Pirenoxine sodium eye drops and solution for eye administration
A technology of pirenoxine sodium and eye drops, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 prepares test solution
[0052] In this example, according to the method described in the general method, a series of solutions for dissolving pirenoxine sodium tablets and pirenoxine sodium tablets were prepared, and a series of test solutions were obtained by mixing and dissolving, as follows:
[0053] (1) Test solution A
[0054] The weight ratio of pirenoxine sodium to betamethasone is 1:0.13 (the concentration of pirenoxine sodium is 0.0053% by weight).
[0055] (2) Test Solution B
[0056] The weight ratio of pirenoxine sodium to hydrocortisone is 1:0.17 (the concentration of pirenoxine sodium is 0.0045% by weight).
[0057] (3) Test solution C
[0058] The weight content ratio of pirenoxine sodium to triamcinolone is 1:0.12 (the concentration of pirenoxine sodium is 0.0051% by weight).
[0059] (4) Test solution D
[0060] The weight content ratio of pirenoxine sodium-fluocinide is 1:0.29 (the concentration of pirenoxine sodium is 0.0055% by wei...
Embodiment 2
[0067] Embodiment 2 bioavailability test
[0068] "Bioavailability" refers to the extent and rate at which a drug is absorbed and utilized in target tissues. "Ocular bioavailability" refers more specifically to the concentration of a drug in the aqueous humor following topical administration of the drug comprising an aqueous eye drop composition.
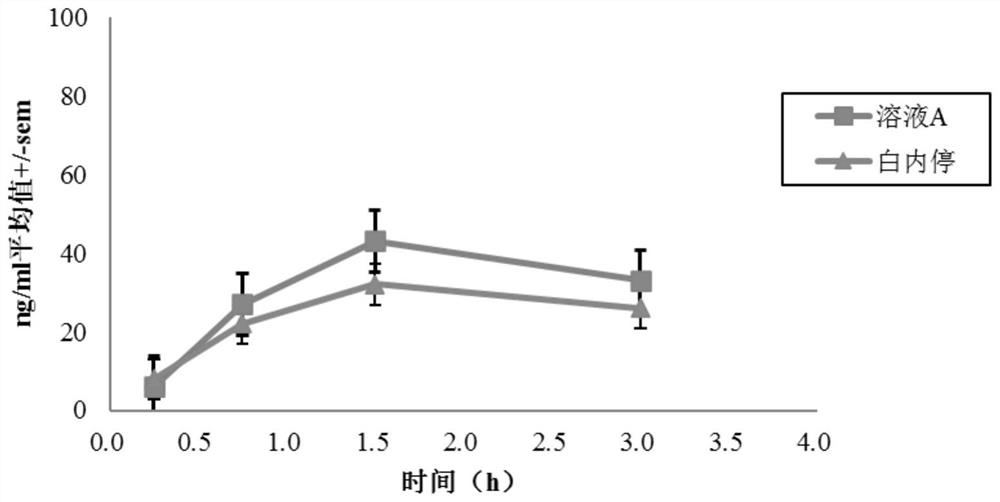
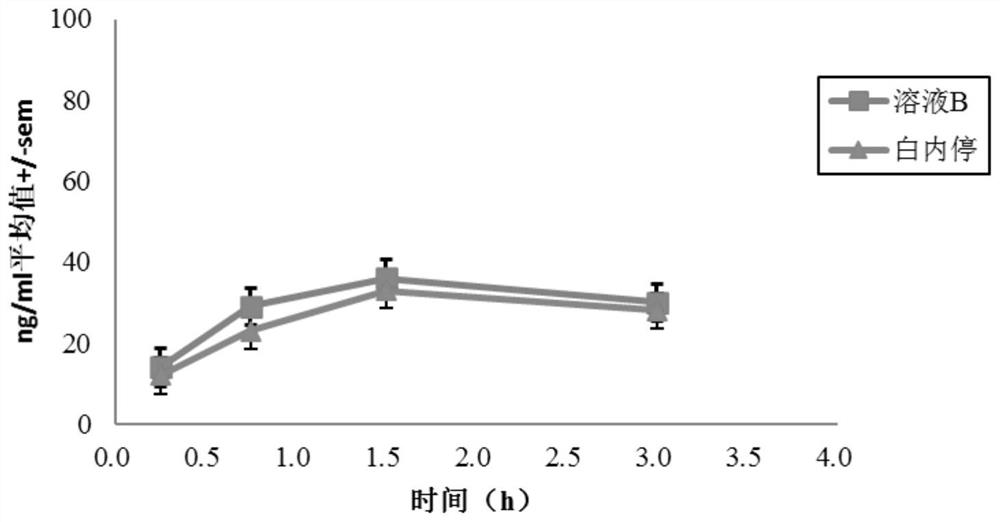
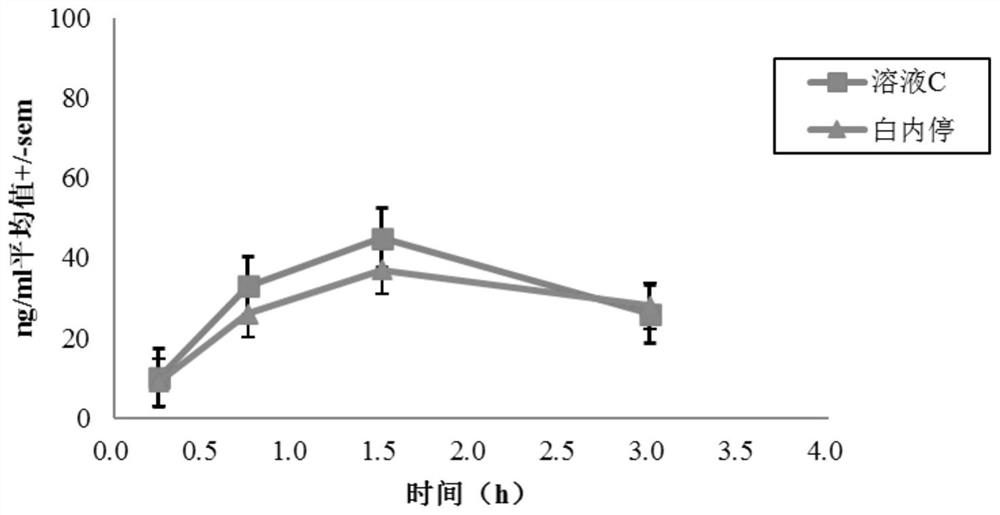
