High molecule bonding adriamycin medicine, nano capsule and preparation thereof
A combination of doxorubicin and doxorubicin, applied in capsule delivery, microcapsules, nanocapsules, etc., can solve the problems of low drug loading and sudden release of drugs, and achieve reduced dosage and low production costs , to avoid the effect of sudden drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of block polymer PEG-PLA-OH
[0040] Add 4 g of polyethylene glycol (PEG-OH) with a number average molecular weight of 400 to a dry ampoule with a water separator, reflux condenser and magnetic stirrer, add 40 ml of anhydrous toluene, and azeotropically remove water for 30 minutes , then cooled to room temperature, 10 g of lactide (LA) monomer recrystallized three times with ethyl acetate was added, the reaction bottle was ventilated three times with high-purity argon, and then octanoic acid sulfide, which was 1 / 500 of the molar number of LA monomer, was added. Tin, stirred and reacted at 110°C for 24h, then dissolved the product in an appropriate amount of dichloromethane, settled with ether to obtain a white product, dried in vacuum at 40°C to obtain a PEG-PLA-OH block polymer, and its number-average molecular weight is 1400 (by 1 Calculated by H NMR).
Embodiment 2
[0041] Example 2: Synthesis of Carboxylated Block Polymer PEG-PLA-COOH
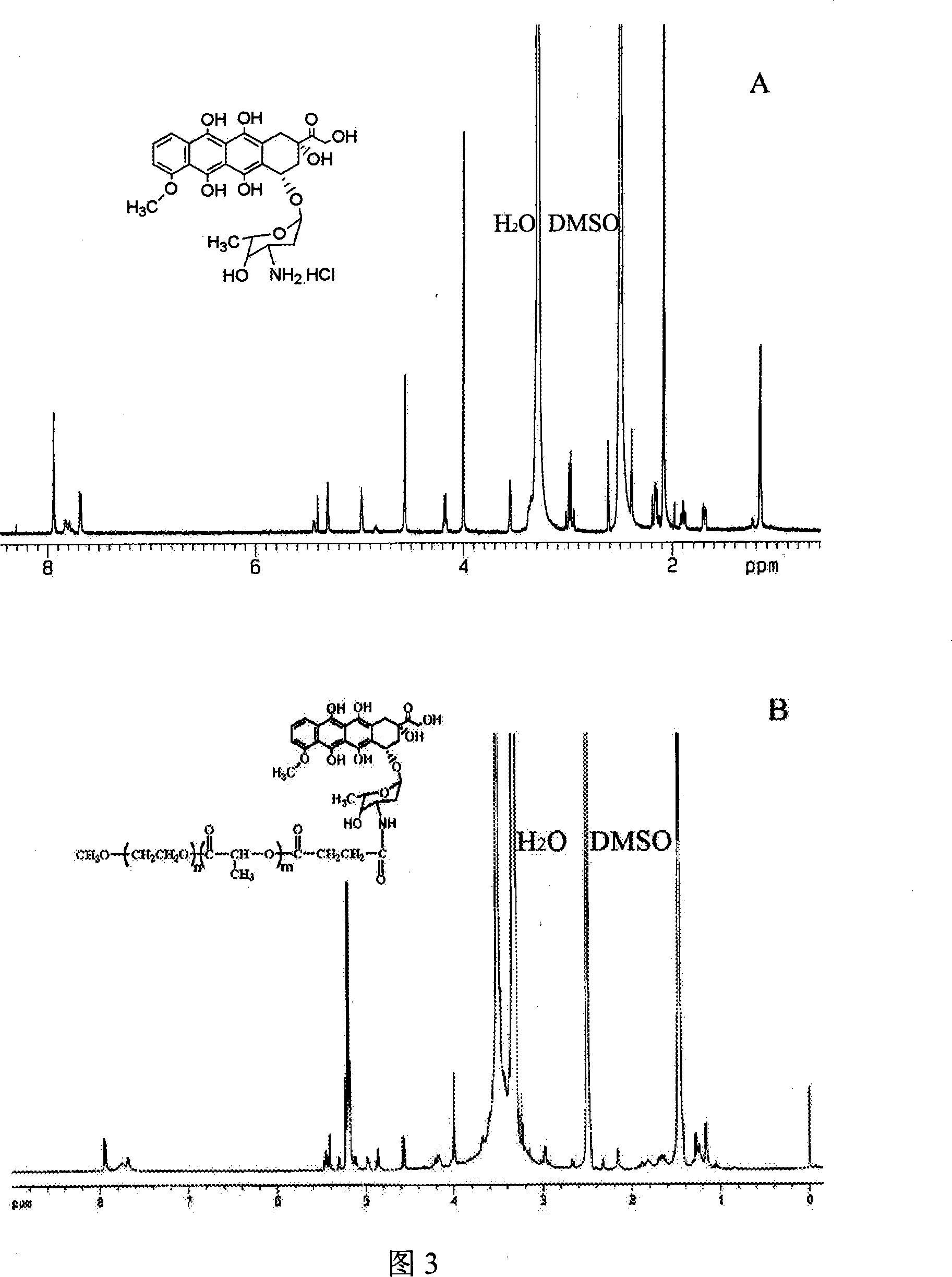
[0042] Dissolve 1.84g of PEG-PLA-OH polymer in 10ml of 1,4-dioxane, add 0.013g of succinic anhydride, then add 0.018ml of triethylamine (TEA) and 0.016g of N,N-dimethyl Base p-aminopyridine (DMAP), react at room temperature for 12h. The salt produced by the reaction was filtered off, and the filtrate was settled with ether to obtain a white product, which was dried under vacuum at 40°C to obtain the carboxylated block polymer PEG-PLA-COOH. Its NMR spectrum is shown in Figure 2.
