Crosslinked hydrogel copolymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Poly(D,L-lactide-co-glycidyl methacrylate)-block-Poly(Ethylene Glycol)-block-Poly(D,L-Lactide-co-Glycidyl Methacrylate) Block Copolymers via Ring-Opening Copolymerization of D,L-Lactide and Glycidyl Methacrylate Initiated from Poly(Ethylene Glycol) (Mn=4600).
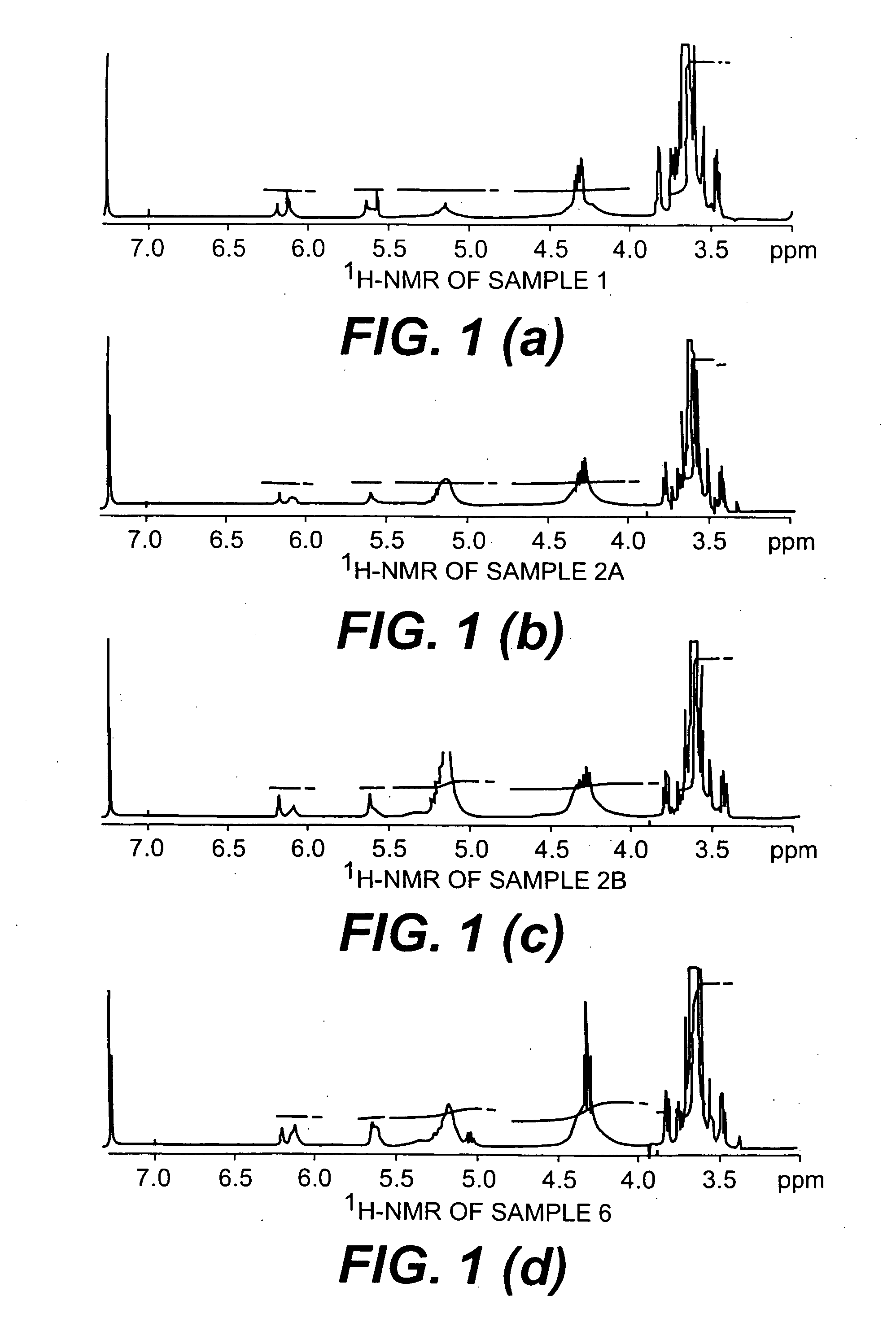
