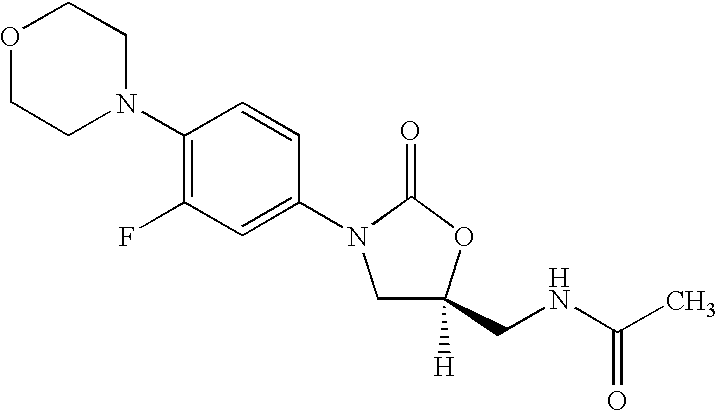
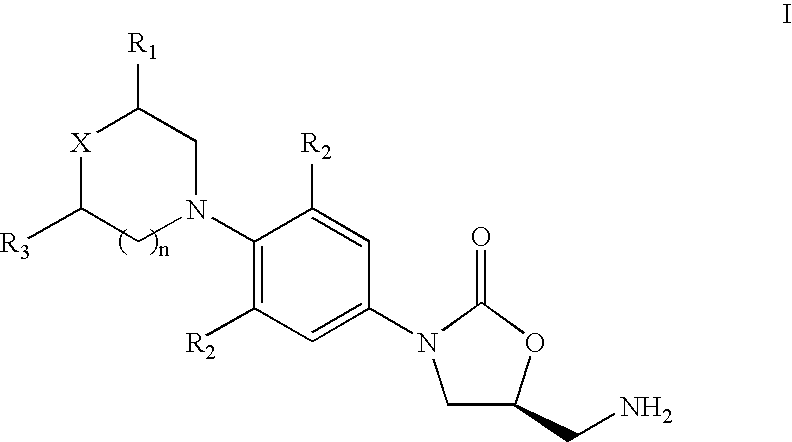
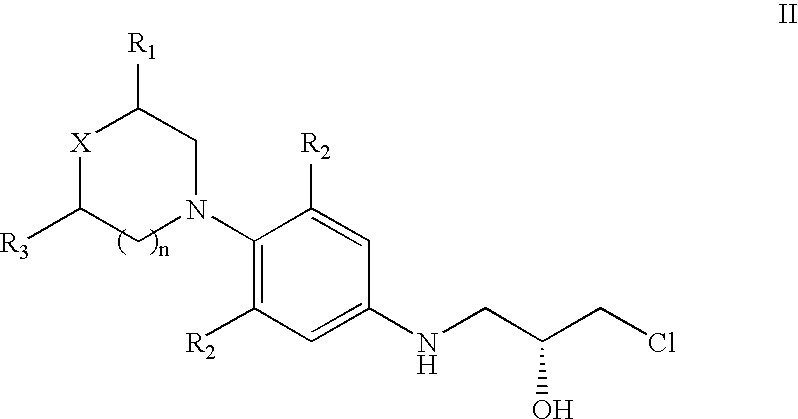
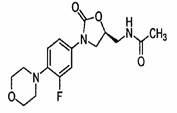
Patents
Literature
228 results about "Linezolid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
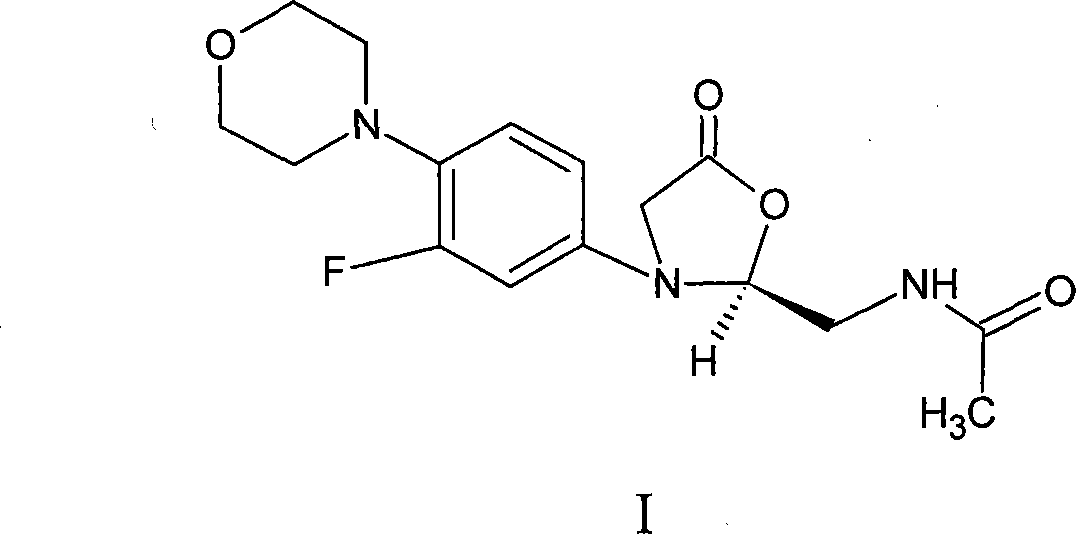
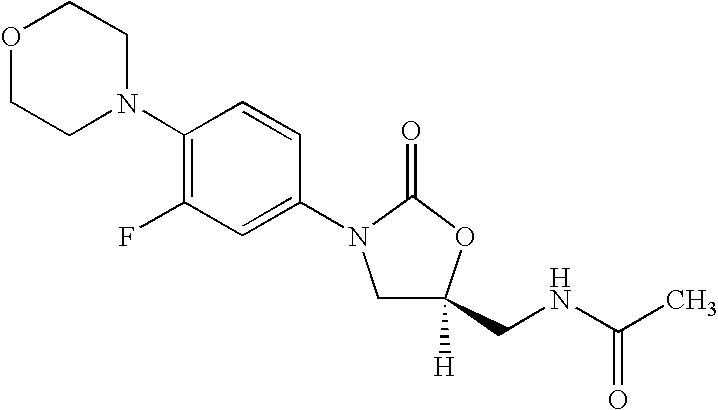
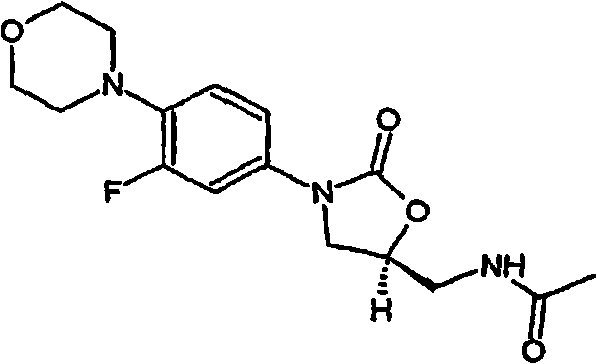
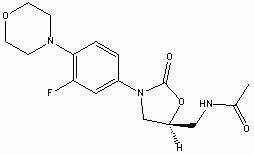
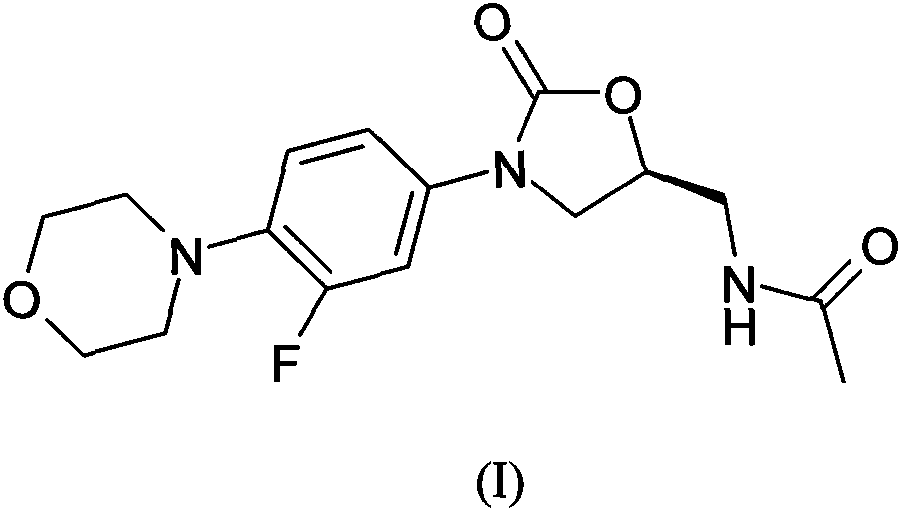
Linezolid is an antibiotic used to treat certain serious bacterial infections.
Tablets of linezolid form iii and processes for their preparation
InactiveUS20070104785A1Reduce gelling tendencyTrend downOrganic active ingredientsPill deliveryLinezolidDissolution
The present invention relates to solid oral dosage forms of linezolid polymorphic Form III with reproducible dissolution profile and processes for their preparation. The solid dosage form includes linezolid Form III, one or more of means to reduce the gelling tendency of linezolid form III, and one or more of pharmaceutically acceptable excipients.
Owner:NAVALE SURYAKANT VAMANRAO +3
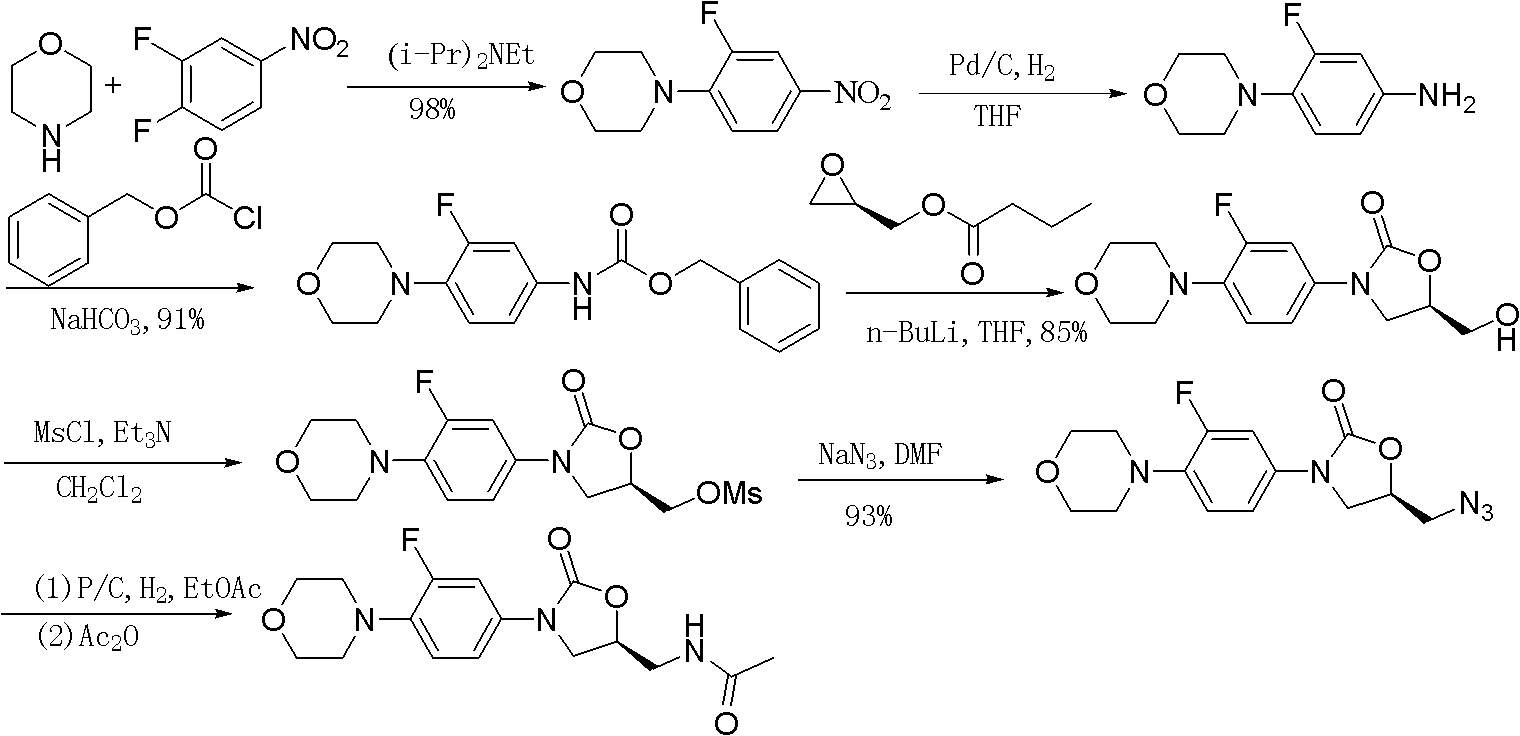
Synthesis of linezolid
InactiveCN101220001ASimple stepsMild reaction conditionsAntibacterial agentsOrganic chemistryEpoxyHalohydrocarbon
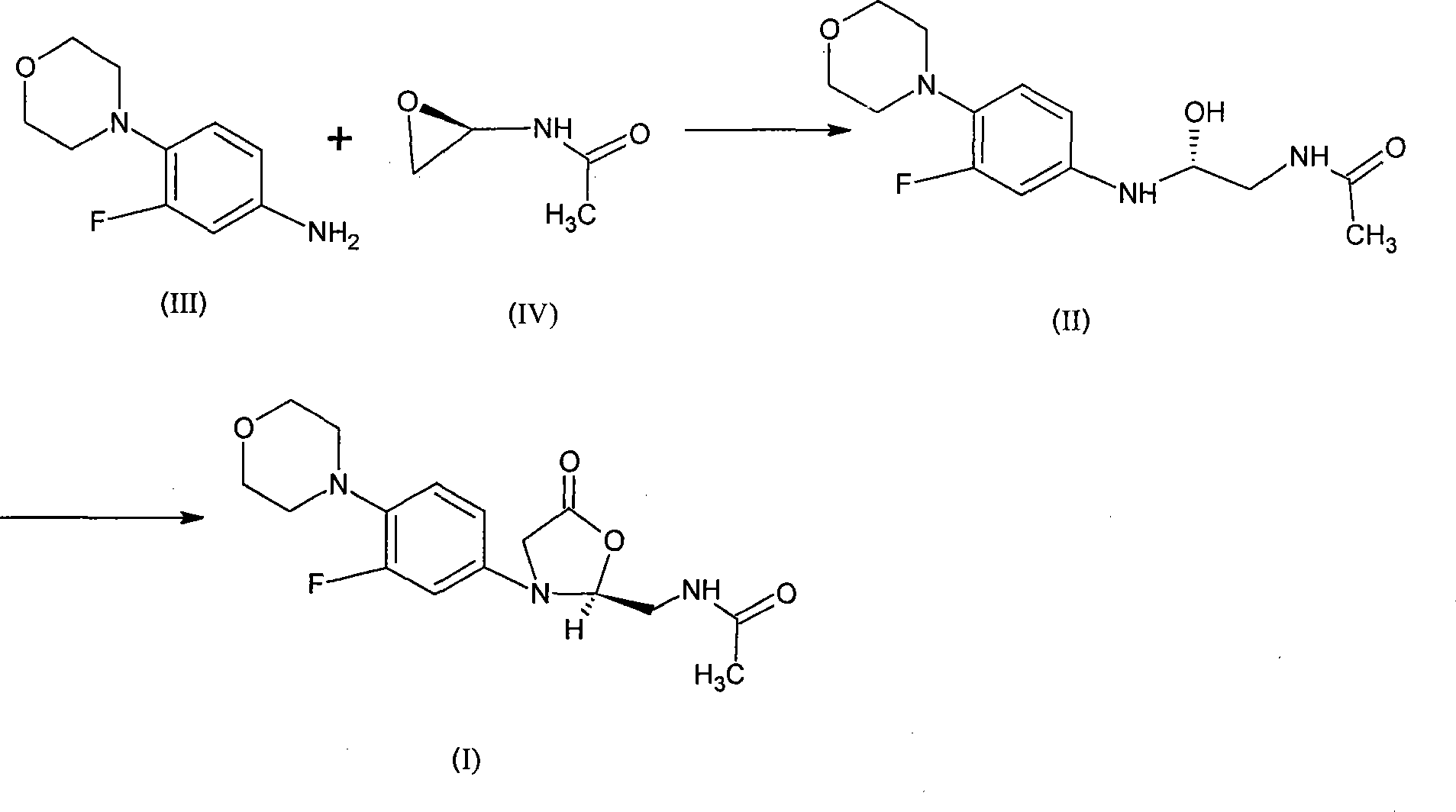
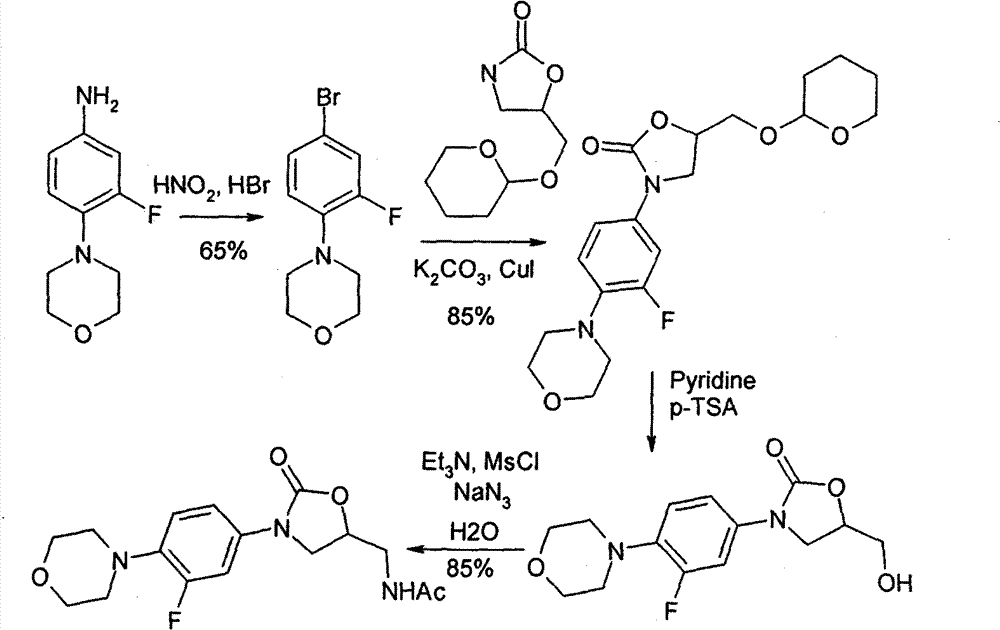
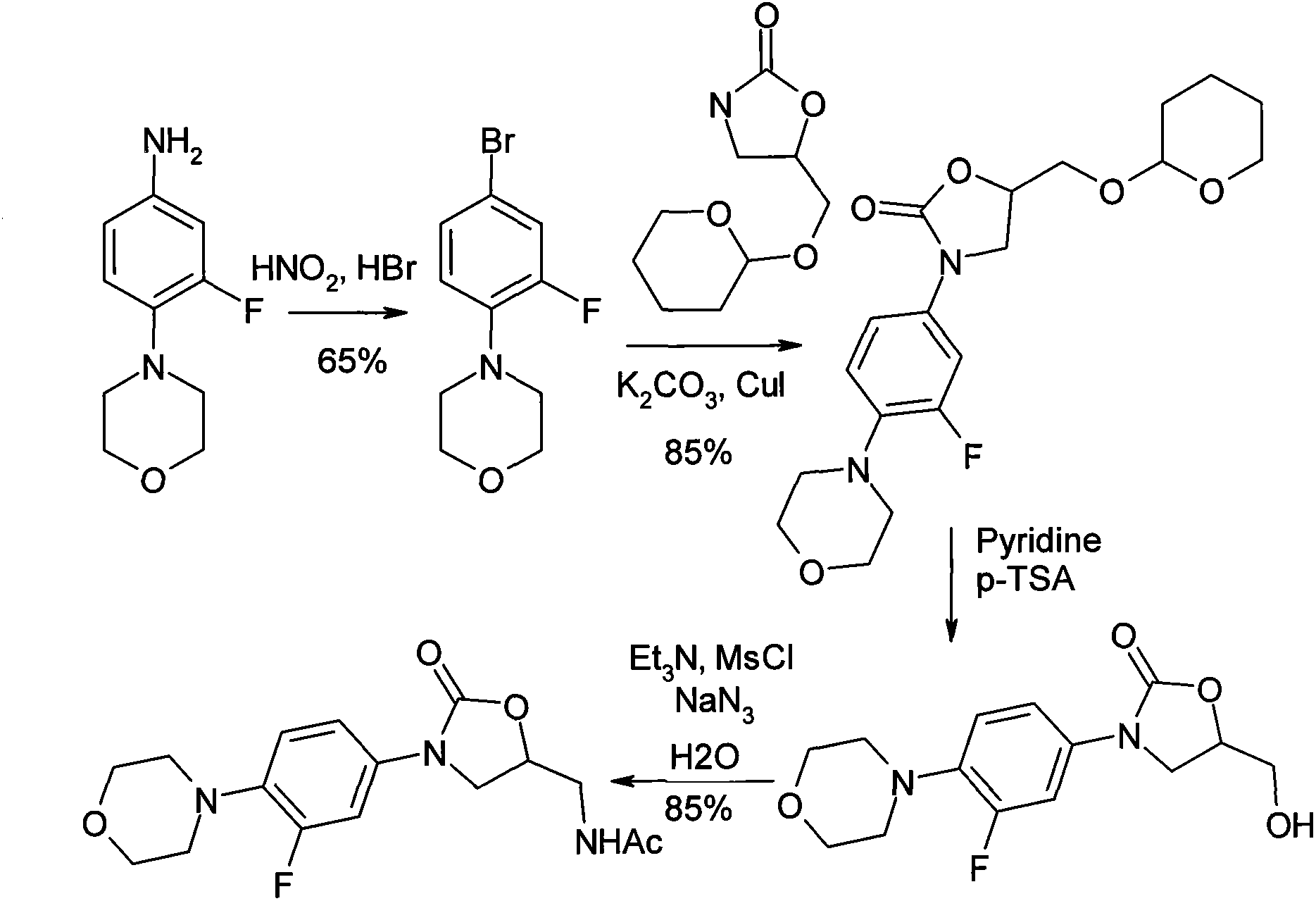
The invention relates to a synthetic method for linezolid, which dissolves a 3-fluoro-4- morpholinyl laniline and an N-2(R)-epoxy acetamide in an organic solvent to react for 10 to 30 hours in a temperature of 20 to 100 DEG C, and acquires an N-{2(R)-2-[(3-fluoro-4 morpholine-4-base phenyl) amino]-2-hydroxy ethyl}; then an acquired compound and a carbonylation agent are dissolved in a halohydrocarbon organic solvent and reacted for 0.5 to 5 hours in a temperature of 0-50 DEG C under the catalysis of a base catalyst, thereby acquiring the linezolid. The method of the invention uses an ordinary reagent and normal plant conditions in industry, the reaction condition is mild and the steps are simple.
Owner:ZHEJIANG BOTAI CHEM
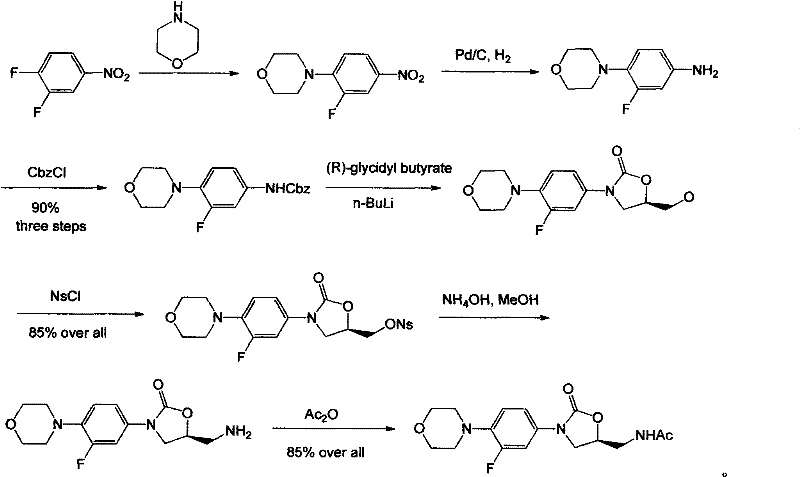
Novel process for the preparation of linezolid and related compounds
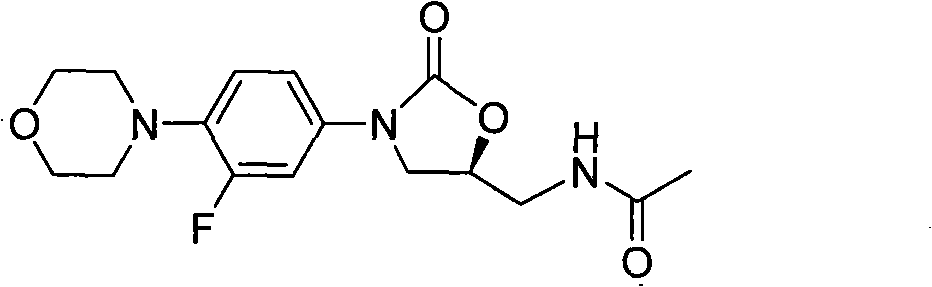
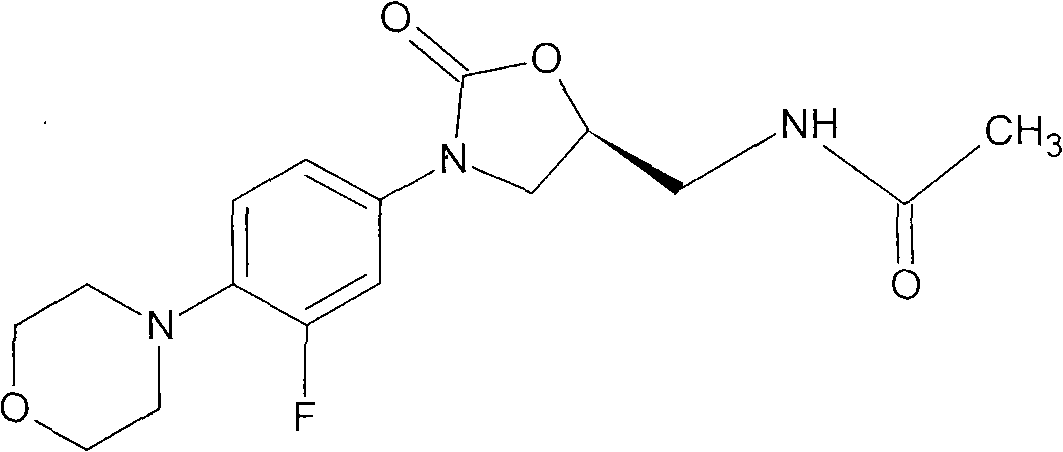
