Patents
Literature
45results about How to "Meet medicinal requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing Linezolid
The invention provides a method for preparing Linezolid. The preparation method has the advantages of few total reaction steps of the synthetic route, high yield, mild reaction of each step of the synthesis, no need of special reagent and device, and simple operation, and is suitable for large scale industrial production. High-purity of Linezolid can be prepared by the method, and medicinal requirements can be satisfied.
Owner:吉林省博大伟业制药有限公司
Crystalline form of androgen receptor inhibitor and preparation method thereof
InactiveCN106518773AMeet the limit requirementsImprove stabilityOrganic active ingredientsOrganic chemistrySolventPhenyl group
The invention relates to a crystalline form of an androgen receptor inhibitor and a preparation method thereof, in particular to a type II crystal of (S)-4-(3-(4-(2,3-hydroxypropoxy)phenyl)-4,4-dimethyl-5-carbonyl-2-trifluoromethyl-1-yl)-2-(trifluoromethyl)benzonitrile (a compound shown as a formula (I)) and a preparation method thereof. The method comprises the following steps: 1) adding the compound shown as the formula (I) of any crystalline form or an amorphous form into a proper amount of organic solvent, and crystalizing after dissolved clarification, wherein the solvent is selected from methanol or ethanol; 2) filtering the crystal, washing and drying. The type II crystal of the compound shown as the formula (II) has the advantages of excellent chemical stability and crystalline form stability, and the used crystal solvent has low toxicity and low residue, and can be better applied to clinical therapy. The formula is shown in the description.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Preparation method of mitiglinide calcium
The invention relates to a preparation method of a bulk drug of mitiglinide calcium used for treating type 2 diabetes mellitus. The mitiglinide calcium is prepared by a series of reaction steps comprising using D-phenylalanine as a starting material, esterification and cis-hexahydroisoindoline reaction. In the reaction, the problem of high cost of special equipment for high pressure hydrogenation and operation as well as chiral catalyst in the traditional process can be solved, the use of chiral resolution and a resolving agent is avoided, and the yield is improved.
Owner:DISHA PHARMA GRP +1
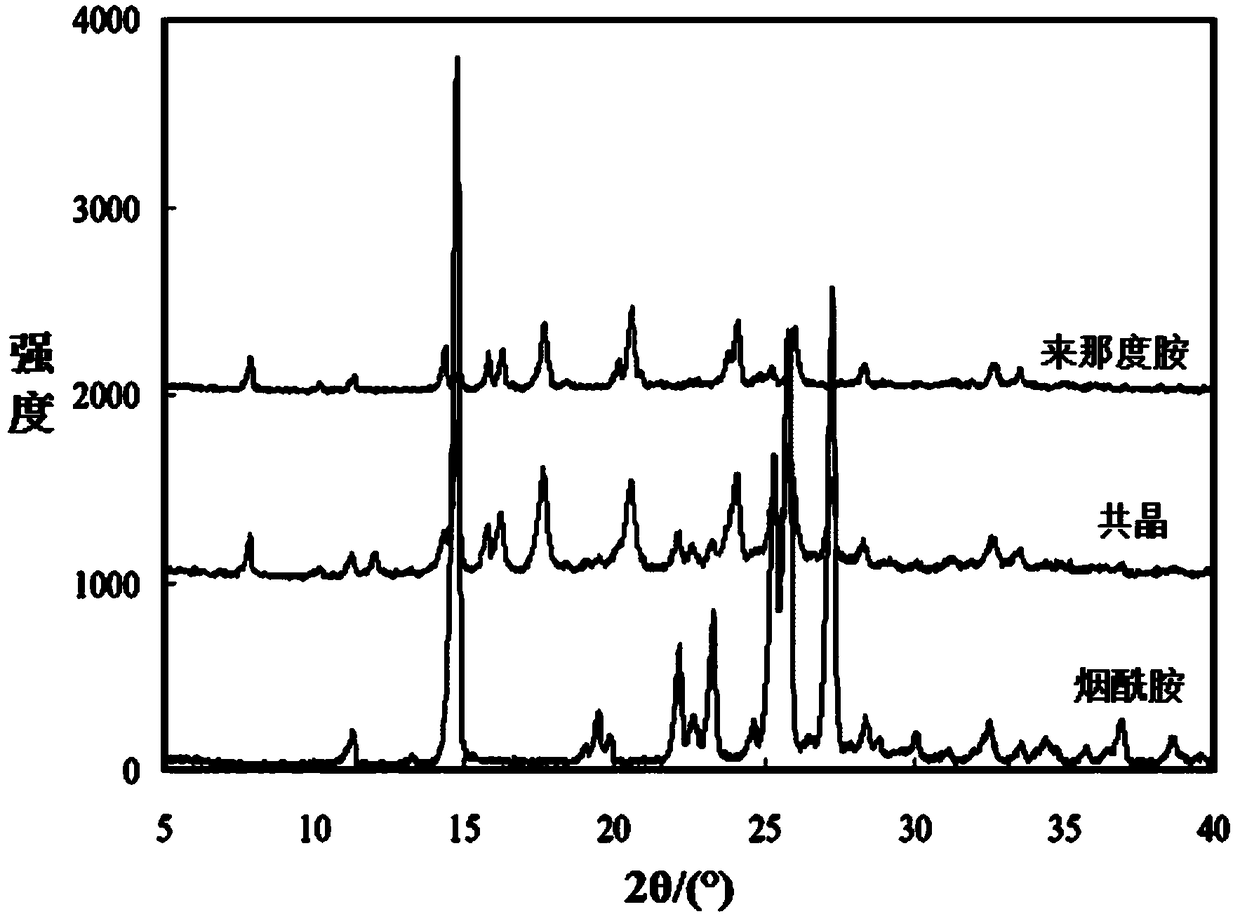
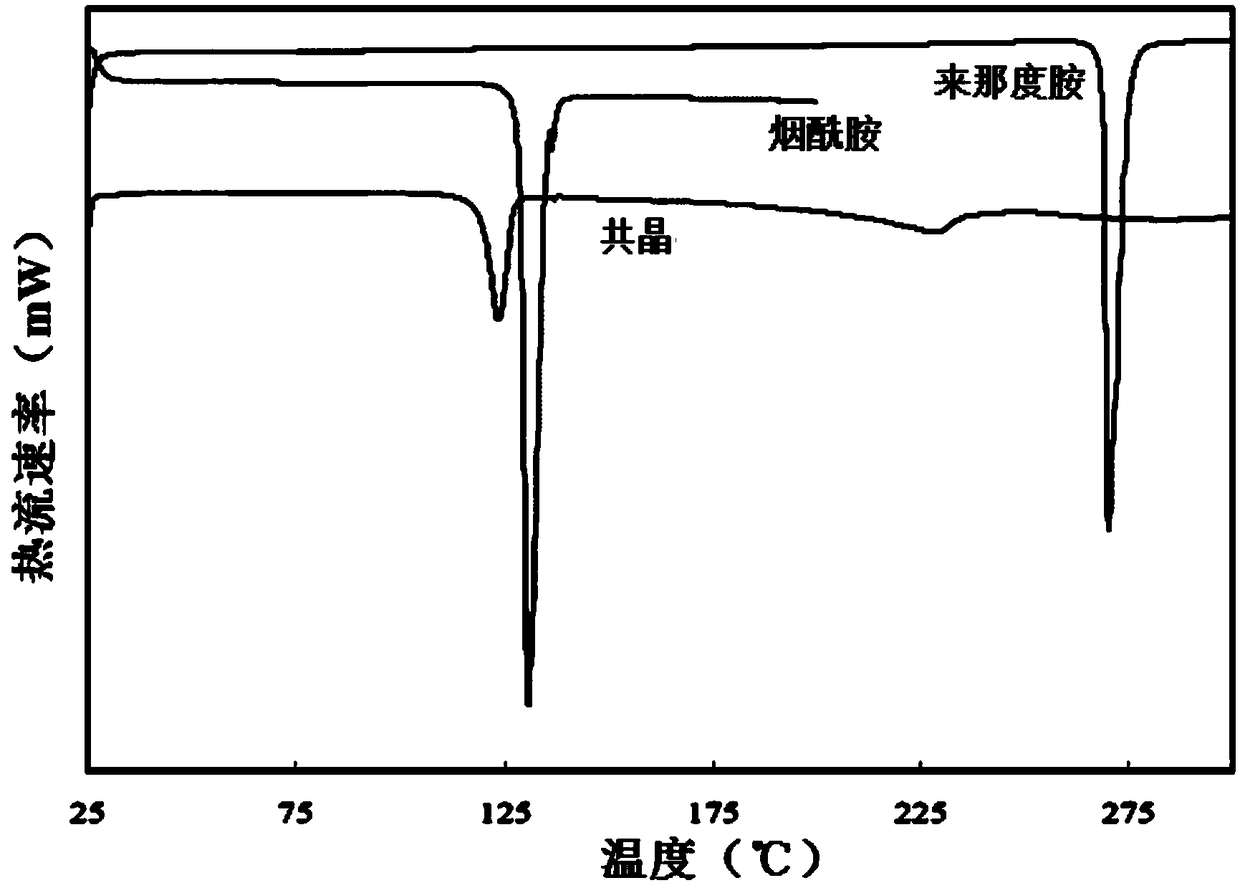
A preparing method of a lenalidomide-nicotinamide eutectic composition
ActiveCN105837556AImprove stabilityImprove solubilityOrganic chemistry methodsSolubilityOrganic solvent
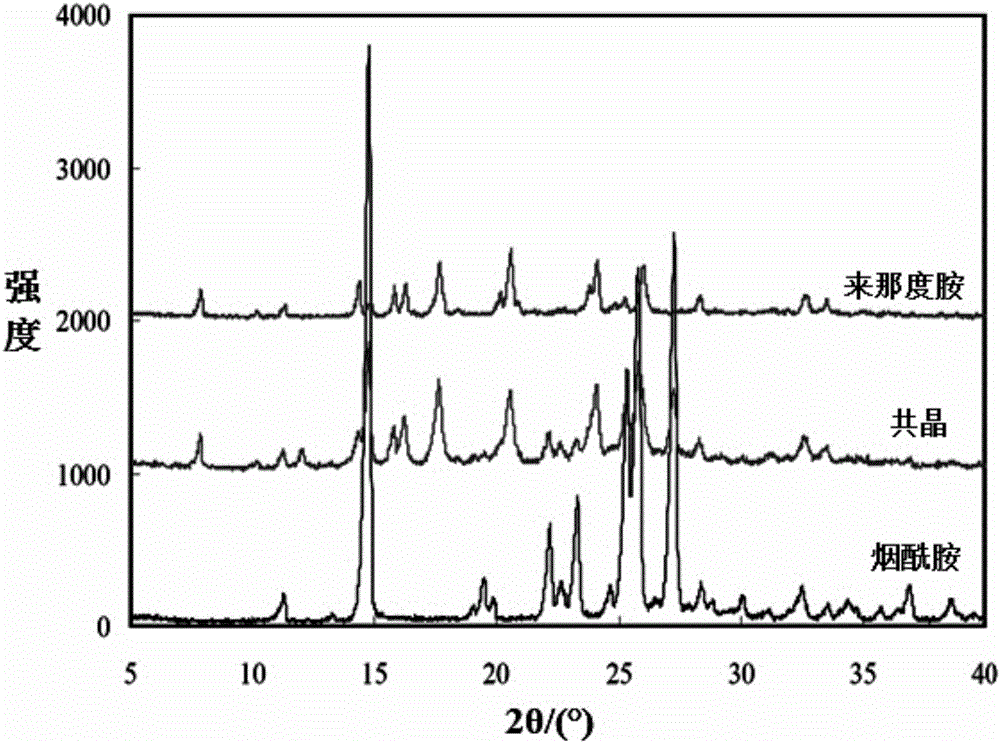
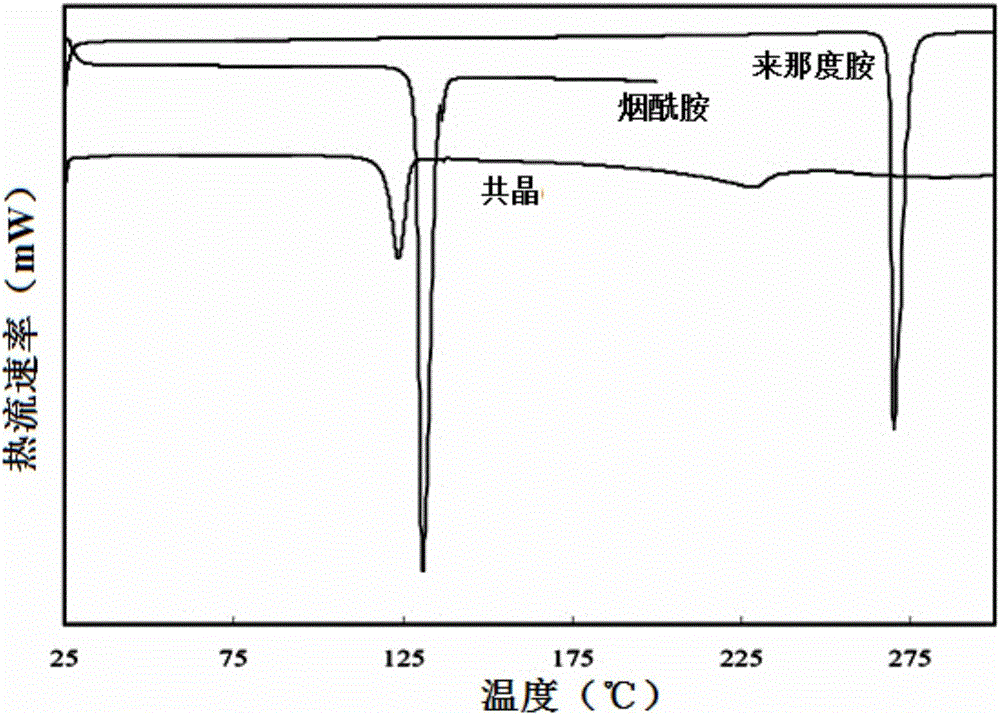
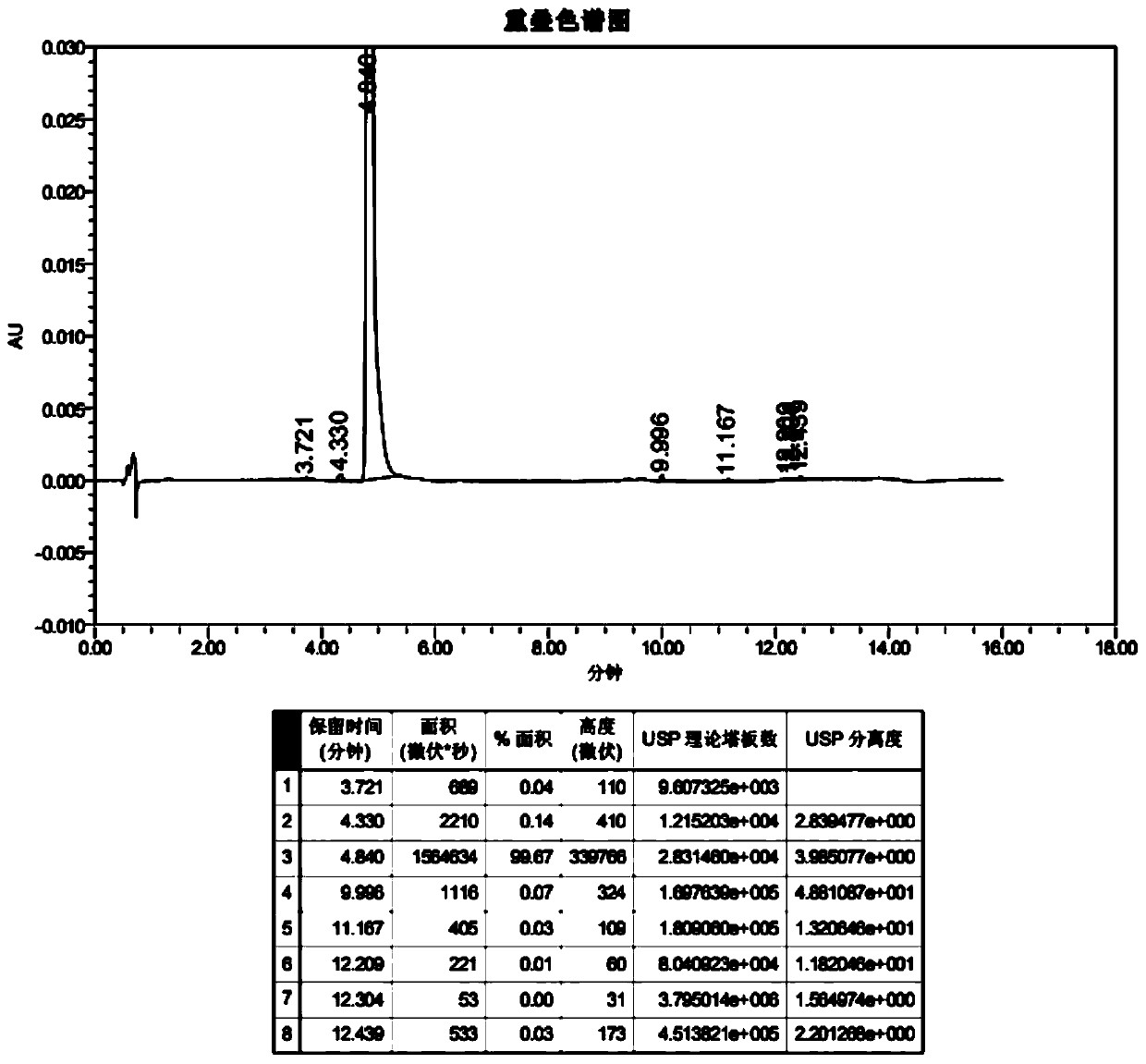
A preparing method of a lenalidomide-nicotinamide eutectic composition is disclosed. The lenalidomide-nicotinamide eutectic composition adopts lenalidomide as a medicine active component, adopts nicotinamide as an eutectic forming component, and is prepared by mixing the lenalidomide and the nicotinamide according to a ratio into an organic solvent, stirring and reacting through adopting a slurry crystallization process. The lenalidomide-nicotinamide eutectic composition prepared by the slurry crystallization process has better stability, higher solubility and a higher dissolution rate than lenalidomide-nicotinamide eutectic compositions prepared through conventional grinding methods, and is hoped to improve bioavailability and medicine effects of the lenalidomide and to meet medicinal requirements of the lenalidomide.
Owner:SHANGHAI UNIV OF ENG SCI
Preparation method of high-purity vinpocetine
The invention discloses a preparation method of high-purity vinpocetine. The preparation method comprises the following steps: S1, preparing vincamine, wherein tabersonine is dissolved, a hydrogenation reaction is performed under the catalysis of palladium and carbon to obtain vincadifformine, the vincadifformine is oxidized to obtain a vincadifformine nitric oxide, and an intermediate vincamine is obtained from the vincadifformine nitric oxide reaction liquid under the catalysis of an acid; S2, dewatering the vincamine by using toluene as a solvent and adding a dewatering agent to obtain apovincamine; and S3, on the basis of the step S2, carrying out a transesterification reaction by taking ethanol as a solvent, using dichloromethane as a cosolvent and using sodium ethoxide / sodium methoxide as a catalyst to obtain a vinpocetine crude product, and re-crystallizing with ethanol to obtain vinpocetine. The process provided by the invention has the advantages of simple production operation, avoidance of high-toxicity reagents, less impurity of the obtained product, high production efficiency, simple and convent operation and environmental friendliness, wherein the purity of the productis more than 99.9%, the quality of the product is higher than the quality of the product obtained by the original research factory, and the medicinal requirements are met.
Owner:GUANGZHOU YIPINHONG PHARMA +4
S-Manidipine hydrochloride crystal form I and preparation method thereof
ActiveCN105924382AGood crystal stabilityStable production processOrganic active ingredientsOrganic chemistry methodsManidipine hydrochlorideX-ray
The invention relates to an S-Manidipine hydrochloride crystal form I and a preparation method thereof. The preparation method includes: dissolving S-Manidipine free alkali into alcohols or alcohol / water, salifying with HCL, and crystallizing to obtain S-Manidipine hydrochloride crystal which is determined as the crystal form I according to X-ray powder diffraction detection. Products of the S-Manidipine hydrochloride crystal form I have excellent temperature and humidity stability, meet quality requirements on residual solvent and moisture and are suitable for preparation process and long-term storage. Pharmaceutical compositions with compounds of the crystal form I serving as active ingredients can be used for treating diseases such as hypertension and the like.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Refining method of castor oil with low acid value and low chroma
A refining method of low-acid-value low-chroma castor oil comprises the following steps: (1) mixing and dissolving commercially available industrial castor oil and an extracting agent according to a certain proportion; (2) adding an adsorbent into the solution obtained in the step (1), stirring and adsorbing; (3) filtering to remove the adsorbent; and (4) cooling the filtrate, standing for layering, performing separation to obtain a lower-layer supernatant, and removing the solvent to obtain a castor oil finished product with low chromaticity and low acid value, wherein the extracting agent comprises any one or a mixture of petroleum ether, normal hexane and normal heptane, and the dosage is 1-8 times of the mass of the castor oil; the adsorbent is any one or a mixture of activated carbonand decolorizing soil, activated clay, diatomite, aluminum oxide and magnesium oxide; the using amount of the activated carbon is 1-10% of the mass of the castor oil, and the mass of the other adsorbents is 1-50% of the mass of the castor oil. The method is simple in process flow and easy for large-scale production, can stably control the acid value of the finished product to be less than or equalto 0.5 mgKOH / g and the chroma to be less than or equal to Y3#, and the application range of the product in the field of medicines is broadened.
