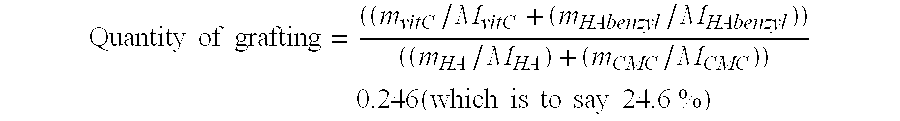Patents
Literature
282results about How to "Avoid rapid degradation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
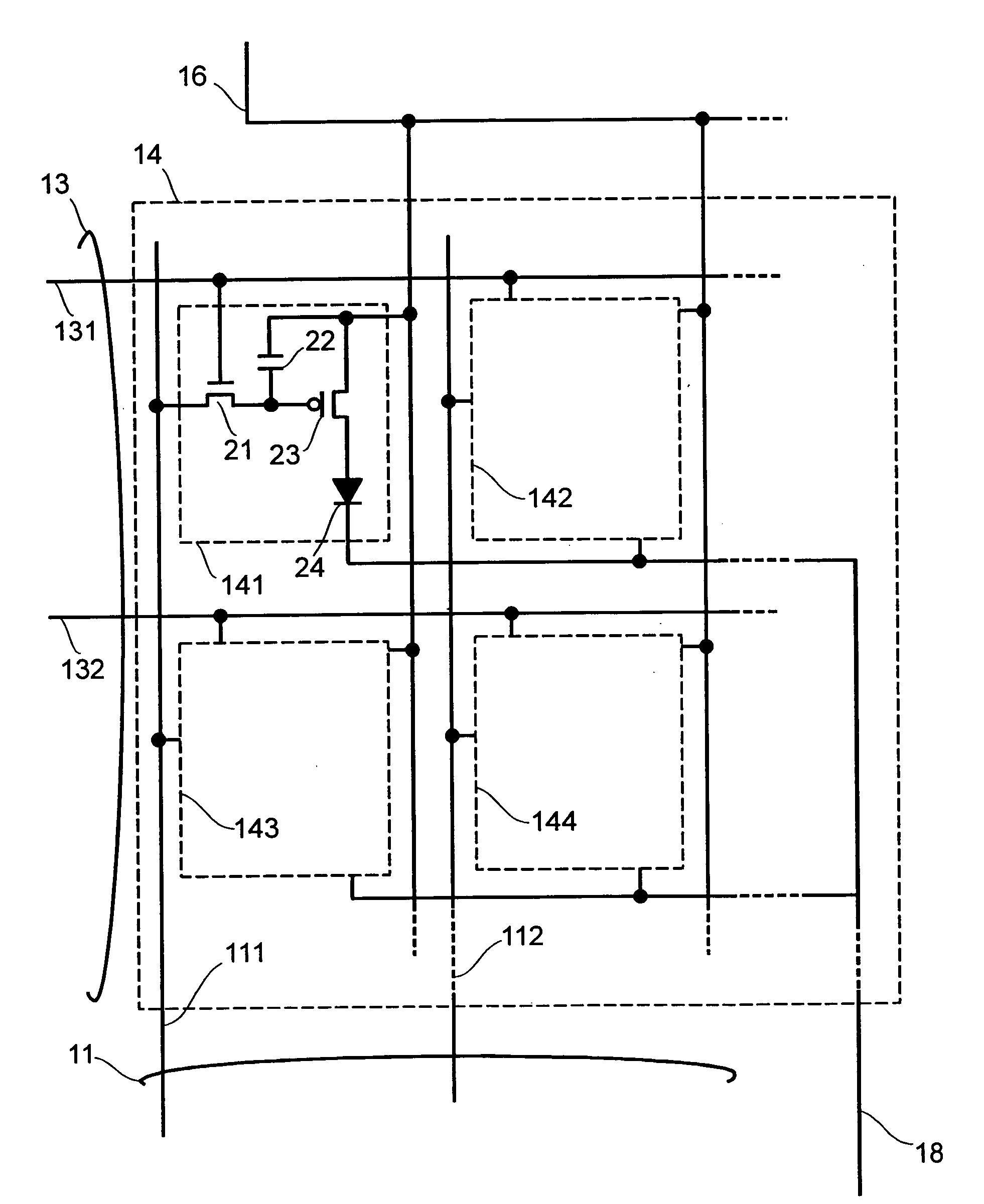
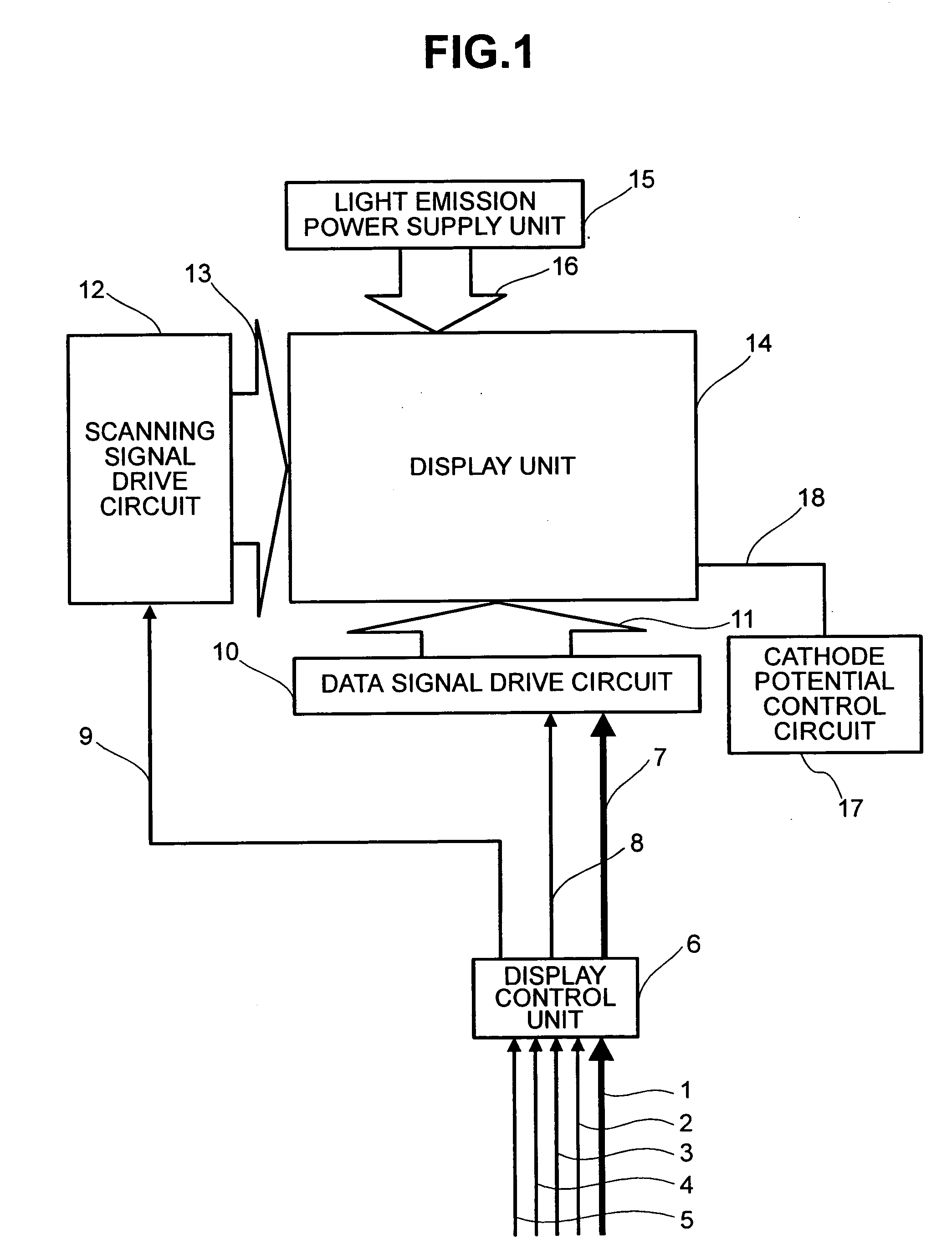
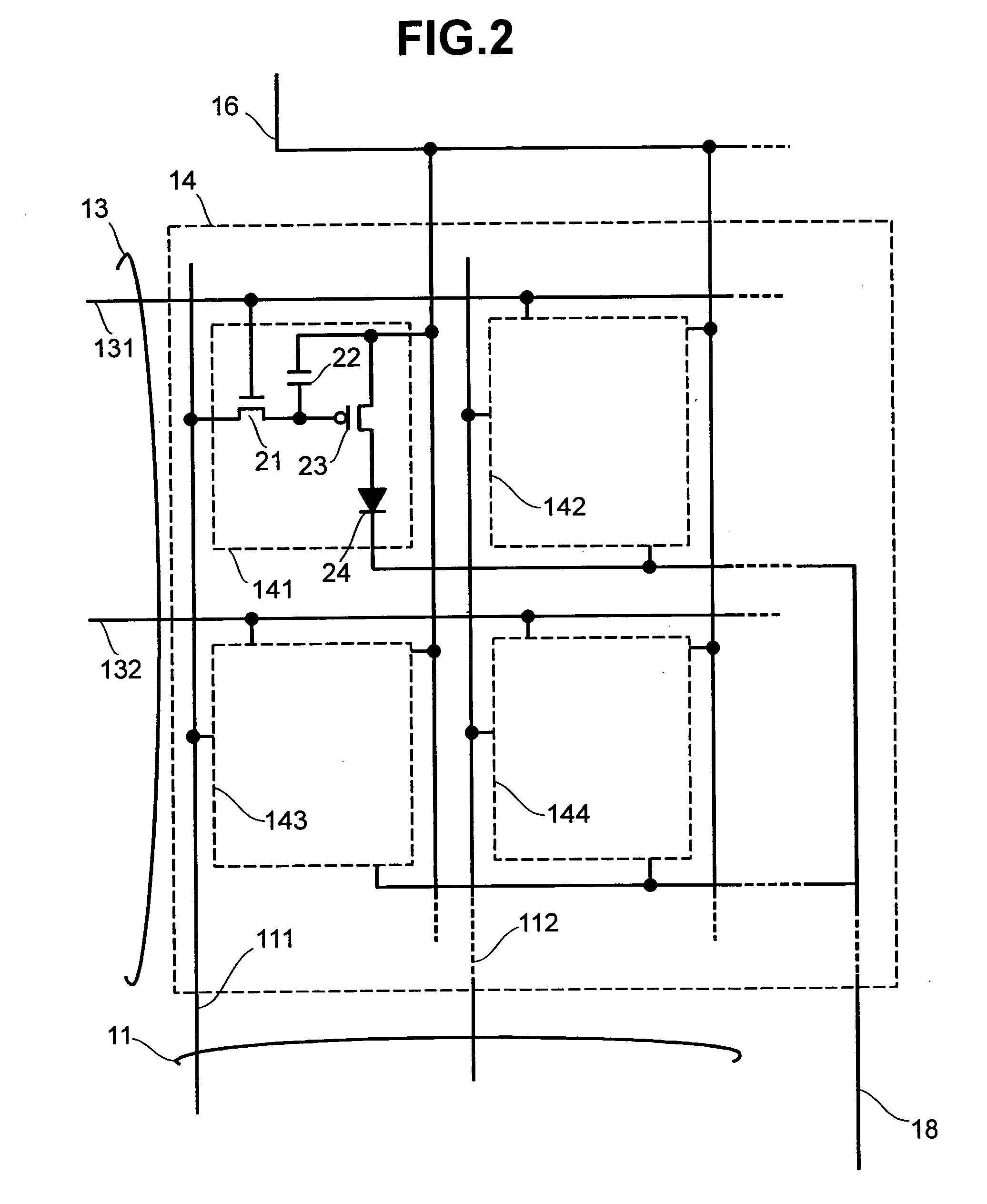
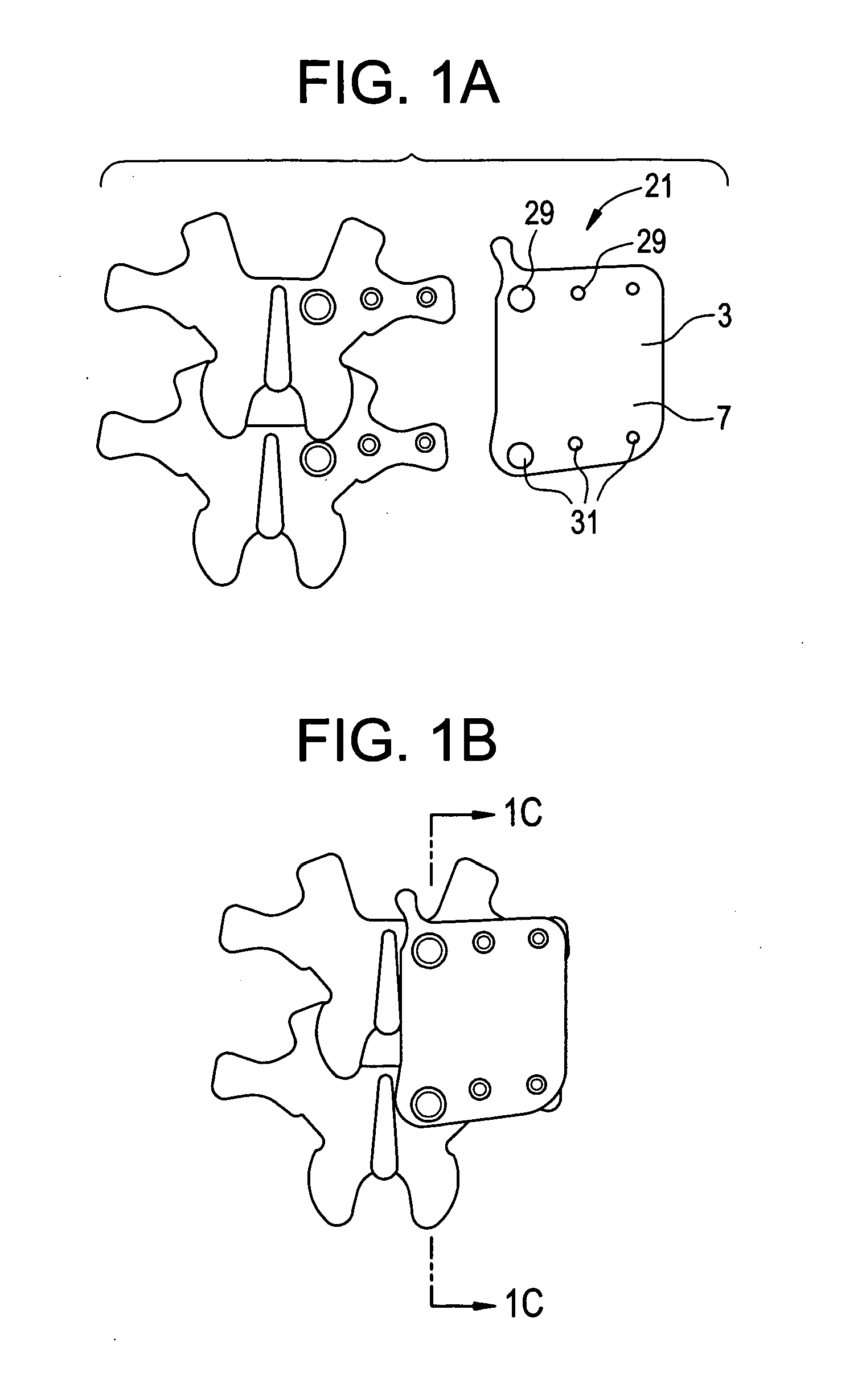
Display apparatus and display control method
ActiveUS20050068270A1Peak luminance can be increasedDisplay luminance can be enhancedCathode-ray tube indicatorsInput/output processes for data processingDriven elementLight emission
The present invention comprises: a display unit having a plurality of pixels arranged therein, each pixel including an organic EL element 24, a switching TFT, and a drive TFT; a data signal drive circuit for receiving image data for each frame period and outputting an image signal based on the image data; a scanning signal drive circuit for outputting a scanning signal for controlling a timing at which the switching element of each of the plurality of pixels receives the image signal; and a current source (a light emission power supply unit and a cathode potential control circuit together) for outputting a current supplied to the light emitting unit of each of the plurality of pixels through its drive element; wherein the current source modulates the value of the output current within each frame period.