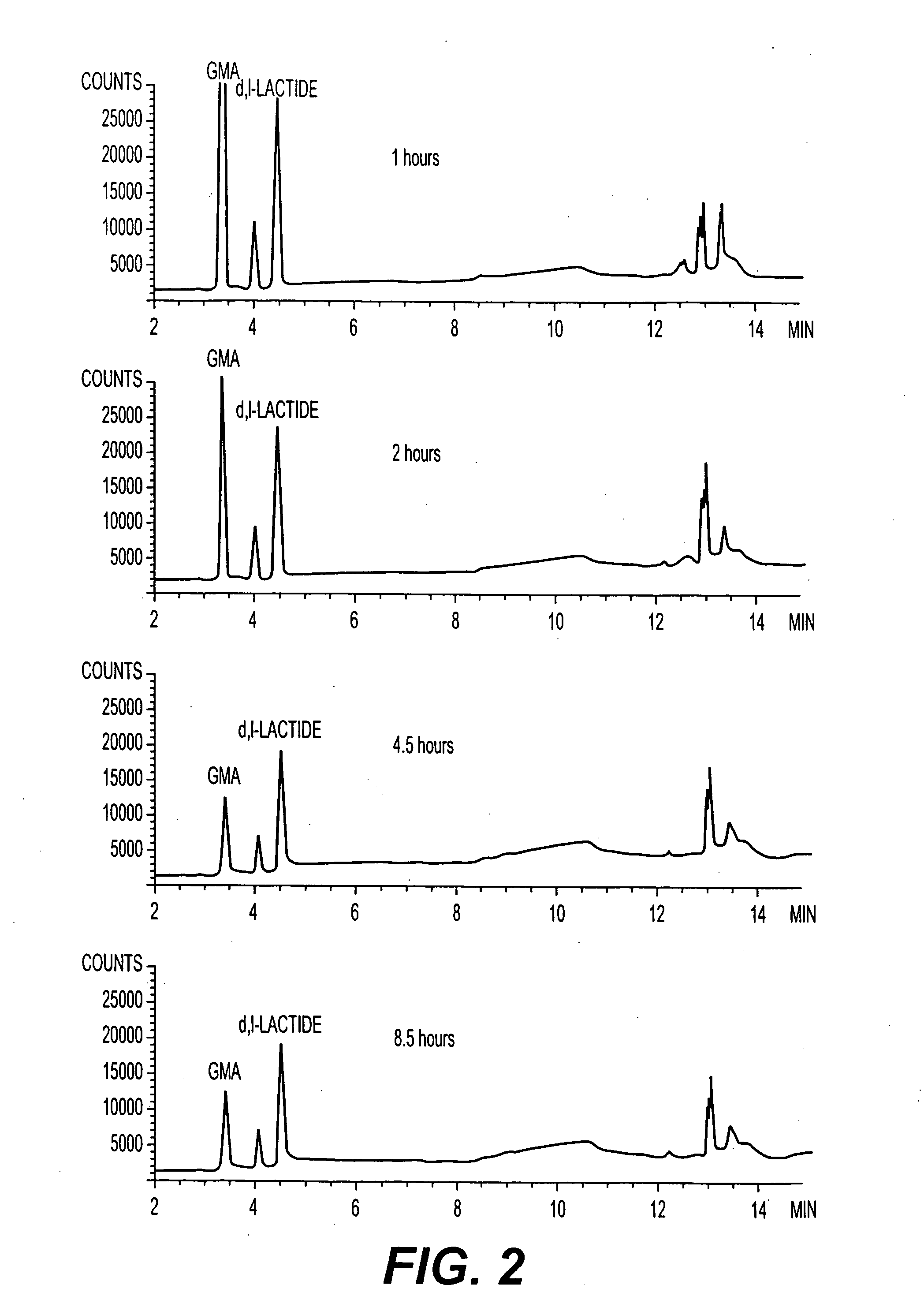
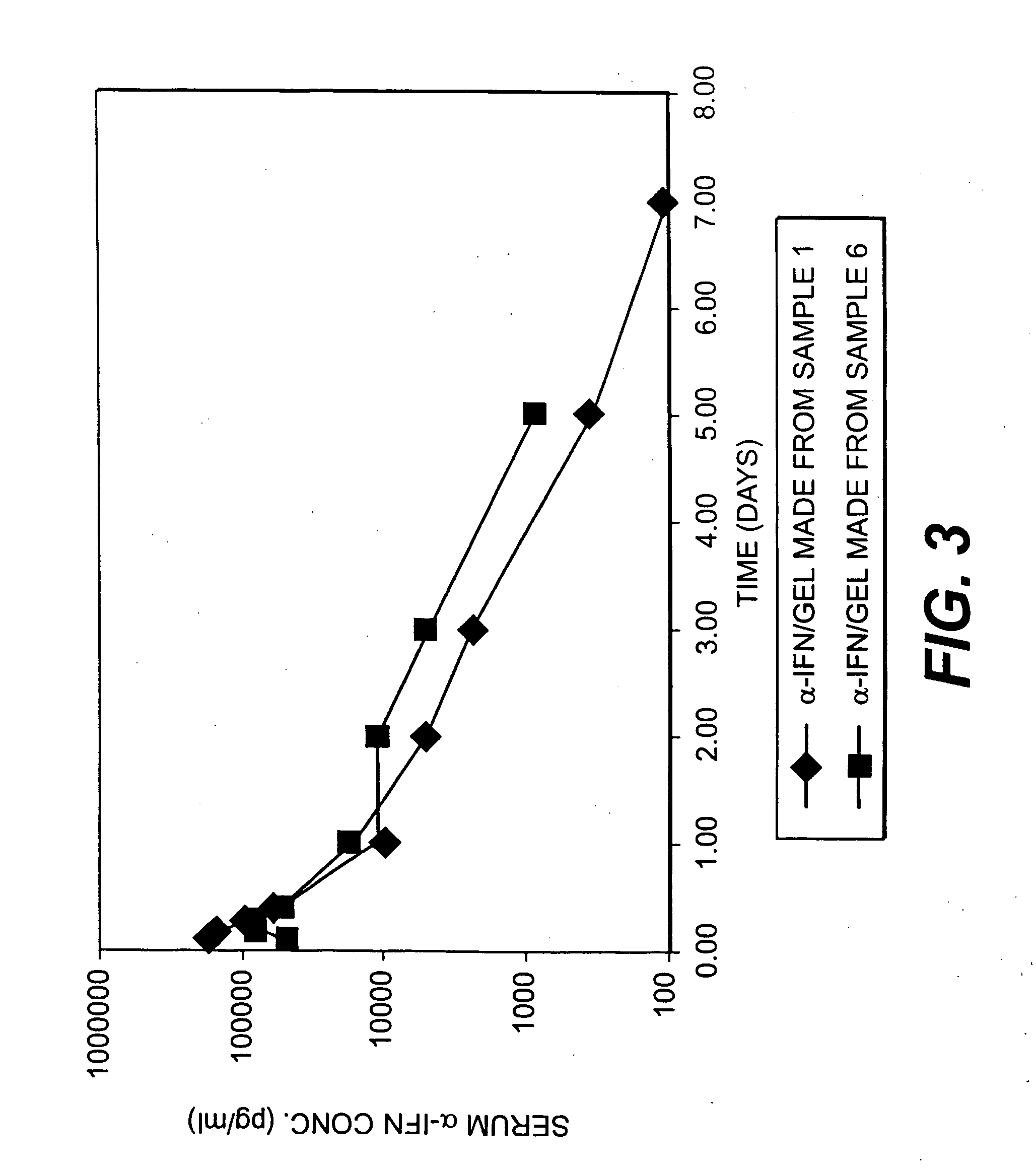
D,L-Lactide (2 g), glycidyl methacrylate (2 mL) and poly(ethylene glycol) (PEG) (8.1 g, Mn=4600) were combined in a necked tube. After melting the reaction mixture at 150° C., 0.2 mL of 1 wt. % solution of 1,4-benzoquinone in dibutyl phthalate as a radical inhibitor and 0.5 mL of a 50 mg / mL solution of stannous octoate in dibutyl phthalate as a catalyst were added to the tube under nitrogen flow. The tube was degassed and sealed under vacuum. The reaction mixture was immersed in an oil bath and held at 175° C. for one hour. After opening the tube following the one hour reaction time, the reaction mixture was dissolved in THF and precipitated in ether. The copolymer that was obtained (Sample 1) was isolated by fil...
