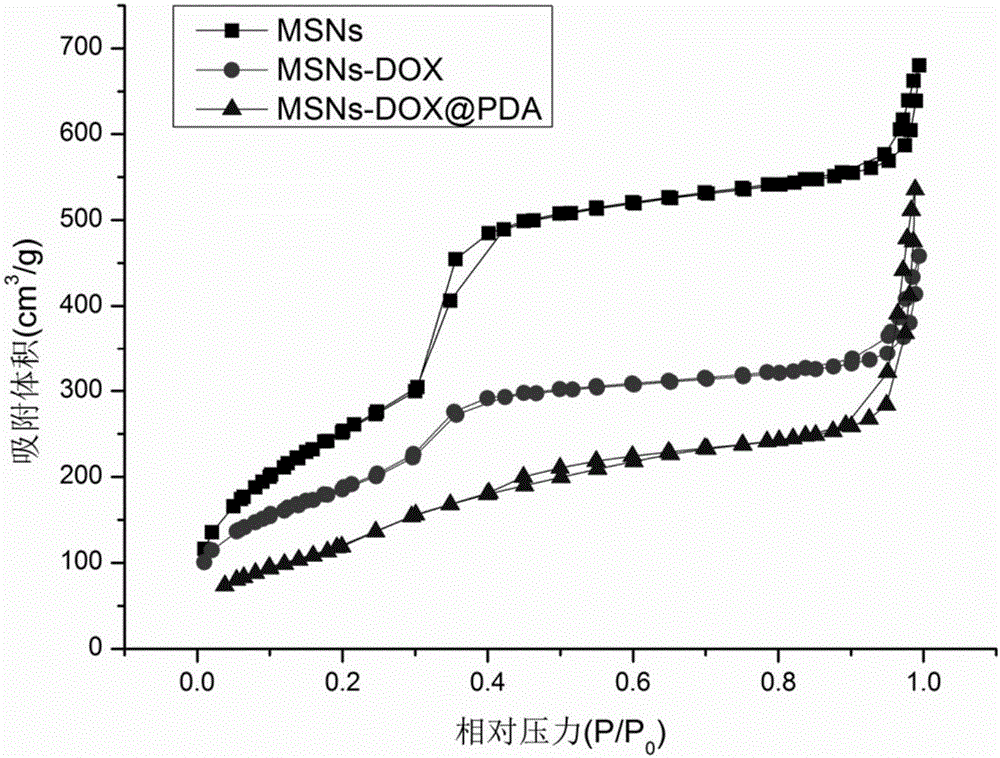
ActiveCN106806344AEasy to prepareGood biocompatibilityPowder deliveryHeavy metal active ingredientsTumor targetingPolythylene glycol
The invention provides a polydopamine and polyethylene glycol vitamin E succinate-modified mesoporous silica nanoparticle as well as a preparation method and application thereof. Specifically, the preparation method of the polydopamine and polyethylene glycol vitamin E succinate-modified mesoporous silicon dioxide nanoparticle comprises the following steps: 1) dissolving mesoporous silicon dioxide and a drug into a solvent, reacting to be complete and separating to obtain a drug-loaded mesoporous silica initial nanoparticle; 2) adding the initial nanoparticle into a solution, adding dopamine hydrochloride, reacting to be complete and separating to obtain a drug-loaded polybutadiene-wrapped mesoporous silica nanoparticle; 3) adding the polybutadiene-wrapped mesoporous silica nanoparticle into a weak base aqueous solution, adding polyethylene glycol 1000 vitamin E succinate, reacting to be complete and separating to obtain a tumor targeting mesoporous silica nanoparticle. The nanoparticle provided by the invention is simple in preparation method, free from pollution and good in biocompatibility and biodegradability, and has a treatment effect on a lung cancer.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Green preparation method of slow-release polycarboxylic-acid high-performance water reducing agent
The invention relates to a green preparation method of a slow-release polycarboxylic-acid high-performance water reducing agent, which comprises the following steps: firstly, dissolving unsaturated polyethenoxy ether in water, adding an oxidant solution, and evenly stirring; then, respectively and dropwisely adding a reducer / chain-transfer agent mixed solution and a comonomer solution, and polymerizing; and finally, adding an alkaline regulator to neutralize and age the solution, thereby obtaining the slow-release polycarboxylic-acid high-performance water reducing agent. In the invention, hydrogen on the terminal hydroxyl group of the original unsaturated polyethenoxy ether is substituted by an allyl group containing unsaturated bond, so that the terminal group of methoxypolyethylene glycol is converted from the original active hydroxyl group into the inert methoxy group at present; and thus, the byproducts of the copolymerization product are greatly reduced, and the molecular weight distribution is narrow, so that the synthesized water reducing agent has the advantages of high water reduction rate and slump resistance. The redox system with low activation energy is used for initiating the monomer copolymerization, so that the polymerization can be implemented at normal temperature, thereby implementing the production of the product by a no heat source method, simplifying the production equipment and lowering the energy consumption and cost.
