Preparation method of high-purity vinpocetine
A vinpocetine and high-purity technology, which is applied in the field of high-purity vinpocetine preparation, can solve the problems of unsatisfactory impurity profiles in consistency evaluation, and achieve the effects of avoiding highly toxic reagents, simple production operation, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
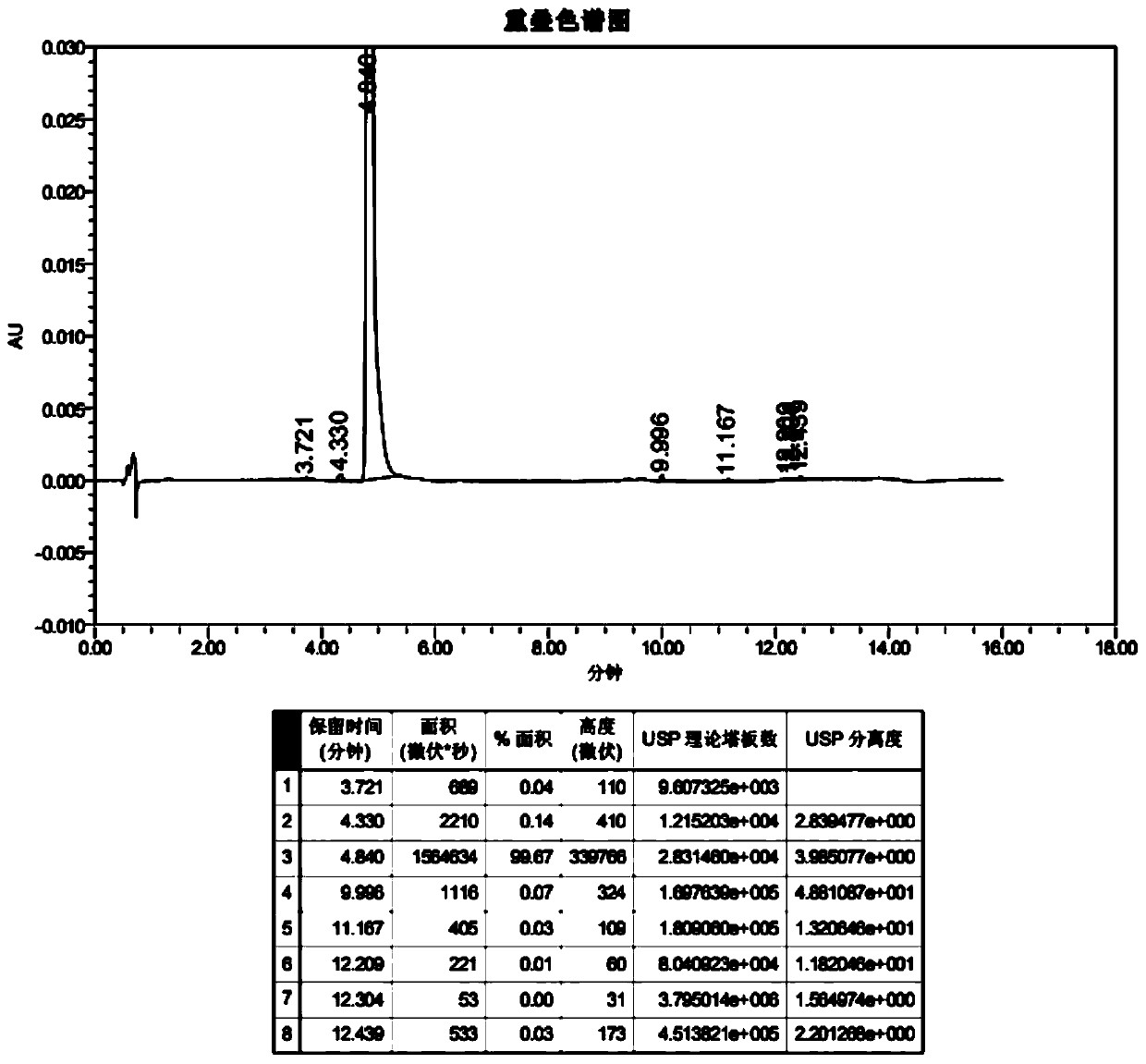
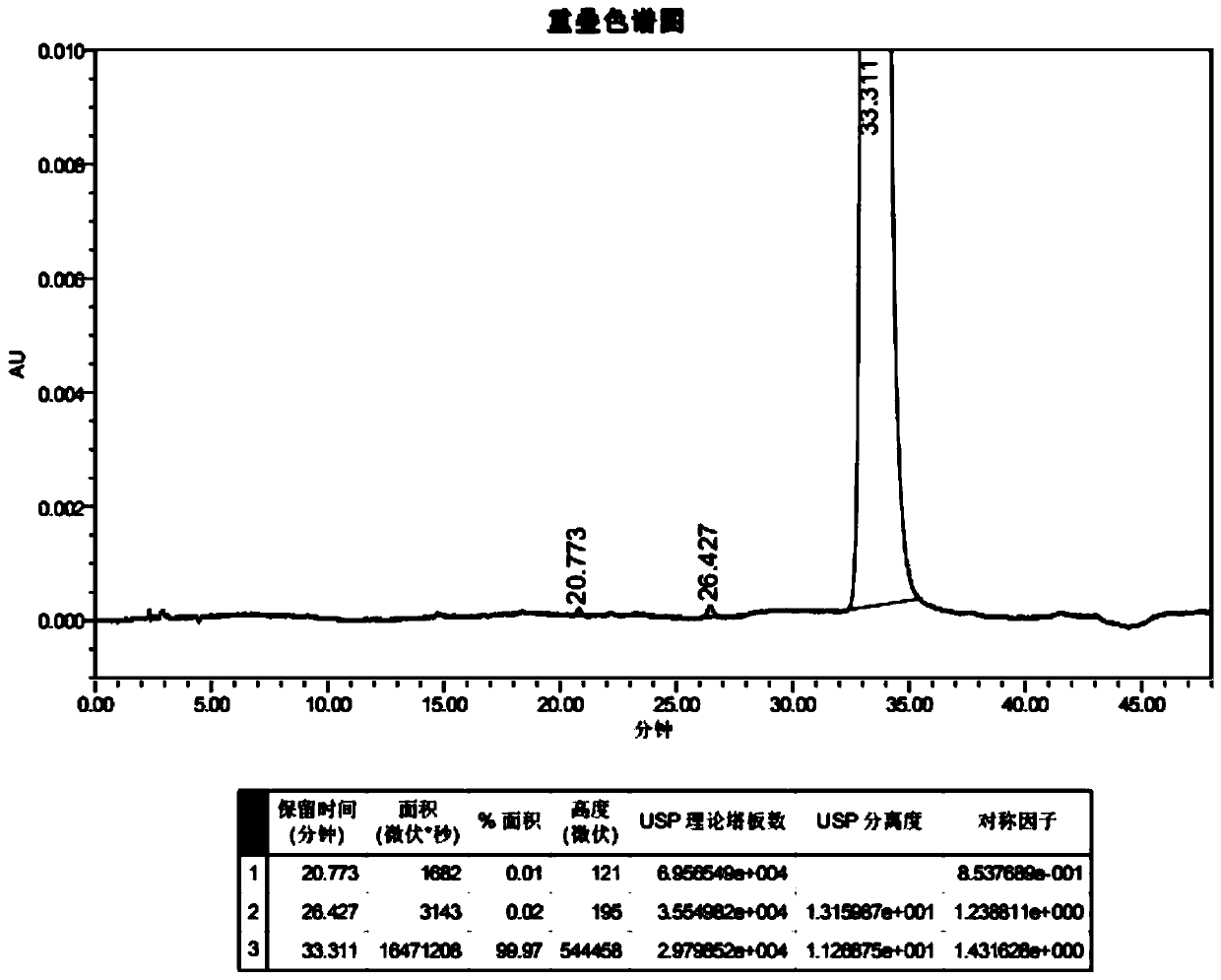
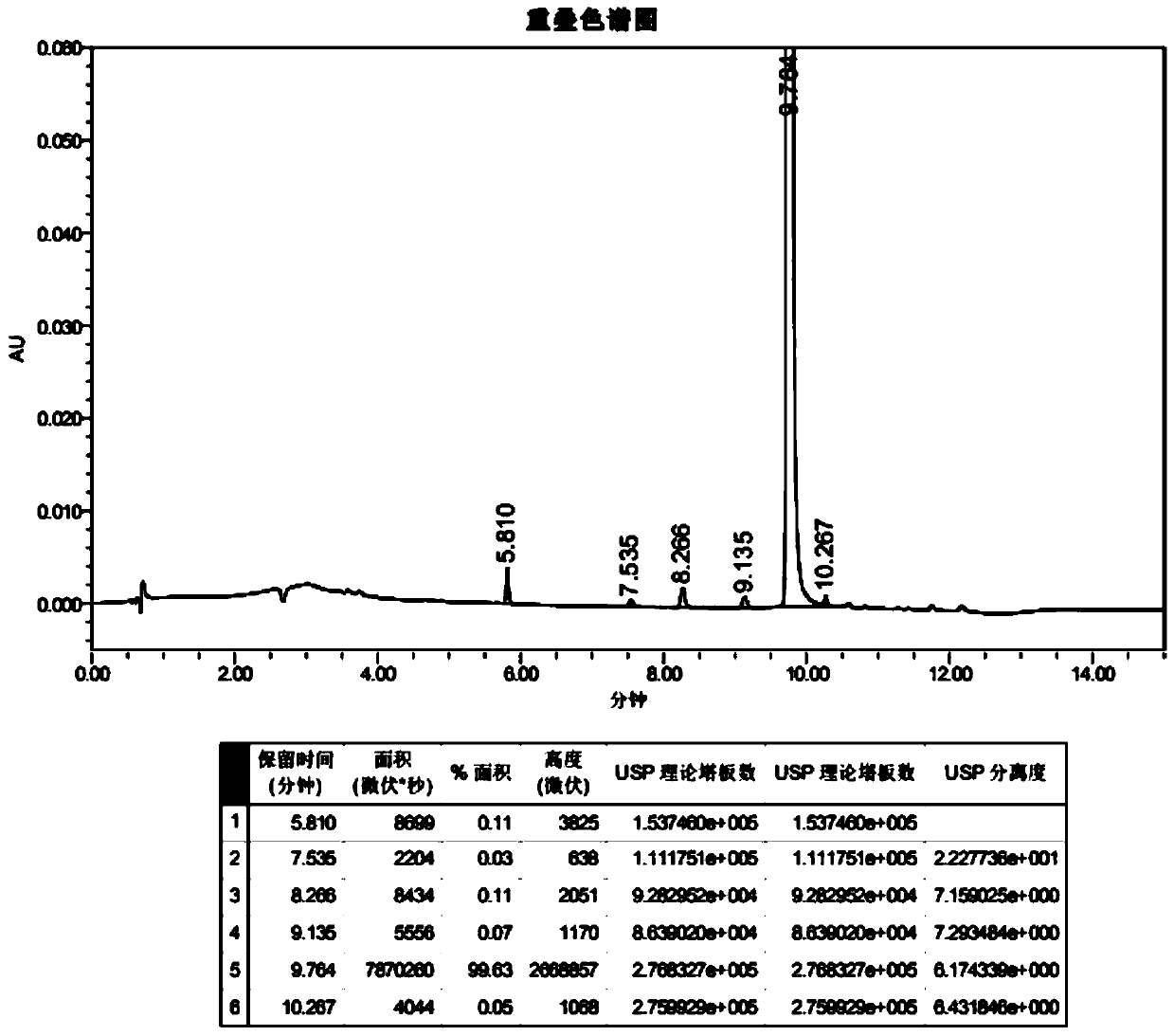
Image
Examples
Embodiment 1
[0044] 1, the preparation of vincamine:
[0045] 1.1 Preparation of 15% (w / w) sodium hydroxide solution: Weigh 17.0kg of purified water and put it into a stainless steel bucket, then add 3.0kg of sodium hydroxide, stir well and let stand for later use.
