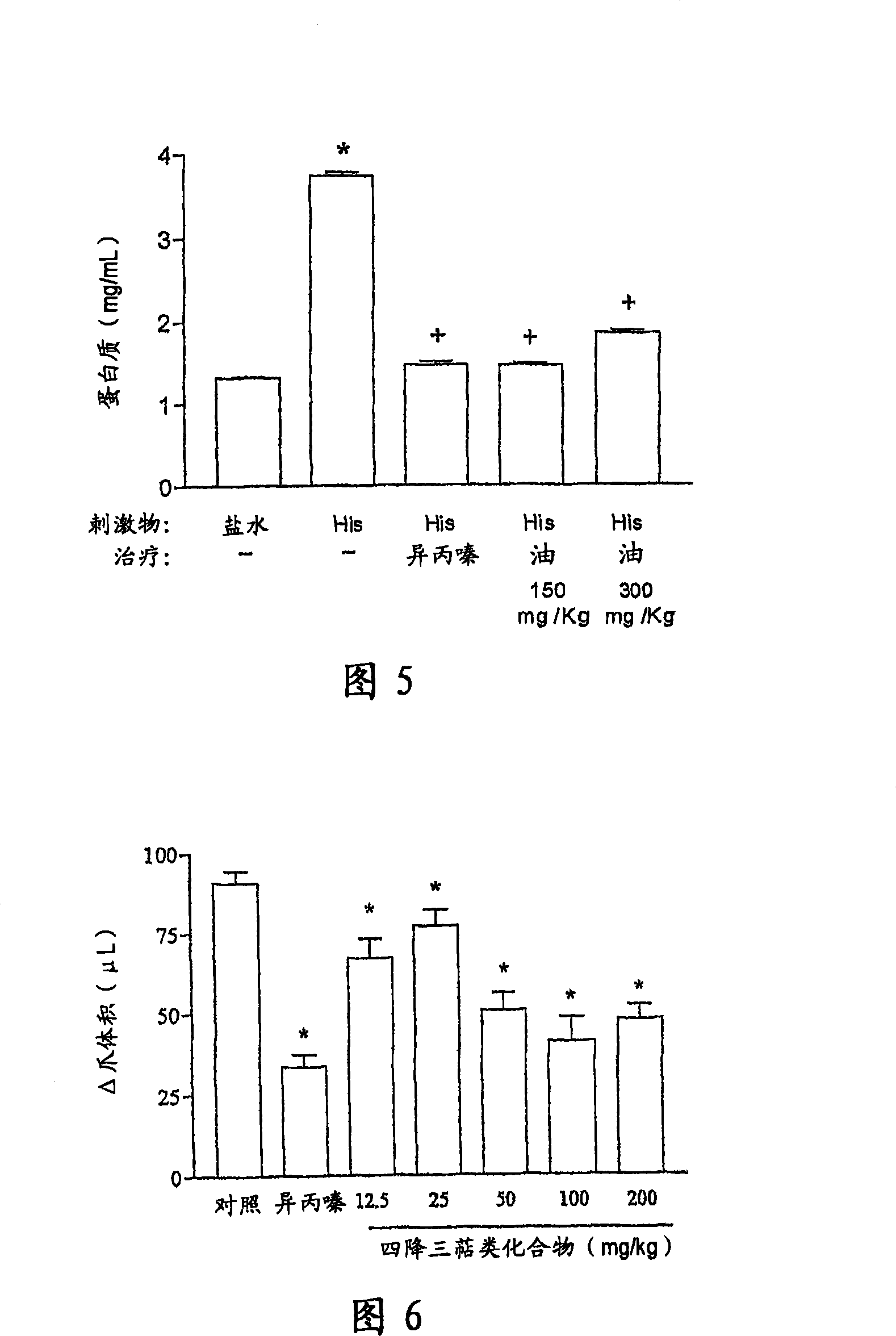
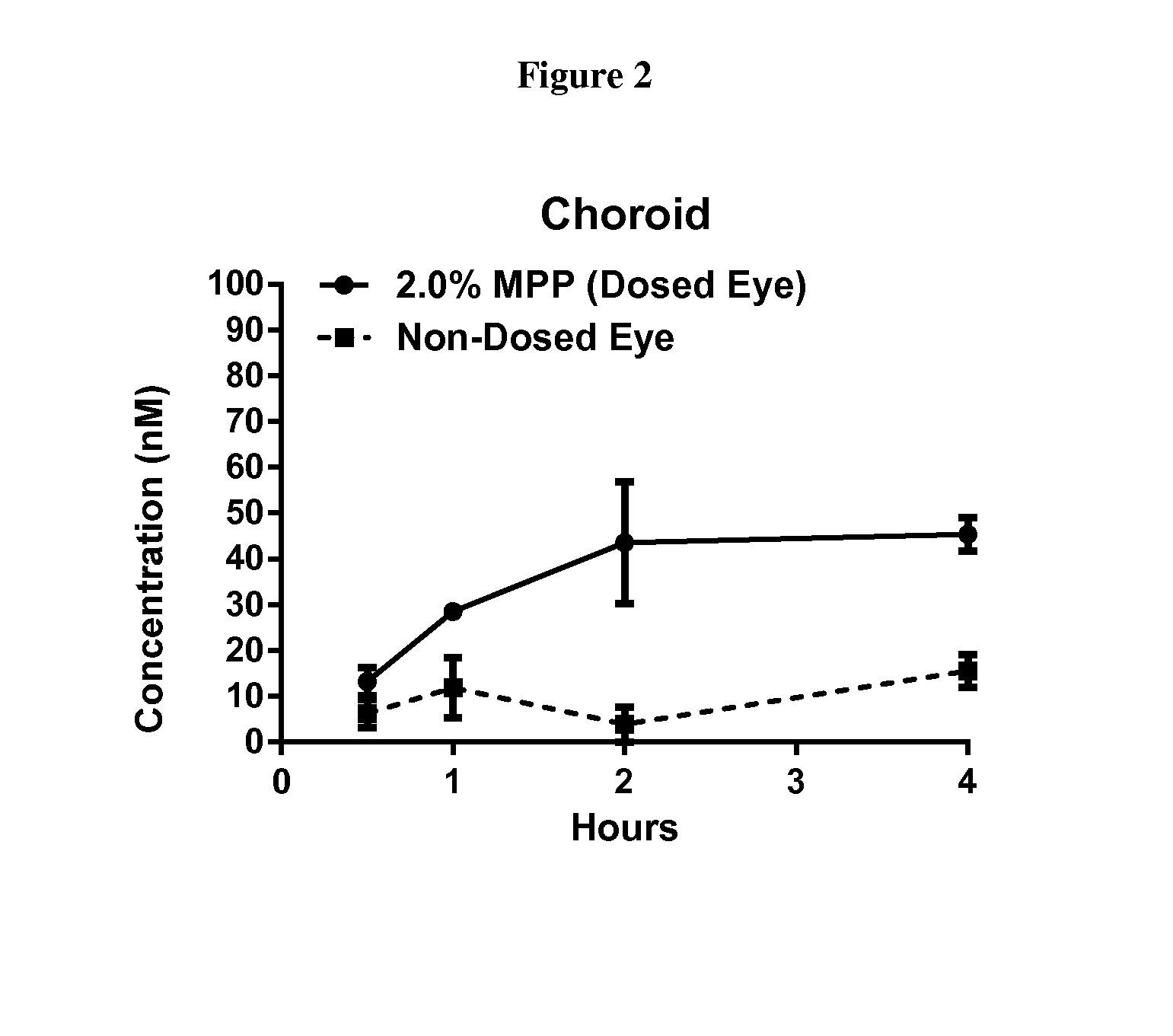
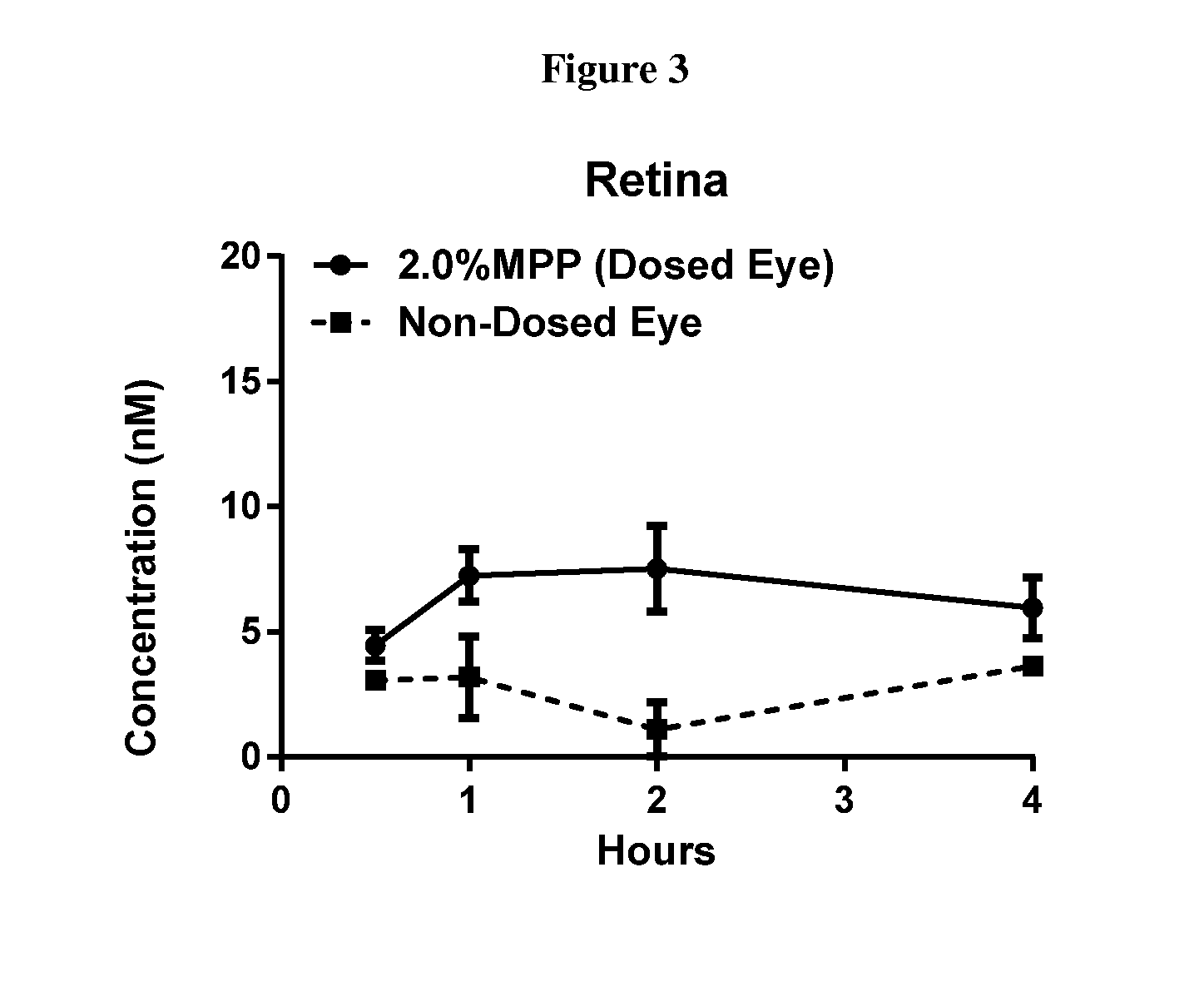
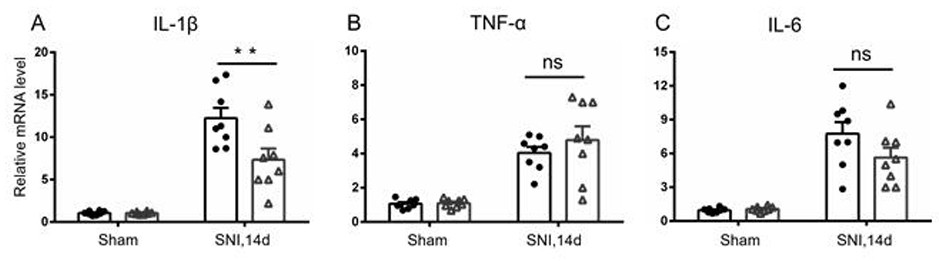
Patents
Literature
31 results about "Postoperative inflammation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
About Postoperative Ocular Inflammation: Postoperative Ocular Inflammation is defined as inflammation of the eye as result of a surgical intervention. The following list of medications are in some way related to, or used in the treatment of this condition.