Owner:SAMSUNG DISPLAY CO LTD +1
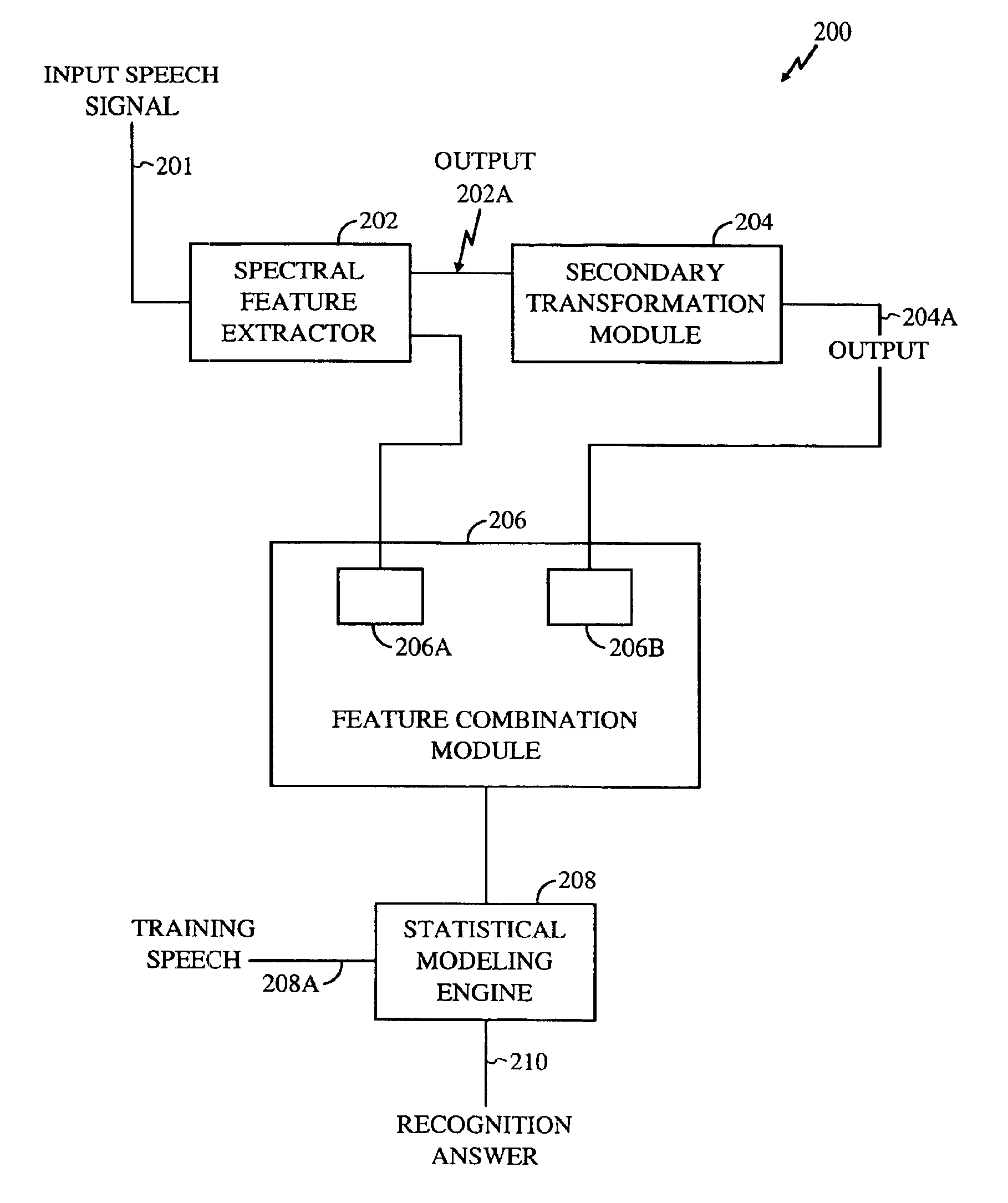
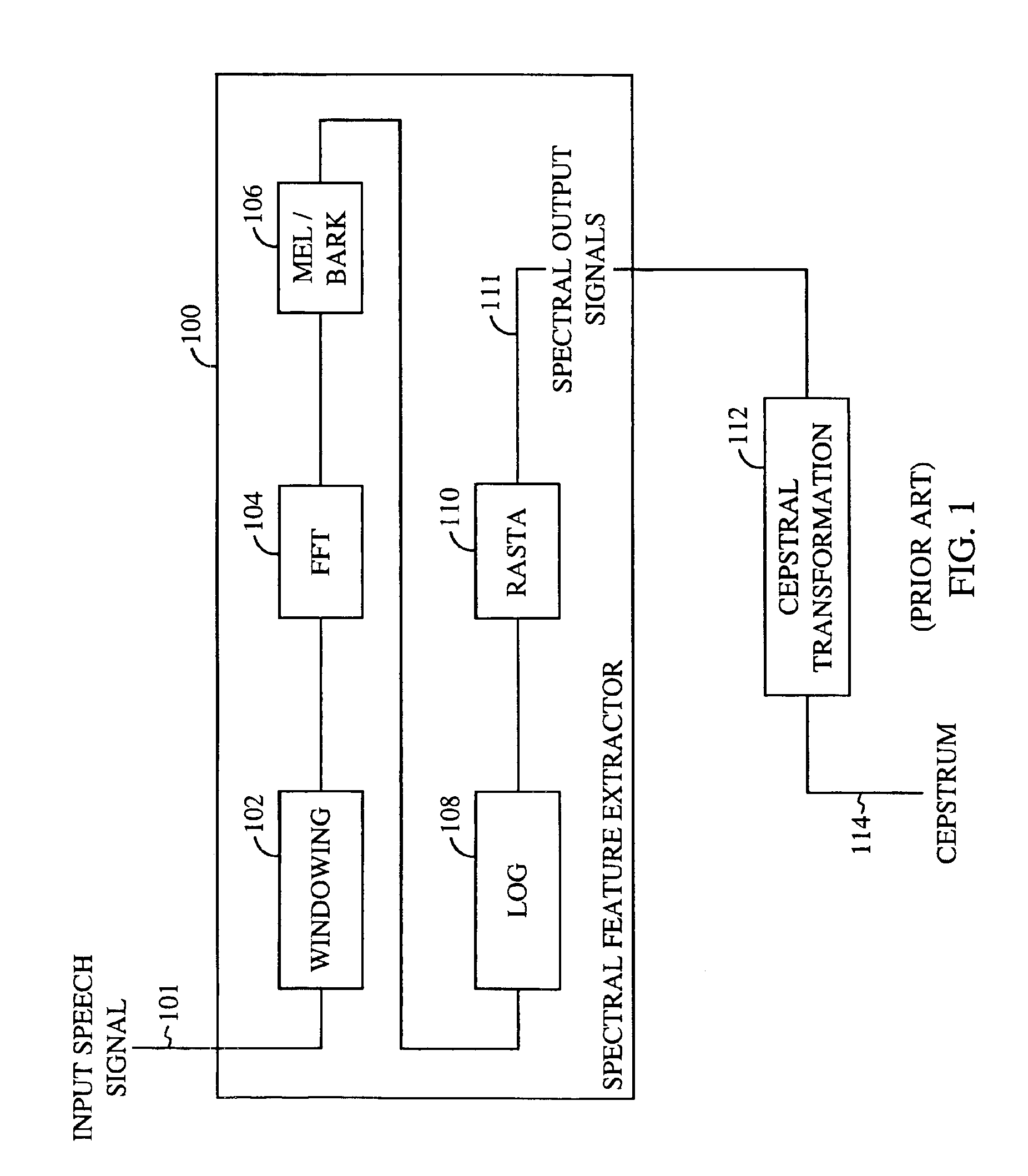
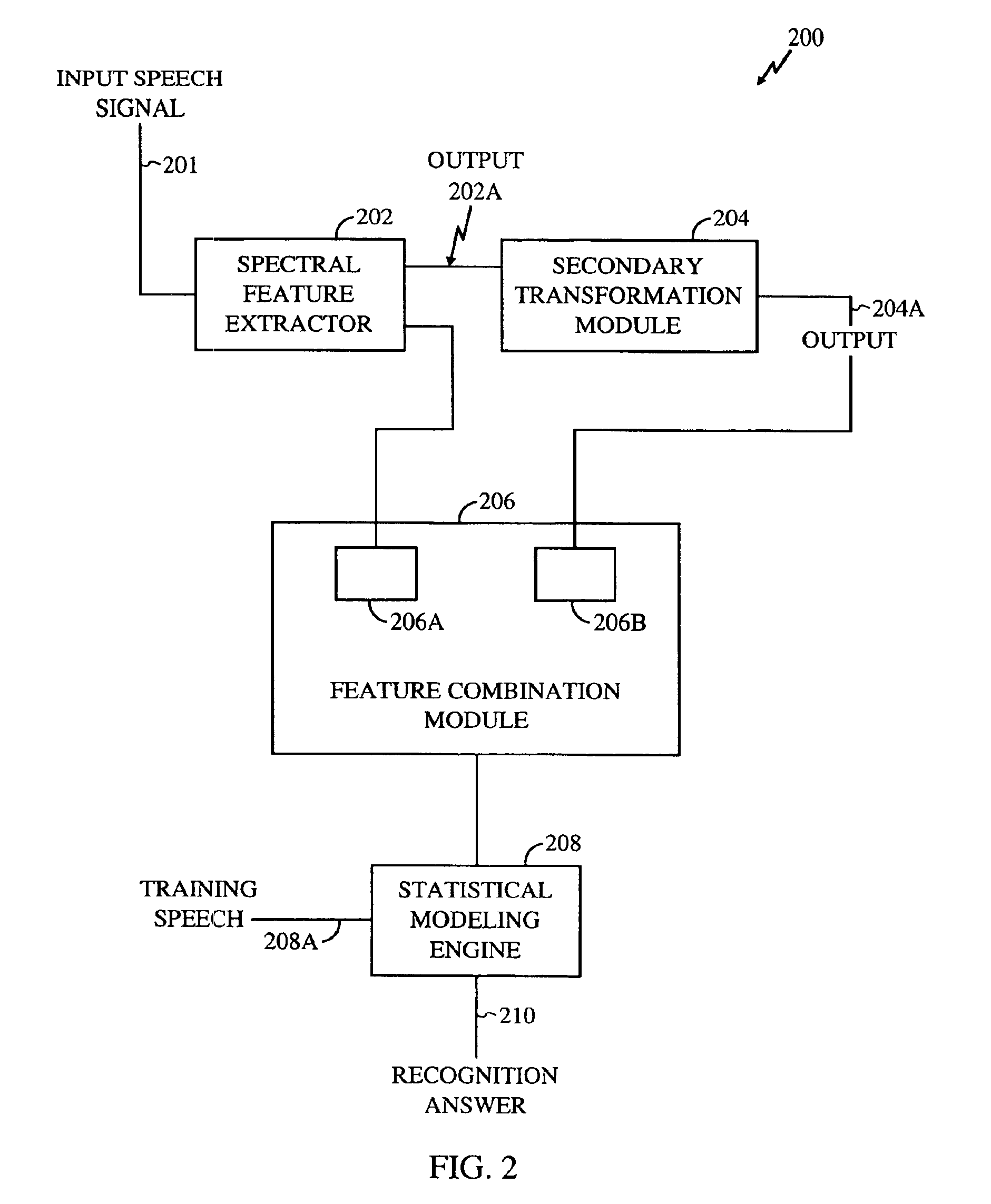
Method for robust voice recognition by analyzing redundant features of source signal
InactiveUS6957183B2Accurate voice recognitionPerformance deteriorationSpeech recognitionPattern matchingSpeech identification
A method for processing digitized speech signals by analyzing redundant features to provide more robust voice recognition. A primary transformation is applied to a source speech signal to extract primary features therefrom. Each of at least one secondary transformation is applied to the source speech signal or extracted primary features to yield at least one set of secondary features statistically dependant on the primary features. At least one predetermined function is then applied to combine the primary features with the secondary features. A recognition answer is generated by pattern matching this combination against predetermined voice recognition templates.
