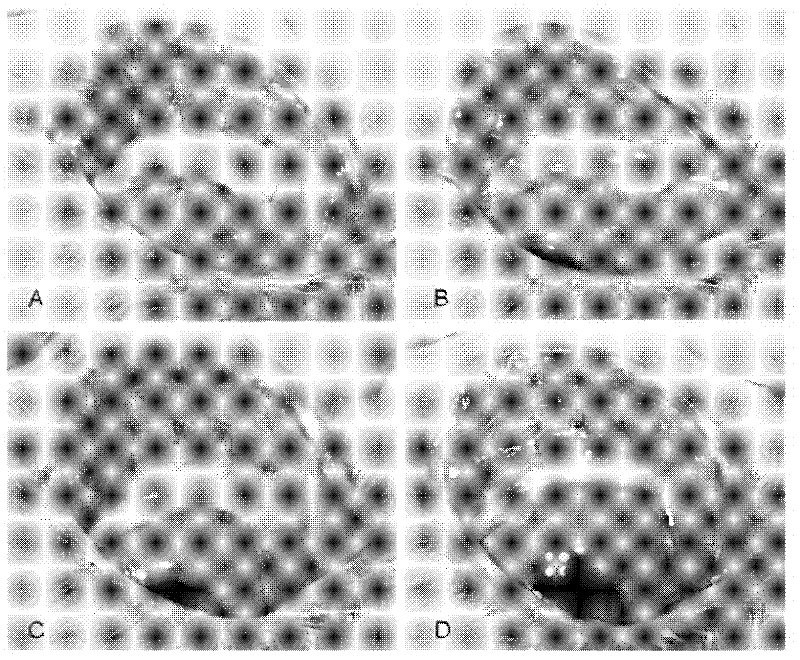
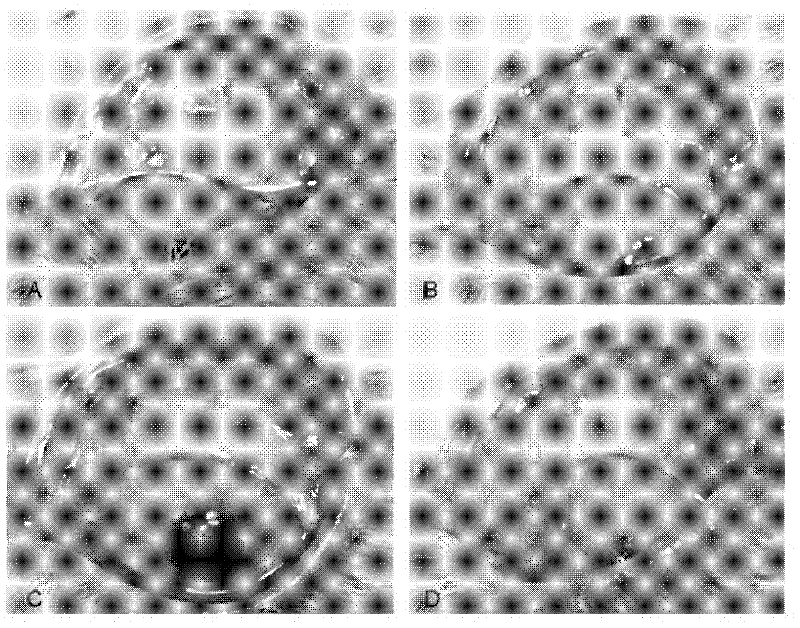
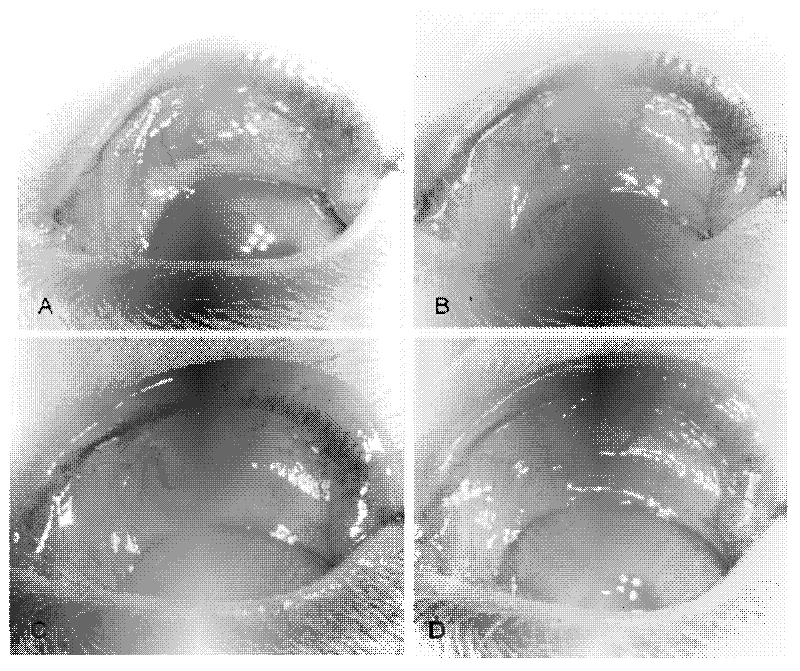
Application of pirfenidone in preparation of medicaments for controlling proliferative diseases after ophthalmologic operation and eye drops thereof
A proliferative disease, ophthalmic surgery technology, applied in blood diseases, drug combinations, extracellular fluid diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Every 100ml of pirfenidone eye drops of the present embodiment is prepared like this: the pirfenidone of 0.1g is moistened with a small amount of water for injection, then dissolves with 3ml 10% (volume fraction) acetic acid aqueous solution, adds water for injection To about 80ml, adjust the pH to about 6 with 1NNaOH, add 0.8g of sodium chloride, 0.2g of sodium citrate, and 0.002g of thimerosal, adjust to pH = 7, add sterile water for injection to 100ml, filter through 0.22μm , aseptically dispensed.
Embodiment 2
[0187] Every 100ml of pirfenidone eye drops of the present embodiment is prepared like this: the pirfenidone of 0.5g is moistened with a small amount of water for injection, then dissolves with 4ml 10% (volume fraction) acetic acid aqueous solution, adds water for injection To about 80ml, adjust the pH to about 6 with 1NNaOH, add 0.75g of sodium chloride, 0.2g of sodium citrate, and 0.002g of thimerosal, adjust to pH = 7, add sterilized water for injection to 100ml, filter through 0.22μm , aseptically dispensed.
Embodiment 3
[0189] Every 100ml of pirfenidone eye drops of the present embodiment is prepared like this: the pirfenidone of 1g is moistened with a small amount of water for injection, then dissolves with 5ml 10% (volume fraction) acetic acid aqueous solution, adds water for injection to About 80ml, adjust the pH to about 6 with 1NNaOH, add 0.7g of sodium chloride, 0.2g of sodium citrate, and 0.002g of thimerosal, adjust to pH = 7, add sterile water for injection to 100ml, filter at 0.22μm, Aseptically dispensed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com