Artificial cornea
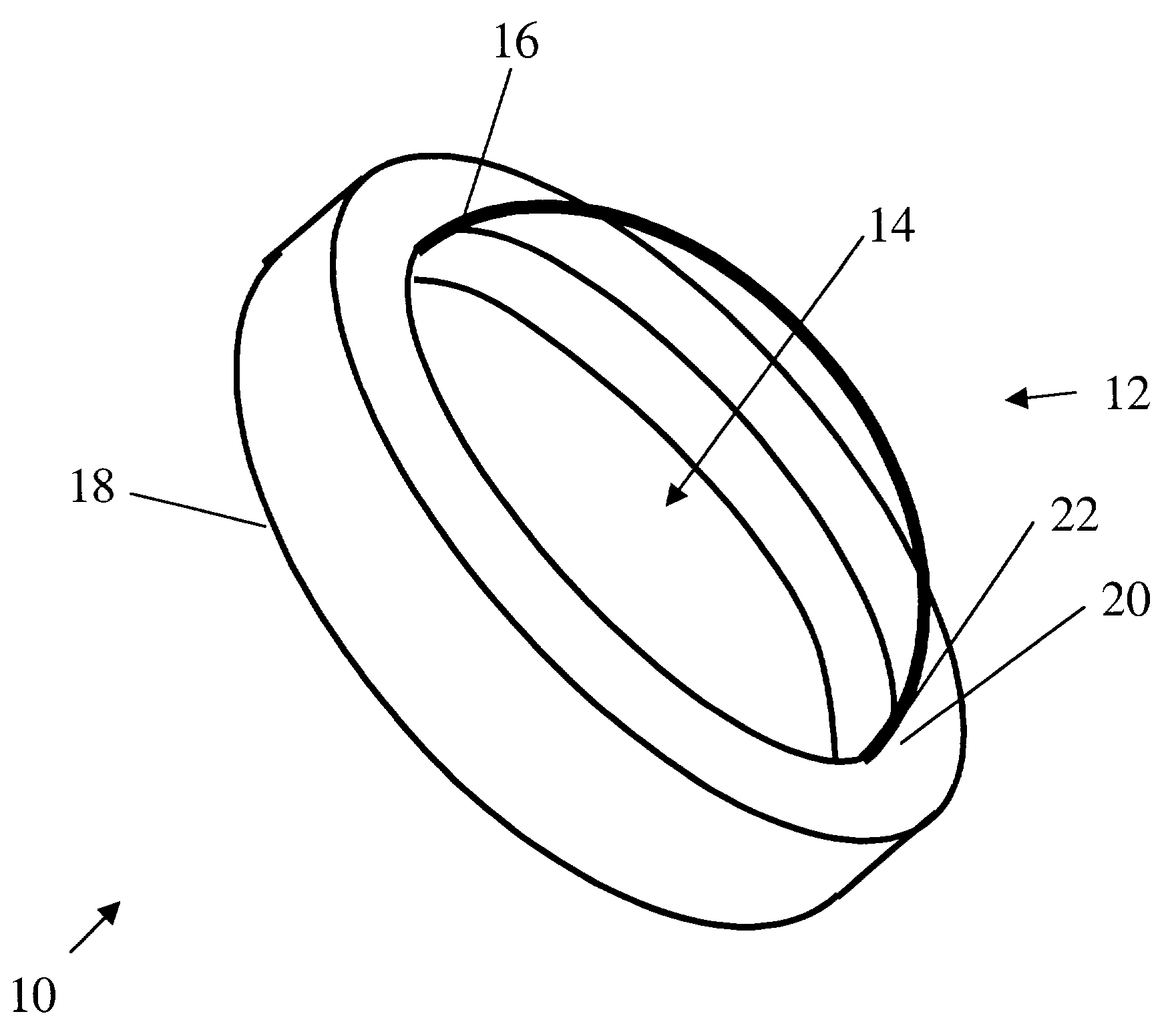
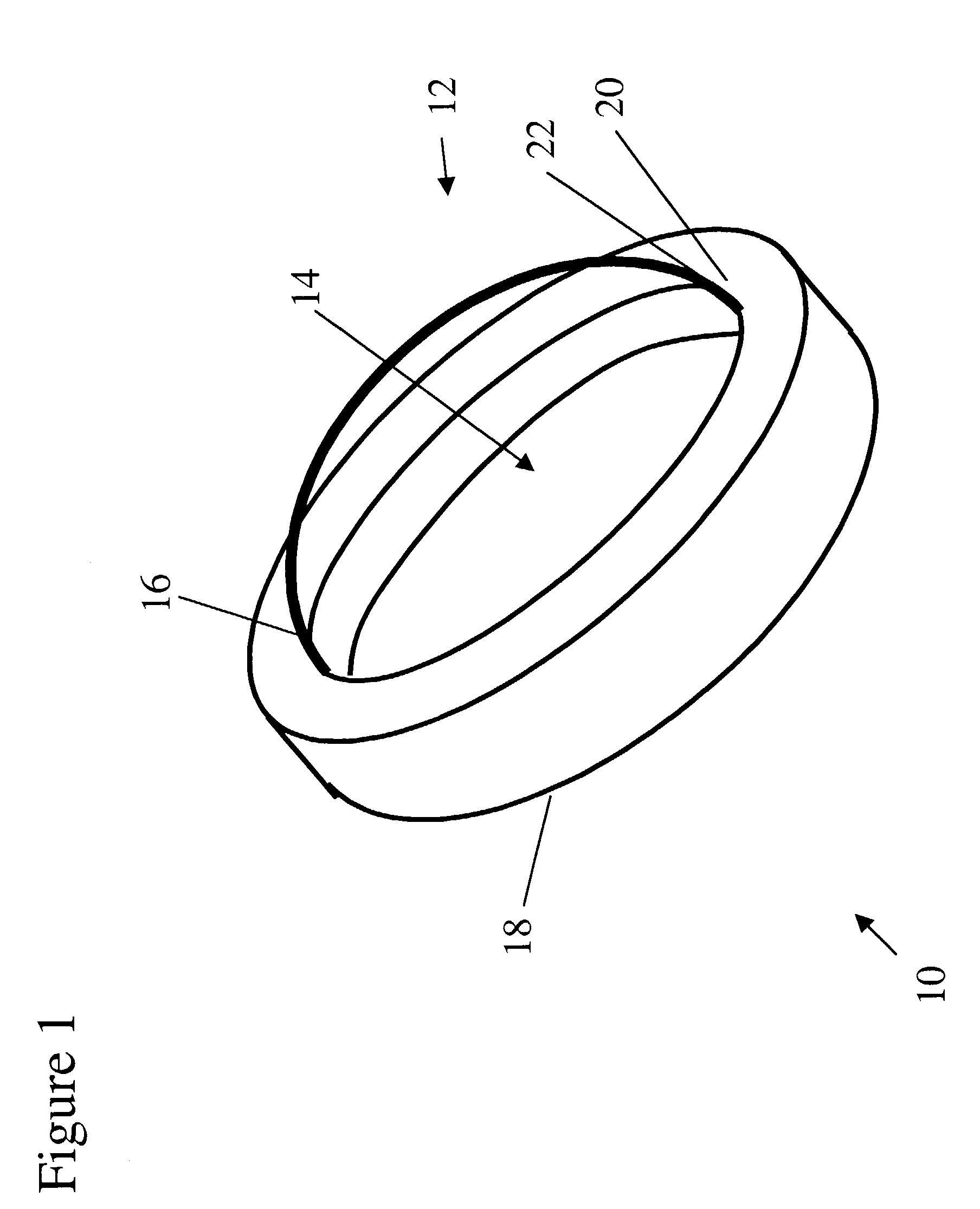
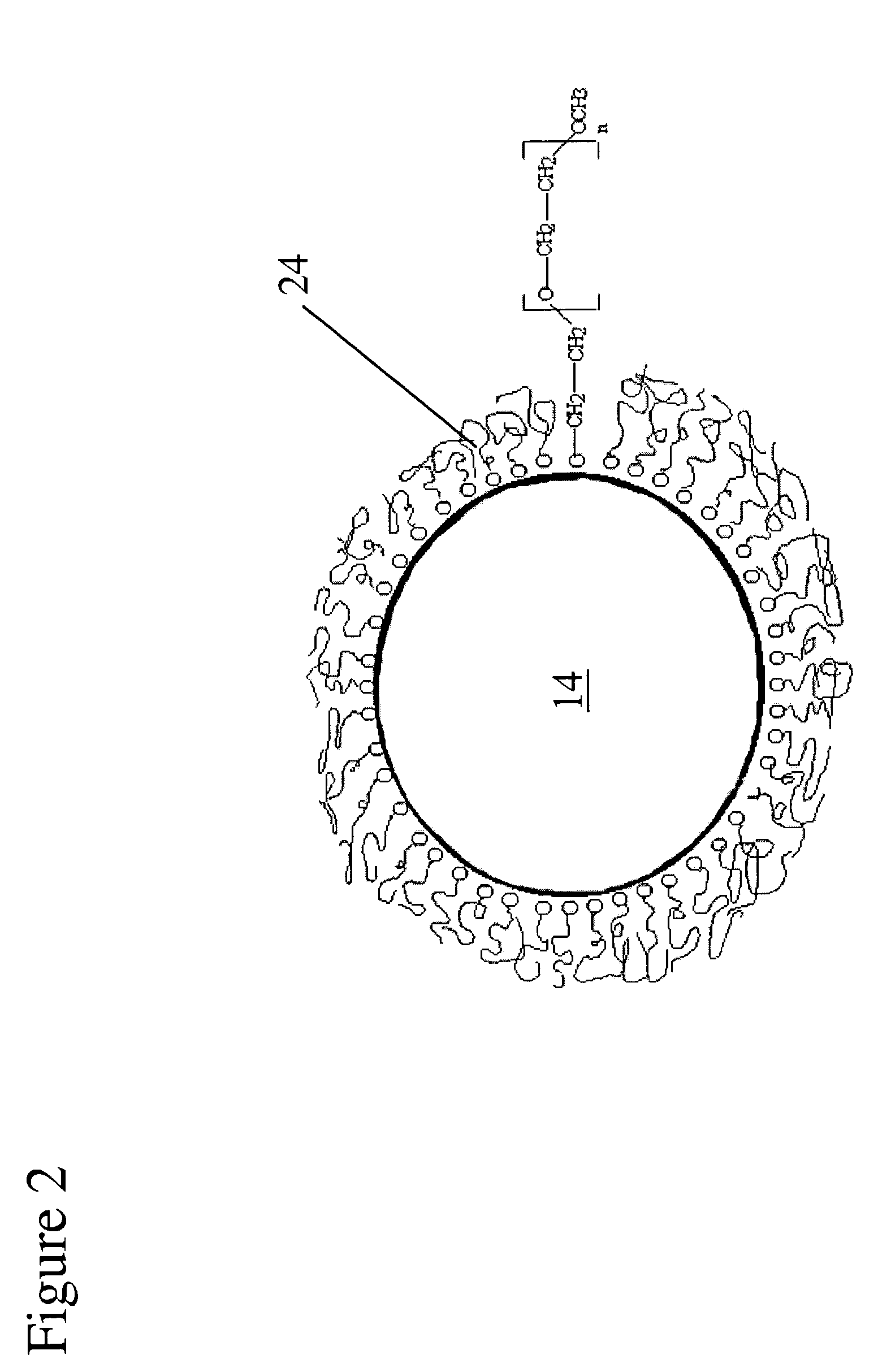
InactiveUS6976997B2Improve mechanical propertiesEasy to suture onto recipient bedMaterial nanotechnologyCoatingsDiseasePostoperative inflammation
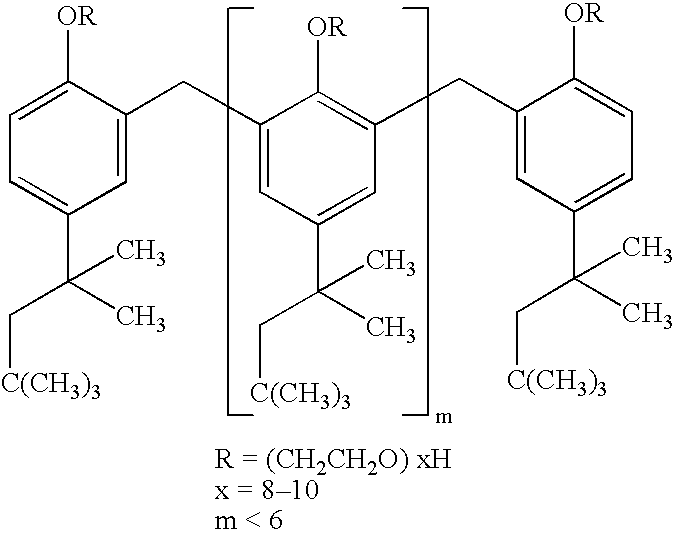
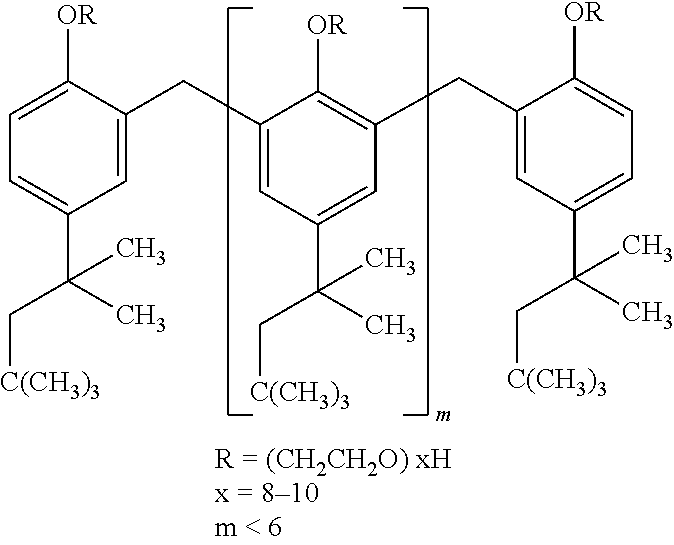
The invention provides implants suitable for use as an artificial cornea, and methods for making and using such implants. Artificial corneas having features of the invention may be two-phase artificial corneas, or may be three phase artificial corneas. These artificial corneas have a flexible, optically clear central core and a hydrophilic, porous skirt, both of which are biocompatible and allow for tissue integration. A three-phase artificial cornea will further have an interface region between the core and skirt. The artificial corneas have a high degree of ocular tolerance, and allow for tissue integration into the skirt and for epithelial cell growth over the surface of the prosthesis. The use of biocompatible material avoids the risk of disease transmission inherent with corneal transplants, and acts to minimize post-operative inflammation and so to reduce the chance or severity of tissue necrosis following implantation of the synthetic cornea onto a host eye.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
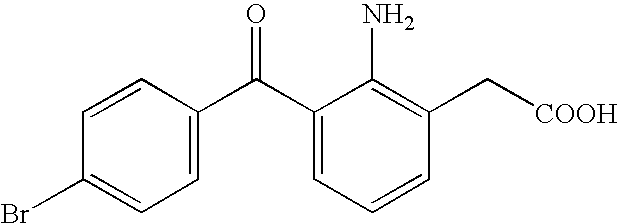
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
ActiveUS20050239895A1No irritationInhibit deteriorationBiocideOrganic active ingredientsScleritisPhenylacetic acid
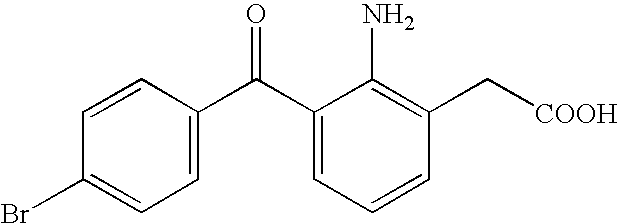
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Methods for Tissue Passivation
ActiveUS20150134049A1Preventing and reducing stenosisElectrotherapyPeptide/protein ingredientsHemodialysisHaemodialysis machine
Methods of tissue passivation are described herein for use in preserving normal tissue architecture, reducing post-surgical inflammation, and reducing or preventing the development of pathogenic collagen bundles and adhesions following surgical procedures. Passivated tissues prepared in accordance with these methods are useful in a variety of therapies including, e.g., cardiac bypass surgery, hemodialysis, etc.