Owner:QUALCOMM INC
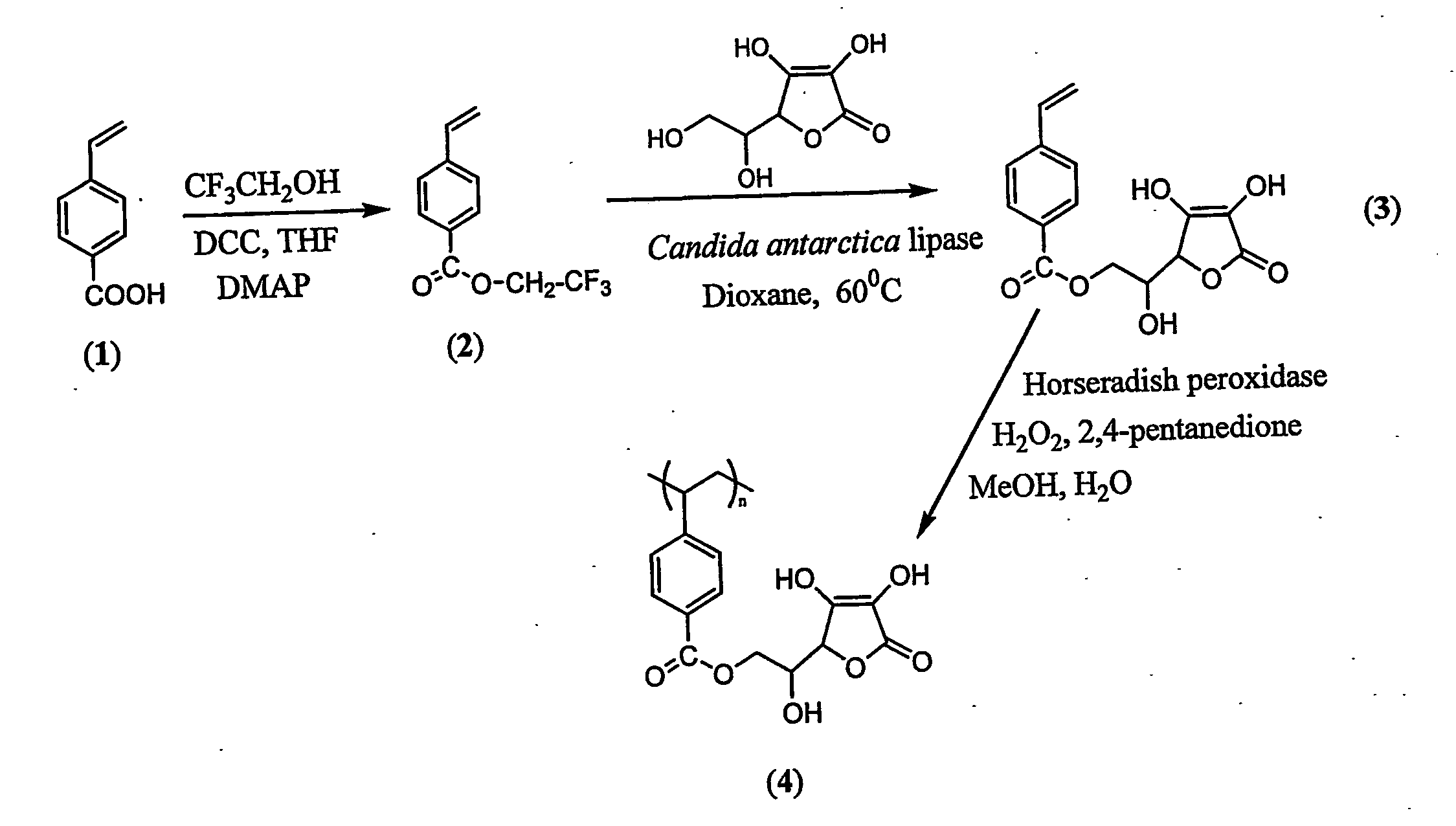
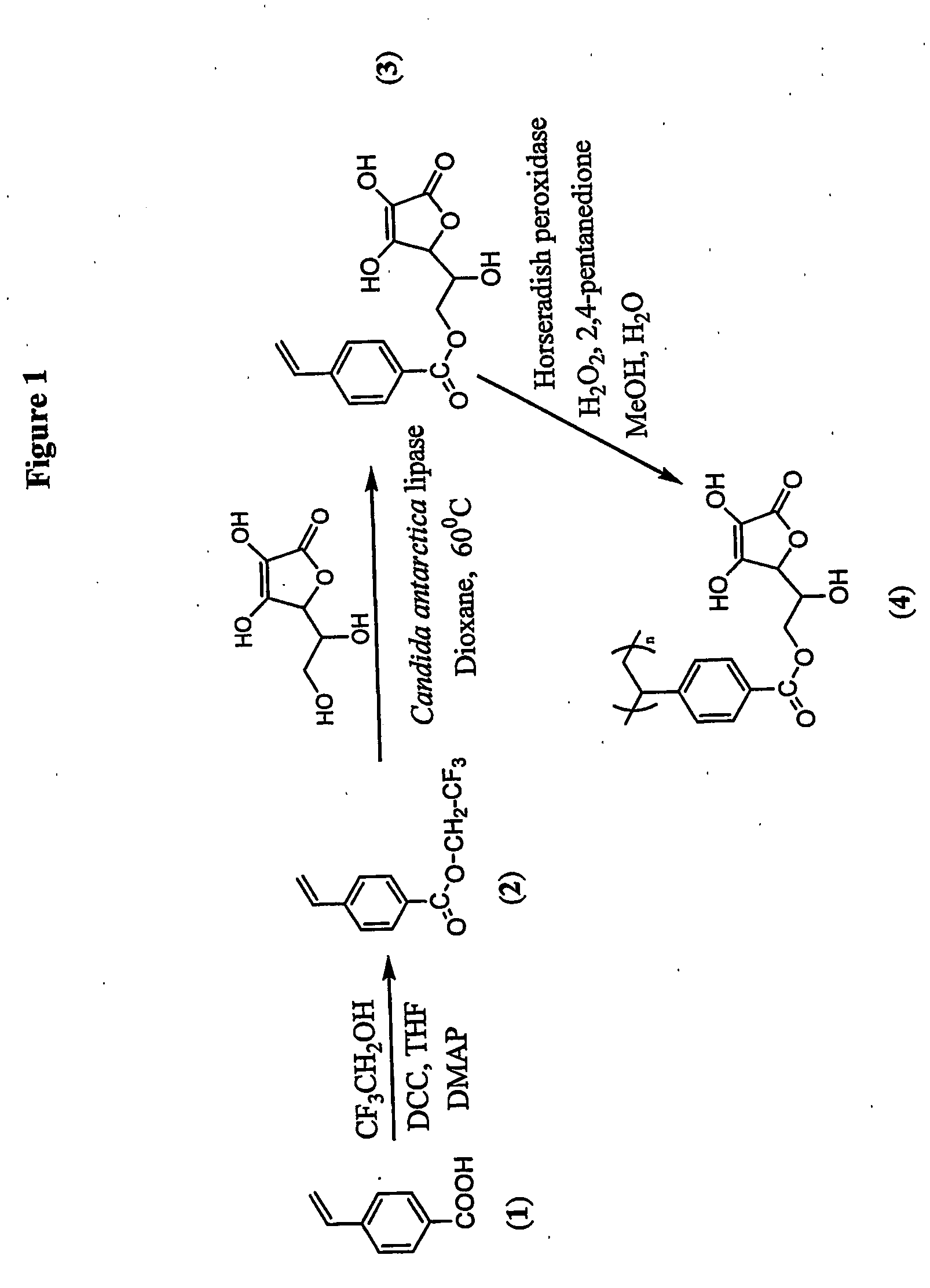
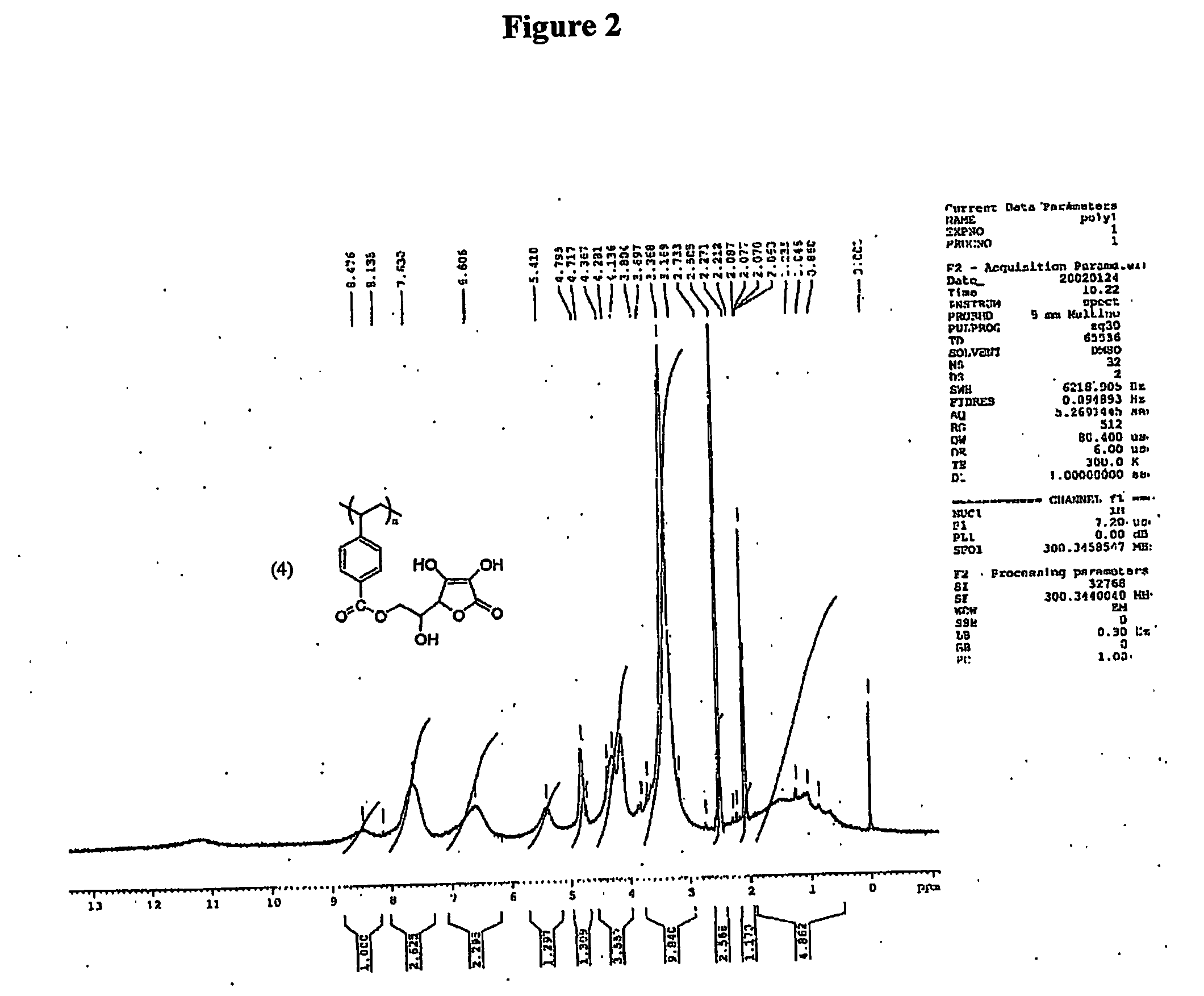
Antioxidant-functionalized polymers
InactiveUS20070010632A1Low yieldReduce sensitivitySuture equipmentsOrganic chemistryAntioxidantOxygen
Methods and compositions are disclosed for the preparation of free radical scavenging polymers and polymer films functionalized with antioxidants. Enzymatic and chemical tailoring of monomers with antioxidants followed by enzymatic polymerization is described. These antioxidant functionalized polymers can increase shelf life and quality of food products, as well as, increase effectiveness of pharmaceutical agents when used as packaging or as coatings on packaging for oxygen sensitive materials. The novel enzymatic covalent coupling of antioxidants to a polymer enhances the free radical scavenging ability of packaging while also inhibiting the escape of the antioxidants, and thus limiting exposure and / or absorption by an individual. In addition to its use in food or pharmaceutical packaging, methods are disclosed for using the antioxidant coupled polymers in a variety of applications including as coatings on the inside of medical devices, such as stents and catheters, which would substantially reduce free radical damage and / or oxygen depletion during medical procedures. Furthermore, through the coupling of antioxidants to biodegradable polymers, controlled delivery and sustained release of an antioxidant to a subject is possible.
Owner:TRUSTEES OF TUFTS COLLEGE
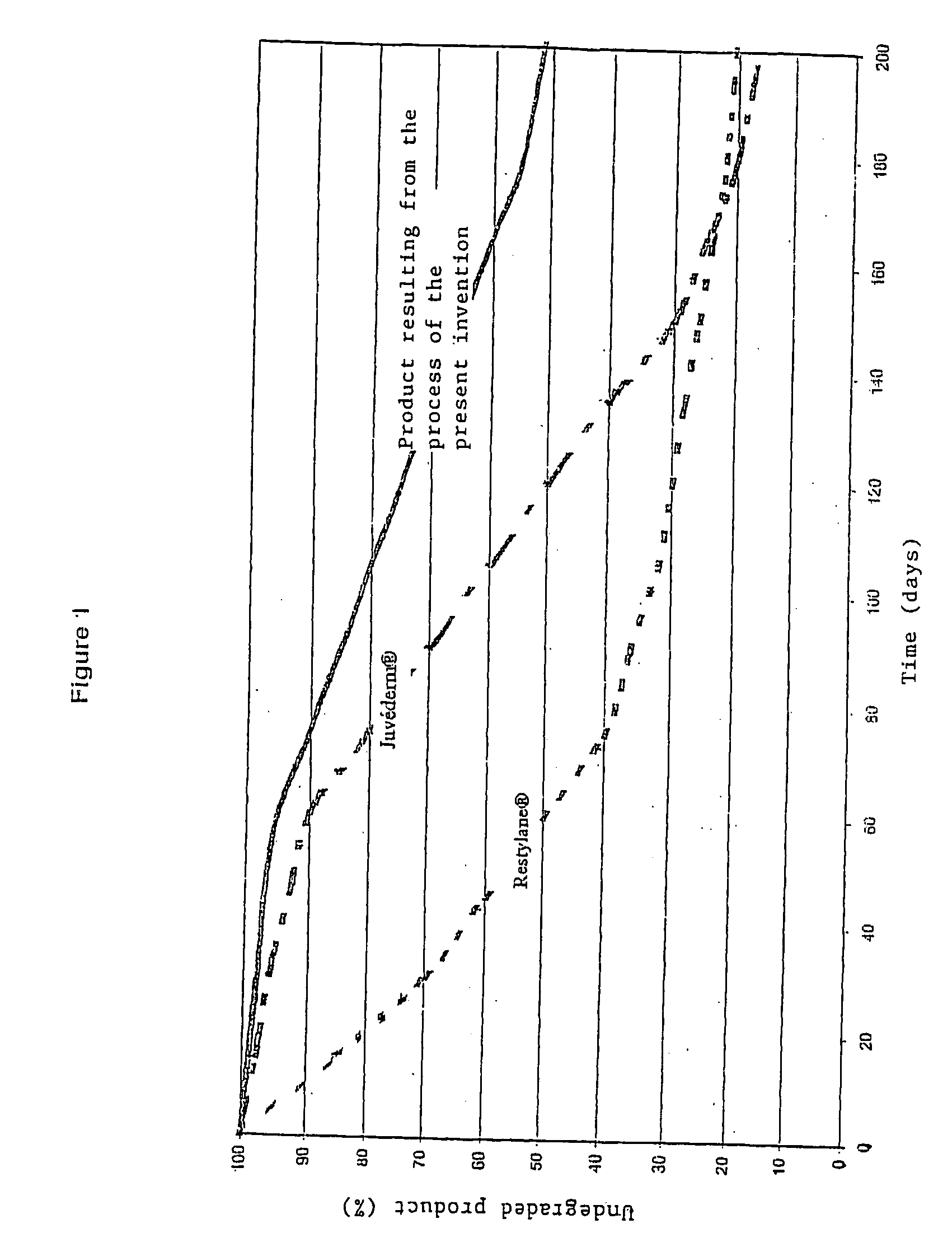
Complex matrix for biomedical use
InactiveUS20060246137A1Easy to useEnhance lipPowder deliveryOrganic active ingredientsCross-linkComplex matrix
A complex matrix is constituted by at least one biocompatible polymer of natural origin, cross linked and to which are grafted small chains of a molecular weight less than 50,000 Da with an amount of grafting of 10 to 40%, as well as a process for preparation of a biocompatible matrix that is partly degradable, constituted by at least one polymer of natural origin, consisting: on the one hand, grafting small chains of molecular weight less than 10,000 Da, with a grafting amount of 10 to 40%, on the other hand, cross linking the principal polymer chains to create a homogeneous matrix.
