Crystalline form of androgen receptor inhibitor and preparation method thereof
A technology of crystallization and interplanar spacing, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve problems such as easy agglomeration, poor product stability, difficult filtration, etc., and achieve repeatable and controllable production process and stable crystal form Good performance and stable production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
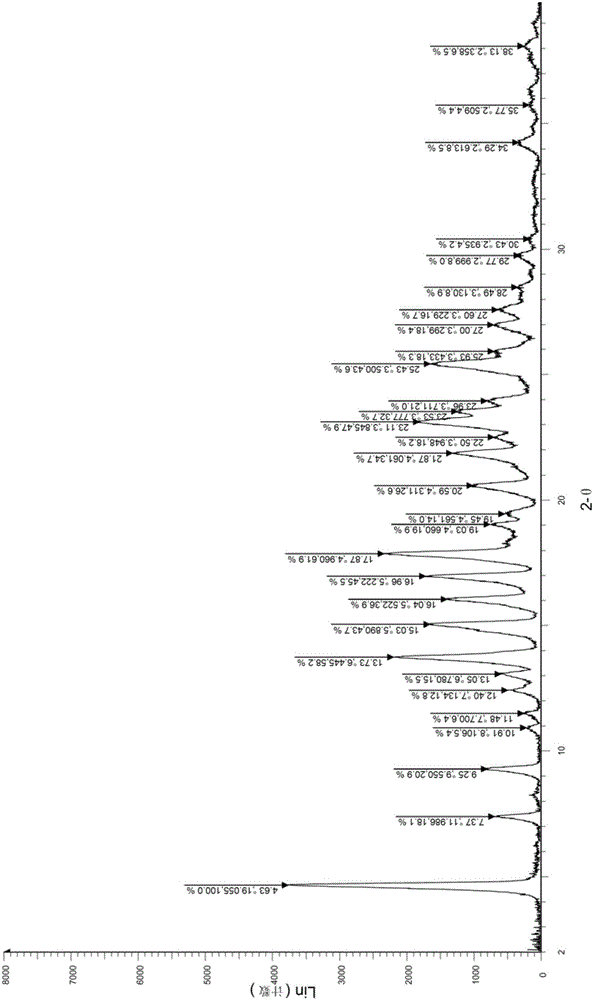
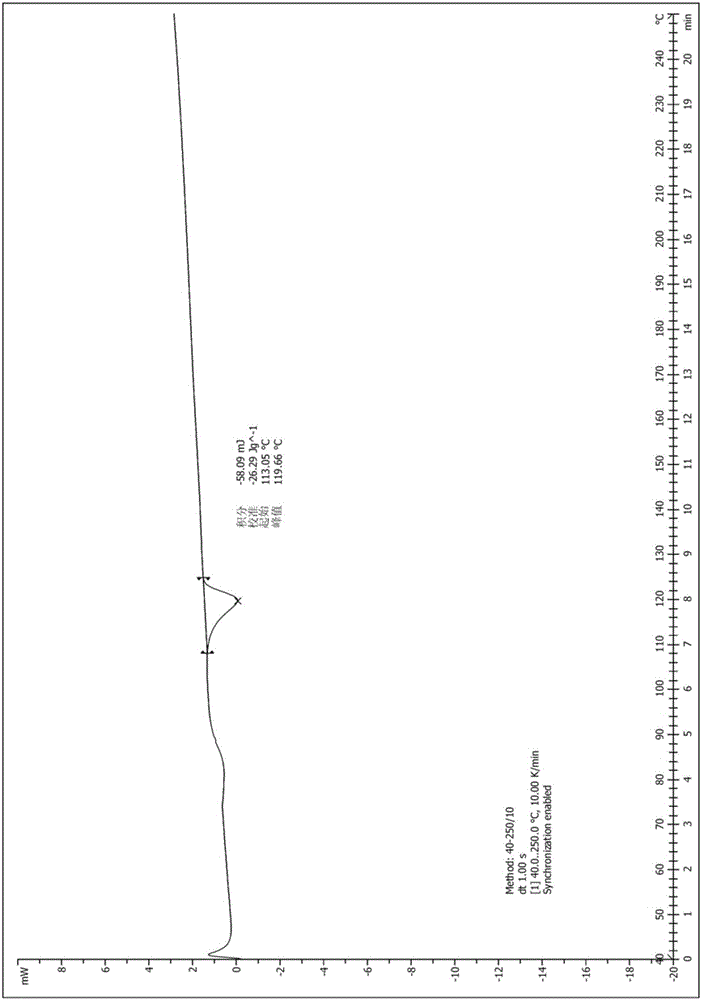
[0035] Take (1.0g, 2.09mmol) the compound represented by formula (I) (prepared according to the method provided by WO2014036897A1) into a 25ml single-necked bottle, add 5.0ml of methanol, heat to reflux to dissolve, continue to reflux for 10min, cool, stir and crystallize, pump Filter and dry to obtain 516mg of solid, the yield is 51.6%. The X-ray diffraction spectrum figure of this crystalline sample is shown in figure 1 . The crystallization at about 4.63 (19.06), 7.37 (11.99), 9.25 (9.55), 10.91 (8.11), 11.48 (7.70), 12.40 (7.13), 13.05 (6.78), 13.73 (6.45), 15.03 (5.89), 16.04 (5.52), 16.96(5.22), 17.87(4.96), 19.03(4.66), 19.45(4.56), 20.59(4.31), 21.87(4.06), 22.50(3.95), 23.11(3.85), 23.53(3.78), 23.96 (3.71), 25.43(3.50), 27.00(3.30), 27.60(3.23), 29.77(3.00) have characteristic peaks. See the DSC spectrum figure 2 , with a sharp melting endothermic peak at 119°C, this crystal form is defined as II crystal form.