Owner:THE GENERAL HOSPITAL CORP
Non-steroid anti-inflammatory medicine for external use for ophthalmology
ActiveCN102058581AGood intraocular penetrationStrong penetrating powerOrganic active ingredientsAntipyreticDiseaseSide effect
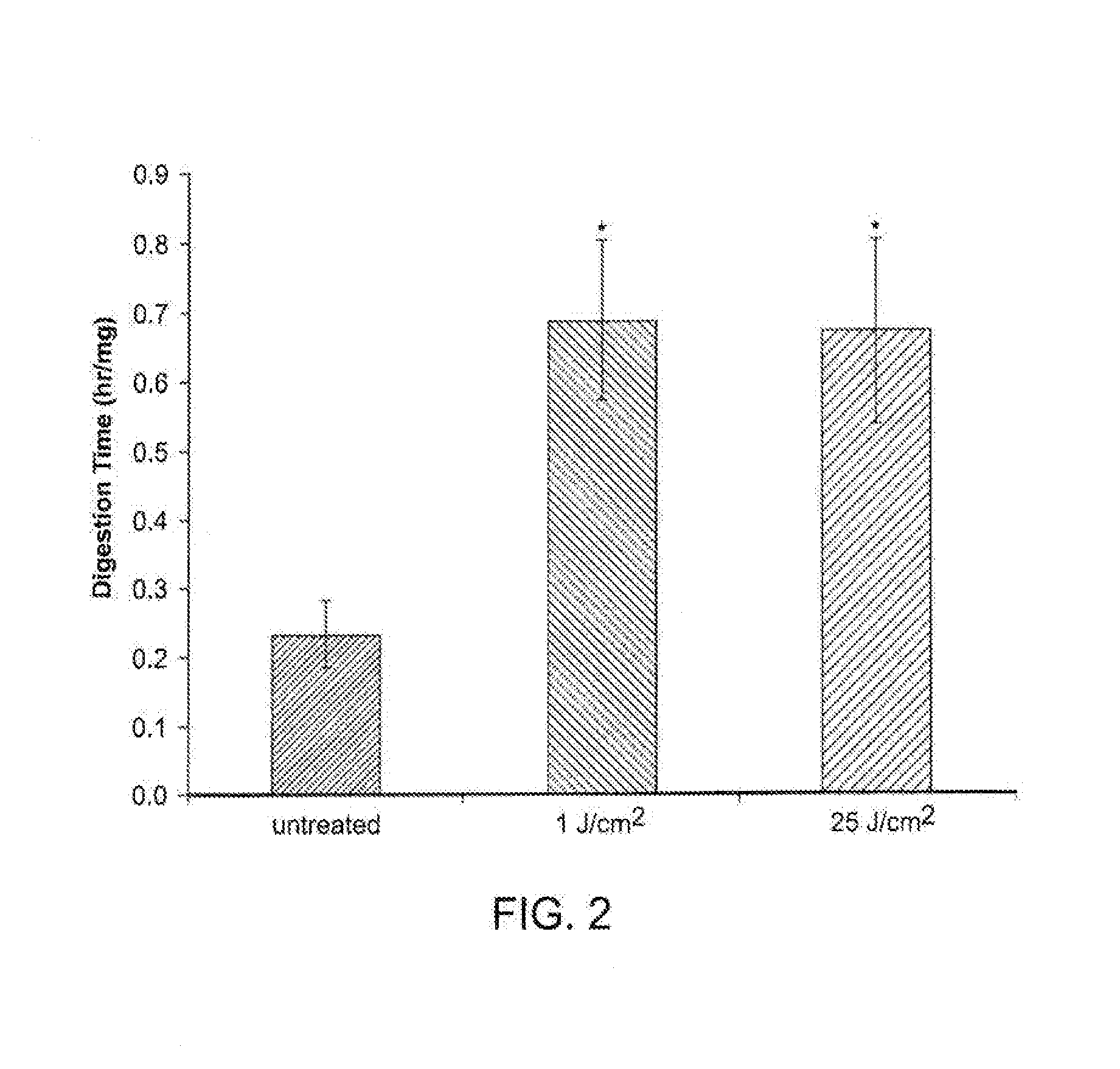
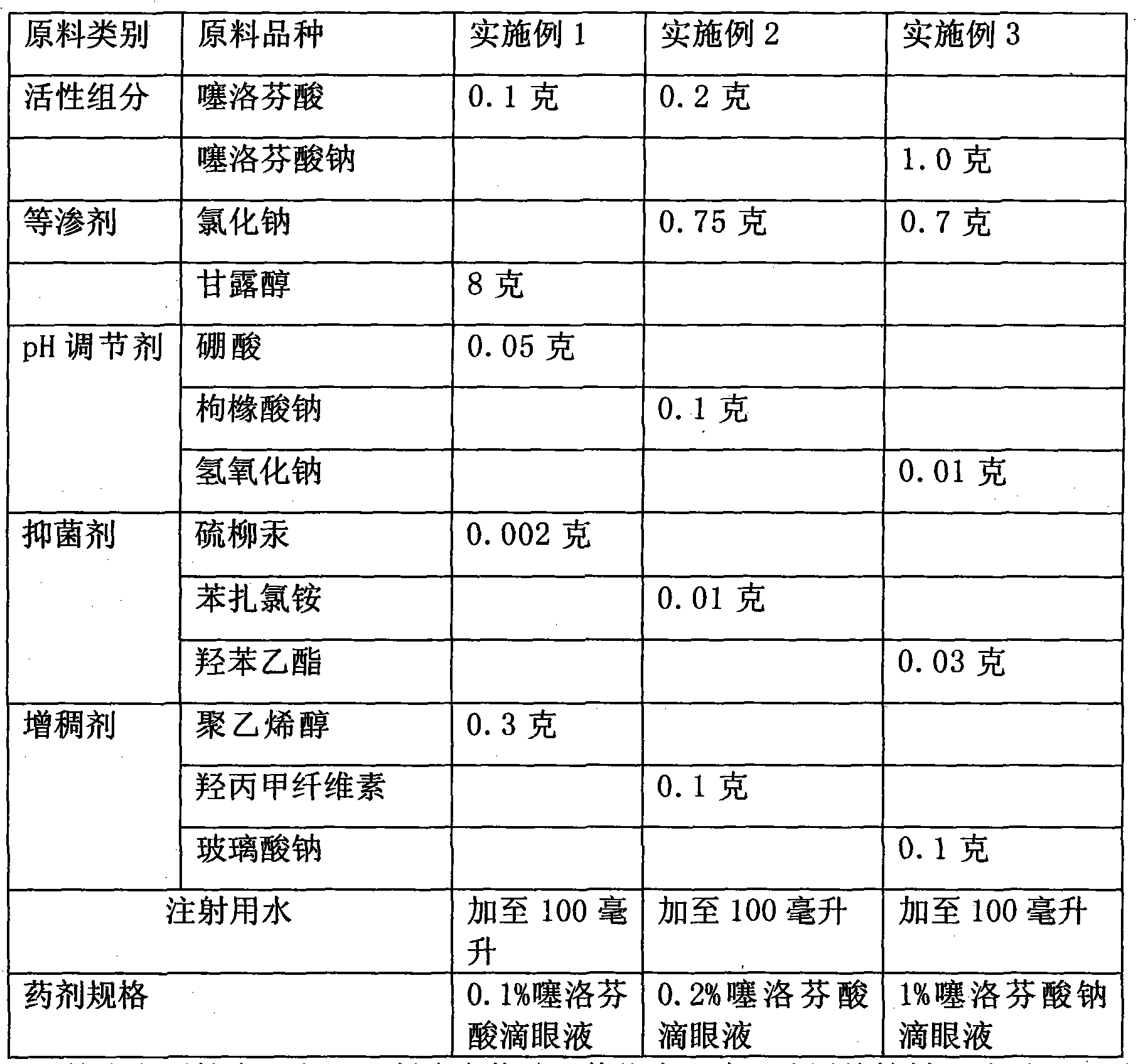
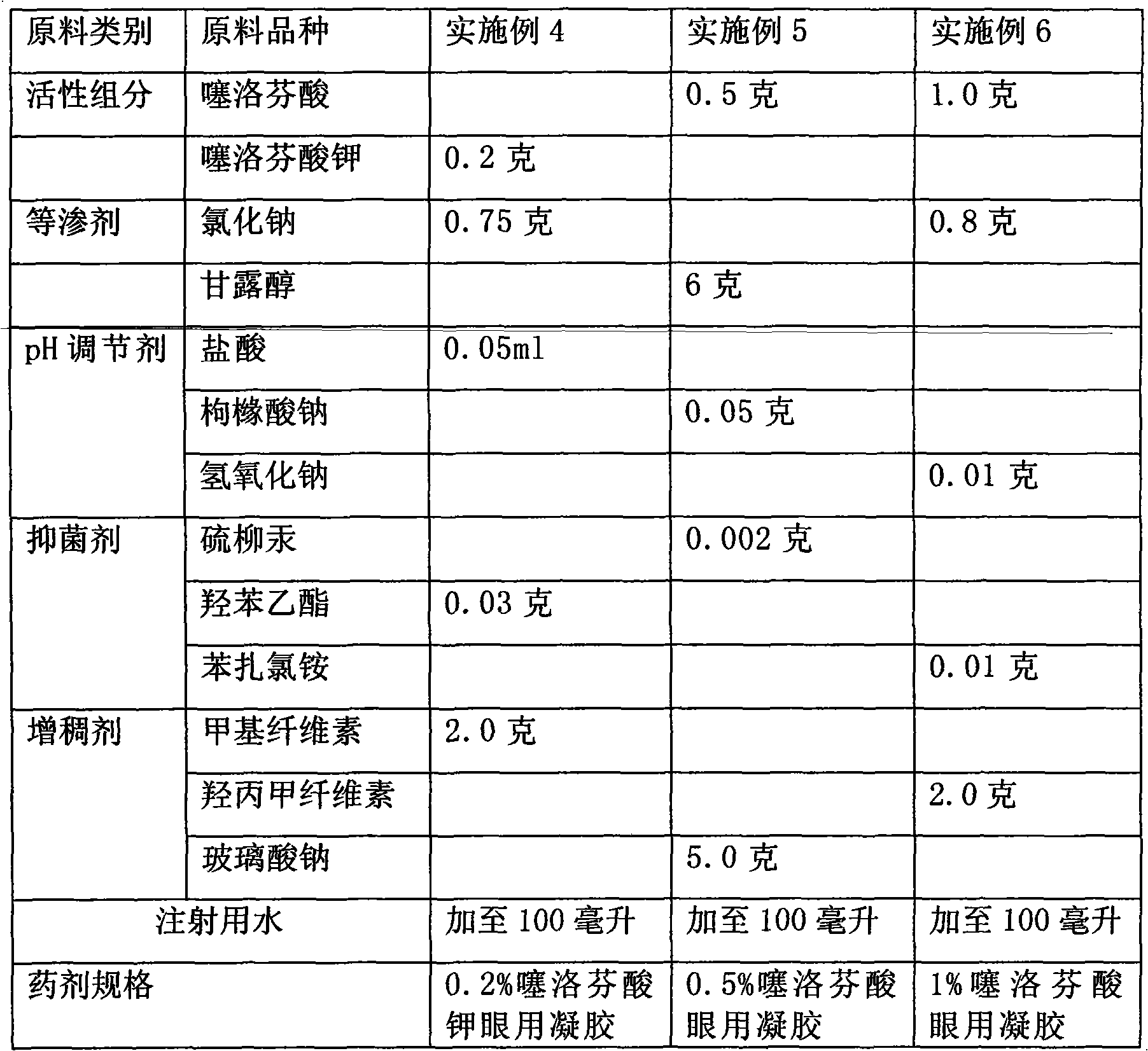
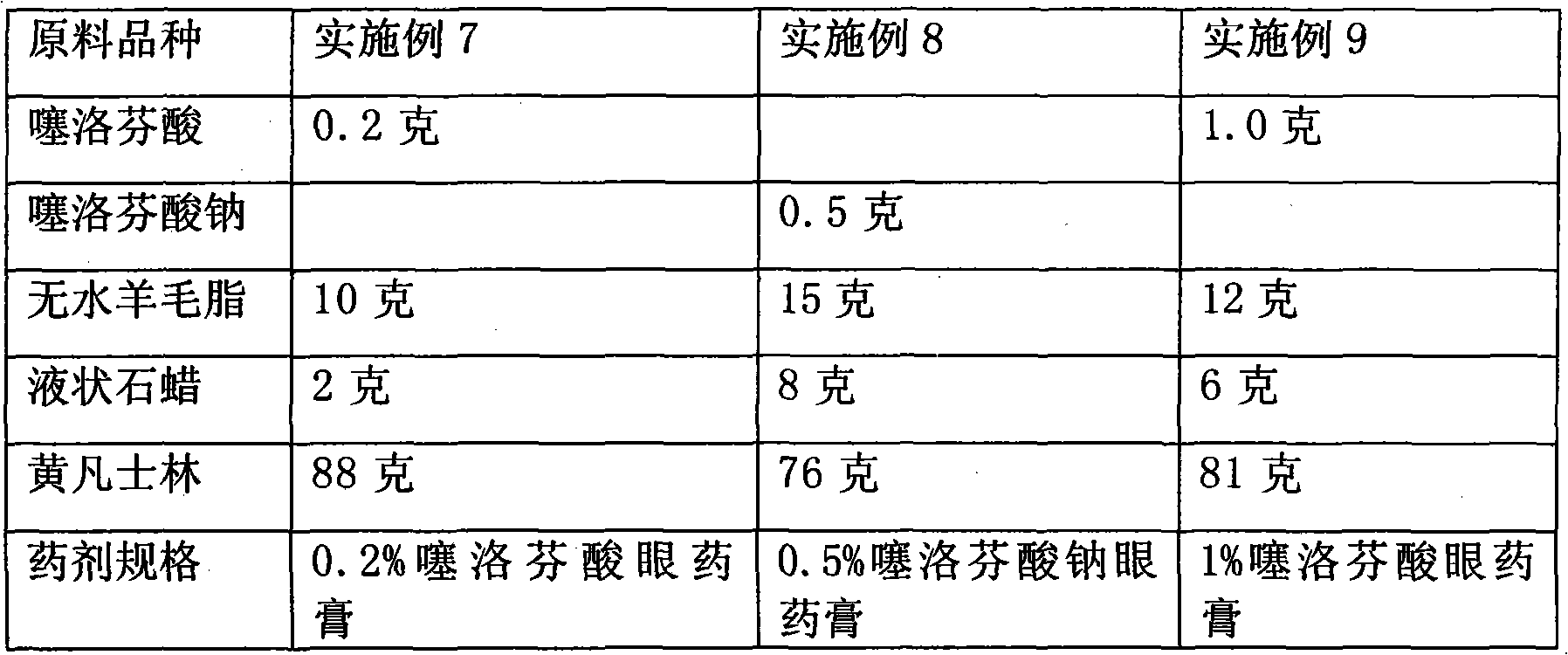
The invention relates to a eye gel. The content of tiaprofenic acid or the salts thereof in every 100 weight parts of the medicine is 0.1-1.0 weight parts. The non-steroid anti-inflammatory medicine for external use for ophthalmology has good permeability in eyes and low toxic and adverse effects, and can be used for treating and preventing diseases of outer eye and anterior segment of eye, caused by non-infectious inflammations, such as blepharitis, conjunctivitis, keratitis, sclerotitis, episcleritis, iridocyclitis and postoperative inflammation.
