miRNA and application of miRNA in preparation of product for diagnosing and treating posterior capsule opacification
A post-onset cataract technology, applied in the direction of DNA / RNA fragments, recombinant DNA technology, gene therapy, etc., can solve the problems of iris hemorrhage, high cost, scope of action, targeting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
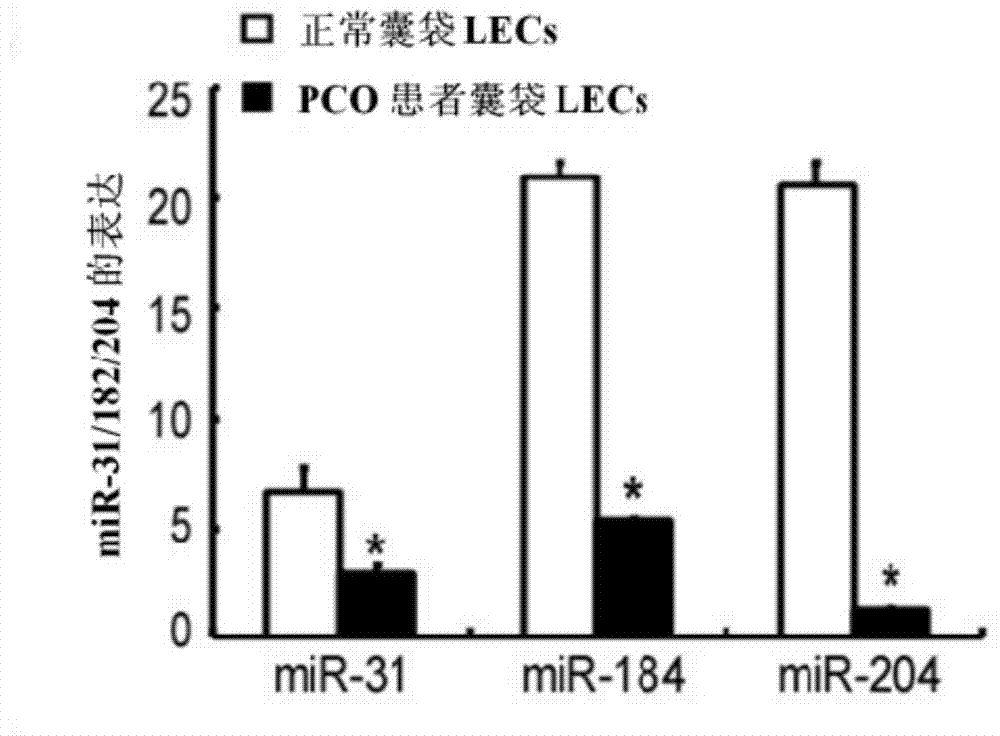
[0017] Example 1: Screening of has-miR-204-5p and determination of target gene SMAD4
[0018] 1. Sample RNA extraction and detection
[0019] Biological material source: lens capsule of normal people and post-cataract patients
[0020] Total RNA including miRNA was extracted according to the instructions of TRIzol (Invitrogen) and miRNeasy mini kit (QIAGEN).
[0021] 1) Collect the crystal capsule and add 1mL TRIzol (10μL β-mercaptoethanol) reagent, after cracking, put it at room temperature
[0022] Set for 5min.
[0023] 2) Add 200 μL of chloroform, shake vigorously for 15 seconds, let stand at room temperature for 2-3 minutes, and centrifuge at 12,000×g for 15 minutes at 4° C.
[0024] 3) Remove the supernatant, add 1.5 times the volume of absolute ethanol, and mix well.
[0025] 4) Transfer the solution and precipitate obtained in step 3 to a 2mL RNeasy Mini spin column, cover the tube gently, centrifuge at ≥8000xg for 15s at room temperature, discard the waste liquid ...
