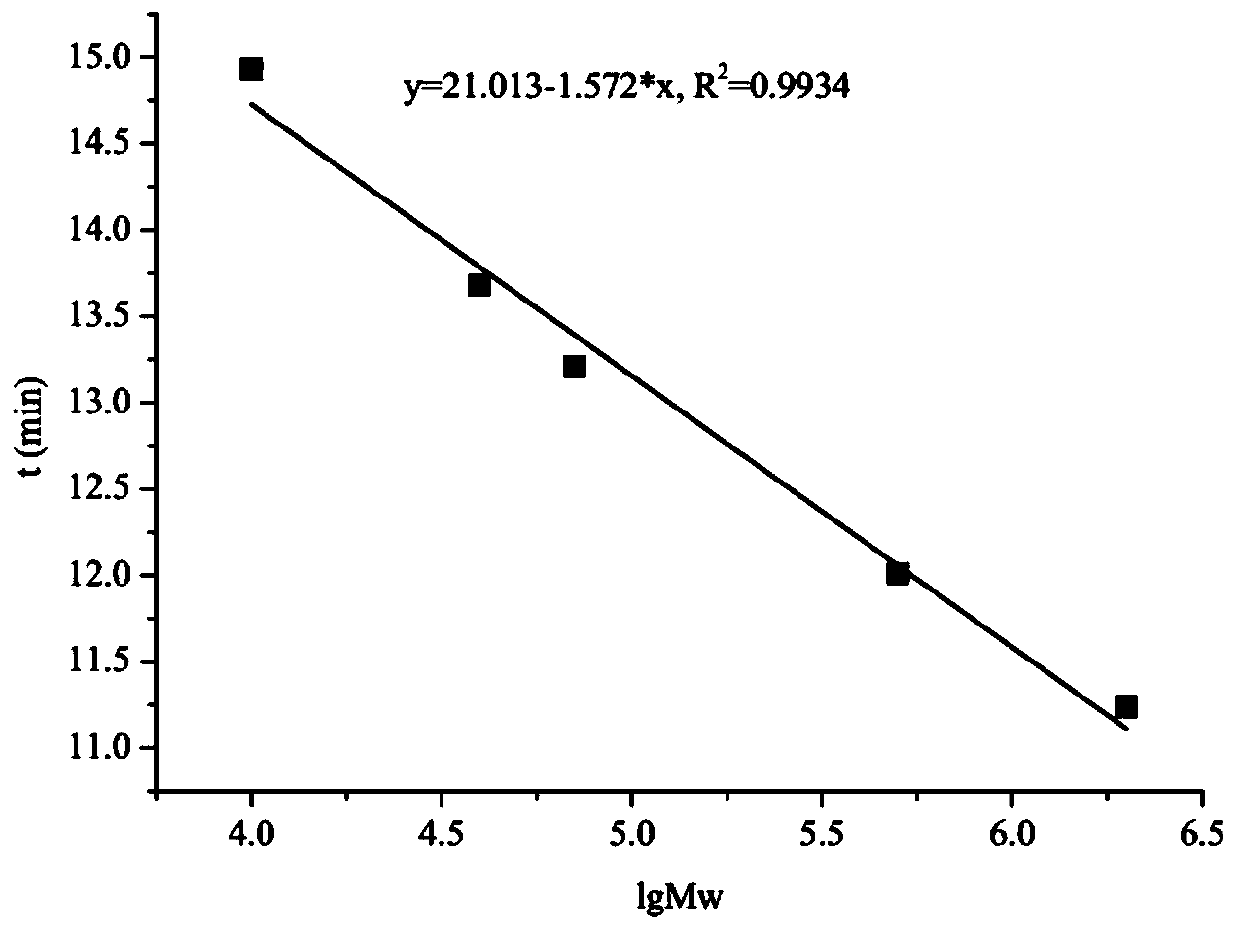
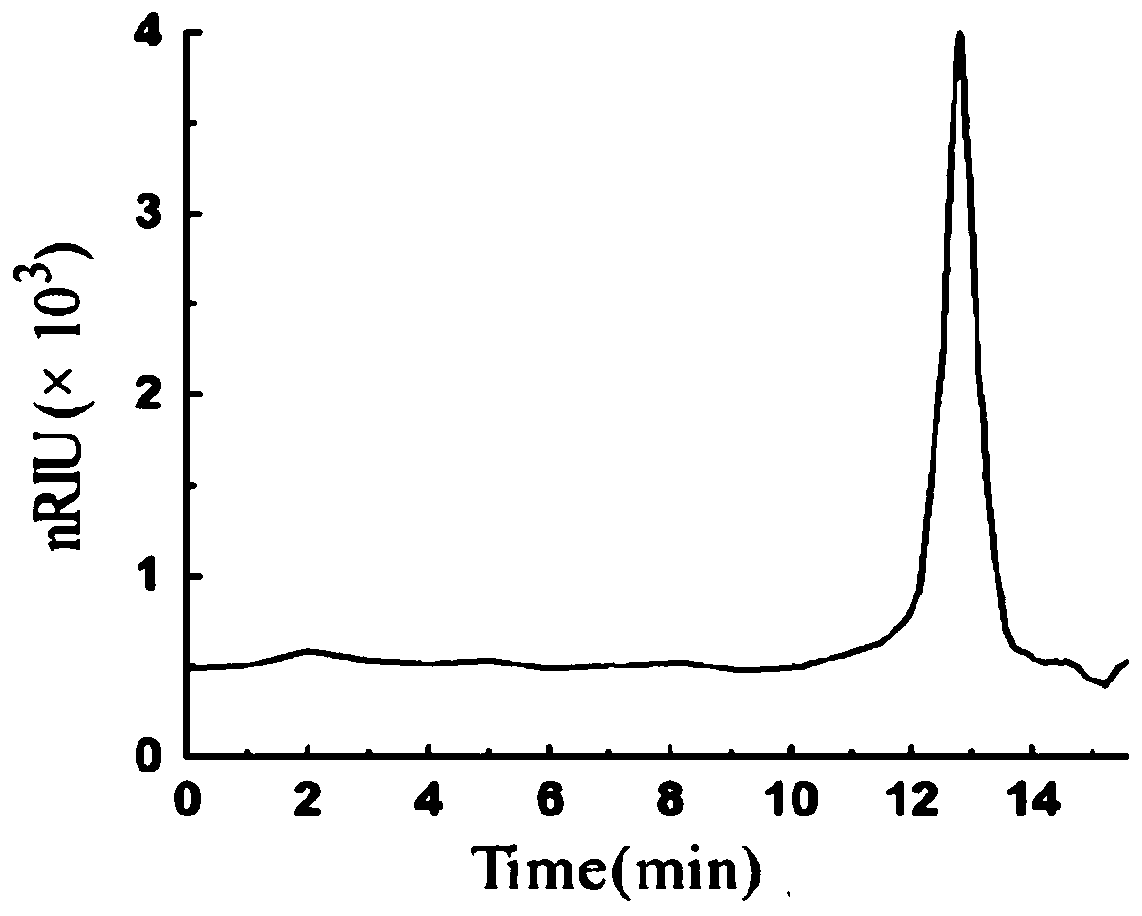
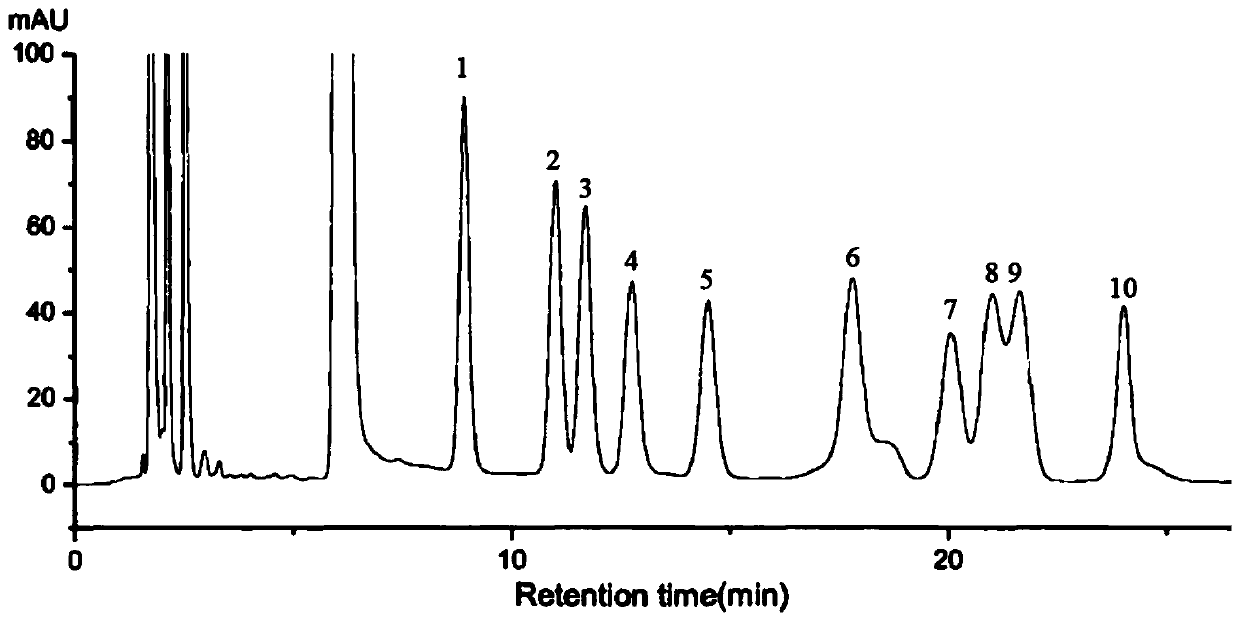
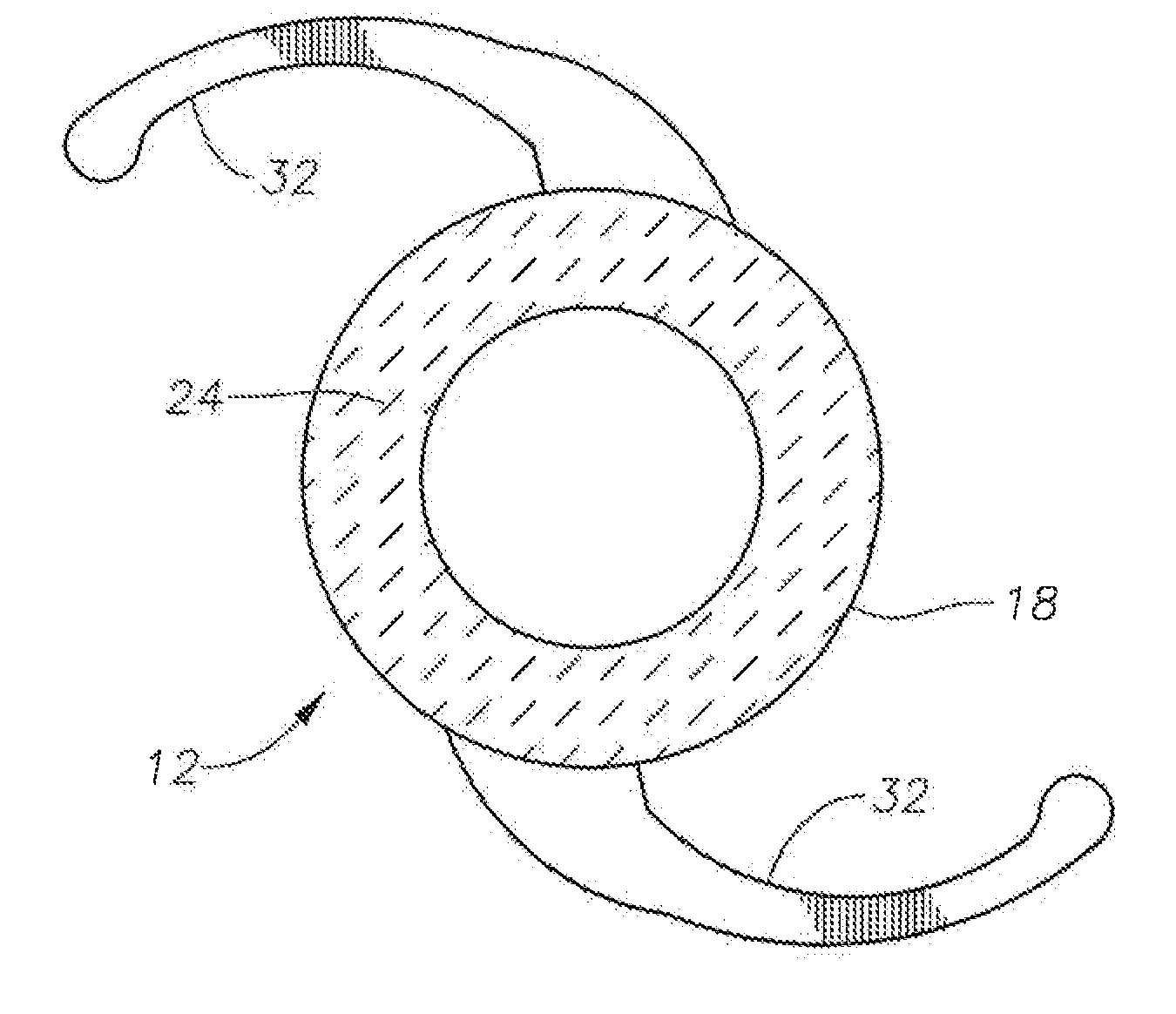
Patents
Literature
35 results about "Posterior capsule opacification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
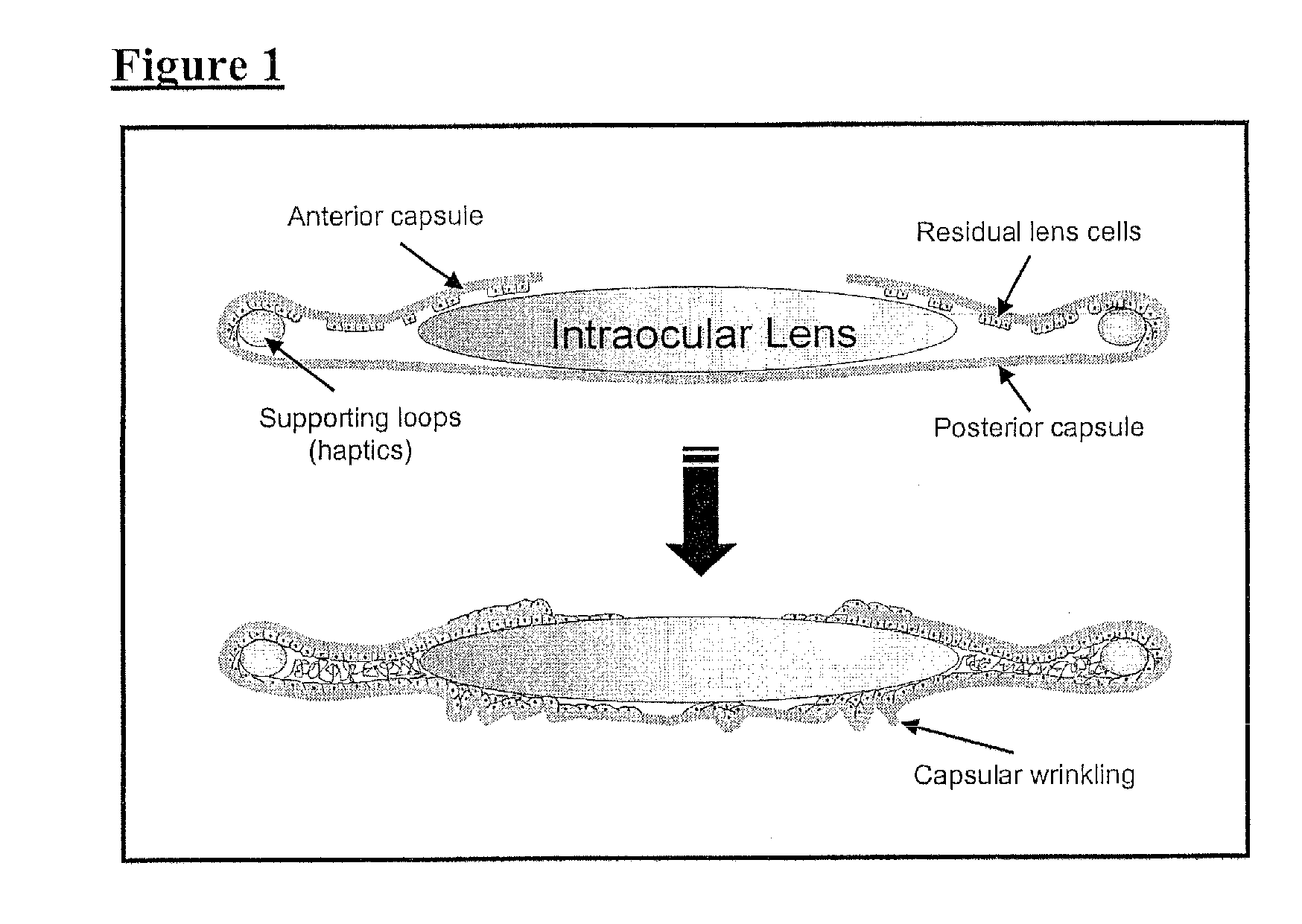
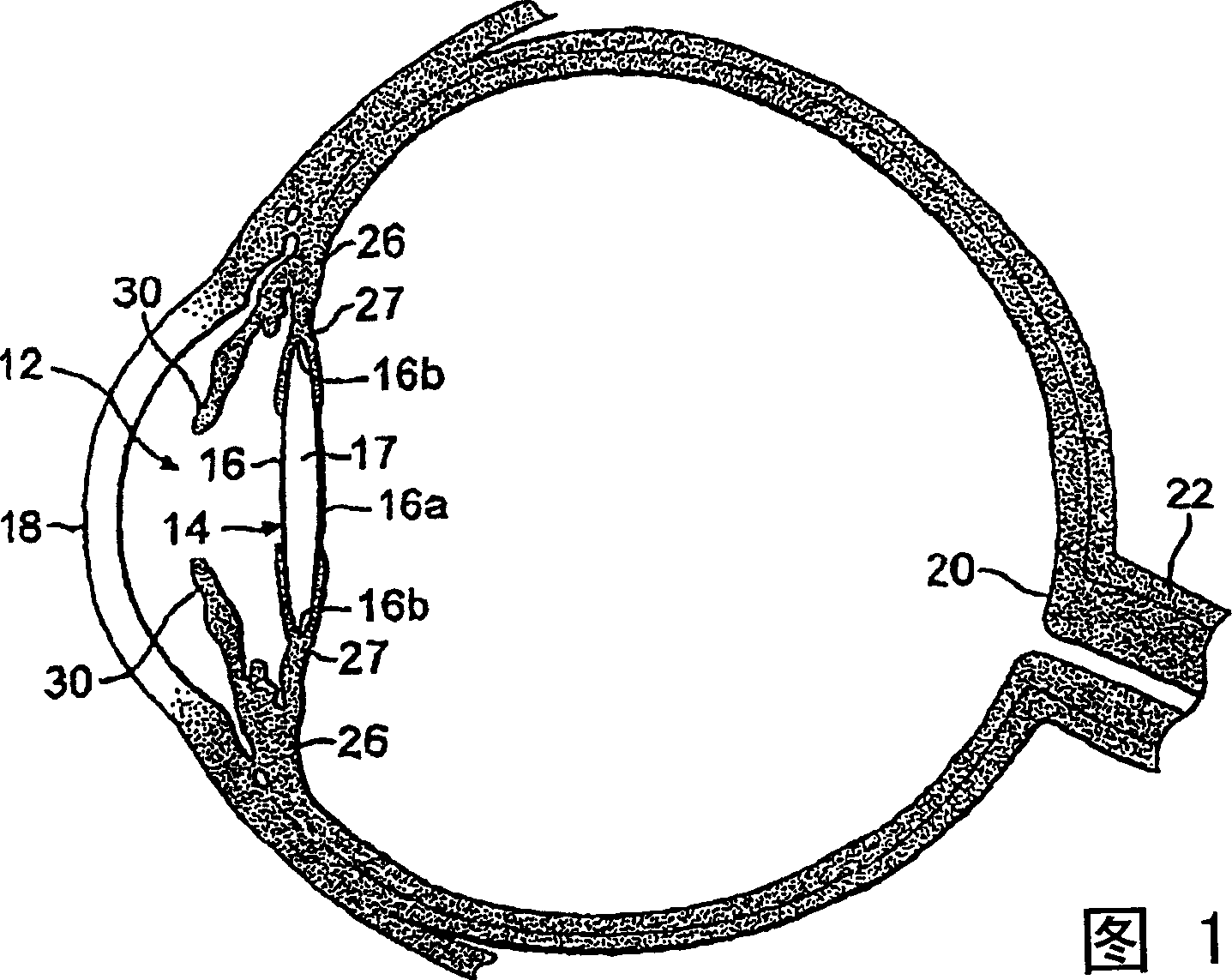
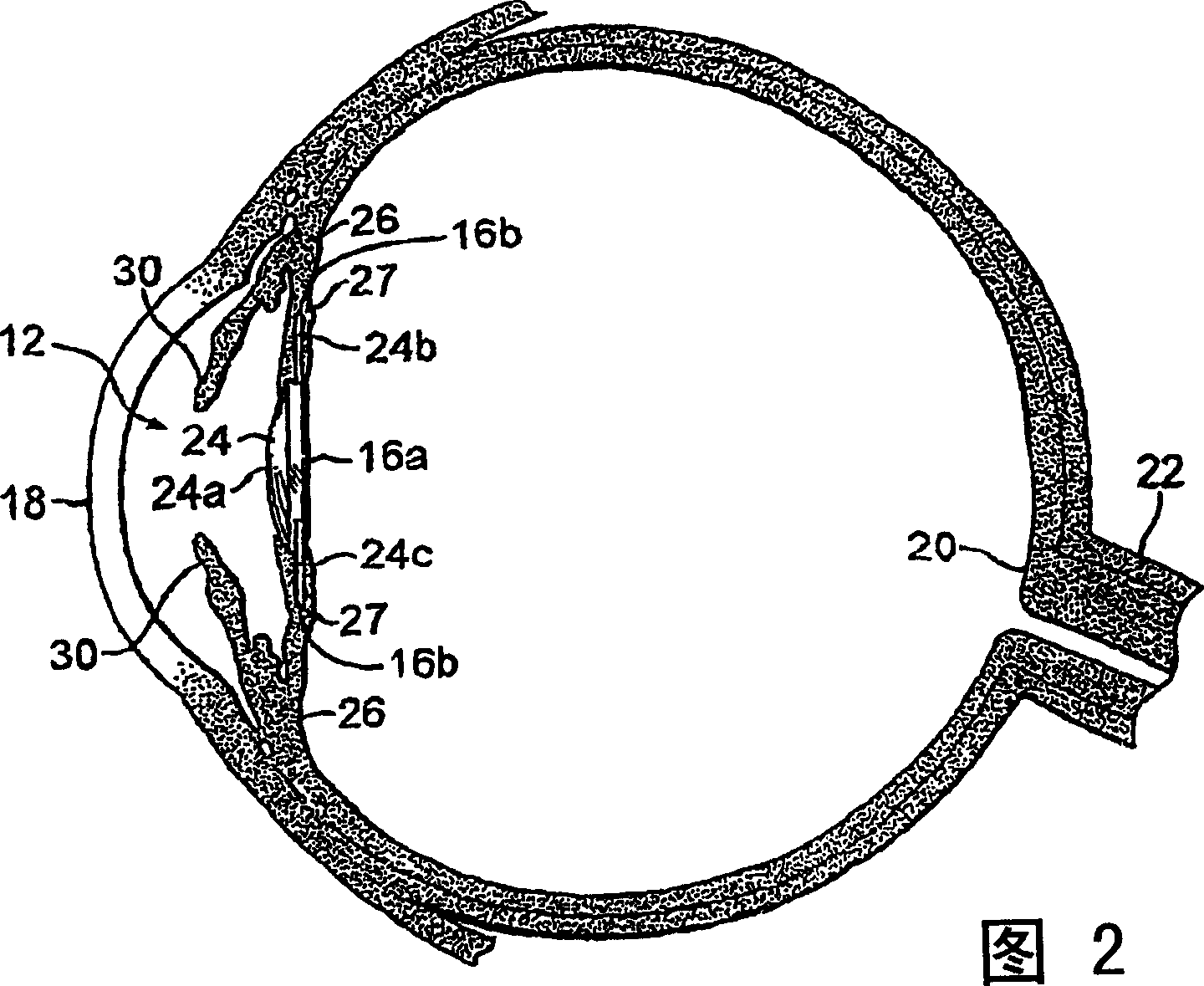
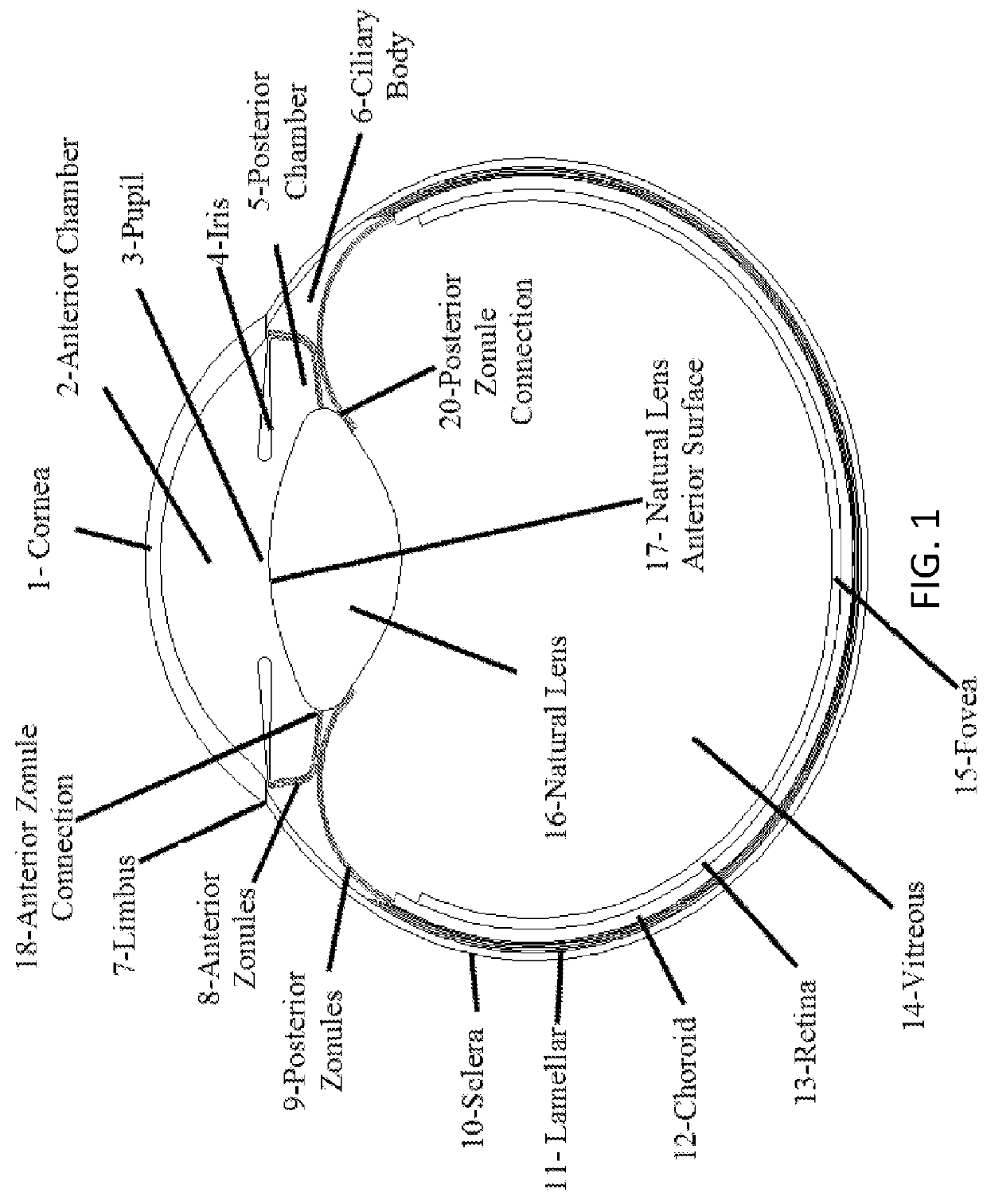
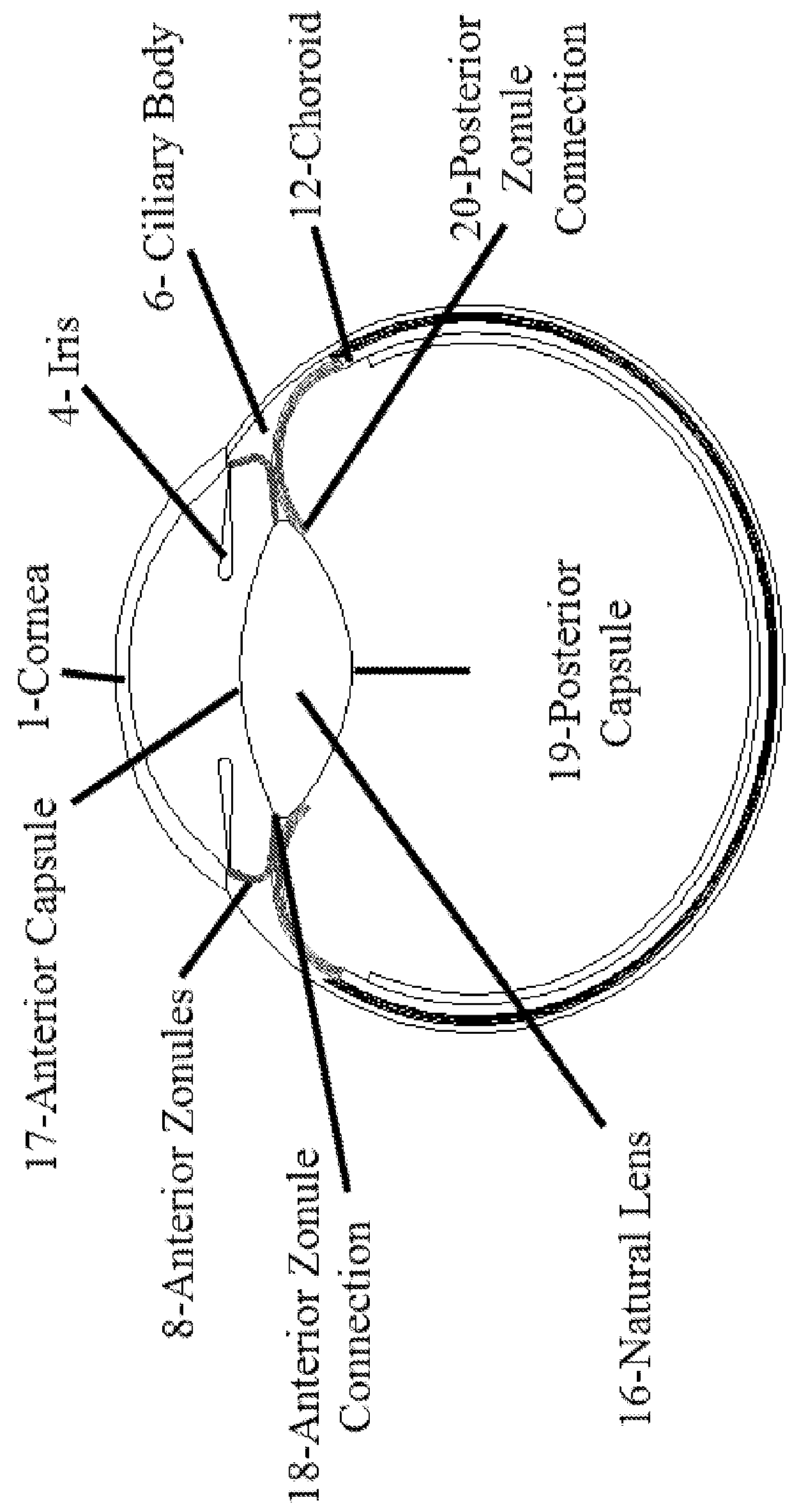
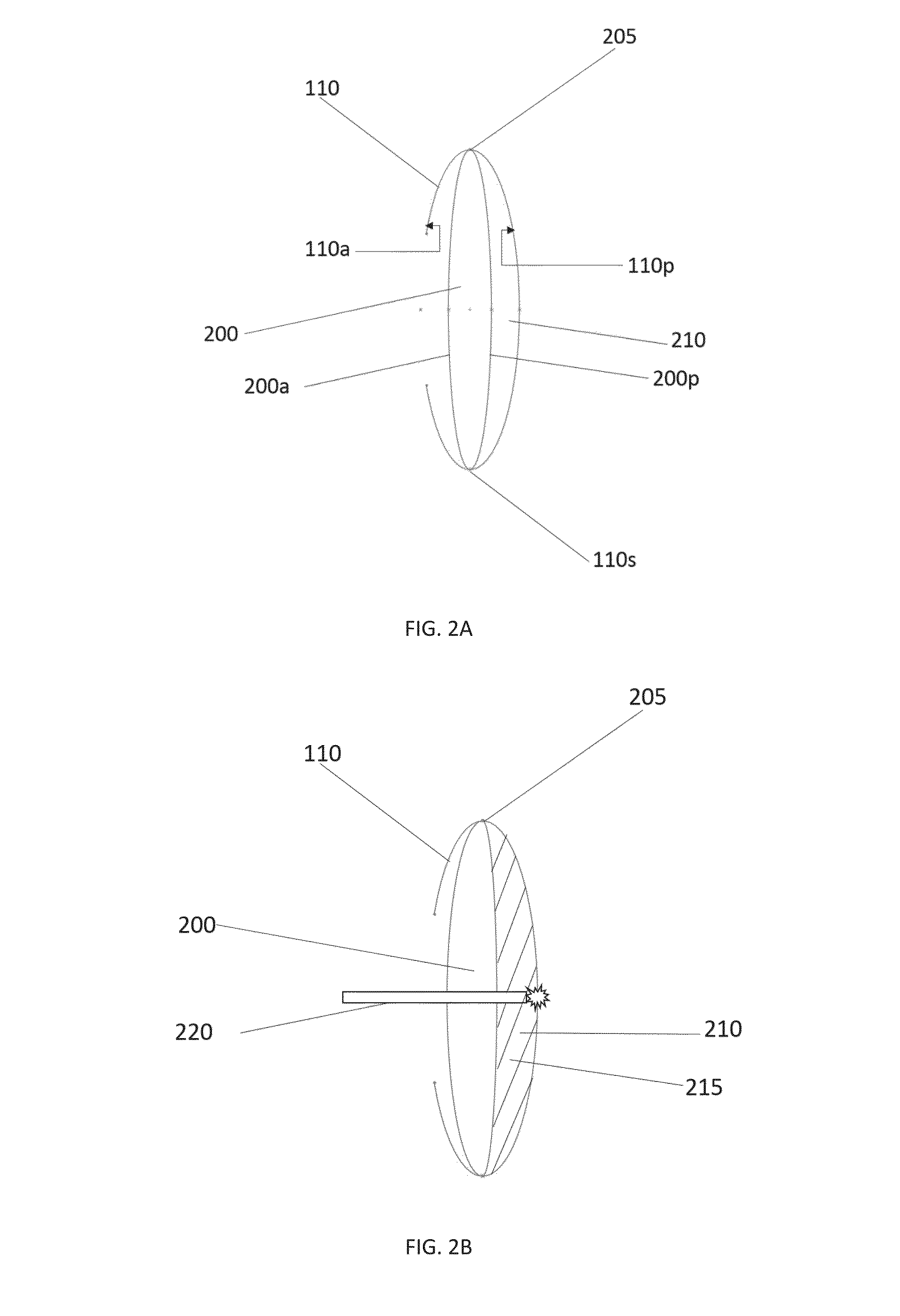
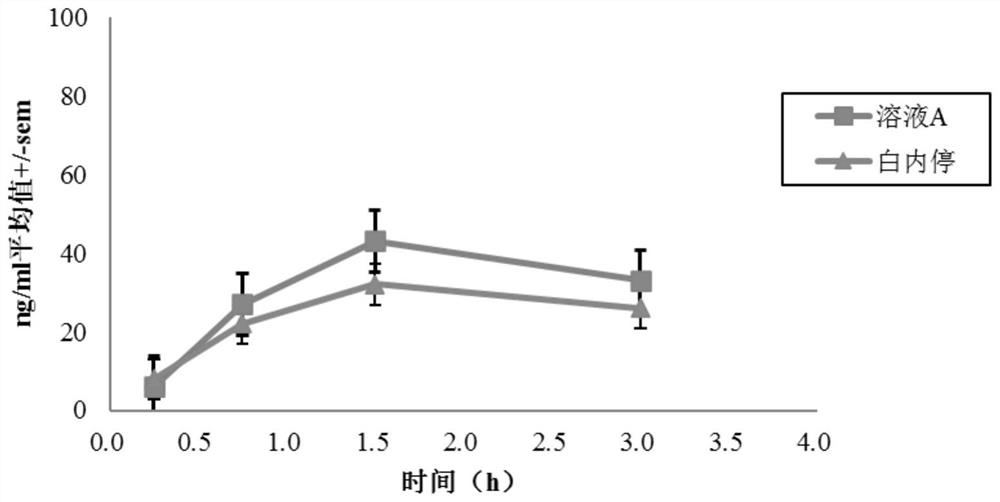
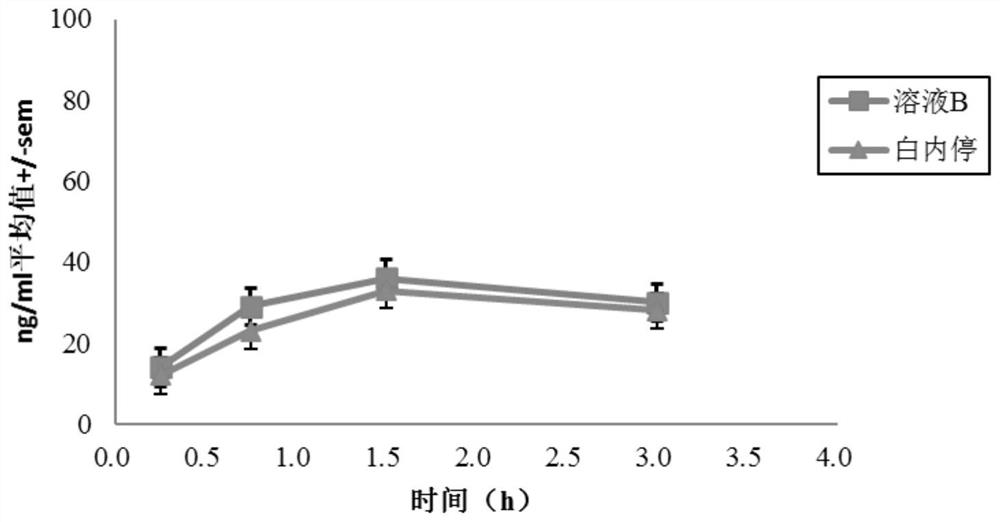
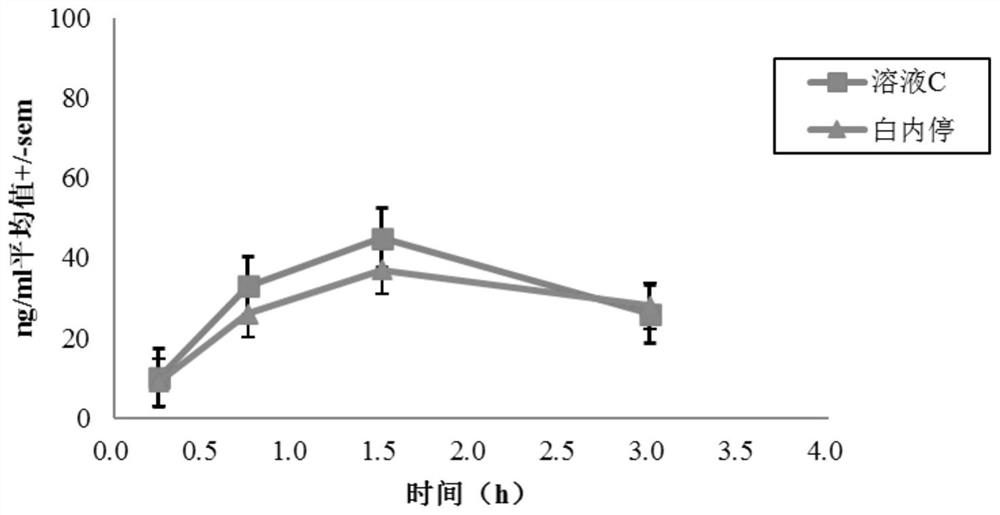
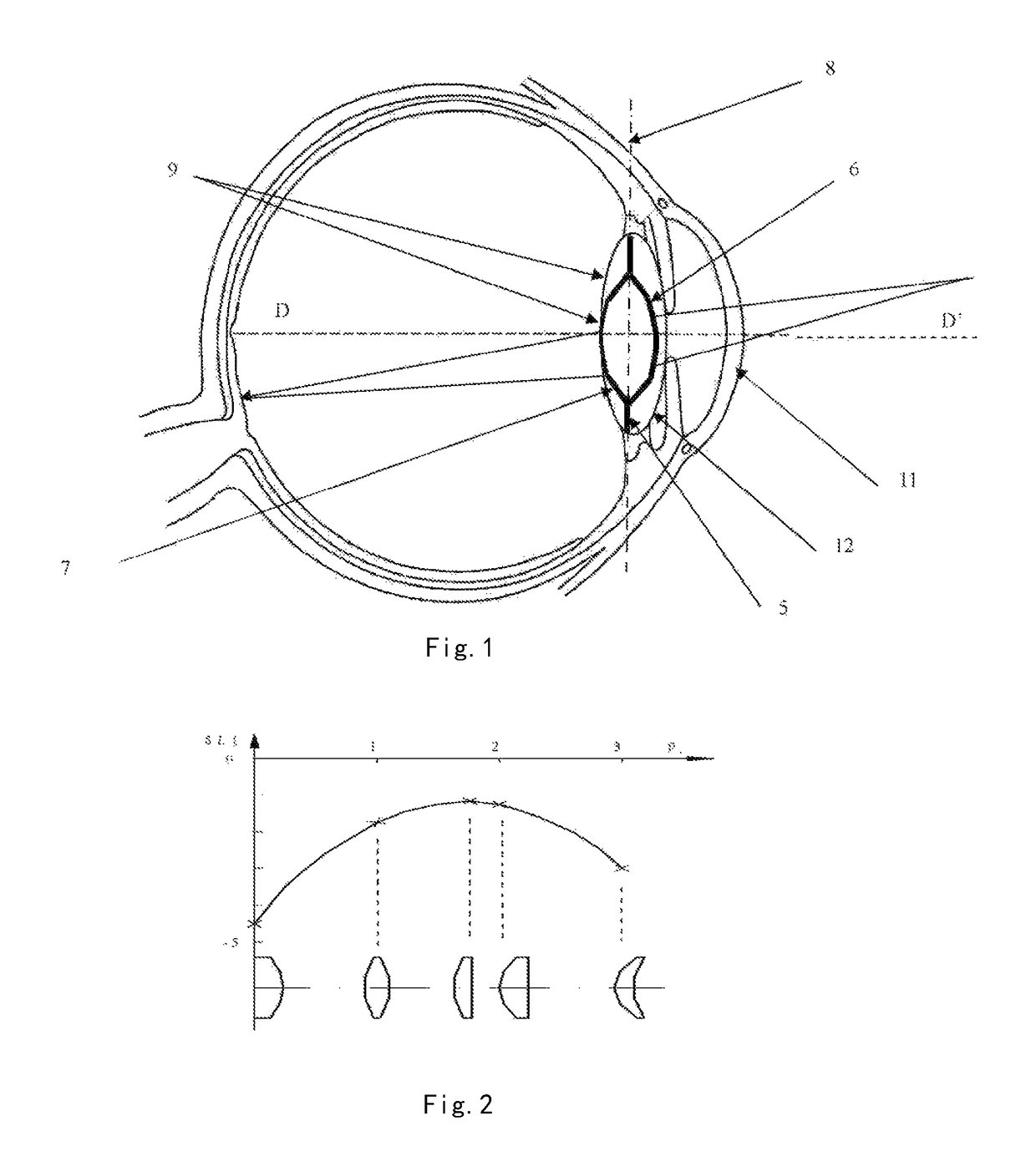
Posterior capsule opacification (PCO) is a fairly common complication of cataract surgery. Sometimes you can develop a thickening of the back (posterior) of the lens capsule which holds your artificial lens in place. This thickening of the capsule causes your vision to become cloudy.
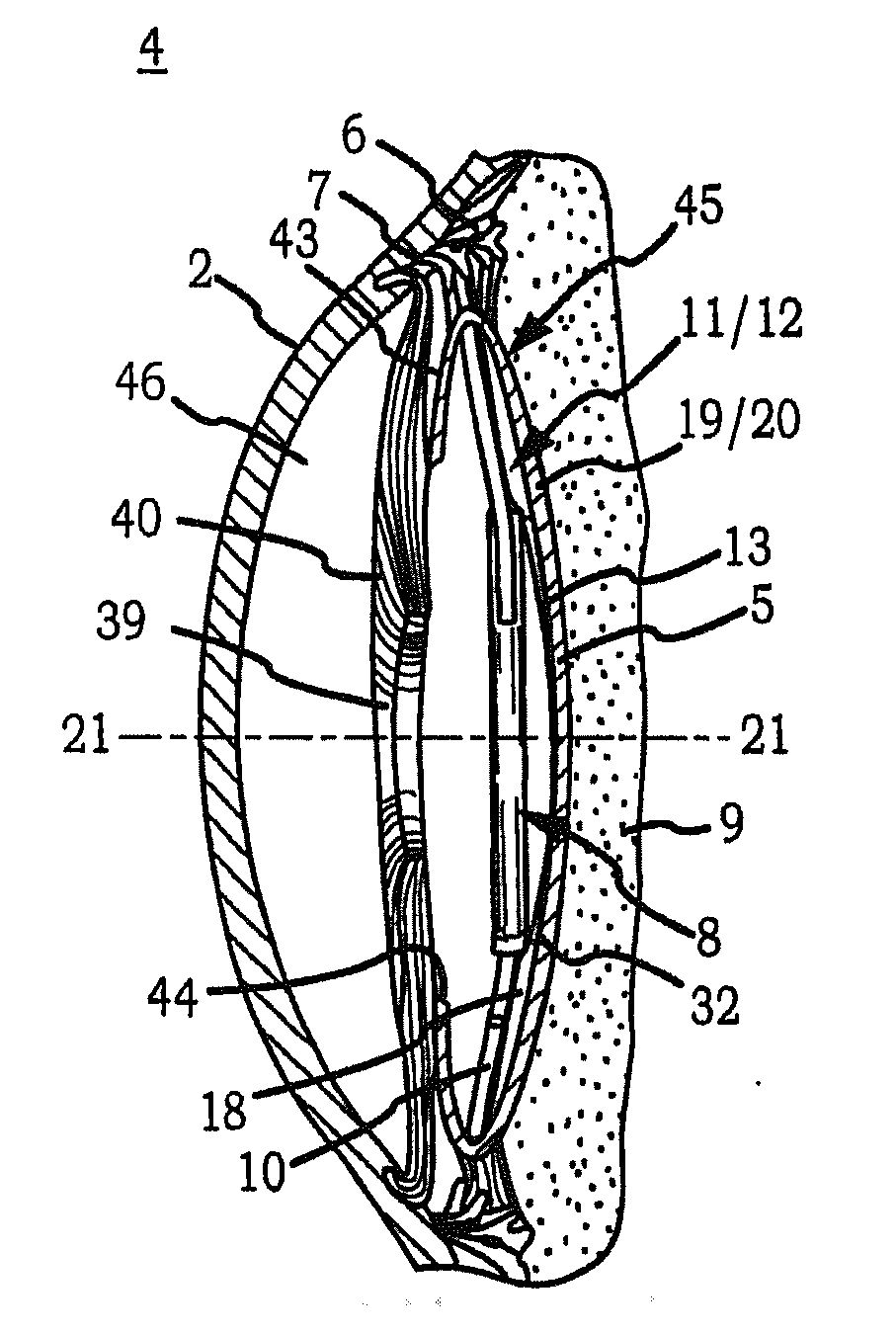