Owner:ANTEIS SA
Polysaccharide sponges for cell culture and transplantation
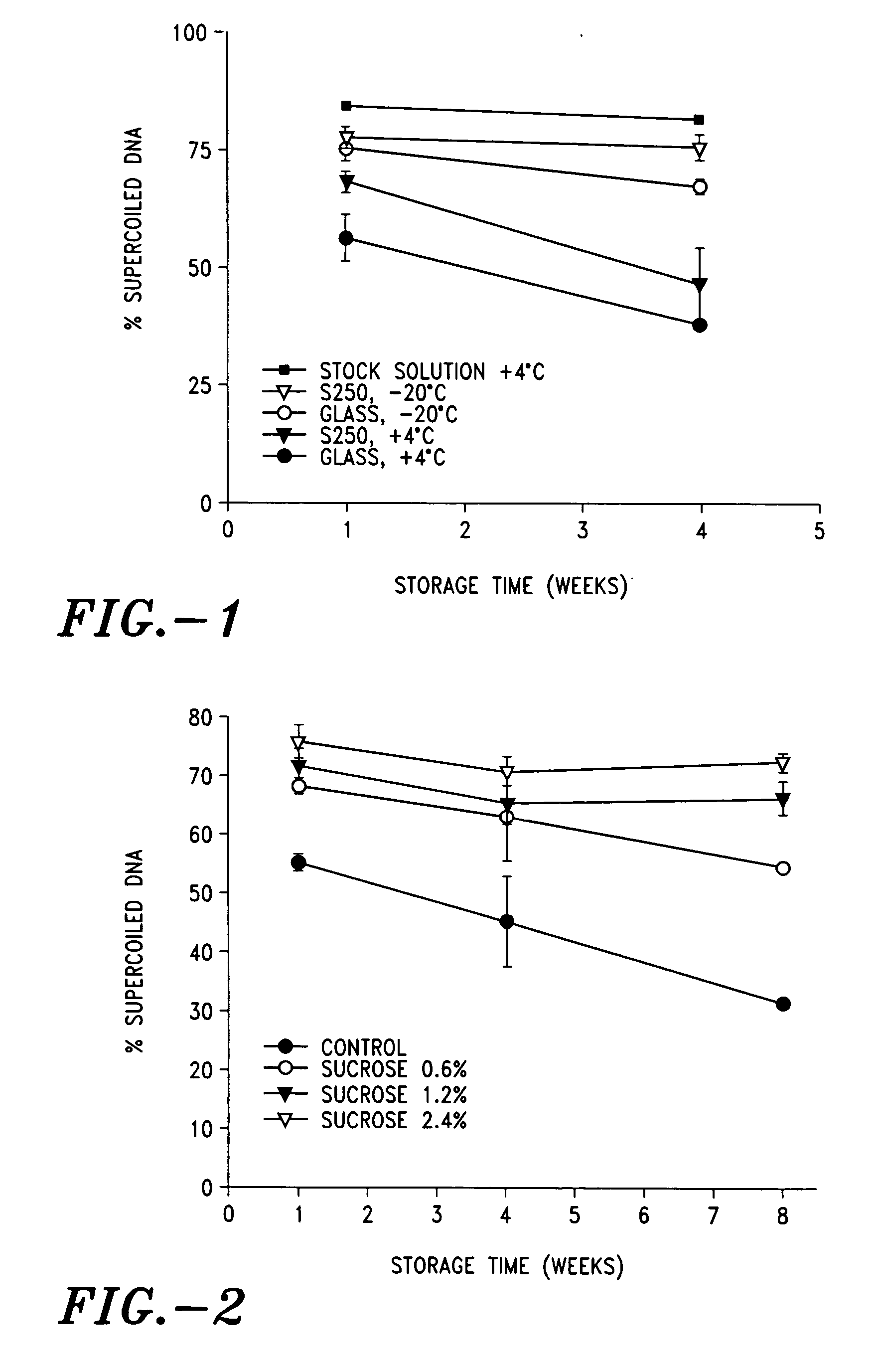
InactiveUS6425918B1Avoid rapid degradationEasy to useSkin implantsUnicellular algaePolysaccharideElastic modulus
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Formulation for Anti-alpha4beta7 antibody
InactiveUS20120282249A1Improve stabilityReduction of formationAntipyreticAnalgesicsReducing sugarDosing regimen
Antibody formulations are described comprising a mixture of a non-reducing sugar, an anti-α4β7 antibody and at least one amino acid. The disclosed formulations have improved stability, reduced aggregate formation, and may retard degradation of the anti-α4β7 antibody therein or exhibit any combinations thereof. The present invention further provides a safe dosing regimen of these antibody formulations that is easy to follow, and which results in a therapeutically effective amount of the anti-α4β7 antibody in vivo.
Owner:MILLENNIUM PHARMA INC
Biodegradable composite wire for medical devices
ActiveUS20110319978A1Vessel patency has been restoredDiffering biodegradation rateStentsSurgeryMechanical integrityPercent Diameter Stenosis
A bimetal composite wire including, in cross-section, an outer shell or tube formed of a first biodegradable material and an inner core formed of a second biodegradable material. When formed into a stent, for example, the first and second biodegradable materials may be different, and may have differing biodegradation rates. In a first embodiment, the first biodegradable material of the shell may degrade relatively slowly for retention of the mechanical integrity of a stent during vessel remodeling, and the second biodegradable material of the core may degrade relatively quickly. In a second embodiment, the first biodegradable material of the shell may degrade relatively quickly, leaving a thinner structure of a second biodegradable material of the core that may degrade relatively slowly. The biodegradation rates may be inherently controlled, such as by selection of materials, and also may be mechanically controlled, such as by material thicknesses and the geometric configuration of the shell, core, or overall device. In any embodiment, the metallic scaffold may also be coated with a drug-eluting, biodegradable polymer, to further inhibit neointimal proliferation and / or restenosis.
Owner:FORT WAYNE METALS RES PROD CORP
Methods and Crosslinked Polymer Compositions for Cartilage Repair
InactiveUS20090117070A1Promote growthGood adhesionBiocidePeptide/protein ingredientsDamages tissueActive agent
A method of repairing damaged cartilage and soft tissue in a patient is provided using a biocompatible, non-immunogenic composition. The composition comprises a hydrophilic polymer and a plurality of crosslinkable components having reactive functional groups. The composition used in the method may be loaded with biologically active agents for delivery to the damaged tissues. Kits for use in carrying out the method of the invention are also provided.
Owner:ANGIOTECH PHARMA US
Dissolvable oxides for biological applications
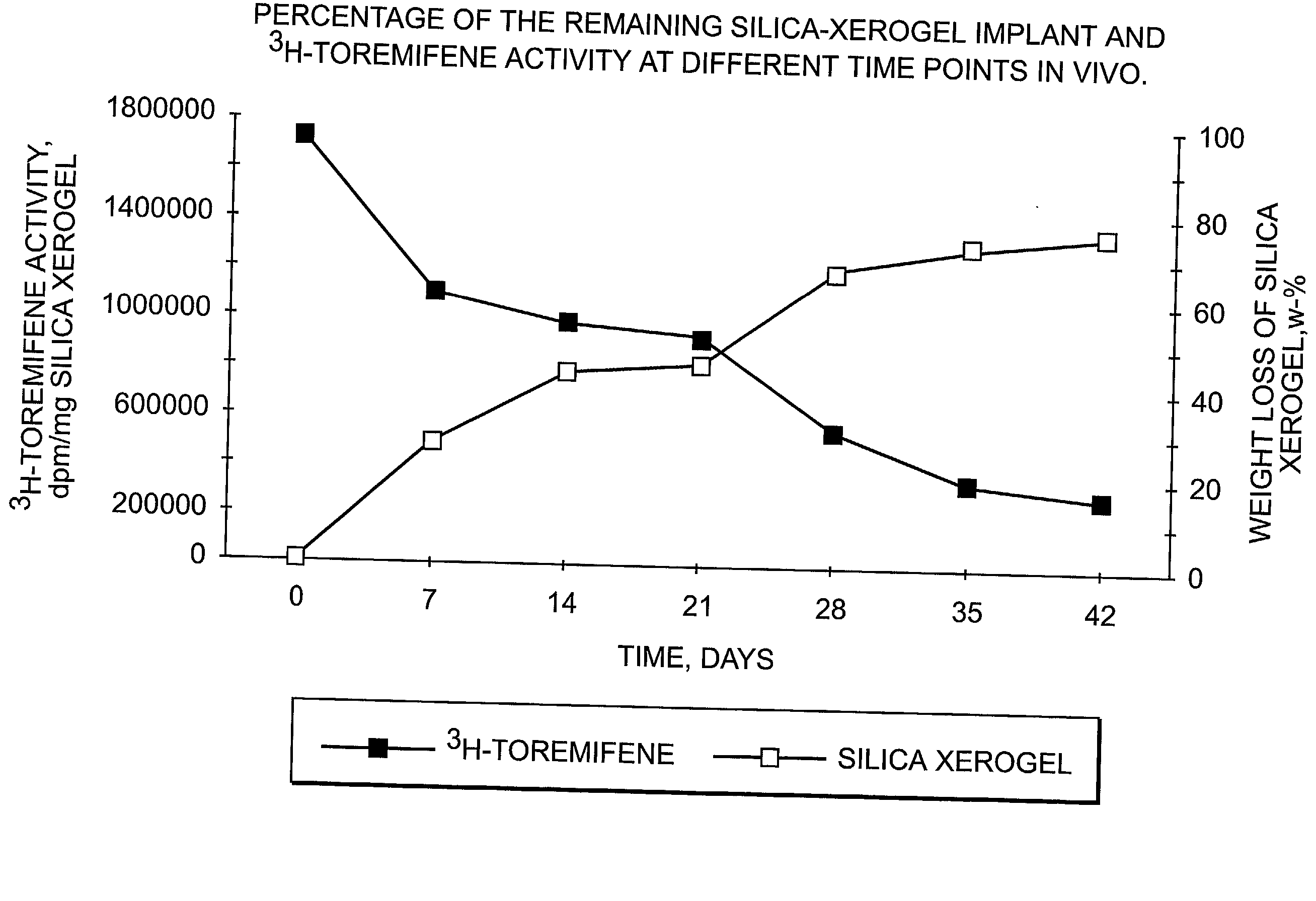
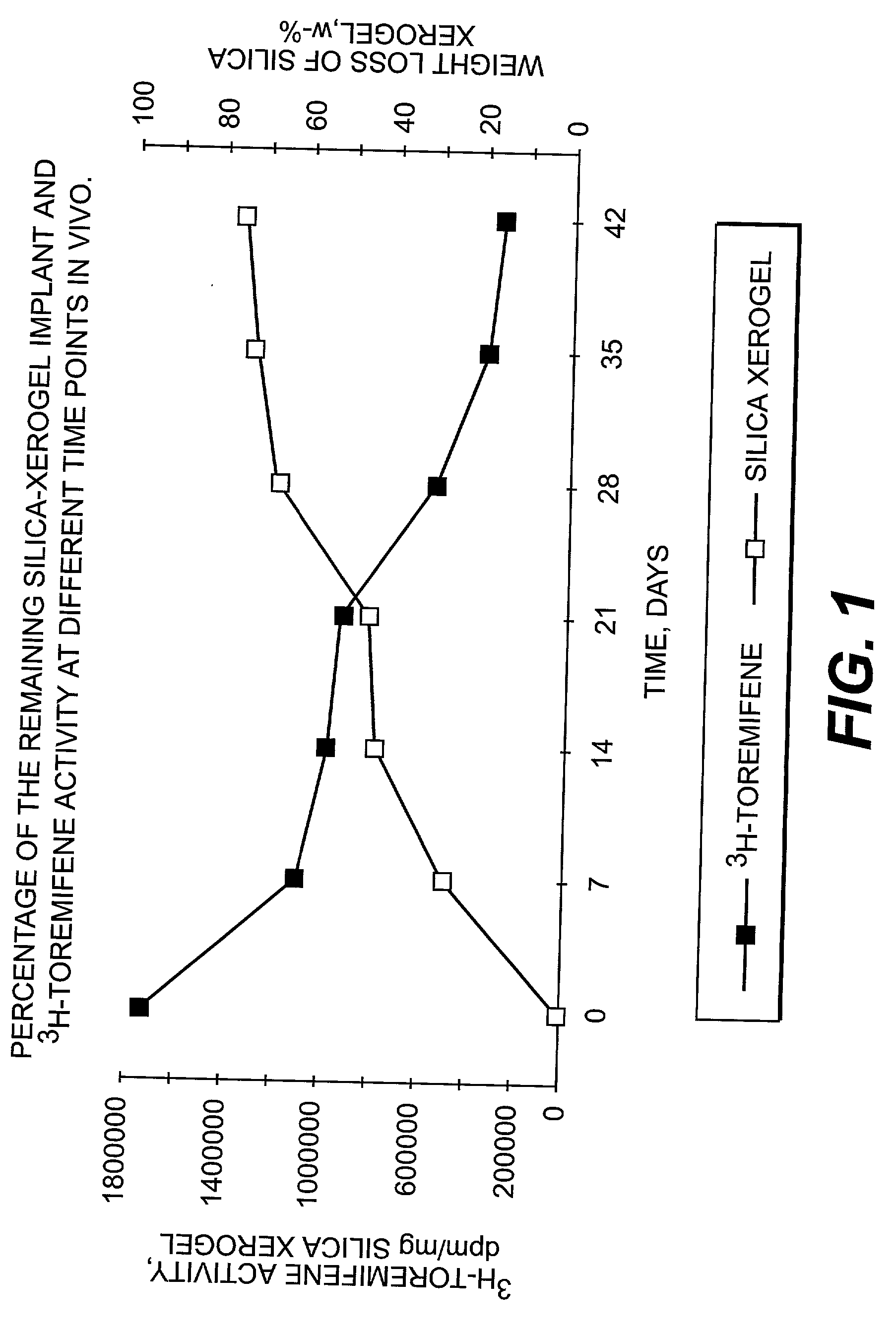
InactiveUS20030021820A1Remarkable effectControlled release ratePowder deliverySilicaActive agentMedicine
The present invention is concerned with controllably dissolvable silica-xerogels prepared via sol-gel process and their use. Specifically, the invention is concerned with a delivery device comprising controllably dissolvable silica-xerogel into which structure a biologically active agent is incorporated. The invention is further concerned with pharmaceutical preparations comprising said delivery device. Further, the invention is directed to medical devices for orthopedic and surgical purposes comprising controllably dissolvable silica-xerogels, which may further comprise a biologically active agent.
Owner:DELSITECH
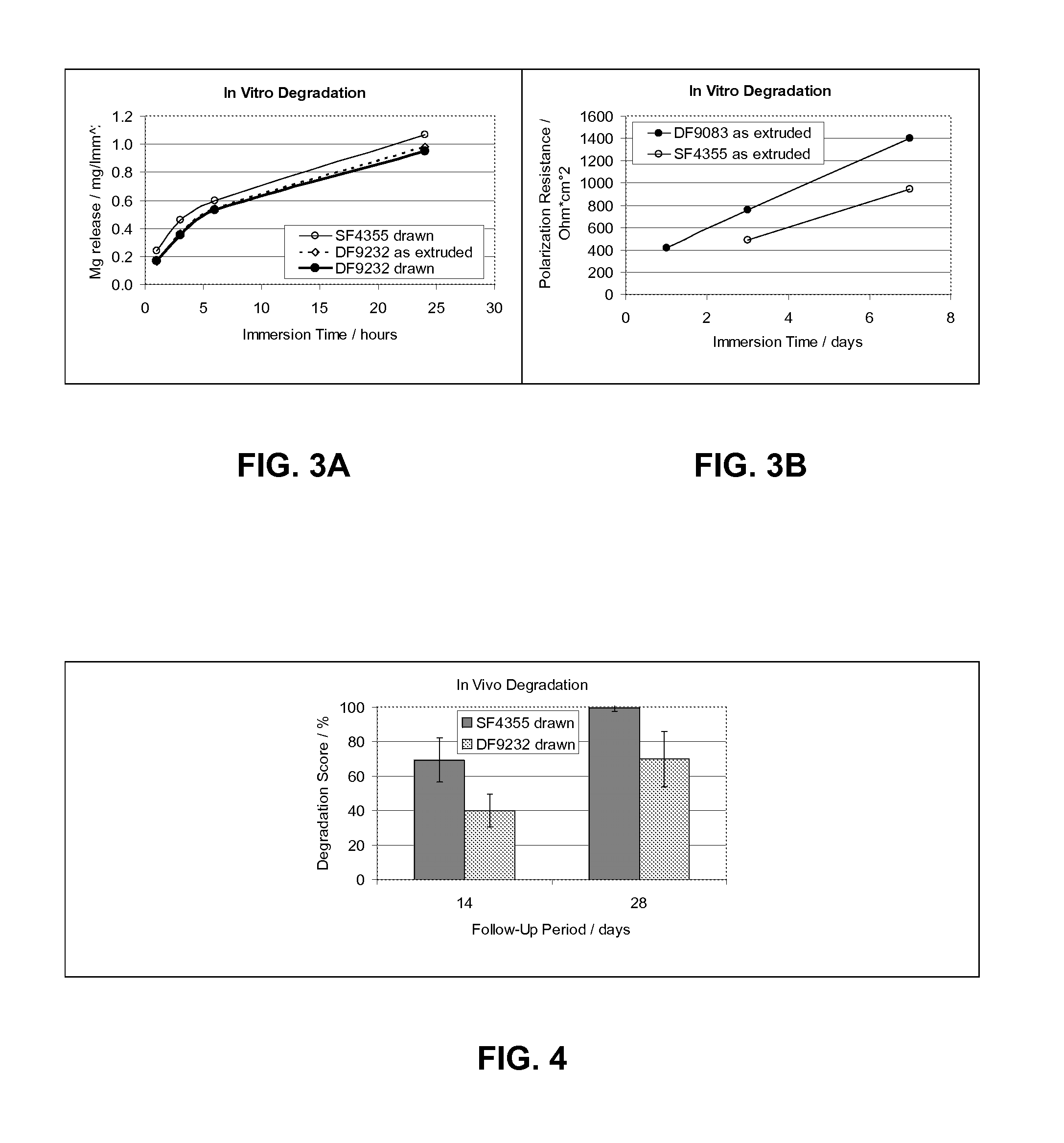
Controlled Degradation of Magnesium Stents
InactiveUS20090240323A1Prolong lifeAvoid rapid degradationSurgeryPharmaceutical containersActive agentTherapeutic effect
Implantable medical devices, more specifically stents, are described herein comprising magnesium based core structures whose elimination times are slowed by the appropriate polymer coating. Appropriate biodegradable polymers are selected which are suitable to provide a specific degradation time for the magnesium based core structure. Bioactive agents are incorporated into the polymer coating in order to aid in the therapeutic effect of the stent.