Owner:GUANGDONG WHOLEWIN TECH
Preparation method of polysialic acid-hyaluronic acid composite gel, obtained product and application
ActiveCN105801870AEasy to makeEasy to operateAerosol deliveryOintment deliveryHalf-lifeBiocompatibility Testing
The invention discloses a preparation method of polysialic acid-hyaluronic acid composite gel, an obtained product and an application. The preparation method comprises steps as follows: in an alkaline aqueous solution environment, polysialic acid is linked with a crosslinking agent, and activated polysialic acid is obtained; the activated polysialic acid is added to a hyaluronic acid solution, so that the polysialic acid is grafted on the hyaluronic acid in an acid environment, and the product is obtained. The reaction process is controllable, few side reactions exist, the obtained product has excellent biocompatibility of the hyaluronic acid and non-immunogenicity of the polysialic acid, can be applied to embedded slow-release of polypeptide or protein drugs and can also applied to coating of crosslinked hyaluronic acid gel particles for injection, the postoperative inflammatory reaction is reduced, skin redness and swelling are relieved, pain of users is relieved, besides, the product stability can be improved, the degradation time can be prolonged, and the half-life period can be prolonged.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
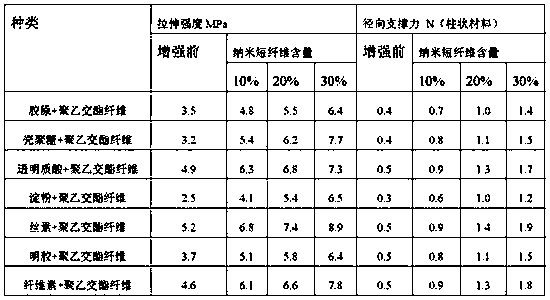
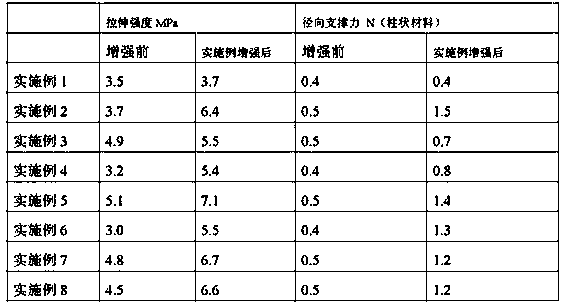
Medical nanofiber reinforced hydrophilic composite material as well as preparation method and application thereof
The invention discloses a medical nanofiber reinforced hydrophilic composite material as well as a preparation method and application thereof. The composite material is prepared from a hydrophilic basis material and nano staple fibers, wherein the nano staple fibers are maintained in crystal morphology and are uniformly dispersed in the hydrophilic basis material; the mass percent of the nano staple fibers in the composite material is 1 to 30 percent; the nano staple fibers have the diameters of 200nm to 800nm and the lengths of 10mu m to 100mu m; according to the preparation method of the medical nanofiber reinforced composite material, which is provided by the invention, the technological design is peculiar; the nano staple fibers are uniformly dispersed in the basis material by utilizing the solubility difference of the basis material and the nano staple fibers; the multicellular spongy composite material is obtained by adopting a freeze drying method; the composite material keeps the respective advantages of two materials; defects are complementary; the composite material which is excellent in mechanical performance, good in biocompatibility and good in biodegradability is obtained, and the medical nanofiber reinforced hydrophilic composite material is moderate in degradation cycle, cannot cause a postoperative inflammation and the postoperative risk of adhesion, is more suitable for clinical demand.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
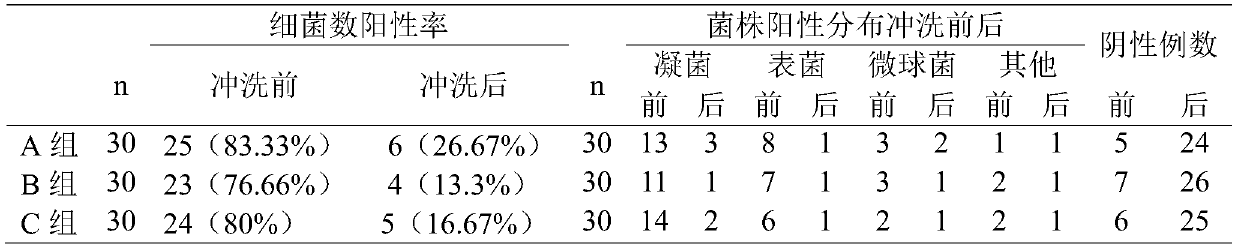
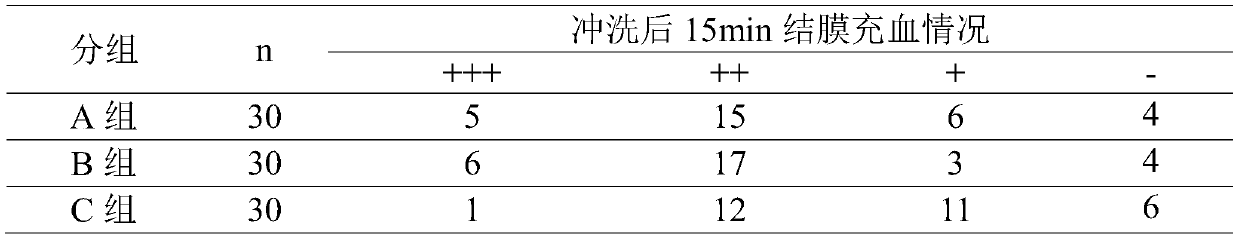
Flushing fluid for external eye operation and preparation method thereof
ActiveCN111001010AImprove stabilityLess irritatingOrganic active ingredientsSenses disorderConjunctivaTissue repair
The invention discloses a flushing fluid for an external eye operation and a preparation method of the flushing fluid, and belongs to the technical field of surgical flushing fluids. The flushing fluid contains the following components: NaCl, KCl, CaCl2H2O, MgCl2.6H2O, C2H3NaO2.3H2O, C6H5Na3O7.2H2O, cocamidopropyl betaine, digitalin, Esculin, an amnion extracting solution, trehalose and hyaluronicacid. Through combined application of all the components, grease in an operation area, tissue debris generated in an operation and the like can be cleaned more easily, the cleanliness of the operation area is improved, the number of flora of conjunctival sacs is reduced, tissue repair can be promoted, postoperative inflammatory response is relieved, and discomforts such as eye edema, hyperemia and dryness are relieved. Meanwhile, the solutions are less irritating, have no toxic or side effect, are low-cost, and are suitable for wide clinical application.
Owner:AFFILIATED HOSPITAL OF WEIFANG MEDICAL UNIV
Application of pirfenidone in preparation of medicaments for controlling proliferative diseases after ophthalmologic operation and eye drops thereof
ActiveCN102349901AImprove stabilityInhibit migrationOrganic active ingredientsBlood disorderPosterior capsule opacificationSide effect
The invention discloses an application of pirfenidone in the preparation of medicaments for controlling proliferative diseases after an ophthalmologic operation and eye drops thereof. According to the invention, pirfenidone is prepared into the eye drops. Experiments show that pirfenidone has good stability and good ocular tissue permeability, can inhibit HLECs migration and propagation, and has no cytotoxicity to HLECs within the action range (0-1mg / ml). With pirfenidone being within the range of 0-1%, the eye drops are continuously applied on eyes within a month with safety and with no obvious toxic and side effect. Pirfenidone can be used to delay the generation of PCO after the rabbit corneal Phaco operation, reduce HLECs propagation and minimize the shield of PCO to an optical region. In addition, there is no obvious ocular surface injury or postoperative inflammation aggravation and severe adverse reaction after PFD is applied in the rabbit corneal Phaco operation. Therefore, pirfenidone can be used to control proliferative diseases after an ophthalmologic operation, especially posterior capsule opacification generated after a cataract surgery.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Ophthalmic compositions and methods of use
ActiveUS10555947B2Small amountEliminate side effectsOrganic active ingredientsSenses disorderHerpes zoster keratitisPostoperative inflammation
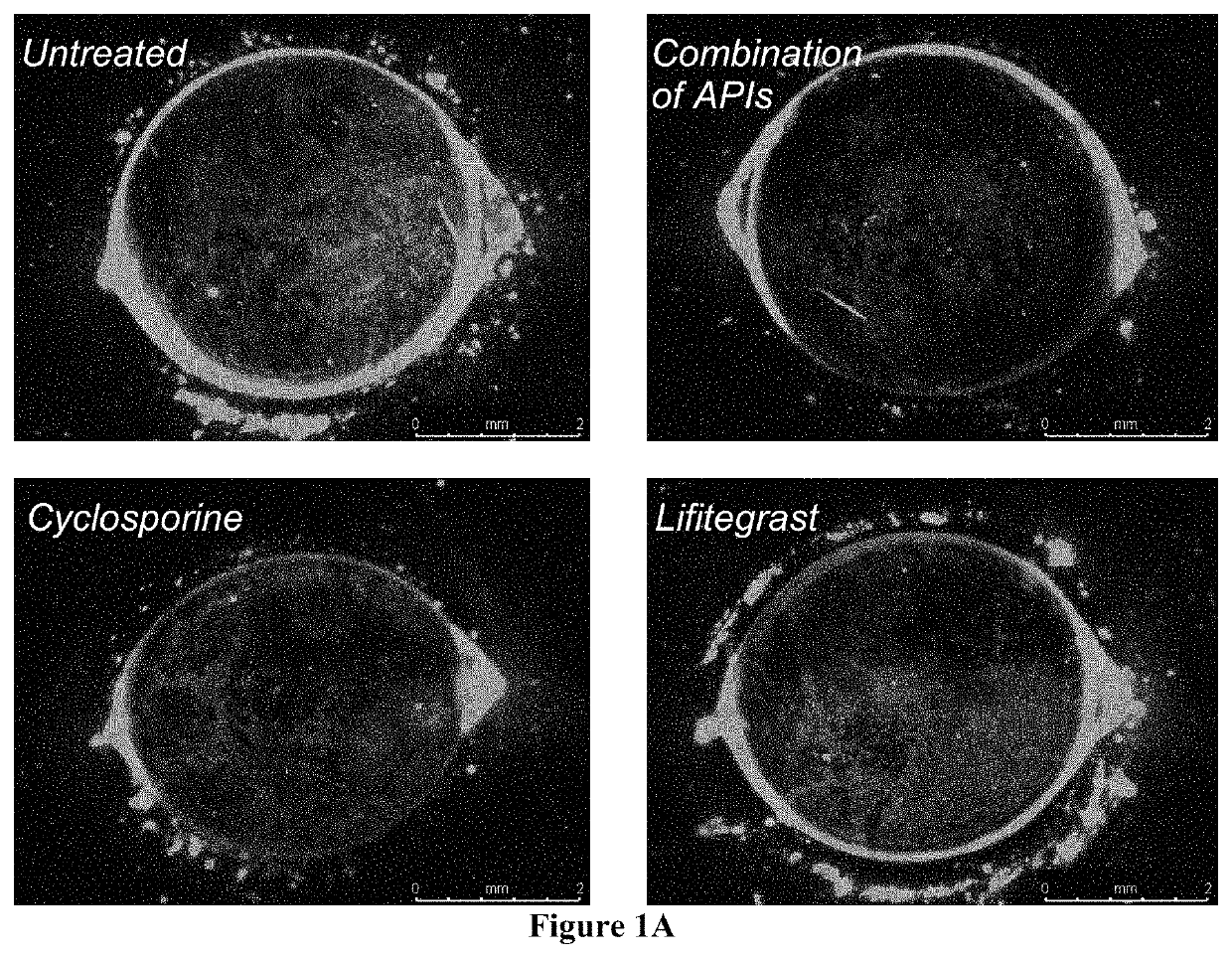
The present invention provides a composition comprising two or more of the following pharmaceutically active compounds: (i) an alpha 2 adrenergic agonist; (ii) a corticosteroid; (iii) a lymphocyte function-associated antigen antagonist; (iv) a non-steroidal anti-inflammatory drug (NSAID); (v) a sodium channel blocker; and (vi) an antibiotic, provided at least one of the pharmaceutically active compound is selected from the group consisting of (i) alpha 2 adrenergic agonist and (ii) corticosteroid. The present invention also provides a method for using such composition to treat an eye disorder such as a dry eye syndrome; ocular graft-versus-host-disease; ocular rosacea; allergic conjunctivitis; autoimmune ocular surface disease; thygeson's superficial punctuate keratopathy; herpes zoster keratitis; Stevens-Johnson syndrome; keratitis; conjunctivitis; blepharitis; blepharochalasis; conjunctivochalasis; blepharoconjunctivitis; blepharokeratoconjunctivitis; post-operative inflammation or pain from ocular surgery; scleritis; episcleritis; anterior uveitis; iritis; cyclitis; ocular surface vascular disorder; ulcerative keratitis; photokeratitis; dacryocystitis; eyelid disorder; congenital alacrima; xerophthalmia; dacryoadenitis; vernal keratoconjunctivitis; pinguecula; and / or ocular surface disorder induced by chemical burns, thermal burns, or physical insult to the ocular surface.