Owner:NANJING WELL CHEM
I type crystal of L-alanine-(14-rubescensin A)-ester trifluoroacetate, and preparation method thereof
InactiveCN105131009AGood crystal stabilityStable production processOrganic active ingredientsOrganic chemistrySolventRubescensin S
The present invention relates to an I-type crystal of L-alanine-(14-oridonin) ester trifluoroacetate and a preparation method therefor.The preparation method comprises crystallizing a L-alanine-(14-oridonin) ester trifluoroacetate solid in any crystal form or amorphous form in a single organic solvent or a mixed organic solvent thereof to obtain the I-type crystal of the L-alanine-(14-oridonin) ester trifluoroacetate.The I-type crystal of the L-alanine-(14-oridonin) ester trifluoroacetate obtained in the present invention has a good crystal form stability and chemical stability, and uses a crystallization solvent which has a low toxicity and low residue, and can be better used in clinical treatments.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Crystal form of extrarenal medulla secretory potassium channel inhibitor and preparation method thereof
ActiveCN109879863BImprove solubilityHigh purityOrganic active ingredientsCardiovascular disorderMedicinal chemistryChemical stability
The invention relates to a crystal form of a renal outer medullary potassium channel inhibitor and a preparation method of the crystal form. Specifically, the invention relates to an IV crystal form of an L-tartrate of a renal outer medullary potassium channel (ROMK) inhibitor and a preparation