[0046] 1.2 Add 3.0kg of tabonin, 0.3kg of activated carbon, and 27.0kg of tetrahydrofuran into a 50L double-layer glass reactor, and stir at room temperature for 2 hours for decolorization. Among them, the addition of activated carbon can adsorb toxic substances and prevent catalyst poisoning.
[0047] 1.3 Remove activated carbon by filtration, rinse with 1.5 kg of tetrahydrofuran (to clean residual active ingredients in activated carbon).
[0048] 1.4 Add 0.3kg of 10% palladium carbon to the filtrate and mix well, then transfer to a 50L magnetic drive reactor, N 2 Replaced three times, H 2 Replace three times (use nitrogen to drive off oxygen, prevent hydrogen and oxygen from mixing, causing danger. Another oxygen is not c...
Embodiment 2
[0090] On the basis of Example 1, in step 3.1: add 1.5kg of apovincamine, 12.0kg of absolute ethanol, 45.0g of sodium ethylate, and 3.0kg of dichloromethane into a 50L double-layer glass reactor, and heat up to 60-70°C React for 1 hour. The same detection method as in Example 1 was tested, and the purity was 99.90%.
Embodiment 3
[0092] On the basis of Example 1, in step 3.1: add 1.5kg of apovincamine, 12.0kg of absolute ethanol, 45.0g of sodium ethylate, and 3.0kg of dichloromethane into a 50L double-layer glass reactor, and heat up to 60-70°C React for 4 hours. The same detection method as in Example 1 was tested, and the purity was 99.92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com