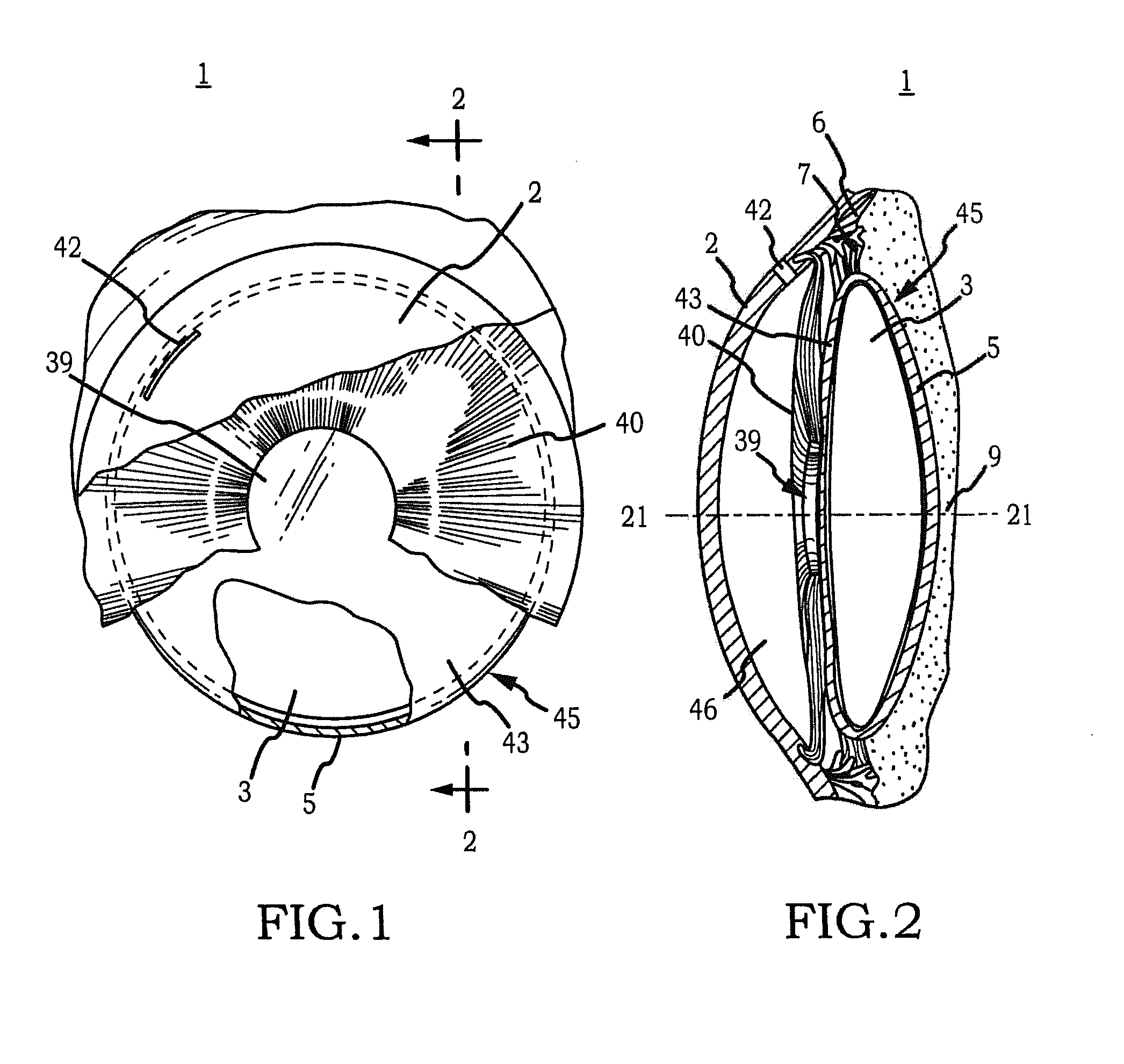
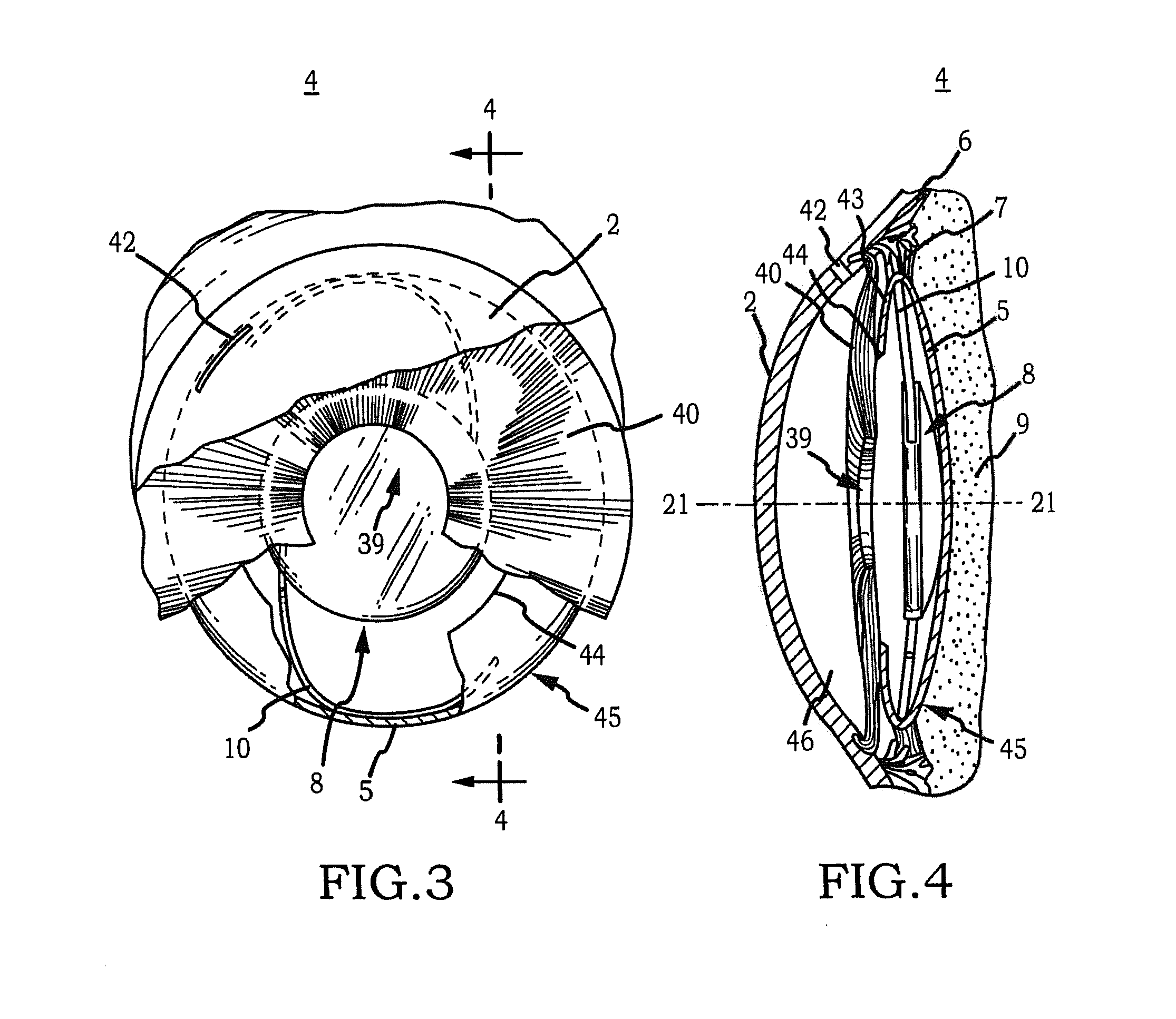
Intraocular Lens Cell Migration Inhibition System
InactiveUS20120232649A1Inhibit migrationReduce posterior capsule opacificationEye treatmentTissue regenerationPosterior capsular opacificationLens epithelial cell
Generally, an intraocular implant and methods for treating an ocular condition. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:INSIGHT INNOVATIONS
Methods for preventing or treating posterior capsular opacification
InactiveUS20160106591A1Preventing and treatingNot to damageLaser surgerySurgical instrument detailsPosterior capsule opacificationRectal epithelium
The present invention relates to methods and apparatus for preventing or treating posterior capsular opacification in a subject in need of prophylaxis or treatment for posterior capsular opacification, including a subject undergoing cataract surgery by ablating epithelial cells on an interior surface of the lens capsule with a multi-photon laser system.