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
Preparation method of linezolid and preparation thereof
InactiveCN101948442AImprove stabilityExtended shelf lifeAntibacterial agentsPowder deliveryFreeze-dryingNitrobenzene
The invention relates to a preparation method of linezolid and a preparation thereof, in particular to a preparation method of a linezolid raw material and a freeze-dried powder injection thereof. The preparation method mainly comprises the following steps: (1) synthesizing 2(S)-1-amino-3-chloro-2-propanol hydrochloride; (2) synthesizing (S)-N-[2-acetoxyl-3-chloropropyl] acetamide; (3) synthesizing 3-fluoro-4-morpholinyl nitrobenzene; (4) synthesizing N-carbobenzoxy-3-fluoro-4-morpholinyl aniline; (5) synthesizing the linezolid; and (6) preparing the linezolid preparation. The freeze-dried powder injection of the linezolid for injection, which is prepared by the invention, effectively improves the stability of medicines so that the medicines have longer shelf life, can be transported more conveniently and are more favorable for clinical applications. Moreover, the method of the invention for preparing the raw material linezolid and the freeze-dried powder injection thereof is simple and effective and is suitable for large-scale industrial production.
Owner:符健
Preparation method of linezolid and intermediate thereof
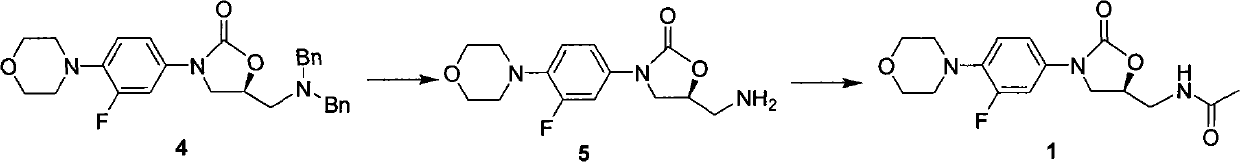
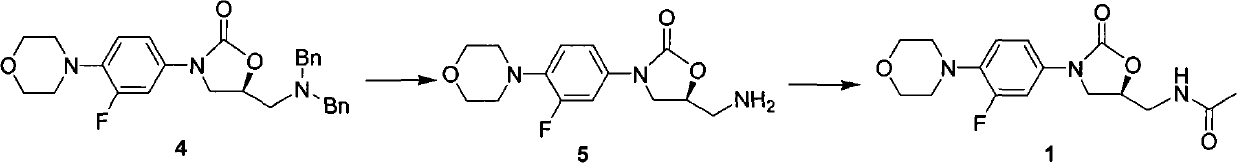
ActiveCN101774978AEasy to purifyChiral raw materials are readily availableOrganic chemistryLinezolidAcetylation
The invention discloses a preparation method of linezolid (a compound 1), which comprises the following steps: (1) carrying out debenzylation reaction on a compound 4 in a solvent for preparing a compound 5; and (2) carrying out amino-group acetylation reaction on the compound 5 prepared in the first step for preparing and obtaining the compound 1. The invention also discloses an intermediate for preparing the compound 1. The preparation method of the invention has the advantages that chiral raw materials can be easily obtained and have low price, the process is simple, the post treatment is simple, both the intermediate product and the final product can be purified easily, the total yield is high, the purity is high, and the industrial production can be easily realized.