Owner:OCUGEN INC
Methods for treatment of postoperative inflammation with reduced intraocular pressure
Methods of treating a subject after ocular surgery are disclosed. The methods include administering to an eye of a subject in need thereof a composition comprising at least one corticosteroid or an ophthalmically acceptable salt thereof in an ophthalmically acceptable vehicle that can provide a sustained release of the at least one corticosteroid or an ophthalmically acceptable salt thereof. Advantageously, the composition does not result in a statistically significant elevation in intraocular pressure in a statistically significant number of subject eyes when administered twice daily over a period of two weeks.
Owner:SUN PHARMA GLOBAL FZE
Pharmaceutical compositions from carapa guianensis
InactiveCN101018558AAntiallergicAnti-inflammatoryNervous disorderDispersion deliveryDiseaseSide effect
The invention refers to pharmaceutical compositions based on oil extracted from the seeds of Carapa guianensis Aubl and / or tetranortriterpenoid compound separated from the oil and bearing its bioactivity, which has following pharmacological properties: allergy resistance, antiphlogistic activity, acesodyne and immunoregulation, reducing the side effect and lowering the cost. The medicine combination is used to treat and prevent or defend human allergy and inflammatory disease by oral or local treatment. In each case, the compound may be liquid or solid form. The inventive compound for local treatment is nontoxic or low toxicity and provided in a semi-solid type (cream). The inventive medicine combination also provides an important replacement therapy to the allergy disease such as skin and respiratory besides the effects to the different inflammation reaction to the allergen and source of infection. Therefore, the combination can treat rheumatoid, inflammation and modification process, a plurality of hurt, pain and postoperative inflammation, acute pain complex.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
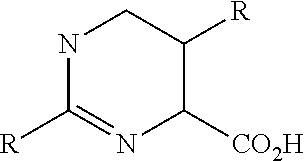
Urea derivatives and uses thereof
The present invention provides novel compounds of any one of Formulae (I)-(III), and pharmaceutical compositions thereof. Also provided are particles (e.g., nanoparticles) comprising compounds of Formula (I)-(III) and pharmaceutical compositions thereof that are mucus penetrating. The invention also provides methods and kits for using the inventive compounds, and pharmaceutical compositions thereof, for treating and / or preventing diseases associated with abnormal or pathological angiogenesis and / or aberrant signaling of a growth factor (e.g., vascular endothelial growth factor (VEGF)), such as proliferative diseases (e.g., cancers, benign neoplasms, inflammatory diseases, autoimmune diseases) and ocular diseases (e.g., macular degeneration, glaucoma, diabetic retinopathy, retinoblastoma, edema, uveitis, dry eye, blepharitis, and post-surgical inflammation) in a subject in need thereof.
Owner:KALA PHARMA
Implantation method of deep flexible brain electrode combined with drug delivery
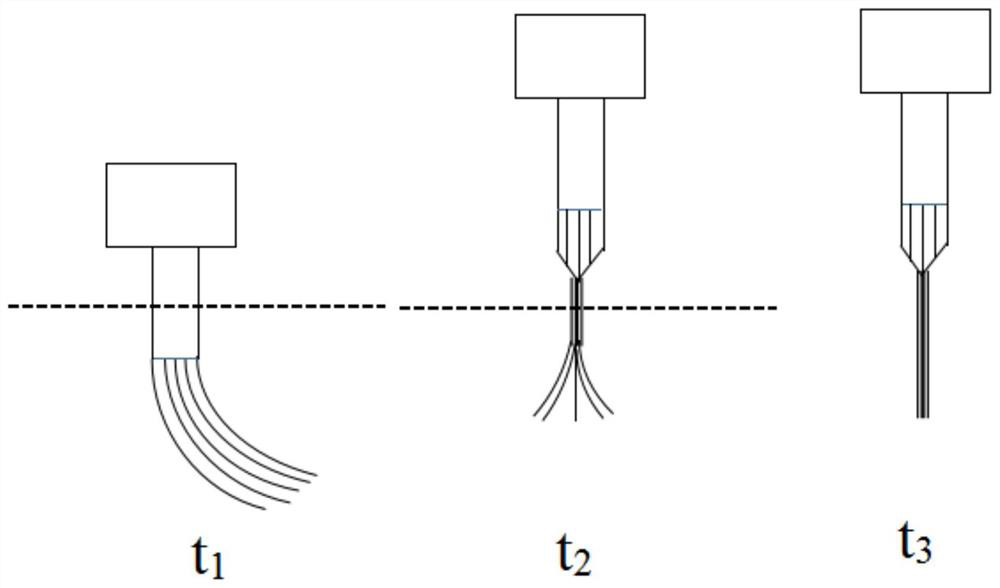
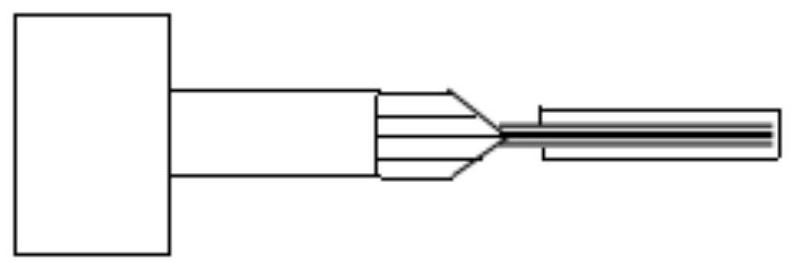
ActiveCN112120696AEasy implantationImprove the success rate of implantationMedical devicesDiagnostic recording/measuringPharmaceutical drugPostoperative inflammation
The invention discloses an implantation method of a deep flexible brain electrode combined with drug delivery. The implantation method comprises a flexible brain electrode pretreatment step and a flexible brain electrode implantation step; the flexible brain electrode pretreatment step specifically comprises the steps of forming a cured protein protection layer on the surface of the flexible brainelectrode, wherein the cured protein protection layer comprises a drug to be delivered. According to the implantation method, the cured protein protection layer is formed on the surface of the flexible brain electrode, so that the Young modulus of the flexible brain electrode can be increased to a degree sufficient to be implanted into the brain, and high biocompatibility and minimally invasive property are achieved. The protein protection layer contains a certain concentration of to-be-delivered drug, so that intraoperative infection and postoperative inflammatory response can be effectivelyinhibited. The implantation operation of the flexible brain electrode is convenient and simple, and the implantation success rate is high.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
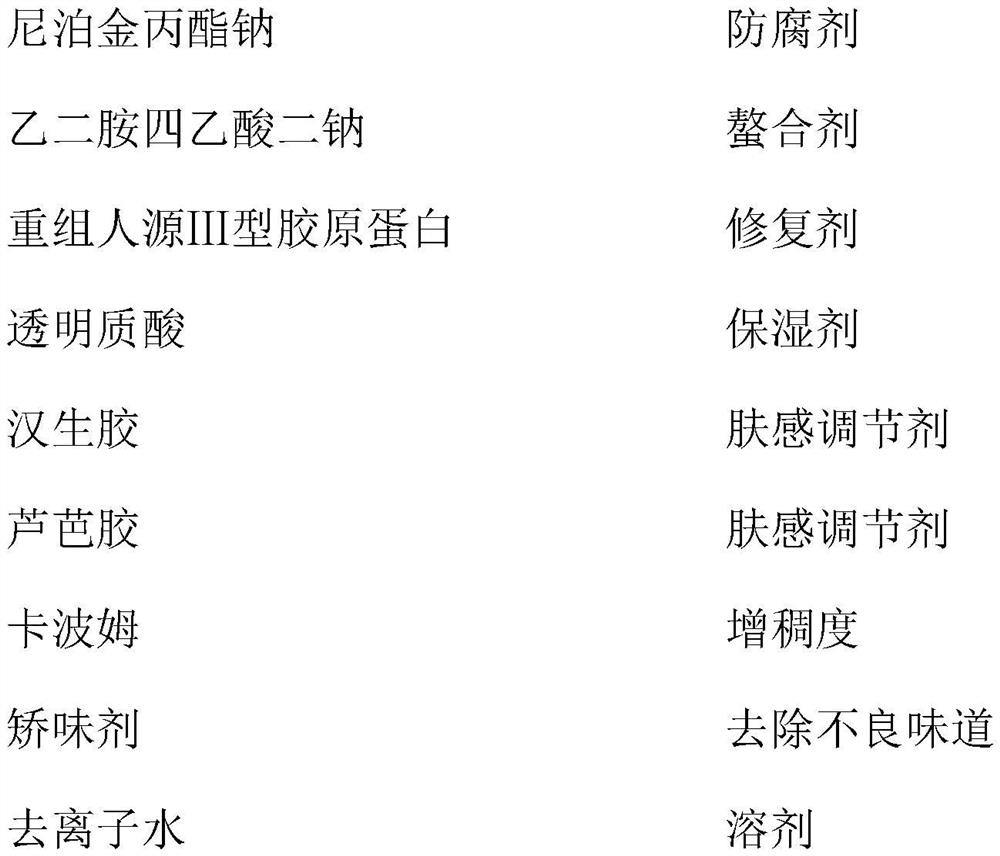
Medical recombinant collagen repair patch, essence of medical recombinant collagen repair patch, and preparation methods of medical recombinant collagen repair patch and essence
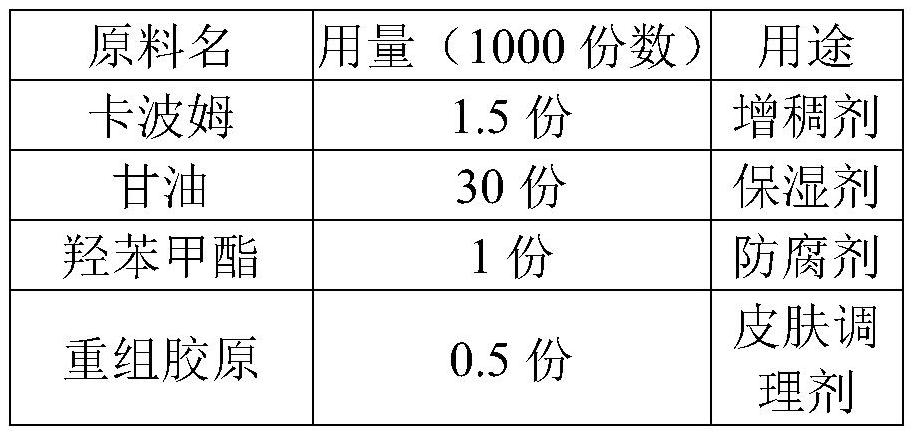
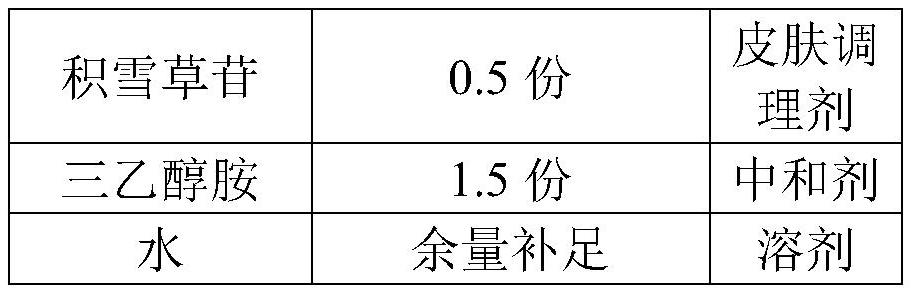
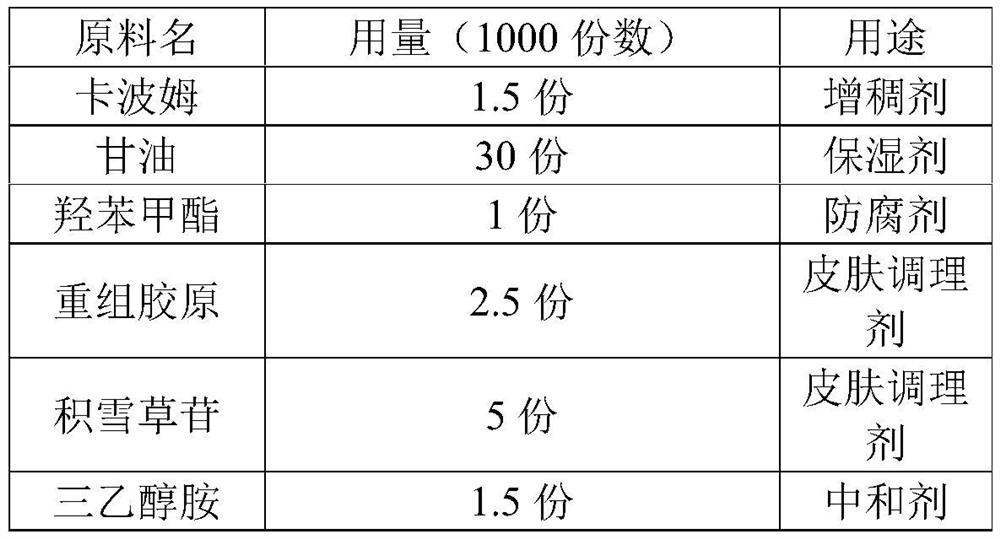
PendingCN113509545AMaintain glossStay flexibleAntibacterial agentsOrganic active ingredientsGlycerolPostoperative inflammation
The invention discloses a medical recombinant collagen repair patch, an essence of the medical recombinant collagen repair patch, and preparation methods of the medical recombinant collagen repair patch and the essence, and belongs to the technical field of medicines. The invention aims to solve the problems of facial acne, hyperpigmentation, superficial scar and laser photon postoperative inflammation. The essence for the medical recombinant collagen repair patch is prepared from, by weight, 1.5 parts of carbomer, 30 parts of glycerol, 1 part of methylparaben, 0.5-5 parts of asiaticoside, 1.5 parts of triethanolamine, 0.5-2.5 parts of recombinant collagen and the balance of water in terms of the total weight of 1000 parts. The repair patch can realize daily face moisturizing and treat light and moderate facial acnes equivalent to I-III levels of acnes, superficial depressed scars, hyperpigmentation, skin allergy, laser photons and the like caused by acne healing and the like are used for treating inflammation such as postoperative erythema, burning heat after edema and the like, and the product is safe and effective.