method of the IV crystal form. The IV crystal form has good chemical stability and crystal form stability, a used crystallization solvent is low in toxicity and residue, and the IV crystal form can be better used for clinical treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +2
Preparation method of cinacalcet hydrochloride
PendingCN111704577AShort stepsReduce manufacturing costOrganic compound preparationOrganic chemistry methodsPalladium on carbonPtru catalyst
The invention relates to a preparation method of cinacalcet hydrochloride. The preparation method comprises the following steps: taking m-trifluoromethyl benzaldehyde, hydantoin and (R)-1-(1-naphthyl)ethylamine as raw materials, performing condensation, hydrolysis, amidation and reduction reaction to prepare cinacalcet, and reacting cinacalcet with hydrochloric acid to prepare cinacalcet hydrochloride. Compared with an existing synthesis method of cinacalcet hydrochloride, the preparation method is short in route and low in raw material cost, the adopted condensing agent is oxalyl chloride and thionyl chloride, which are low in price, the adopted reducing agent is sodium borohydride, which is low in price, a precious metal catalyst (palladium on carbon) is not used, the hydrogenation reaction step is avoided, the requirement for equipment is low, normal-pressure reaction operation can be adopted, and the method is suitable for large-scale industrial production.
Owner:NORTH CHINA UNIV OF WATER RESOURCES & ELECTRIC POWER
Multiple crystal forms of S-Montelukast sodium and preparation method thereof