Owner:LIANHE CHEM TECH
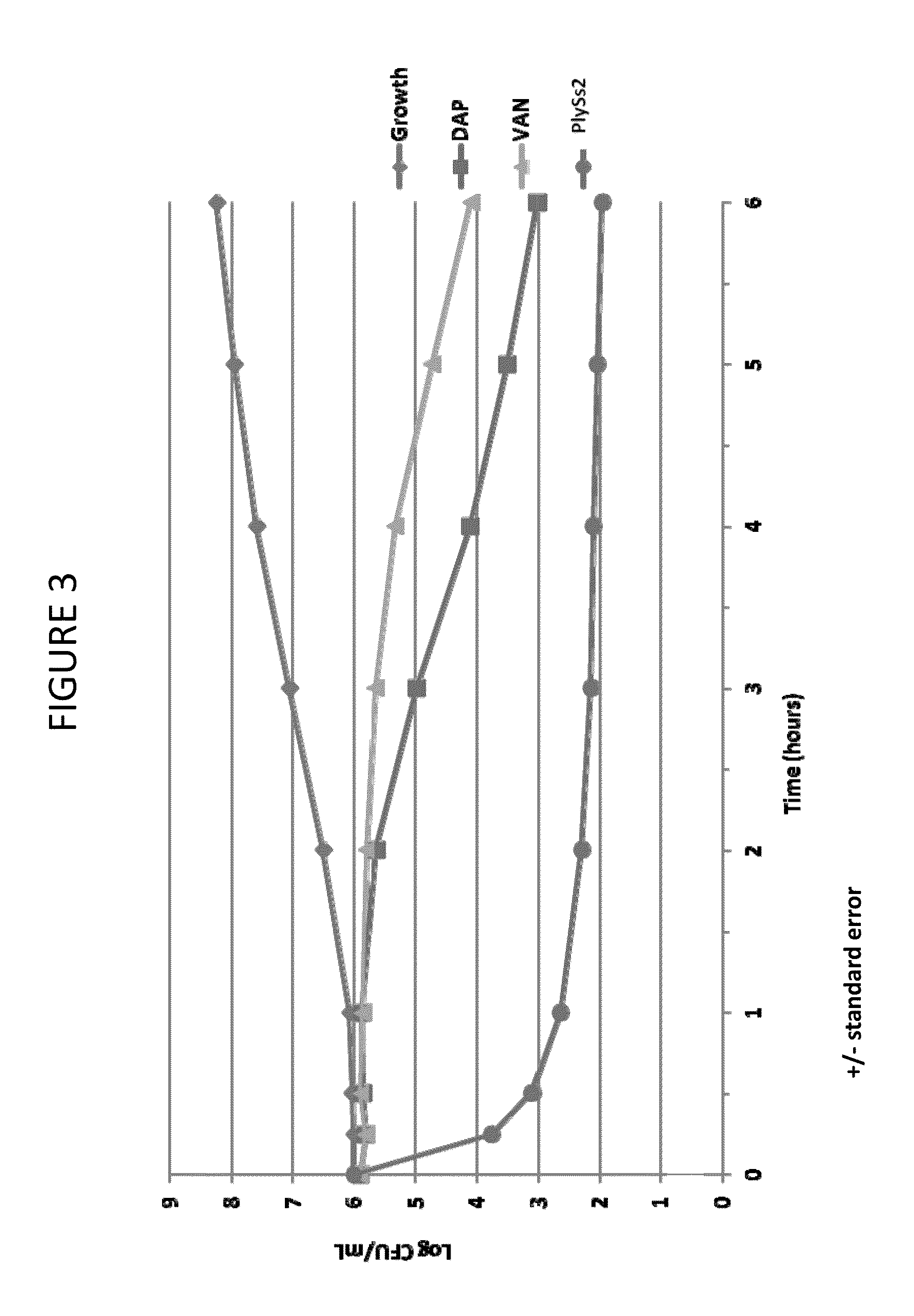
Bacteriophage lysin and antibiotic combinations against gram positive bacteria
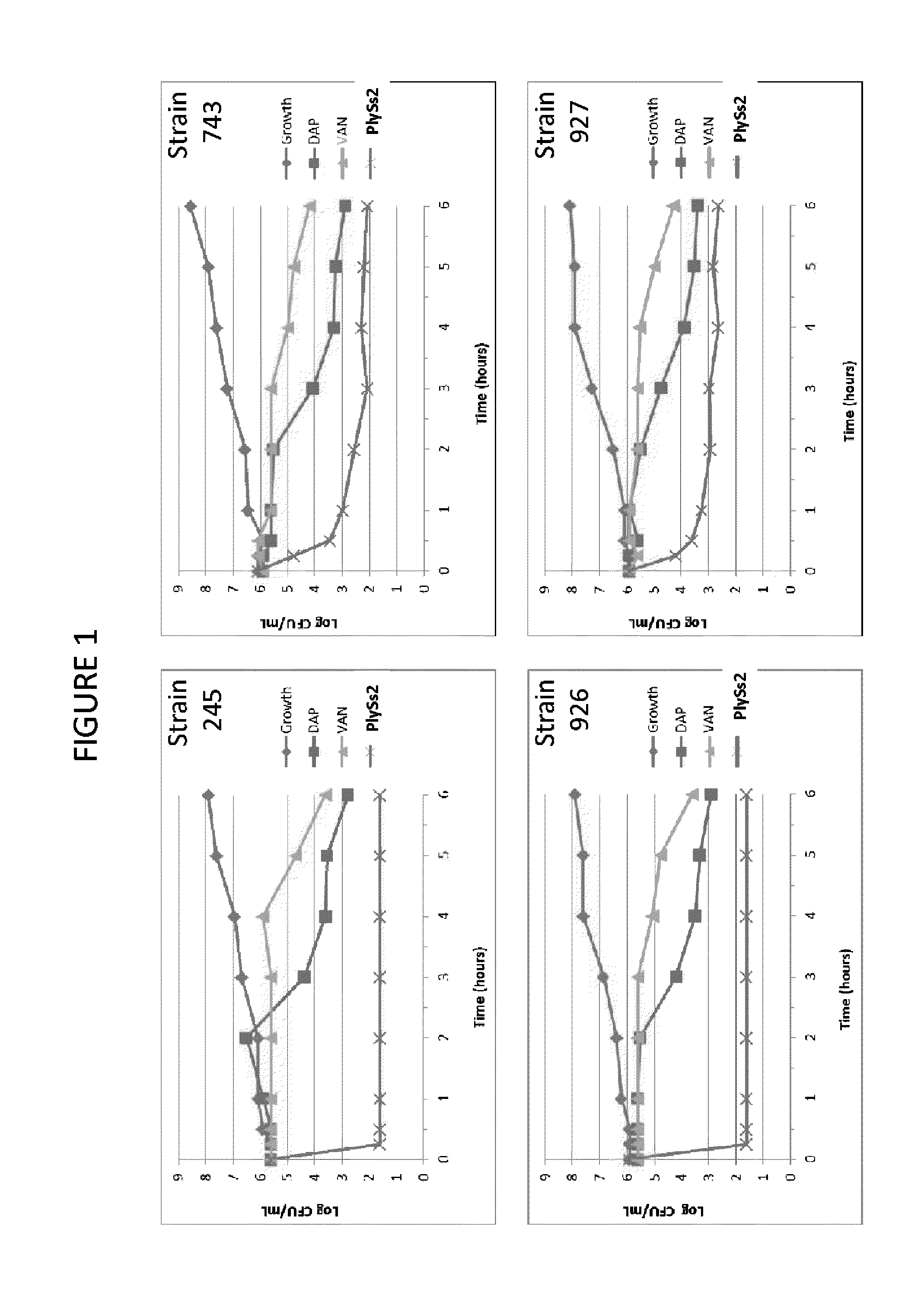
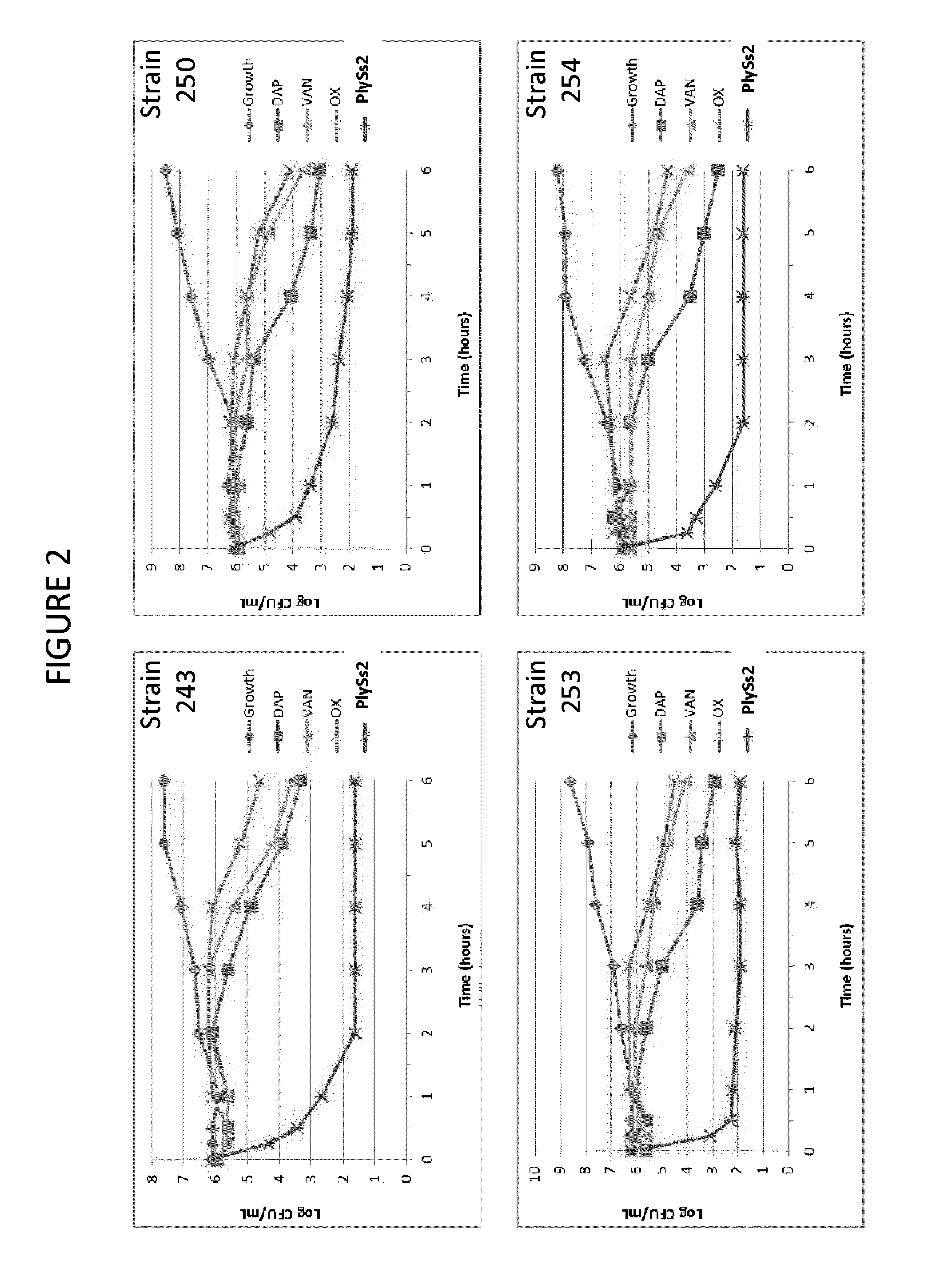
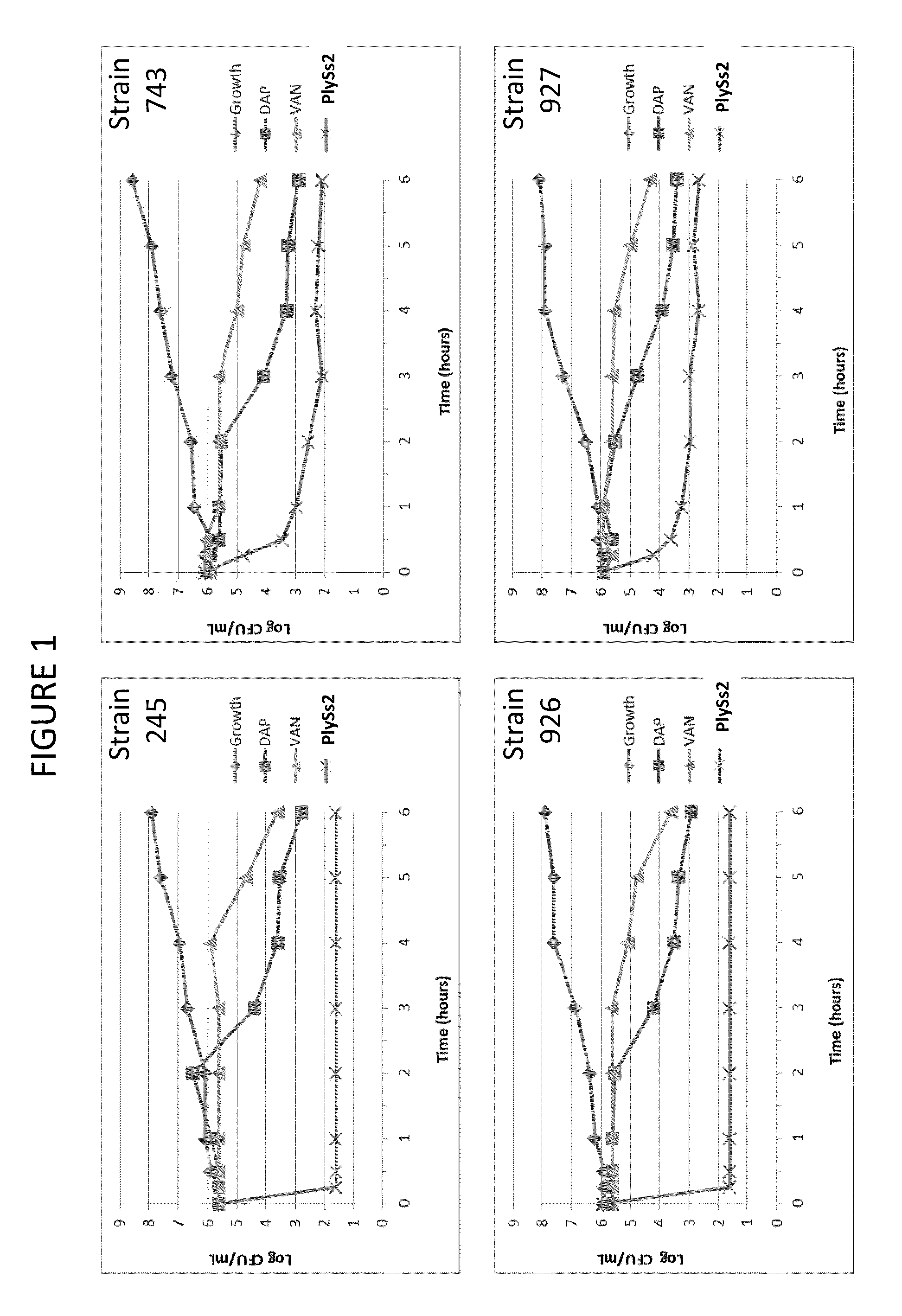
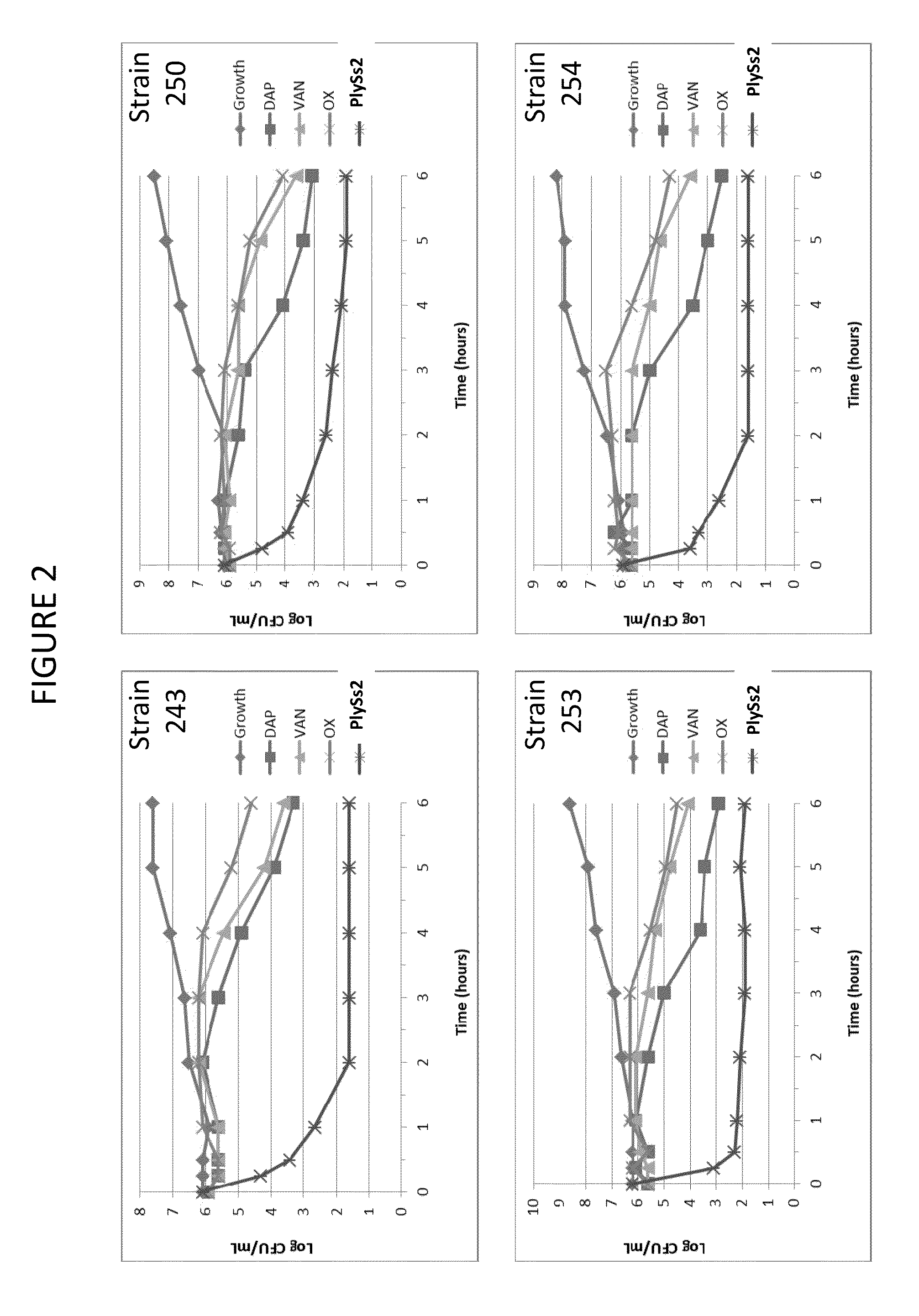
ActiveUS20130302306A1Quick killBroad killing activityAntibacterial agentsOrganic active ingredientsLysinLinezolid
The present invention provides compositions and methods for prevention, amelioration and treatment of gram positive bacteria, particularly Staphylococcal bacteria, with combinations of lysin, particularly Streptococcal lysin, particularly the lysin PlySs2, and one or more antibiotic, including daptomycin, vancomycin, oxacillin, linezolid, or related antibiotic(s).