Owner:NEXXTVISIONLLC
Amphiphilic block copolymers and their use
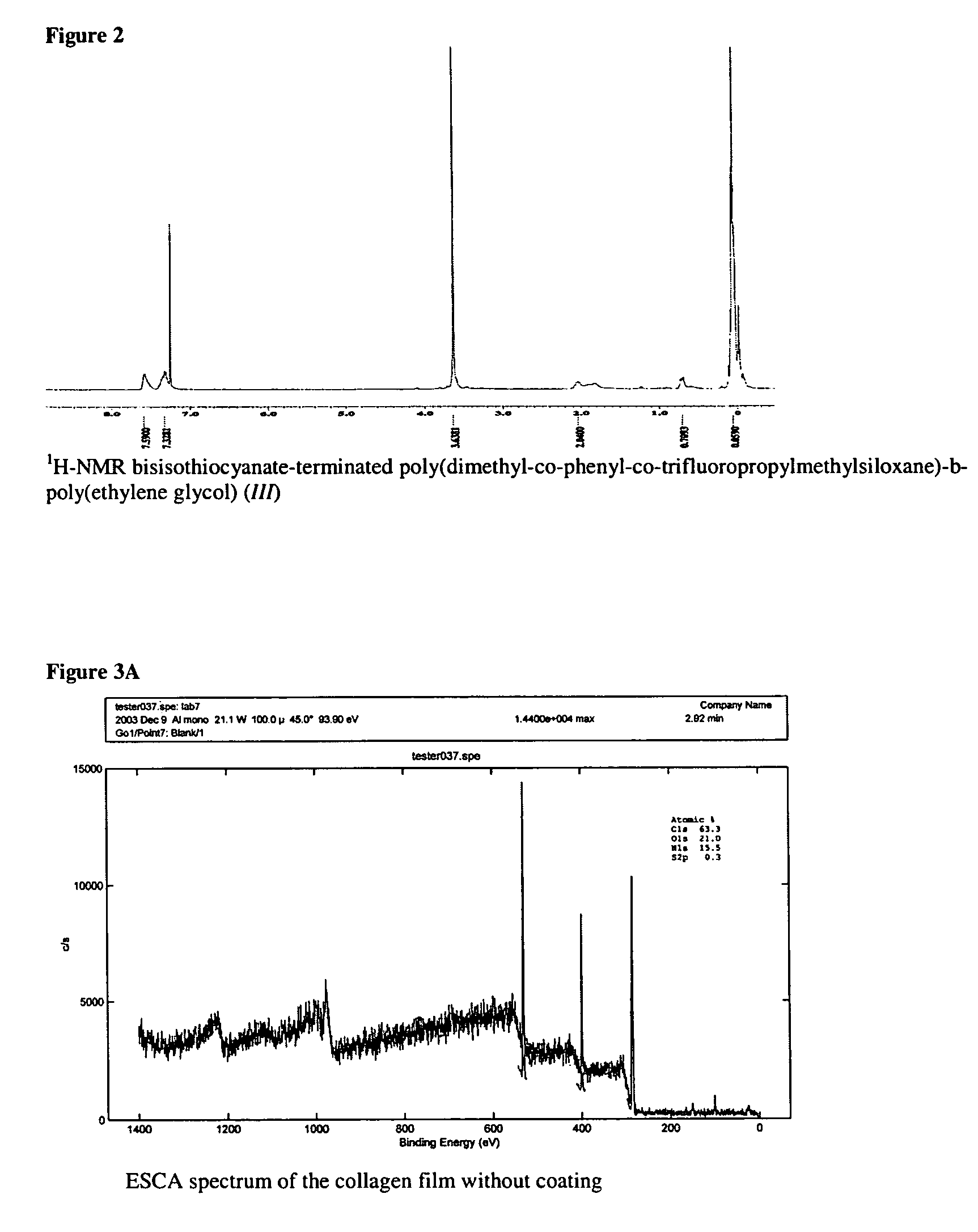
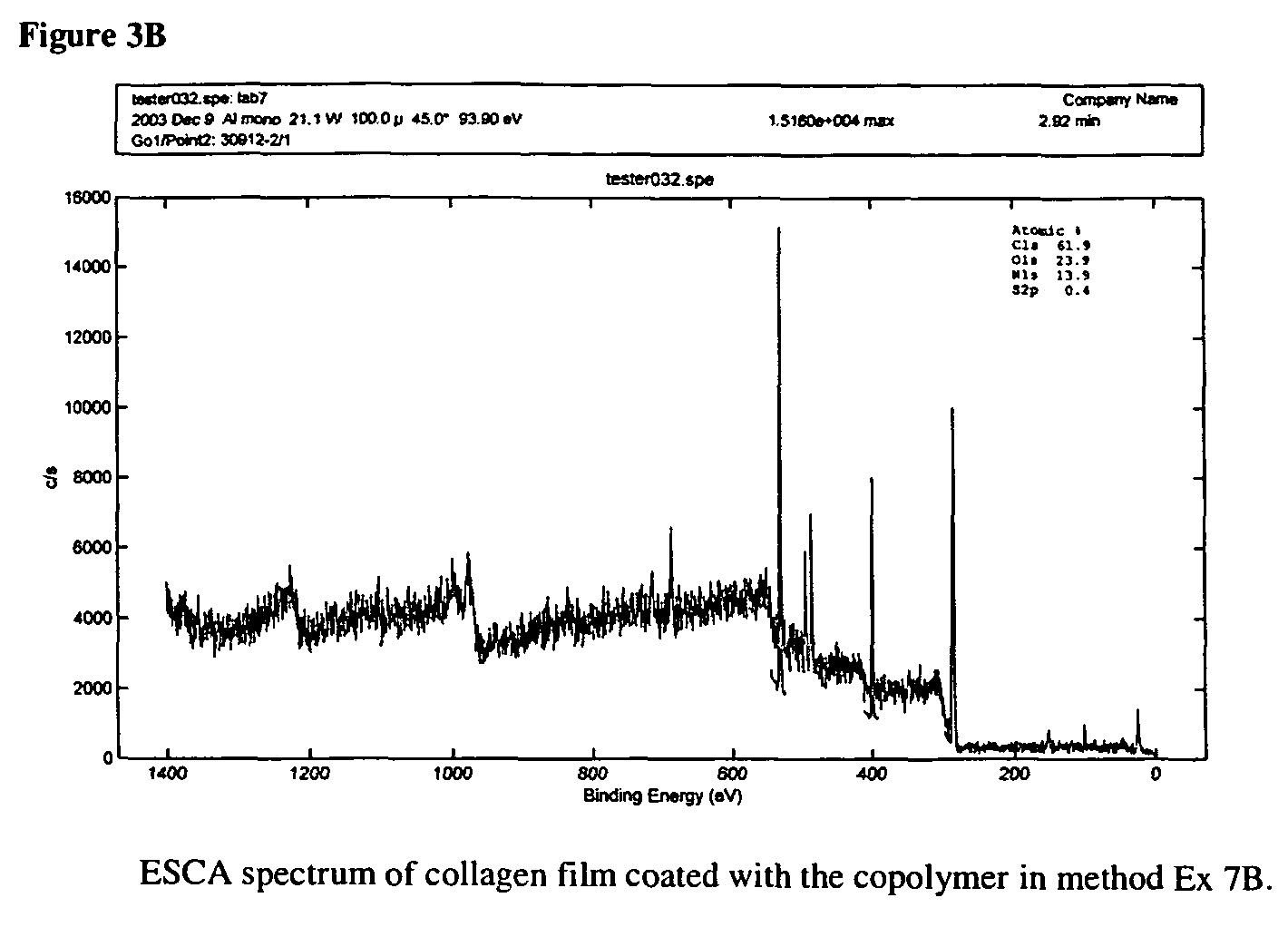
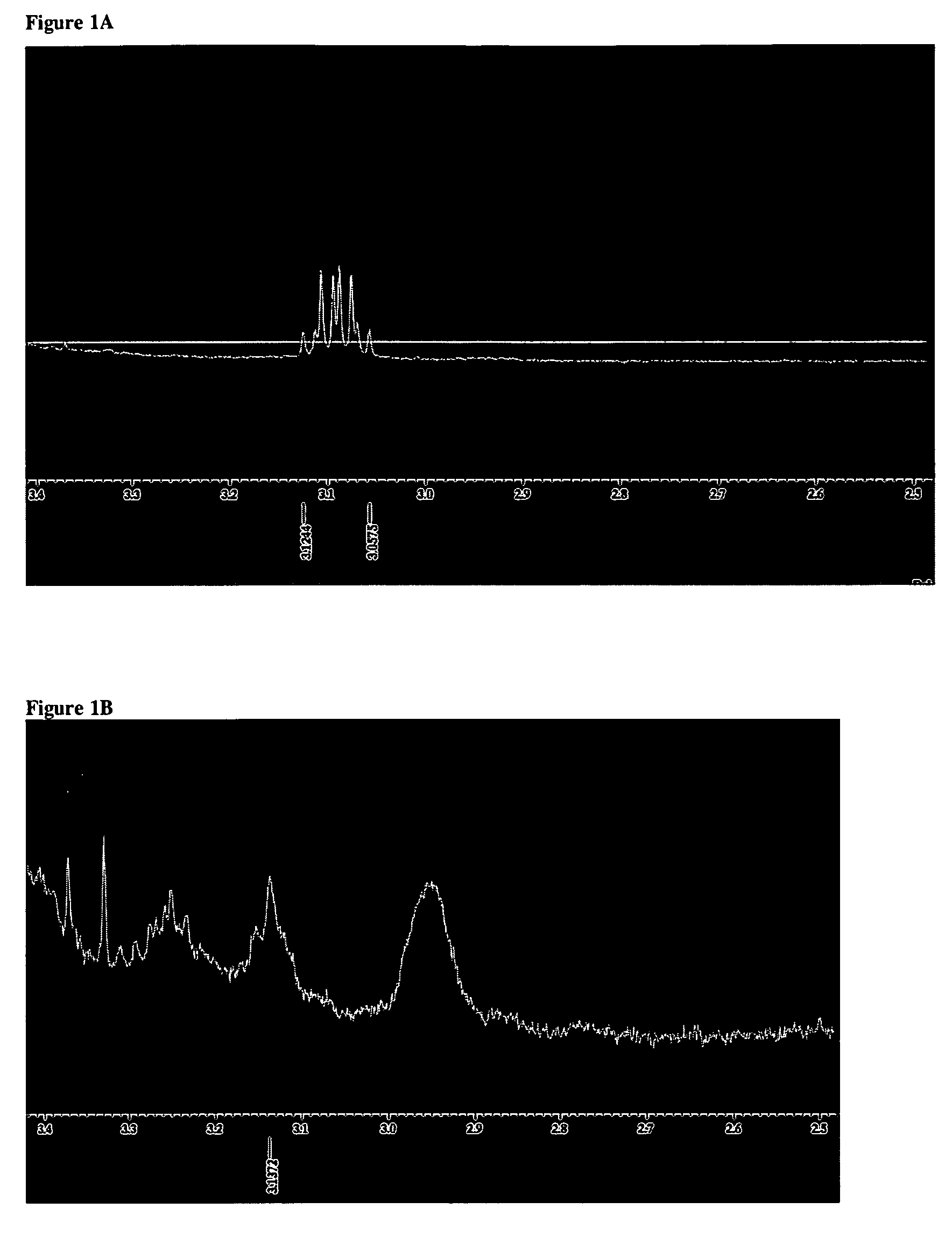
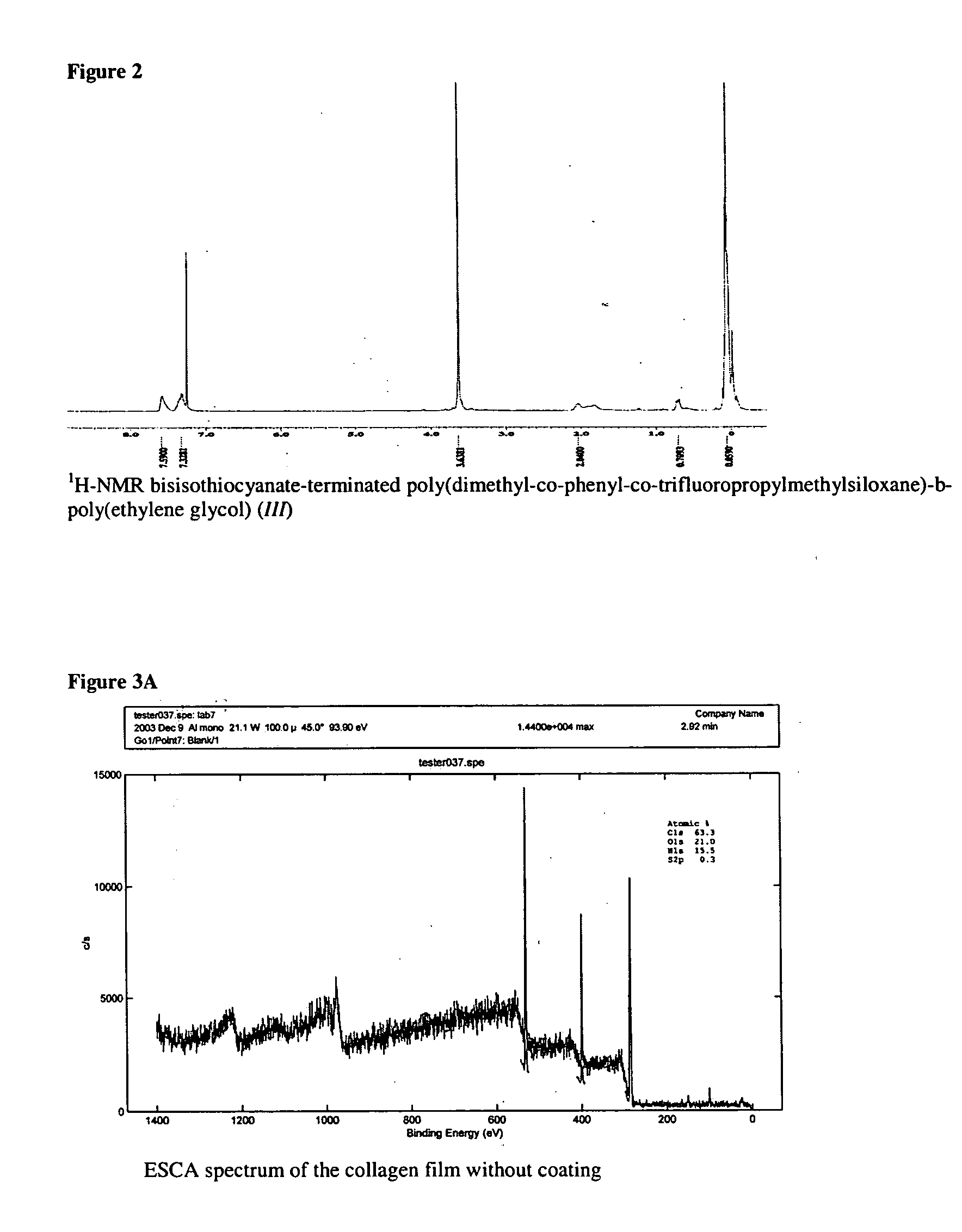
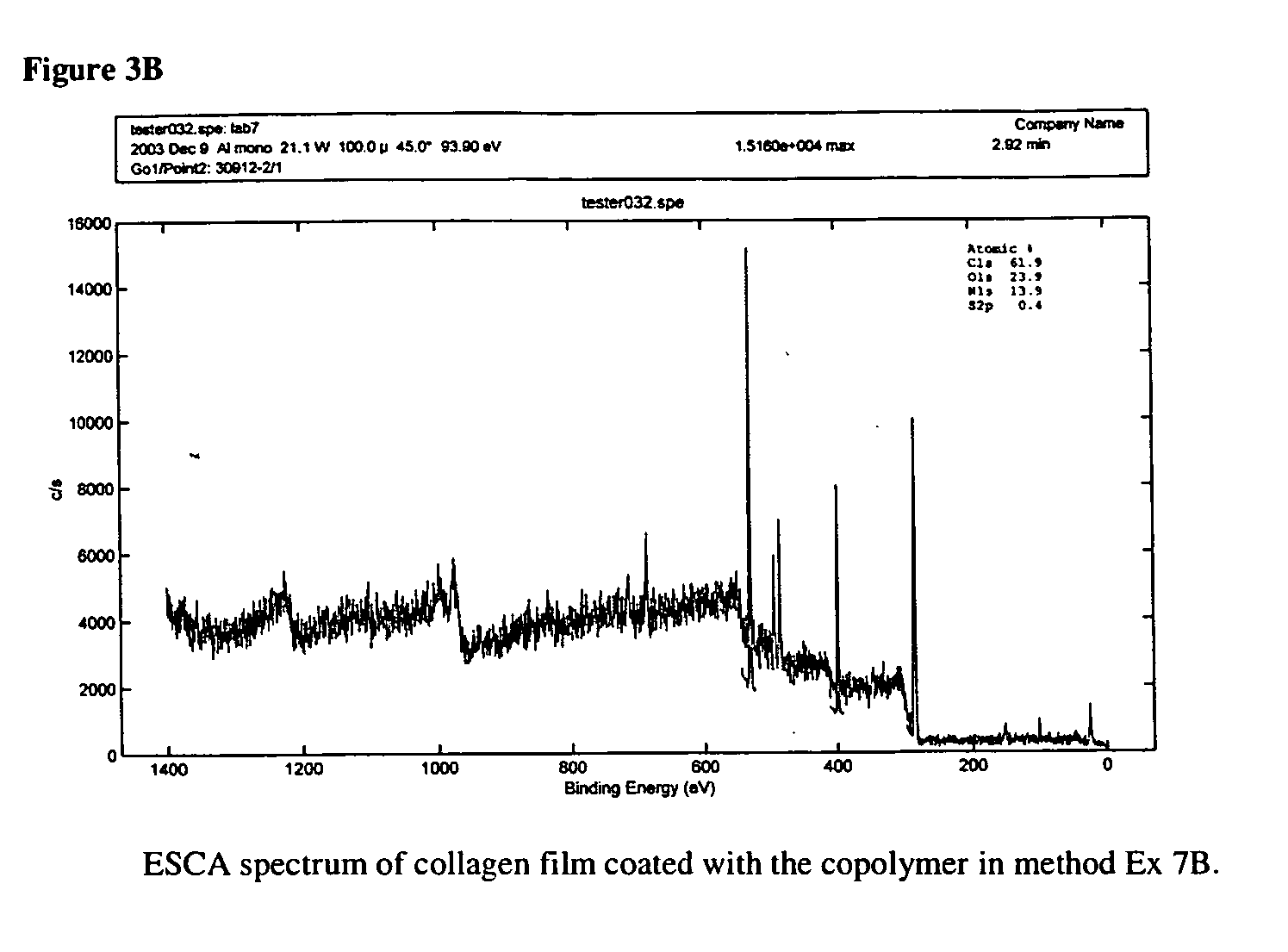
ActiveUS7847025B2Preventing secondary cataractSilicon organic compoundsBiocidePosterior capsule opacificationIntraocular lens
The present invention relates to amphiphilic block copolymers comprising at least one block of hydrophilic units and at least one block of hydrophobic units, wherein at least one hydrophobic block contains siloxane units. The present invention may be particularly useful as a tissue adhesive or as a coating for an intraocular lens (IOL). As an IOL coating, copolymers according to the invention may be used, for example, to promote tissue adhesion for the prevention of posterior capsule opacification.