Owner:哈尔滨运美达生物科技有限公司
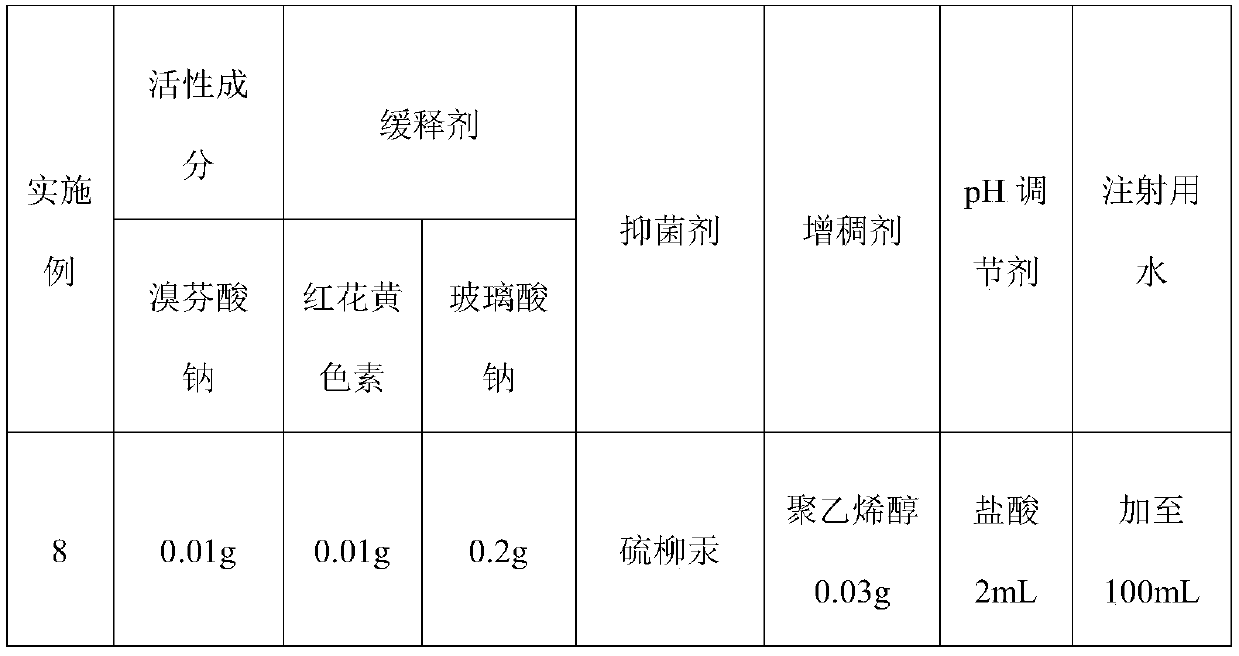
Sustained-release bromfenac sodium ophthalmic preparation
ActiveCN110538138AImprove securityLess irritatingOrganic active ingredientsSenses disorderOcular bioavailabilitySide effect
Owner:合肥华威药业有限公司
Traditional Chinese medicine decoction for auxiliary treatment of appendicitis and preparation method thereof
InactiveCN105055599AAccelerated dissipationReduce inflammationAntipyreticAnalgesicsAppendicitisRemove blood
The invention discloses a traditional Chinese medicine decoction for auxiliary treatment of appendicitis; the traditional Chinese medicine decoction is prepared from the following raw material medicines in parts by weight: 30-40 parts of honeysuckle, 10-14 parts of liquorice, 10-14 parts of dandelion, 8-10 parts of safflower, 6-8 parts of radix aucklandiae, 13-17 parts of dried tangerine or orange peel and 3-7 parts of fructus cinnamomi. The invention also provides a preparation method of the decoction. The decoction has the advantages of promoting blood circulation to remove blood stasis, improving postoperative intestinal blood circulation, and promoting dissipation of inflammation, can reduce the patient postoperative inflammation, bleeding and other symptoms, reduces the pain of patients, and accelerates the postoperative recovery of the patients.
Owner:吴倩颖
Sustained-release coating of degradable medicaments coating bracket
InactiveCN101259295ASmooth releaseDuring release, the drug releases at a uniform rateStentsSurgerySide effectDrug-Coated Stents
The invention belongs to a drug release coating of a degradable drug-coated stent of coronary stents in the medicinal material field. The drug release coating is composed of drug for inhibiting thrombosis and tissue hyperplasia and a polymer carrier. The drug can be rapamycin, tacrolimus or paclitaxel, wherein, the polymer carrier in the drug release coating is poly ortho ester (POE). The invention is the drug release coating of the degradable drug-coated stent which is especially applied to coronary interventional operations. With the poly ortho ester used as the carrier, the stent can lead to the long-acting release of the drug coated on the surface of the stent, improve the stability of the drug, achieve more ideal effects of the drug release, prolong the physical activity of the drug and reduce the toxic and side effects of the drug, thereby preventing the occurrence of postoperative inflammation effectively.
Owner:天津市凯迪亚医疗器械有限公司
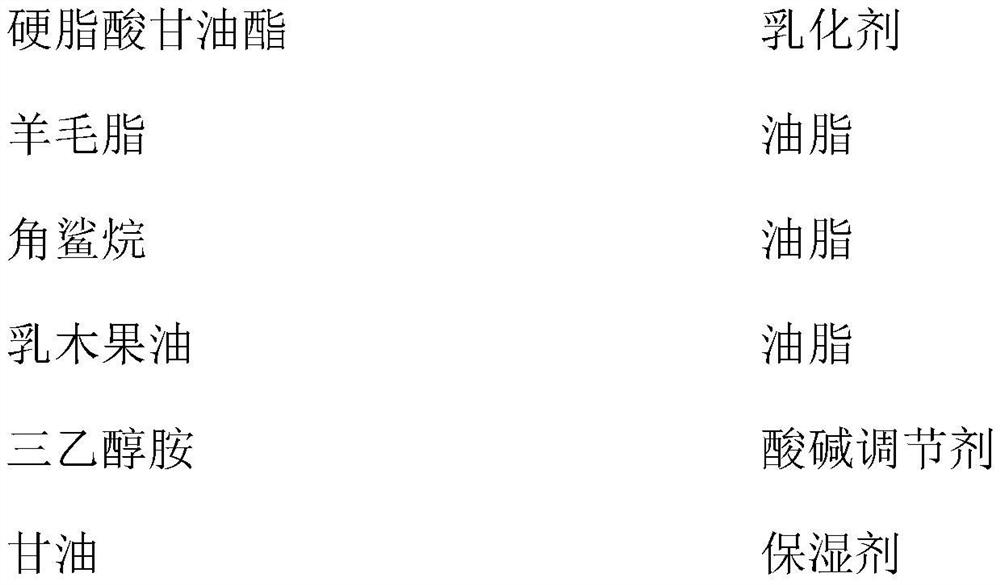
Medical skin repair nursing film and preparation method thereof
PendingCN113274337AAnti agingPromote growthCosmetic preparationsToilet preparationsSkin repairGlycerol
The invention provides a medical skin repair nursing film. The medical skin repair nursing film comprises 2% of stearin, 3% of lanolin, 6% of squalane, 1.5% of shea butter, 0.15% of triethanolamine, 5% of glycerin, 0.2% of sodium propylparaben, 0.02% of disodium ethylenediamine tetraacetate, 0.8% of recombinant human-derived type III collagen, 0.1% of hyaluronic acid, 0.1% of xanthan gum, 2% of lubrajel, 0.15% of carbomer, 0.1% of a flavoring agent and the balance of deionized water. The medical skin repair nursing film provided by the invention can not only carry out daily facial moisturizing, but also treat mild and moderate facial acne (equivalent to I-III level of acne), superficial sunken scar caused by acne healing and other reasons, pigmentation, skin allergy, laser photon and other postoperative inflammation such as erythema, edema and scorching hot, and is safe and effective.
Owner:吉林省国大生物工程有限公司
Application of pirfenidone in preparation of medicaments for controlling proliferative diseases after ophthalmologic operation and eye drops thereof
ActiveCN102349901BImprove stabilityInhibit migrationOrganic active ingredientsPosterior capsule opacificationSide effect
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Ophthalmic drug delivery method
ActiveUS10842669B2Easy to processPromote recoveryPowder deliveryOrganic active ingredientsDiabetic retinopathyUveitis
A method of treatment is disclosed herein. The method includes administering to a patient in need thereof a polymer implant device containing a biocompatible drug, or a plurality of nanoparticles or microparticles conjugated with the biocompatible drug, the biocompatible drug comprising one or more Rock inhibitors, one or more Wnt inhibitors, one or more integrin inhibitors, and / or one or more glycogen synthase kinase 3 (GSK-3) inhibitors, the patient having a medical condition selected from the group consisting of dry eye, glaucoma, retinal detachment, retinal degeneration, age-related macular degeneration, a cataract, uveitis, a corneal genetic disease, postoperative inflammation, immune-related inflammatory processes, diabetic retinopathy, a side effect occurring after cataract surgery, a side effect occurring after refractive surgery, and combinations thereof. The administration of the biocompatible drug to the patient treats the medical condition, reduces the symptoms associated with the medical condition, enhances nerve regeneration, and / or alleviates the medical condition.
Owner:PEYMAN GHOLAM A
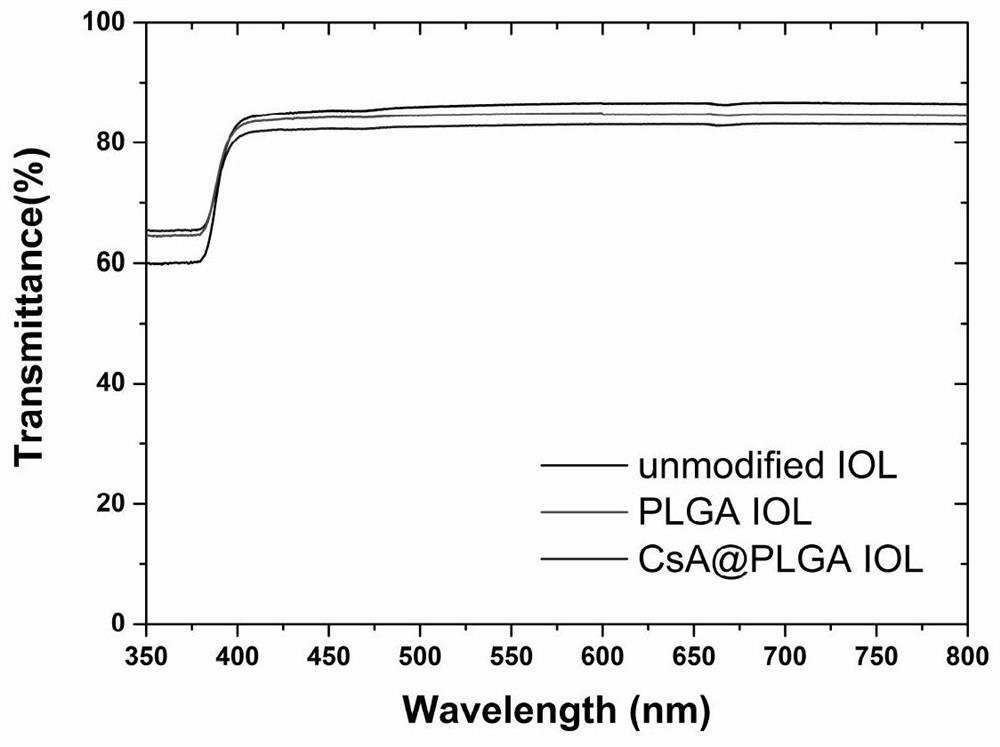
Intraocular lens with concentric ring pattern and surface modified by degradable drug sustained-release coating and preparation method of intraocular lens with concentric ring pattern and surface modified by degradable drug sustained-release coating
PendingCN113413237AAvoid complicationsAvoid turbidityMedical devicesIntraocular lensOphthalmology departmentIntraocular inflammation
The invention relates to the field of medical implant materials and instrument surface modification in the ophthalmology department, in particular to a foldable intraocular lens with a concentric ring pattern, drug sustained release function and a surface modified by a degradable drug sustained-release coating, and a preparation method of the foldable intraocular lens. The degradable drug sustained-release coating with the concentric ring pattern is formed by spin-coating a sustained-release drug composition on the surface of the intraocular lens, and the sustained-release drug composition comprises a degradable polymer, a drug and an organic solvent. The spin-coating technology is applied, the drug sustained-release coating has the special concentric ring pattern with the thin center and the thick periphery, the influence on refraction, stability and foldability of the intraocular lens is small, and the drug sustained-release coating is not prone to falling off and can be stably attached to the surface of the intraocular lens, through drug reasonable selection, the intraocular lens can be used for effectively preventing various common complications after cataract phacoemulsification surgery, such as posterior capsular opacity, intraocular inflammation and other postoperative inflammatory reactions, and the surgery effect is guaranteed.