Embodiment 2
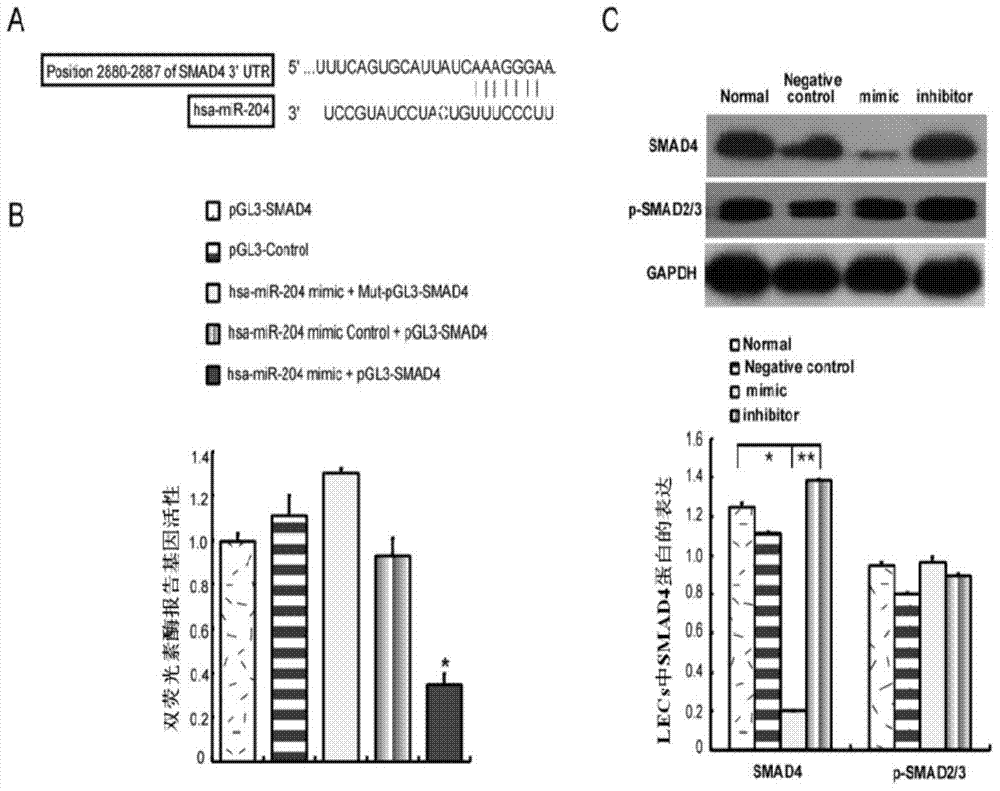
[0045] Example 2: Determination of has-miR-204 target gene SMAD4
[0046] According to bioinformatics analysis, it was found that has-miR-204 binds to the 2880-2887bp of the 3'-UTR region of the SMAD4 gene, such as figure 2 As shown in A.
[0047] Amplify the 3'-UTR region and Mut-SMAD4-3'-UTR region of SMAD4 gene, the sequence is 359bp, add BglII in the upstream and MluI restriction site in the downstream respectively, the sequence is shown in Table 1, and the pGL3-Basic Vector (Promega) as the vector backbone, pGL3-SMAD4, pGL3-SMAD4-3'-UTR and Mut-pGL3-SMAD4-3'-UTR vectors were constructed.
[0048] As a chemically synthesized mature miRNA mimic, miRNA mimic can mimic endogenous miRNA to function after transfection into cells. As a chemically synthesized mature miRNA inhibitor, miRNA inhibitor can specifically inhibit the function of miRNA after transfection into cells. Therefore, under in vitro culture conditions, miRNA mimic and miRNA inhibitor are used to simulate the...
Embodiment 3
[0051] Example 3: has-miR-204-5p inhibits the occurrence of EMT in a PCO model cultured in vitro
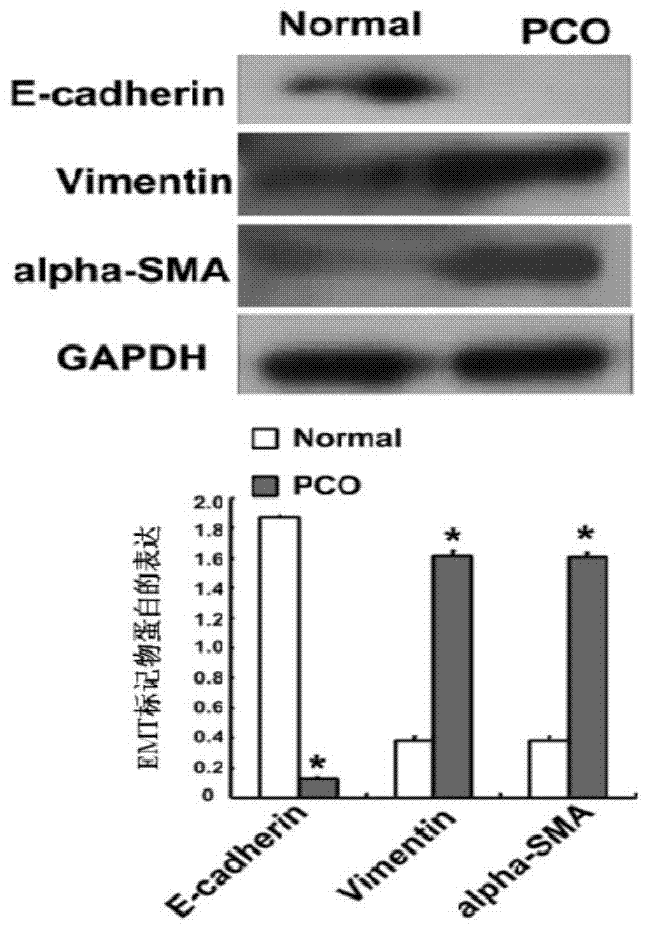
[0052] 1. Detection of EMT marker gene expression from PCO patient materials
[0053] In line with the relevant national policies and regulations, and on the basis of the consent of the sampling subjects, the capsular bag materials of patients with post-cataract PCO and normal people were selected, and Western blotting was performed to detect the expression of EMTmarker genes E-cadherin, a-SMA, and Vimentin. It was found that Compared with normal people, the expression of E-cadherin was significantly down-regulated, and the expression of a-SMA and Vimentin were up-regulated, such as image 3 shown.
[0054] 2. Up-regulation of has-miR-204-5p expression inhibits EMT
[0055] In accordance with the relevant national policies and regulations, and on the basis of the consent of the sampling subjects, the pouch materials of PCO patients and normal people were selected, and TGF- Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com