Owner:AMO GRONINGEN
Nanometer fluorouracil coat artificial crystalloid and the preparing method
InactiveCN101036804ASuppress turbidityLow toxicityCoatingsIntraocular lensPosterior capsule opacificationChitosan nanoparticles
The invention relates to a intraocular lens. At present the Poly-Methyl Methacrylate (PMMA) intraocular lens conventional for clinical treatment of cataract always causes inflammatory treaction after implantation. Posterior capsule opacification is also a major compalication after cataract surgery. The invention selects fluorouracil to solve the above problems. Based on weak penetrating force of chitosan nanoparticles in eyes and the relation between the phagocytosis amount of conjunctival epithelial cells to nanoparticles and the particle size of nanoparticles, the fluorouracil nanoparticles preparation is prepared using chitosan-poly(acrylic acid) as carrier, composite coated on the surface of PMMA intraocular lens. The invantion also provides a preparation method thereof. The said intraocular lens not only increases the biocompatibility of the intraocular lens, but also inhibites posterior capsule opacification pafter cataract surgery. The said intraocular lens can also prevent anterior membrane after implantation of intraocular lens and after-cataract.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Micropatterned Intraocular Implant
InactiveUS20150342725A1Inhibit migrationInhibit growth and migrationTissue regenerationIntraocular lensPosterior capsule opacificationLens epithelial cell
Generally, an intraocular implant having on the external surface a plurality of pattern surface elements disposed in spaced apart relation defining a tortuous pathway adapted to control a flow of fluid, or a flow of particles suspended in a fluid, or inhibits the growth or migration of cells. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits growth or migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:SHARKLET TECH +2
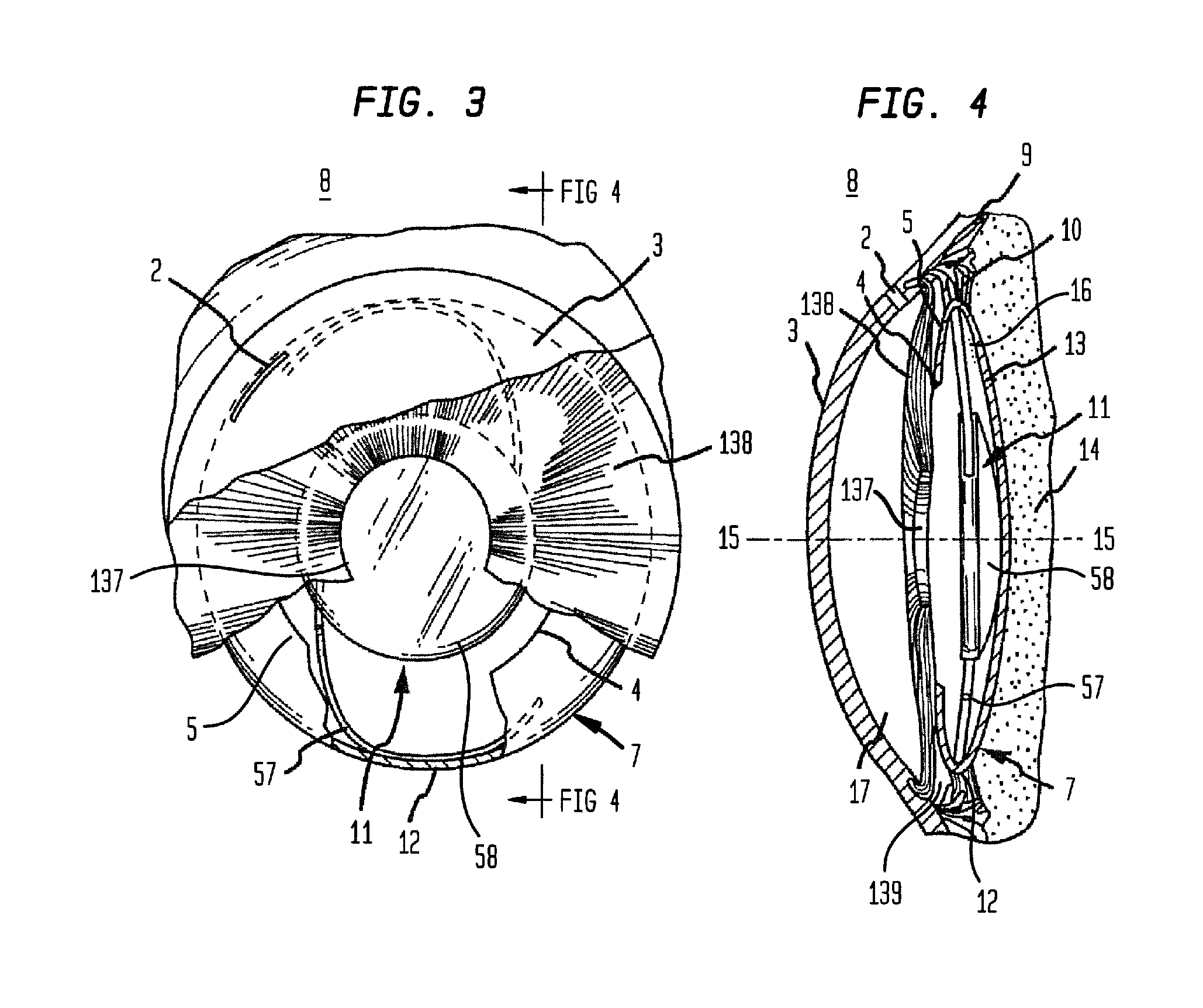
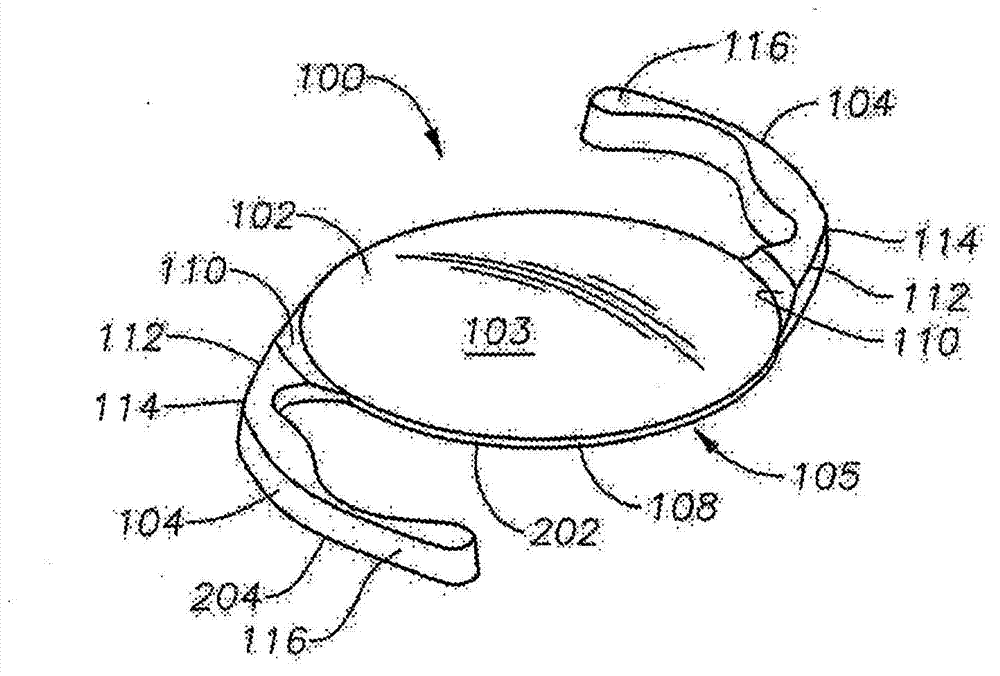
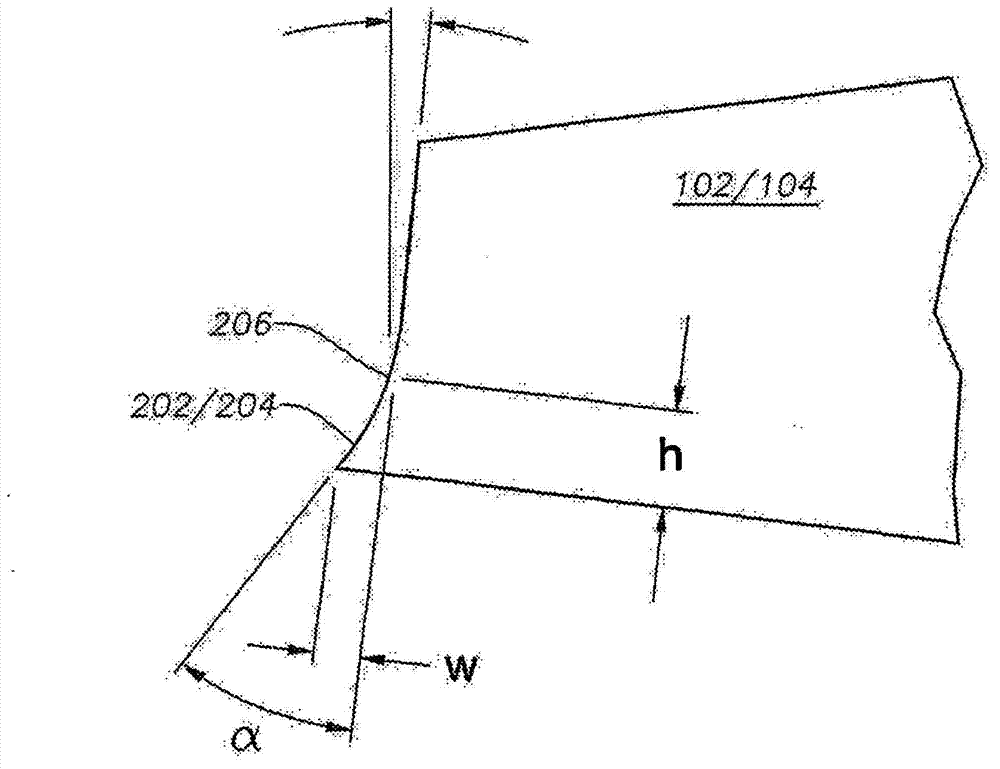
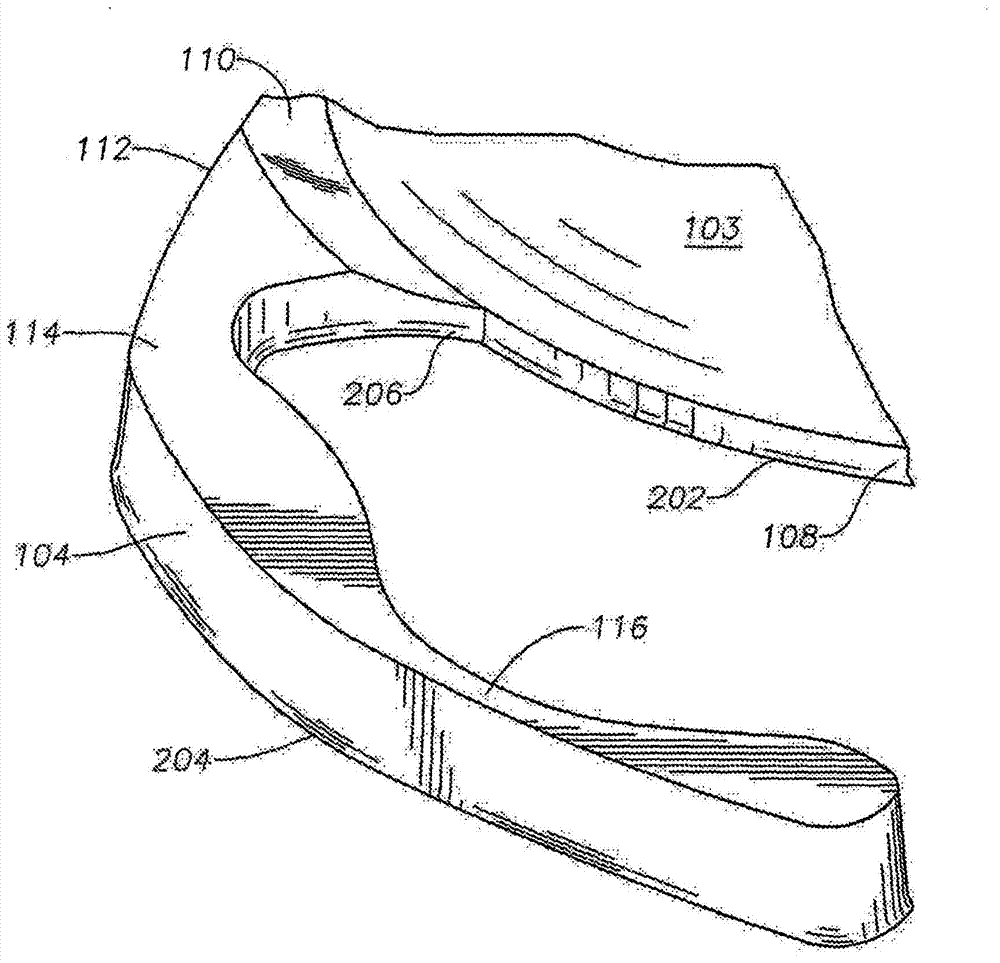
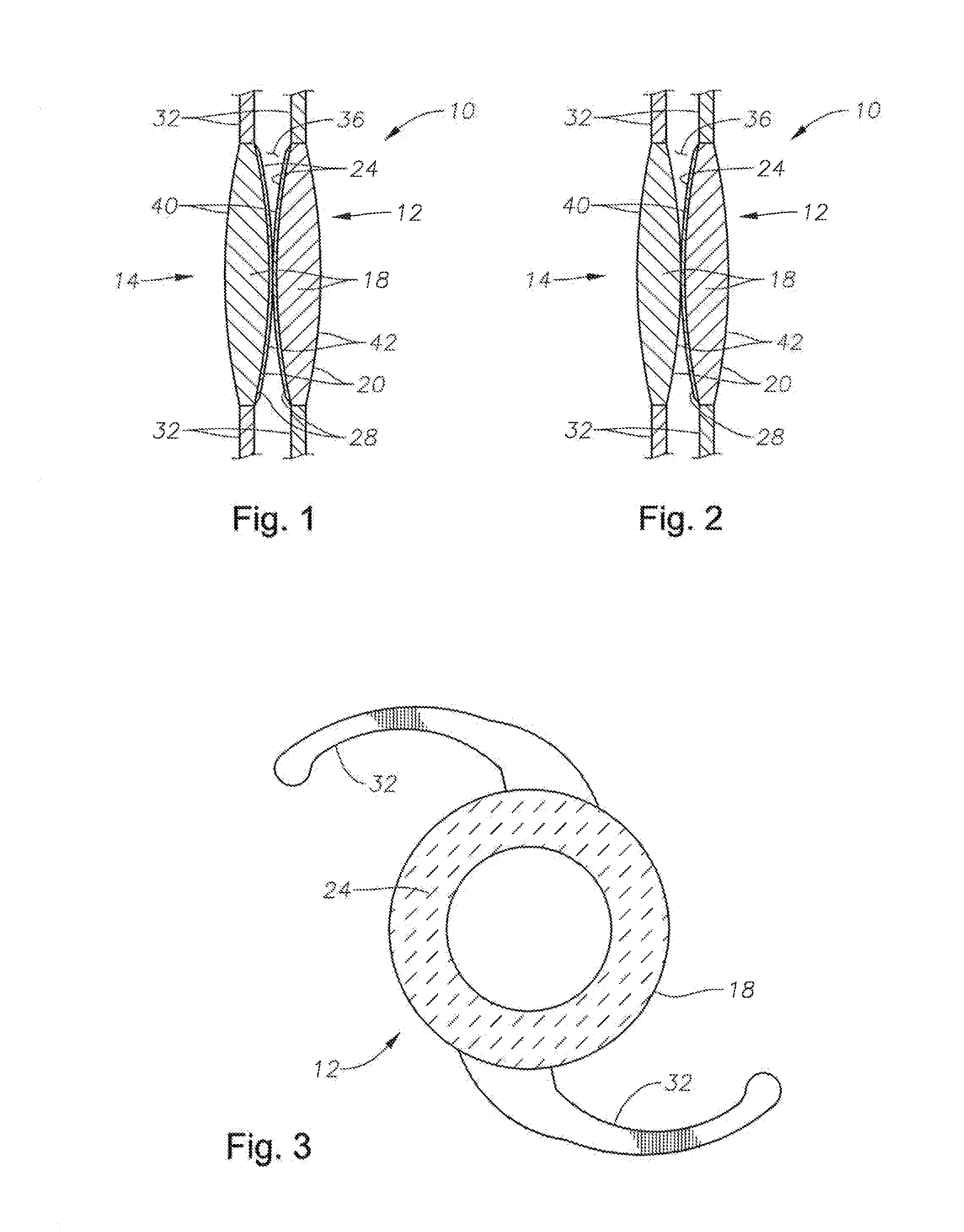
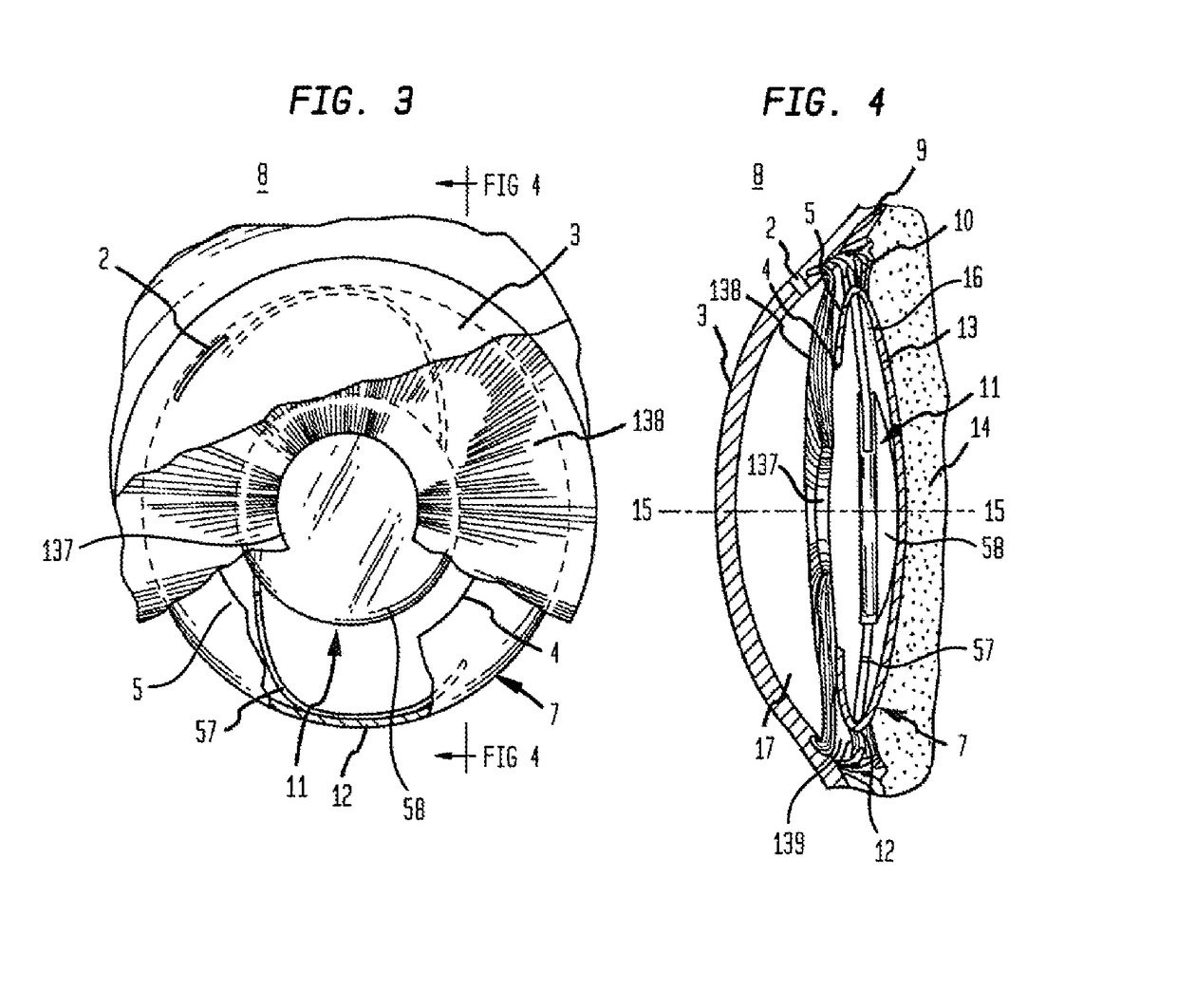
Intraocular lens having edge configured to reduce posterior capsule opacification
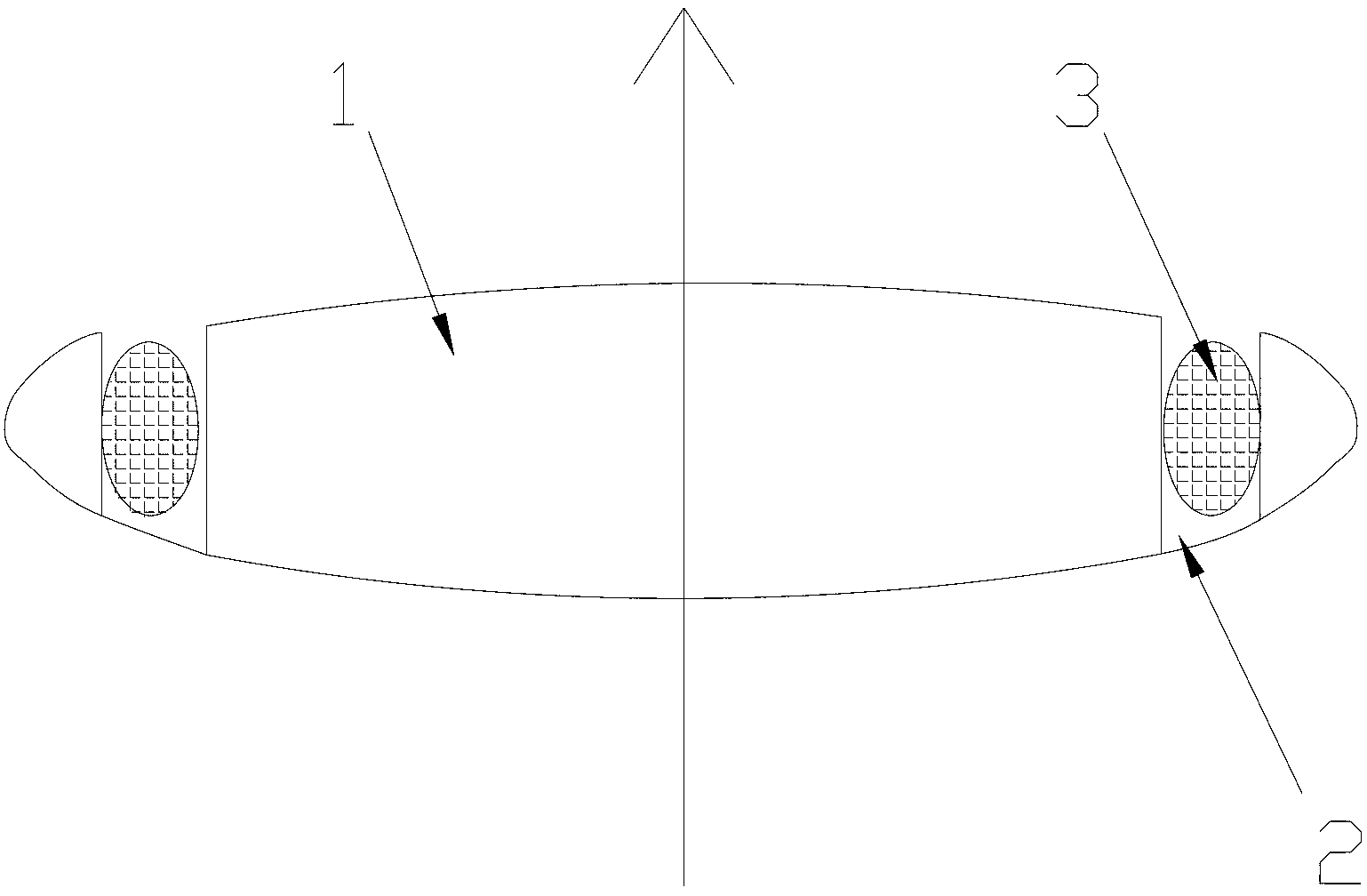
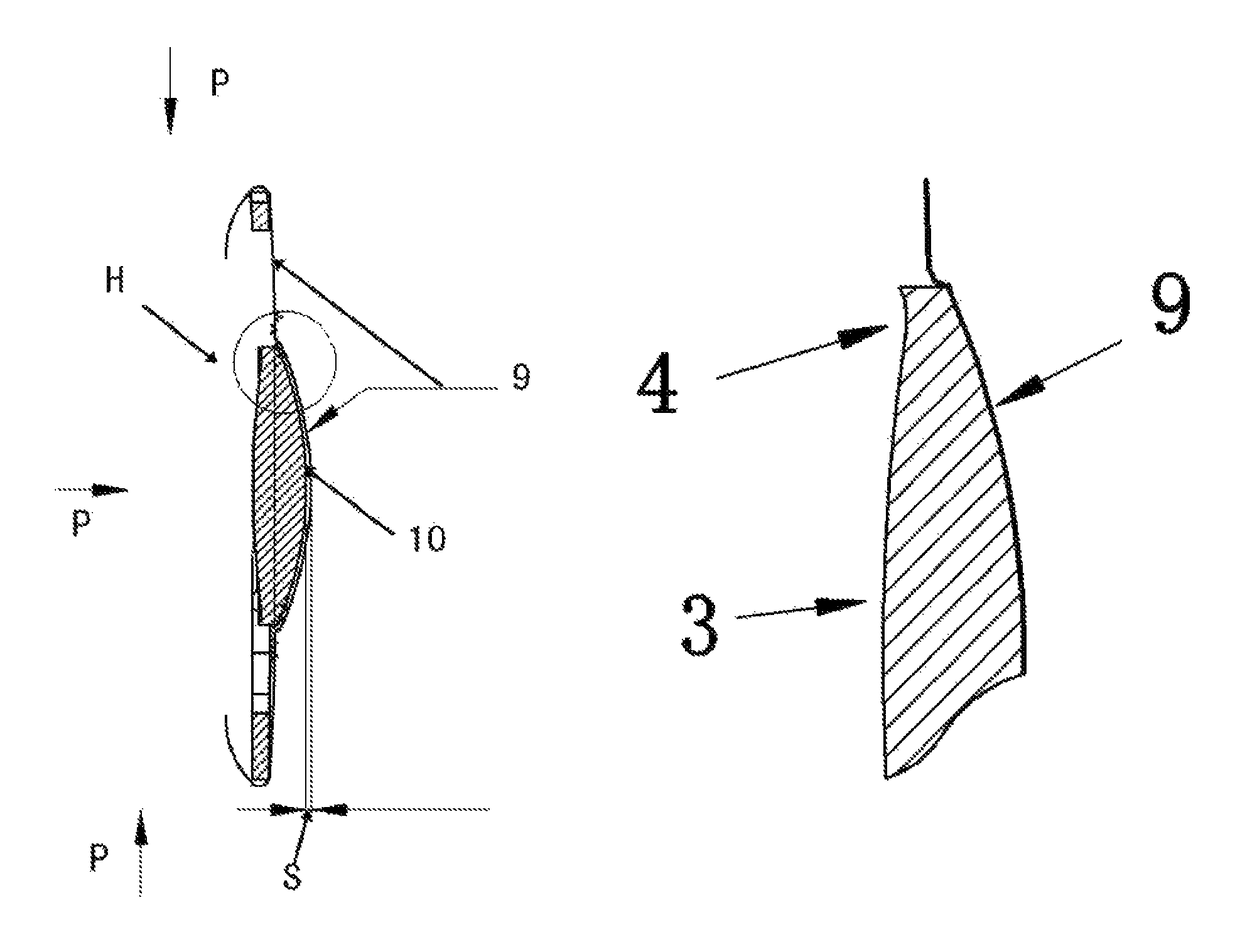
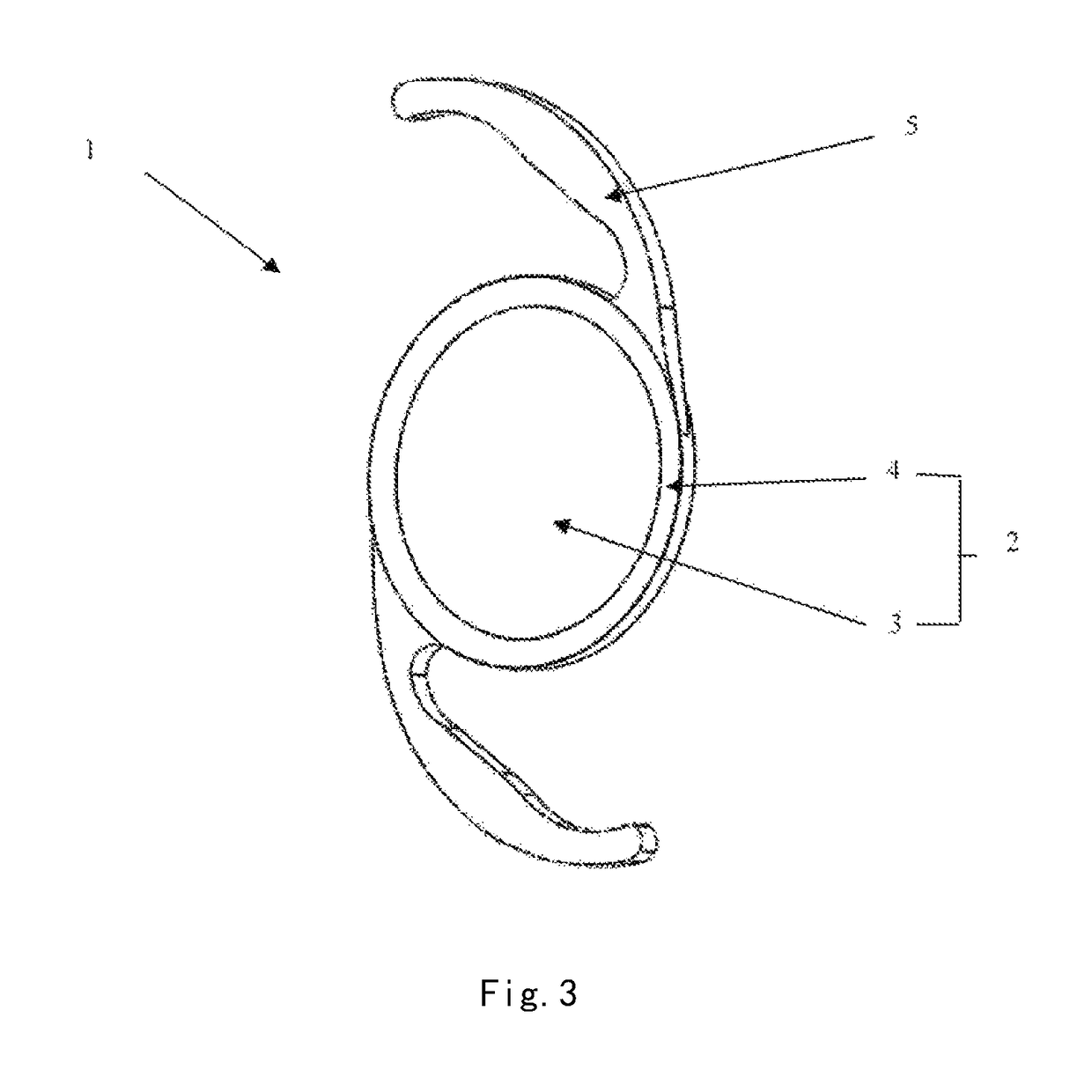
An intraocular lens (IOL) for implantation within a capsular bag includes an optic and a plurality of haptics. The optics has an anterior optic face and a posterior optic face joined by a peripheral wall. The peripheral wall includes a straight portion of uniform width extending posteriorly from the anterior optic face to a flare point and a flared optic edge. The flared optic edge extends posteriorly and widens from the flare point and meets the posterior optic face at a sharp optic corner. Each of the haptics is coupled to the optic at the peripheral wall at respective haptic-optic junctions. The flared optic edge surrounds the peripheral wall between the haptic-optic junctions.