Owner:MEDTRONIC VASCULAR INC
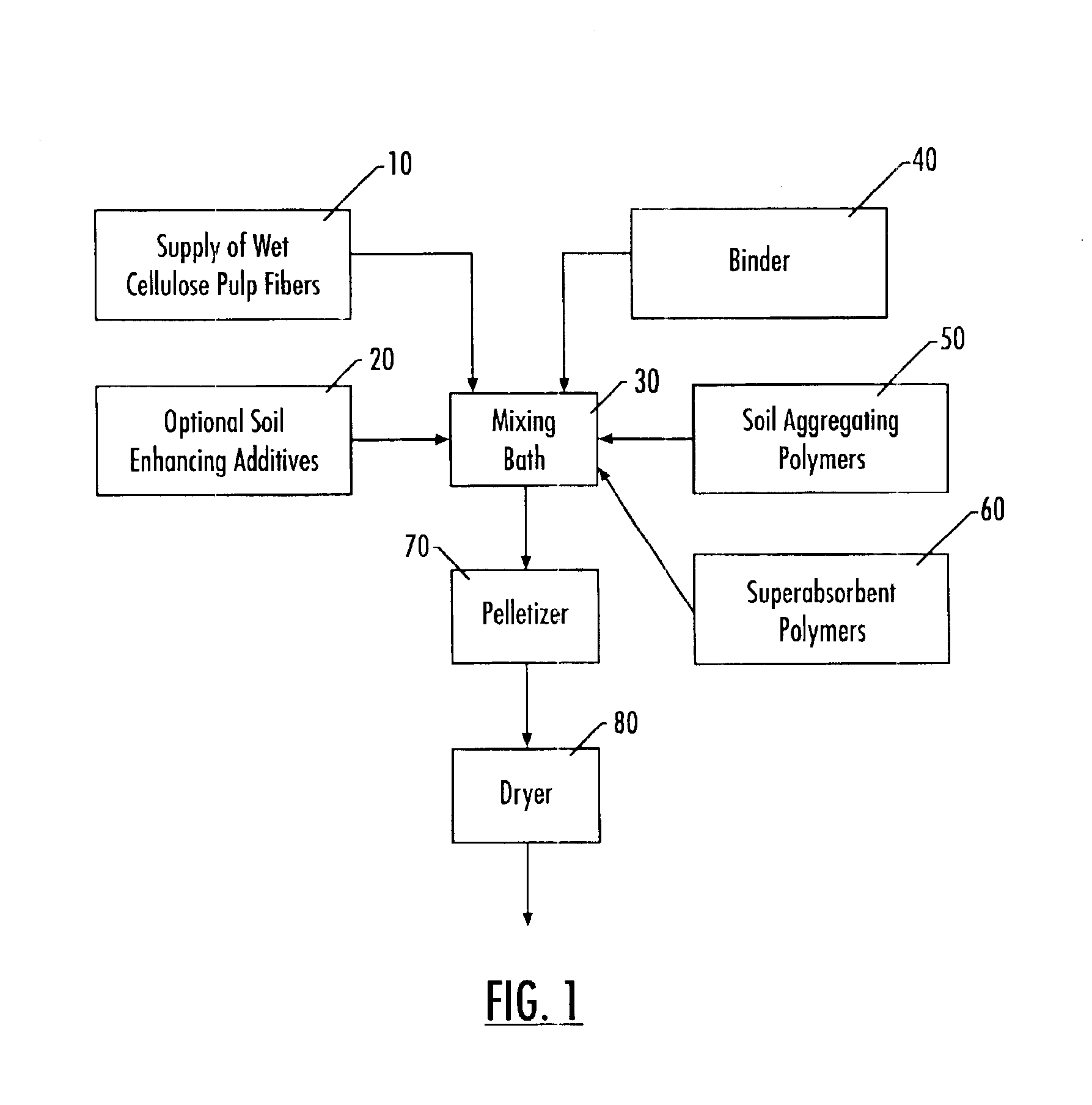
Lignocellulose fiber composite with soil conditioners
InactiveUS6855182B2Conveniently applyEasily disperseAmmonium nitratesOrganic fertilisersFiber matrixFertilizer
A pellet for use in conditioning soil made of a lignocellulose fiber matrix having up to 20% by weight of a soil conditioning material incorporated therein. The soil conditioning material comprises one or more synthetic soil conditioning polymers and, optionally, additional soil enhancing additives such as fertilizers, gypsum, and calcium salts. The preferred lignocellulose materials are pulp fibers with an alpha-cellulose purity of greater than about 75% by weight, with preferred lignin content of no higher than 10%. The soil conditioning polymers are preferably polyacrylamides (PAMs) or modified PAMs, but may be a combination of other soil conditioning polymers. The pellet is produced by dispersing the polymers into a bath of lignocellulose fibers. The dispersion is then formed into pellets with a pelletizing machine such that the polymer and other soil conditioning material is interspersed within the matrix formed by the pelletized lignocellulose.
Owner:RAYONIER PERFORMANCE FIBERS
Compositions of stabilized DNA for coating microprojctions
InactiveUS20050090009A1Reduce degradationAvoid rapid degradationMicroinjection basedNon-active genetic ingredientsCompound (substance)Biology
Owner:ALZA CORP
Preparation of functional gel particles with a dual crosslink network
ActiveUS8367051B2Improve surface propertiesAvoid rapid degradationPowder deliveryCosmetic preparationsCrosslinked polymersReactive agent
Functional gel particle formed from a crosslinked polymeric network including a fraction of stable crosslinks and a second fraction of cleavable crosslinks are disclosed. Functional compounds may be chemically or physically encapsulated within and / or released from the gel particle by selective cleavage of the cleavable crosslinks. The functional compounds may be delivered and released to a pre-selected target site. Peripheral or other accessible functionality on the surface of the gel particle allows attachment of a surface reactive agent, thereby modifying one or more surface properties of the gel particle. Processes of preparing the gel particles and processes of delivering the functional compounds to a target site are also disclosed.
Owner:CARNEGIE MELLON UNIV
In-situ formed posterolateral fusion system
ActiveUS20060004358A1Avoid rapid degradationAvoiding permanenceInternal osteosythesisJoint implantsPosterolateral fusionBones fusion
A formed in-place spinal implant comprising a hardenable, resorbable, bone fusion-promoting composition, wherein the implant may be rigidly connected to adjacent vertebrae until fusion occurs.
Owner:DEPUY SPINE INC (US)
Biodegradable stents
InactiveUS20070050018A1Faster rate of degradationFaster rateStentsBlood vesselsInsertion stentGenetic Materials
A stent comprising a matrix and a fiber reinforcement about which the matrix is chemically or mechanically attached. The matrix is provided with heavier loads of pharmaceutically active ingredients or genetic materials as a result of the increased strength and mechanical characteristics provided to the stent by the fiber reinforcement. The fiber reinforcement can be comprised of a plurality of mono-filament fibers spaced and oriented in a flat weave pattern to which the matrix is chemically or mechanically attached. Degradation rates of the materials that comprise the matrix and the fiber reinforcement can be varied to vary the time period in which the stent maintains its mechanical characteristics or releases the pharmaceutically active ingredients or genetic materials therefrom. Multiple stage release profiles can be provided by providing multiple layers of matrices and fiber reinforcements, whereby different pharmaceutically active ingredients or genetic materials or different concentrations thereof, can be released according to the degradation profiles of the matrix and fiber reinforcment.
Owner:WAINWRIGHT JOHN
Composites of thermosetting resins and carbon fibers having polyhydroxyether sizings
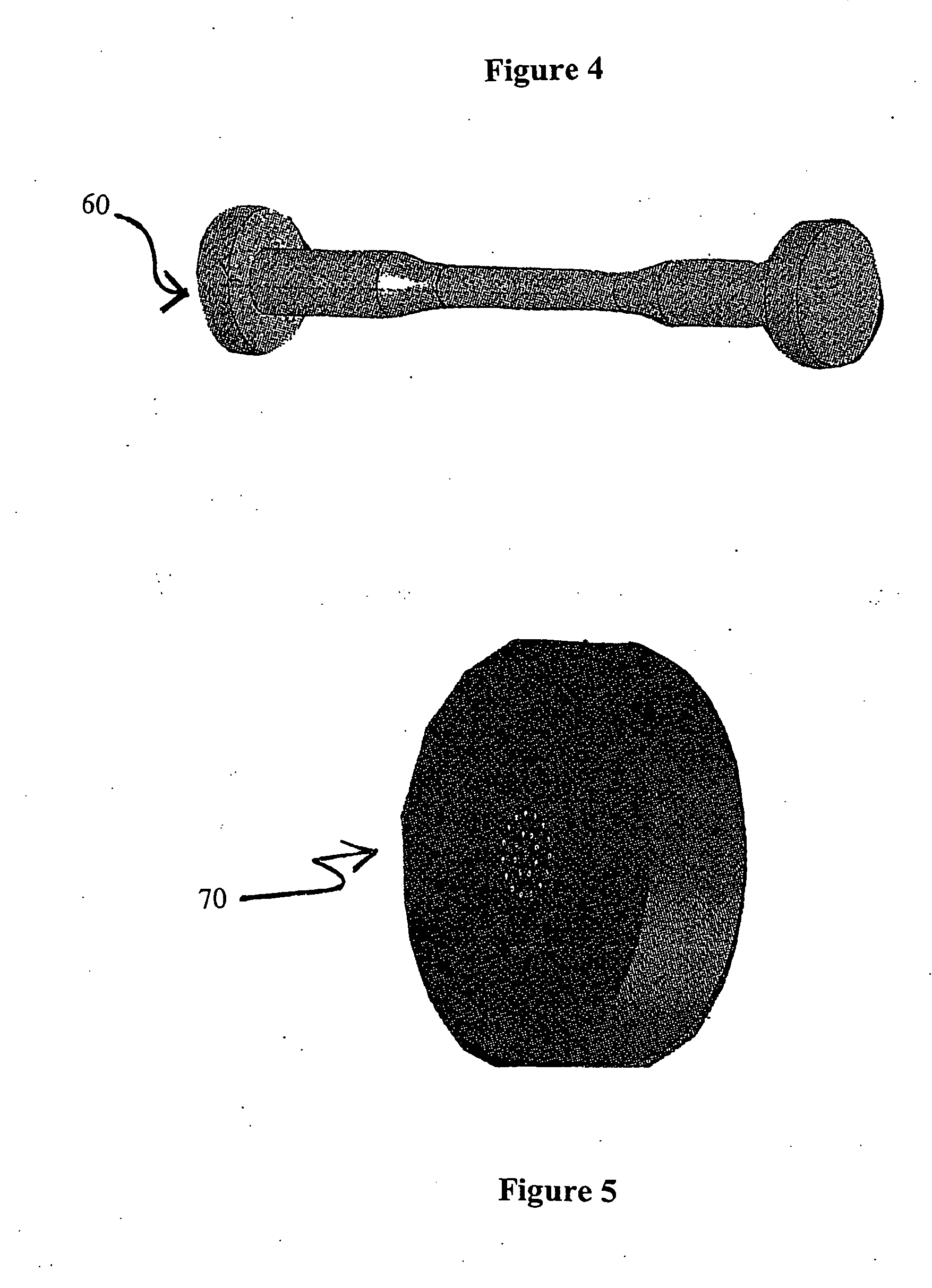
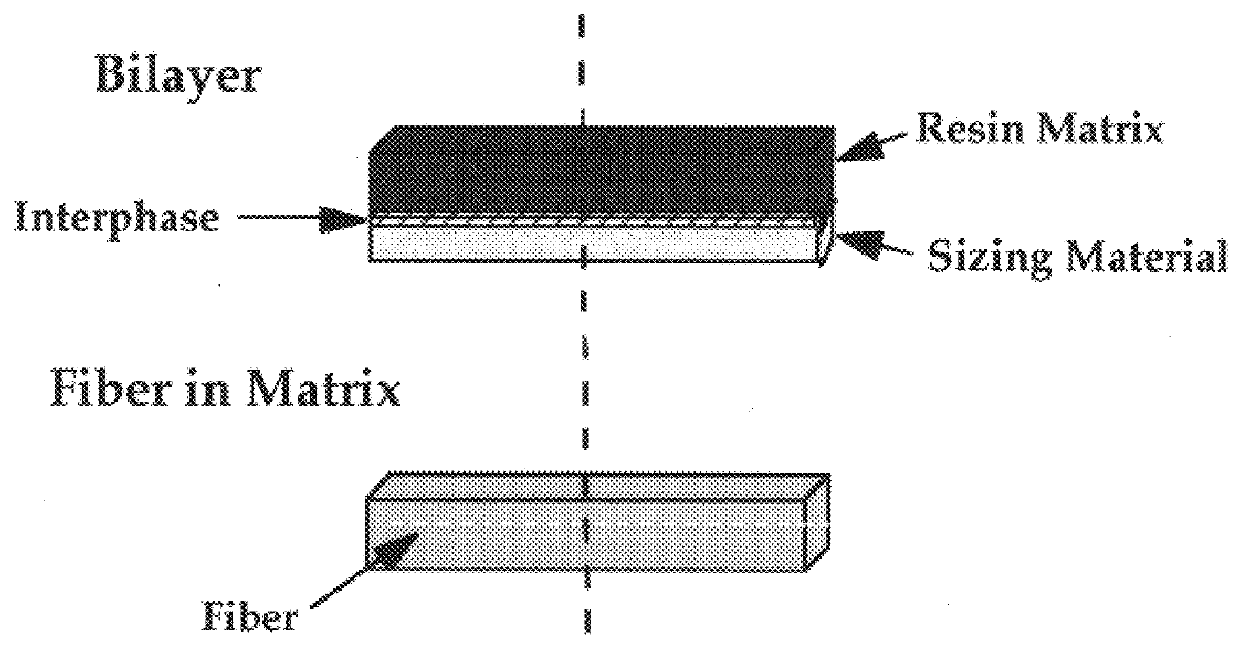
InactiveUS6020063AImprove adhesionIncreased durabilityGlass/slag layered productsNatural mineral layered productsFiberPolymer science
Composite material having carbon fibers embedded in a polymeric matrix comprising a thermoset resin, where the carbon fibers are precoated with a sizing agent comprising a saturated polyurethane or polyhydroxyether before being embedded in the resin. The invention also contemplates processes for making this composite material and intermediate products thereof.