ActiveCN106083698AGood crystal stabilityStable production processOrganic active ingredientsOrganic chemistry methodsActive componentMontelukast Sodium
The invention relates to multiple crystal forms of S-Montelukast sodium and a preparation method thereof, in particular to a crystal form II, a crystal form III and a crystal form IV of S-Montelukast sodium, the preparation method of the crystal forms, pharmaceutical composition containing the crystal forms and a medical application of the crystal forms and the pharmaceutical composition. The crystal form II, the crystal form III and the crystal form IV of S-Montelukast sodium have excellent physicochemical properties and good stability, and are suitable for a preparation technological process and long-term storage. The pharmaceutical composition adopting compounds in the crystal forms as active components can be used for treating diseases such as hypertension and the like.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
I-type crystallization and preparation method of l-alanine-(14-oridonin A) ester trifluoroacetate
ActiveCN105636964BStable production processGood crystal stabilityOrganic active ingredientsOrganic chemistryOrganic solventTrifluoroacetic acid
The present invention relates to an I-type crystal of L-alanine-(14-oridonin) ester trifluoroacetate and a preparation method therefor.The preparation method comprises crystallizing a L-alanine-(14-oridonin) ester trifluoroacetate solid in any crystal form or amorphous form in a single organic solvent or a mixed organic solvent thereof to obtain the I-type crystal of the L-alanine-(14-oridonin) ester trifluoroacetate.The I-type crystal of the L-alanine-(14-oridonin) ester trifluoroacetate obtained in the present invention has a good crystal form stability and chemical stability, and uses a crystallization solvent which has a low toxicity and low residue, and can be better used in clinical treatments.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
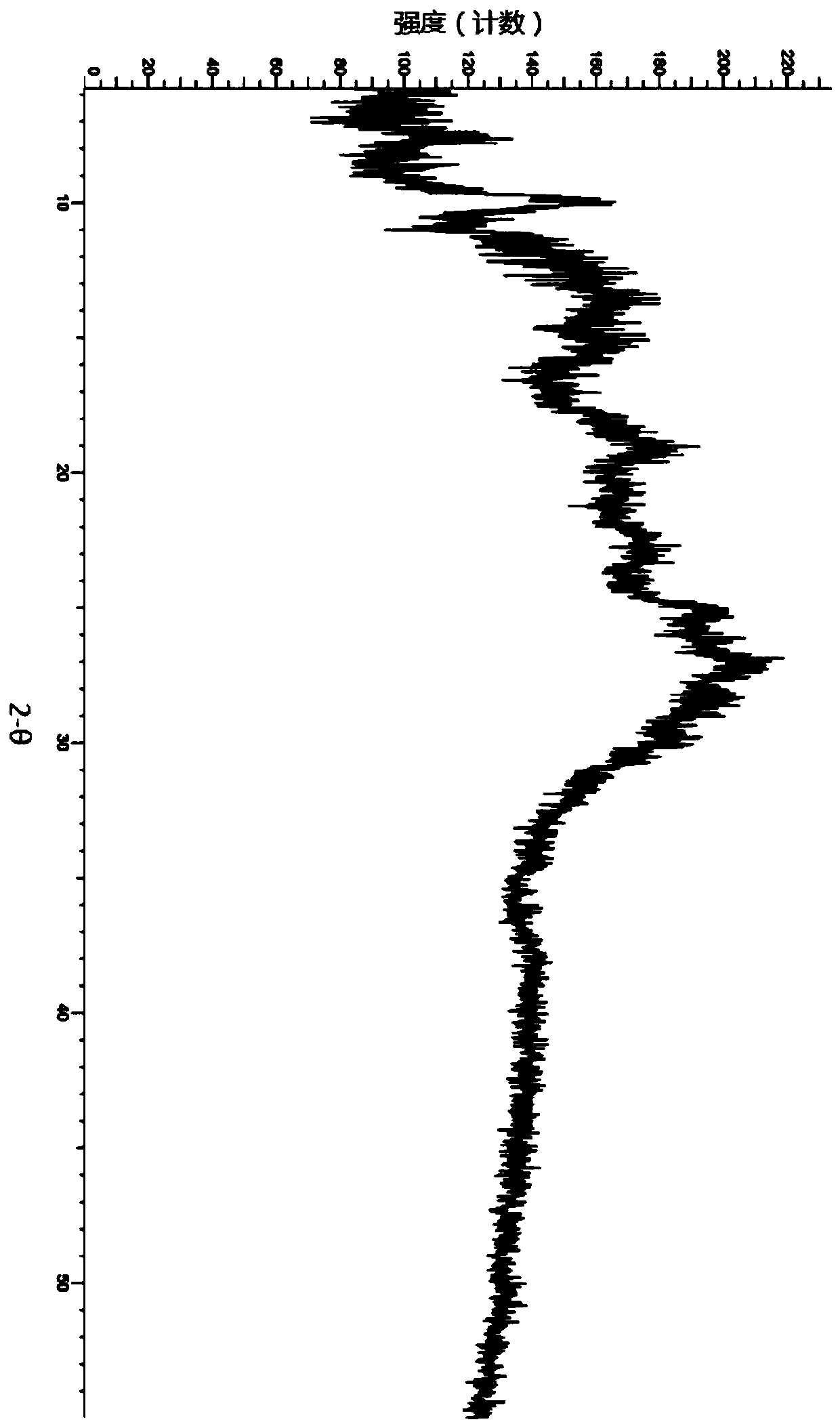
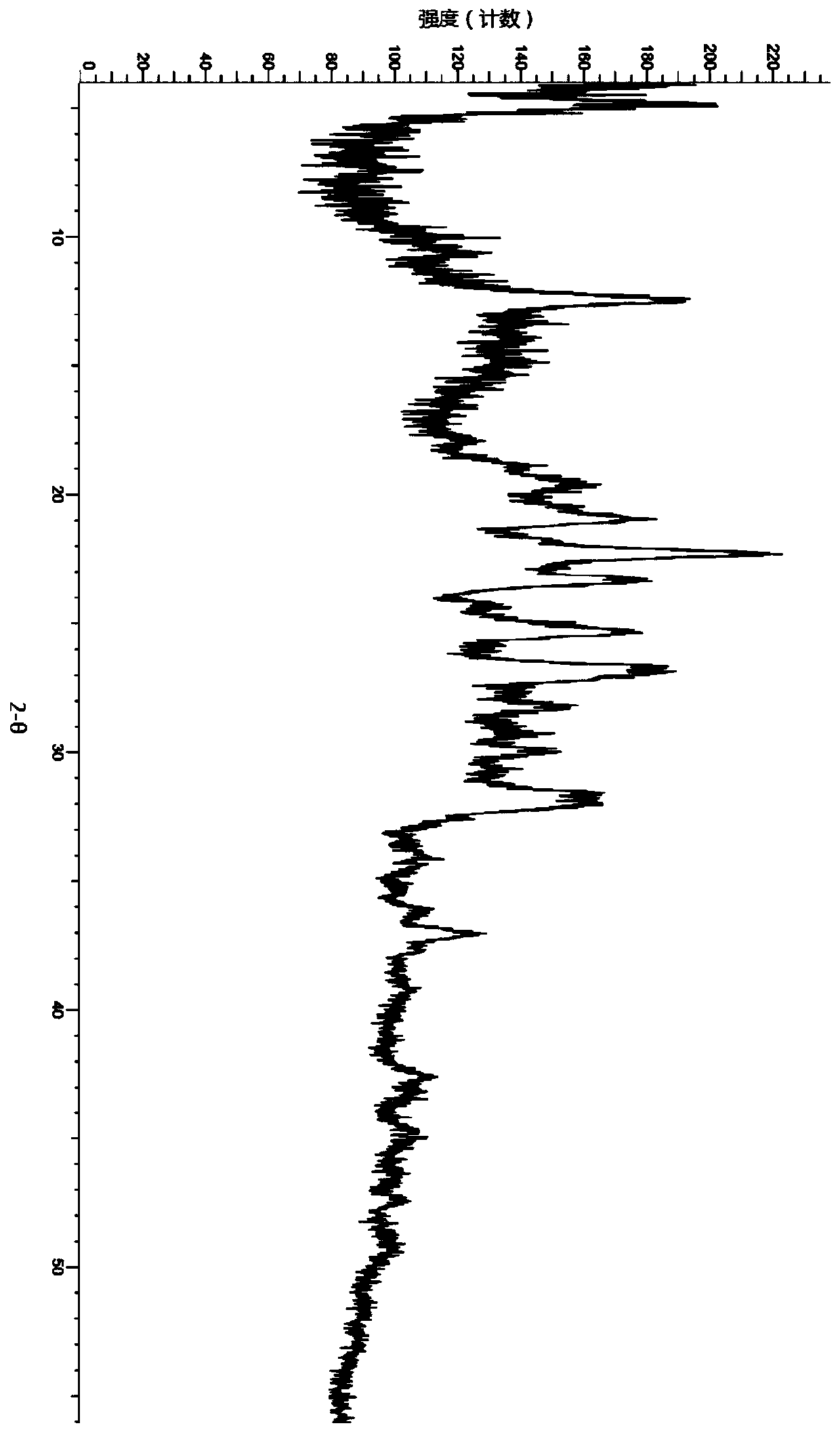
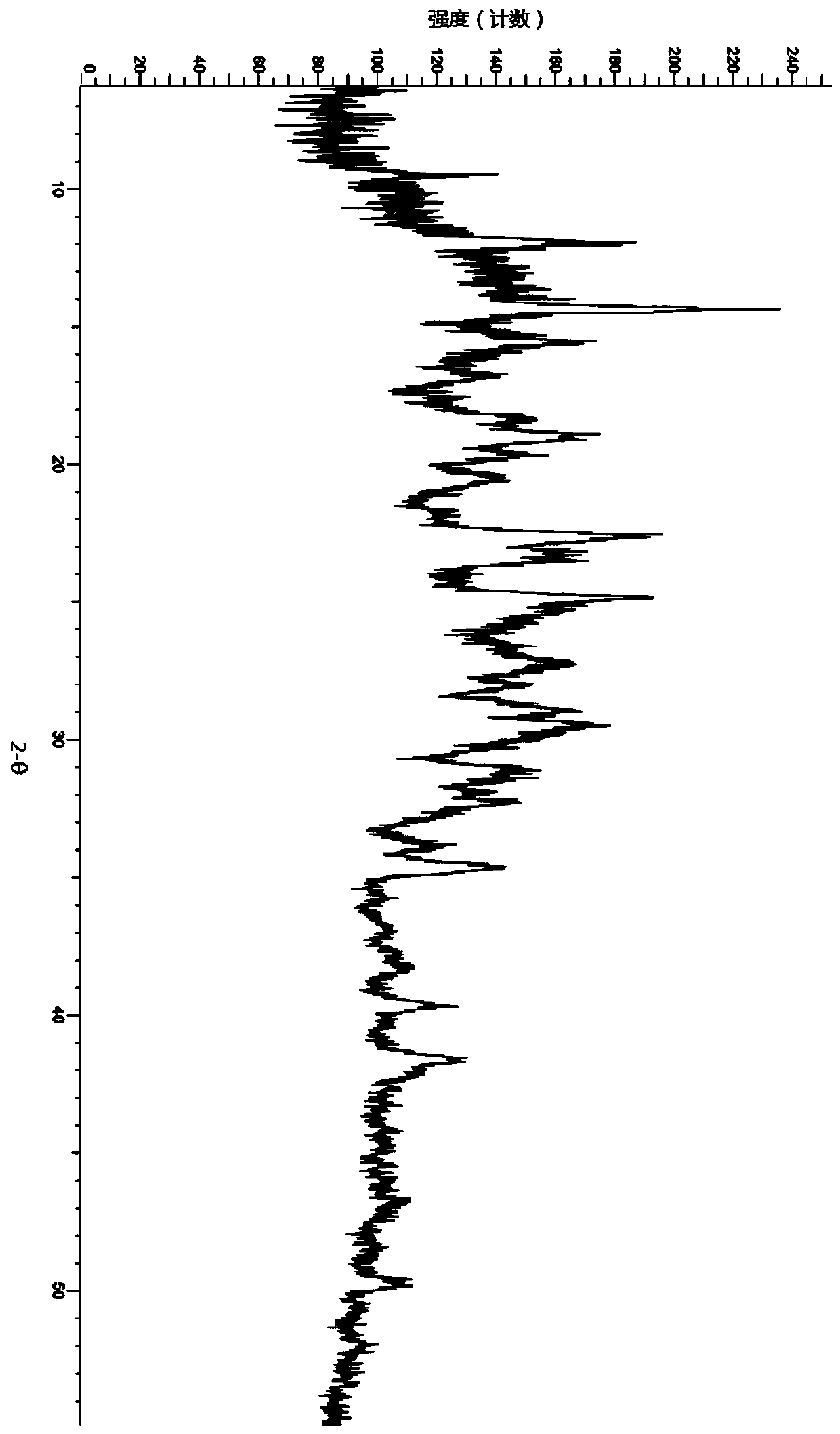
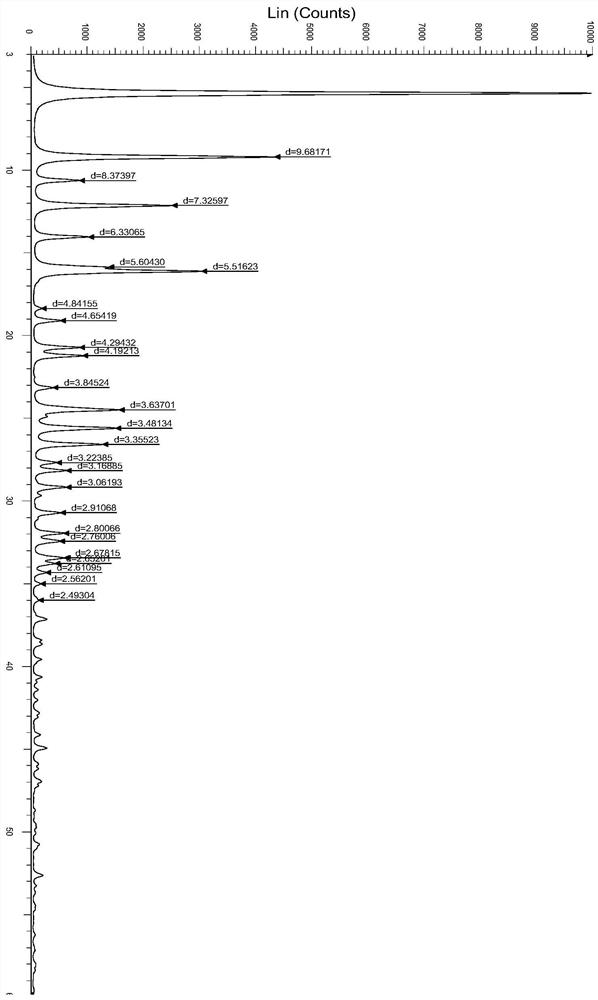
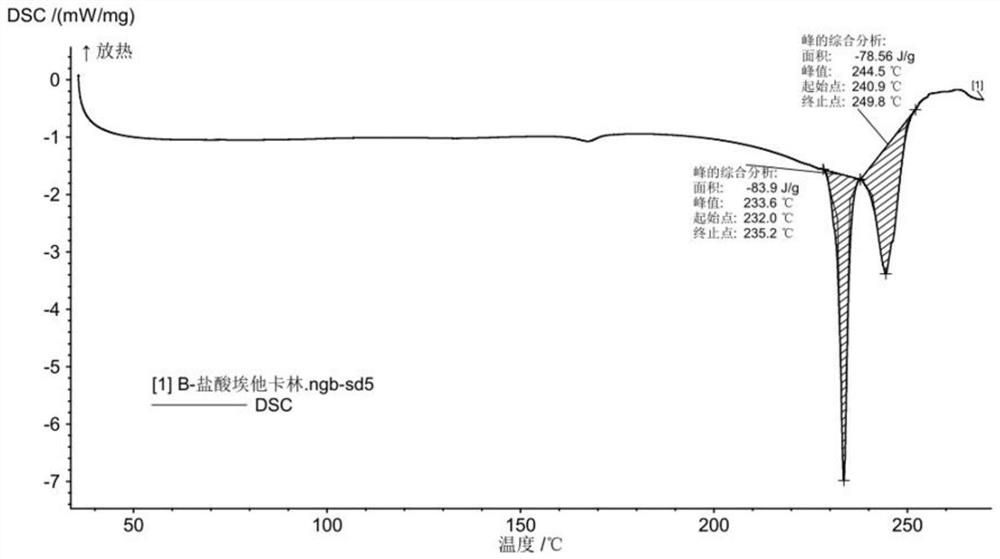
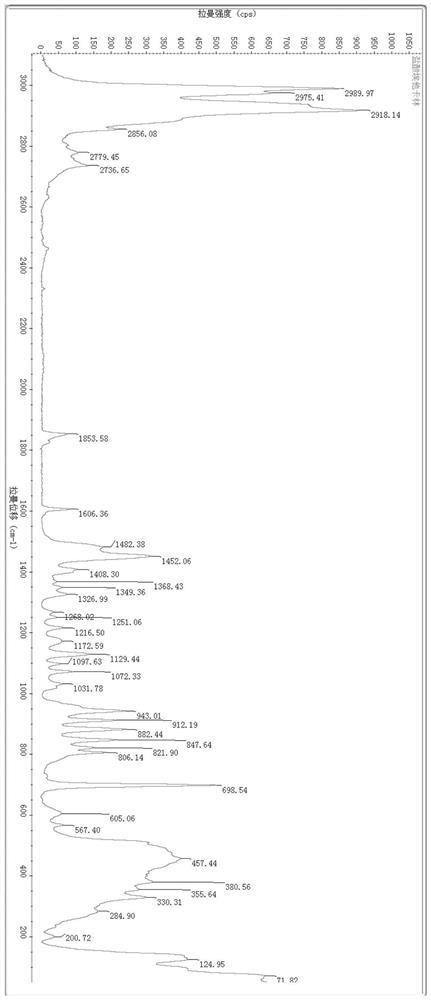
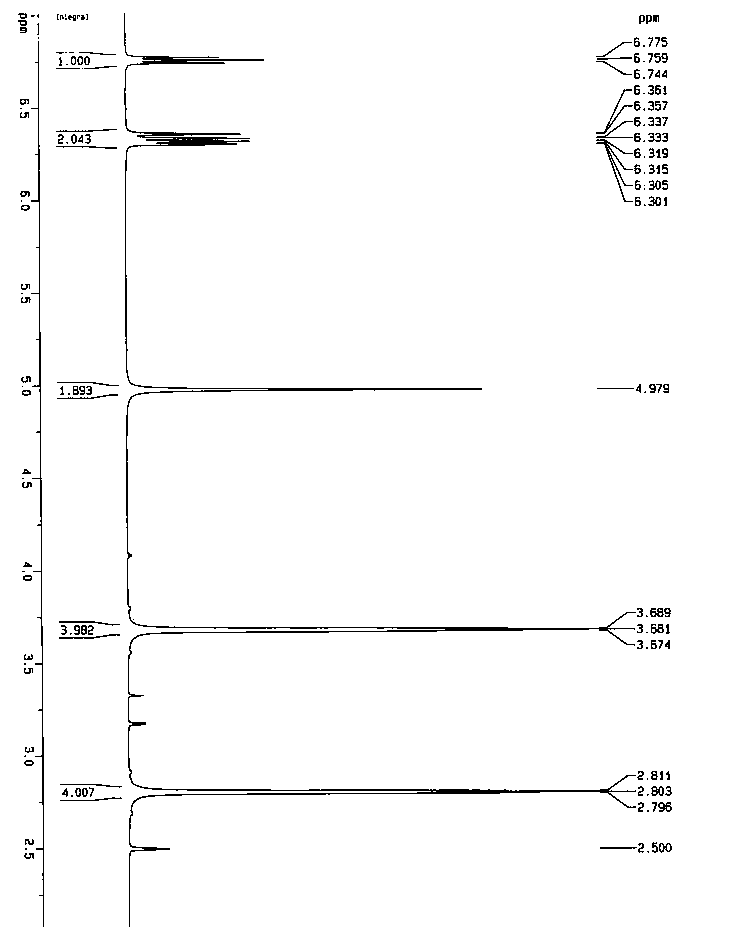
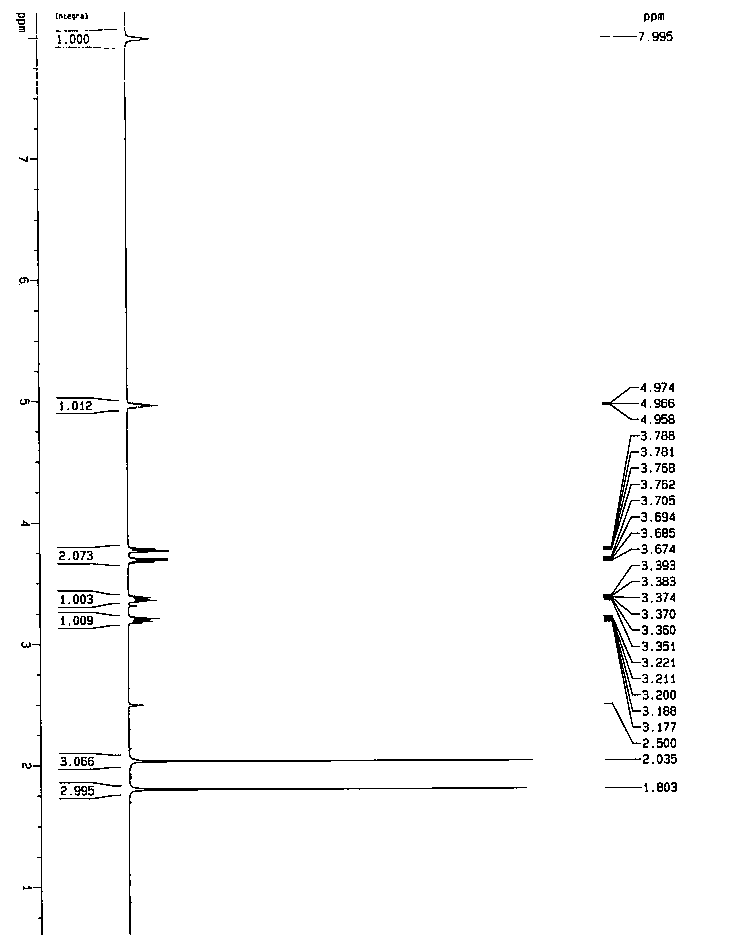
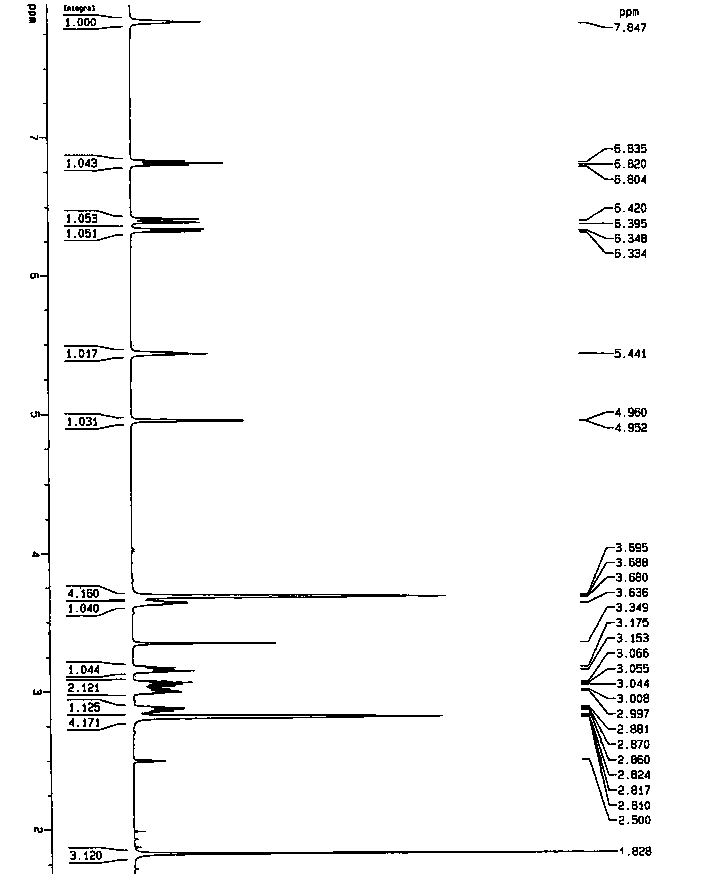
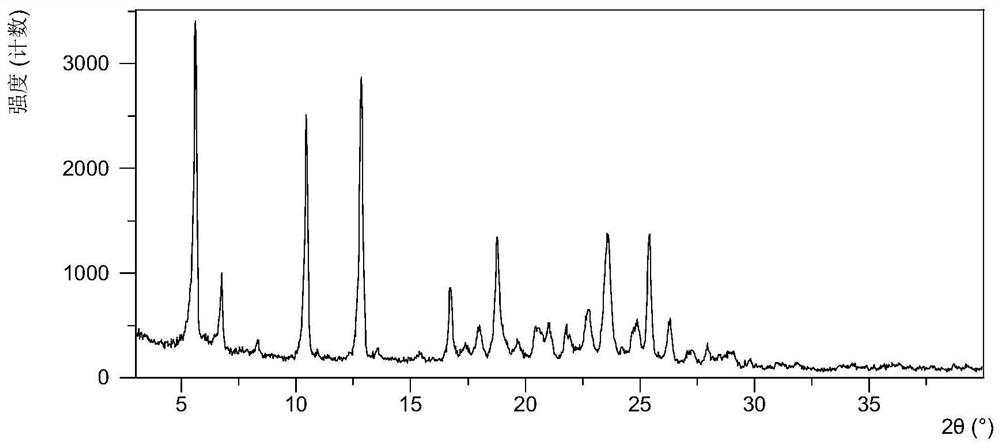
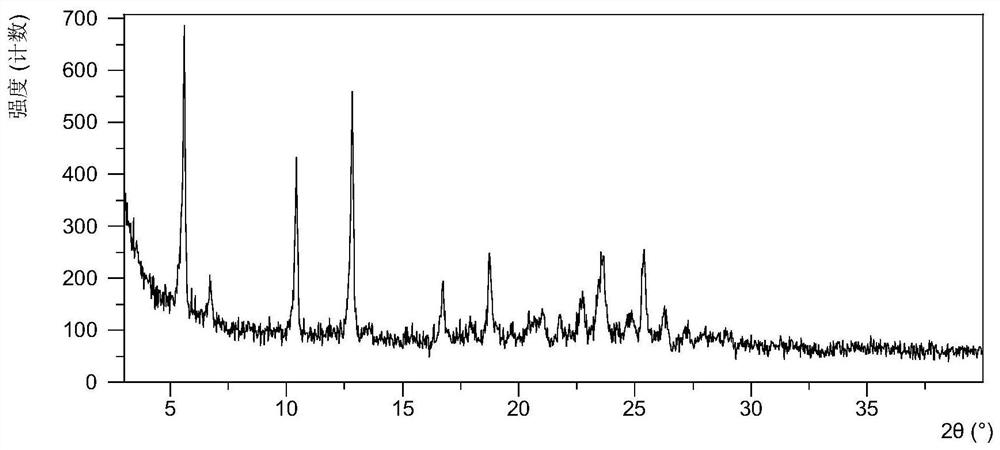
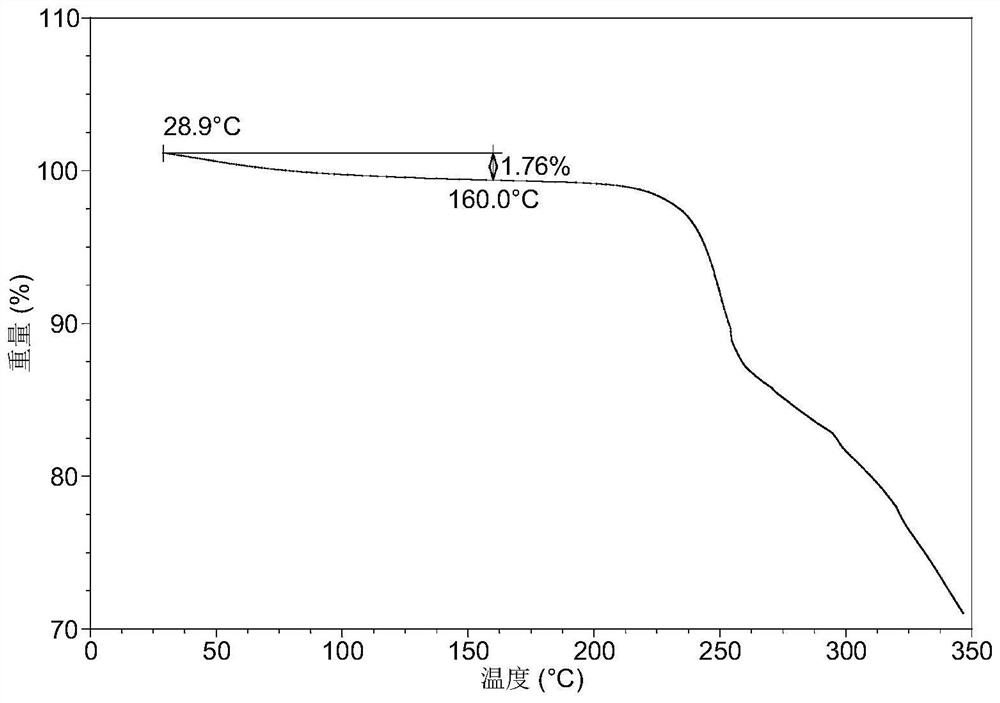
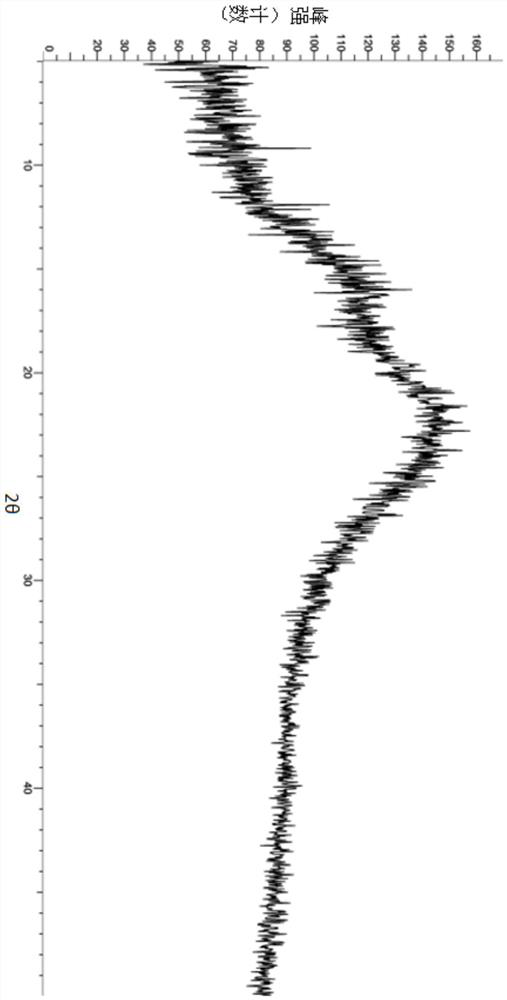
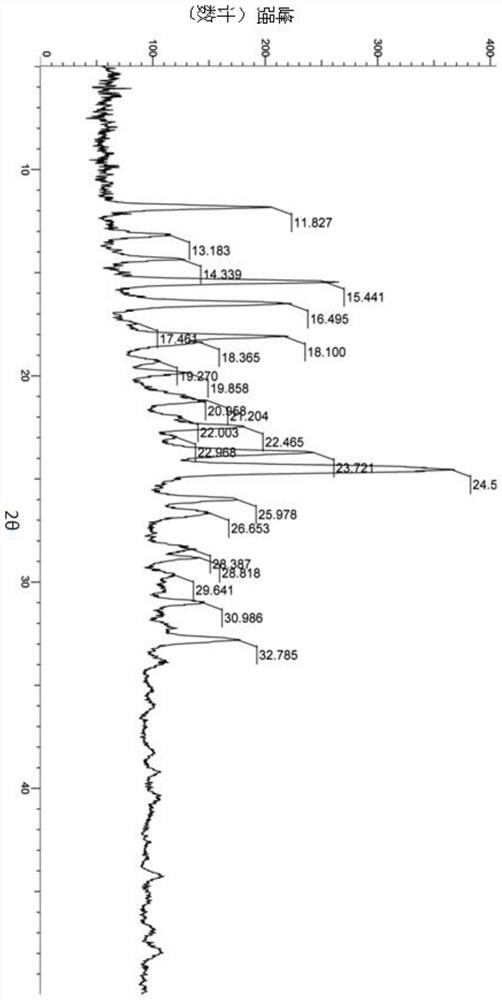
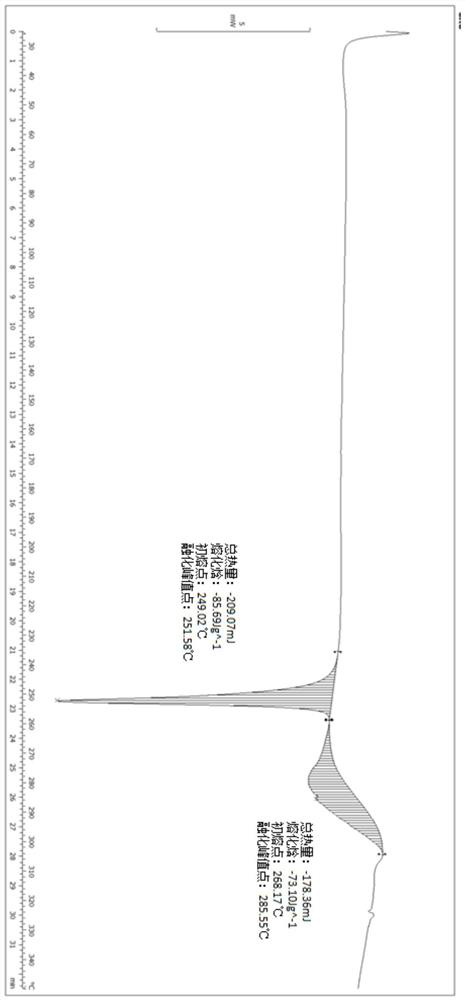
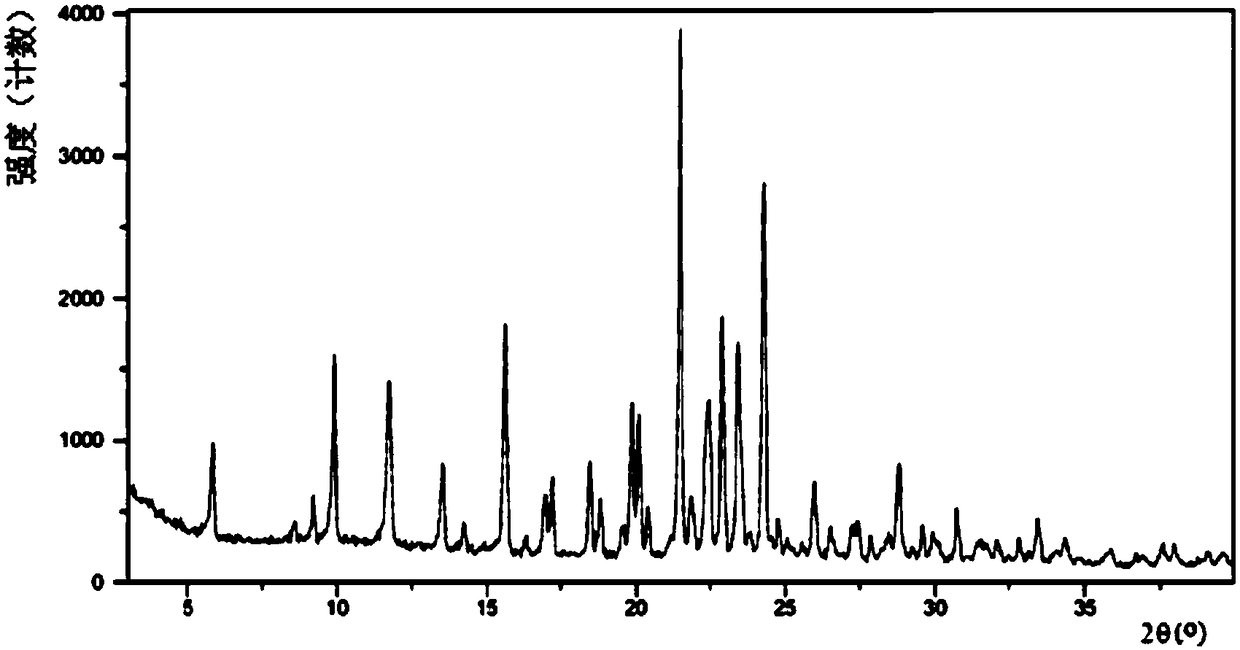
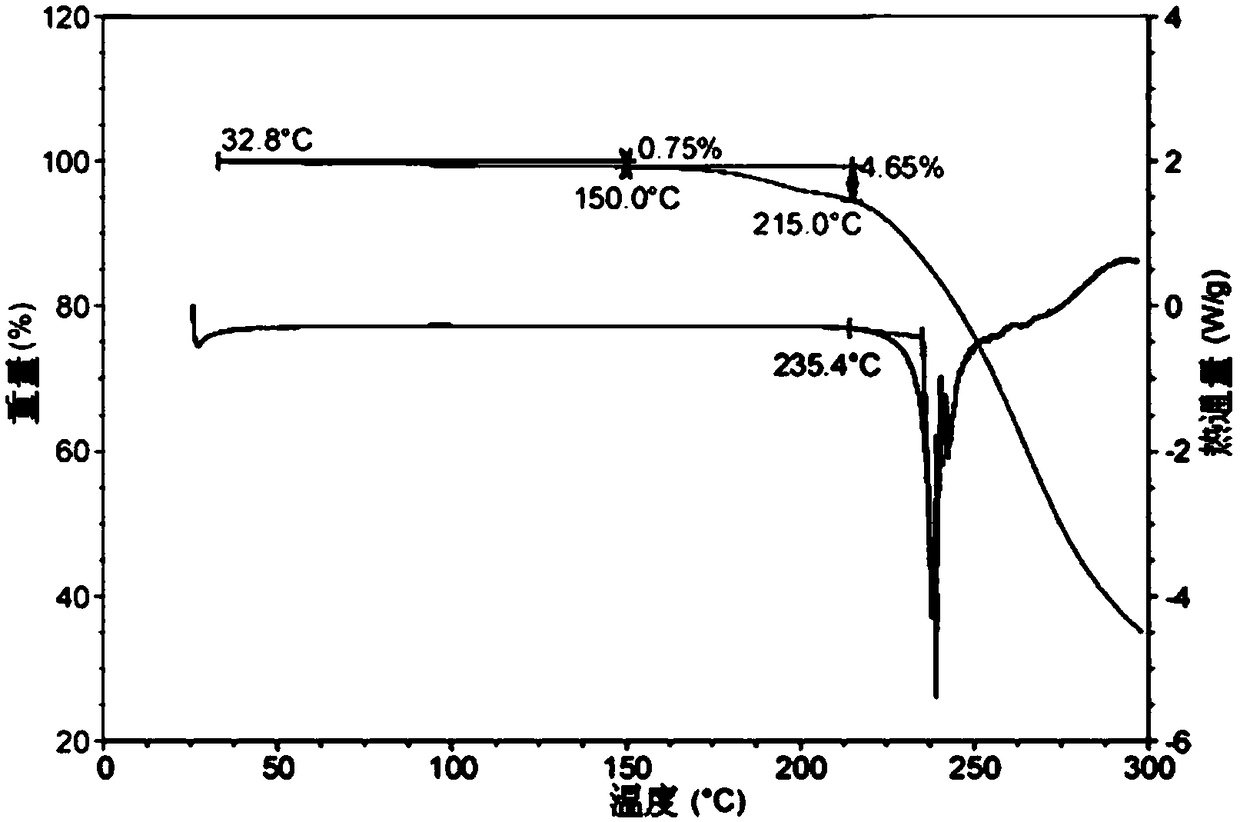
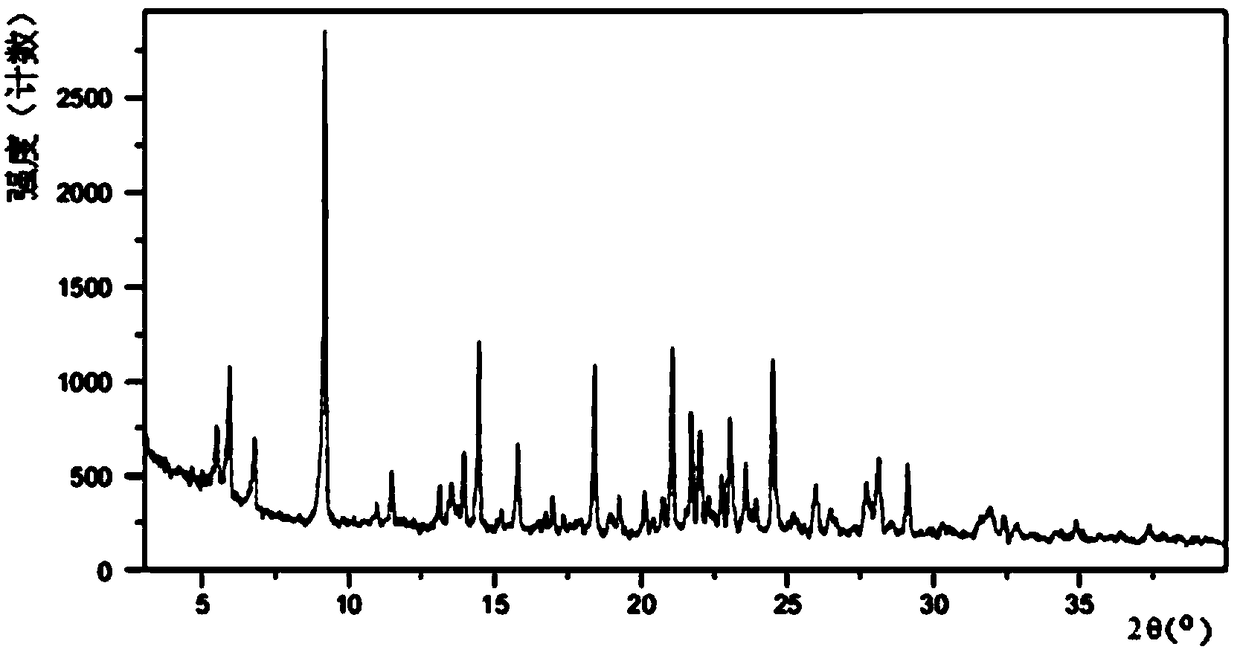
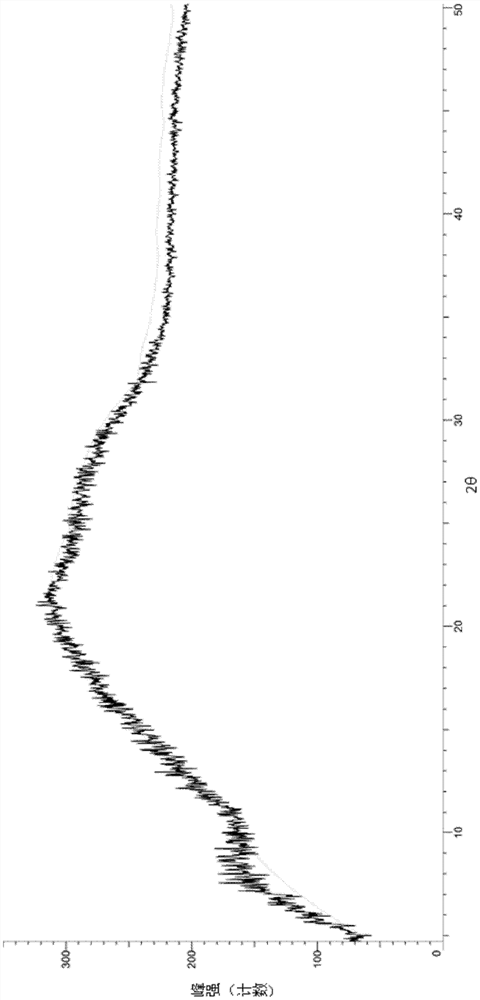
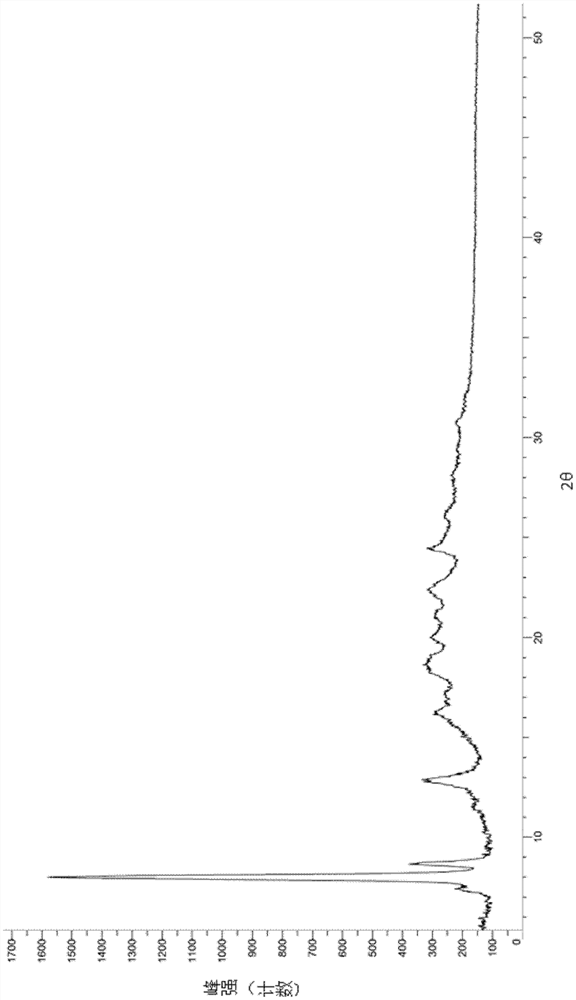
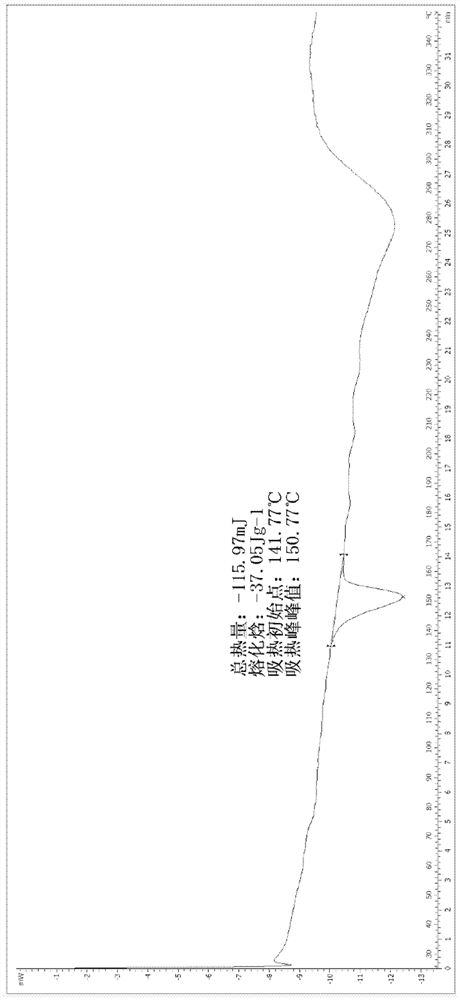
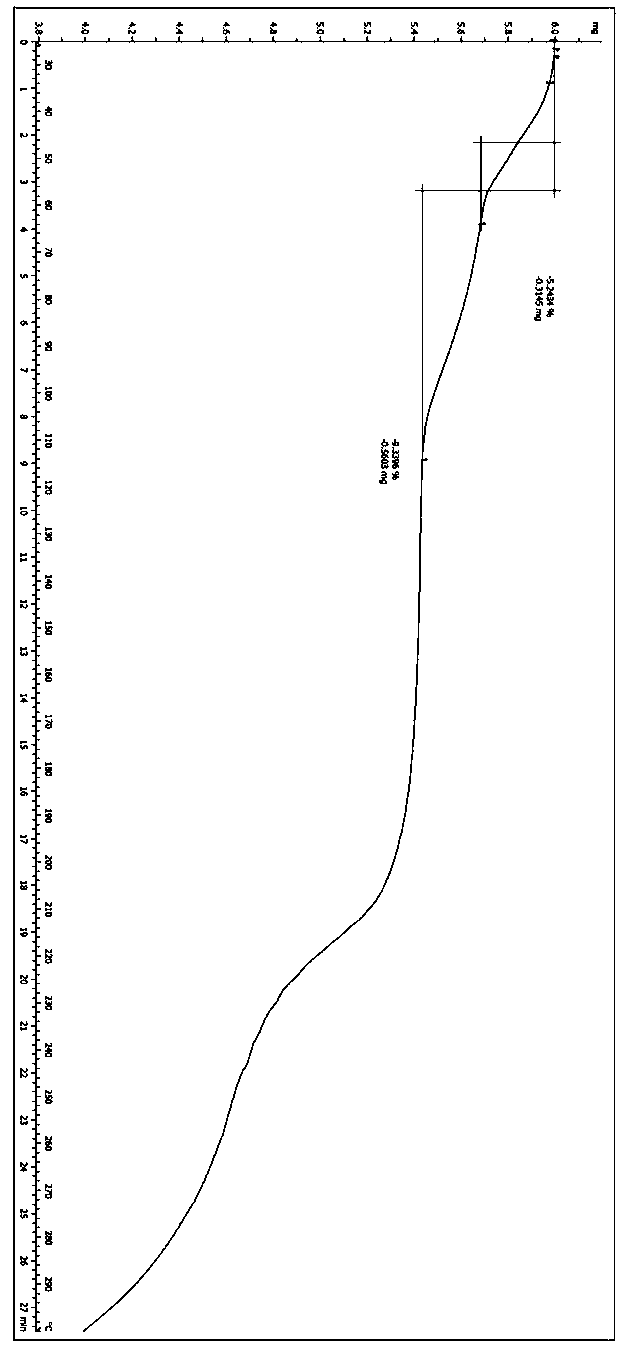
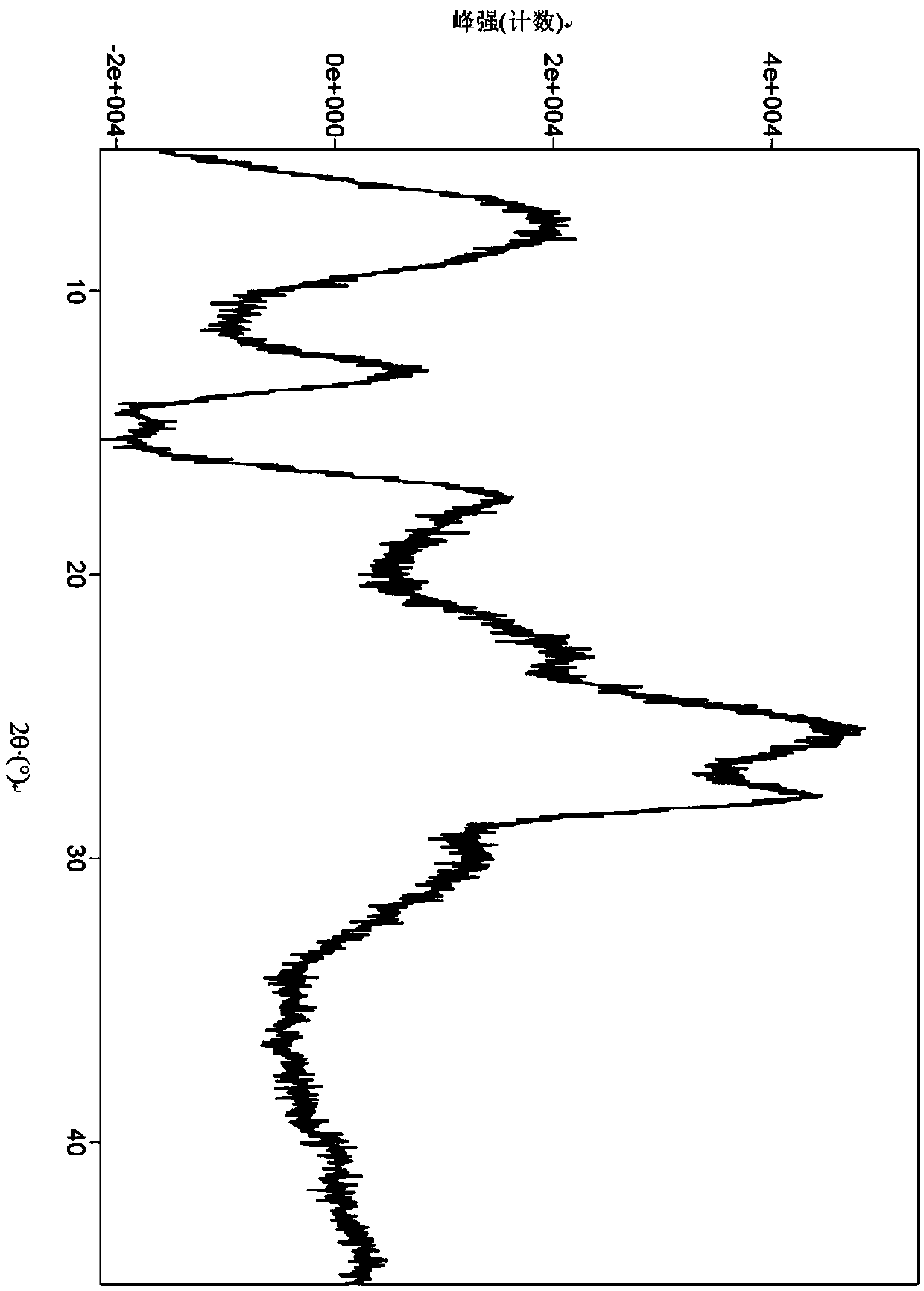
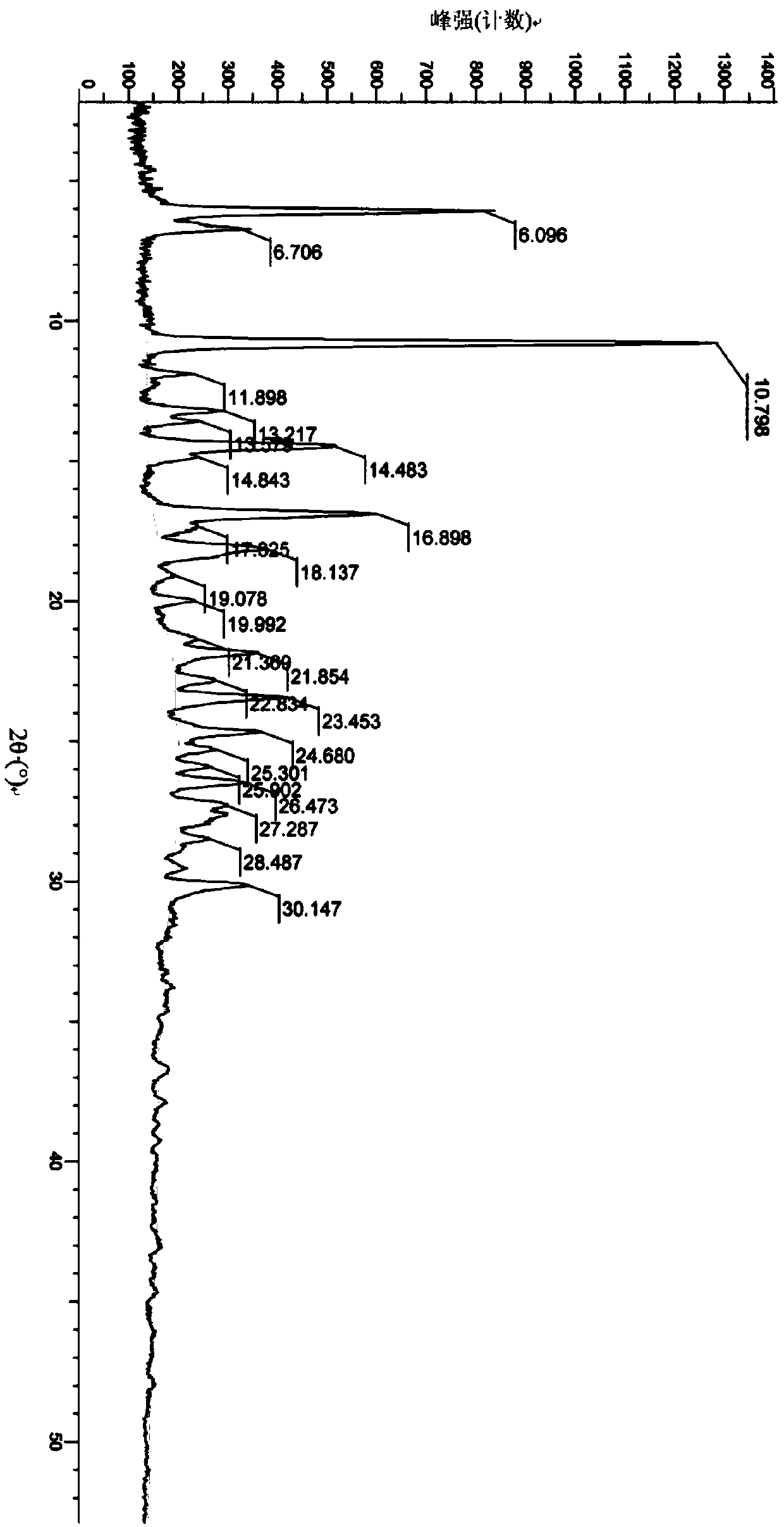
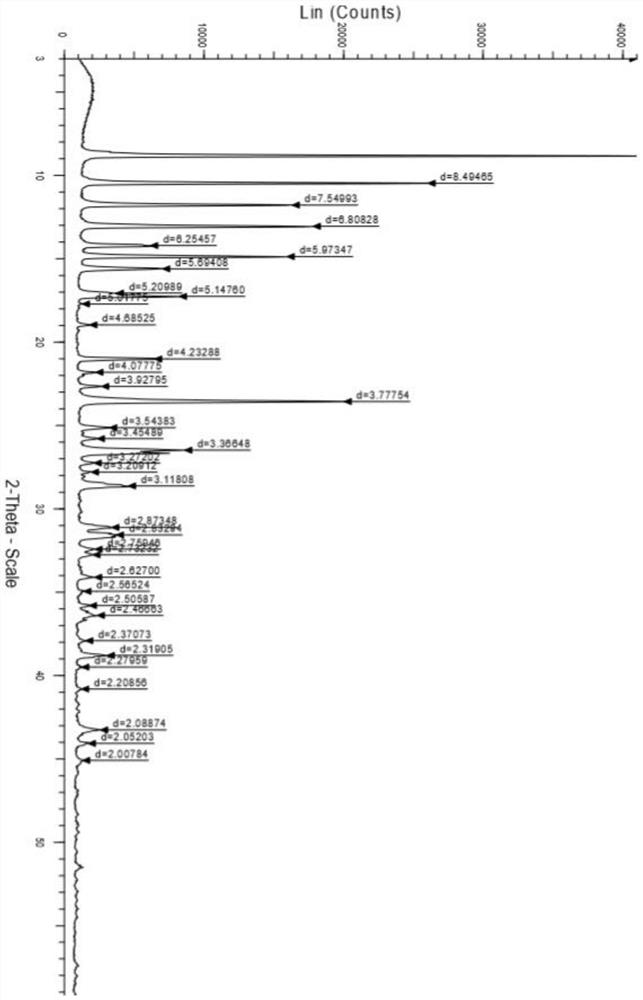
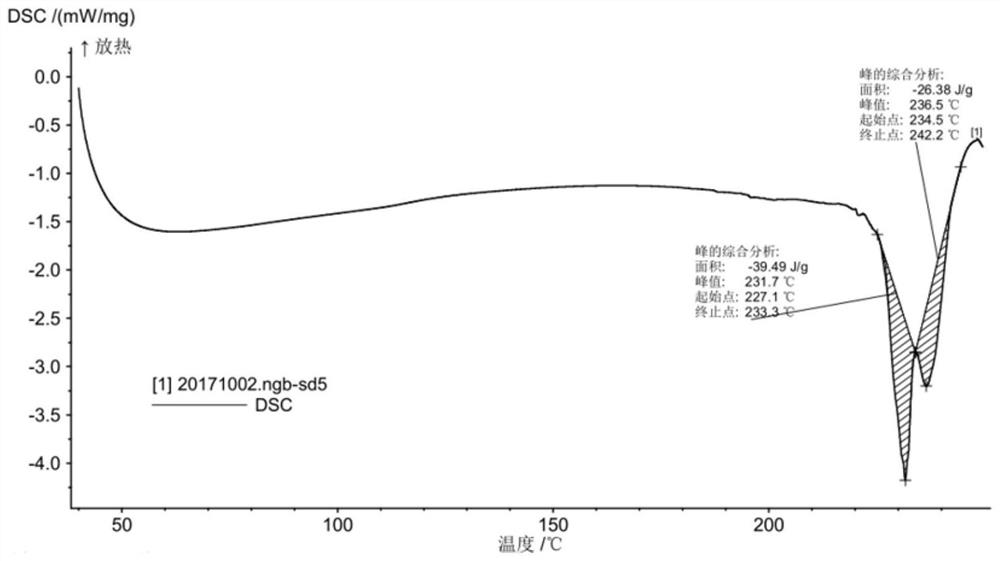