Owner:ALCON RES LTD
Amphiphilic block copolymers and their use
ActiveUS20060134177A1Avoid problemsPreventing secondary cataractSilicon organic compoundsBiocideIntraocular lensPosterior capsule opacification
The present invention relates to amphiphilic block copolymers comprising at least one block of hydrophilic units and at least one block of hydrophobic units, wherein at least one hydrophobic block contains siloxane units. The present invention may be particularly useful as a tissue adhesive or as a coating for an intraocular lens (IOL). As an IOL coating, copolymers according to the invention may be used, for example, to promote tissue adhesion for the prevention of posterior capsule opacification.
Owner:AMO GRONINGEN
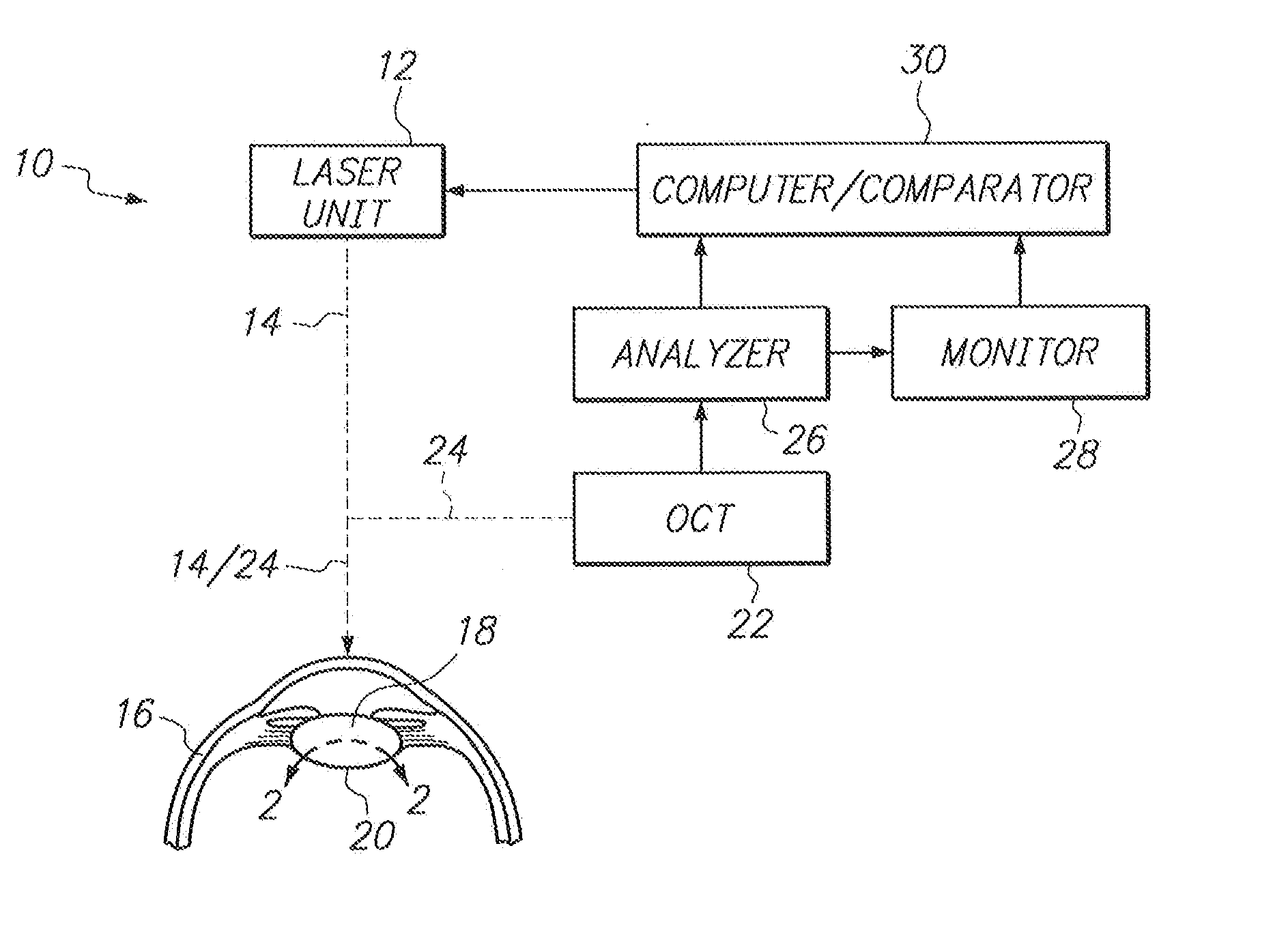
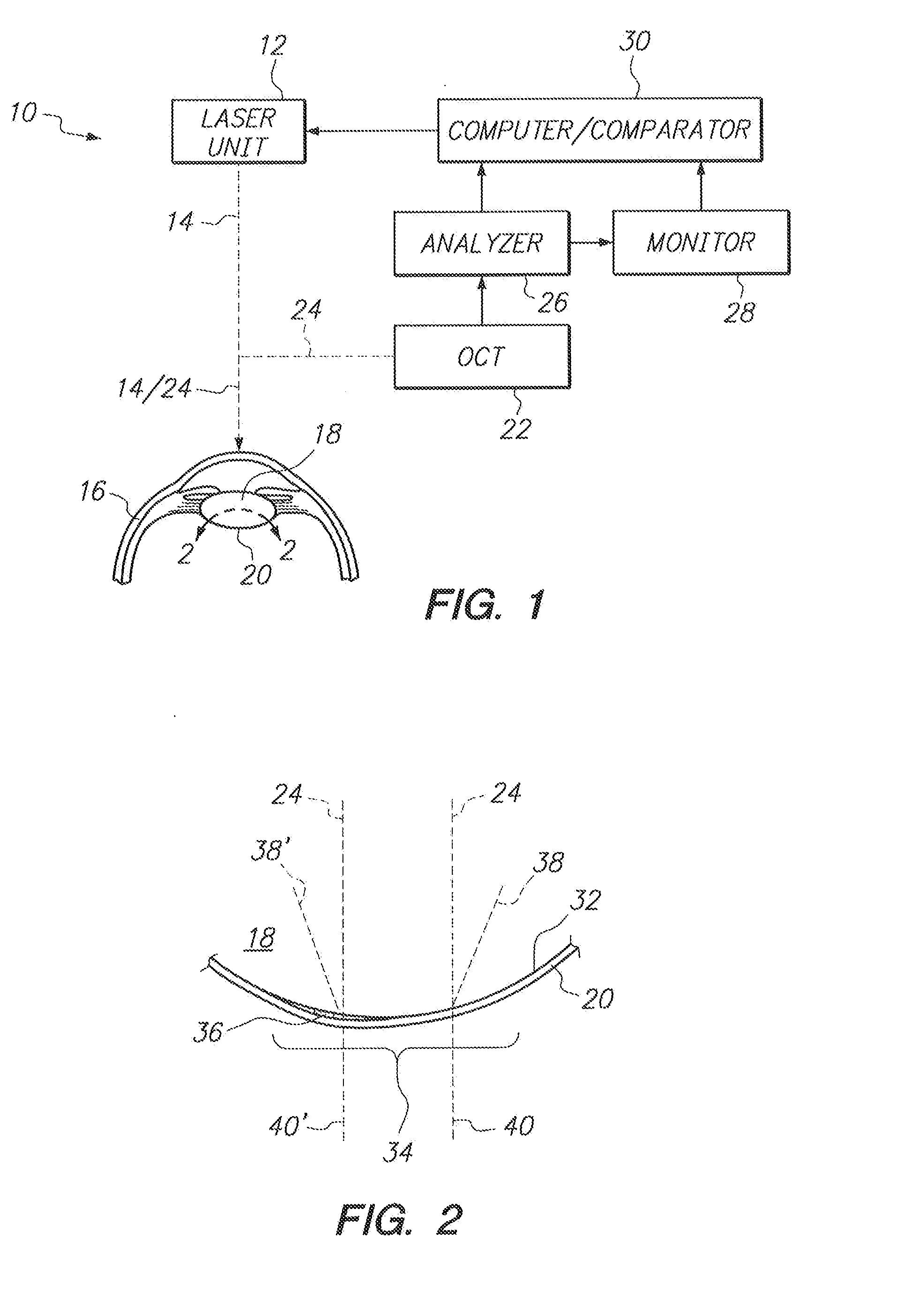
System and Method for Obviating Posterior Capsule Opacification
InactiveUS20130103012A1Avoid potential damageAppropriate distanceLaser surgerySurgical instrument detailsPosterior capsule opacificationFemto second laser
A system and method are provided for obviating Posterior Capsule Opacification (PCO) which require an Optical Coherence Tomography (OCT) device for imaging the interface surface between the posterior surface of an intraocular lens (IOL) and the capsular bag. Further, the OCT device is used to identify areas of relative opacity caused by a biological growth on the interface surface in the optical zone of the IOL. A laser unit is then used to direct the focal point of a femtosecond laser beam onto the areas of relative opacity to ablate the biological growth by Laser Induced Optical Breakdown (LIOB) to thereby obviate the PCO.
Owner:BAUSCH & LOMB INC
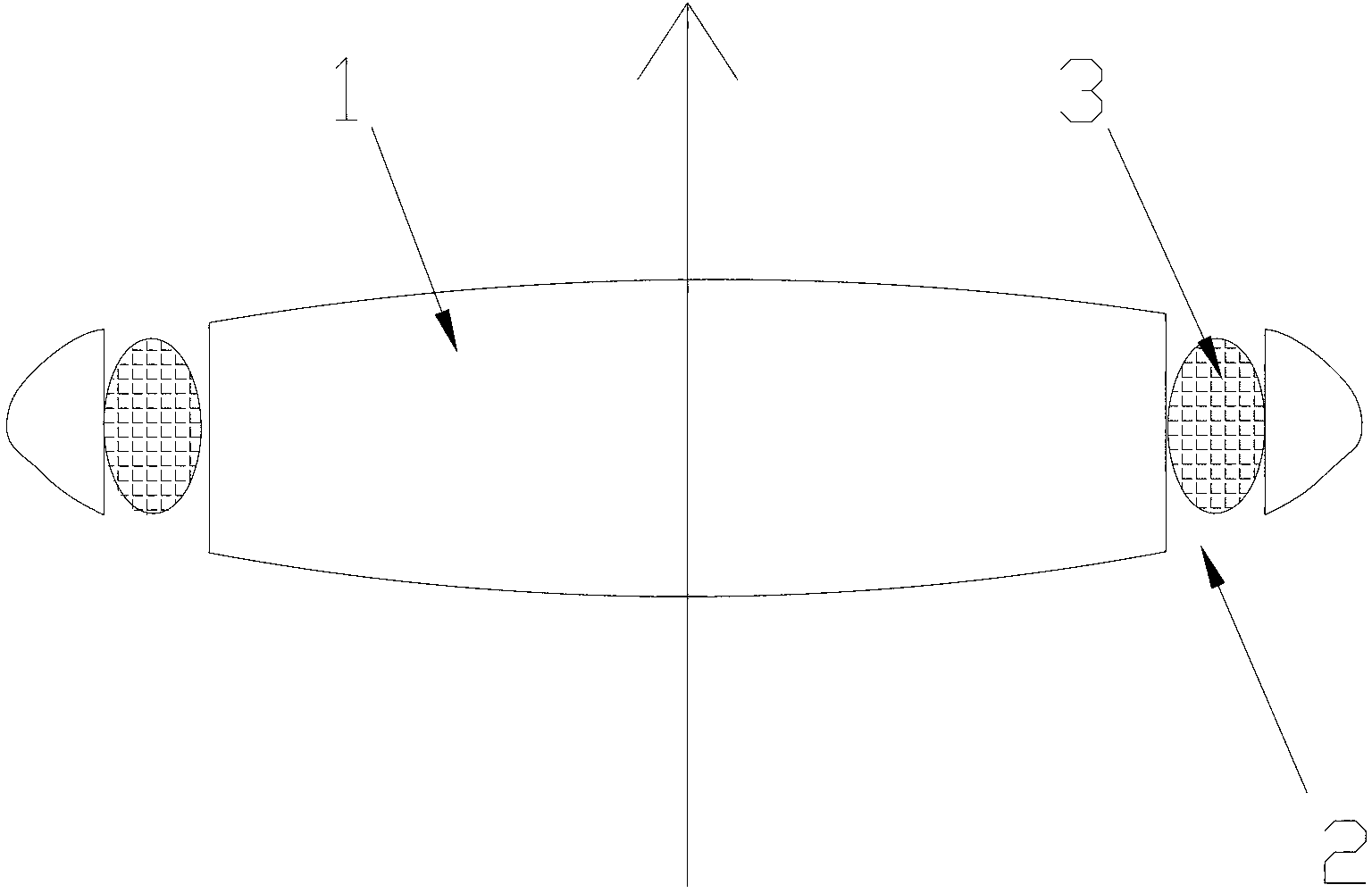
Posterior chamber intraocular lens
ActiveUS20140358225A1Increase spacingPromote lowerIntraocular lensPosterior capsule opacificationAnterior surface
The present invention relates to a posterior chamber intraocular lens (IOL), comprising: an optic consisting of an effective optical area and an effective optical area edge; at least two haptics connected to the optic, wherein a posterior surface of the effective optical area is a convex surface, and a basic spherical surface thereof has a radius of curvature in a range of 6.6 mm-80.0 mm. The effective optical area of the posterior chamber IOL adopts a design with the posterior surface obviously convex, which reduces the distance between the posterior surface of the effective optical area of the IOL and the posterior capsule, improves the stability of a spatial position of the IOL in a capsule bag, and reduces an incidence rate of posterior capsule opacification (PCO) after implantation of the IOL; since the effective optical area anterior surface is relatively flat, the IOL haptics will not be tightly pressed on the effective optical area anterior surface upon folding, the haptics are more easily unfolded after implantation into the eye and the support haptics are not mutually adhered to the effective optical area, and meanwhile the IOL imaging quality can be improved and / or the visual quality of the astigmatism sufferer is enhanced.
Owner:EYEBRIGHT MEDICAL TECH BEIJING
Treatment of fibrotic eye disorders
InactiveUS20110223177A1Slowly compositionAvoid rapid degradationBiocideSenses disorderMyosinPosterior capsule opacification
Inhibitors of myosin activity are used to treat or prevent a fibrotic disorder of the eye, for example posterior capsule opacification (PCO).
Owner:UNIVERSITY OF EAST ANGLIA
Nanometer fluorouracil coat artificial crystal and the preparing method
InactiveCN100553692CSuppress turbidityLow toxicityCoatingsIntraocular lensChitosan nanoparticlesPosterior capsule opacification
The present invention relates to intraocular lenses. At present, the polymethyl methacrylate (PMMA) intraocular lens commonly used in clinic for the treatment of cataract often causes inflammation after being implanted in the human body. Posterior capsule turbidity is also the main complication after cataract surgery. To solve these problems, the present invention selects antineoplastic drugs According to the weak penetrability of chitosan nanoparticles in the eye and the characteristics that the phagocytosis of conjunctival epithelial cells on nanoparticles is related to the size of the nanoparticles, chitosan-polyacrylic acid was used as a carrier to prepare fluorouracil nanoparticles Formulation, mixed coating on the surface of PMMA intraocular lens. The present invention also provides the preparation method of the above-mentioned intraocular lens. The invention not only increases the biocompatibility of the intraocular lens, inhibits the opacity of the posterior capsule after the implantation of the intraocular lens, but also prevents the anterior membrane and post-cataract after the implantation of the intraocular lens.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Intraocular lens for inhibiting pco and aco
InactiveCN1856281AInhibit migrationSimple structureIntraocular lensPosterior capsule opacificationOphthalmology
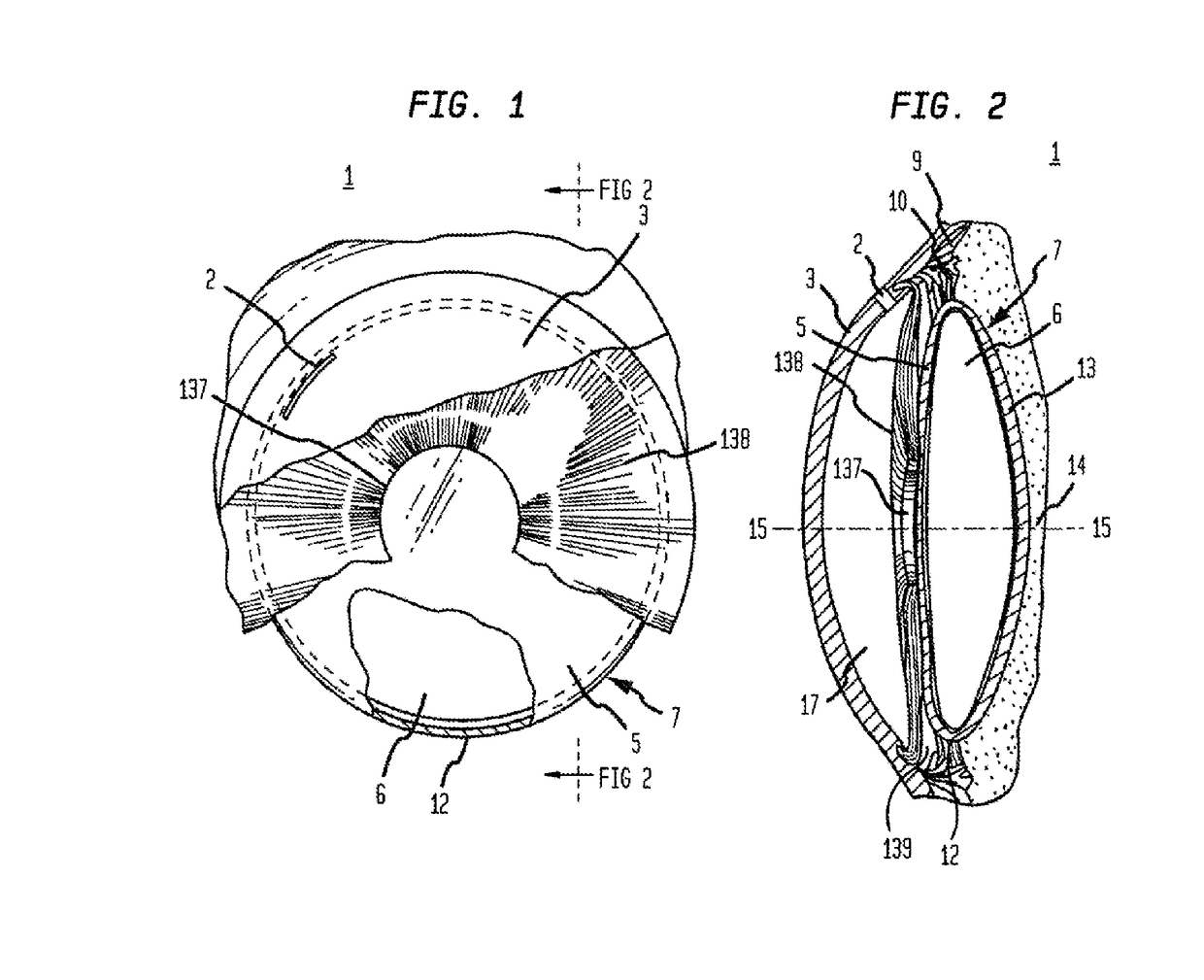
An intraocular lens for inhibiting posterior and anterior capsular opacification, or secondary cataract, includes an optic having a periphery provided with sharp bevels extending along arc segments defined by the juncture of the haptic-optic points of attachment and adjacent both the anterior and posterior optic surfaces.