Owner:VIRGINIA TECH INTPROP INC
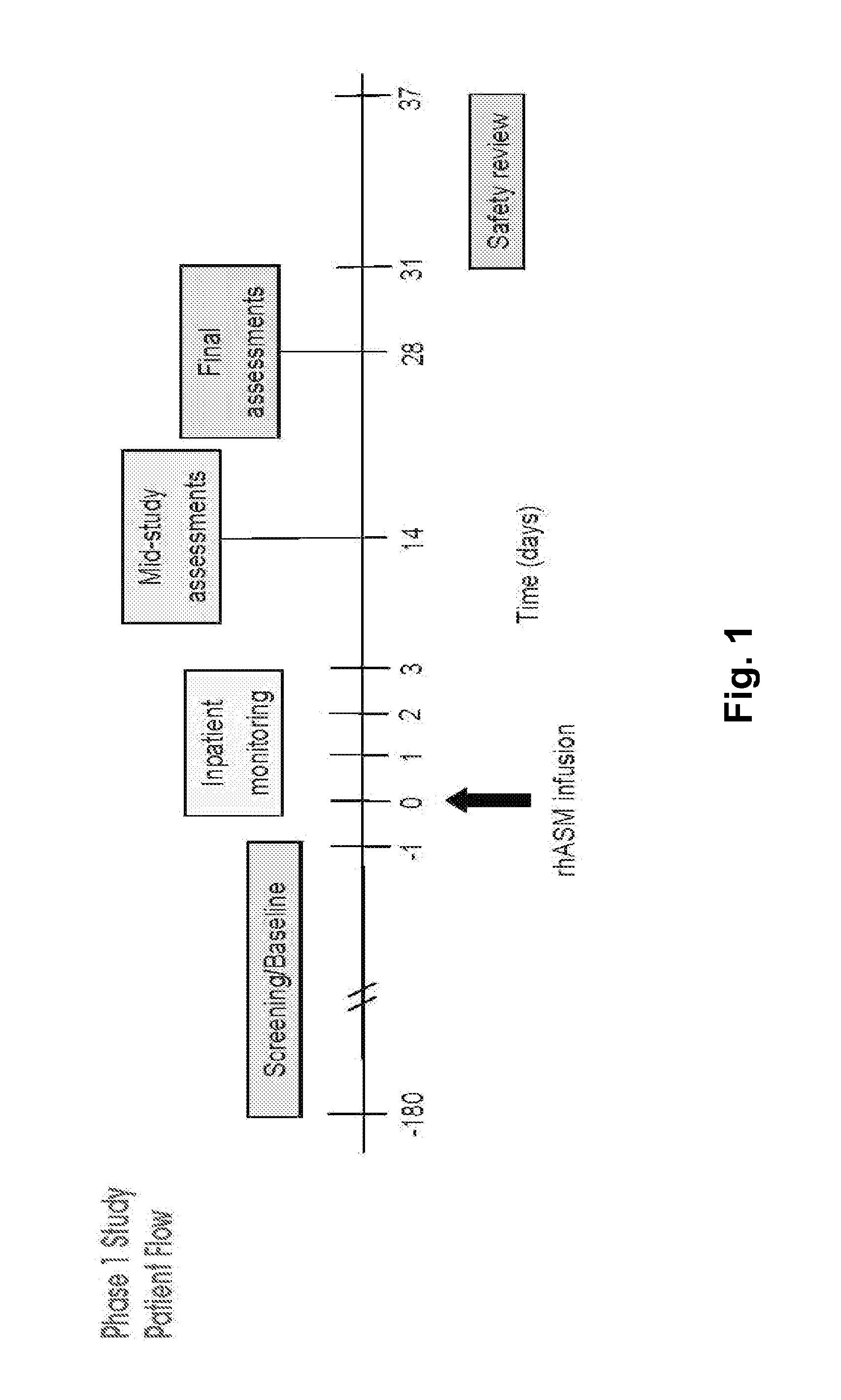
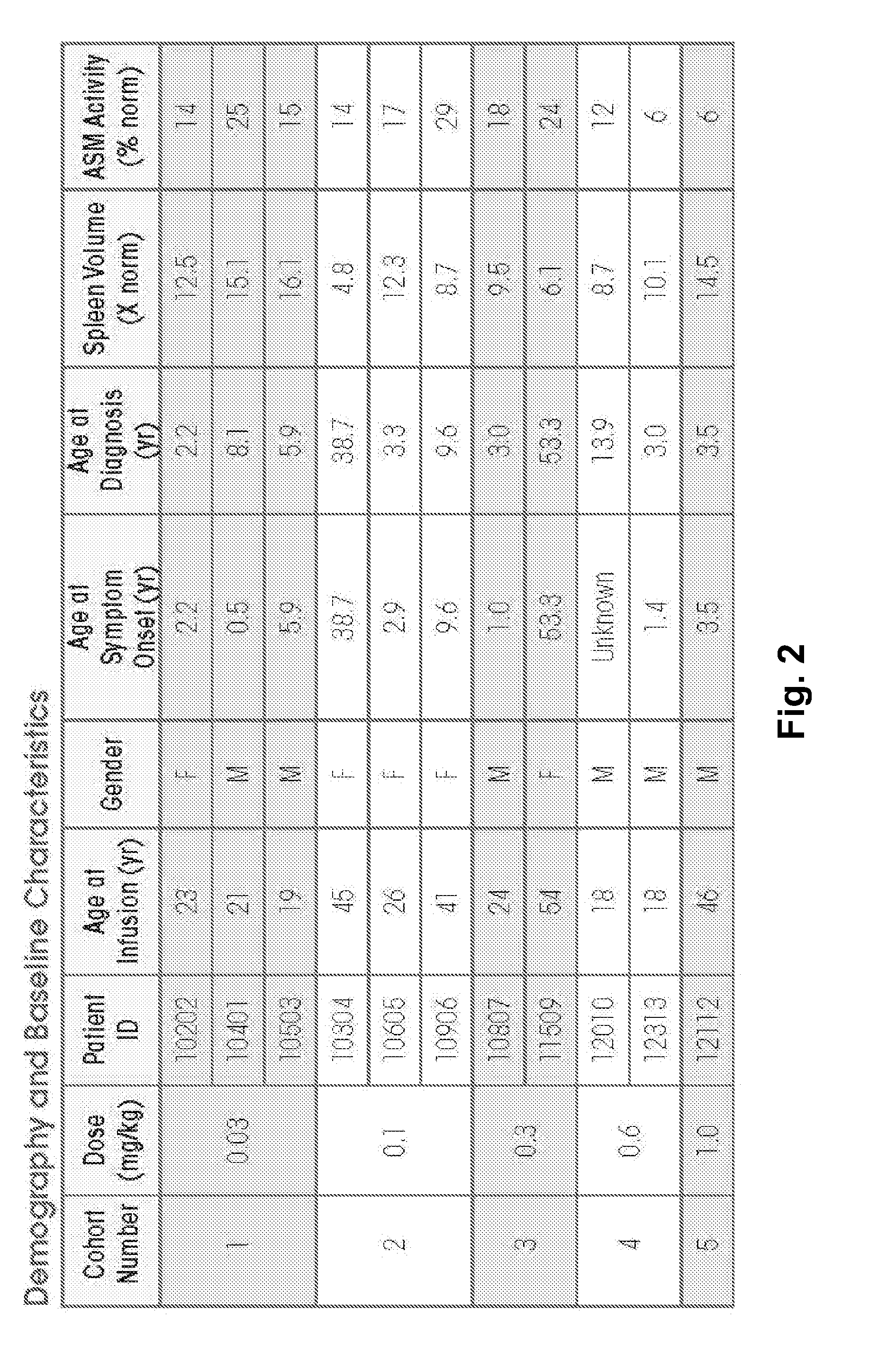
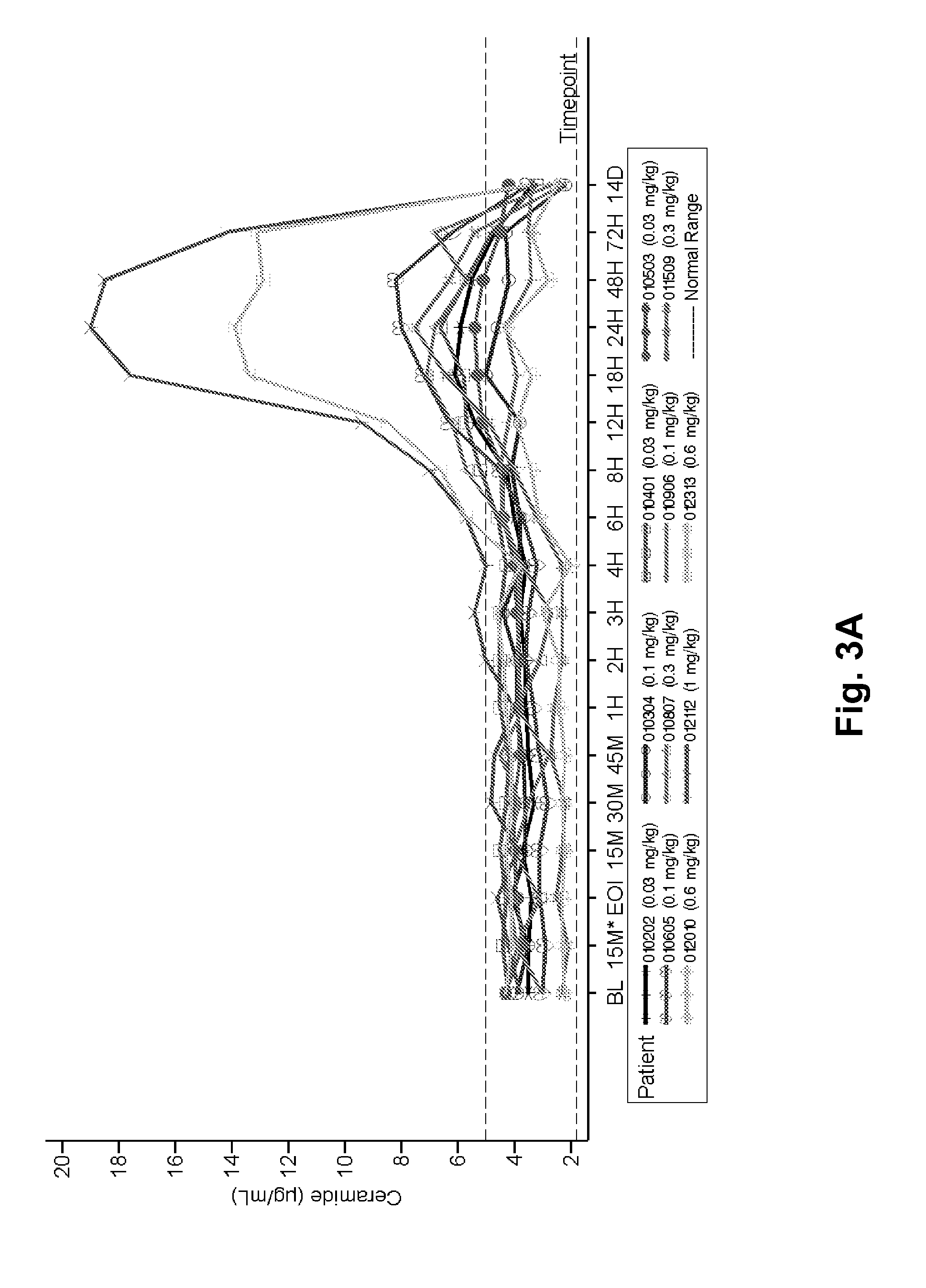
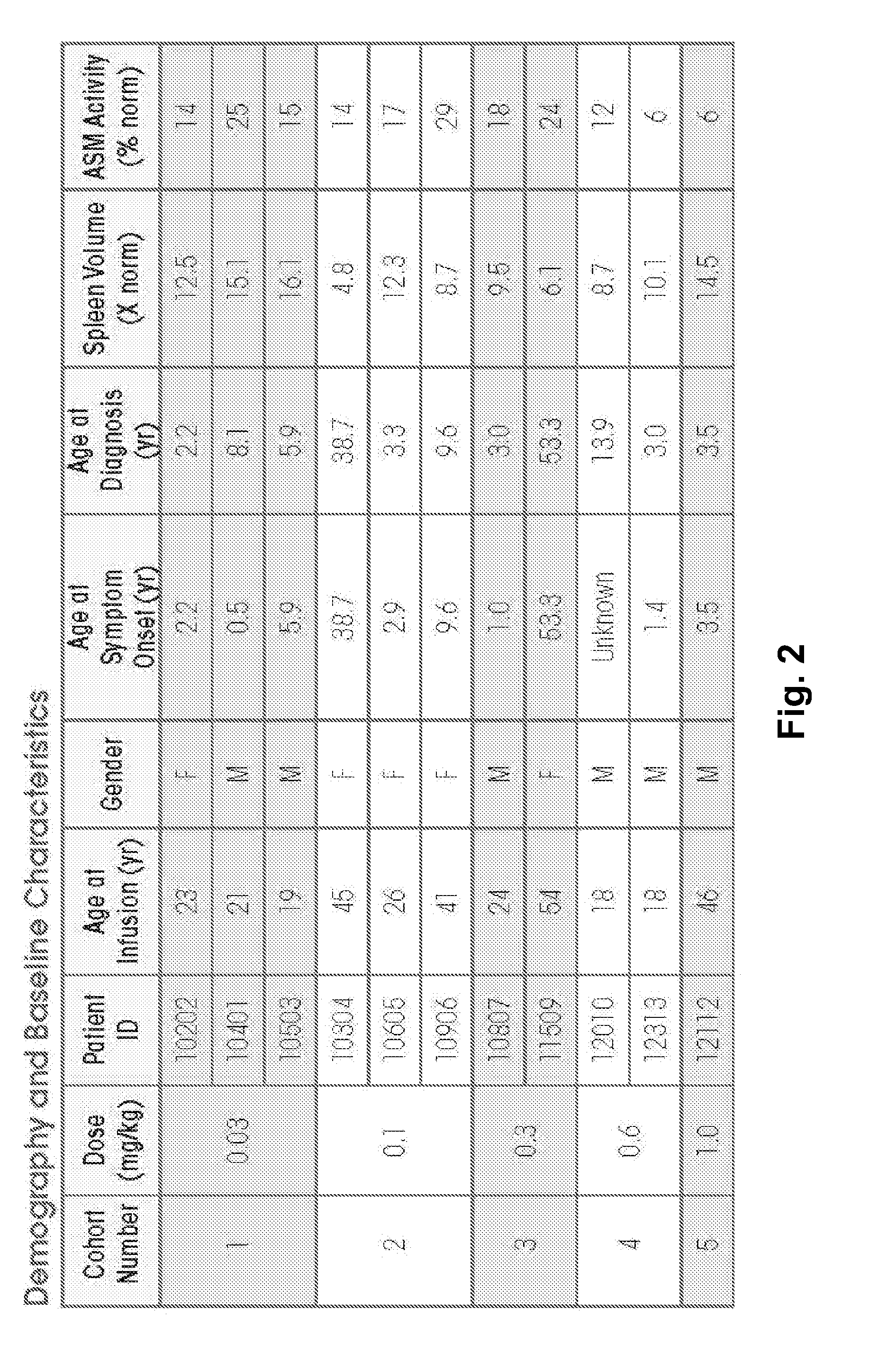
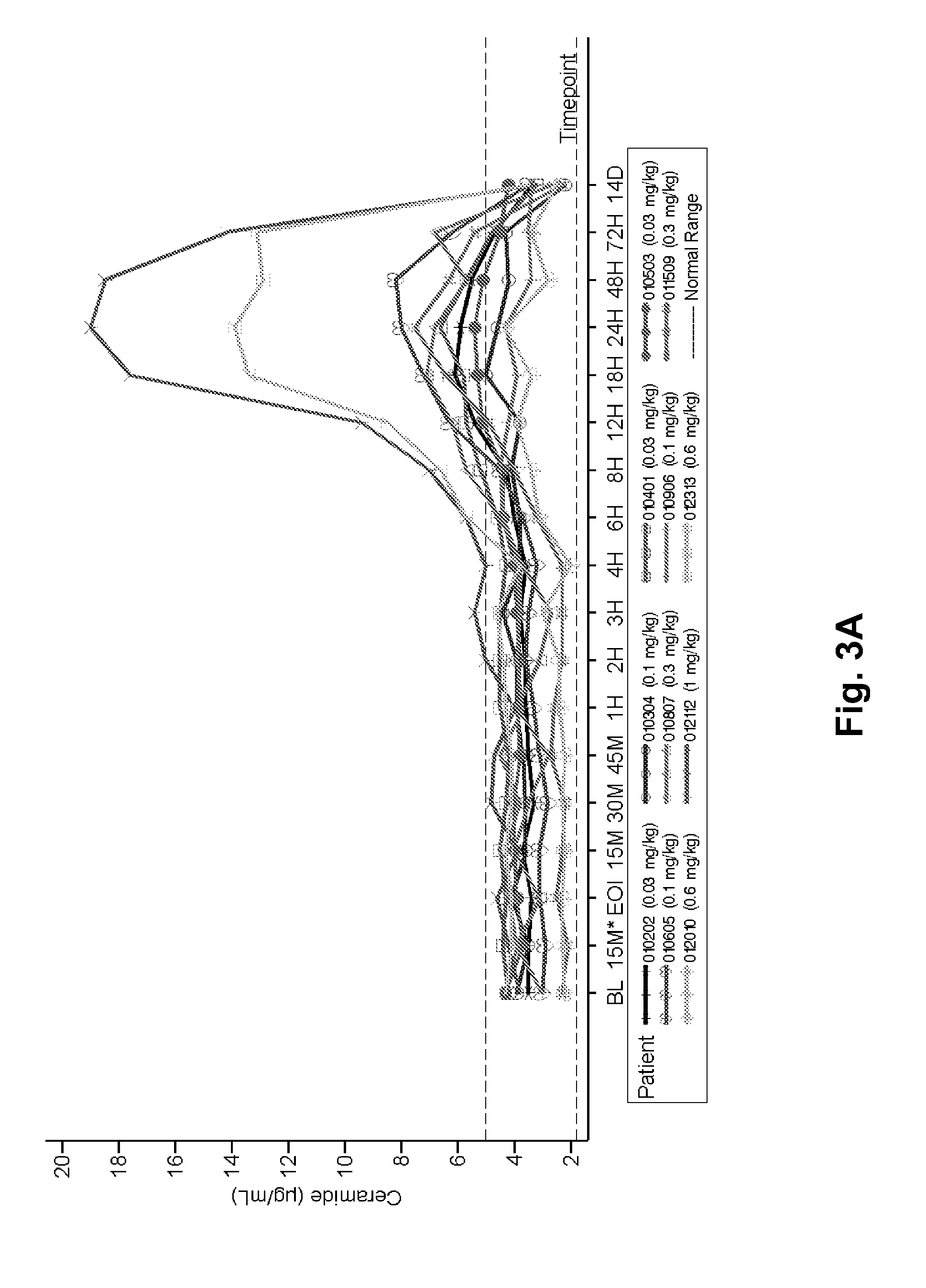
Dose escalation enzyme replacement therapy for treating acid sphingomyelinase deficiency
ActiveUS20110052559A1Avoid rapid degradationLess side effectsNervous disorderHydrolasesNeurological manifestationDose escalation
The invention relates to dose escalation enzyme replacement therapy using acid sphingomyelinase (ASM) for the treatment of human subjects having acid sphingomyelinase deficiency (ASMD), and, in particular, patients with non-neurological manifestations of Niemann-Pick Disease (NPD), and in certain embodiments, NPD type B.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Stable pharmaceutical compositions without a stabilizer
InactiveUS6893660B2Need can be overcomeAvoid rapid degradationOrganic active ingredientsBiocideControlled releaseBULK ACTIVE INGREDIENT
Stabilized controlled release pharmaceutical preparations are disclosed in which active ingredient degradation is prevented without the use of a stabilizer. The active ingredient is sealed away from excipients that can adversely affect stability by sealing the excipients rather than the active ingredient. The preparations are substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:ANDRX PHARMA INC
Cosmetic treatment method including the projection of an image onto the zone to be treated
InactiveUS20120029417A1Precise positioningReduce riskCosmetic preparationsElectrotherapyBiomedical engineering
Owner:LOREAL SA
Implant Made of a Biodegradable Magnesium Alloy
ActiveUS20100082092A1Easy to processImprove material performanceStentsSurgeryBiodegradable magnesium
An implant made in total or in parts of a biodegradable magnesium alloy consisting of Y: 2.0-6.0% by weight, Nd: 1.5-4.5% by weight, Gd: 0-4.0% by weight, Dy: 0-4.0% by weight. Er: 0-4.0% by weight, Zr: 0.1-1.0% by weight, Li:0-0.2% by weight, Al: 0-0.3% by weight, under the condition that a) a total content of Er, Gd and Dy is in the range of 0.5-4.0% by weight and b) a total content of Nd, Er, Gd and Dy is in the range of 2.0-5.5% by weight, the balance being magnesium and incidental impurities up to a total of 0.3% by weight.
Owner:BIOTRONIK AG
Formulation for Anti-alpha4beta7 antibody
ActiveUS20140341885A1Minimising immunogenicityImprove stabilityMetabolism disorderAntipyreticDosing regimenAntioxidant