Owner:NANJING RUIDI HIGH TECH
Curable composition containing reactive (METH) acrylate polymer and cured products thereof
InactiveUS20110077334A1Excellent surface hardnessIncrease flexibilityPlastic/resin/waxes insulatorsPolyureas/polyurethane adhesivesEthyl groupAcrylate polymer
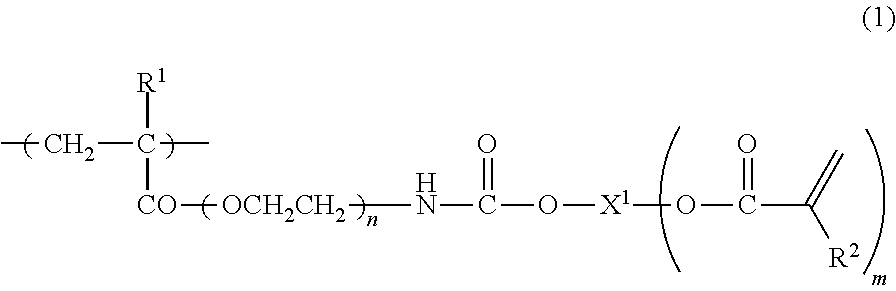
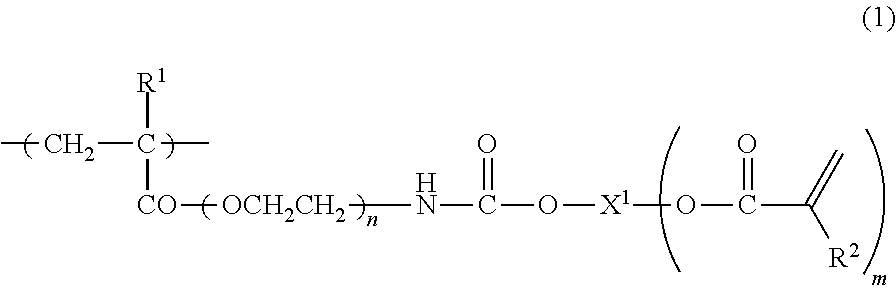
It is an object of the present invention to provide a curable composition capable of forming a heat-resistant cured film which is excellent in surface hardness, is good in flexibility and bending properties, and has strength and flexibility that are compatible with each other, and a cured product (film) of the composition. The curable composition includes a reactive (meth)acrylate polymer (A) having a monomer unit represented by the following formula (1), a polymerization initiator (B) and a reactive monomer(C):wherein R1 is a hydrogen atom, a methyl group or an ethyl group, R2 is a hydrogen atom or a methyl group, X1 is a straight-chain or branched hydrocarbon group of 2 to 6 carbon atoms or an alcohol residue of polyethylene glycol, polypropylene glycol or caprolactone-modified both-terminal diol, n is an integer of 2 to 4, and m is an integer of 1 to 5.