Owner:BAUSCH & LOMB INC
Drug-carrying type artificial lens capable of being opened by laser
InactiveCN103169548ASmall regulationEasily exposedMedical devicesIntraocular lensDiseaseMultiple injection
The invention discloses a drug-carrying type artificial lens capable of being opened by a laser. The drug-carrying type artificial lens capable of being opened by the laser comprises an artificial lens main body, a plurality of drug storing holes with preset intervals are formed in the peripheral surface of the artificial lens main body, and micro-capsules loading active drug and capable of being punched through or resolved by the laser are embedded in the drug-storing holes. A plurality of drug micro-capsules are embedded into the peripheral portion of the artificial lens, the lens is opened to release by the laser on a suitable opportunity to achieve a treatment goal, and relative to a drug sustained releasing system, side effects brought by long-term sustained drug releasing is avoided. Besides the treatment goal of post-operation inflammatory resistance and posterior capsule opacification resistance, the drug-carrying type artificial lens capable of being opened by the laser can be embedded to achieve the purpose of controlling eye diseases aiming at certain diseases of age related macular degeneration, diabetic retinopathy and the like together with the complication of a cataract, and the complications caused by multiple injections of a vitreous cavity and the side effects of systemic administration are avoided or reduced.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
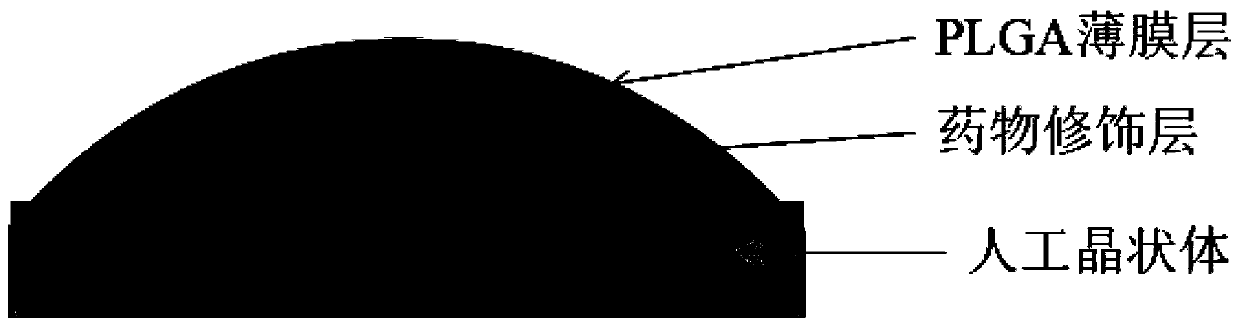
Drug-modified intraocular lens and preparation method and application thereof
InactiveCN110215519AGood preventive treatmentGood biocompatibilityOrganic active ingredientsSenses disorderPosterior capsule opacificationLens epithelial cell proliferation
The invention discloses a drug-modified intraocular lens and a preparation method and application thereof. According to the intraocular lens, by researching the effect of a signal path on proliferation and migration of lens epithelial cells, a drug with a prevention and treatment effect on posterior capsule opacification after cataract surgery is screened out. It is found that an inhibitor of a ROCK signal path has an influence on the proliferation and migration of the lens epithelial cells, and the drug-modified intraocular lens is provided by improving a drug feeding mode. The intraocular lens can relatively controllably release the explosive drug in the early stage; moreover, by slowly releasing the drug in the later stage, the intraocular lens integrally has the advantages of great biocompatibility and good prevention and treatment effect.
Owner:THE EYE HOSPITAL OF WENZHOU MEDICAL UNIV
Intraocular lens having edge configured to reduce posterior capsule opacification
Owner:ALCON RES LTD
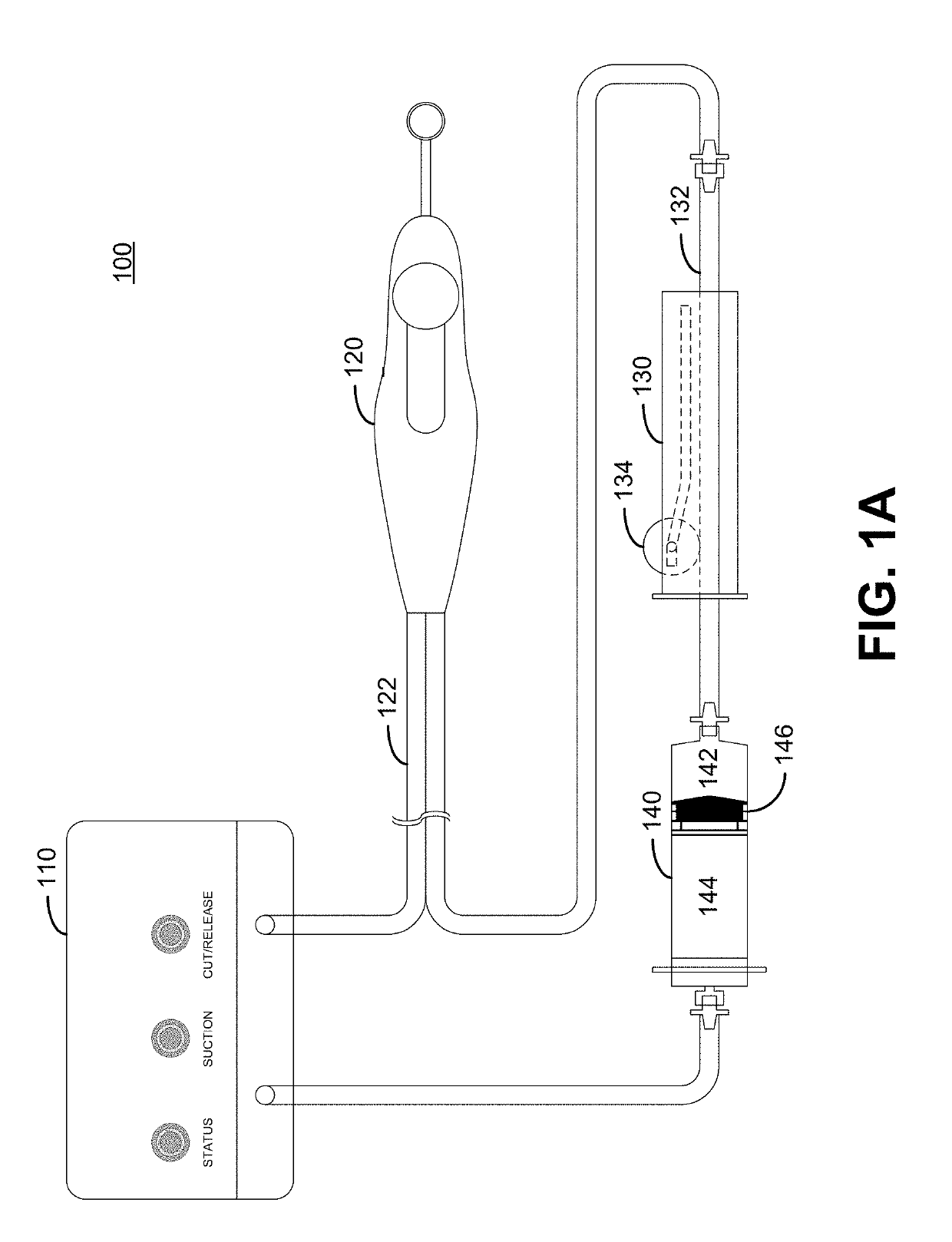
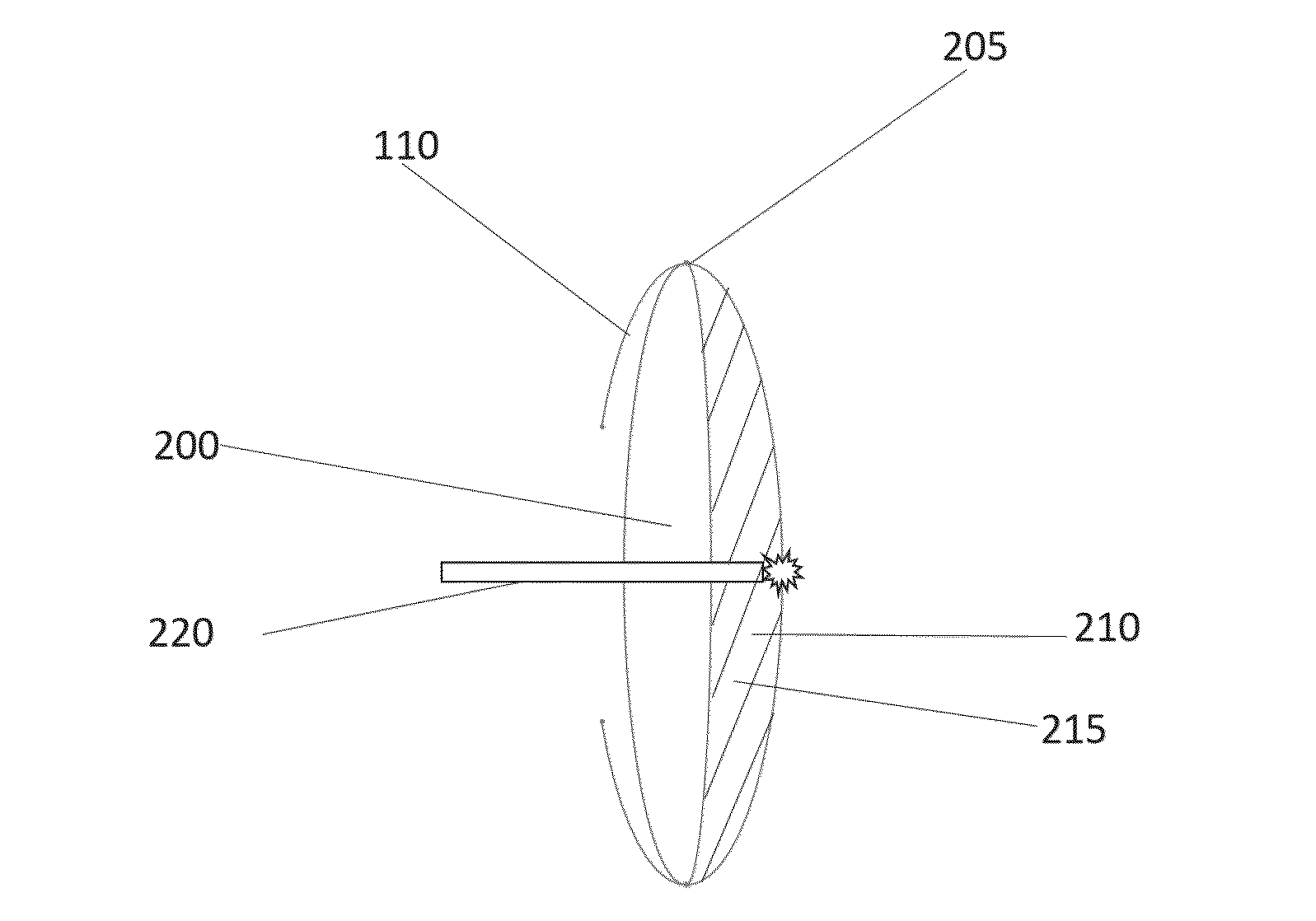
Hydrodissection and posterior capsule opacification prevention during capsulotomy procedure
ActiveUS20190231593A1PCO prevention and reductionEasy to disassembleLaser surgerySurgical instruments for heatingPosterior capsule opacificationCapsulotomy
Embodiments of the invention provide hydrodissection and / or PCO prevention or reduction in a patient undergoing eye surgery. In one embodiment, the invention is a surgical device for cutting and excising a portion of tissue, for example in performing a lens capsulotomy. A capsulotomy tip is inserted into an eye through an incision in the surface of the eye. The capsulotomy tip includes a suction cup to provide suction to the lens capsule. Then suction is applied via the suction cup to secure the capsulotomy tip to the eye. In some embodiments, after the capsulotomy tip is secured to the lens capsule, a cutting element of the capsulotomy tip is used to cut a tissue of the eye. Fluid is pushed through the capsulotomy tip and the capsulotomy tip is removed from the eye. Moreover, disclosed is an intraocular lens (IOL) to be used in conjunction with the surgical device.
Owner:MYNOSYS CELLULAR DEVICES INC
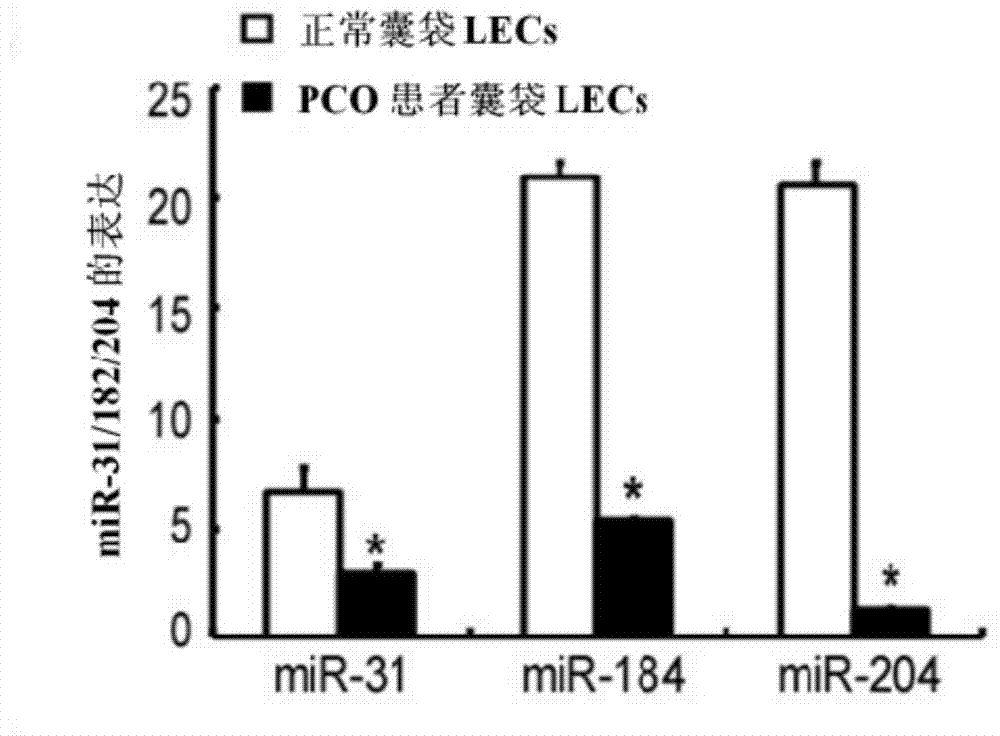
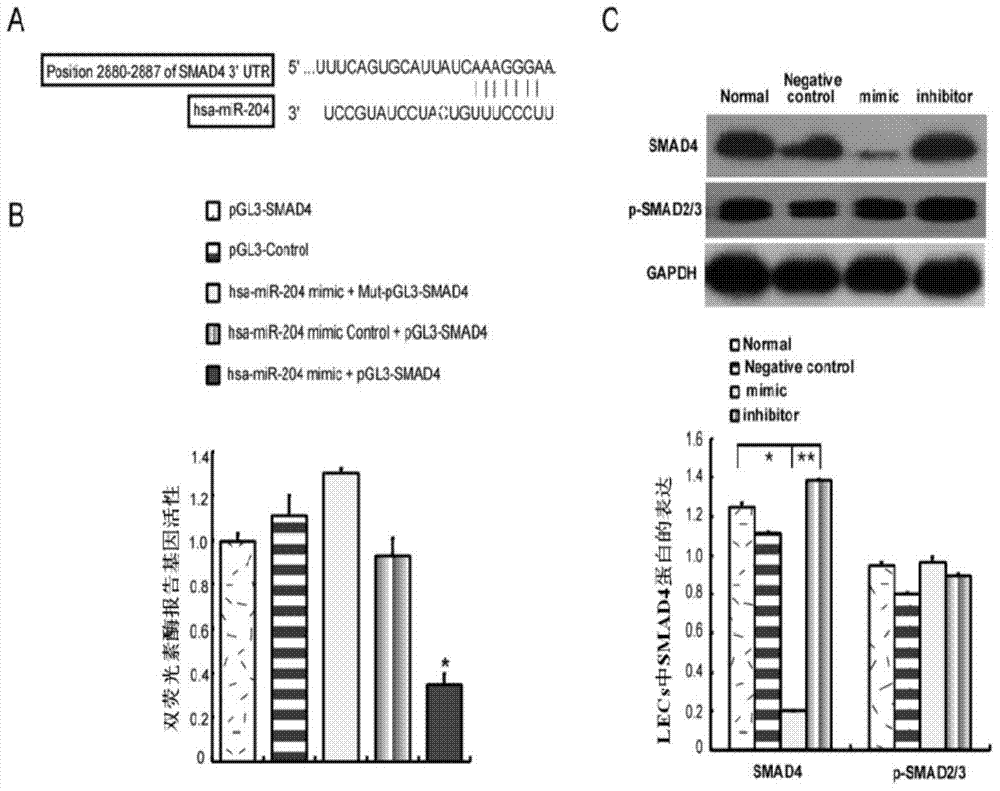
miRNA and application of miRNA in preparation of product for diagnosing and treating posterior capsule opacification
InactiveCN103773764ARegulate expressionInhibition of epithelial-mesenchymal transitionSenses disorderGenetic material ingredientsPosterior capsule opacificationMiRNA Gene
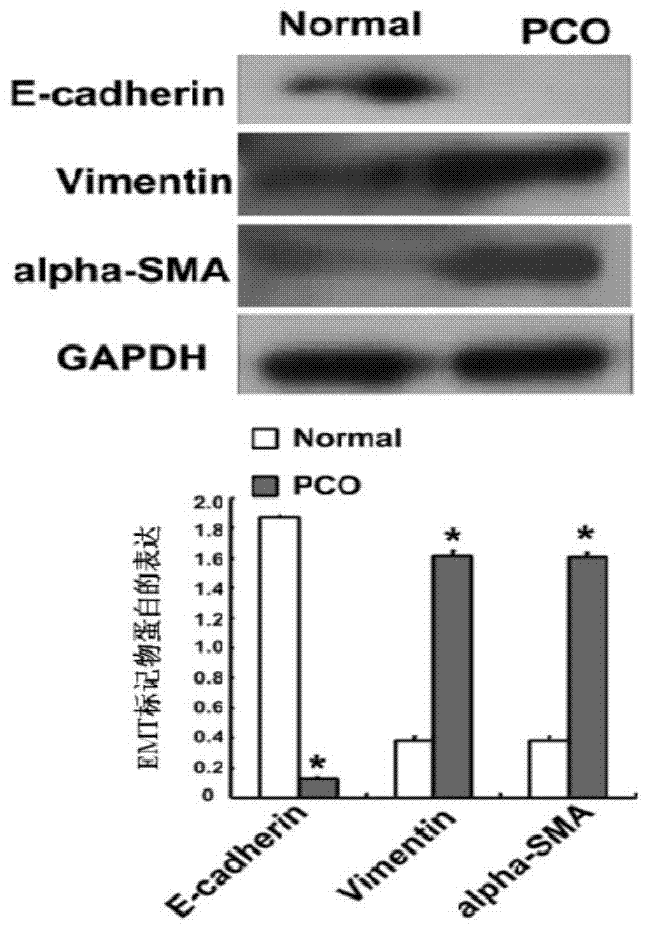
The invention aims to provide a miRNA and an application of miRNA in preparation of a product for diagnosing and treating posterior capsule opacification. The miRNA is miR-204-5p and the RNA sequence is SEQ ID NO:1. A miRNA gene product can be used for adjusting the expression of an SMAD4 gene, blocking a TGF (Transforming Growth Factor)-beta / Smads signal channel and inhibiting epithelial-mesenchymal transition (EMT), and has the potential of preparing the diagnosing and treating product for the posterior capsule opacification PCO.