Owner:CONTRAFECT CORP
Novel intermediates for linezolid and related compounds
The present invention provides a novel process for preparation of 5 aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus, the key intermediate of linezolid is prepared by a) reacting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline with potassium phthalimide; b) subjecting N-[3-pthalimido-2-(R)-hydroxypropyl]-3-fluoro-4-(morpholinyl) aniline produced in the above step to carbonylation; and c) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidiriyl]methyl]phthalimide produced in the above step with hydrazine hydrate to produce (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]amine.
Owner:HETERO USA INC
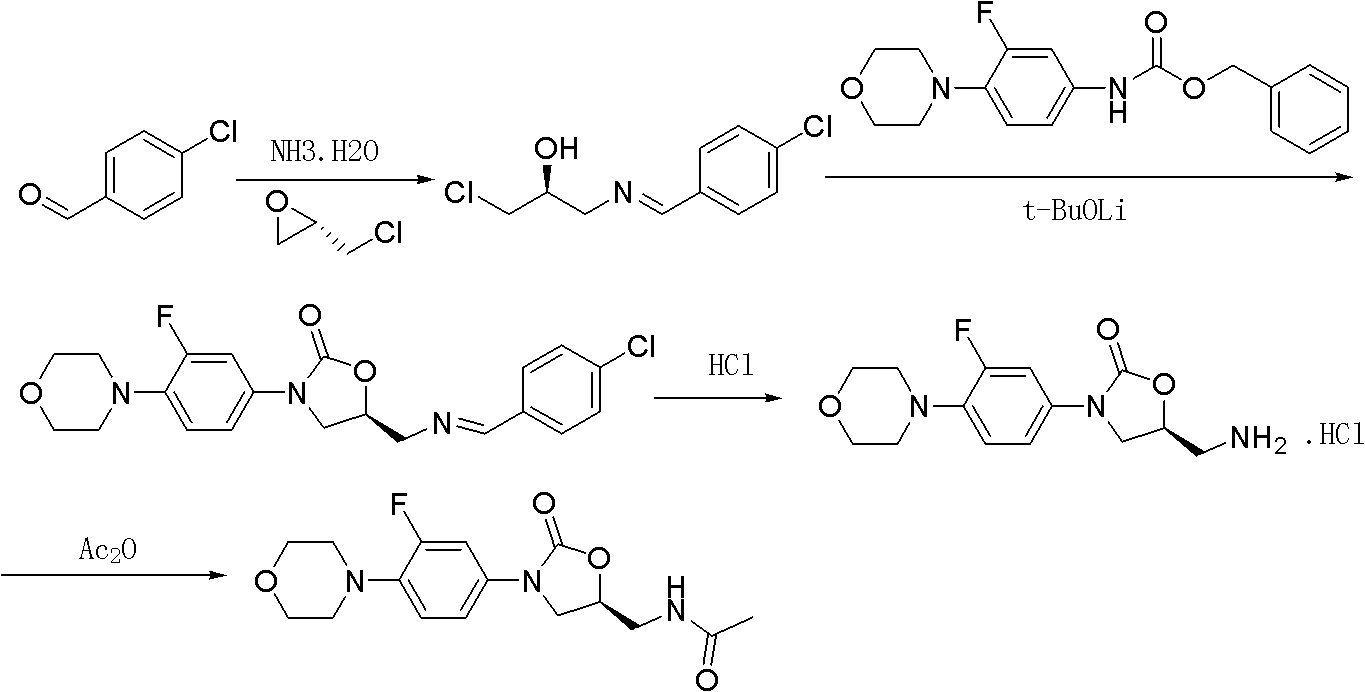
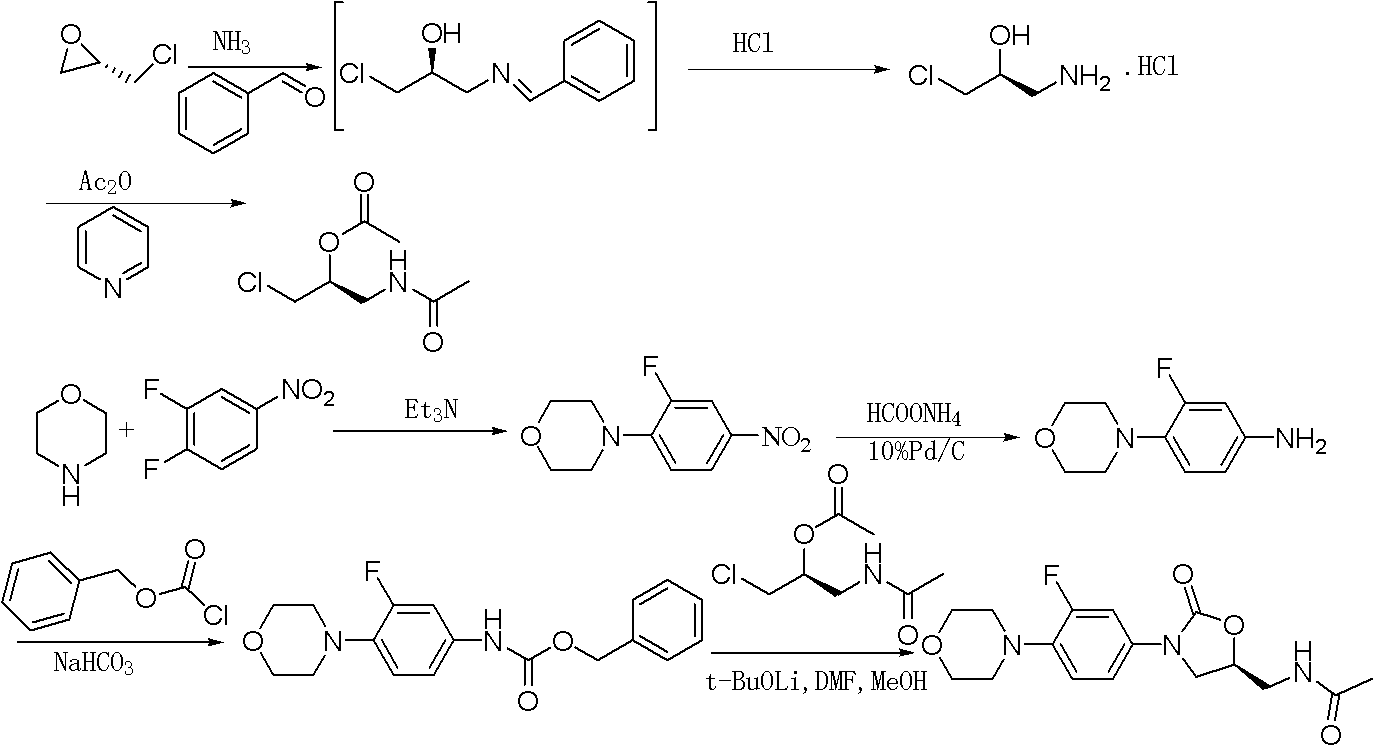
Preparation method for antibacterial drug linezolid
The invention relates to a preparation method for linezolid, which comprises the following steps of: carrying out condensation reaction on N-benzyloxyl hydroxyl-3-fluoro-4-morpholinyl aniline and (S)-N-[2-acetoxyl-3-chloropropyl] acetamide in a solvent and purifying to obtain linezolid, wherein a condensating agent is lithium tert-butoxide, the solvent is a mixed solvent of dimethyl formamide, methanol and dichlormethane in volume ratio of (7-9):(0.8-1.2):(75-85). The preparation method has the advantages of simple operation, shorter reaction time and high yield, and is suitable for industrial production.
Owner:湖北省医药工业研究院有限公司
Intermediates for linezolid and related compounds
The present invention provides a novel process for preparation of 5 aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus, the key intermediate of linezolid is prepared by a) reacting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline with potassium phthalimide; b) subjecting N-[3-pthalimido-2-(R)-hydroxypropyl]-3-fluoro-4-(morpholinyl) aniline produced in the above step to carbonylation; and c) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidiriyl]methyl]phthalimide produced in the above step with hydrazine hydrate to produce (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]amine.
Owner:HETERO USA INC
Process for the preparation of linezolid and related compounds
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
Preparation method of IV crystal linezolid tablets having high drug loading capacity and capable of quickly dissolving
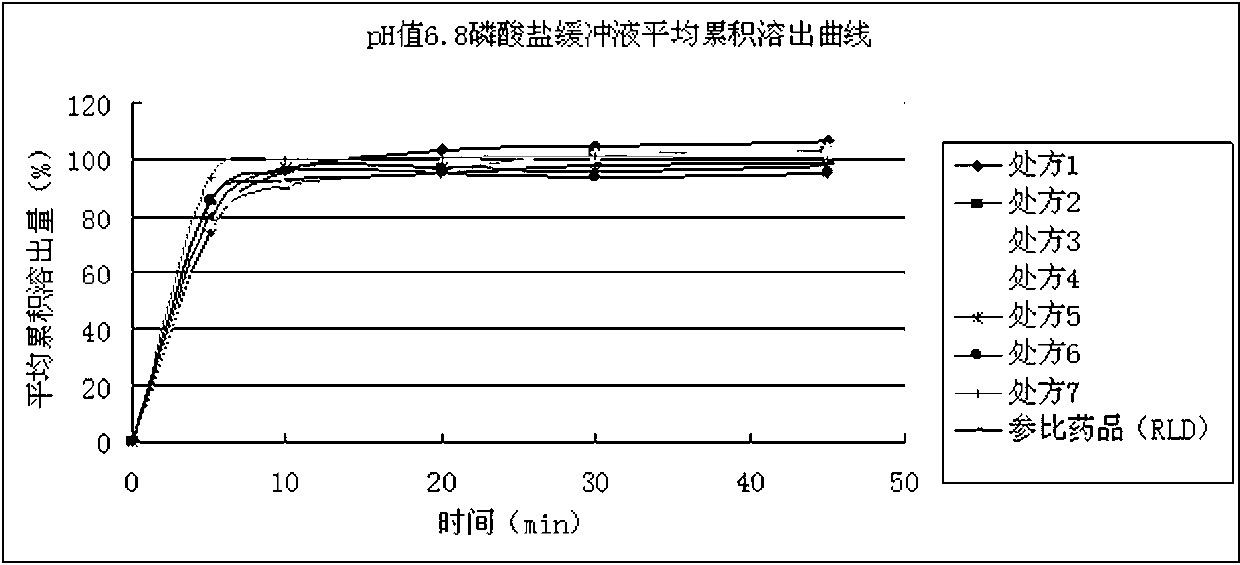
The invention discloses a preparation method of IV crystal linezolid tablets having high drug loading capacity and capable of quickly dissolving. The preparation method comprises the following steps of preprocessing, premixing, mixing, granulating, drying, arranging granulates, centrally controlling, tabletting and coating. According to the preparation method, corn starch and silica are matched with each other, therefore, the tablets with hardness more than 30kg can be quickly cracked, and the in-vitro dissolution similarity ranging from 50 to 100 with respect to a referred product, as well as high drug loading capacity, can be realized.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Evaporation crystallization process for linezolid with crystal form I
The invention discloses an evaporation crystallization process for linezolid with crystal form I. The method comprises the steps of: dissolving 1 weight part of linezolid in 3 to 5 weight parts of alcohol solvents, evaporating 60 to 70 weight percent of alcohol solvents at the temperature of between 70 and 100 DEG C, inducing crystal nuclei of the linezolid with crystal form I in a boiling state,reducing the temperature by 10 to 20 DEG C, adding the evaporated alcohol solvents again, precipitating crystals, filtering, washing a filter cake by using n-heptane, and performing vacuum-drying at the temperature of between 40 and 50 DEG C and under the pressure of -0.08 to -0.09 MPa to obtain the linezolid with crystal form I. In the evaporation process, the using amount of the solvents is small, the evaporation speed is high, evaporated liquor does not contain crystals, and the evaporated liquor can be directly recycled; and the linezolid is not decomposed and deteriorated, yield is high and the quality is good.