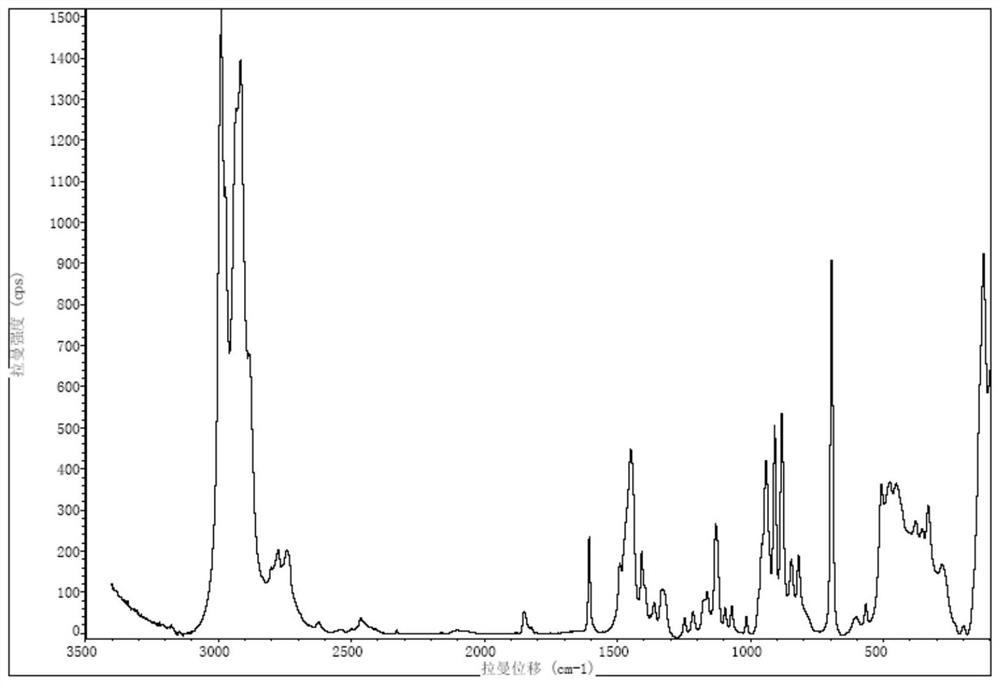
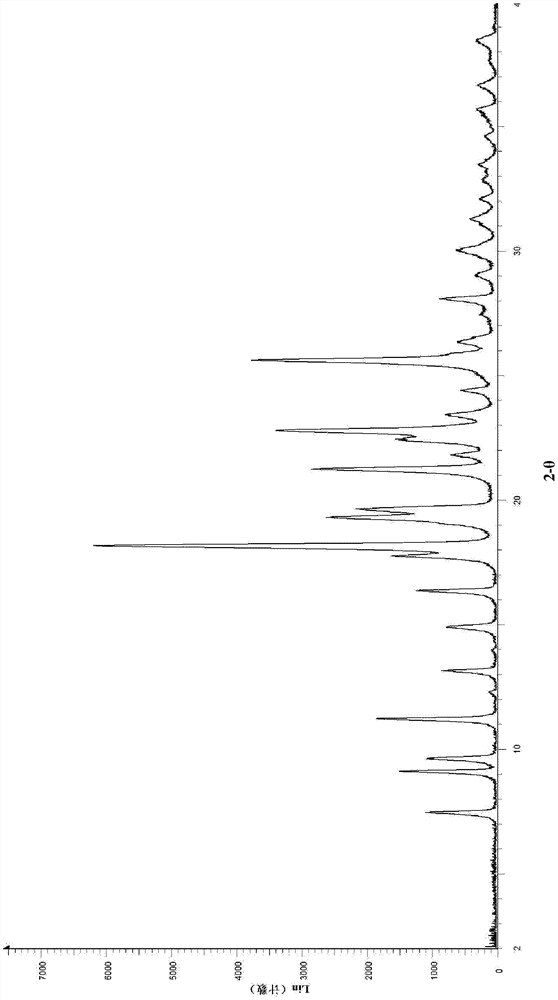
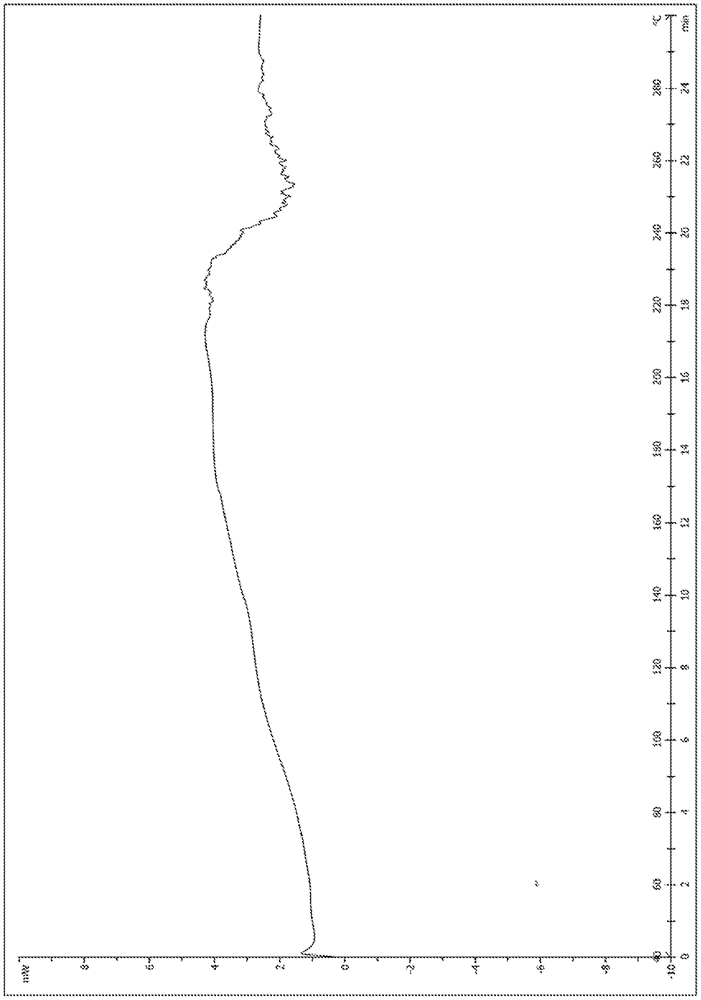
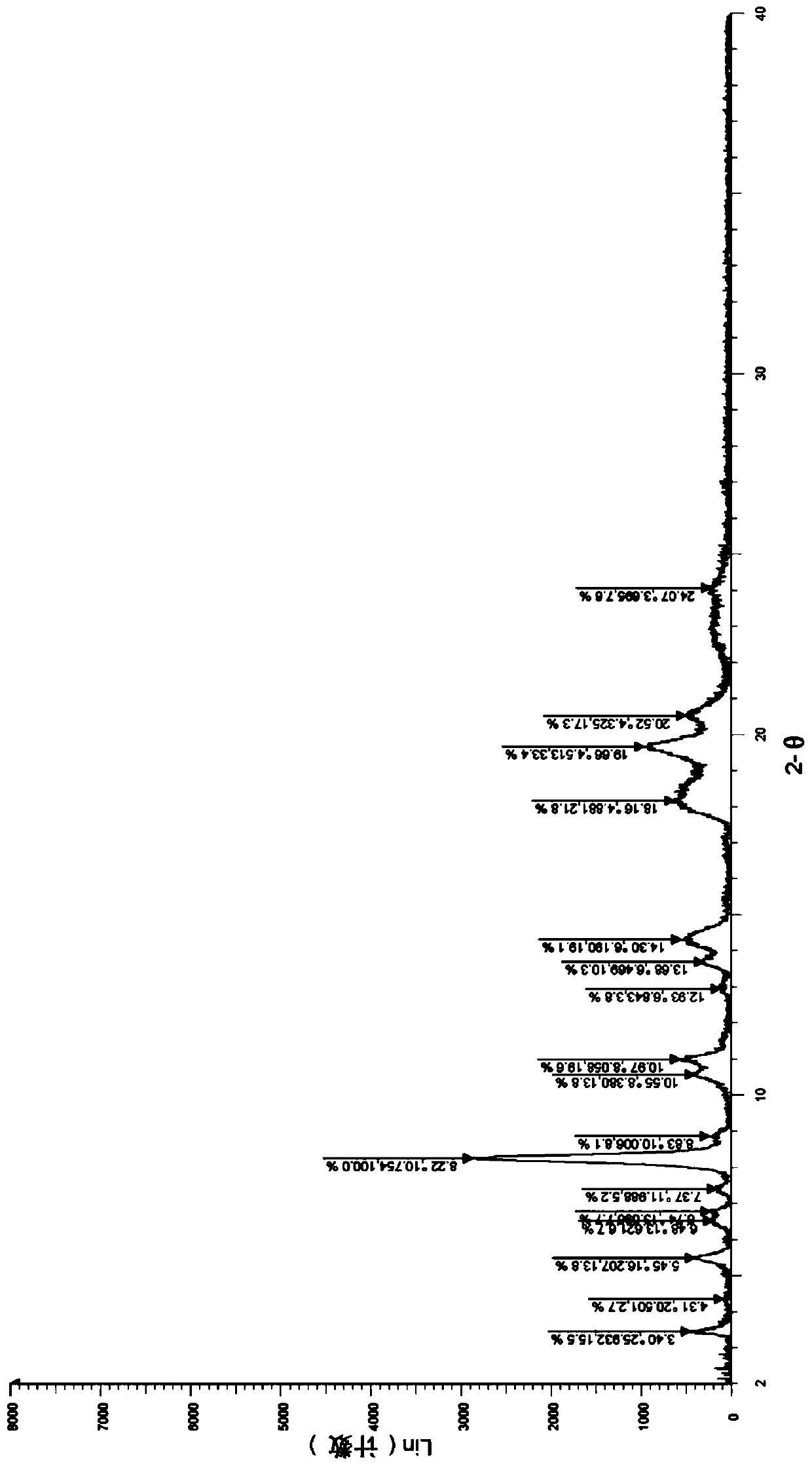
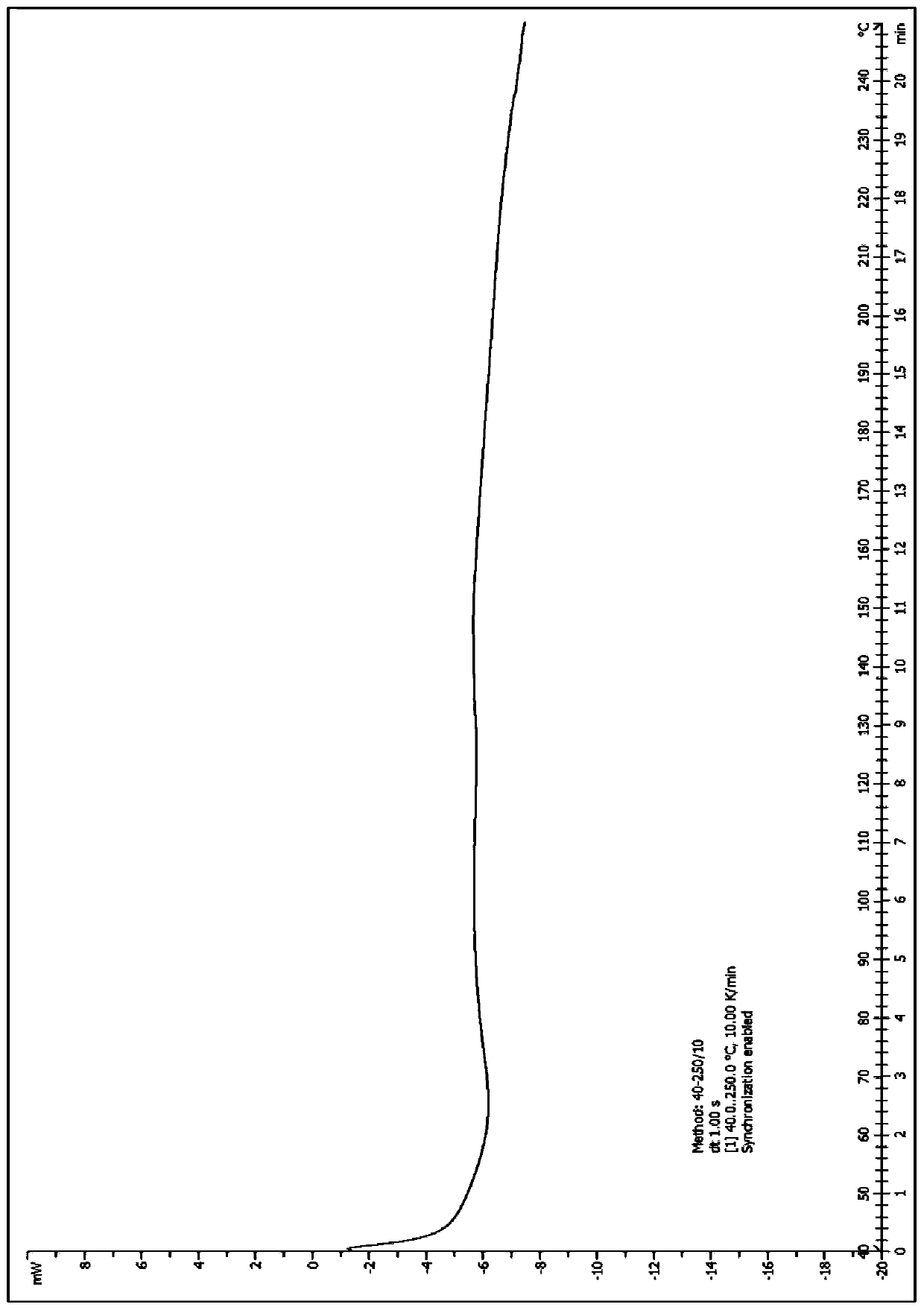
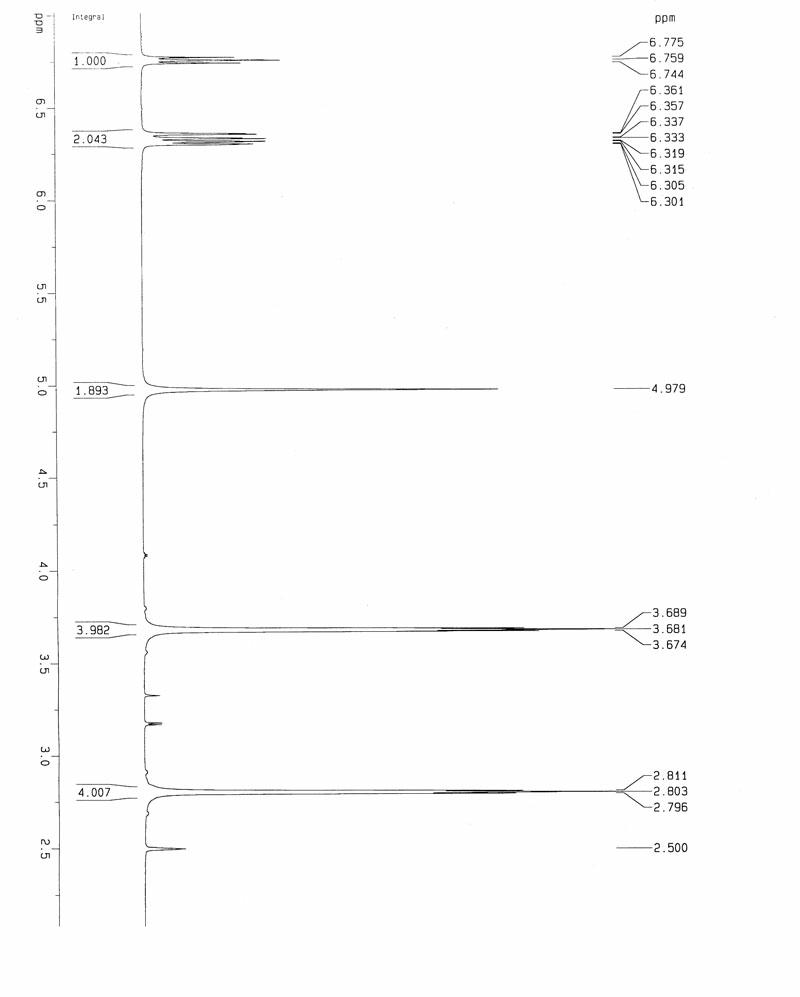
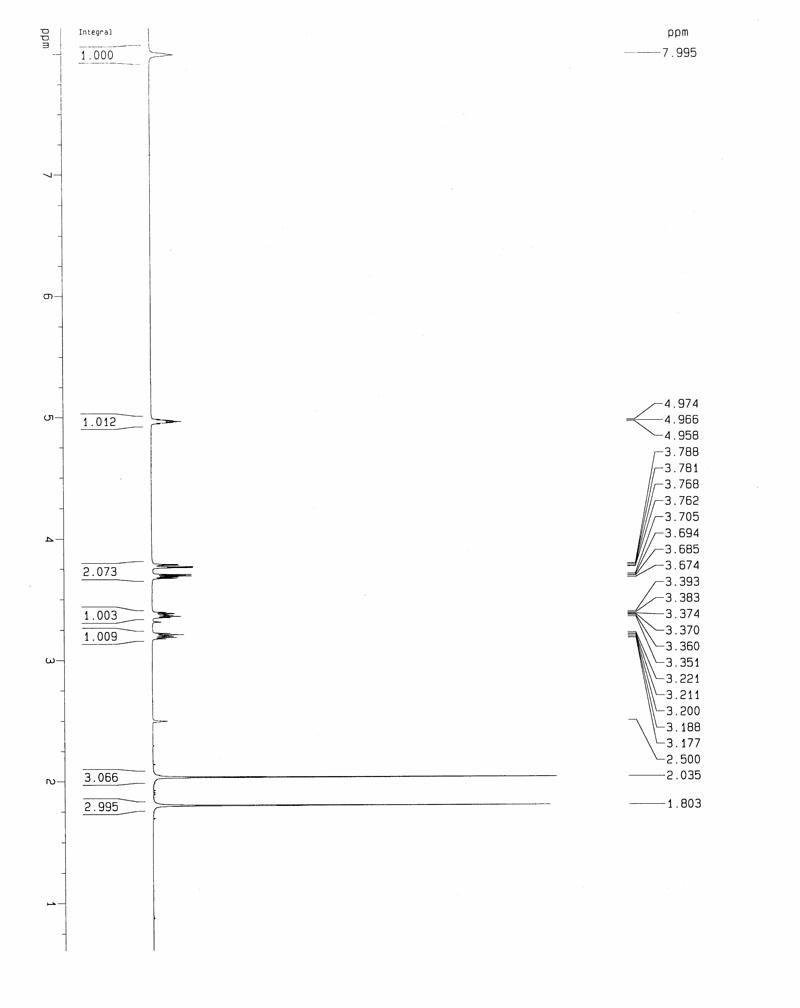
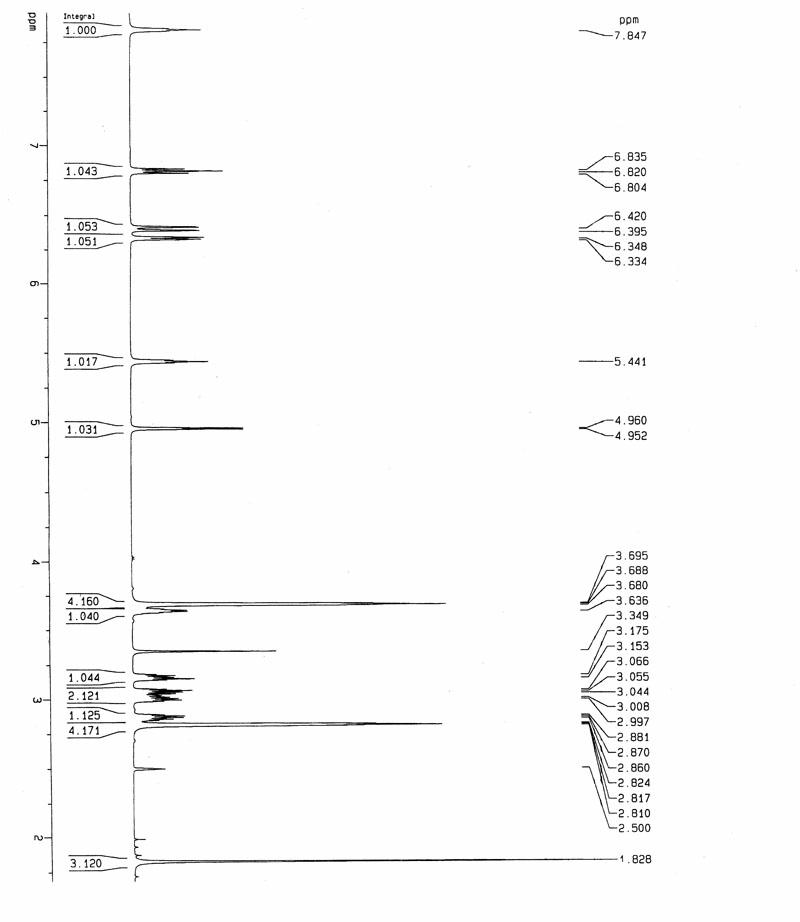
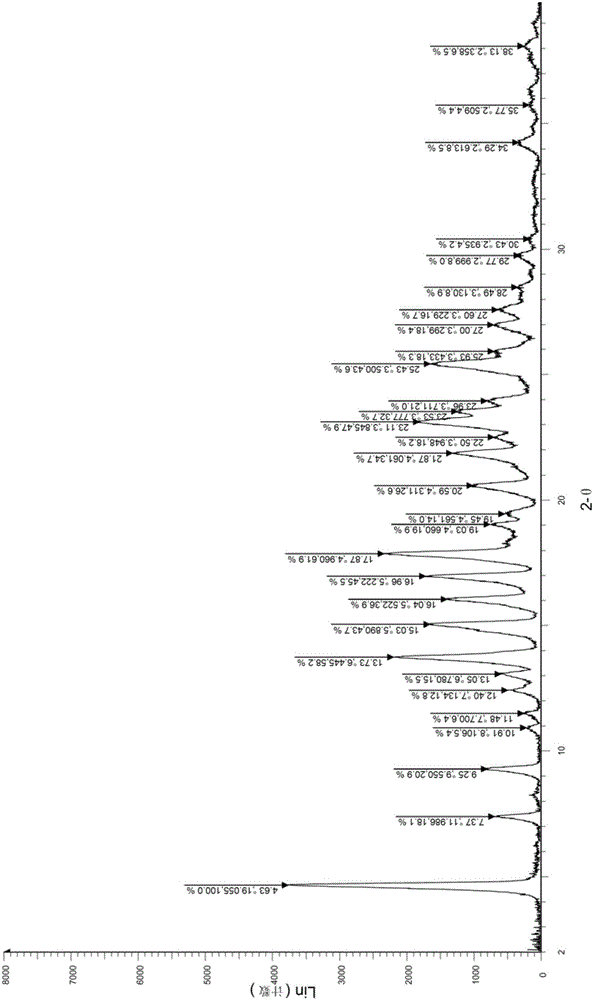
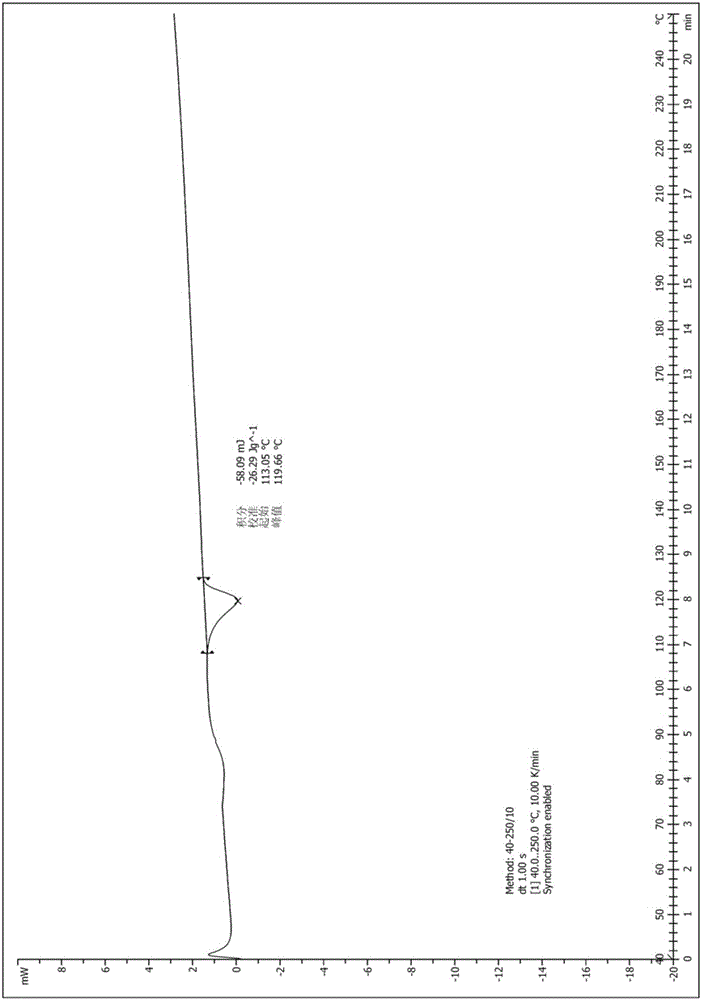
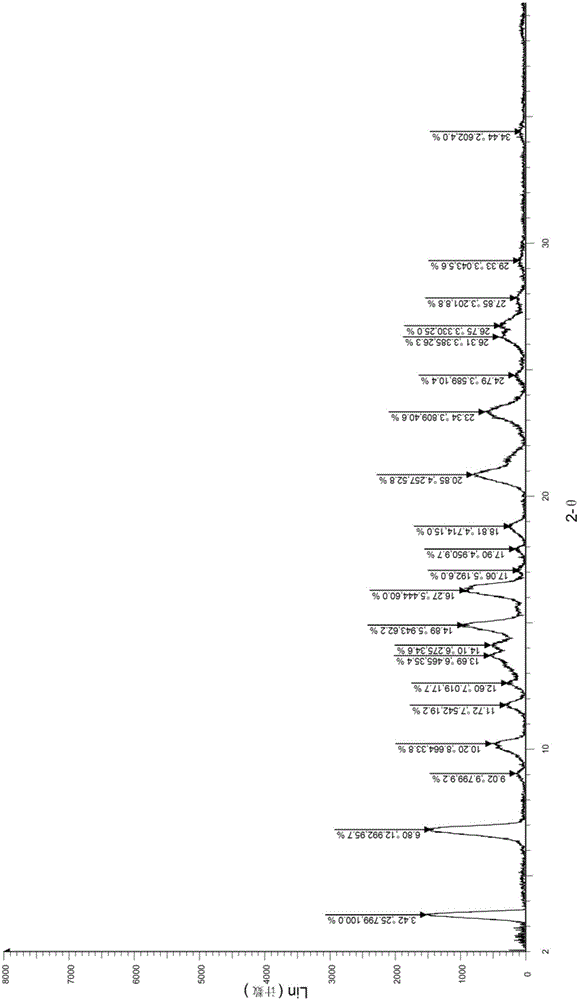
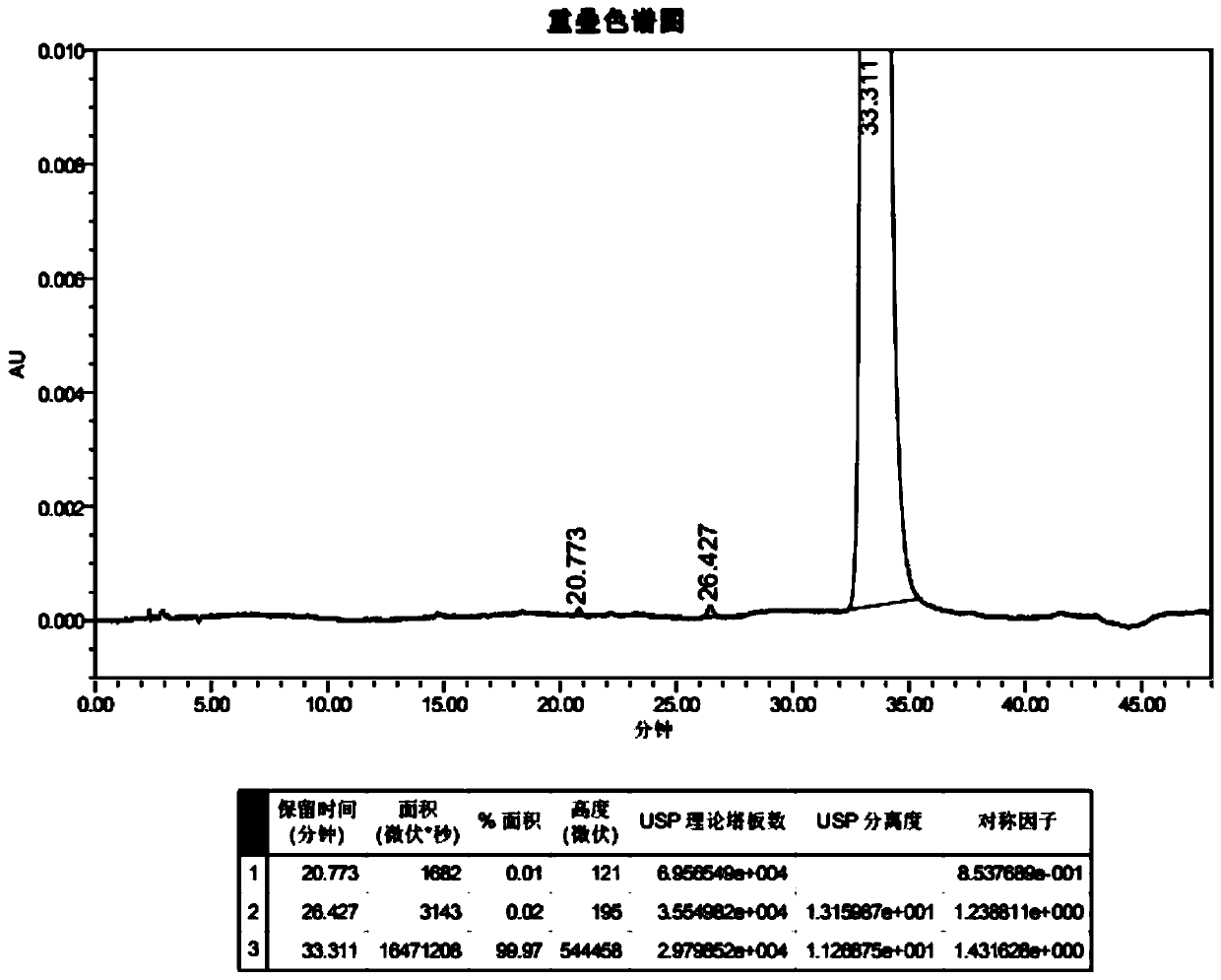
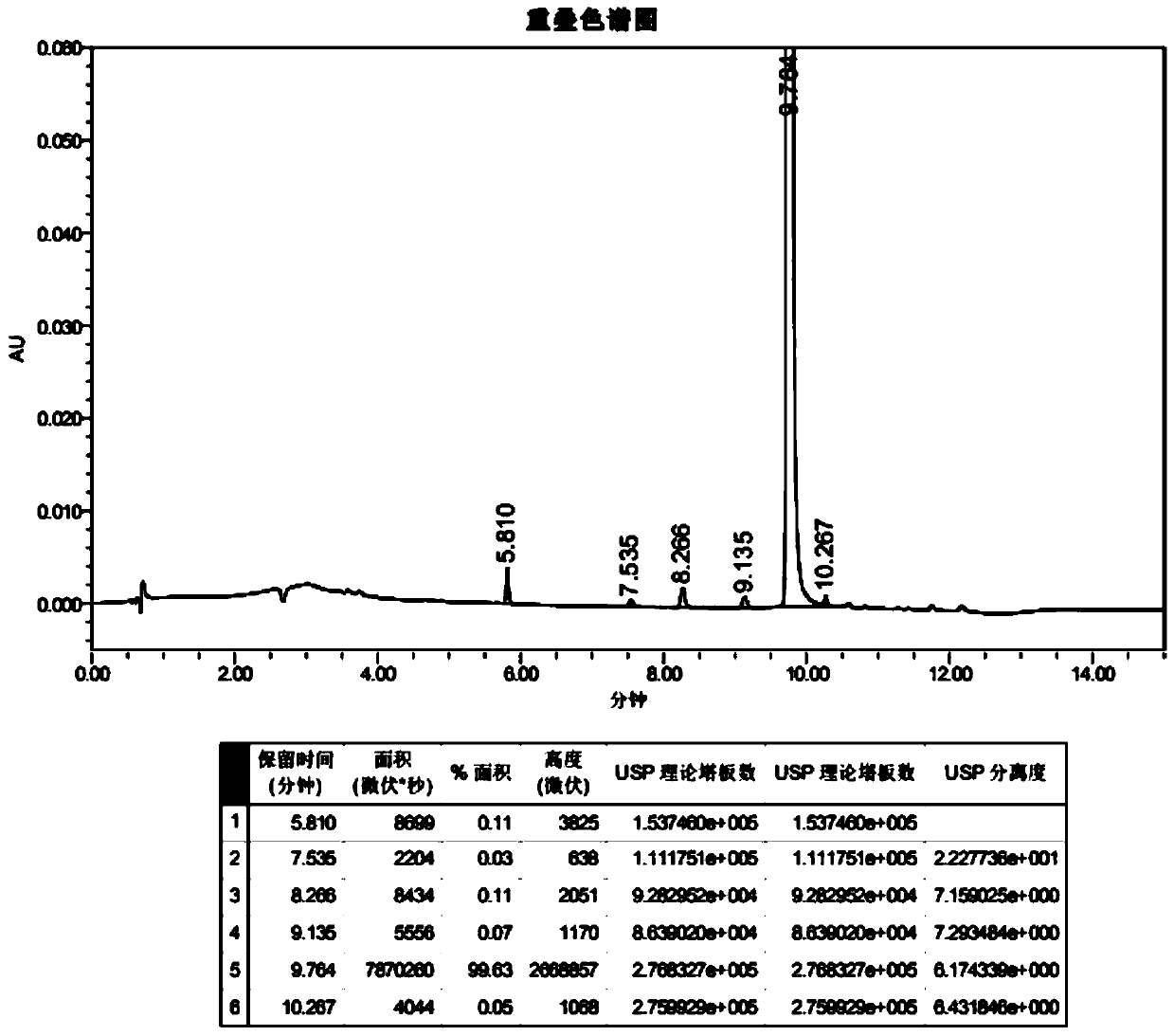
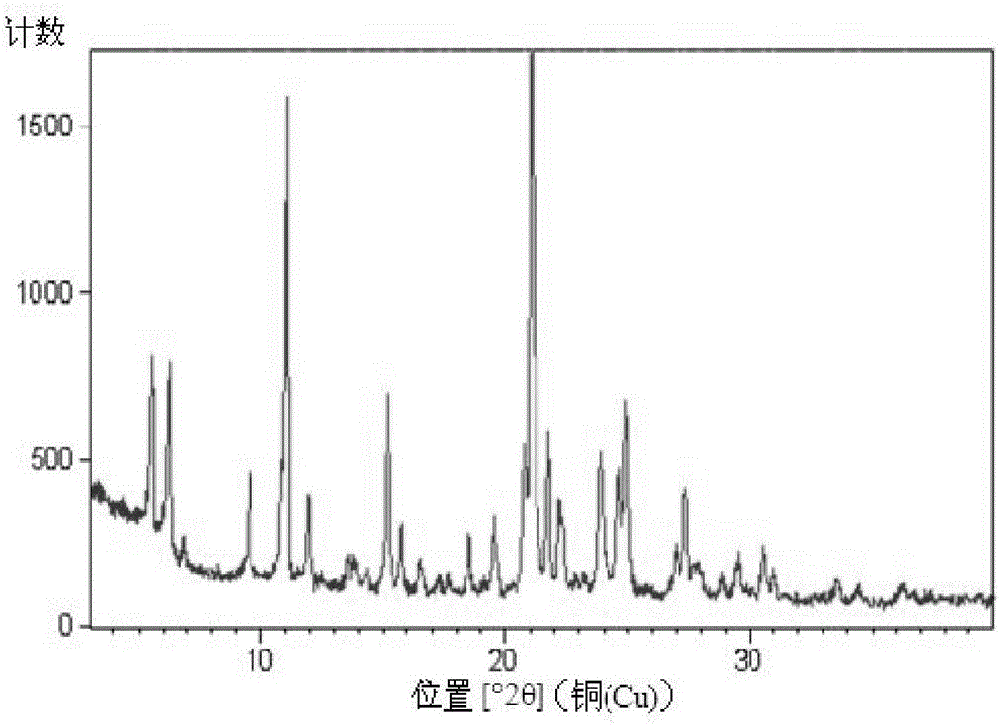
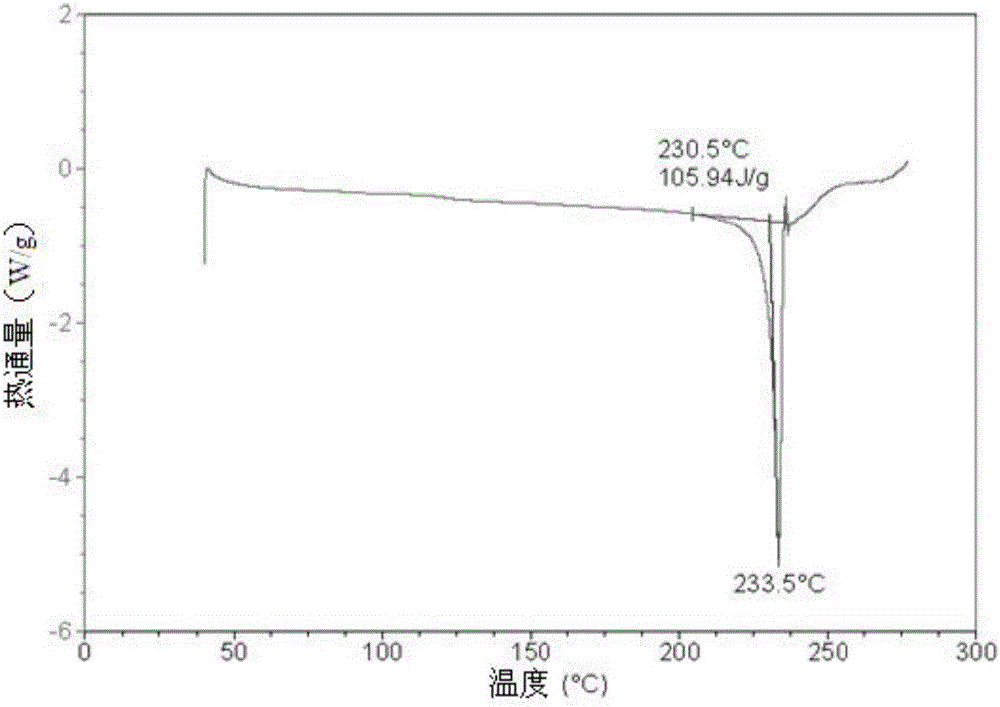
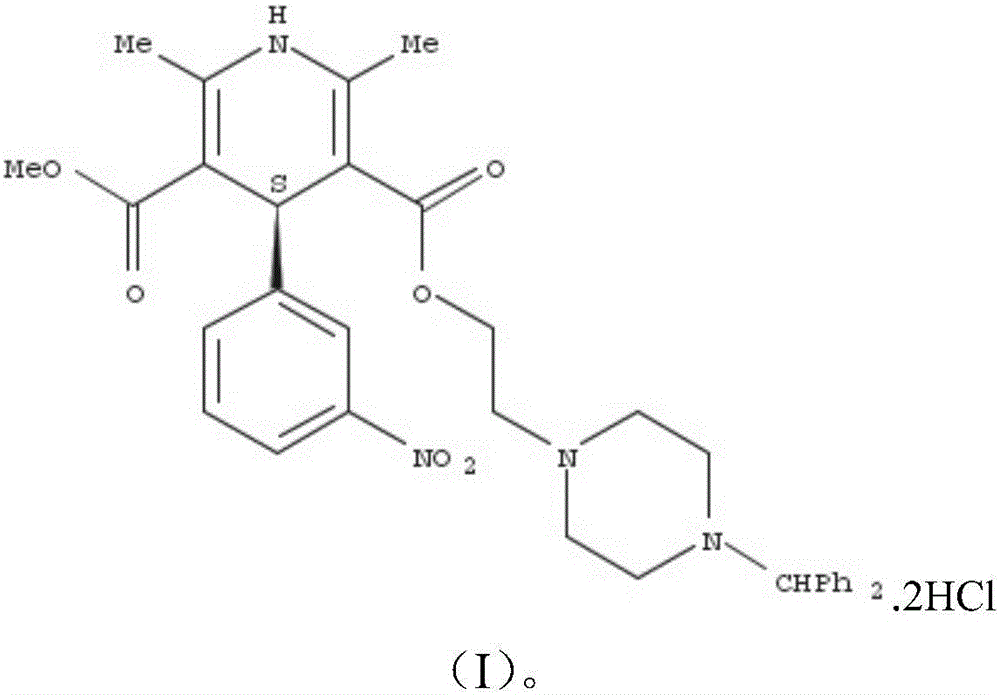
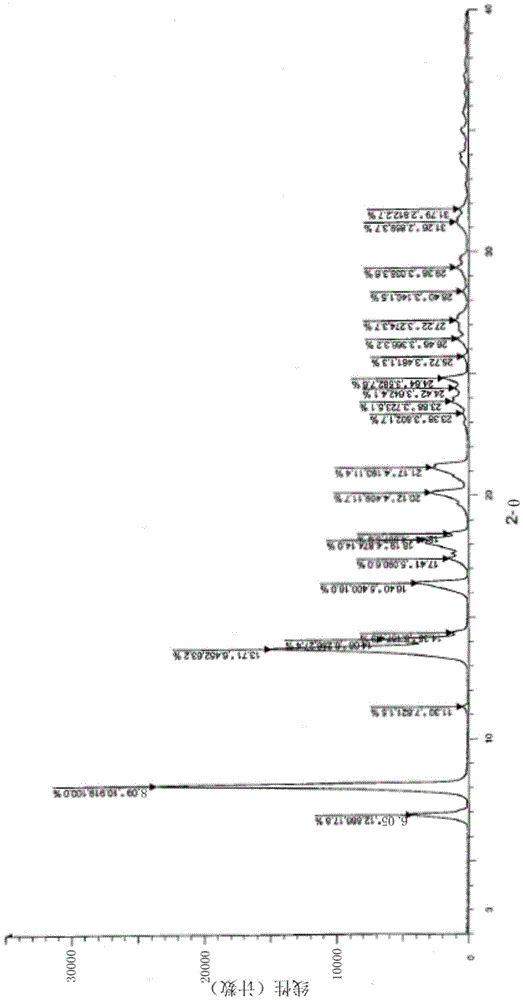
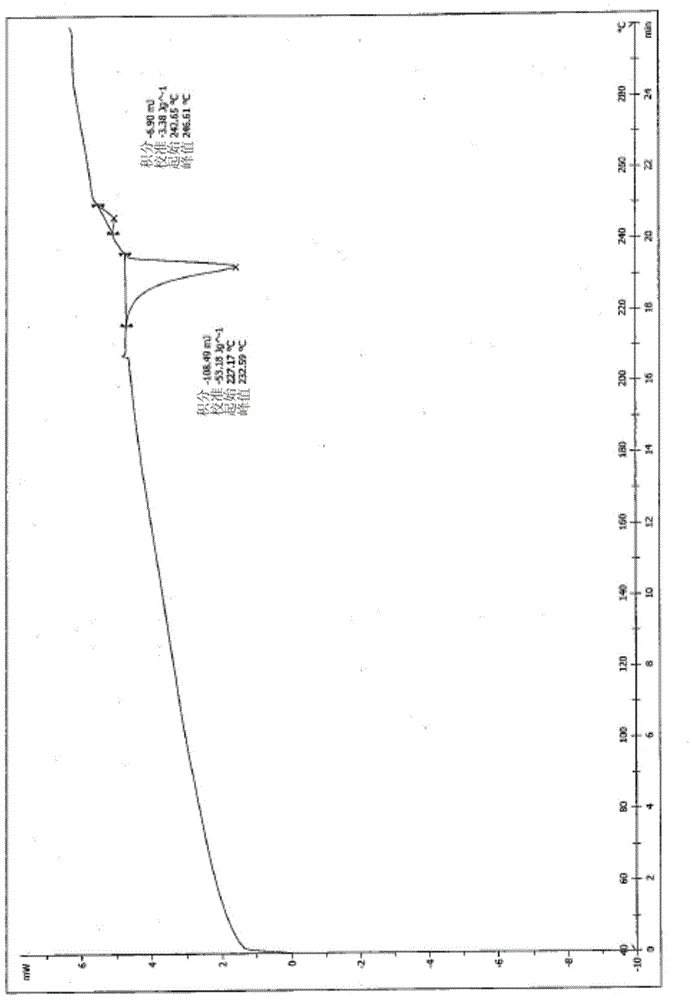
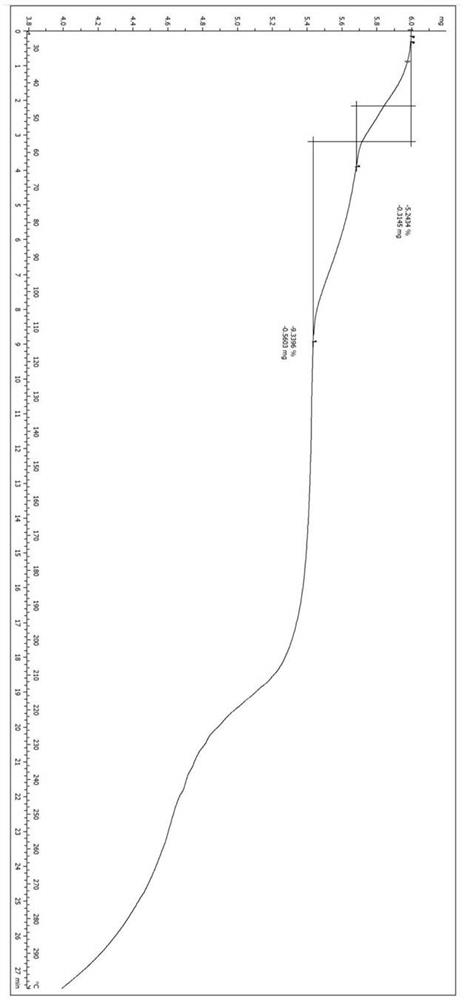
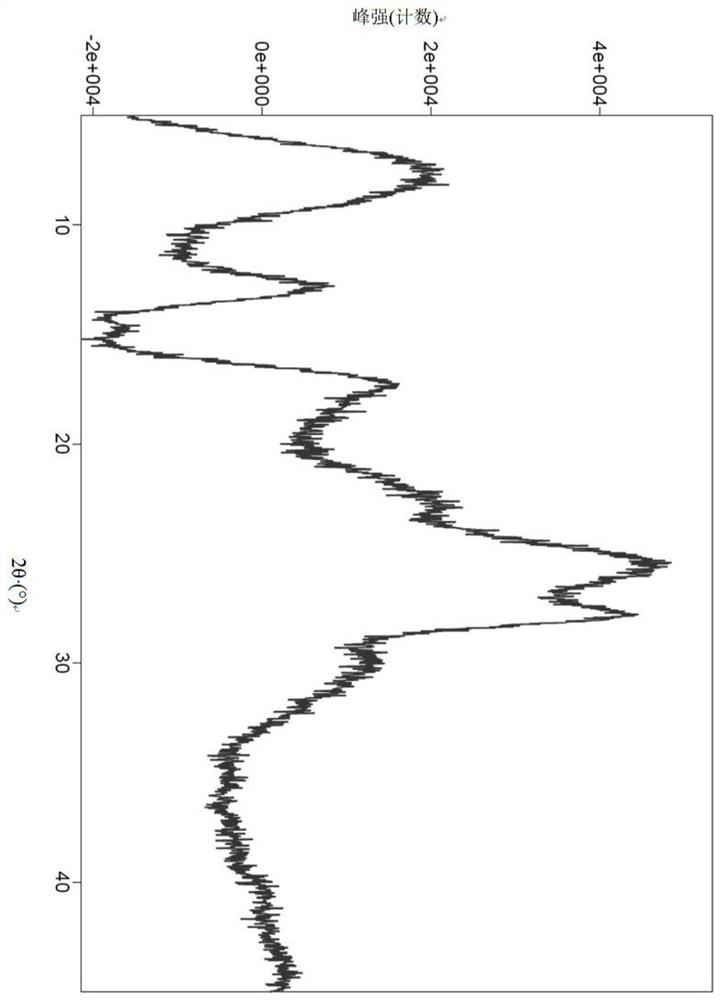
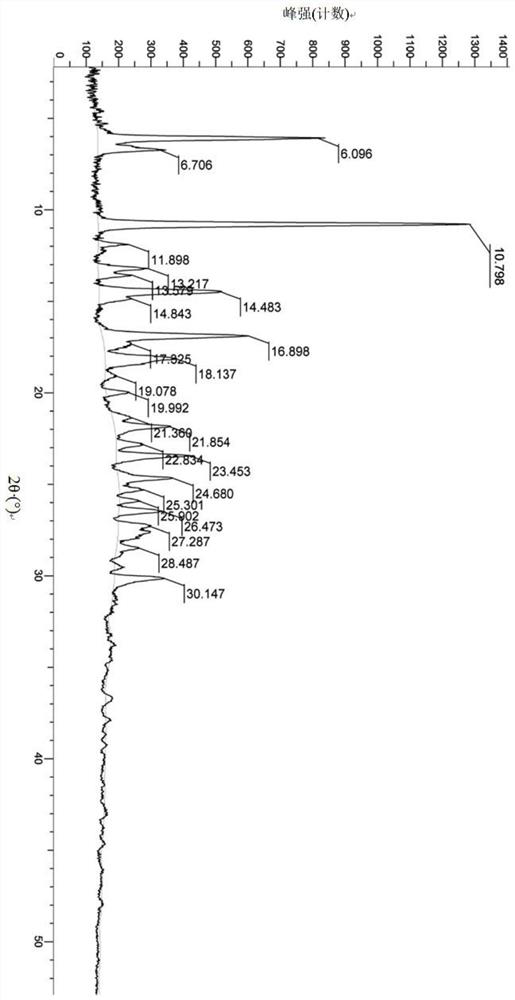
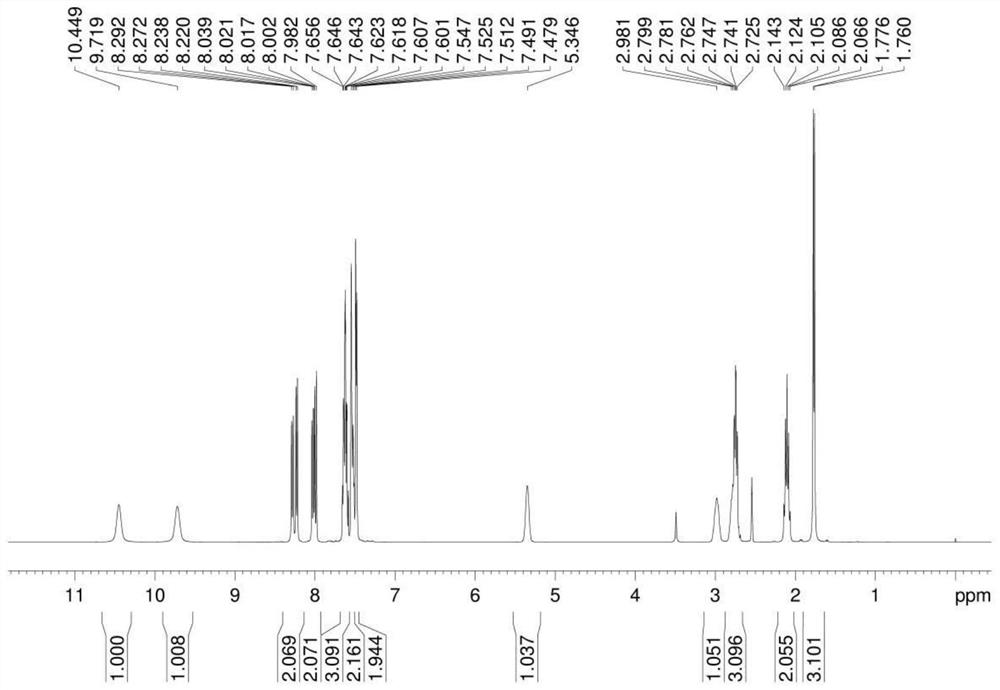
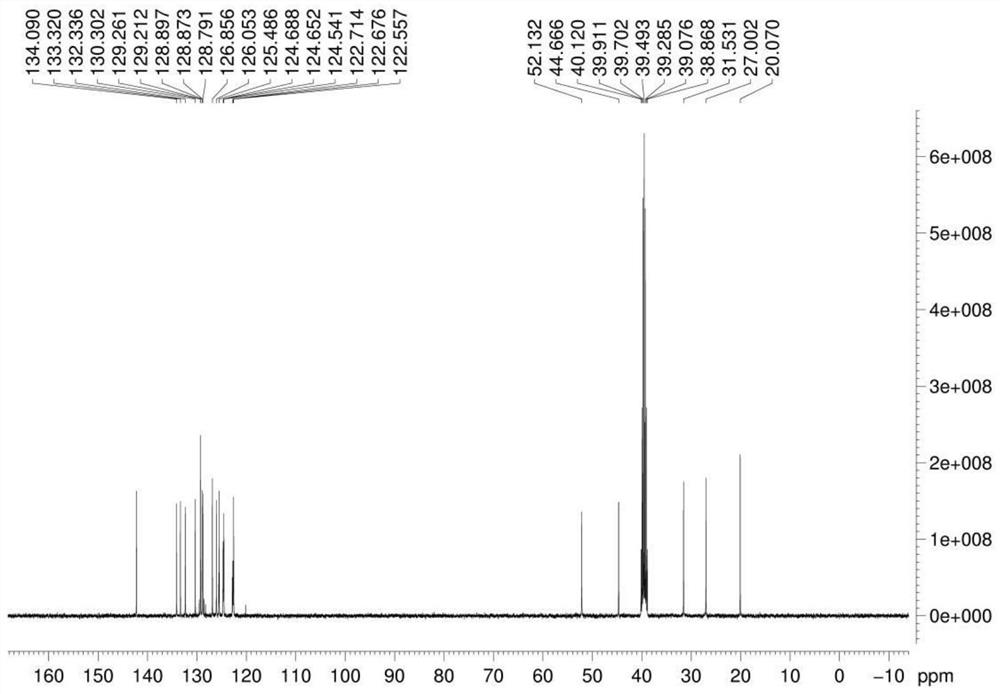
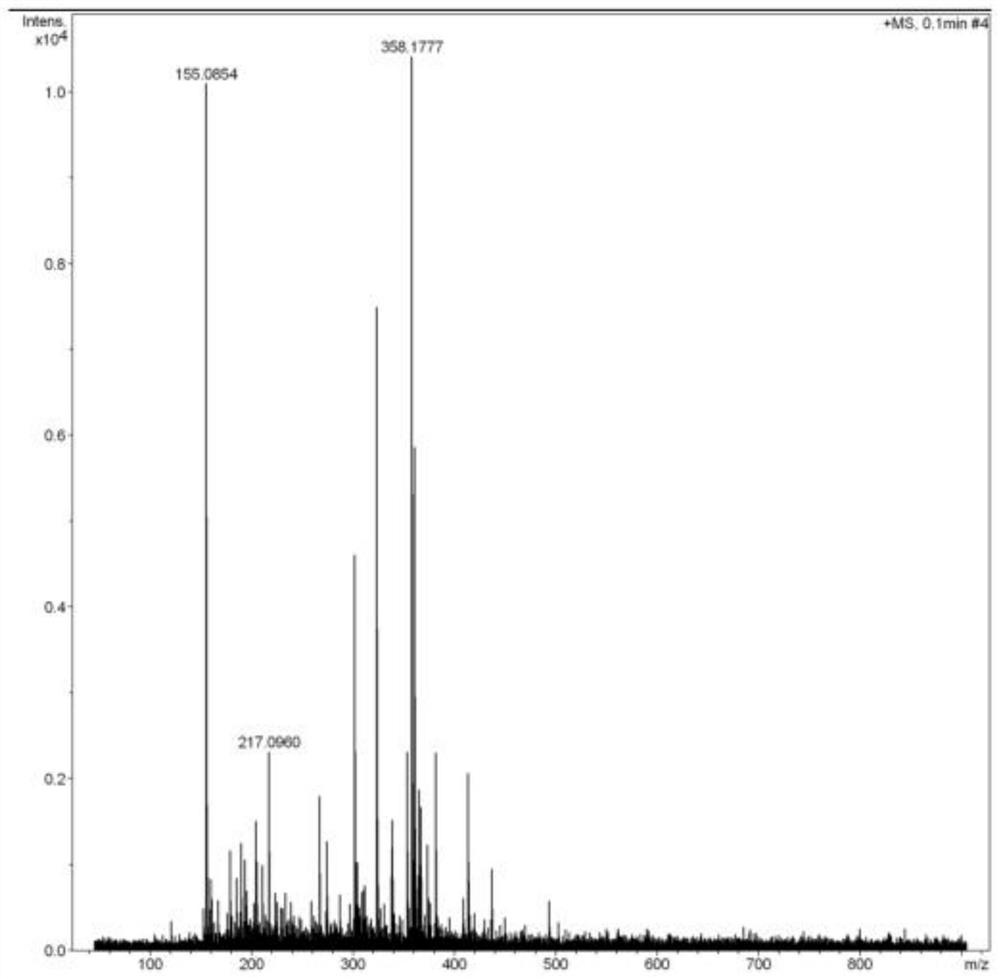
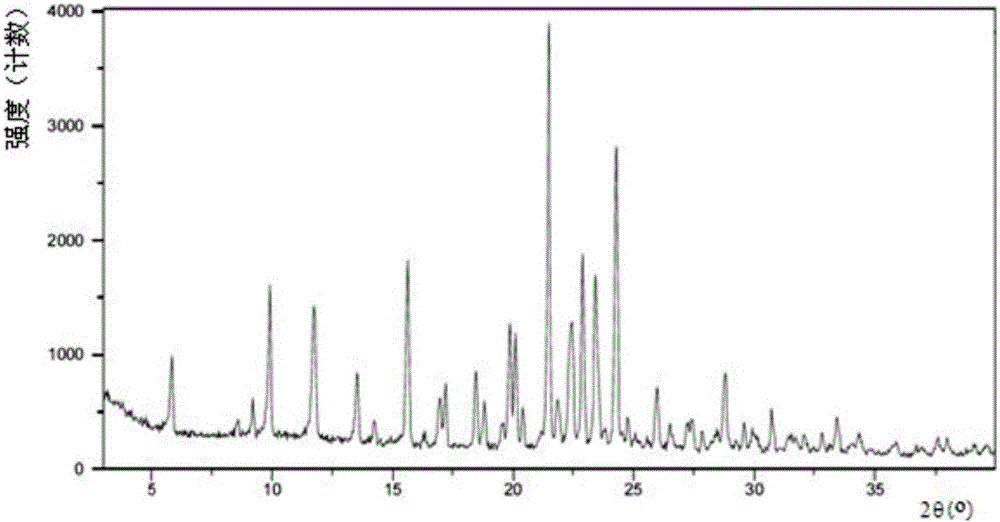
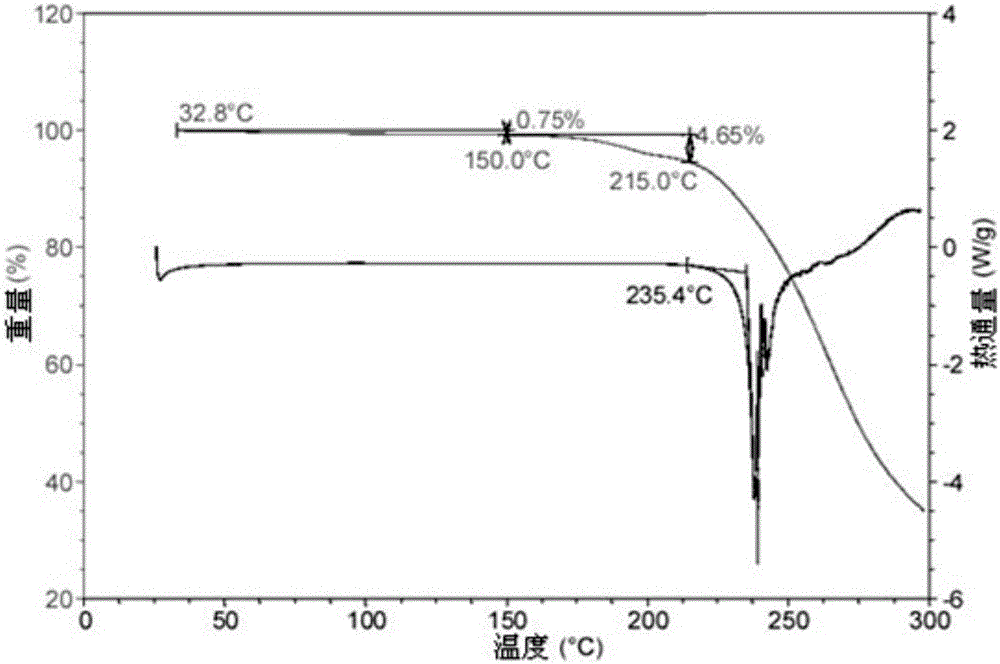
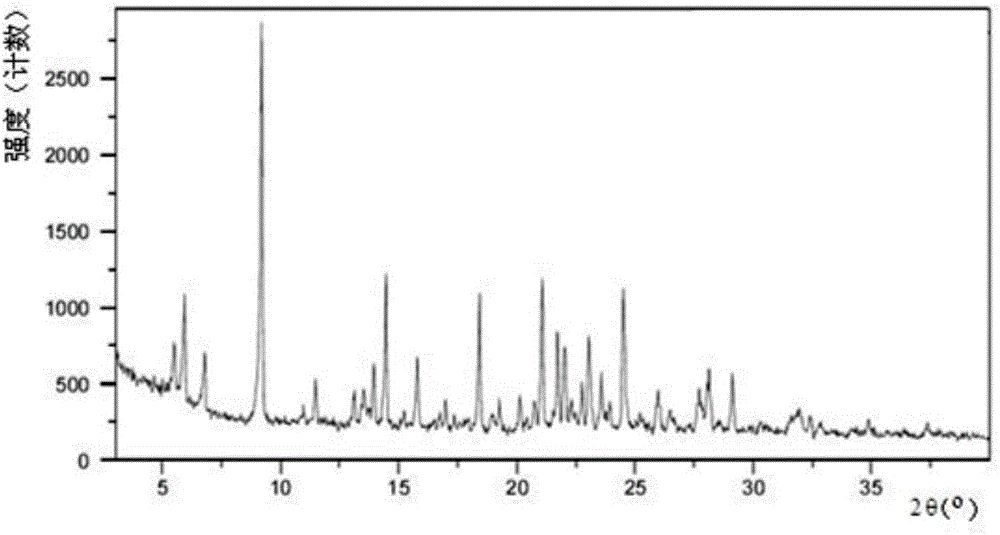
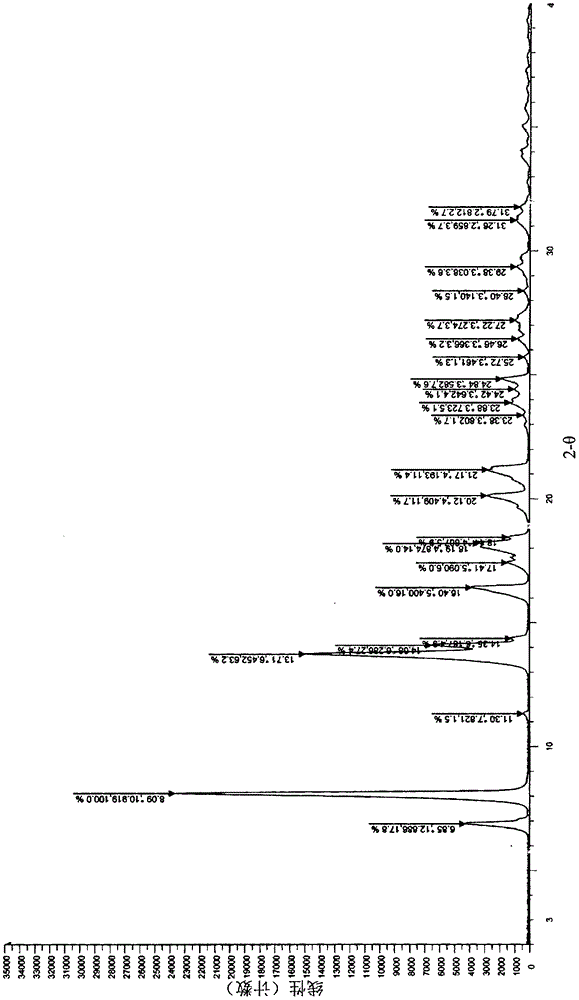
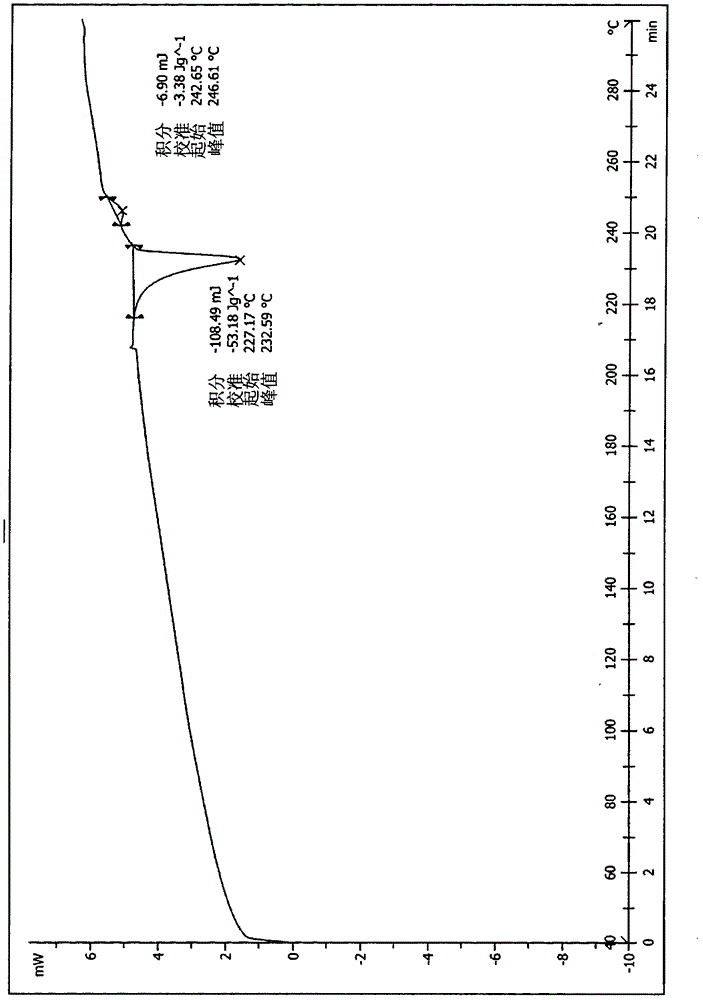
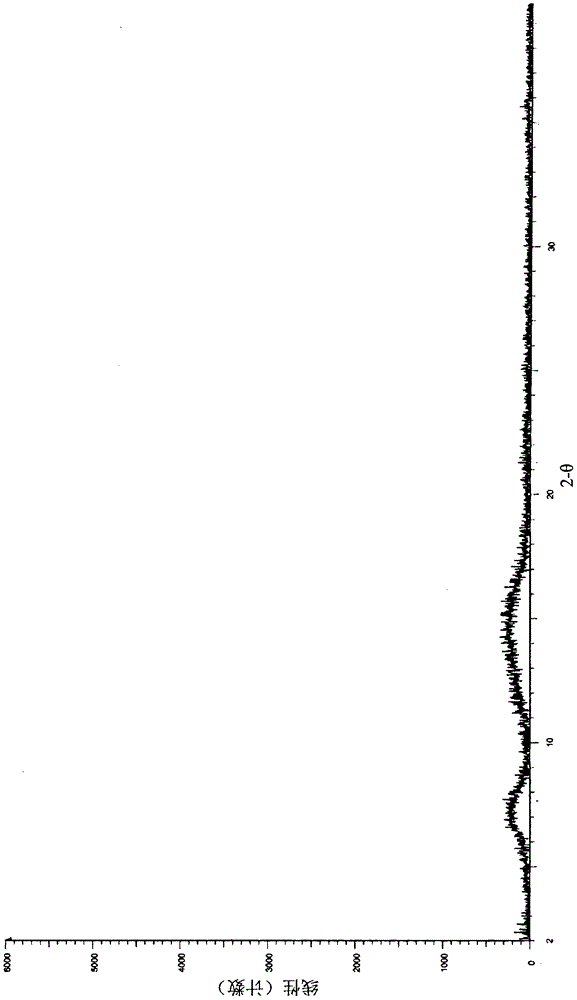
A salt of heteroarylo[4,3-c]pyrimidin-5-amine derivatives, crystal form of the salt and preparation method thereof
ActiveCN110903291BSolubility advantageStability advantageOrganic active ingredientsNervous disorderArylCombinatorial chemistry
