Sustained-release bromfenac sodium ophthalmic preparation
A technology of bromfenac sodium and ophthalmic preparations, applied in the field of sustained-release bromfenac sodium ophthalmic preparations, can solve the problems of large side effects, easy occurrence of corneal hyperemia, etc., and achieves small side effects, good intraocular penetration, Achieve the effect of industrial mass production
Active Publication Date: 2019-12-06
合肥华威药业有限公司
View PDF4 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] At present, there are many kinds of bromfenac sodium ophthalmic preparations on the market in China. The original research is bromfenac sodium eye drops (trade name: Pronaac) produced by Senshou Pharmaceutical Co., Ltd., but its side effects are relatively large. Dosage and method of use sho
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
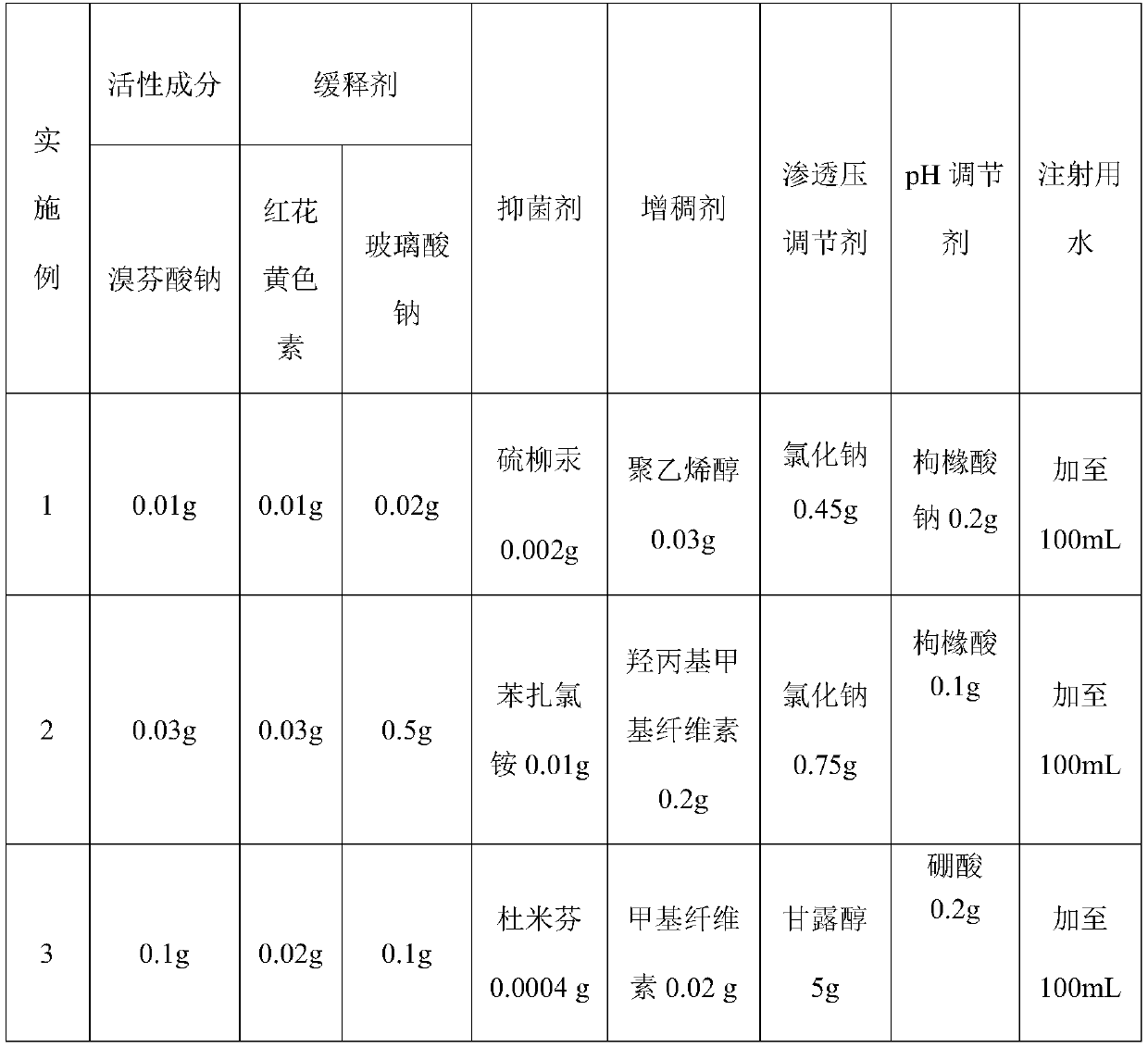
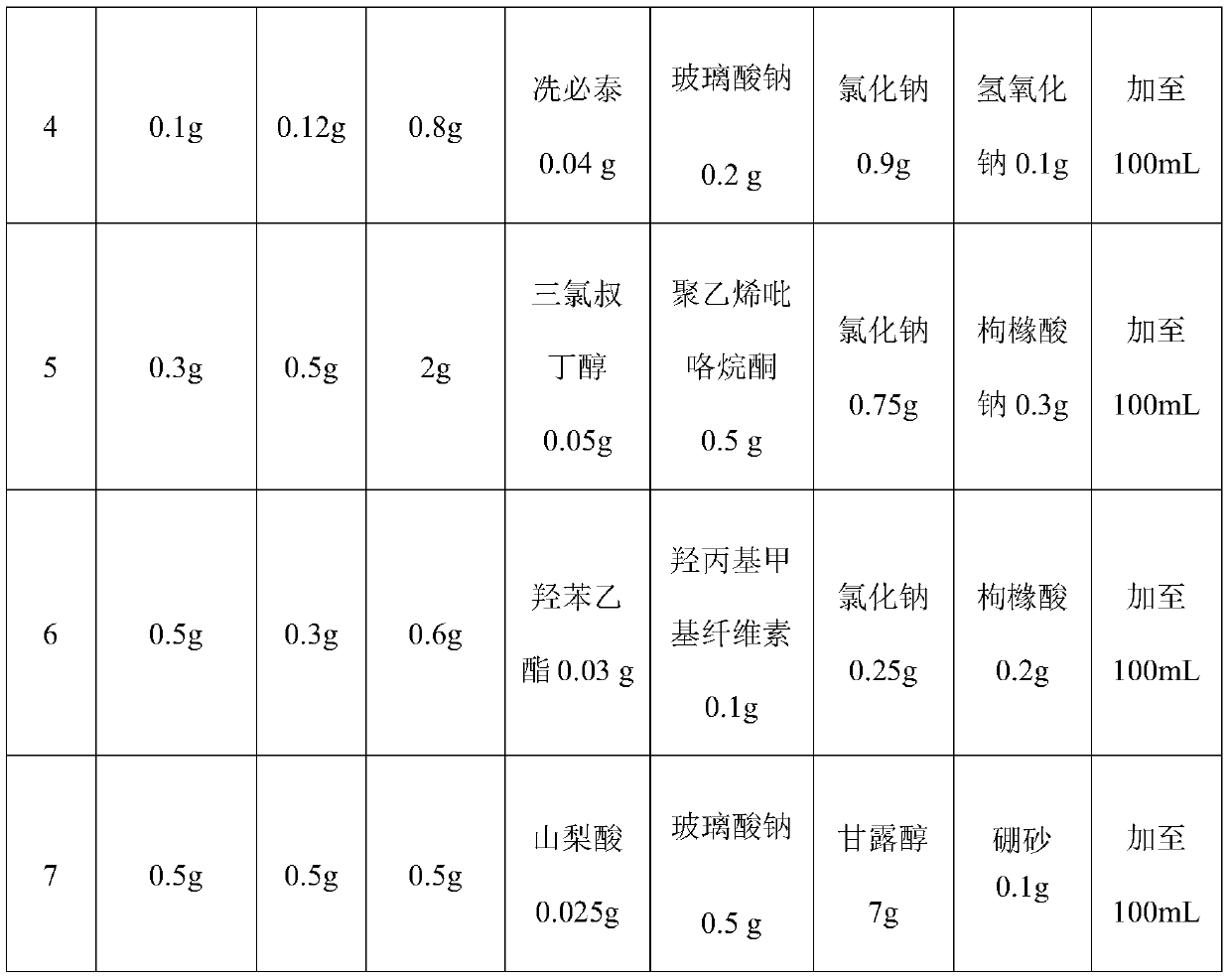
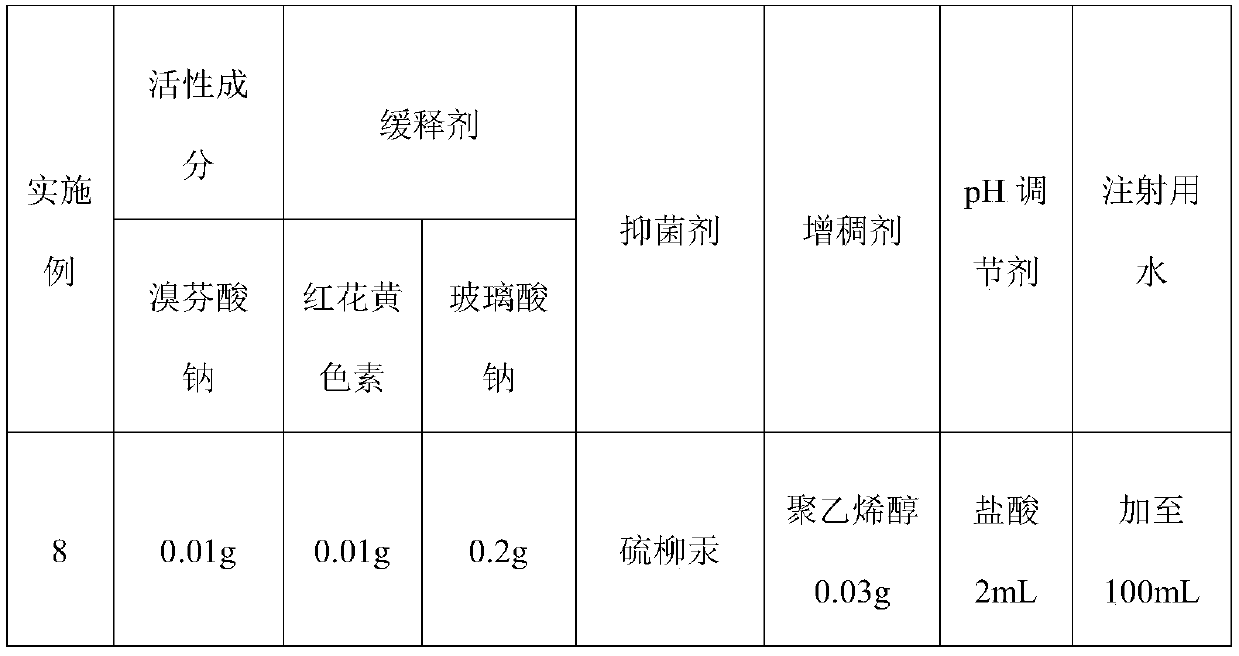