Owner:SHOWA DENKO KK
Process for preparing SBA-15 surfical sulfonic group modified mesoporous molecular sieve
InactiveCN1736598AEasy to operateEasy to manufactureMolecular sieve catalystsMolecular sievePolyethylene glycol
Disclosed is a method for preparation of molecular sieve modified by SBA- 15 surface sulfuric acid group, which contains the step: with polymer of the polyethylene glycol- polyglycerol- polyethylene glycol as the template agent, 3-mercaptopropyl-trimethoxysilane condensing ethyl orthosilicate in a acid condition, and by the oxidization of hydroperoxide, the molecular sieve modified by propanesulfonic acid being synthesized. The method for preparation is simple, the operation is easy, and the reaction condition is mild; the acid containing and the structure of the molecular can be regulated and controlled by regulating the matching of materials and processing parameter, the acid quantity being among 0.48- 1.15mmolH+ / g, specific surface being among 445- 680m2 / g, and the pore diameter being among 5.47- 7.58nm.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Preparation method for high-slump-retention polycarboxylate water reducer
InactiveCN103450411AIncreased steric hindranceSolve problems such as difficult pumpingMeth-Ptru catalyst
The invention discloses a preparation method for a high-slump-retention polycarboxylate water reducer. The preparation method comprises the following steps of: esterifying methoxylpolyethylene glycol monomethyl ethers with different molecular weights with methacrylic acid and a mixed polymerization inhibitor for a period of time under the action of a catalyst and at a certain temperature, then adding allyl alcohol in one time, further esterifying in the same conditions to obtain an esterified macromonomer MP containing crosslinking monomer allyl methacrylate; then performing aqueous solution polymerization on the MP, unsaturated sulfonate, (meth)acrylate unsaturated monomers and a chain transfer