Embodiment 2
[0037]Take (1.0g, 2.09mmol) the compound shown in formula (I) (prepared according to Example 1) in a 25ml single-necked bottle, add 5.0ml of ethanol, heat to reflux to dissolve, continue to reflux for 10min, cool, stir and crystallize, and suction filter , and dried to obtain 633 mg of solid, with a yield of 63.3%. Its X-diffraction and DSC patterns are researched and compared, and it is determined that the product is in the II crystal form.
Embodiment 3
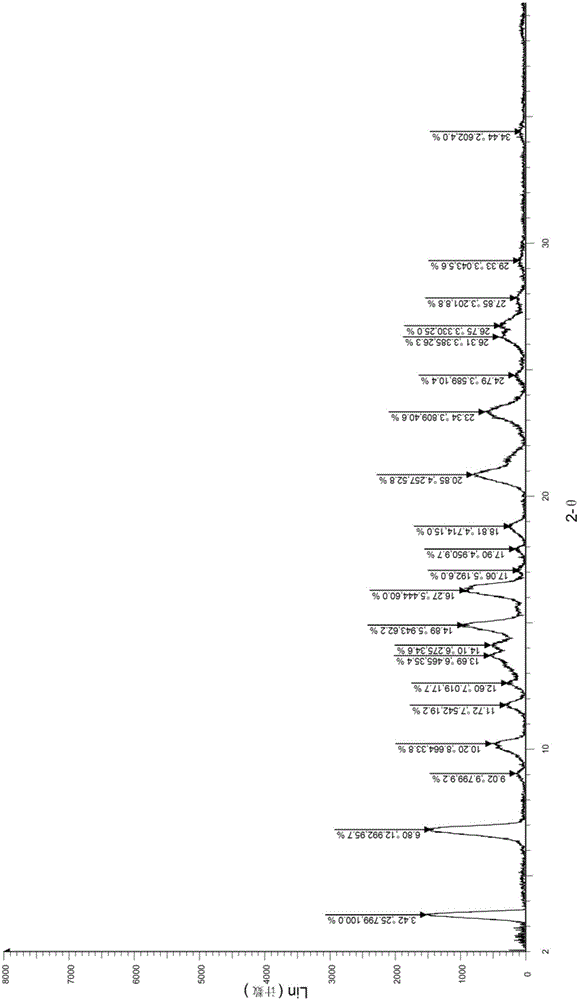
[0039] Repeat all the operations of WO2014036897A1 Example 44, (R)-4-(3-(4-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)benzene Base)-4,4-dimethyl-5-keto-2-thioimidazolin-1-yl)-2-(trifluoromethyl)benzonitrile (2.2g, 4.20mmol) was dissolved in 100mL acetic acid, Add 50mL of water, raise the temperature to 70°C and stir for 1h. The reaction solution is concentrated under reduced pressure to remove acetic acid, 100mL of water and 100mL of ethyl acetate are added, and the layers are separated. The organic phase is washed with saturated sodium bicarbonate solution and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure and purified by silica gel chromatography to obtain crystals of Compound I (1.1 g, 55.0%). The X-ray diffraction and DSC spectra of the crystalline sample were studied and compared, and it was confirmed that it was not the II crystal form, and it was defined as the III crystal form here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com