The invention relates to an ophthalmic pharmaceutical preparation for treating conjunctivitis, scleritis and eye postoperative inflammations, in particular to a sustained-release bromfenac sodium ophthalmic preparation. The sustained-release bromfenac sodium ophthalmic preparation provided by the invention is prepared from bromfenac sodium, carthamin yellow, sodium hyaluronate and a pharmacologically acceptable carrier. The sustained-release bromfenac sodium ophthalmic preparation provided by the invention has the advantages of good intraocular penetrability, high intraocular bioavailability,high penetration, high targeting effect, small toxic and side effects and the like, and is suitable for treating inflammatory eye diseases and used for treating conjunctivitis, scleritis and eye postoperative infection.
Description
technical field [0001] The invention relates to an ophthalmic pharmaceutical preparation for treating conjunctivitis, scleritis and eye postoperative inflammation, in particular to a sustained-release bromfenac sodium ophthalmic preparation. Background technique [0002] Inflammation is the manifestation of host tissue response to damaged blood vessels and cells. Tissue damage may be caused by physical or chemical causes, such as pathogen invasion, ischemia, allergies or improper (autoimmune) functioning of immune mechanisms. At this time, arachidonic acid is released from the phospholipids of the cell membrane, and under the catalysis of cyclooxygenase, it forms ring peroxides (PGG2 and PGH2), and then converts them into prostaglandins (PGE2, PGF2, PGD2) through the action of isomerase. ), and prostaglandins are the most powerful ocular inflammatory substances known to date, which can cause damage to the blood-aqueous humor barrier in the eyes, thereby making proteins, var...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/06A61K47/36A61K47/26A61K31/196A61P27/02A61P29/00A61P31/00
CPCA61K9/0048A61K9/06A61K31/196A61K47/26A61K47/36A61P27/02A61P29/00A61P31/00
Inventor 黄凌云王延东
Owner 合肥华威药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com