Owner:WENZHOU MEDICAL UNIV
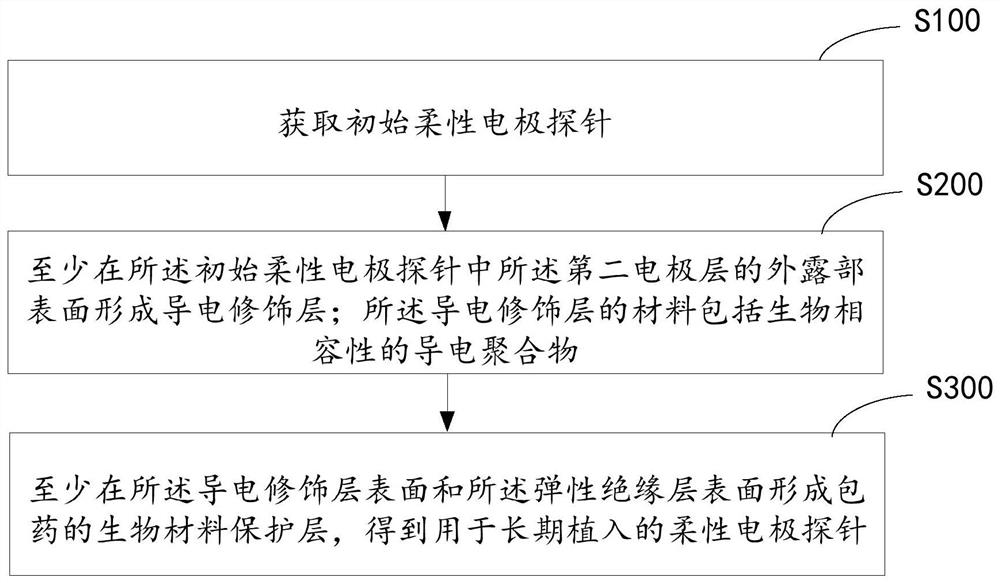
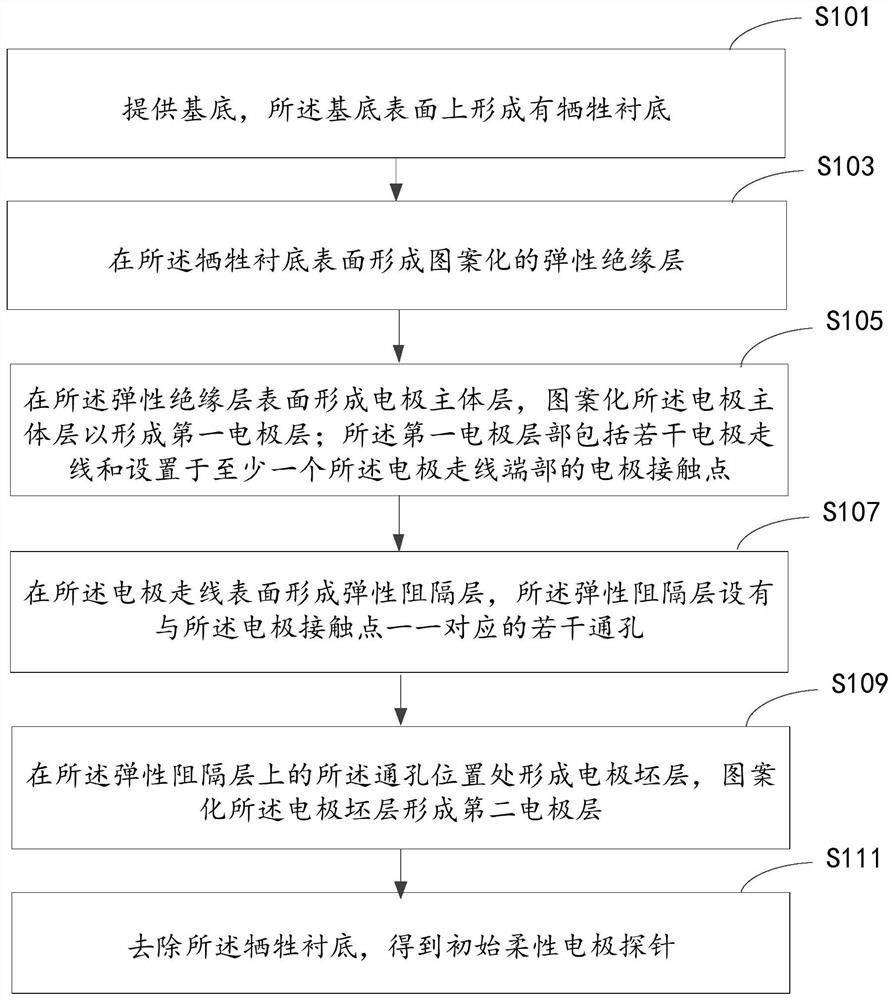
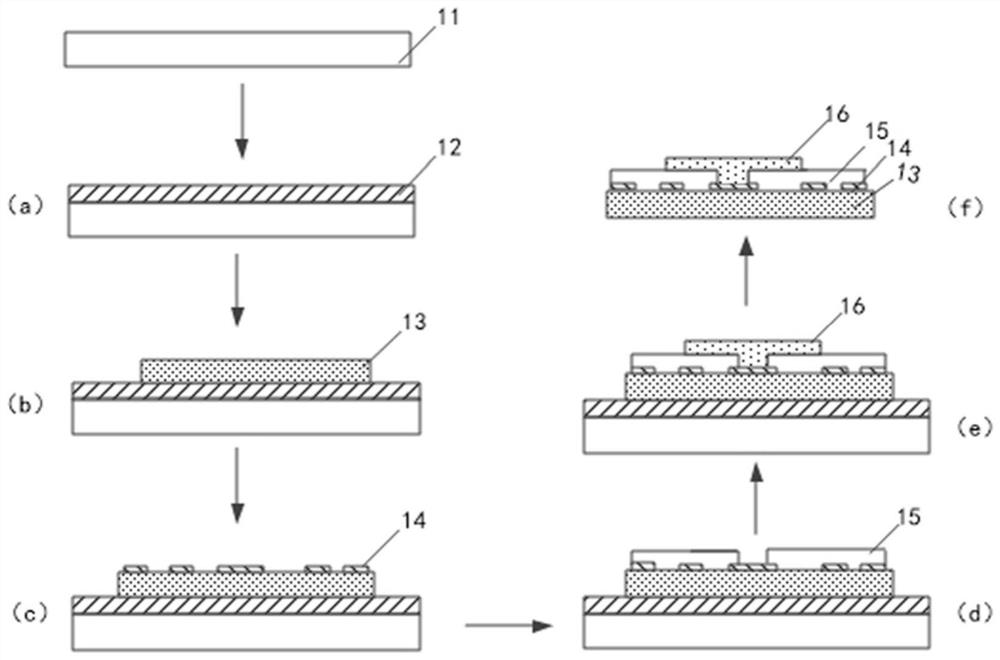
Flexible electrode probe for long-term implantation and its preparation method and device
ActiveCN112244839BReduce mechanical lossInhibitory reactivityDiagnostic recording/measuringSensorsConductive polymerPostoperative inflammation
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Treating postoperative mechanical stress with an ectoine
InactiveUS20160106747A1Enhance water accretionStabilize helical structure of proteinBiocideOrganic active ingredientsDamages tissuePostoperative inflammation
A method of treatment of a patient suffering from postoperative inflammatory stress and pain caused by mechanical impact exerted on portions of the body of the patient that causes damaged tissue wherein the inflammatory stress and pain of the operation outlast the healing of damaged tissue and which is not related to, or caused by, uncontrolled proteolysis. The treatment comprises administering a tetrahydropyrimidine selected from ectoine and / or hydroxyectoine to the patient.
Owner:BITOP
Application of HAP1 in screening neuroinflammation treatment drugs
PendingCN114668861AReduce activation levelInhibition releaseCompounds screening/testingMicrobiological testing/measurementInflammatory factorsPostoperative inflammation
The invention discloses application of HAP1 in screening neuroinflammation treatment drugs, and belongs to the technical field of biological medicines. According to the invention, an HAP1 wild type mouse is selected as a control group, an HAP1 heterozygote mouse is selected as an experimental group, neuropathologic acute pain is constructed, and postoperative inflammatory responses of the two groups of mice are respectively detected. Results show that by reducing the expression of the HAP1, the inflammatory response can be obviously reduced, the activation level of glial cells is reduced, and the release of inflammatory factors is inhibited; and finally, the effect of controlling neuroinflammation is achieved. The discovery can provide a new molecular target for pain research.
Owner:NANTONG UNIVERSITY
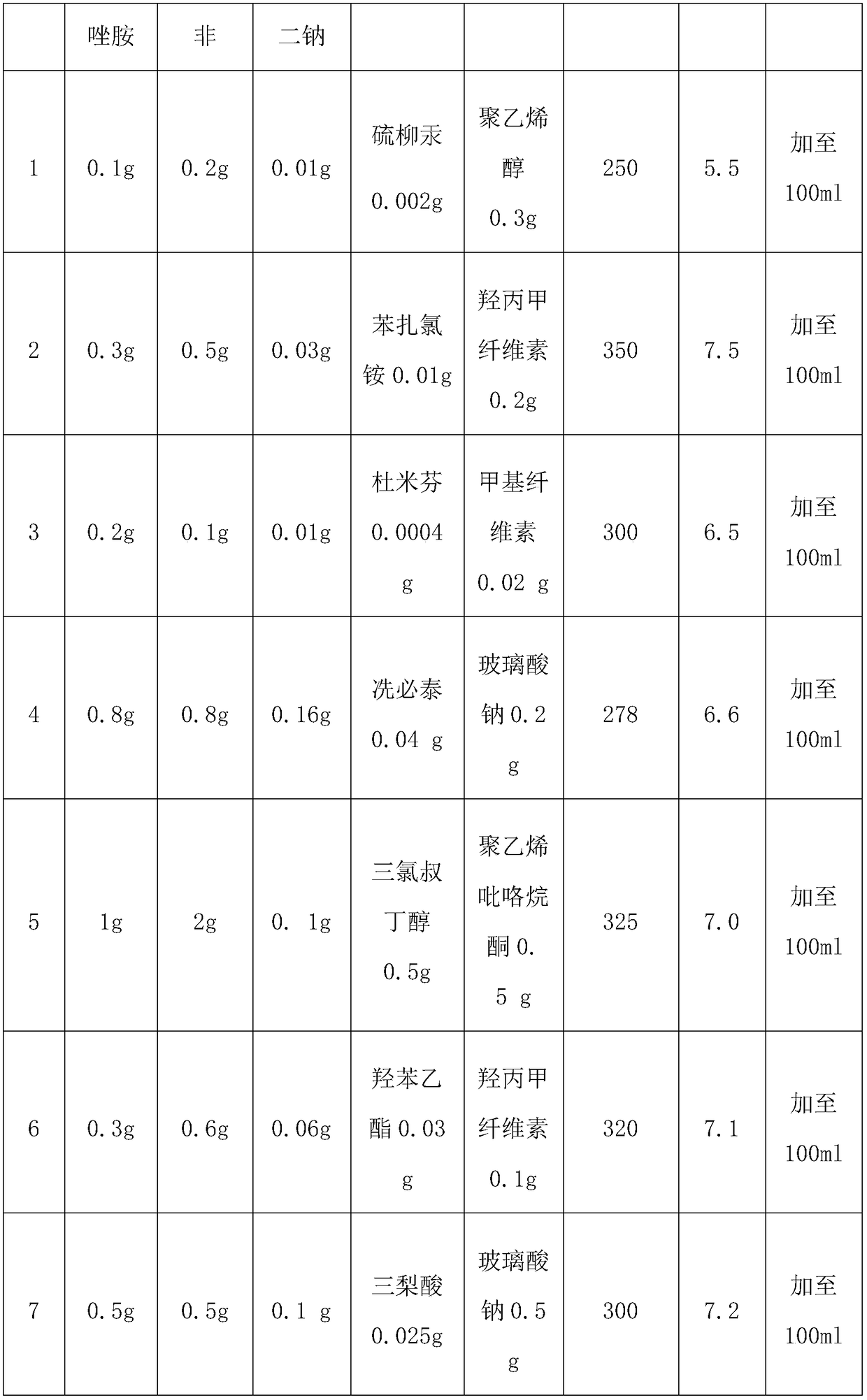
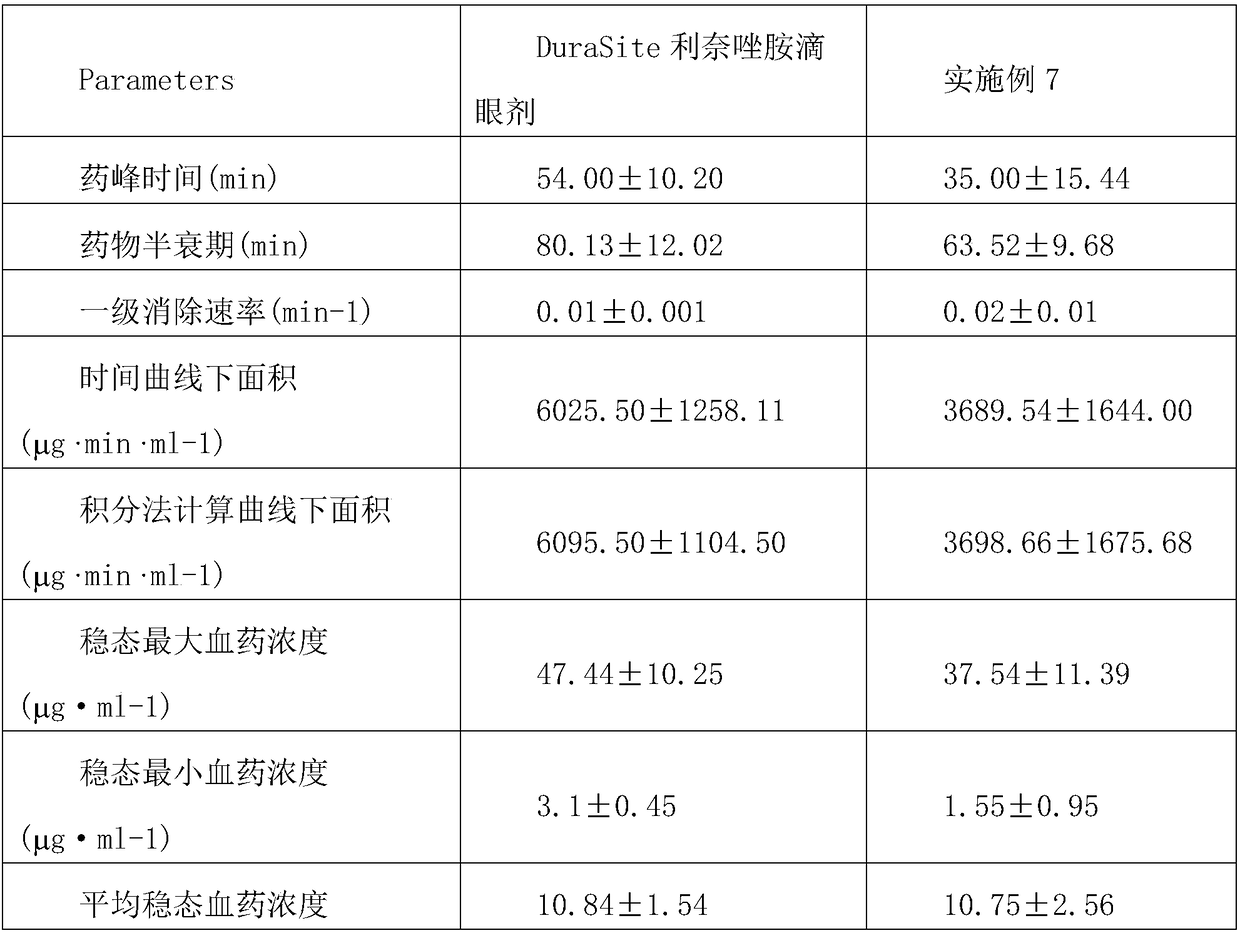
Slow release type linezolid preparation for eyes as well as preparation method and application thereof
InactiveCN108553407ASignificant and long-lasting effectGood intraocular penetrationAntibacterial agentsOrganic active ingredientsOcular bioavailabilityDisease
The invention relates to a slow release type linezolid preparation for eyes as well as a preparation method and application thereof. The slow release type linezolid preparation is prepared from the following components: linezolid, polycarbophil, disodium edetate, an auxiliary material and water for injection, wherein the mass ratio of linezolid to polycarbophil to disodium edetate is 1 to (0.5 to2) to (0.05 to 0.2); the mass percent of linezolid is 0.1 percent to 1 percent. Dosage form of the preparation comprise eye drops, eye ointment and eye gel. The slow release type linezolid preparationhas a remarkable and long-lasting pharmaceutical effect, has good intraocular penetrability and relatively high intraocular bioavailability and has the advantages of strong penetration force, strongtargeting effect and small toxic and side effects; the slow release type linezolid preparation is suitable for treating and preventing outer eye and anterior segment diseases caused by non-infection inflammations and postoperative inflammations, and has good antibacterial and inflammation-diminishing effects, small irritation and high safety; raw materials are easy to obtain, a preparation processis simple and the cost is low; industrialized large-scale production can be realized and the economic benefit is remarkable.