Owner:SHANDONG EYE INST
Long-ncting heparin intraocular slow-released system
InactiveCN1433775APrevent proliferationInhibition of chemotaxisOrganic active ingredientsSenses disorderPosterior capsule opacificationLong acting
The present invention provides a long-acting heparin intraocular slow-releasing system, including heparin and medicinal carrier; their weight ratio is 0.1:0.9-0.9:0.1. Said medicinal carrier can be synthetic biodegradable high-molecular material, natural biodegradable high-molecular material or their mixture. The medicine-releasing period of said invented long-acting heparin slow-releasing systemis one week to one year. It can be used for inhibiting formation of antithrombase, resisting conversion of fibrinogen into insoluble fibrin, inhibiting proliferation and chemotaxis of fibrolast, reducing postoperative fibrinous exudation and preventing postcapsule opacity so as to inhibit the production of after-cataract.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Application of pirfenidone in preparation of medicaments for controlling proliferative diseases after ophthalmologic operation and eye drops thereof
ActiveCN102349901AImprove stabilityInhibit migrationOrganic active ingredientsBlood disorderPosterior capsule opacificationSide effect
The invention discloses an application of pirfenidone in the preparation of medicaments for controlling proliferative diseases after an ophthalmologic operation and eye drops thereof. According to the invention, pirfenidone is prepared into the eye drops. Experiments show that pirfenidone has good stability and good ocular tissue permeability, can inhibit HLECs migration and propagation, and has no cytotoxicity to HLECs within the action range (0-1mg / ml). With pirfenidone being within the range of 0-1%, the eye drops are continuously applied on eyes within a month with safety and with no obvious toxic and side effect. Pirfenidone can be used to delay the generation of PCO after the rabbit corneal Phaco operation, reduce HLECs propagation and minimize the shield of PCO to an optical region. In addition, there is no obvious ocular surface injury or postoperative inflammation aggravation and severe adverse reaction after PFD is applied in the rabbit corneal Phaco operation. Therefore, pirfenidone can be used to control proliferative diseases after an ophthalmologic operation, especially posterior capsule opacification generated after a cataract surgery.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
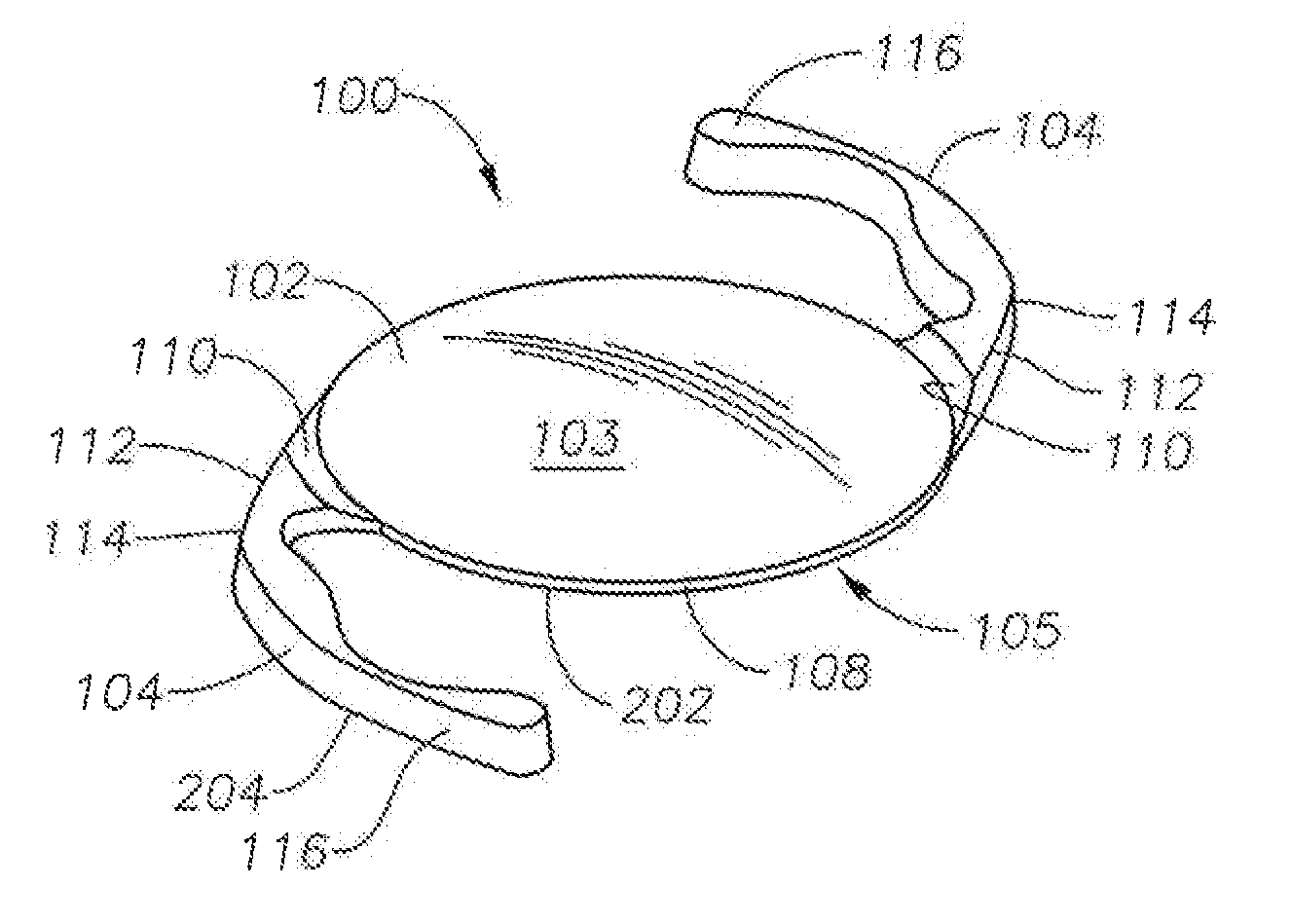
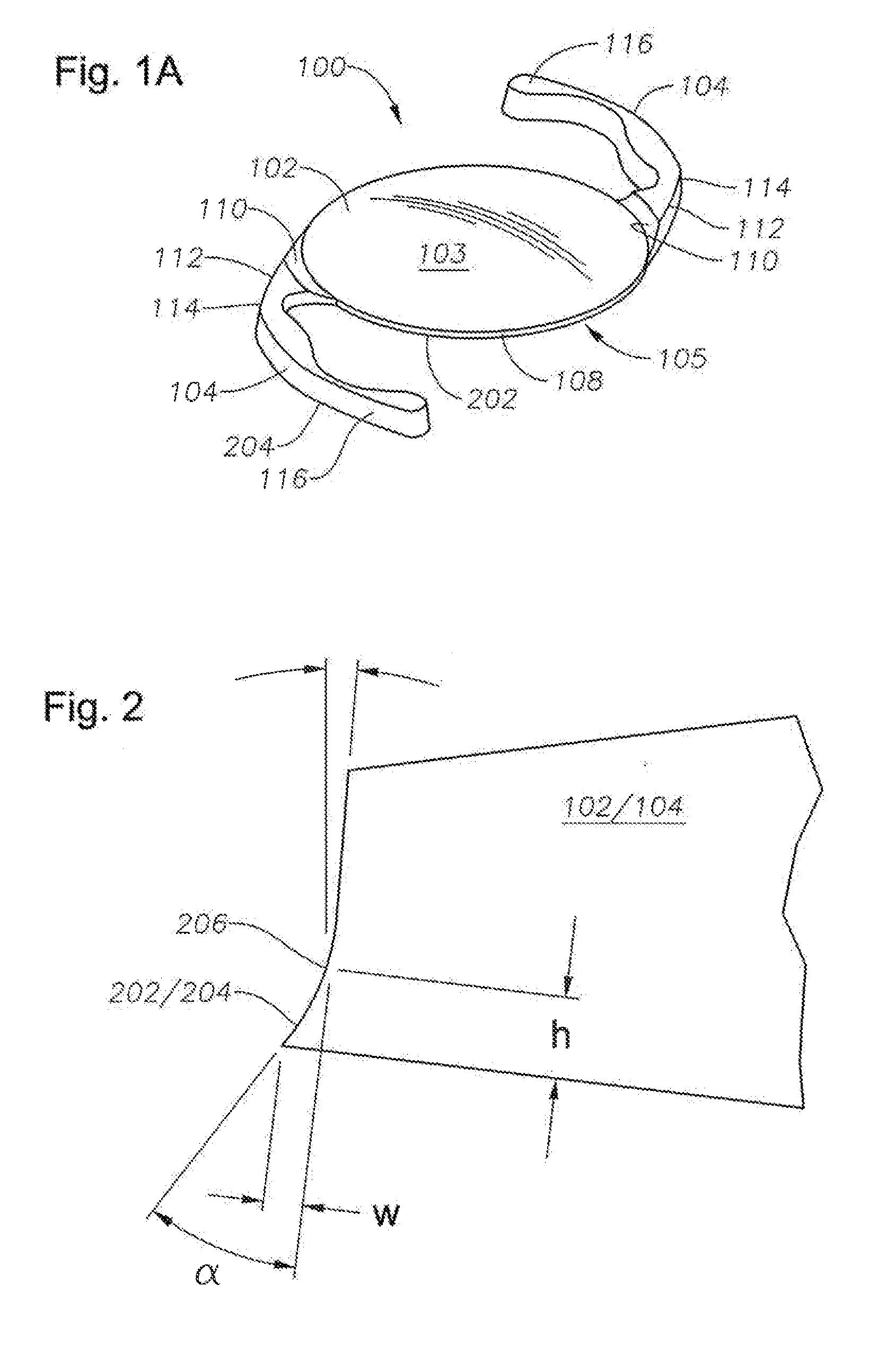
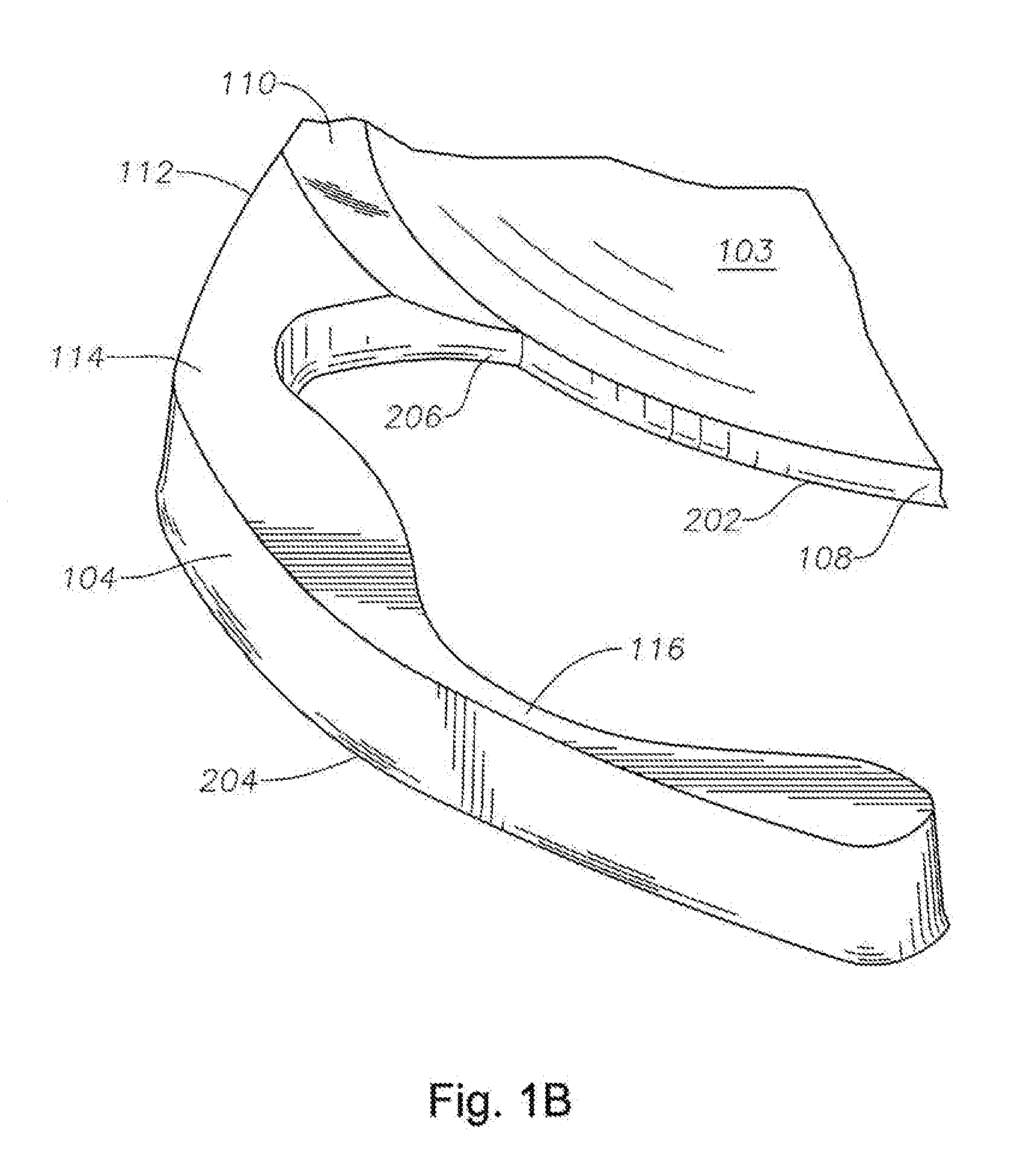
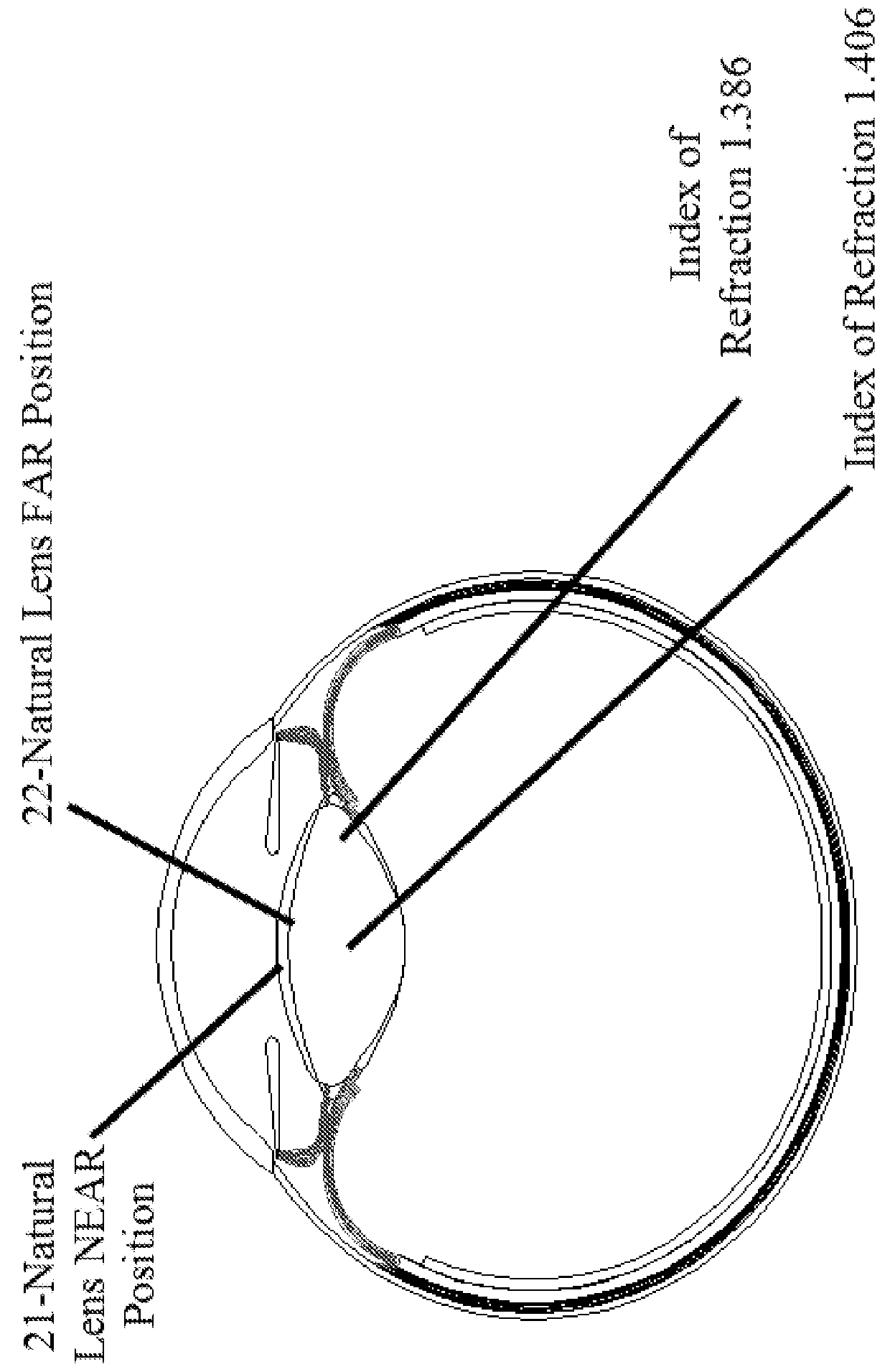
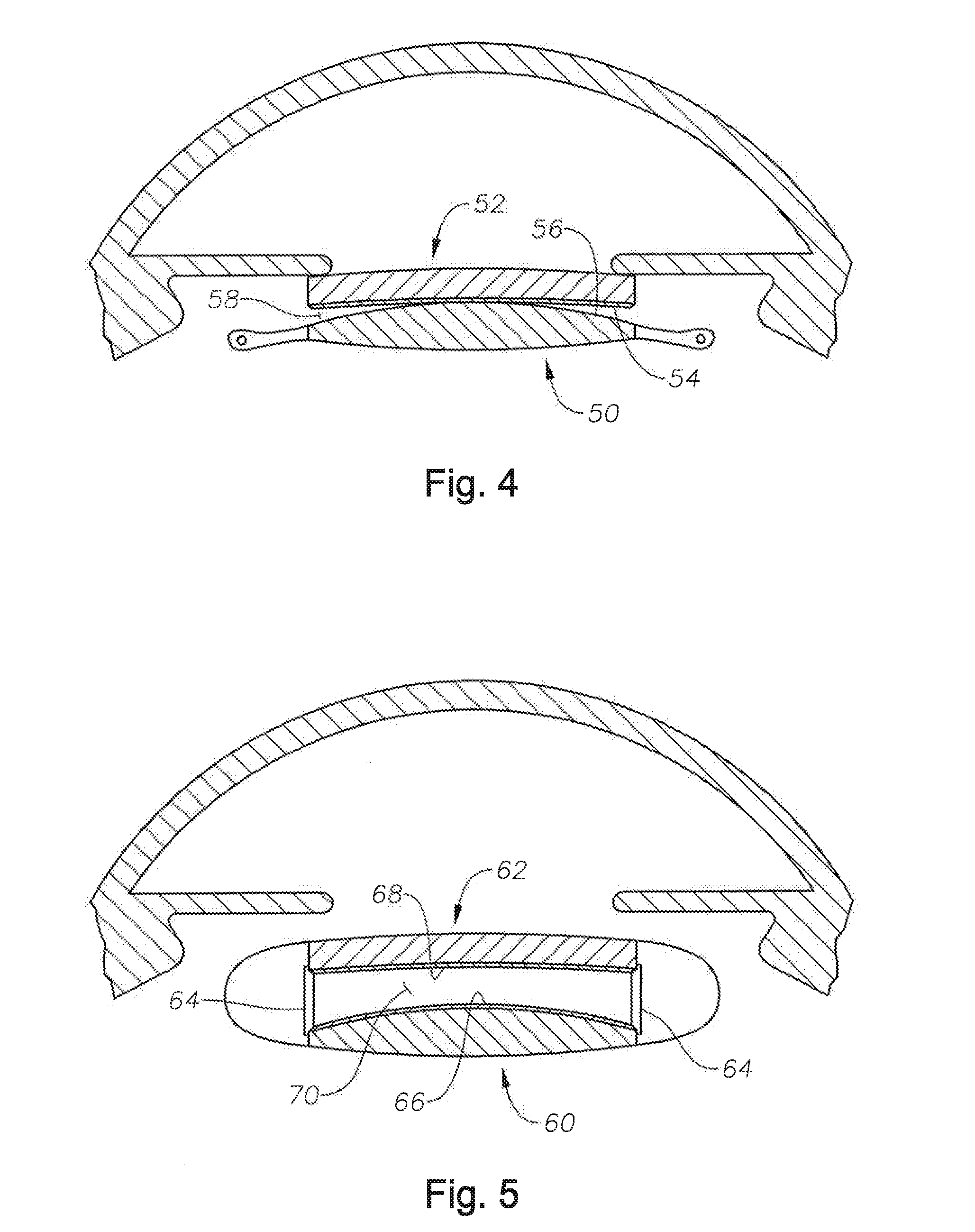
Accommodative intraocular lens that ejects post capsular opacification and self-centers
ActiveUS20180104047A1Reduces potential warpageGood removal effectIntraocular lensPosterior capsule opacificationAnterior surface
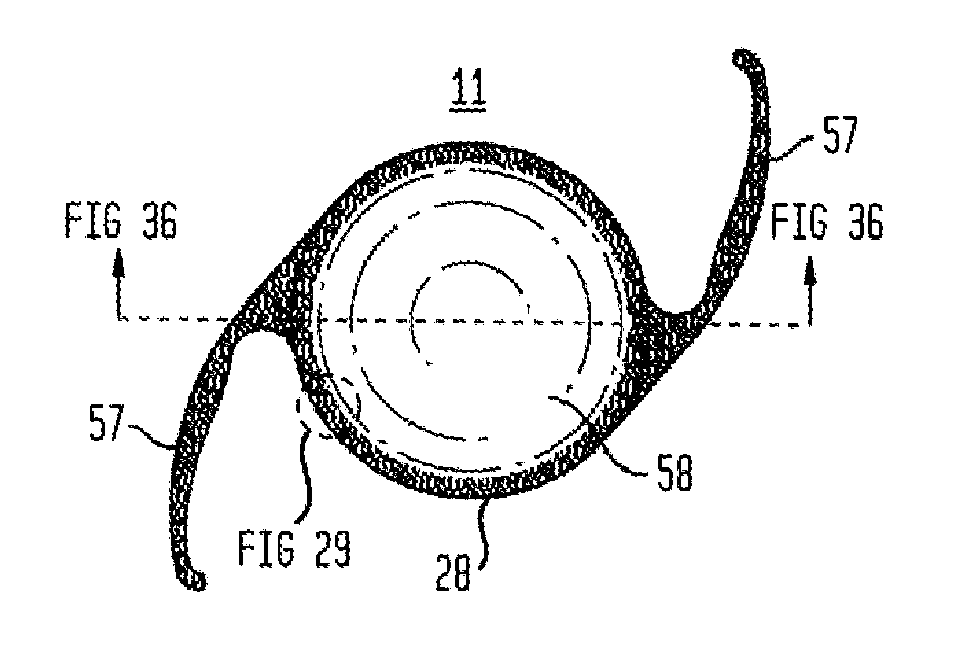
Described is an accommodating intraocular lens with a bi-convex, bi-aspheric, smooth surfaced optic held inside an anterior annulus via tabs. A second larger diameter annulus is positioned posteriorly and connects via a sloped surface to where the annuluses are at a maximum separation when viewing NEAR objects and minimum separation in the FAR position. The sloped surface is cut into ribbons, tabs and / or other annuluses without pushing the surfaces into the capsule when implanted; therefore, only the anterior and posterior annuluses have a force component against the capsule. The proximal edge of the anterior annulus is anterior to the apex of the anterior surface of the optic. The anterior capsule resting on the annulus leaves space for hydration of the capsule and reduces potential warpage of the optic. The annulus edge is designed to scrape posterior capsular opacification from the capsule.
Owner:CALLAHAN WAYNE B
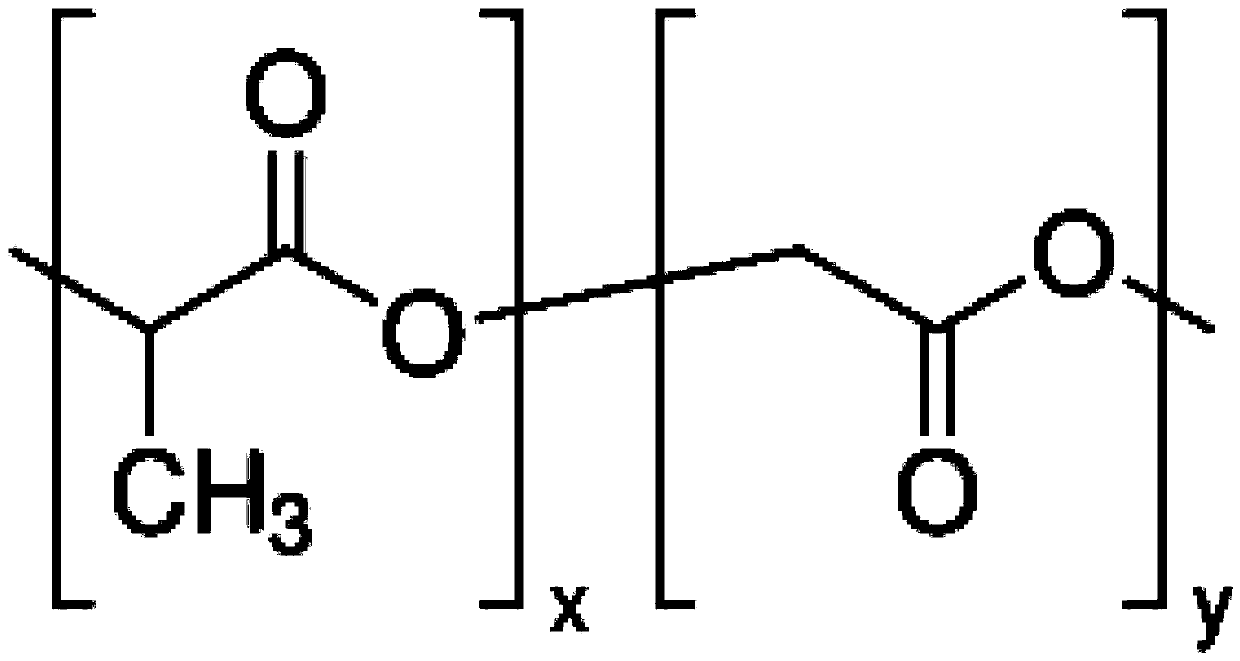
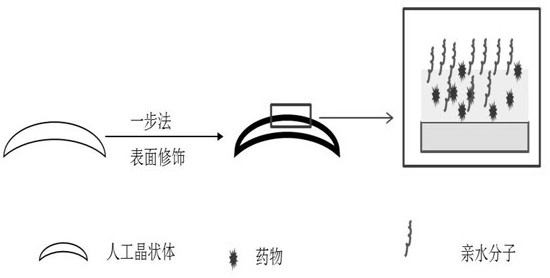
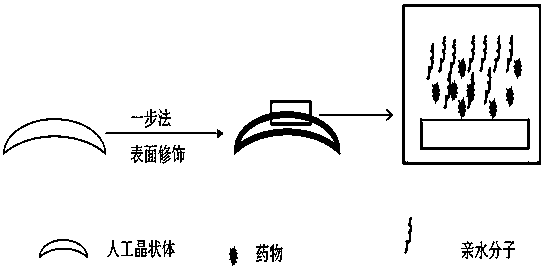
A kind of intraocular lens with hydrophilic-drug sustained-release synergistic function and preparation method thereof
ActiveCN107754018BEasy to disinfectEasy to packCoatingsIntraocular lensCell adhesionPosterior capsule opacification
The invention relates to an artificial lens with a hydrophilic-medicine sustained release synergistic function, and a preparation method thereof. A medicine-loading-hydrophilic multifunctional coatingis modified on the surface of the artificial lens, and the synergistic effect of resisting cell adhesion at an early stage after implantation and resisting lens epithelia cell proliferation at middleand late stage is achieved, so that the occurrence rate of posterior capsule opacification can be reduced and a high-biocompatibility artificial lens is obtained; a hydrophilic-medicine-loading multifunctional modification layer is constructed on the surface of the artificial lens by a one-pot method, the manufacturing process is simple and low in cost, the tedious steps required by surface modification in the past are avoided, and the artificial lens can be applied to the surface of a three-dimensional implantable device with various complex shape structures. The manufactured zwitterionic modified artificial lens is convenient to disinfect, packaging and transport, and is an artificial lens industrialized product which is low in cost, convenient and practical.
Owner:WENZHOU MEDICAL UNIV
Active isaria cicadae miquel substance and application thereof in prevention, delaying or treatment of cataract
InactiveCN110279724AReduce turbidityImprove antioxidant capacitySenses disorderFungiPosterior capsule opacificationLiquid medium
The invention provides an active isaria cicadae miquel substance and an application thereof in prevention, delaying or treatment of cataract, and belongs to the technical field of active substances. A preparation method of the active isaria cicadae miquel substance comprises steps as follows: isaria cicadae miquel strains activated in a PDA (potato dextrose agar) slant medium are inoculated into a seed solution medium for culture, and a seed solution is obtained; the seed solution is inoculated into a PDA liquid medium containing traditional Chinese medicine extract and Cajanonic acid A for culture, then mycelia and fermentation liquor are dried and smashed, and a fermented product containing the active substance is obtained; the fermented product is extracted in distilled water, an extracting solution is obtained and concentrated into extractum, the extractum is dried, and the active isaria cicadae miquel substance is obtained. Active ingredients in the active isaria cicadae miquel substance obtained with the preparation method can play the gain role, and the active isaria cicadae miquel substance has a protecting effect, delays the pathological process of posterior capsule opacification, delays development of cataract and is expected to be applied to prevention and treatment of age-related cataract and after cataract.
Owner:兰溪市立顺生物有限公司
Intraocular lenses with interlenticular opacification resistance
InactiveUS20120059466A1Avoid turbidityPharmaceutical containersPretreated surfacesPosterior capsule opacificationIntraocular lens
The present invention is directed to an intraocular lens, an intraocular lens system and a method of producing and / or implanting the lens or system in an eye wherein at least one intraocular lens includes a coating that aids in resisting opacification (e.g., posterior capsule opacification (PCO), interlenticular opacification (ILO) or the like). The material of the coating is preferably hydrophilic or super-hydrophobic.
Owner:NOVARTIS AG
Artificial lens with hydrophilic-medicine sustained release synergistic function, and preparation method thereof
ActiveCN107754018AEasy to disinfectEasy to packCoatingsIntraocular lensPosterior capsule opacificationCell adhesion
The invention relates to an artificial lens with a hydrophilic-medicine sustained release synergistic function, and a preparation method thereof. A medicine-loading-hydrophilic multifunctional coatingis modified on the surface of the artificial lens, and the synergistic effect of resisting cell adhesion at an early stage after implantation and resisting lens epithelia cell proliferation at middleand late stage is achieved, so that the occurrence rate of posterior capsule opacification can be reduced and a high-biocompatibility artificial lens is obtained; a hydrophilic-medicine-loading multifunctional modification layer is constructed on the surface of the artificial lens by a one-pot method, the manufacturing process is simple and low in cost, the tedious steps required by surface modification in the past are avoided, and the artificial lens can be applied to the surface of a three-dimensional implantable device with various complex shape structures. The manufactured zwitterionic modified artificial lens is convenient to disinfect, packaging and transport, and is an artificial lens industrialized product which is low in cost, convenient and practical.