agent in a low-temperature condition and within a redox initiation system; and finally adding caustic soda liquid to neutralize, so as to obtain the polycarboxylate water reducer with a certain concentration. The high-slump-retention polycarboxylate water reducer prepared by the preparation method disclosed by the invention is a high-performance water reducer which is prepared at a low temperature by virtue of a molecular structure design, as well as is high in slump retention, excellent in dispersing performance, good in adaptability, simple in process, pollution-free, low in energy consumption, and easy to realize industrialized production.
Owner:KZJ NEW MATERIALS GROUP CO LTD
Thermogelling polymer blends for biomaterial applications
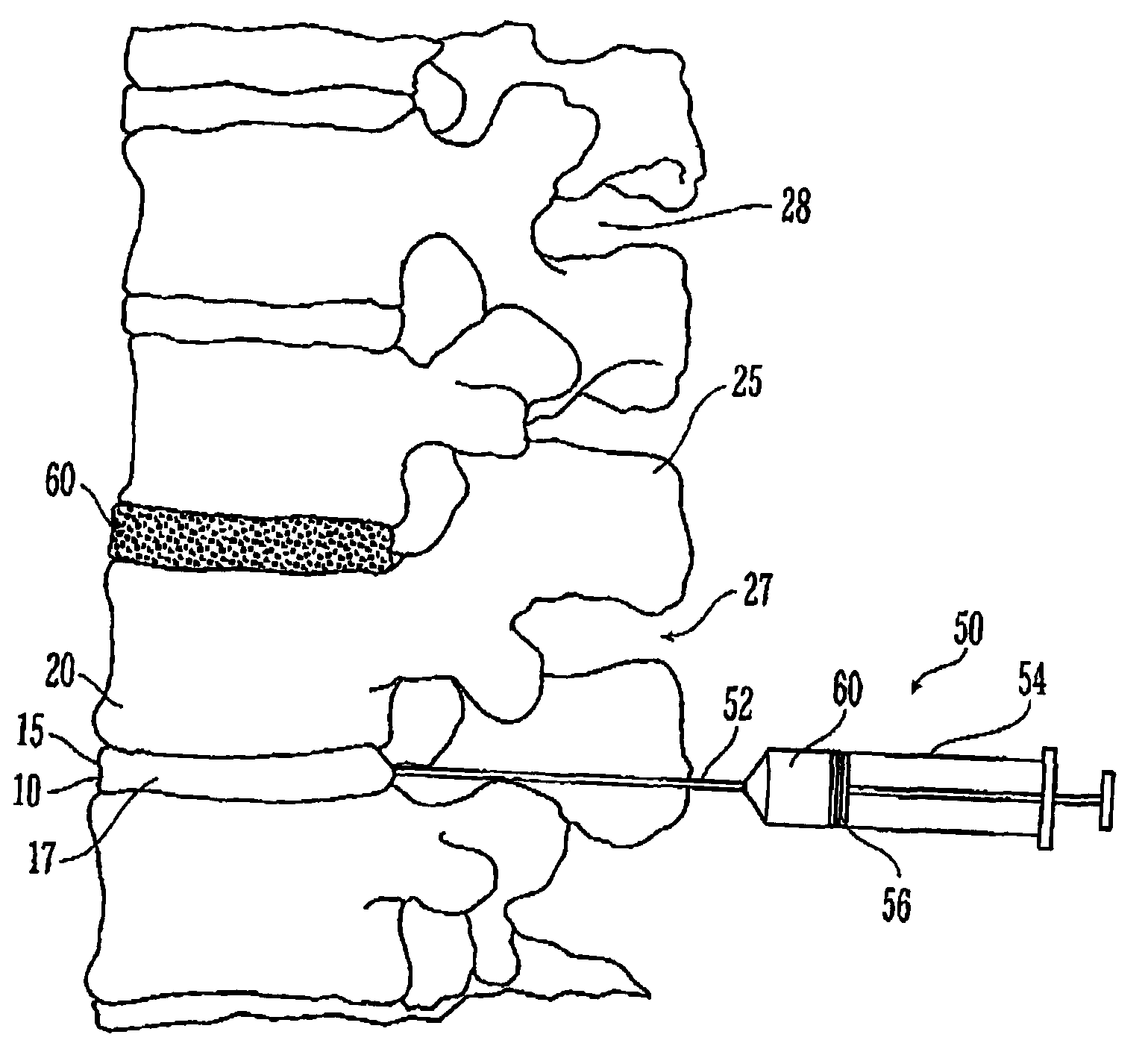
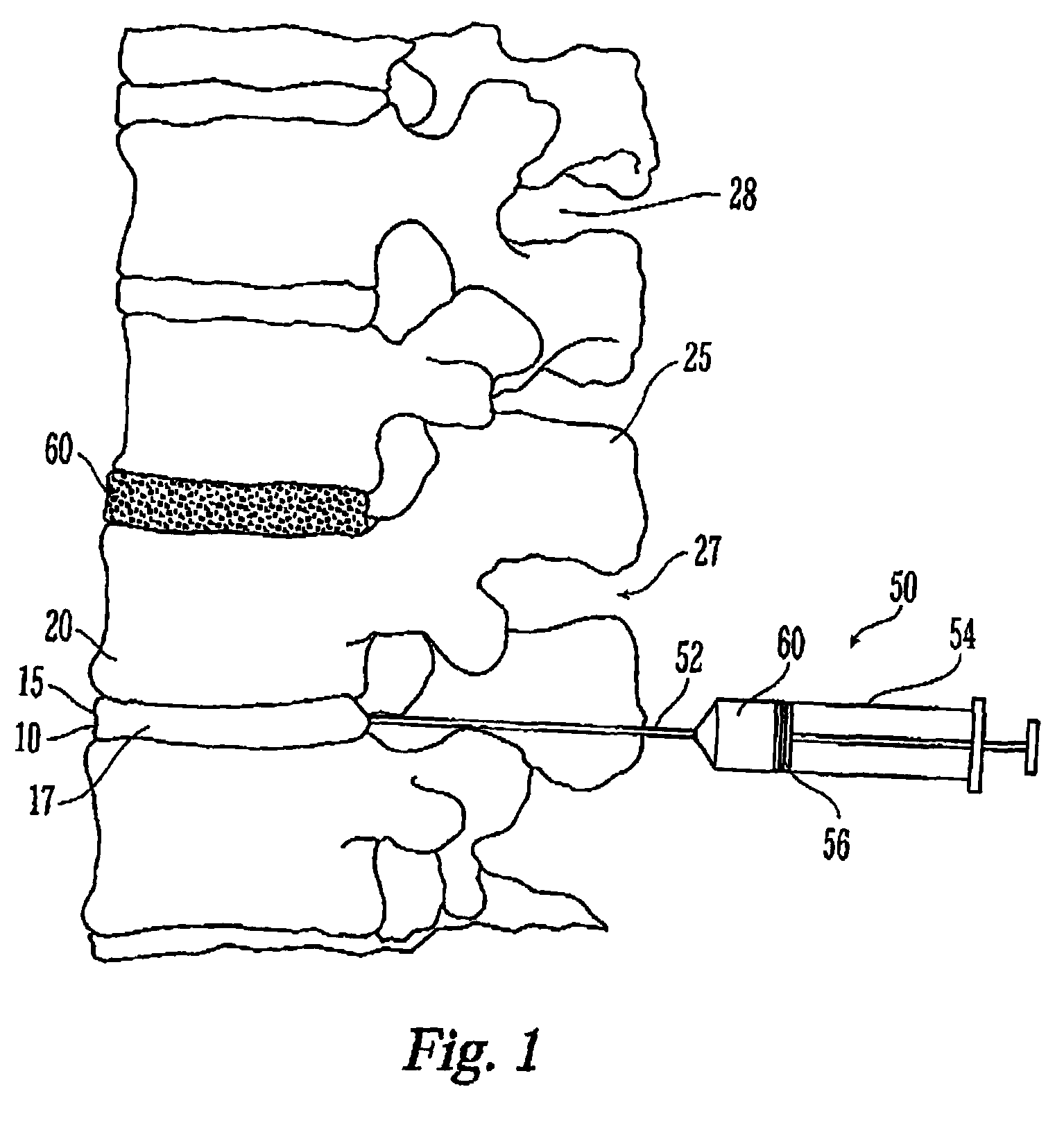
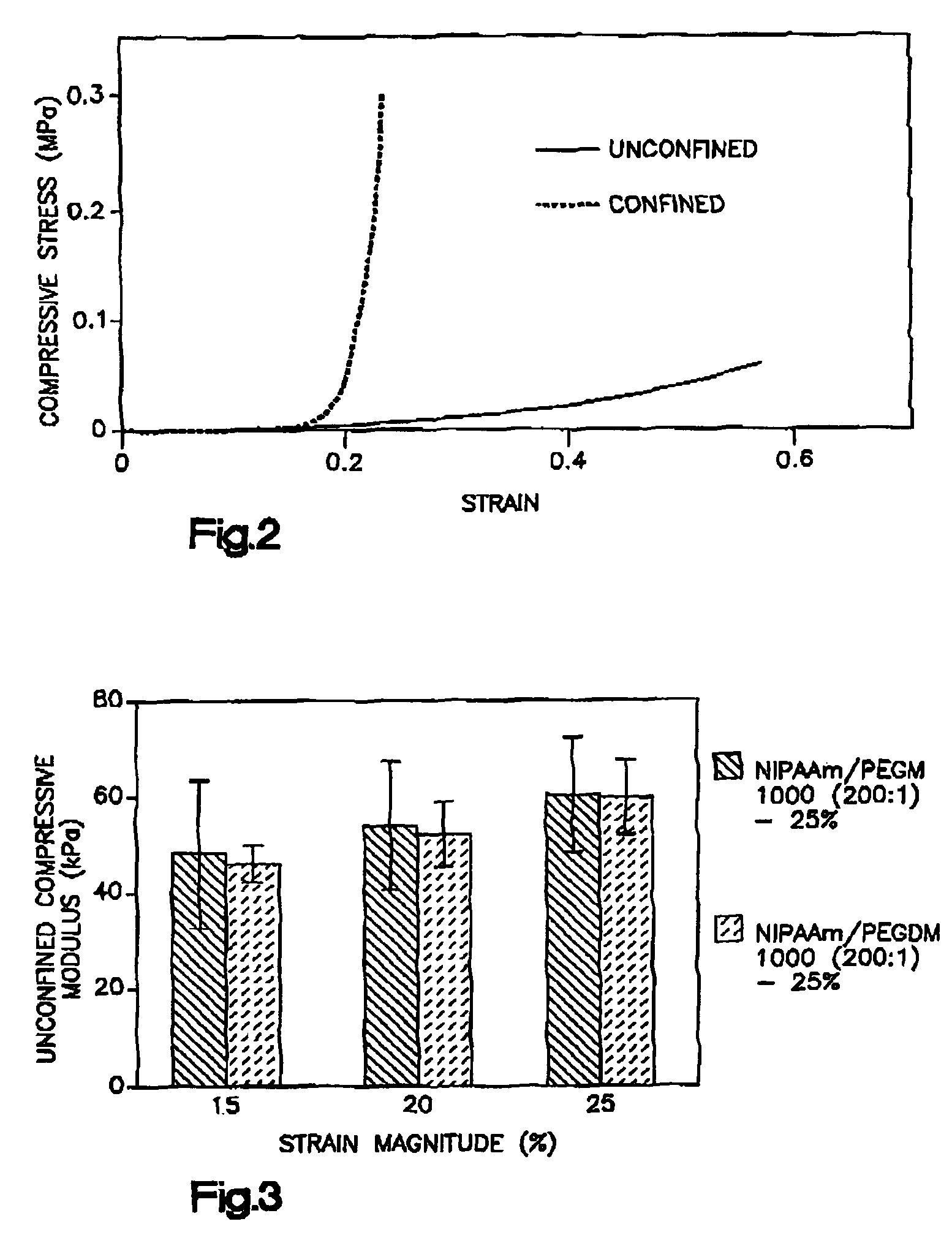