Antibody formulations are described comprising a mixture of an anti-α4β7 antibody, an antioxidant or chelator, and at least one free amino acid. The disclosed formulations may have improved stability, reduced aggregate formation, or both. The present invention further provides a safe dosing regimen of these antibody formulations that is easy to follow, and which results in a therapeutically effective amount of the anti-α4β7 antibody in vivo.
Owner:MILLENNIUM PHARMA INC +2
Plasma torch for microwave induced plasmas
InactiveUS20050242070A1Avoid rapid degradationReduce sensitivityMaterial analysis using microwave meansArc welding apparatusMicrowaveEngineering
A Plasma torch (10) for microwave induced plasma spectrochemical analysis of a sample includes a nozzle (30) in an inlet (18) for the main plasma gas flow between outer tube (12) and intermediate tube (14) of the torch (10). The nozzle (30) increases the gas flow velocity in the sheathing gas layer for the plasma which is provided by the gas flow from the annular gap (22) between the tubes (12 and 14). The increased velocity of the gas in the sheathing gas layer “stiffens” that layer and thus better confines the microwave induced plasma (such better confinement not being necessary for an ICP torch). Thus the torch is of improved durability for a microwave induced plasma compared to an ICP torch. The sample injection (inner) tube (16) may have a reduced diameter outlet at its end (34) which is substantially level with the end (35) of intermediate tube (14) to improve injection of a sample into the microwave induced plasma. The inlet end (26) of the sample injection tube (16) may include a heater (36) to assist in preventing blockages in tube (16) near its outlet end.
Owner:VARIAN AUSTRALIA PTY LTD
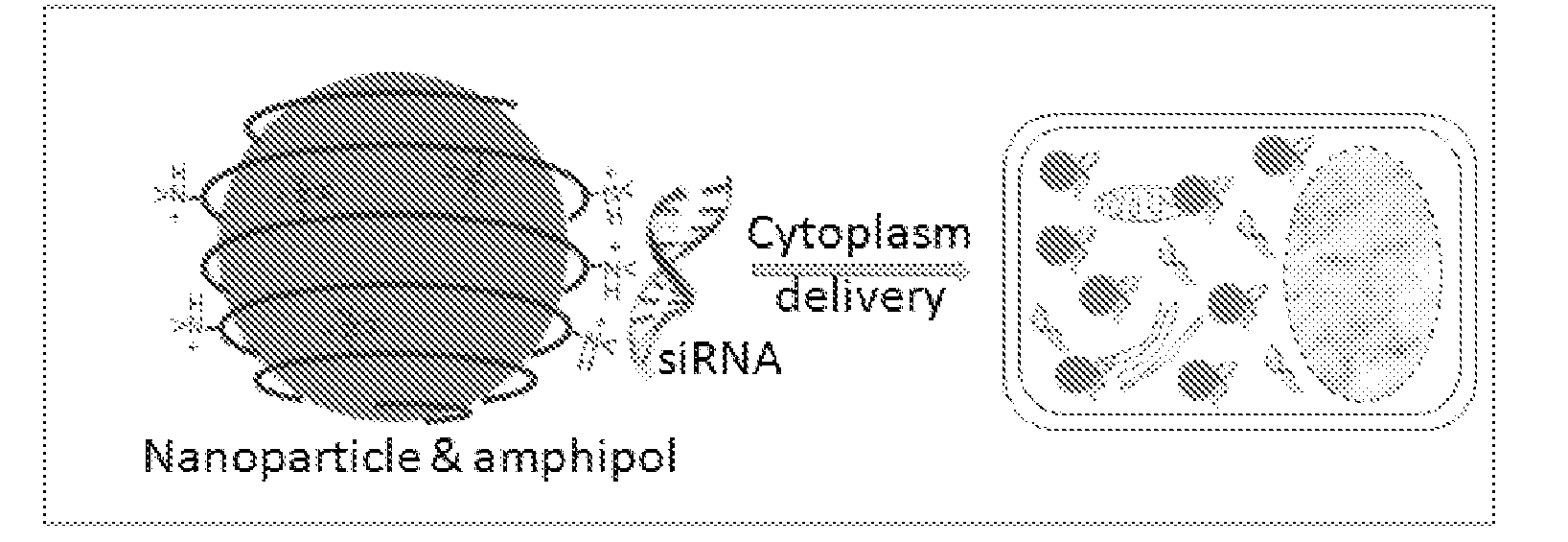
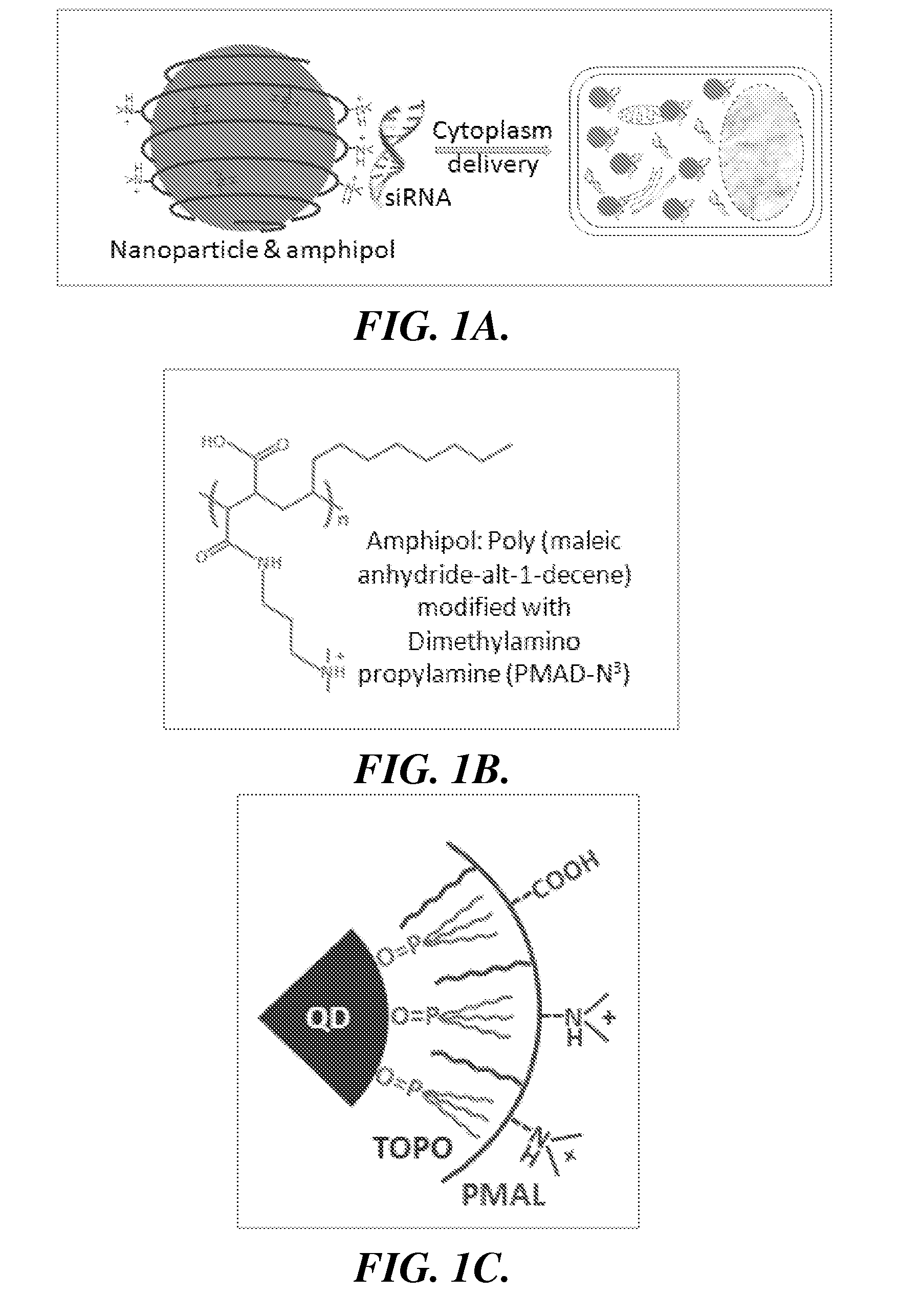
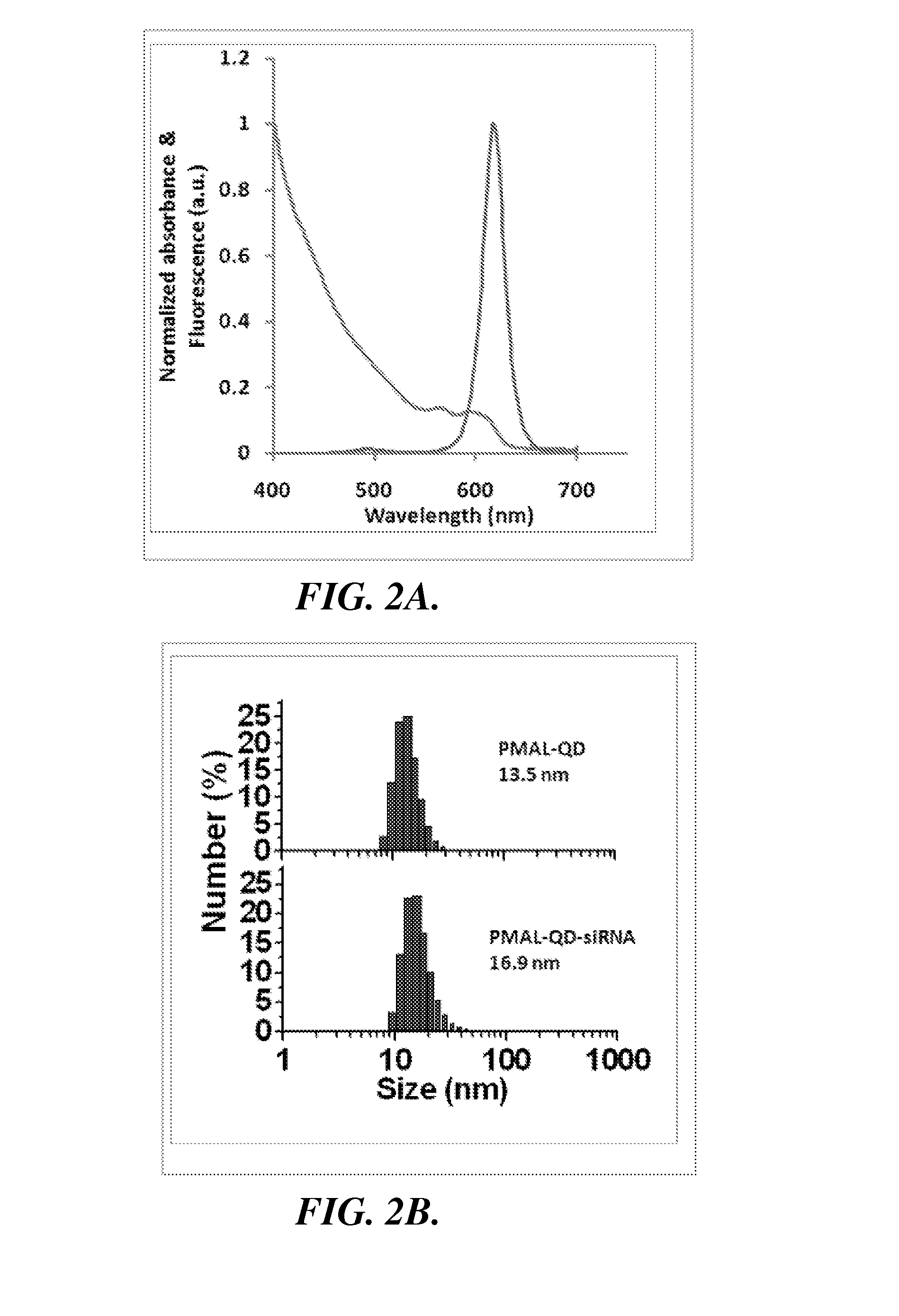
Nanoparticle-amphipol complexes for nucleic acid intracellular delivery and imaging
InactiveUS20090322327A1Strong absorption capabilityFacilitated releaseRadiation measurementNanomedicineNanoparticlePolymer
Owner:UNIV OF WASHINGTON
Pepper plants
InactiveUS7642423B2Avoid rapid degradationLong harvest periodData processing applicationsTissue cultureCapsicum annuumAgronomy
The present invention relates to novel plants, in particular to Capsicum annuum plants capable of producing fruits with extended storability after full coloring of the fruit, and to seeds and fruits of said plants. The present invention also relates to methods of making and using such plants and their fruits. In particular, fruits of plants of the present invention retain marketability over extended periods of time compared to presently available peppers.