Owner:WENZHOU MEDICAL UNIV
Application of pirfenidone in preparation of medicaments for controlling proliferative diseases after ophthalmologic operation and eye drops thereof
ActiveCN102349901BImprove stabilityInhibit migrationOrganic active ingredientsPosterior capsule opacificationSide effect
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Systems, intraocular lenses, and methods for treatment of posterior capsule opacification
InactiveUS20160089232A1Prevent and substantially eliminate damageResist damageLaser surgerySurgical instrument detailsPosterior lens capsulePosterior capsule opacification
In various embodiments, ocular treatment systems, intraocular lenses, and treatment methods are utilized for the treatment or prevention of posterior capsule opacification of the eye by, for example, targeting portions of the posterior lens capsule for ablation while minimizing damage to implanted intraocular lenses.
Owner:DEBOER CHARLES +4
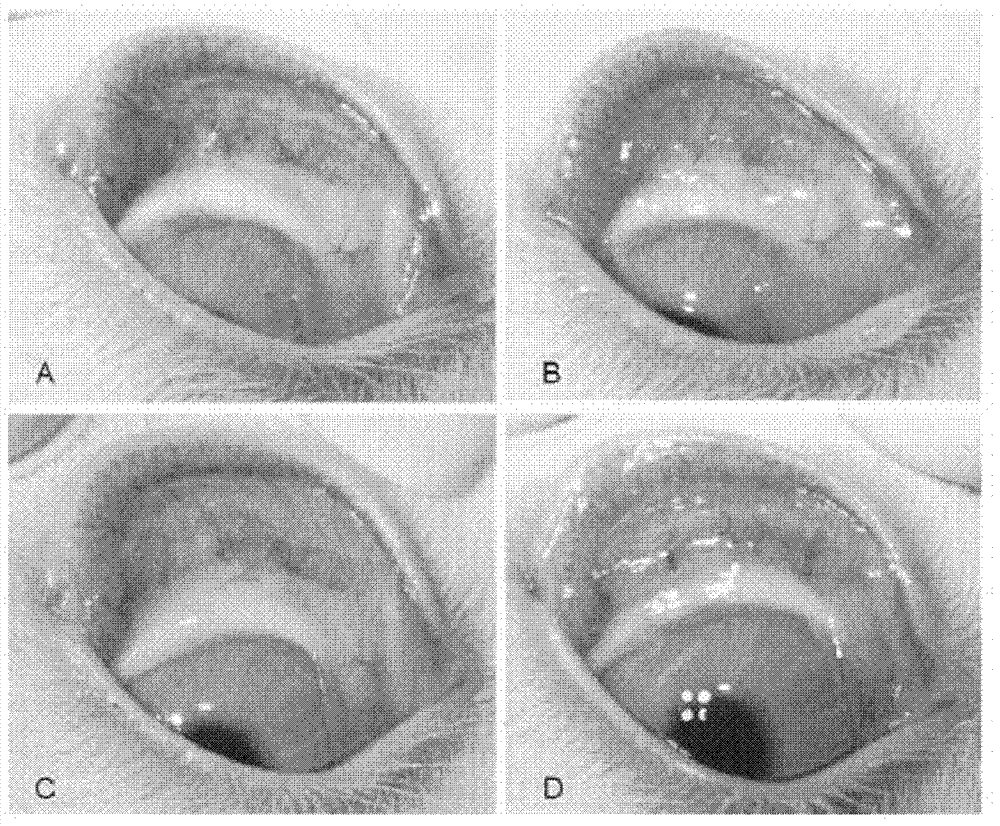
Pirenoxine sodium eye drops and solution for eye administration
ActiveCN112823784ACompatibleGood treatment effectOrganic active ingredientsSenses disorderPosterior capsule opacificationCataract extraction
The invention provides pirenoxine sodium eye drops. According to an embodiment of the invention, the pirenoxine sodium eye drops comprise a solvent, an anti-inflammatory agent, and a solid preparation, wherein the solid preparation contains pirenoxine sodium and pharmaceutically acceptable auxiliary materials. According to the embodiment of the invention, the pirenoxine sodium eye drops contain the anti-inflammatory agent and the pirenoxine sodium at the same time, so that the pirenoxine sodium eye drops can be applied to eye administration in the initial stage after an operation of a patient, the eye infection can be effectively prevented, the incidence rate of posterior capsule opacification after the operation can be reduced, especially, the occurrence rate of posterior capsule opacification after extracapsluar cataract extraction is reduced, the curing time of eye cataract of the patient can be shortened, the pain of the patient is reduced, and the living quality of the patient is improved.
Owner:湖北远大天天明制药有限公司
Posterior chamber intraocular lens
ActiveUS9855136B2Increase spacingPromote lowerIntraocular lensPosterior capsule opacificationImaging quality
The present invention relates to a posterior chamber intraocular lens (IOL), comprising: an optic consisting of an effective optical area and an effective optical area edge; at least two haptics connected to the optic, wherein a posterior surface of the effective optical area is a convex surface, and a basic spherical surface thereof has a radius of curvature in a range of 6.6 mm-80.0 mm. The effective optical area of the posterior chamber IOL adopts a design with the posterior surface obviously convex, which reduces the distance between the posterior surface of the effective optical area of the IOL and the posterior capsule, improves the stability of a spatial position of the IOL in a capsule bag, and reduces an incidence rate of posterior capsule opacification (PCO) after implantation of the IOL; since the effective optical area anterior surface is relatively flat, the IOL haptics will not be tightly pressed on the effective optical area anterior surface upon folding, the haptics are more easily unfolded after implantation into the eye and the support haptics are not mutually adhered to the effective optical area, and meanwhile the IOL imaging quality can be improved and / or the visual quality of the astigmatism sufferer is enhanced.
Owner:EYEBRIGHT MEDICAL TECH BEIJING
Method for reducing the risk of posterior capsular opacification
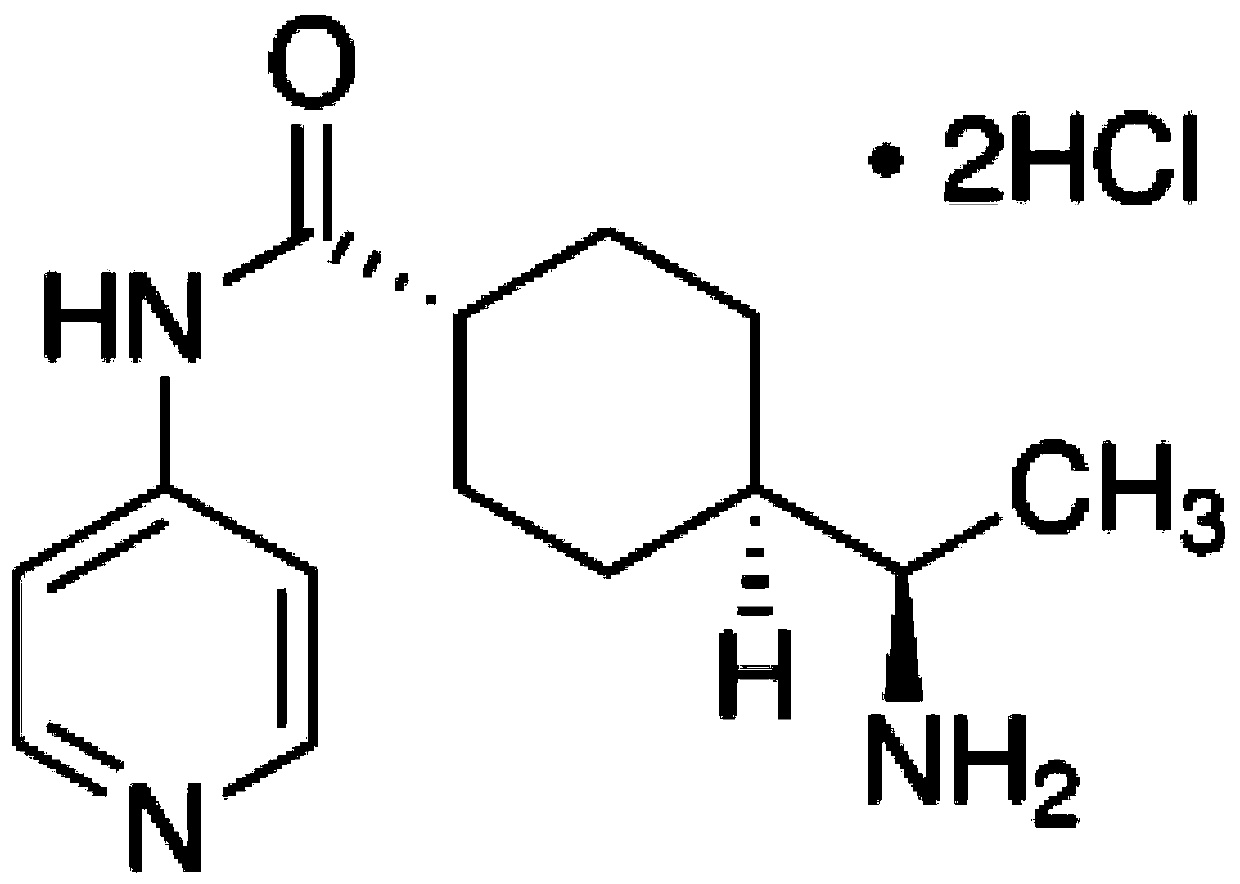
InactiveUS20060271062A1Easy to disassemblePrevent proliferationEye surgeryTissue regenerationPosterior capsule opacificationLens epithelial cell
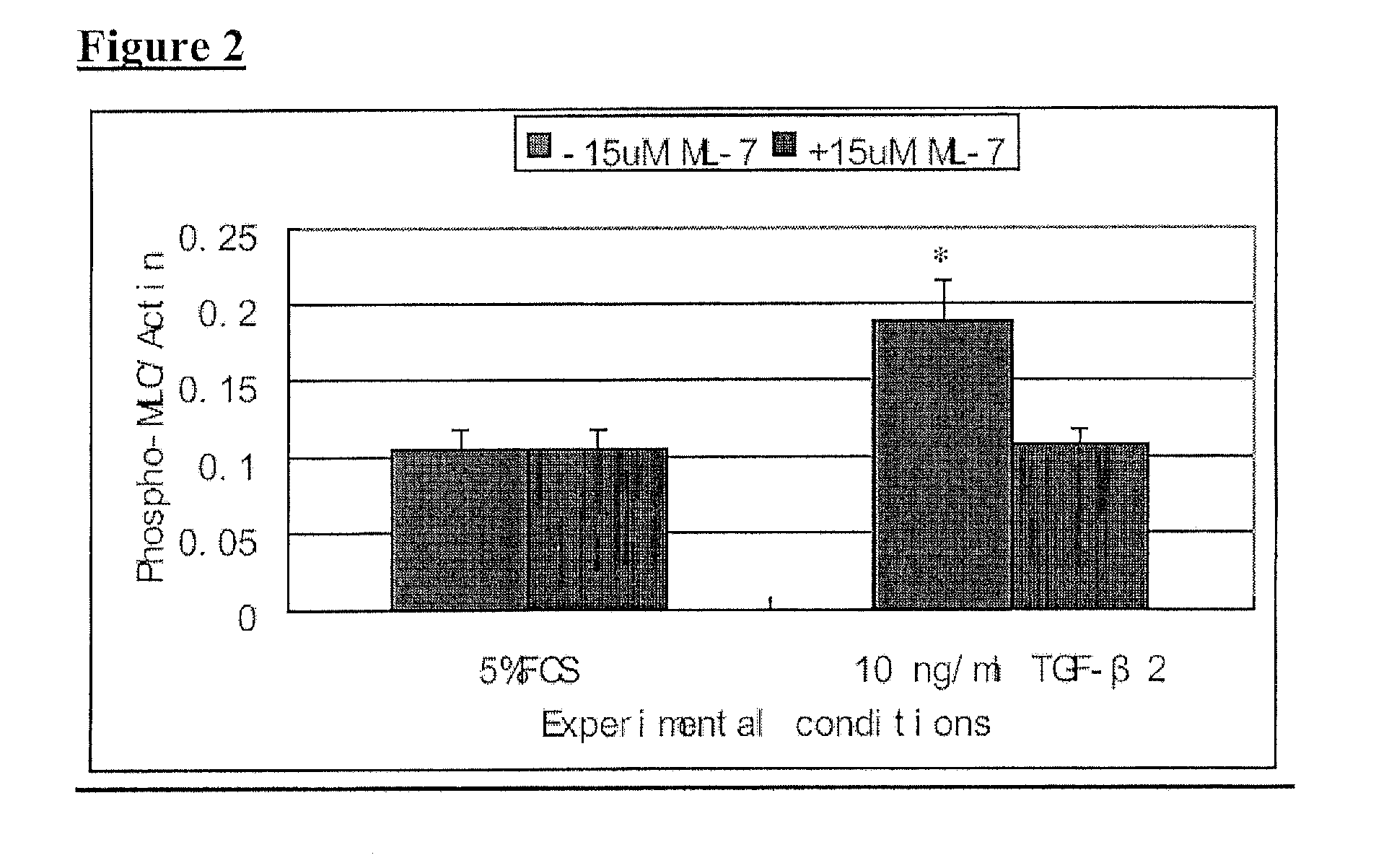
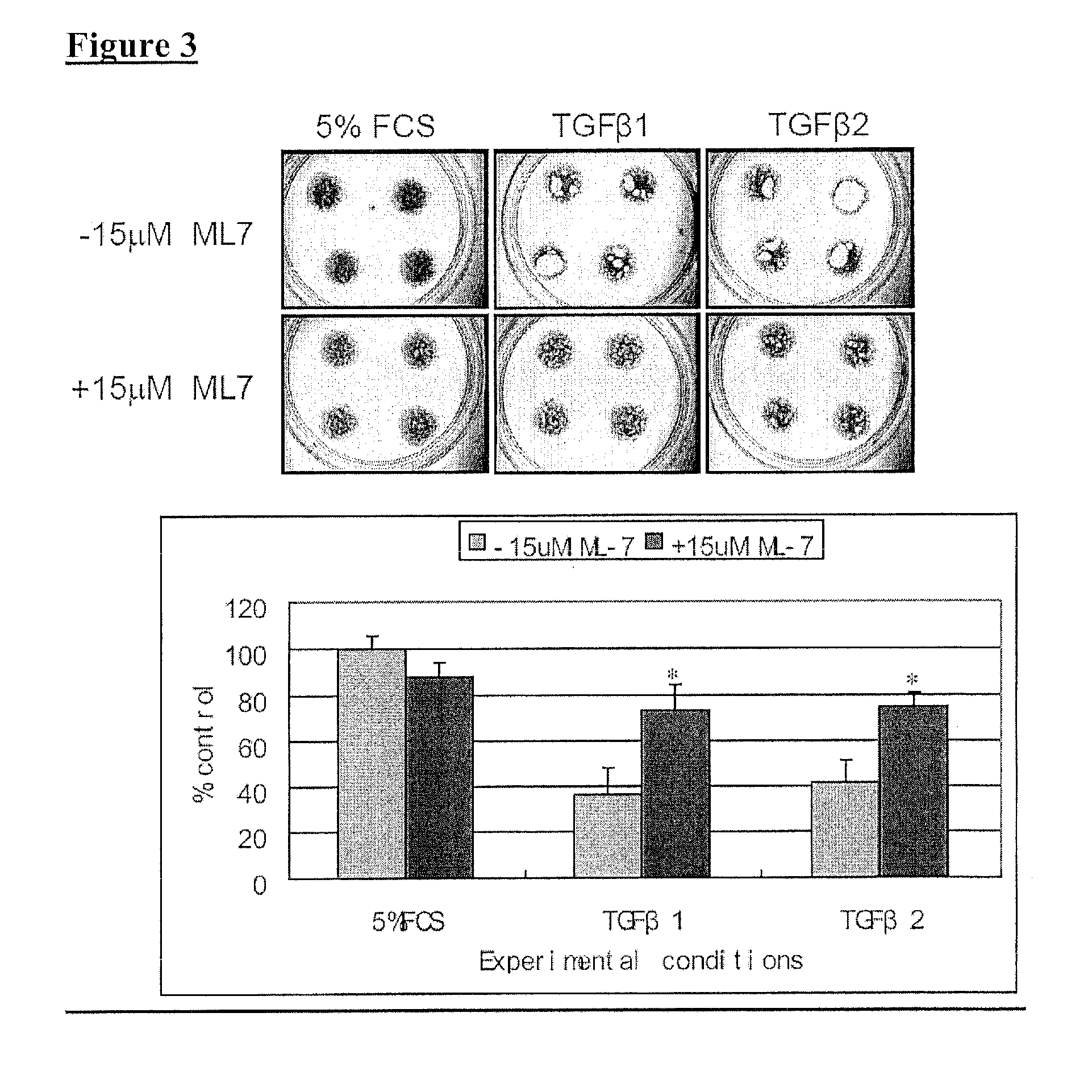
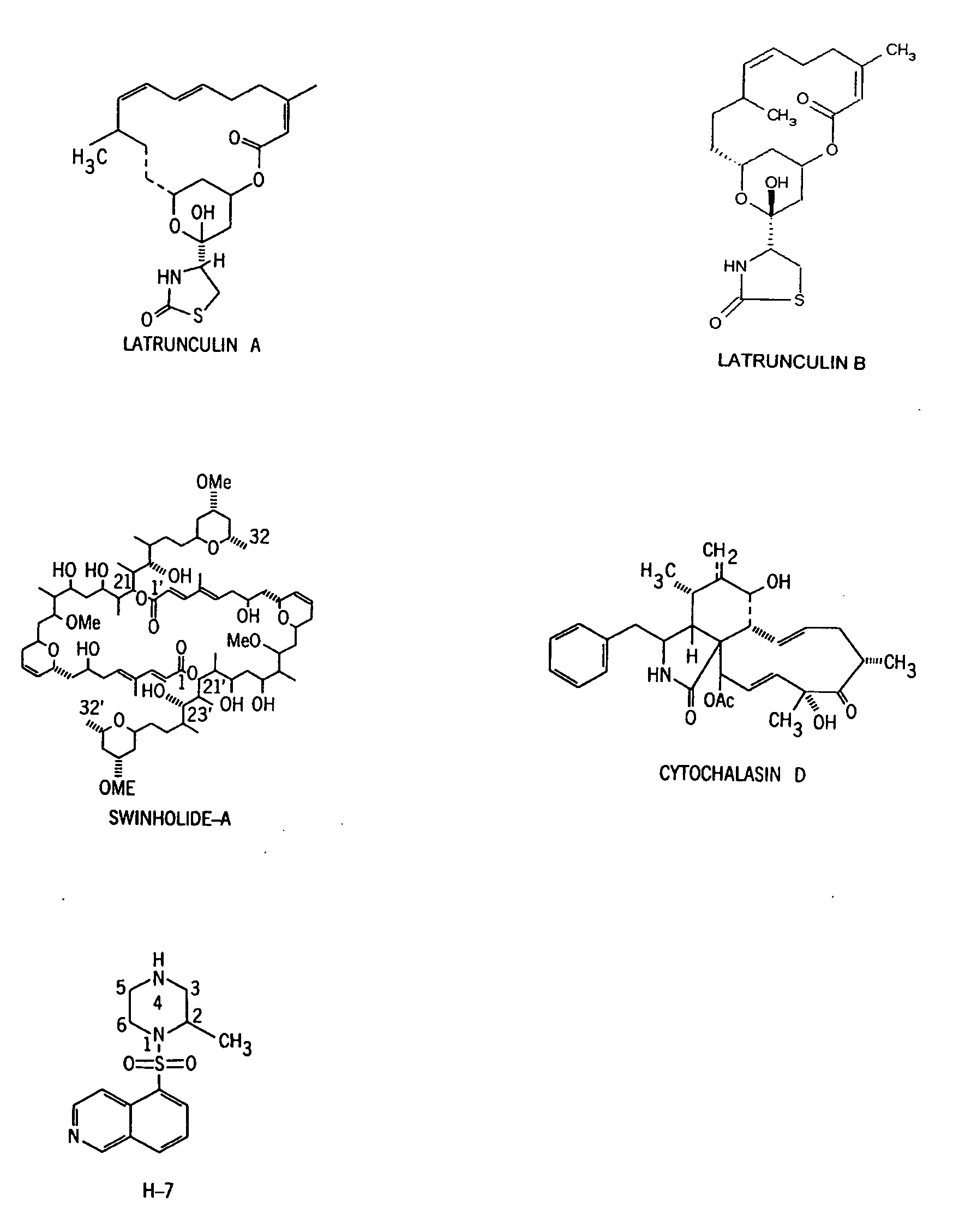
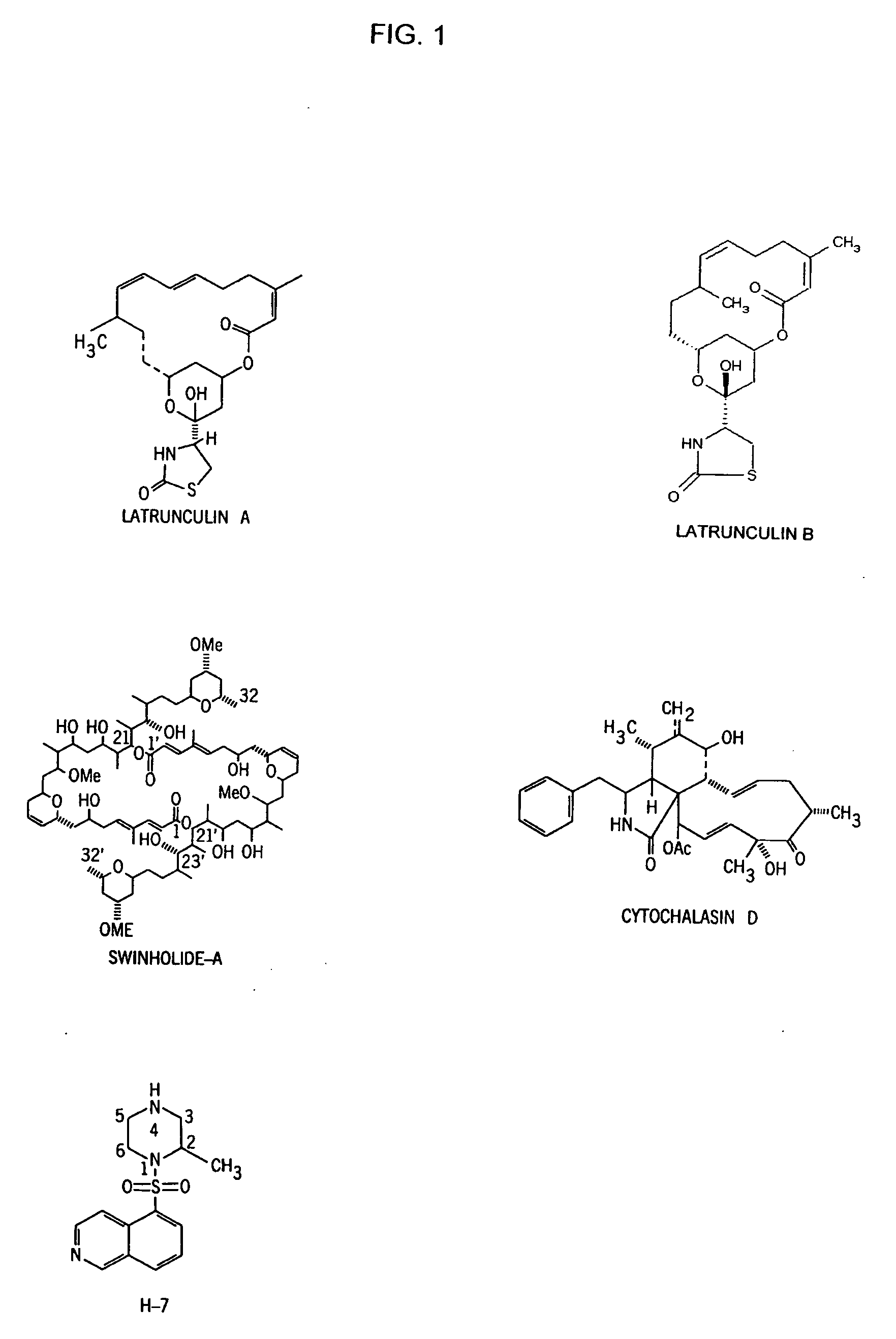
It is disclosed here that actin-disrupting compounds and 1-(5-isoquinolinyl-sulfonyl)-2-methylpiperazine can be used to facilitate the removal of lens epithelial cells from the lens capsule during cataract surgery and they may also be used to inhibit lens epithelial cells' proliferation and / or migration after surgery to reduce the risk or severity of posterior capsular opacification. Various related methods, kits, and ocular implants for reducing the risk or severity of posterior capsular opacification are provided.
Owner:WISCONSIN ALUMNI RES FOUND
Micropatterned intraocular implants
InactiveUS20180228600A1Reduce travel requirementsFix and reduce travelEye treatmentTissue regenerationPosterior capsule opacificationIntraocular lens
Generally, an intraocular implant having on the external surface a plurality of pattern surface elements disposed in spaced apart relation defining a tortuous pathway adapted to control a flow of fluid or a flow of particles suspended in a fluid or inhibit the growth or migration of cells is provided. In particular, an intraocular implant that is implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits growth or migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com