The invention relates to a salt of a heteroarylo[4,3-c]pyrimidine-5-amine derivative, a crystal form of the salt and a preparation method. Specifically, the present invention relates to a salt of a compound of formula I, a crystal form of the salt and a preparation method. The crystal form of the salt of the compound of formula I in the present invention has good crystal stability and can be better used clinically.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Pharmaceutical salts, crystal form and preparation method of prolyl hydroxylase inhibitor
ActiveCN110240562ALittle change in purityGood chemical stabilityOrganic compound preparationOrganic chemistry methodsMedicinal chemistryProlyl-Hydroxylase Inhibitors
The invention relates to pharmaceutical salts, a crystal form and a preparation method of a prolyl hydroxylase inhibitor, in particular to the pharmaceutical salts, the crystal form and the preparation method of the compound shown in formula (I). The new pharmaceutical salts and the new crystal form have good stability, thus being better applied to clinic treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Iptakalim hydrochloride B crystal form and preparation method thereof
ActiveCN112174834AHigh crystal purityHigh purityOrganic active ingredientsAmino compound purification/separationBioavailabilityMedicinal chemistry
The invention relates to an iptakalim hydrochloride B crystal form and a preparation method thereof, and the obtained iptakalim hydrochloride B crystal form has good crystal form stability and chemical stability, has good bioavailability, and can be better used for clinical treatment.