Owner:SYNGENTA PARTICIPATIONS AG
Stable pharmaceutical compositions without a stabilizer
InactiveUS20050142195A1Avoid rapid degradationBiocideOrganic active ingredientsControlled releaseBULK ACTIVE INGREDIENT
Stabilized controlled release pharmaceutical preparations are disclosed in which active ingredient degradation is prevented without the use of a stabilizer. The active ingredient is sealed away from excipients that can adversely affect stability by sealing the excipients rather than the active ingredient. The preparations are substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:ANDRX PHARMA INC
Dose escalation enzyme replacement therapy for treating acid sphingomyelinase deficiency
ActiveUS8349319B2Avoid rapid degradationLess side effectsNervous disorderHydrolasesNeurological manifestationAcid sphingomyelinase
Owner:MT SINAI SCHOOL OF MEDICINE +1
Chemical recycling of pla by hydrolysis
ActiveUS20120142958A1Simple processImprove impactOrganic compound preparationPlastic recyclingSolventHydrolysis
Owner:FUTERRO SA
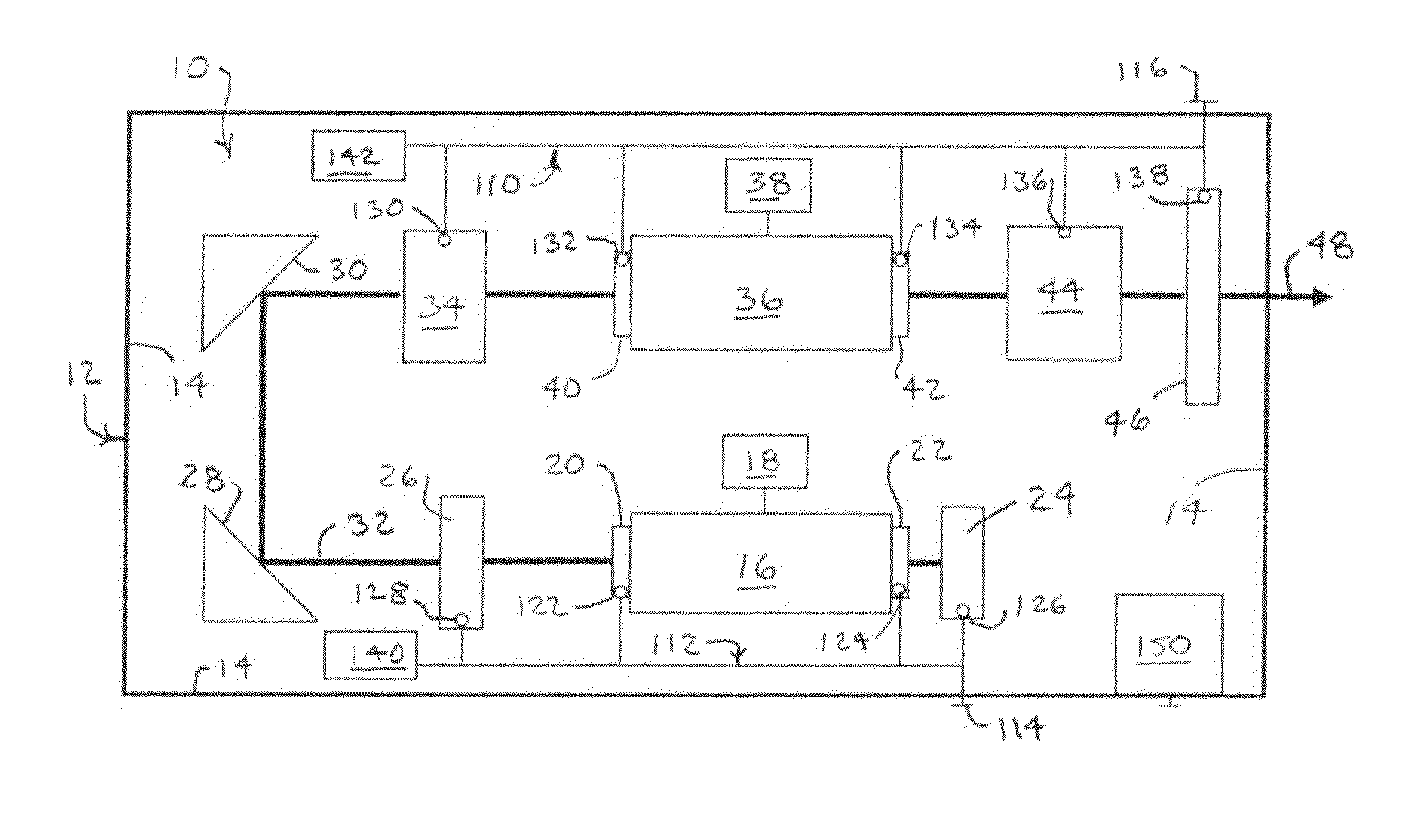
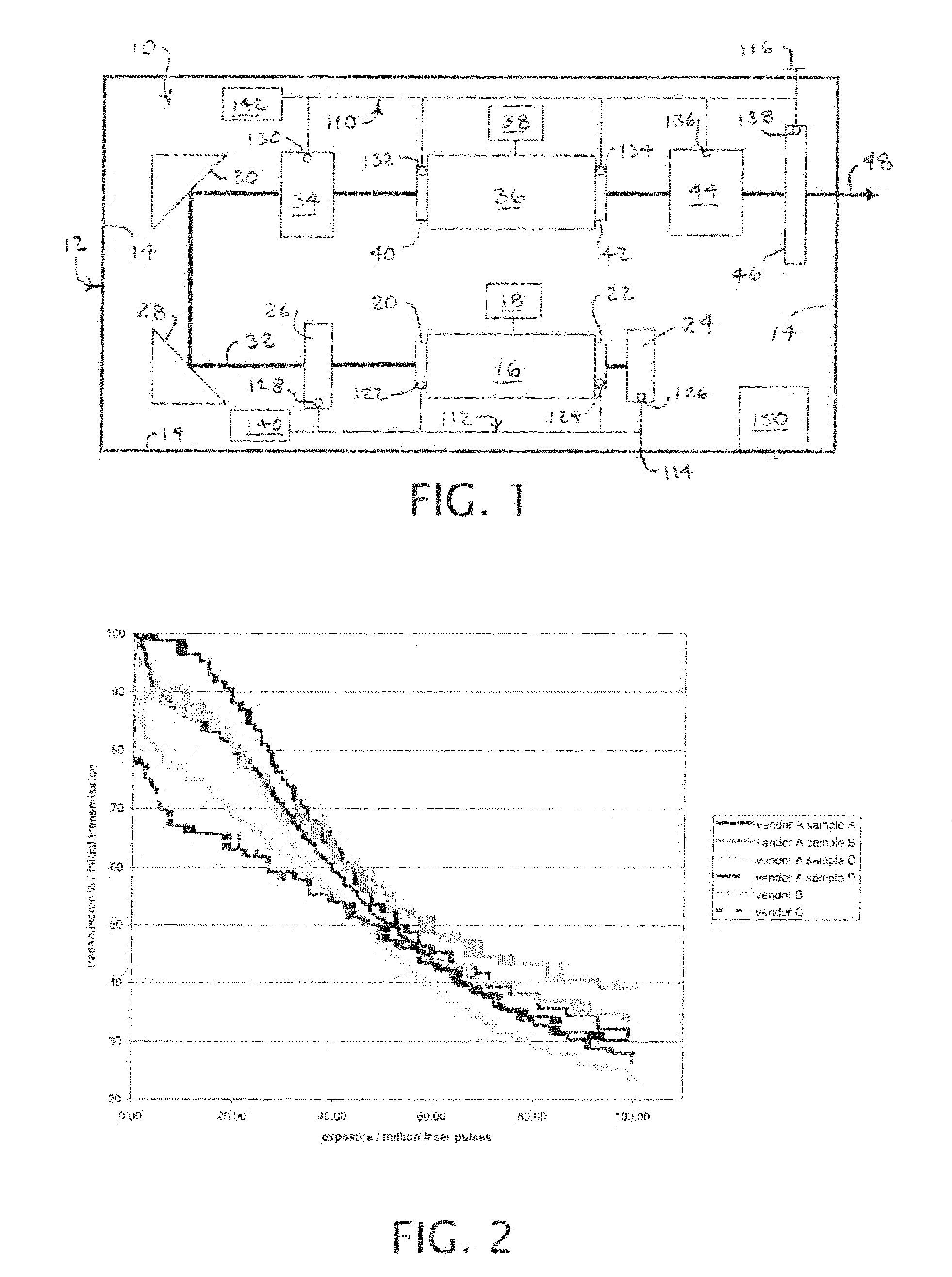
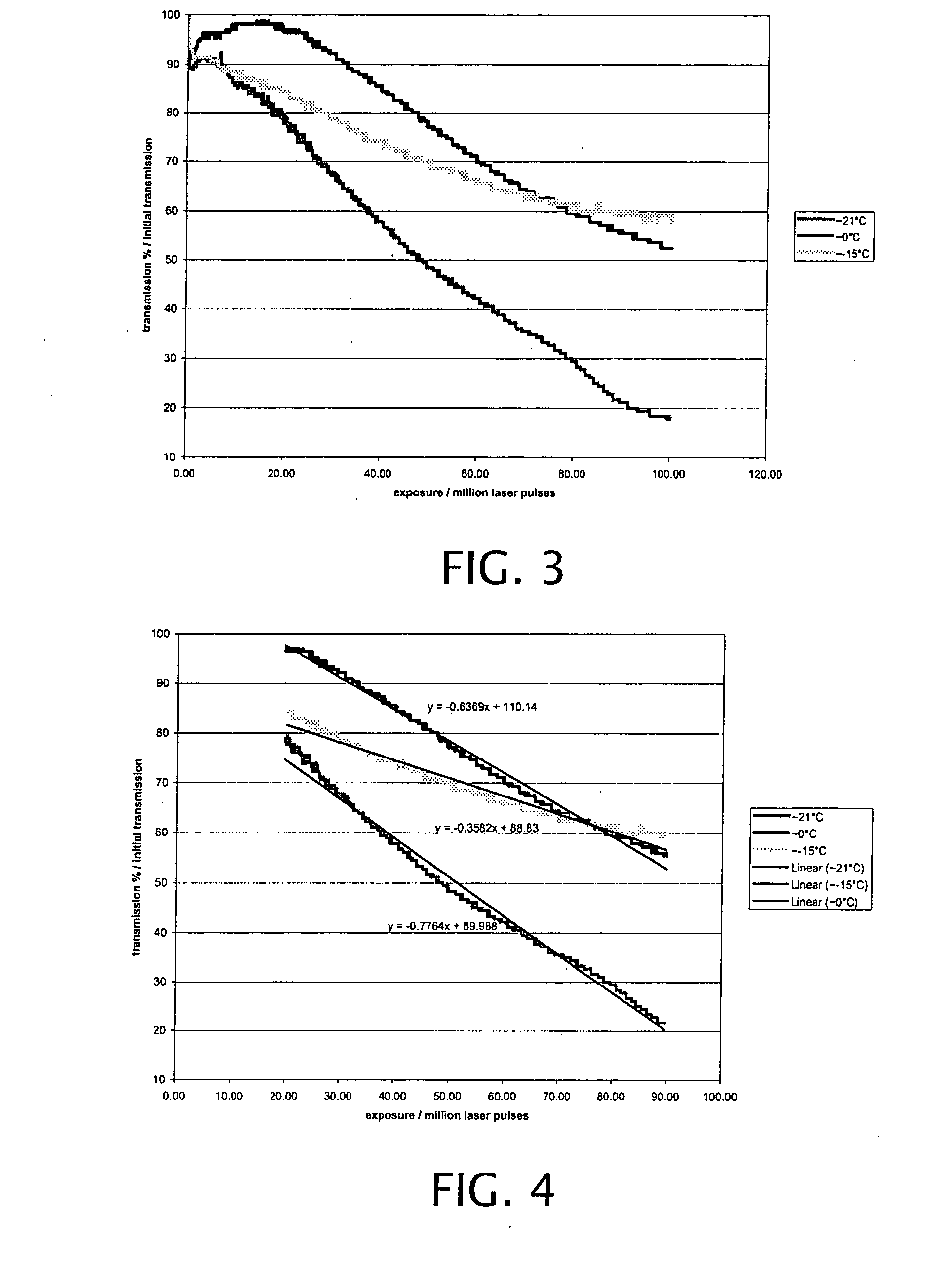
Active cooling of crystal optics for increased laser lifetime
InactiveUS20080198882A1Reduce degradationLow costPhotomechanical apparatusMountingsActive coolingTransmittance
A laser beam is generated and transmitted within an enclosed pathway through at least one crystal optic at a power density that progressively degrades transmissivity of the crystal optic with accumulating fluence. The crystal optics are cooled below normal operating temperatures to slow the progressive degradation in the transmissivity of the crystal optics with the accumulating fluence or to accommodate a higher power density without correspondingly increasing the progressive degradation in transmissivity.
Owner:CORNING INC
Chemical recycling of pla by alcoholysis
ActiveUS20120029228A1Simple processImprove impactPreparation from carboxylic acid halideOrganic compound preparationSolventPolymer
Owner:FUTERRO SA
Ink jet printing apparatus, ink jet printing method, program, and printing medium
InactiveUS6896348B2Extended service lifeAvoid rapid degradationDigitally marking record carriersDigital computer detailsImaging dataElectrical and Electronics engineering
The print head is scanned over a printing medium in a sub-scanning direction different from a direction in which the nozzles are arranged. The printing medium is conveyed by a predetermined amount K (where K=a×L (a is a natural number and L is the size of the gradation patterns in the direction in which the nozzles are arranged) or K=L / b (b is a natural number)) in a direction in which the nozzles are arranged, between a preceding scan and a next scan of the print head. Correspondences between image data and the plurality of nozzles are shifted in the direction in which the nozzles are arranged. The operated dot patterns are changed so as to allow the selective use of a plurality of different dot patterns indicating the same gradation value.
Owner:CANON KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com