Method for preparing Linezolid
A technology of linezolid and carbonyldiimidazole, applied in the synthesis and purification of intermediates, and the field of preparation of linezolid, which can solve the problems of difficult to meet the requirements of medicine, complex reaction mechanism, and incomplete reaction, etc., and achieve fewer reaction steps , high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 3-fluoro-4-morpholine aniline
[0042]
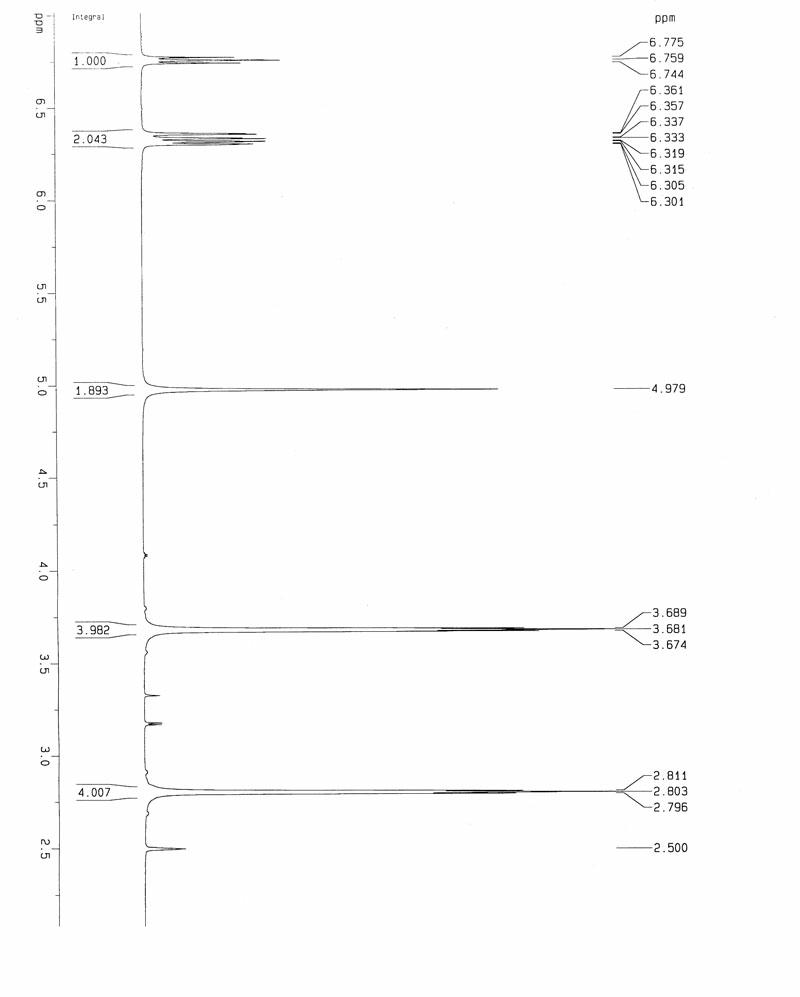
[0043] Dissolve 40.23 grams of 3-fluoro-4-morpholine nitrobenzene in 100 milliliters of tetrahydrofuran and 400 milliliters of methanol containing 44.28 grams of ammonium formate; % palladium carbon, the reaction system was protected with nitrogen, and the reaction was stirred for 2 hours. After the reaction is complete, the reaction liquid is filtered, and the filter cake is washed successively with 40 ml of tetrahydrofuran and 80 ml of ethyl acetate. Extract with 2×100 ml of ethyl acetate, combine the organic phases, wash with 300 ml of saturated brine; dry over anhydrous magnesium sulfate, filter out the desiccant, and evaporate to dryness to obtain 30.41 g of brown product, yield: 87%. 1 H NMR (DMSO-d 6 ,600MHz): 6.77(t,1H) 6.36(m,2H) 4.98(s,2H) 3.69(br s,4H) 2. 81(br s,4H), see figure 1 .
[0044] (S)-3-Chloro-2-acetoxyacetylpropylamide
[0045]
[0046] Suspend and dissolve 100.23 g of (S)-3-chloro-2-hydroxypro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com