Owner:ZHEJIANG LEPU PHARMA CO LTD
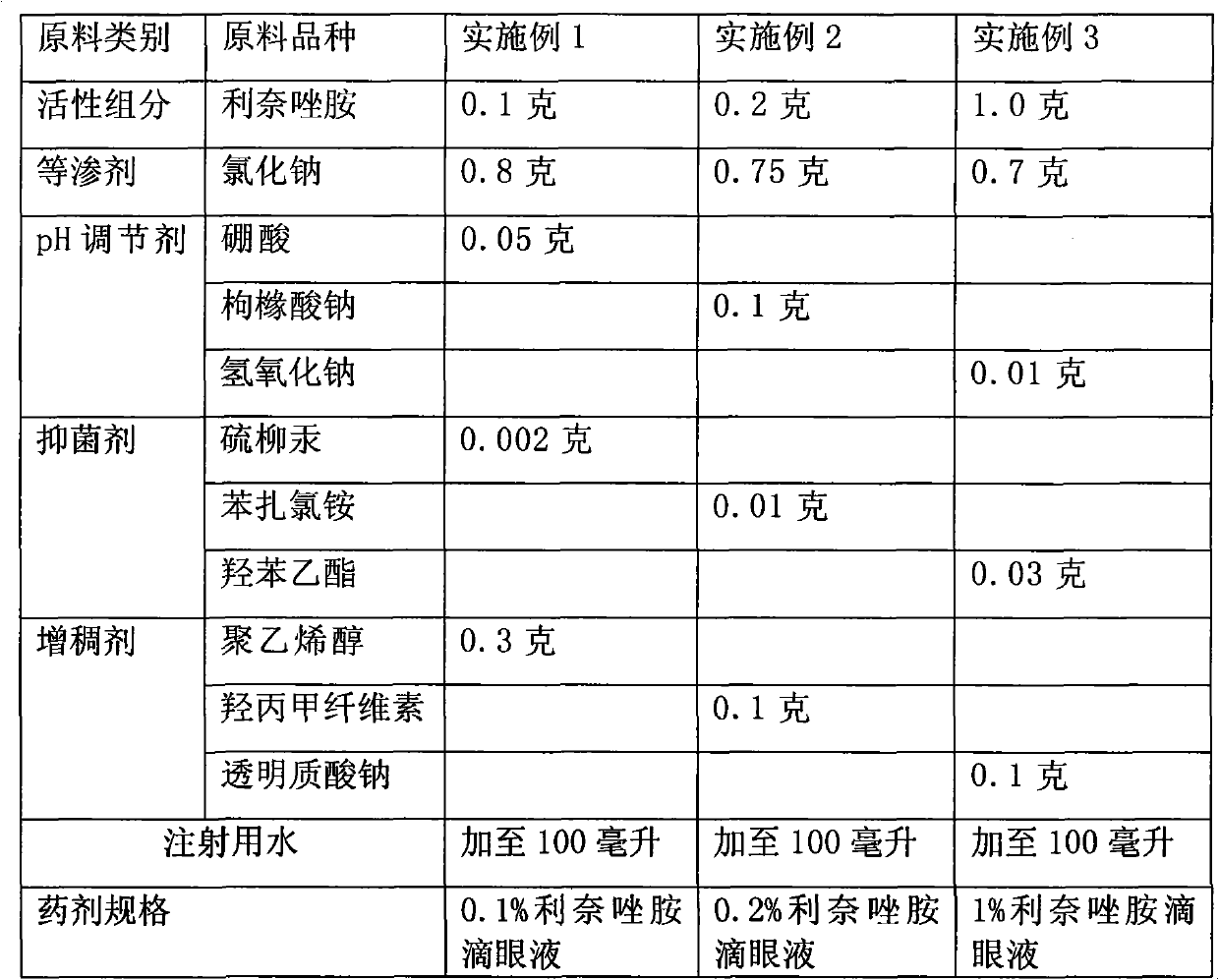
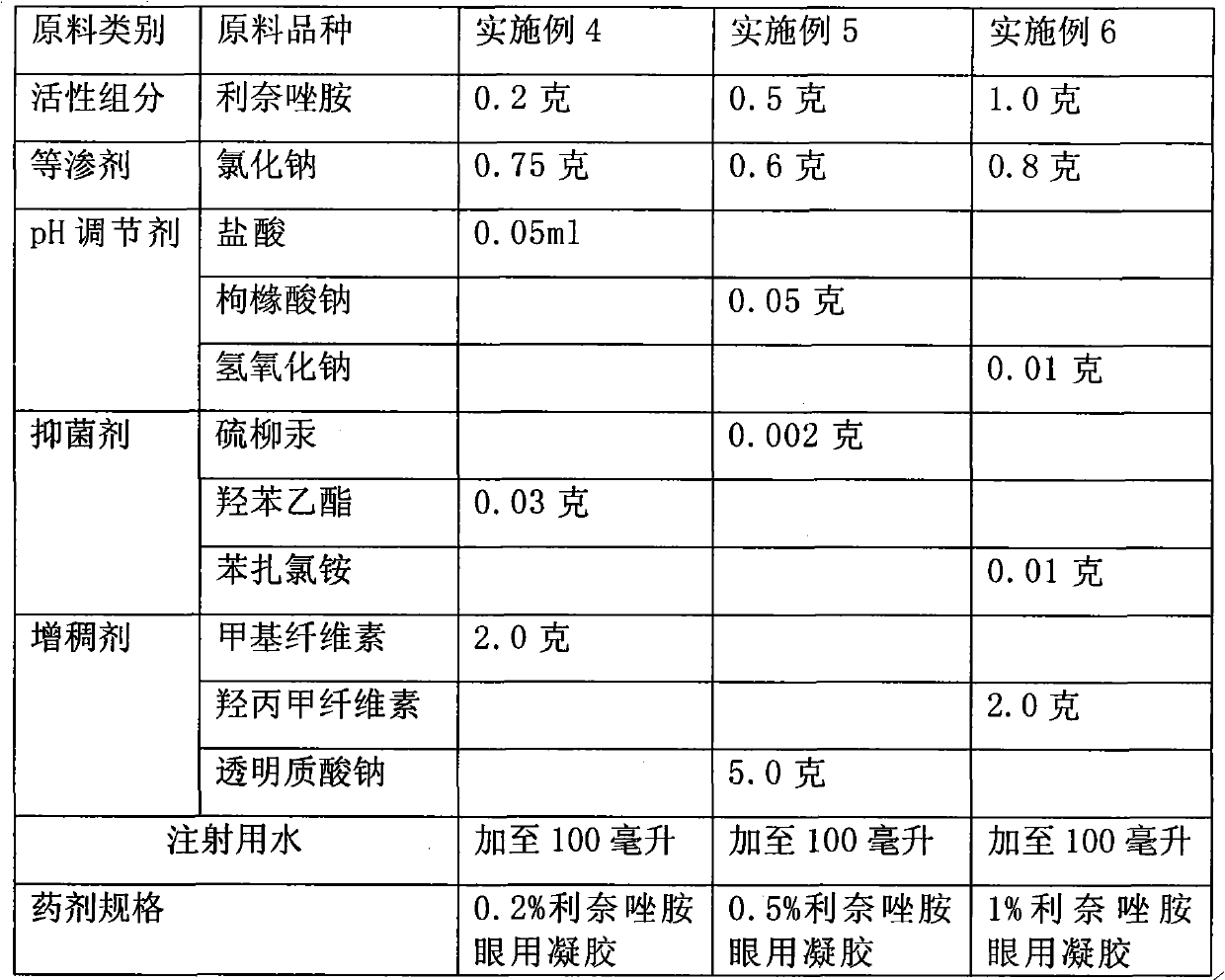
Ophthalmic bacterial-infection resisting medicine for external use
ActiveCN101766628AGood intraocular penetrationInhibit or kill growthAntibacterial agentsSenses disorderDiseaseSide effect
The invention provides ophthalmic bacterial-infection resisting medicine for external use. Linezolid is adopted as medicative raw materials to prepare eye drops, ophthalmic gel and eye ointments. The content of the linezolid in every 100 parts by weight of a medicament is within 0.1 to 1.0 parts by weight. The medicine is for external use at partial positions of eyes, and has good intraocular penetrability and mild toxic as well as side effects; moreover, the medicine is suitable for treating as well as preventing bacterial infection at partial positions of the eyes, including diseases of conjunctivitis, keratitis, keratohelcosis, iritis, ocular traumas, chemical injuries, ophthalmic postoperative infections and the like.
Owner:GUANGDONG WHOLEWIN TECH
Processes for the preparation of linezolid intermediate
The present invention provides improved methods of converting R-N-(4-morpholiyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl azide (III) to the intermediate S-N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine (II) that involve the production of fewer by-products than previous methods. The amine (II) may then be converted into linezolid (I) of high chemical purity with respect to the inactive R-enantiomer and bis-linezolid (IV), and is in high yield, without the need for tedious, complicated purification steps, such as chromatography.
Owner:TEVA PHARMA IND LTD
Method for preparing Linezolid
The invention provides a method for preparing Linezolid. The preparation method has the advantages of few total reaction steps of the synthetic route, high yield, mild reaction of each step of the synthesis, no need of special reagent and device, and simple operation, and is suitable for large scale industrial production. High-purity of Linezolid can be prepared by the method, and medicinal requirements can be satisfied.
Owner:吉林省博大伟业制药有限公司
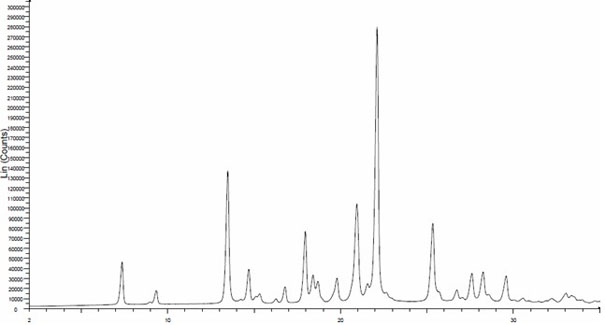
New crystal form of linezolid and preparation method and application thereof
ActiveCN102174027AReduce storage costsSimple preparation processAntibacterial agentsOrganic active ingredientsMorpholinePhenyl group
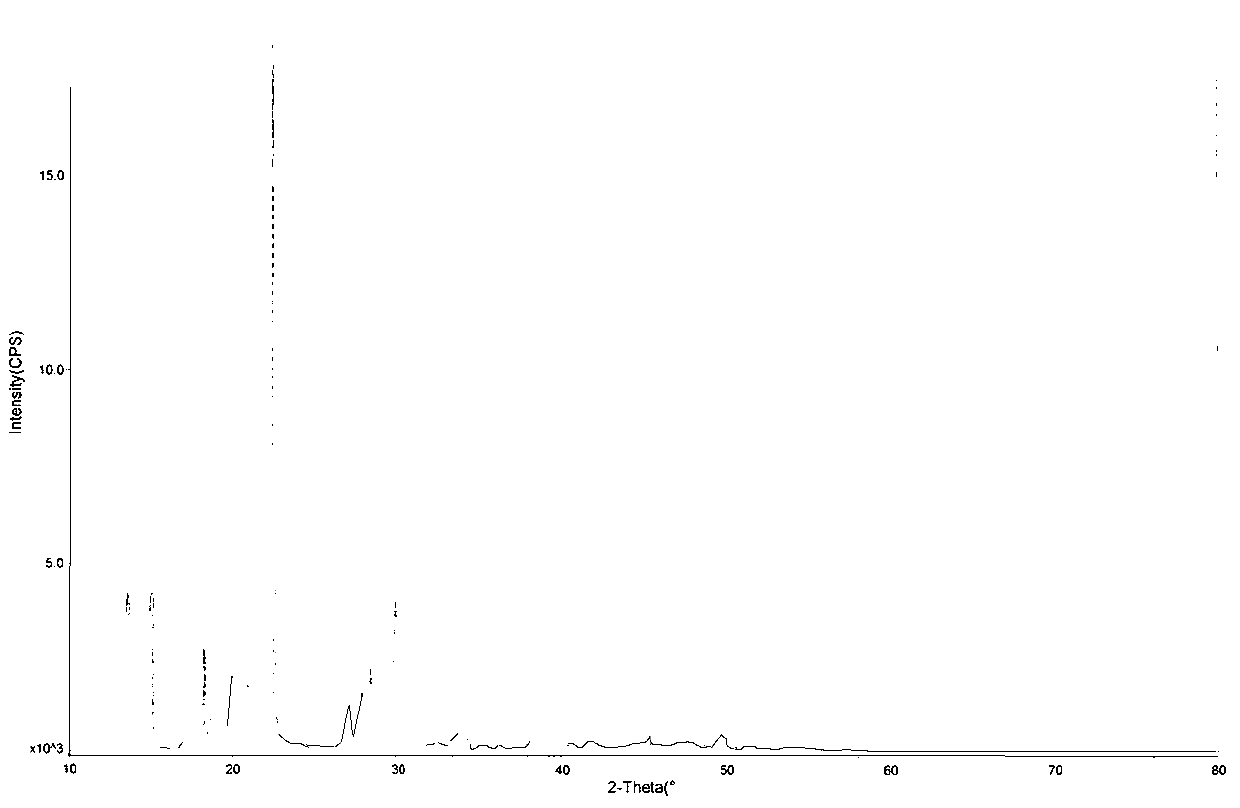
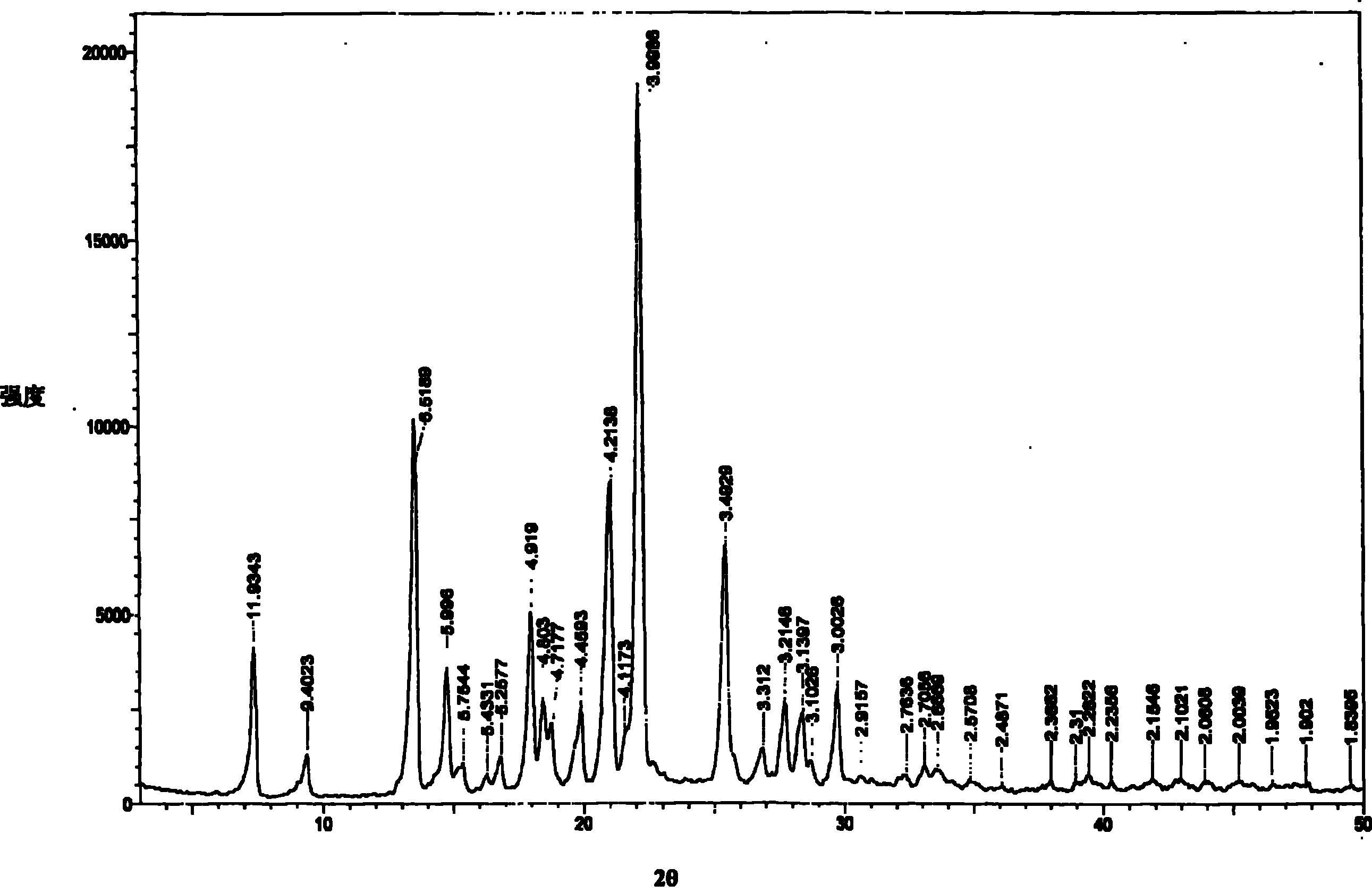
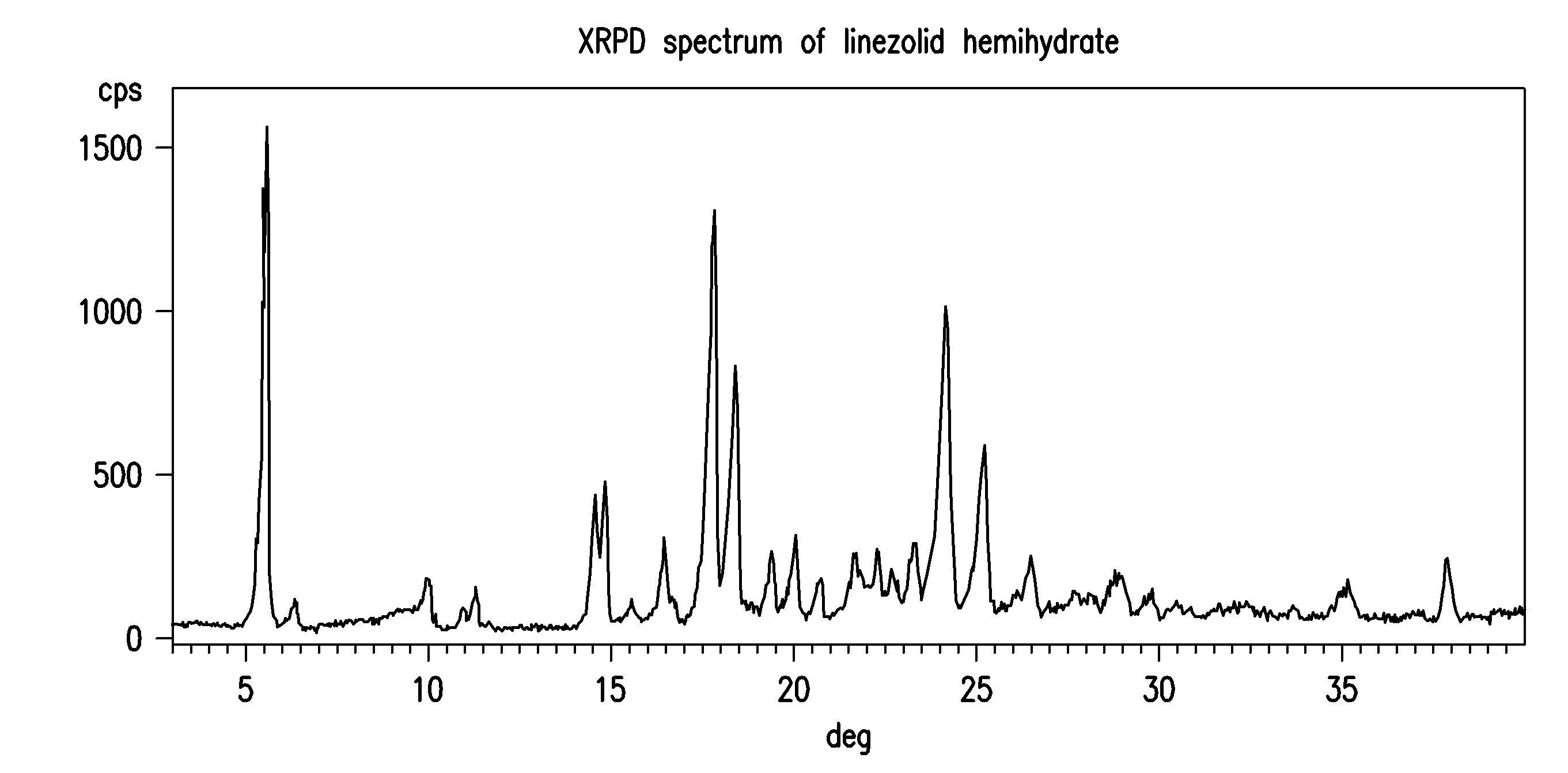
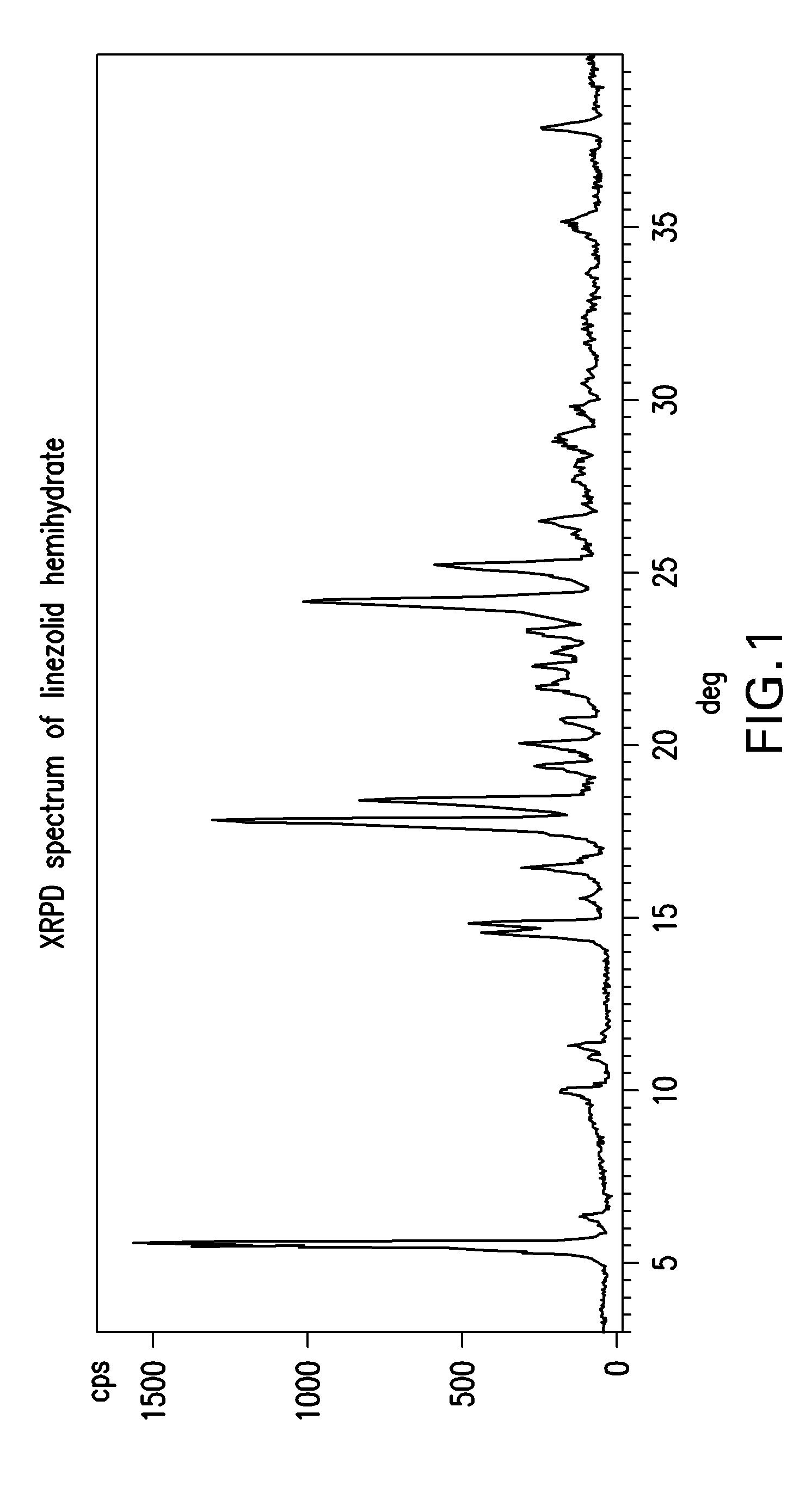
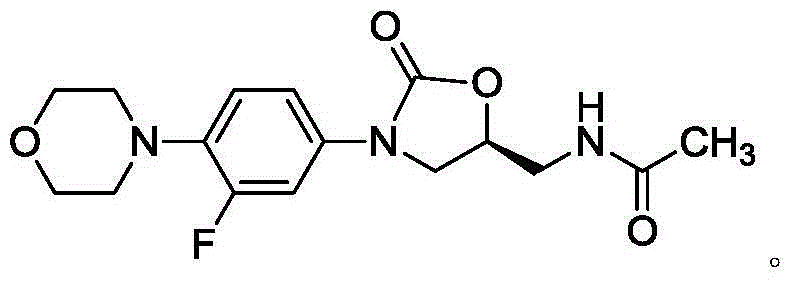
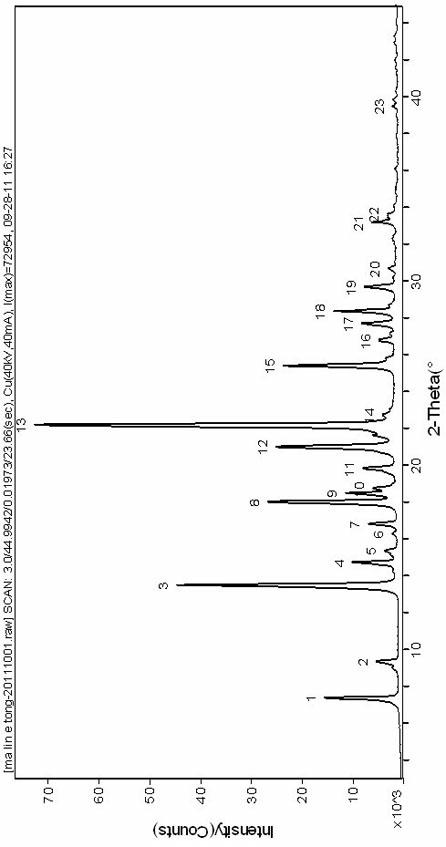
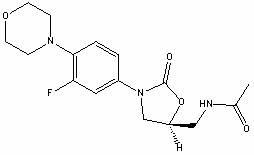
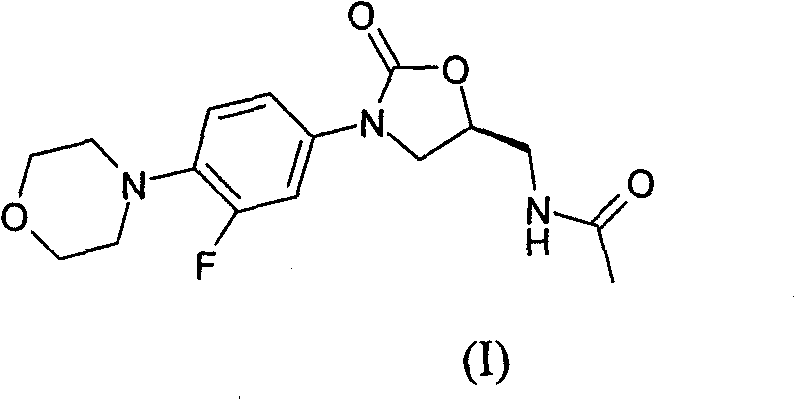
The invention provides a new crystal form V of an oxazolidinone antibacterial, namely (S)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide (linezolid). Characteristic absorption peaks appear on the crystal X-ray powder diffraction pattern of the crystal at the following reflection angle 2 theta: 7.40 degrees, 13.45 degrees, 14.64 degrees, 17.92 degrees, 19.80 degrees, 21.07 degrees, 22.10 degrees, 25.42 degrees, 27.65 degrees, 28.35 degrees and 29.65+ / -0.2 degrees. The invention also provides a preparation method and application of the material in the new crystal form and a pharmaceutical composite containing the material in the crystal form. The material in the crystal form has the advantages of better stability, simple preparation process and lower preparation cost, and the industrial production of the material in the crystal form can be realized.
Owner:四川美大康佳乐药业有限公司
Bacteriophage lysin and antibiotic combinations against gram positive bacteria
ActiveUS20150290299A1Quick killBroad killing activityAntibacterial agentsOrganic active ingredientsLysinLinezolid
