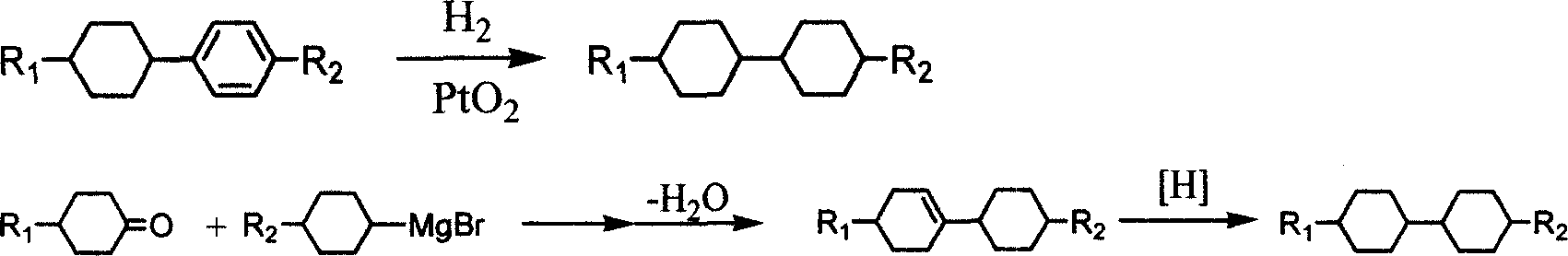
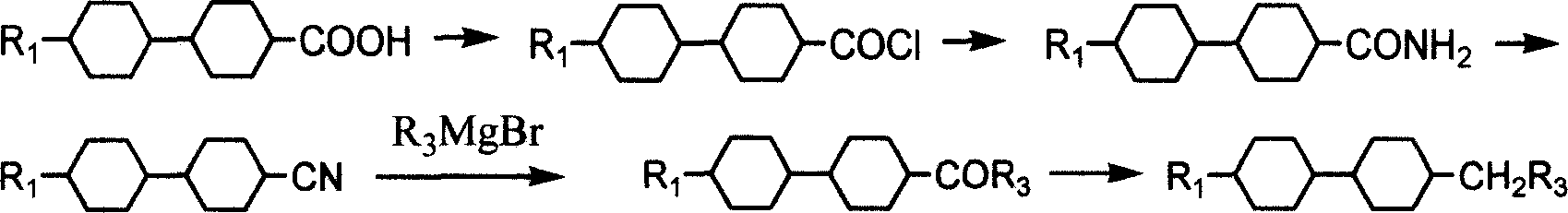
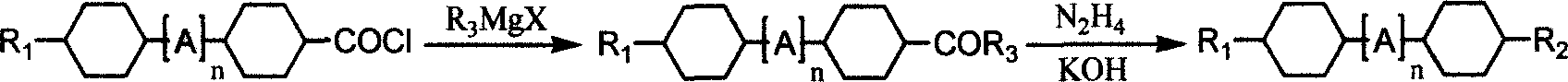Method for preparing bis cyclohexane monomer liquid crystal using grignard reaction
A bicyclohexane and Grignard reaction technology, which is applied to liquid crystal materials, chemical instruments and methods, and hydrocarbon production from oxygen-containing organic compounds, can solve problems such as rising raw material costs and production costs, and declining product yields. Effects of synthesis cycle, reduction of reaction steps, and reduction of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (Trans, trans)-4-ethyl-4'-propyl-1,1'-bicyclohexane [structural formula: ] preparation
[0025] Put 128g (0.5mol) (trans, trans)-4-(4'-ethylcyclohexyl)cyclohexylformyl chloride and 400ml of toluene into a 2-liter reaction flask, drop the temperature in a water bath, and add 0.5mol dropwise to keep the internal temperature at 20°C C 3 h 7 The 500ml THF solution of MgBr was incubated at 20°C for 2 hours after the dropwise addition, then 700ml of water and 20ml of concentrated hydrochloric acid were added, the organic phase was separated, washed 3 times with water until neutral, and the toluene was removed to obtain 133g of white solid. Transfer to a 2-liter three-necked flask, add 640ml of diethylene glycol, 98g of potassium hydroxide, and 125g of hydrazine hydrate, raise the temperature to 130°C and keep it for 3 hours, then raise the temperature and distill to 200°C and keep it for 3 hours, cool down to below 100°C, add 400ml of toluene and 700ml of water, stirred f...
Embodiment 2
[0029] (Trans, trans)-4-propyl-4'-propyl-1,1'-bicyclohexane [structural formula: ] preparation
[0030] Put 135g (0.5mol) (trans, trans)-4-(4'-propylcyclohexyl)cyclohexylformyl chloride and 400ml of toluene into a 2-liter reaction flask, cool down in a water bath, and add 0.5mol dropwise to keep the internal temperature at 20°C C 3 h 7 The 500ml THF solution of MgBr, after the dropwise addition, was incubated at 20°C for 2 hours, then added 700ml water and 20ml concentrated hydrochloric acid, separated the organic phase, washed 3 times with water until neutral, and removed toluene to obtain 140g white solid, which was transferred to 2 In the there-necked flask, add 600ml of diethylene glycol, 92g of potassium hydroxide, and 125g of hydrazine hydrate, raise the temperature to 130°C and keep it for 3 hours, then raise the temperature and distill to 200°C and keep it for 3 hours, cool down to below 100°C, add 400ml of toluene and 700ml of water , stirred for 10 minutes, left ...
Embodiment 3
[0035] Operation is the same as in Example 2, using trans-4-(2-(trans-4'-ethylcyclohexyl) ethyl) cyclohexylformyl chloride and C 4 h 9 MgBr can make 1-(trans-4-ethylcyclohexyl)-2-(trans-4'-pentylcyclohexyl)ethane [structural formula: ]. GC: 99.9%, mp: 2.5°C, cp: 76°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com