example 2
Synthesis of Poly(D,L-Lactide-co-Glycidyl Methacrylate)-block-Poly(Ethylene Glycol)-block-Poly(D,L-Lactide-co-Glycidyl Methacrylate) Block Copolymers Containing Different Weight Ratios of D,L-Lactide and PEG (Mn=4600).
Poly(D,L-lactide-co-glycidyl methacrylate)-block-poly(ethylene glycol)-block-poly(D,L-lactide-co-glycidyl methacrylate) block copolymers with various D,L-lactide to poly(ethylene glycol) ratios were prepared according to the synthesis procedure described in Example 1, where PEG=poly(ethylene glycol) (Mn=4600); DLLA=D,L-lactide; and GMA=glycyidyl methacrylate. The results are summarized in Table 1.
TABLE 1PEG:DLLA:GMAPEG:DLLA:GMAPEG:DLLA:GMASample(wt. %:wt. %:wt. %)(mol:mol:mol)(mol:mol:mol)MwIVNo.FeedFeed1H NMR(g / mol)Mw / Mn(dL / g)166.5:16.4:17.11.0:8.2:9.1 1.0:1.3:1.8 10,3001.090.182A66.7:24.7:8.6 1.0:12.7:4.51.0:8.1:1.3 10,7001.090.17 2B*44.3:44.3:11.41.0:34.5:9.11.0:22.4:2.111,7001.080.202C66.5:33.5:0.0 1.0:16.1:0.01.0:14.0:0.010,1001.110.17
*Reaction time of 2 hour...
example 3
Synthesis of Poly(D,L-Lactide-co-Glycidyl Methacrylate)-block-Poly(Ethylene Glycol)-block-Poly(D,L-Lactide-co-Glycidyl Methacrylate) Block Copolymers Containing PEG with Different Molecular Weights.
Poly(D,L-lactide-co-glycidyl methacrylate)-block-poly(ethylene glycol)-block-poly(D,L-lactide-co-glycidyl methacrylate) block copolymers with various PEGs of different molecular weights were prepared according to the synthesis procedure described in Example 1, where PEG=poly(ethylene glycol) of the molecular weight indicated; DLLA=D,L-lactide; and GMA=glycyidyl methacrylate. All reaction times were 2 hours at 175° C. The results are summarized in Table 2.
TABLE 2PEG:DLLA:GMAPEG:DLLA:GMAPEG:DLLA:GMASolubility inSamplePEG(wt. %:wt. %:wt. %)(mol:mol:mol)(mol:mol:mol)Water atNo.(Mn)FeedFeed1H NMR20 wt. %3A10,00044.3:44.3:11.41.0:69.4:18.11.0:26.7:2.3Soluble2B4,60044.3:44.3:11.41.0:34.5:9.1 1.0:22.4:2.1Soluble3C3,35044.3:44.3:11.41.0:23.3:6.1 1.0:13.5:1.0Soluble3D1,50044.3:44.3:11.41.0:10....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrophilicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com