The present invention provides compositions and methods for prevention, amelioration and treatment of gram positive bacteria, particularly Staphylococcal bacteria, with combinations of lysin, particularly Streptococcal lysin, particularly the lysin PlySs2, and one or more antibiotic, including daptomycin, vancomycin, oxacillin, linezolid, or related antibiotic(s).
Owner:CONTRAFECT CORP
Tablet containing linezolid crystal form III
ActiveCN103893138AGood compressibilityModerate intensityAntibacterial agentsOrganic active ingredientsLinezolidLactose
The invention relates to a tablet containing a linezolid crystal form III. The tablet contains 67.0-75.0wt% of linezolid, 2.0-4.8wt% of lactose, 9.0-18.0wt% of microcrystalline cellulose as well as disintegrating agent, binding agent, lubricant and other medicinal excipients, wherein preferably, the tablet contains 67.0-75.0wt% of linezolid, 2.0-4.8wt% of lactose, 9.0-18.0wt% of microcrystalline cellulose, 2.0-10.0wt% of disintegrating agent, 1.2-4.0wt% of binding agent and 0.3-2.0wt% of lubricant. The tablet containing the linezolid crystal form III of the prescription, in an extremely limited additive adding range, guarantees characteristics of good compressibility, excellent formability and rapid dissolution.
Owner:CHENGDU GUOHONG PHARMA
Use of oxazolidinone compound for resisting biofilm
ActiveCN104873510APrevent or inhibit the formation ofDecreases the amount of extracellular matrixAntibacterial agentsOrganic active ingredientsBiofilmDisinfectant
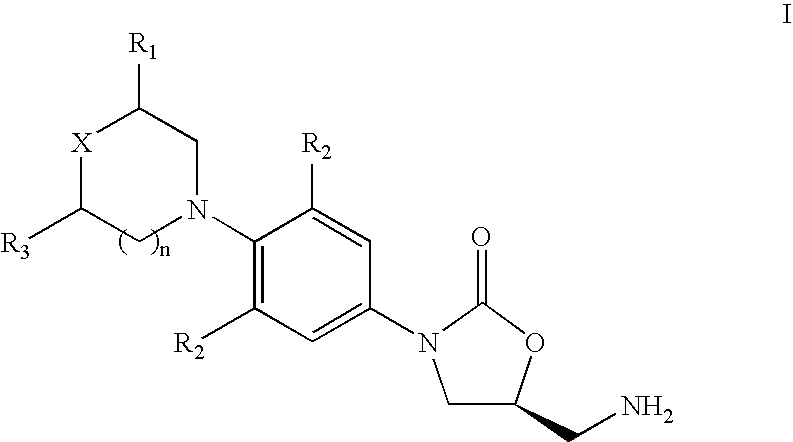
The invention provides a use of an oxazolidinone compound shown in the formula I in preparation of a drug, a disinfectant or a daily chemical article for preventing or inhibiting bacterial biomembrane formation or removing a bacterial biofilm. The oxazolidinone compound can effectively prevent or inhibit bacterial biofilm formation or removing a bacterial biofilm, can effectively reduce biomass and viable count of a biofilm, has biofilm resistance activity obviously superior to that of a classical drug linezolid, and provides a better choice for treatment or prevention of biofilm-caused infection.
Owner:SICHUAN GOODDOCTOR PHARMA GRP +1
Preparation method of linezolid
The invention provides a preparation method of linezolid which is an antimicrobial medicament with a brand-new structure and has an obvious effect in the aspects of treating drug-resistant gram-positive bacteria and Mycobacterium tuberculosis infections. The invention relates to a synthesis method of linezolid. The method comprises the following steps: carrying out a reaction on S-epichlorohydrinand sodium azide to obtain 1-azido-3-chloro-2-propanol; cyclizing 1-azido-3-chloro-2-propanol with N-(3-fluoro-4-morpholinophenyl) urethane; and finally, reducing and acetylizing to obtain the linezolid. The method provided by the invention has the advantages of cheap-price and available raw materials, simple technology process and high product yield, provides a new way for industrial production of linezolid.
Owner:CHINA RESOURCES SAIKE PHARMA
A kind of preparation method of linezolid
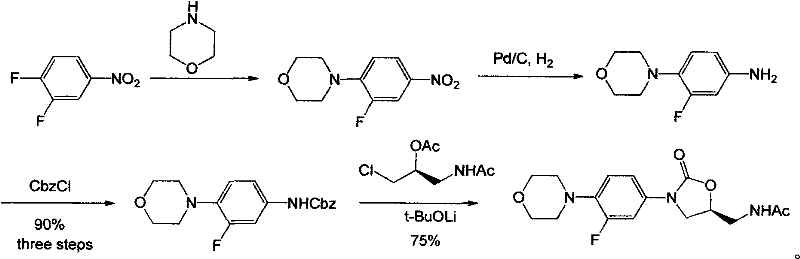
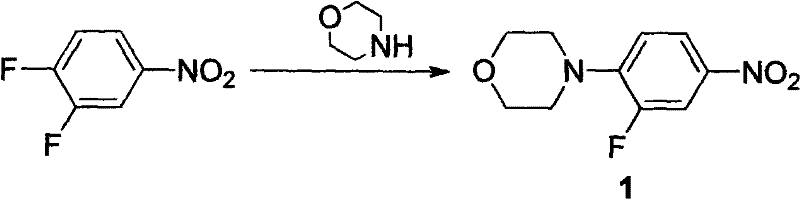
The invention discloses a preparation method of linezolid. The method comprises reacting 3,4-difluoronitrobenzene to generate 3-fluoro-4-morpholine nitrobenzene, and then adding a small amount of catalyst Pd / C, In the case of hydrogen, 3-fluoro-4-morpholine aniline is obtained, which does not undergo separation and purification on a silica gel column, and reacts to generate N-Cbz-3-fluoro-4-morpholine aniline, and then reacts with the intermediate 3-chloro- 2-Acetoxyacetylpropylamide reacts to form linezolid. The preparation method of the invention not only reduces numerous purification steps in the reaction, but also reduces the amount of catalyst used, thereby making the preparation of linezolid more effective and more environmentally friendly.