Embodiment 3
[0043] Example 3: Bonding of PEG-PLA-COOH and Adriamycin
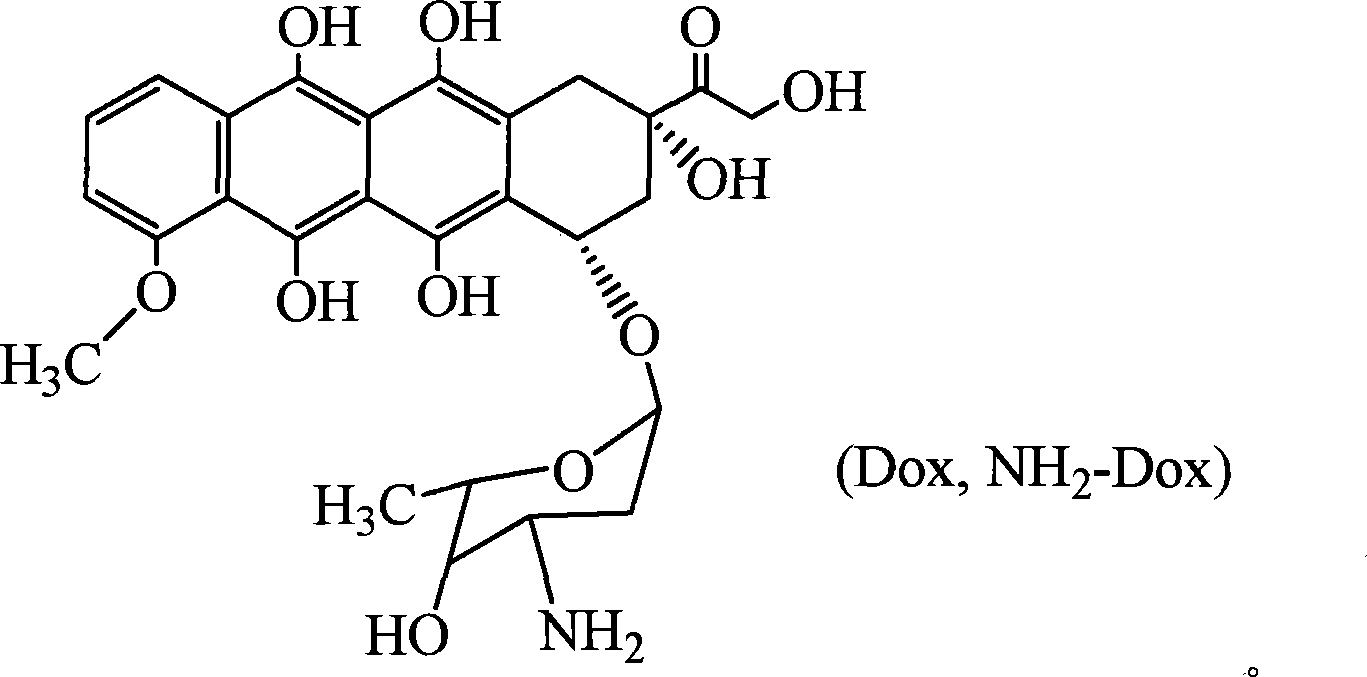
[0044] Add 0.38g carboxyl-terminated block polymer PEG-PLA-COOH to a 50ml ampoule, then add 20ml anhydrous dimethyl sulfoxide, after the polymer dissolves, add 27.2mg doxorubicin, 0.007ml TEA and 6mg DMAP was reacted at 0°C for 12 hours, and the precipitate formed during the reaction was filtered off, and the filtrate was settled with anhydrous ether to obtain a white precipitate, which was dried in vacuo. The mass content of doxorubicin in the bonded drug is 12%. Its NMR spectrum is shown in Figure 3(B). For comparison, the NMR spectrum of doxorubicin is given in Fig. 3(A).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com