Thermogelling polymers are described containing poly (n-isopropyl acrylamide). Solutions of this polymer, copolymers or mixtures of the polymer with a second polymer such as poly(ethylene glycol), poly (vinyl pyrrolidone) or poly(vinyl alcohol) are liquids at room temperature and solids at body temperature. Thus, also provided are methods of implanting a hydrogel into a mammal by injecting the solution as a liquid at a temperature below body temperature into a selected site in the mammal at a temperature below body temperature, which then undergoes thermal phase transition to form a solid hydrogel in situ in the body as the implant warms to body temperature. Methods for using these thermal gelling materials in various applications including nucleus pulposus replacement / augmentation, wound care, disk replacement, cartilage replacement, joint replacement, surgical barriers, gastrointestinal devices, cosmetic and reconstructive surgery, and breast enlargement are also provided.
Owner:SYNTHES USA +1
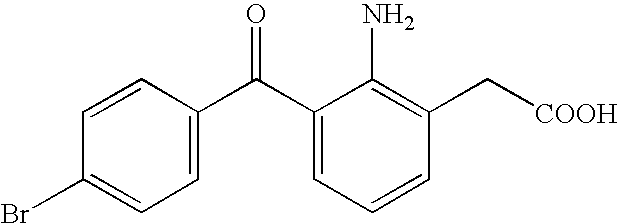
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com