Owner:SHANDONG LUKANG PHARMA
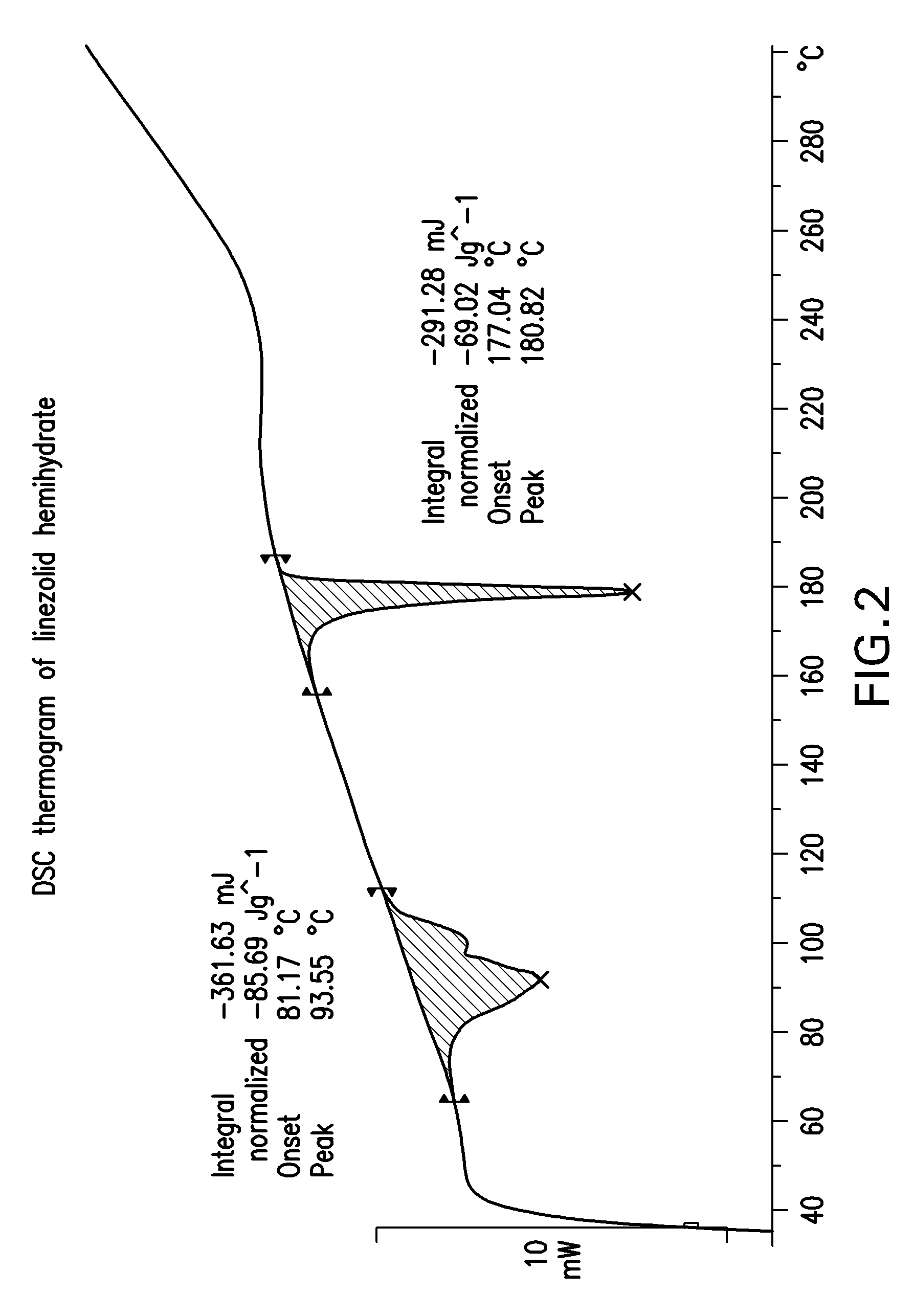
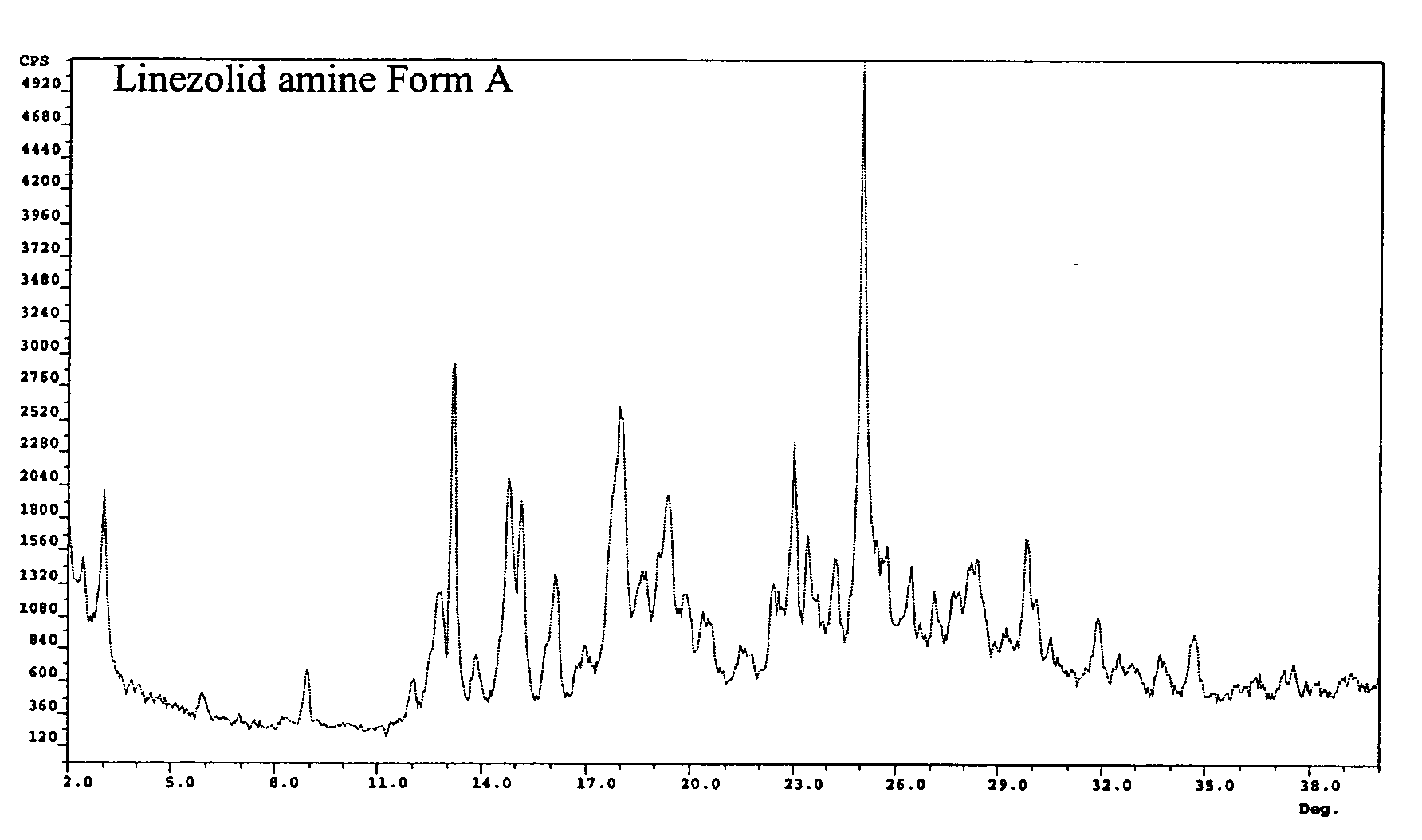
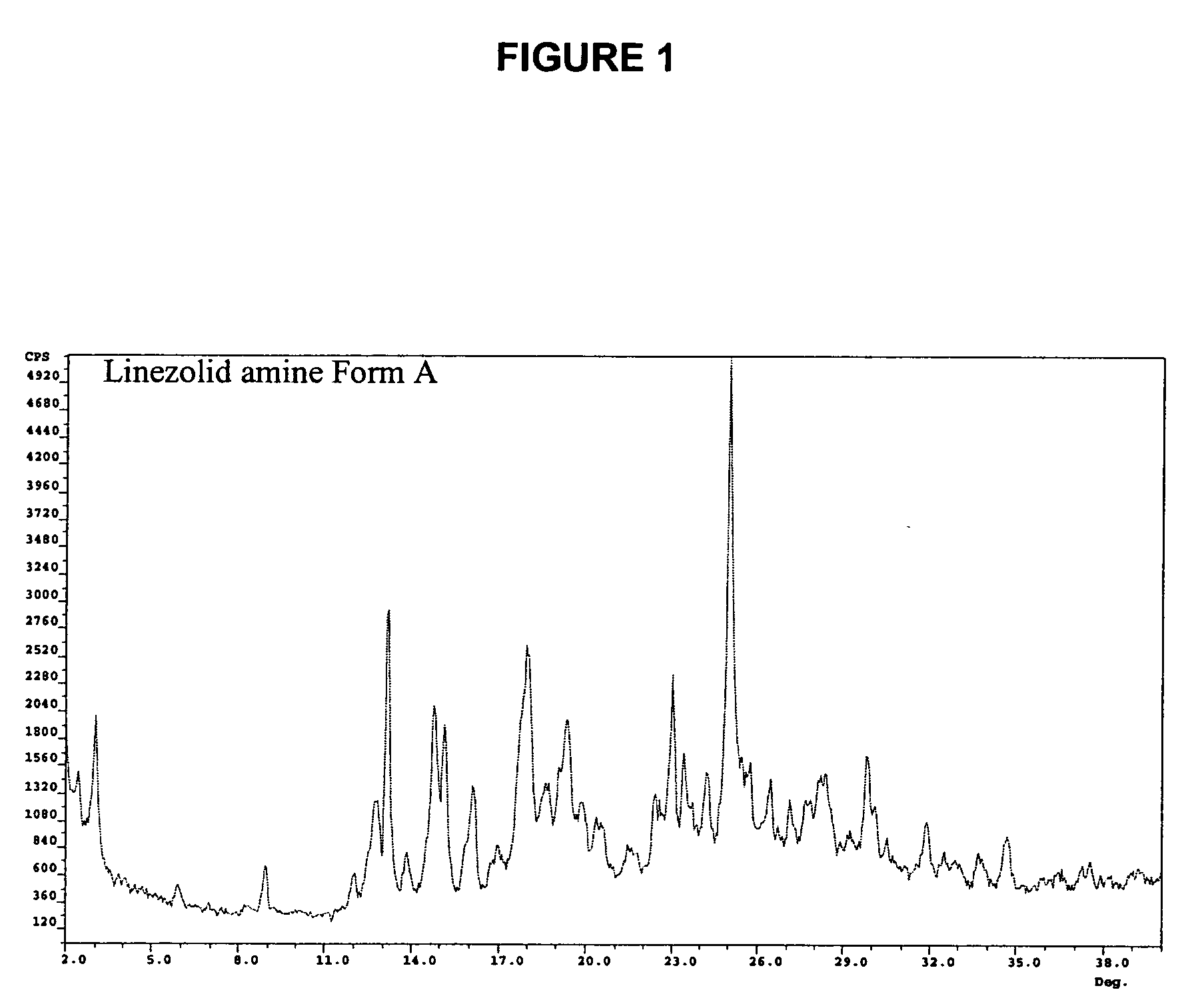
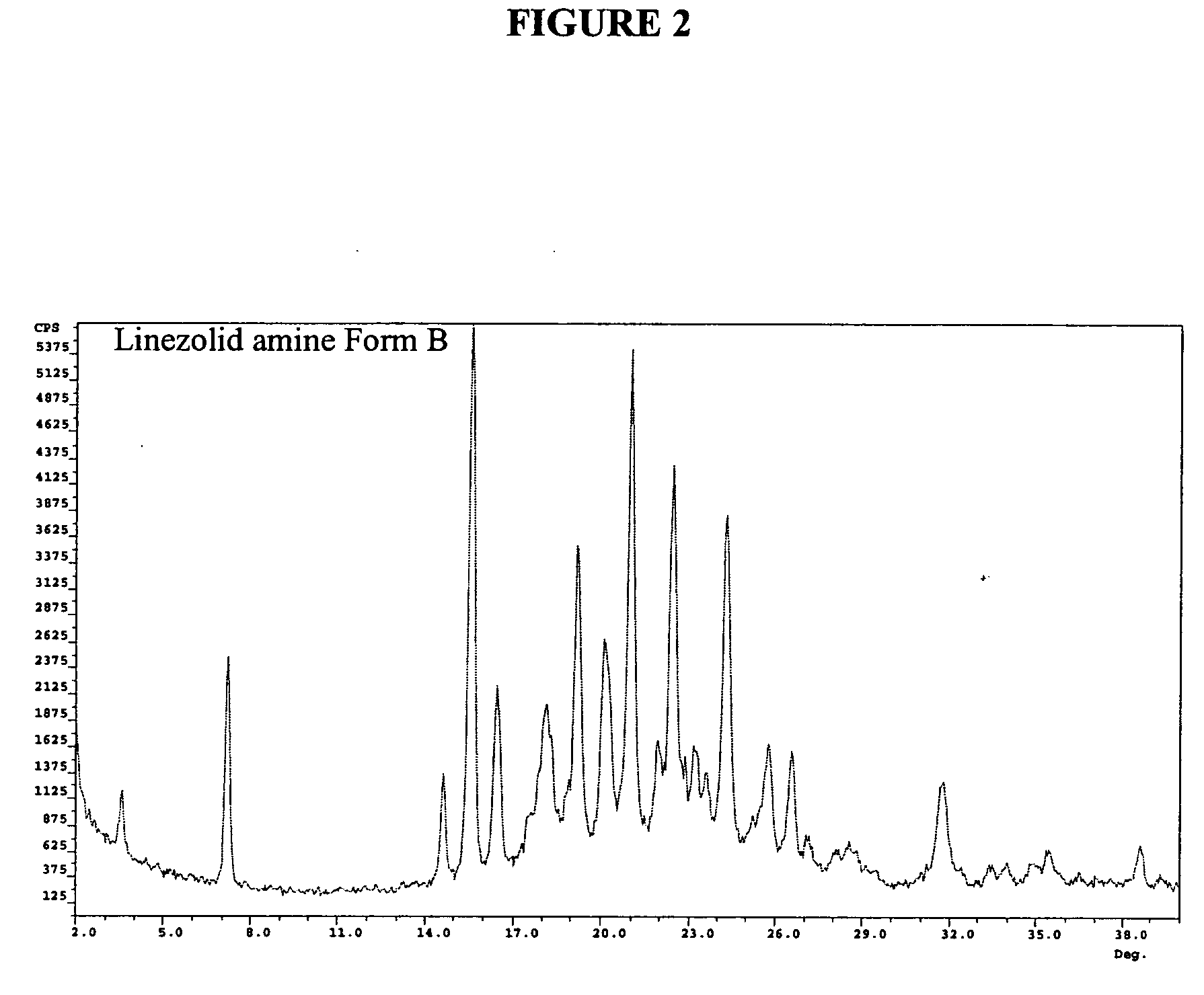
Crystalline forms of linezolid intermediate
The present invention relates to novel crystalline forms of the linezolid intermediate S—N-(4-morpholinyl-3-fluorophenyl)-2-oxo-5-oxazolidinyl-methyl amine referred to herein as Form A, Form B, and Form C.
Owner:TEVA PHARM USA INC
Linezolid-containing anti-infection pharmaceutical composition and preparation method thereof
The invention discloses a linezolid-containing anti-infection pharmaceutical composition and a preparation method thereof. The pharmaceutical composition contains 1.0-5.0% (g / ml) of linezolid and is composed of an ethosome drug-loading system and a hydrogel system; and the pharmaceutical composition can be prepared into a linezolid-ethosome gel spray, and can be externally applied to anti-infection treatment.