Owner:江苏恩华赛德药业有限责任公司 +1
Method for preparing Linezolid
The invention provides a method for preparing Linezolid. The preparation method has the advantages of few total reaction steps of the synthetic route, high yield, mild reaction of each step of the synthesis, no need of special reagent and device, and simple operation, and is suitable for large scale industrial production. High-purity of Linezolid can be prepared by the method, and medicinal requirements can be satisfied.
Owner:吉林省博大伟业制药有限公司
Crystal form of quinazoline compound and preparation method thereof
PendingCN113717111AEfficient selectionImprove stabilityOrganic active ingredientsOrganic chemistry methodsSolubilityPharmaceutical Substances
The invention relates to a crystal form C of (2E)-N-[4-[(3-chloro-4-fluorophenyl) amino]-7-methoxy-6-quinazolinyl]-4-(1-piperidinyl)-2-buteneamide, a preparation method thereof and a medicine containing the crystal form C. The crystal form C is an anhydrous substance, Cu-K alpha radiation is used, and the crystal form C has an X-ray powder diffraction pattern with characteristic peaks at diffraction angles 2theta of 5.6 + / - 0.2 degrees, 10.5 + / - 0.2 degrees and 12.8 + / - 0.2 degrees. The crystal form C is the anhydrous substance and has obvious advantages in medicine development compared with an existing solvate, in addition, the solubility of the crystal form C is remarkably improved compared with that of the existing solvate on the premise that good stability is kept.
Owner:CRYSTAL PHARMATECH CO LTD
Rapid melting misoprostol vaginal composition as well as preparation method and application of same
ActiveCN102697746BGood content uniformityAccurate doseOrganic active ingredientsPharmaceutical non-active ingredientsGynecologyFiller Excipient
Owner:REGENEX PHARMA LTD
A kind of preparation method of lenalidomide and nicotinamide co-crystal
ActiveCN105837556BImprove stabilityImprove solubilityOrganic chemistry methodsSolubilityOrganic solvent
Owner:SHANGHAI UNIV OF ENG SCI
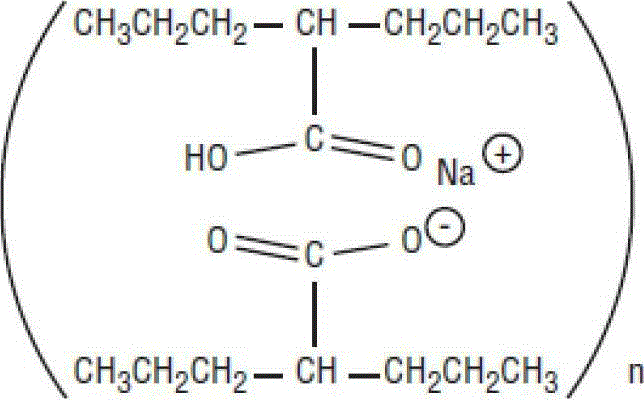
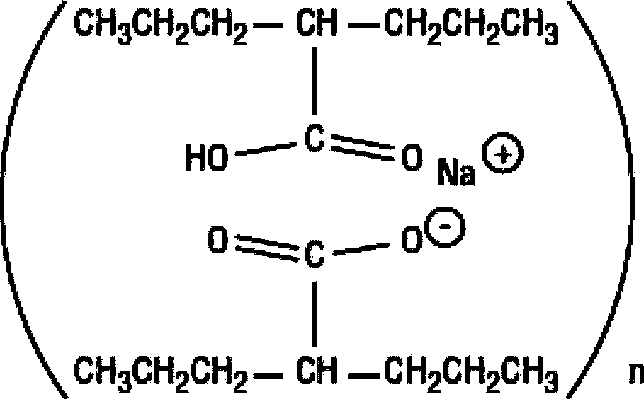
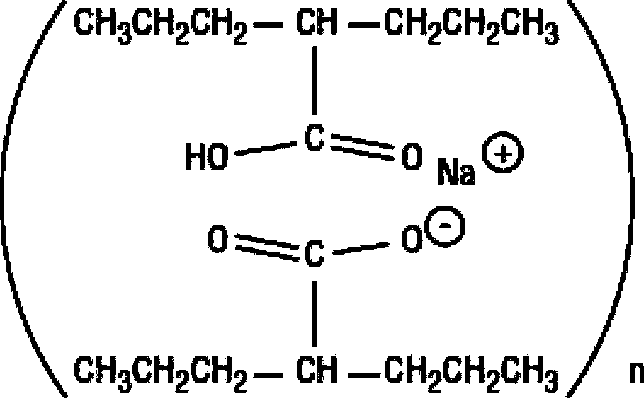
The method for recycling divalproex sodium crystallization mother liquor
ActiveCN103664574BHigh purityEfficient recyclingPreparation from carboxylic acid saltsOrganic layerFractionation
The invention provides a method of recycling divalproex sodium crystallization mother liquor. The method comprises the following steps: (1), concentrating the divalproex sodium crystallization mother liquor to obtain a solid-state mixture containing divalproex sodium; (2), carrying out acidizing treatment onto the solid-state mixture obtained in the step (1), controlling pH to 0.5-4.5 to obtain an organic layer; and (3), carrying out fractionation onto the organic layer obtained in the step (2) to obtain valproic acid. Yield of the valproic acid obtained by the method disclosed by the invention can be as high as 95%, and purity of the valproic acid is not lower than 99.1%, so that medicinal requirements can be satisfied. Moreover, the method disclosed by the invention is simple and easy to implement and easy to control in a reaction process, lowers actual production cost, and satisfies large-scale industrial production requirements.
Owner:NEW FOUNDER HLDG DEV LLC +2
Crystal form of benzimidazole derivative and preparation method thereof
ActiveCN112745268AA crystal form stability advantageAdvantages of crystal stabilityOrganic active ingredientsSenses disorderBenzimidazole derivativePharmaceutical drug
The invention relates to a crystal form of a benzimidazole derivative and a preparation method thereof. Specifically, the disclosure relates to a crystal form A of a compound shown in a formula I and a preparation method thereof. The crystal form A of the compound shown in the formula I has the advantages in the aspects of stability and hygroscopicity, is more suitable for medicine development, can meet the medicinal requirements of production, transportation and storage, and is stable in production process, repeatable and controllable and suitable for industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method of recycling divalproex sodium crystallization mother liquor
ActiveCN103664574AReduce manufacturing costHigh yieldPreparation from carboxylic acid saltsDivalproex SodiumSolid-state
The invention provides a method of recycling divalproex sodium crystallization mother liquor. The method comprises the following steps: (1), concentrating the divalproex sodium crystallization mother liquor to obtain a solid-state mixture containing divalproex sodium; (2), carrying out acidizing treatment onto the solid-state mixture obtained in the step (1), controlling pH to 0.5-4.5 to obtain an organic layer; and (3), carrying out fractionation onto the organic layer obtained in the step (2) to obtain valproic acid. Yield of the valproic acid obtained by the method disclosed by the invention can be as high as 95%, and purity of the valproic acid is not lower than 99.1%, so that medicinal requirements can be satisfied. Moreover, the method disclosed by the invention is simple and easy to implement and easy to control in a reaction process, lowers actual production cost, and satisfies large-scale industrial production requirements.
Owner:新方正控股发展有限责任公司 +2
S-manidipine hydrochloride polymorph and preparation method thereof
ActiveCN106083698BGood crystal stabilityStable production processOrganic active ingredientsOrganic chemistry methodsManidipine hydrochlorideActive component
The invention relates to multiple crystal forms of S-Montelukast sodium and a preparation method thereof, in particular to a crystal form II, a crystal form III and a crystal form IV of S-Montelukast sodium, the preparation method of the crystal forms, pharmaceutical composition containing the crystal forms and a medical application of the crystal forms and the pharmaceutical composition. The crystal form II, the crystal form III and the crystal form IV of S-Montelukast sodium have excellent physicochemical properties and good stability, and are suitable for a preparation technological process and long-term storage. The pharmaceutical composition adopting compounds in the crystal forms as active components can be used for treating diseases such as hypertension and the like.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Crystal form and preparation method of a kind of oxopyridine amide derivative
ActiveCN112004810BSolubility advantageStability advantageOrganic active ingredientsOrganic chemistry methodsPerylene derivativesPyridine
The invention relates to a crystal form and a preparation method of oxopyridine amide derivatives. Specifically, the present invention relates to crystal forms A, B, C, D, E, and F of the compound of formula (I) and a preparation method thereof. The crystal form of the compound of formula (I) of the present invention has good crystal stability and can be better used in clinic.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Crystal form of renal outer medullary potassium channel inhibitor and preparation method of crystal form
ActiveCN109879863AImprove solubilityHigh purityOrganic active ingredientsCardiovascular disorderRENAL OUTER-MEDULLARY POTASSIUM CHANNELPotassium
The invention relates to a crystal form of a renal outer medullary potassium channel inhibitor and a preparation method of the crystal form. Specifically, the invention relates to an IV crystal form of an L-tartrate of a renal outer medullary potassium channel (ROMK) inhibitor and a preparation method of the IV crystal form. The IV crystal form has good chemical stability and crystal form stability, a used crystallization solvent is low in toxicity and residue, and the IV crystal form can be better used for clinical treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +2
Itakaline hydrochloride A crystal form and preparation method thereof
ActiveCN112174833BHigh crystal purityHigh purityOrganic active ingredientsAmino compound purification/separationCombinatorial chemistryBioavailability
The present invention relates to a crystal form A of ertacarline hydrochloride and a preparation method thereof. The crystal form A of ertacarline hydrochloride obtained in the present invention has good crystal form stability and chemical stability, and has good bioavailability It can be better used in clinical treatment.
Owner:江苏恩华赛德药业有限责任公司 +1
A kind of preparation method of chloromethyl hexafluoroisopropyl ether
ActiveCN108689808BHigh purityReduced content of acetal impuritiesOrganic compound preparationEther preparationPolymer scienceIsopropyl ether
The invention relates to a preparation method of chloromethyl hexafluoroisopropyl ether. Specifically, the present invention relates to a method for preparing chloromethyl hexafluoroisopropyl ether, wherein the solvent used in the reaction contains chloromethyl hexafluoroisopropyl ether. The preparation method introduces its own product as a solvent, which not only solves the solidification phenomenon of the reaction system during the reaction process, but also greatly improves the purity of the product, which is beneficial to industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +2
A kind of crystal form of bisulfate salt of jak kinase inhibitor and preparation method thereof
ActiveCN108779122BImprove solubilityHigh purityOrganic active ingredientsOrganic chemistryThiadiazolesPyrrole
The present invention relates to a crystal form of bisulfate of a JAK kinase inhibitor and a preparation method therefor. Specifically, the present invention relates to a crystal form III of (3aR,5s,6aS)-N-(3-methoxy-1,2,4-thiadiazole-5-base)-5-(methyl(7H-pyrrolo[2,3-d]pyrimidine-4-base)amino)hexahydrocyclopenta[c]pyrrole-2(1H)-formamide bisulfate, and a preparation method therefor. The crystal form III of a compound represented by formula (I) in the present invention has good crystal stability, and a used crystallization solvent has low toxicity and low residue, and can be better used in clinical treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Recycling method of divalproex sodium crystallization mother liquor
ActiveCN103664570AHigh purityEfficient recyclingPreparation from carboxylic acid saltsOrganic compound preparationInorganic saltsFractionation
The invention provides a recycling method of divalproex sodium crystallization mother liquor. The method comprises the following steps: 1) concentrating the divalproex sodium crystallization mother liquor to obtain a solid-state mixture containing divalproex sodium; 2) mixing the solid-state mixture obtained in the step 1) with an alkaline solution to dissolve the solid-state mixture to further obtain a mixed solution; 3) mixing the mixed solution obtained in the step 2) with an inorganic salt to produce a valproate precipitate, and separating to obtain the valproate precipitate; 4) mixing the valproate precipitate obtained in the step 3) with an acid solution to obtain an organic layer, and performing fractionation on the organic layer to obtain valproic acid. Through the method provided by the invention, the yield of obtained valproic acid can be as high as 93%, the purity is not lower than 99.6%, and valproic acid can meet medicinal requirements; the method provided by the invention has the advantages of simplicity, easiness in operation, easiness in control of reaction process and effect of reducing actual production cost, and is in line with requirements of industrial mass production.
Owner:NEW FOUNDER HLDG DEV LLC +2
A sulfate salt of an intestinal type 2b sodium phosphate cotransporter inhibitor and its crystalline form
ActiveCN107082773BImprove stabilityReduced stabilityOrganic active ingredientsMetabolism disorderIntestinal typeSodium phosphates
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
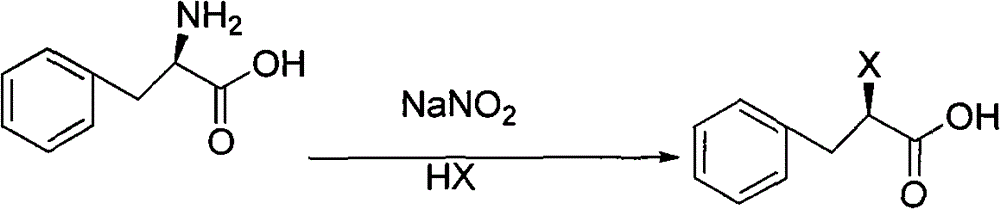
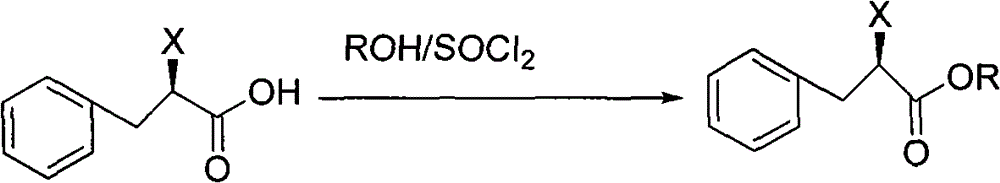
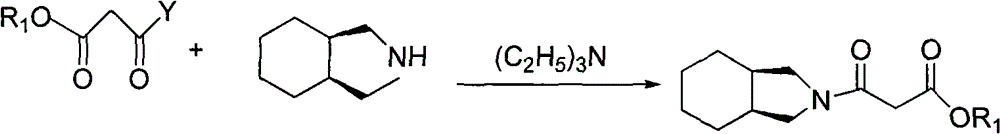
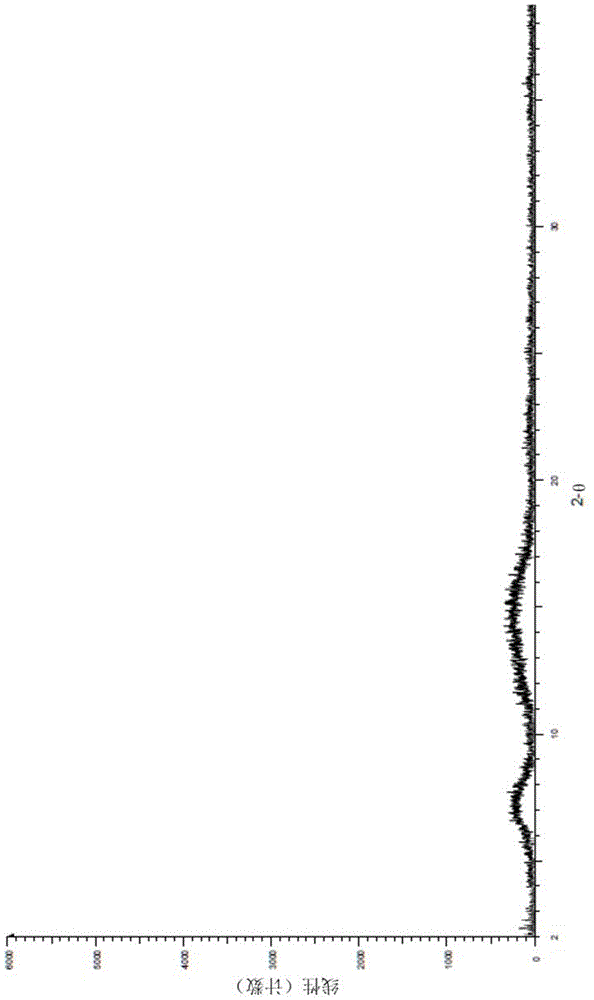
![A salt of heteroarylo[4,3-c]pyrimidin-5-amine derivatives, crystal form of the salt and preparation method thereof A salt of heteroarylo[4,3-c]pyrimidin-5-amine derivatives, crystal form of the salt and preparation method thereof](https://images-eureka.patsnap.com/patent_img/0f75bd90-3bce-45d1-9dcf-222c698b67ae/HDA0002200280960000011.png)
![A salt of heteroarylo[4,3-c]pyrimidin-5-amine derivatives, crystal form of the salt and preparation method thereof A salt of heteroarylo[4,3-c]pyrimidin-5-amine derivatives, crystal form of the salt and preparation method thereof](https://images-eureka.patsnap.com/patent_img/0f75bd90-3bce-45d1-9dcf-222c698b67ae/HDA0002200280960000021.png)
![A salt of heteroarylo[4,3-c]pyrimidin-5-amine derivatives, crystal form of the salt and preparation method thereof A salt of heteroarylo[4,3-c]pyrimidin-5-amine derivatives, crystal form of the salt and preparation method thereof](https://images-eureka.patsnap.com/patent_img/0f75bd90-3bce-45d1-9dcf-222c698b67ae/HDA0002200280960000031.png)