[0069] Male albino rabbits of the New Zealand white strain were used, and one of the test solutions A-G was topically applied to one eye of each rabbit, and a reference solution (Bai Nei Ting) was applied to the other eye, wherein solutions A to E Pyrenol The concentration of pirenoxine sodium is the same as that of "Bai Nei Ting", while the concentration of pirenoxine sodium in solutions F and G is about 60% of that of "Bai Nei Ting".
[0070] Rabbits were sacrificed at various time points after dosing (5 animals were treated per time point) and aqueous humor samples were collected. The concentration of pirenoxine sodium was dete...
Embodiment 3
[0072] Example 3 Observation of postoperative capsule turbidity
[0073] 90 male albino rabbits of New Zealand white strain were continuously injected with d-galactose 125 mg / kg / day for 42 days to form a cataract model, and were randomly divided into three groups, 30 rabbits in each group, for extracapsular cataract extraction. The pirenoxine sodium eye drops (pirenoxine sodium-halometasone weight content ratio 1:0.13 (0.0053% by weight of pirenoxine sodium concentration)) given to the group after operation was instilled 2 times a day, 2 times each time. -4 drops, while the control group began to instill "Bai Nei Ting" eye drops on the third day after operation, while the control group was given normal saline, and 10 rabbits in each group were executed at 1, 2 and 3 months respectively. A total of 20 eyes, the eyeball specimens were taken and fixed with 10% formaldehyde, the intraocular lens was taken out, half of the eyeball was embedded in paraffin, sectioned, and stained wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com