Owner:YABANG PHARMA +1
Suspension crystallization method for preparing crystal form I of linezolid
The invention discloses a suspension crystallization method for preparing a crystal form I of linezolid. The method comprises the following steps of: mixing 1 to 10 weight part of water and 1 weight part of linezolid, heating to the temperature of between 95 and 105DEG C to obtain suspension, crystallizing at the temperature of between 70 and 100DEG C, filtering at the temperature of between 0 and 90DEG C, and performing vacuum drying on a filter cake at the temperature of between 30 and 90DEG C to obtain the crystal form I of the linezolid. The method has the advantages that: water is taken as a suspension solvent, organic solvents are not used at all, the method is green, safe, environment-friendly and pollution-free, the product yield is high, the filter cake is not required to be washed, the filtered mother solution can be repeatedly used, the volume of the mother solution cannot be increased due to reuse, the energy consumption in the production process is obviously reduced, and clean production can be realized.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Method for preparing linezolid
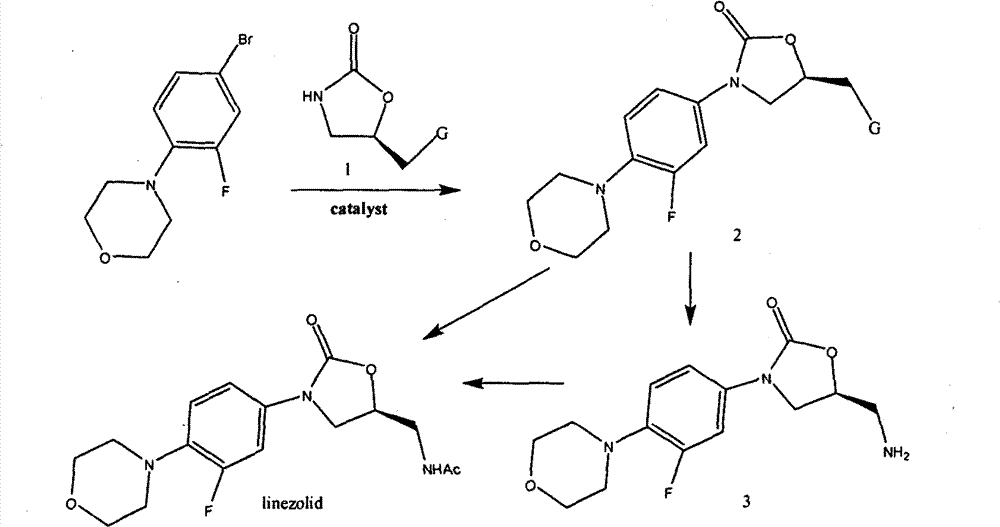
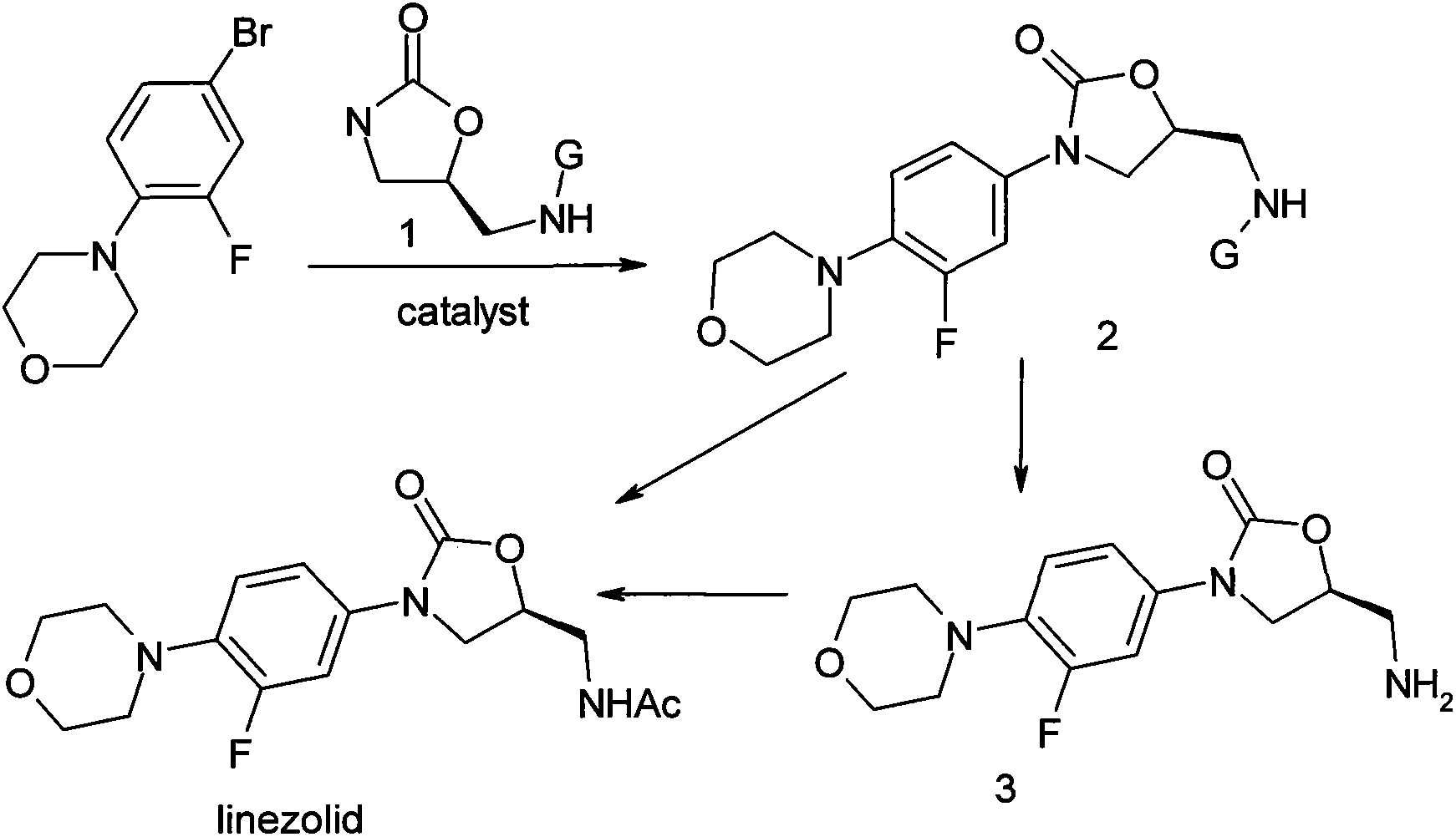
The invention relates to a method for preparing linezolid. The method comprises the following steps of: reacting 3-fluorine-4-morpholinyl bromobenzene used as a raw material, with a chiral oxazolidinone compound in the presence of a catalyst, so as to directly obtain the linezolid, or to obtain the linezolid after conversion. Compared with the existing method for synthesizing linezolid, the method disclosed by the invention is simple in route, convenient to operate, slight in pollution and stable in yield.
Owner:CHONGQING MEDICAL UNIVERSITY
Method for preparing linezolid
InactiveCN102850288AThe synthesis method is simpleFew reaction stepsOrganic chemistryLinezolidRaw material
The invention relates to a method for preparing linezolid. The method is characterized by reacting 3-fluoro-4-morpholinyl bromobenzene as raw material with a chiral oxazolidinone compound under the action of a catalyst to obtain linezolid. Compared with the prior art, the inventive method has the advantages of simple synthesis path, simple operation, less pollution and stable yield.
Owner:袁建勇 +1
Porphyromonas gingivalis culture medium for tartar specimens
InactiveCN103789390AImprove separation rateReliable detectionMicrobiological testing/measurementPorphyromonas gingivalisHemin
The invention discloses a Porphyromonas gingivalis culture medium for tartar specimens and a preparation method thereof. The invention is characterized in that every 1000ml of culture medium contains malt extract, egg white, hemin solution, vitamin K1 ethanol solution, magnesium citrate, agar, synercid, telithromycin, linezolid, asparagine and the balance of distilled water. The preparation method of the optimal formula comprises the following steps: dissolving the malt extract, egg white, hemin, agar, magnesium citrate, calcium citrate tetrahydrate, synercid, garlic press juice and L-cysteine in a rhizoma polygonati boiling solution, evenly mixing, adding water to the constant volume, sterilizing and packaging. Compared with the prior art, the culture medium has the characteristics of high detection reliability and high Porphyromonas gingivalis separation rate.
Owner:武改莲
Novel antimicrobials
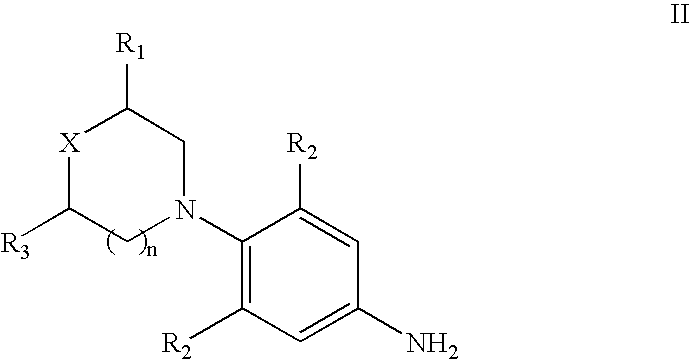
InactiveUS20110245258A1Management such as prophylaxis and amelioration and treatmentAntibacterial agentsBiocideMetaboliteMulti drug resistant bacteria
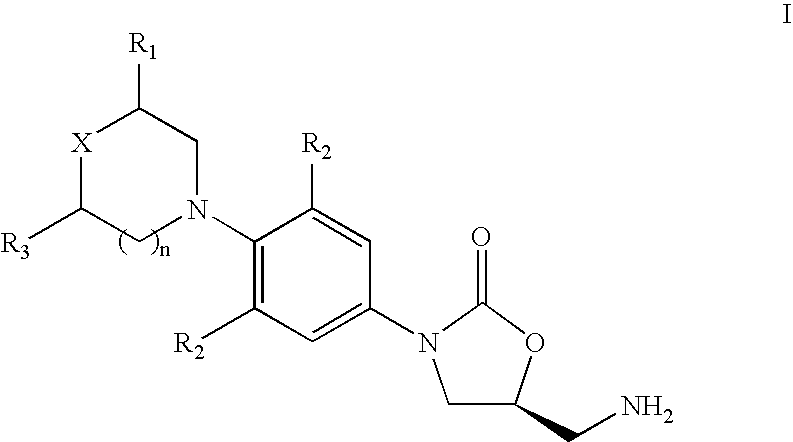
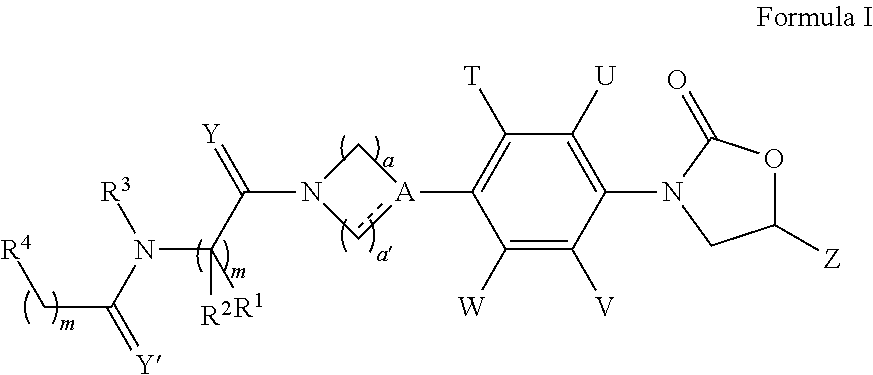
The present invention relates to novel phenyl oxazolidinone compounds of formula I, their pharmaceutically acceptable analogs, tautomeric forms, stereoisomers, polymorphs, prodrugs, metabolites, salts or solvates thereof. The invention also relates to the processes for the synthesis of novel compounds of formula I or their pharmaceutically acceptable analogs, tautomeric forms, stereoisomers, polymorphs, prodrugs, metabolites, salts or solvates thereof. The present invention also provides pharmaceutical compositions comprising novel compounds of formula I and methods of using them. The compounds of the present invention are useful as antimicrobial agents, effective against a number of aerobic and / or anaerobic Gram positive and / or Gram negative pathogens such as multi drug resistant species of Staphylococcus, Streptococcus, Enterococcus, Bacterioides, Clostridia, H. influenza, Moraxella, acid-fast organisms such as Mycobacterium tuberculosis as well as Linezolid resistant species of Staphylococcus and Enterococcus.
Owner:PANACEA BIOTEC
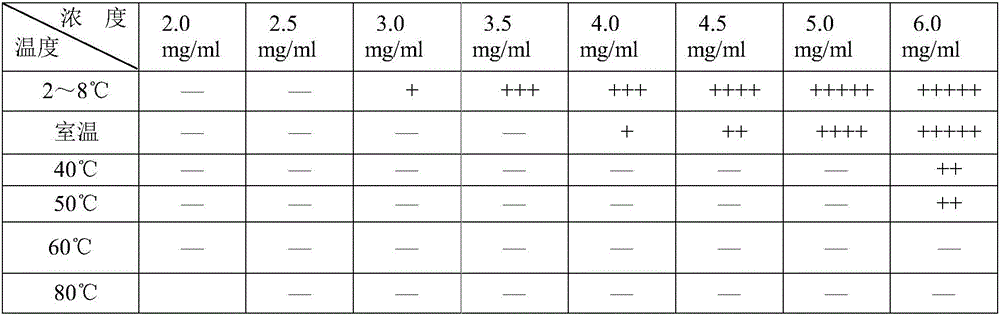
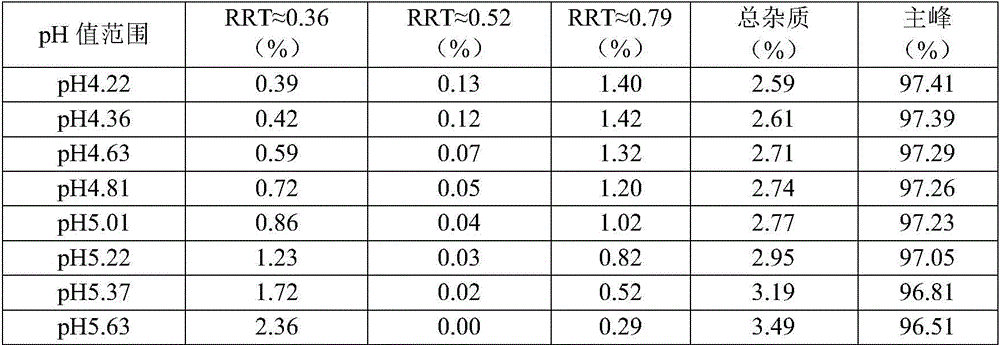
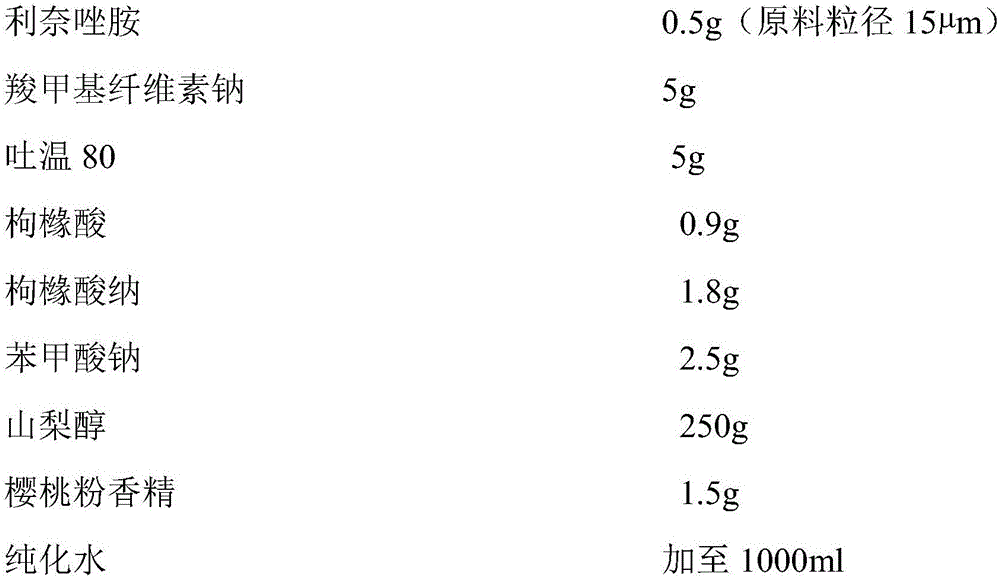
Linezolid oral suspension and preparation method theroef
ActiveCN105853351AEffective anti-corrosion effectGood physical and chemical compatibilityAntibacterial agentsOrganic active ingredientsCarboxymethyl cellulosePreservative
The invention belongs to the field of pharmaceutic preparation, and specifically a linezolid oral suspension and a preparation method thereof. The linezolid oral suspension comprises the following components by weight: 0.5-2.0 parts of linezolid, 0.5-5.0 parts of sodium carboxymethyl cellulose, 0.05-0.5 part of polysorbate 80, 0.15-0.20 part of sodium citrate, 0.08-0.10 part of citric acid, 0.22-0.28 part of a preservative, 22-28 parts of sweetener, 1.2-1.8 parts of a correctant and an aqueous medium. The present invention fills the market blank of only containing tablet or injection, and provides a novel dosage form choice. The linezolid oral suspension product has good quality, can be accurately divided into the dosage based on body weight, and is easy to swallow. The preparation process is simple, feasible and reproducible, and can consistently produce linezolid oral suspension with quality meeting the requirements.
Owner:CHONGQING HUAPONT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com