Owner:广州君博医药科技有限公司
Beta-tricalcium phosphate bearing tetracycline and preparation method thereof
InactiveCN101618231AHigh drug loadingAvoid violent releaseProsthesisSolid componentTetracycline Hydrochloride
The invention provides a Beta-tricalcium phosphate bearing tetracycline. The tetracycline is absorbed on the surface of the Beta-tricalcium phosphate, and the mass ratio of the tetracycline and the Beta-tricalcium phosphate is (0.001-0.1):1. The invention also provides a preparation method of the Beta-tricalcium phosphate bearing tetracycline, comprising the following steps: dissolving teline powder in a tetracycline solution formed in deionized water, uniformly mixing the calcined Beta-tricalcium phosphate powder and the tetracycline solution, extracting a solid component in the mixed solution, finally drying the solid component, and preparing and obtaining Beta-tricalcium phosphate powder bearing tetracycline on the surface. The Beta-tricalcium phosphate prepared by the method has better tetracycline bearing capacity, is used for repairing and replacing osseous tissues and also effectively eliminates postoperative inflammation.
Owner:HUAZHONG UNIV OF SCI & TECH
Stapling staple with anti-inflammatory function and preparation method thereof
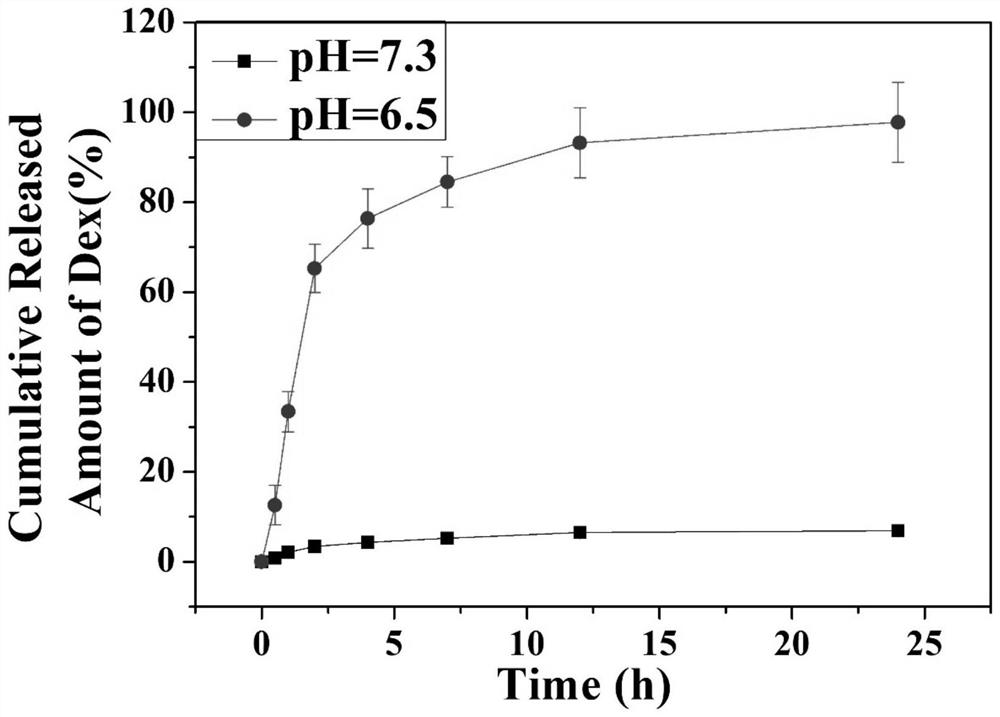
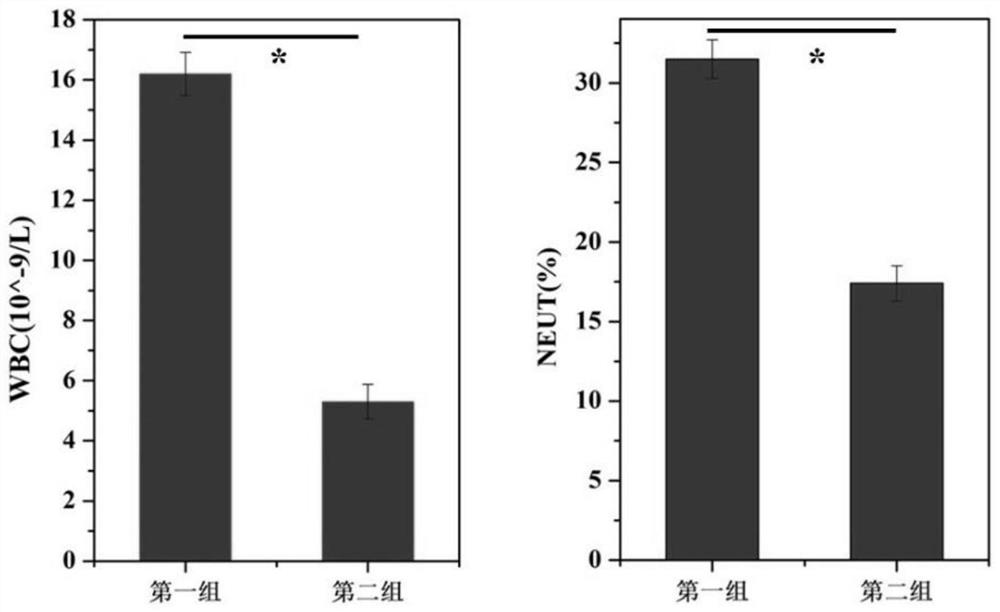
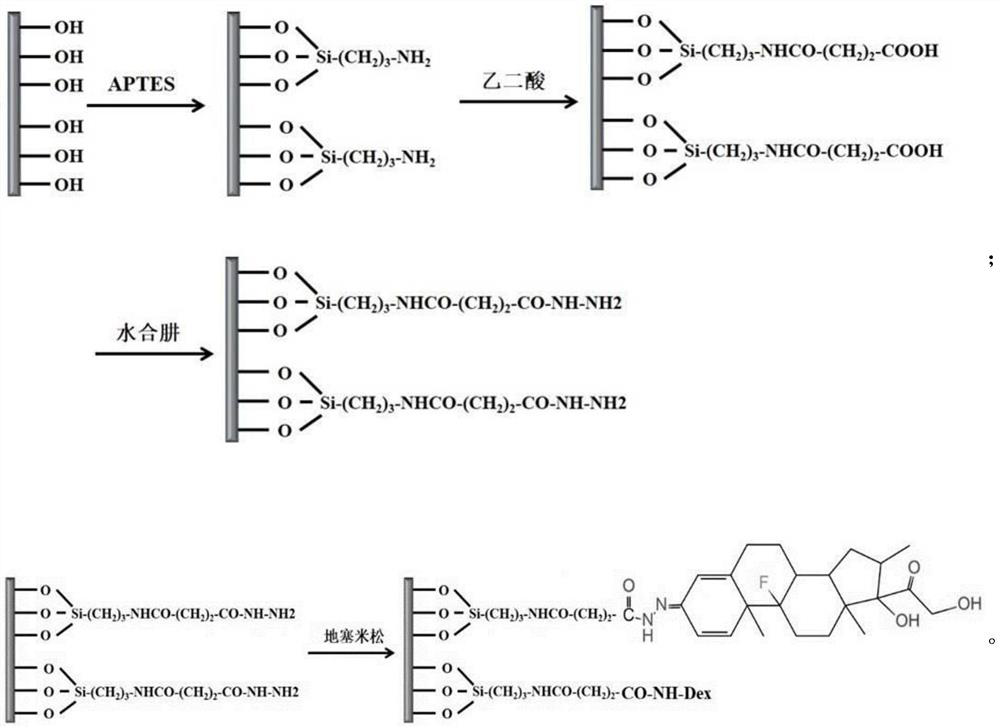
ActiveCN112190772BImprove the quality of lifePlay a timely anti-inflammatory effectSurgeryMetallic material coating processesDexamethasoneHydrazone
The invention discloses a staple with anti-inflammatory function and a preparation method thereof. The surface of the staple is coupled with a pH-responsive anti-inflammatory agent release layer; the preparation method comprises: using a plasma surface treatment instrument to carry out surface hydroxylation treatment on the staple; Then, the anastomotic surface is subjected to surface hydrazide treatment; finally, the surface hydrazide staple is soaked in an ethanol solution containing excess dexamethasone to prepare a staple with anti-inflammatory function. The staple with anti-inflammatory function provided by the present invention does not change the mechanical properties, but since the surface is modified with anti-inflammatory drugs such as dexamethasone through hydrazone bonds, it can release dexamethasone at the inflammatory site when postoperative inflammation occurs, playing a role The effect of timely anti-inflammatory is much higher than that of oral and injection anti-inflammatory drugs, and it will not bring systemic side effects and improve the quality of life of patients.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Split type device for healing patellar claw
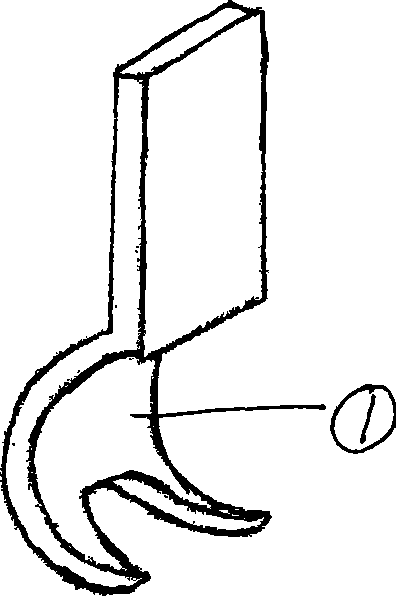
InactiveCN101411641ARelieve painAvoid flexion restrictionInternal osteosythesisPostoperative inflammationOrthodontics
The invention relates to a split-type patella jaw contractor, which comprises a cambered jaw the shape of which corresponds to that of a split-type patella jaw, wherein the cambered jaw is provided with a left cambered jaw and a right cambered jaw which correspond to each other; the left cambered jaw is provided with two jaws; the right cambered jaw is provided with a jaw; a vertical mounting plate is fixed on the upper end of each cambered jaw; a rectangular rod is vertically fixed on the mounting plate of the left cambered jaw; a strip-shaped surface of the rectangular rod is provided with continuous teeth to form a toothed rack; a hole and a sleeve for the rectangular rod to pass through are arranged on the mounting plate of the right cambered jaw; and a spring knob for braking and loosening the rectangular rod is arranged on the sleeve. The spit-type patella jaw contractor has the advantages of small volume, convenient operation, avoidance of bending limitation after healing of the knee joint, avoidance of postoperative inflammation and pain, short surgical time, simultaneously capability of performing pressurization between broken ends well and tightly combining the patella jaw and the patella, and short healing time.
Owner:李志海 +3
Implantation method of deep flexible brain electrodes combined with drug delivery
ActiveCN112120696BEasy implantationImprove the success rate of implantationMedical devicesDiagnostic recording/measuringPharmaceutical drugPostoperative inflammation
The invention discloses an implantation method of a deep flexible brain electrode combined with drug delivery. The implantation method includes a flexible brain electrode pretreatment step and a flexible brain electrode implantation step. The flexible brain electrode pretreatment step specifically includes: A cured protein protective layer is formed on the surface of the flexible brain electrode, wherein the cured protein protective layer includes drugs to be delivered. The present invention forms a solidified protein protective layer on the surface of the flexible brain electrode, and its Young's modulus can be raised enough to be implanted in the brain, and has good biocompatibility and minimal invasiveness; Containing a certain concentration of the drug to be delivered can effectively inhibit intraoperative infection and postoperative inflammatory response; the implantation operation of the flexible brain electrode of the present invention is relatively convenient and simple, and the implantation success rate is high.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com