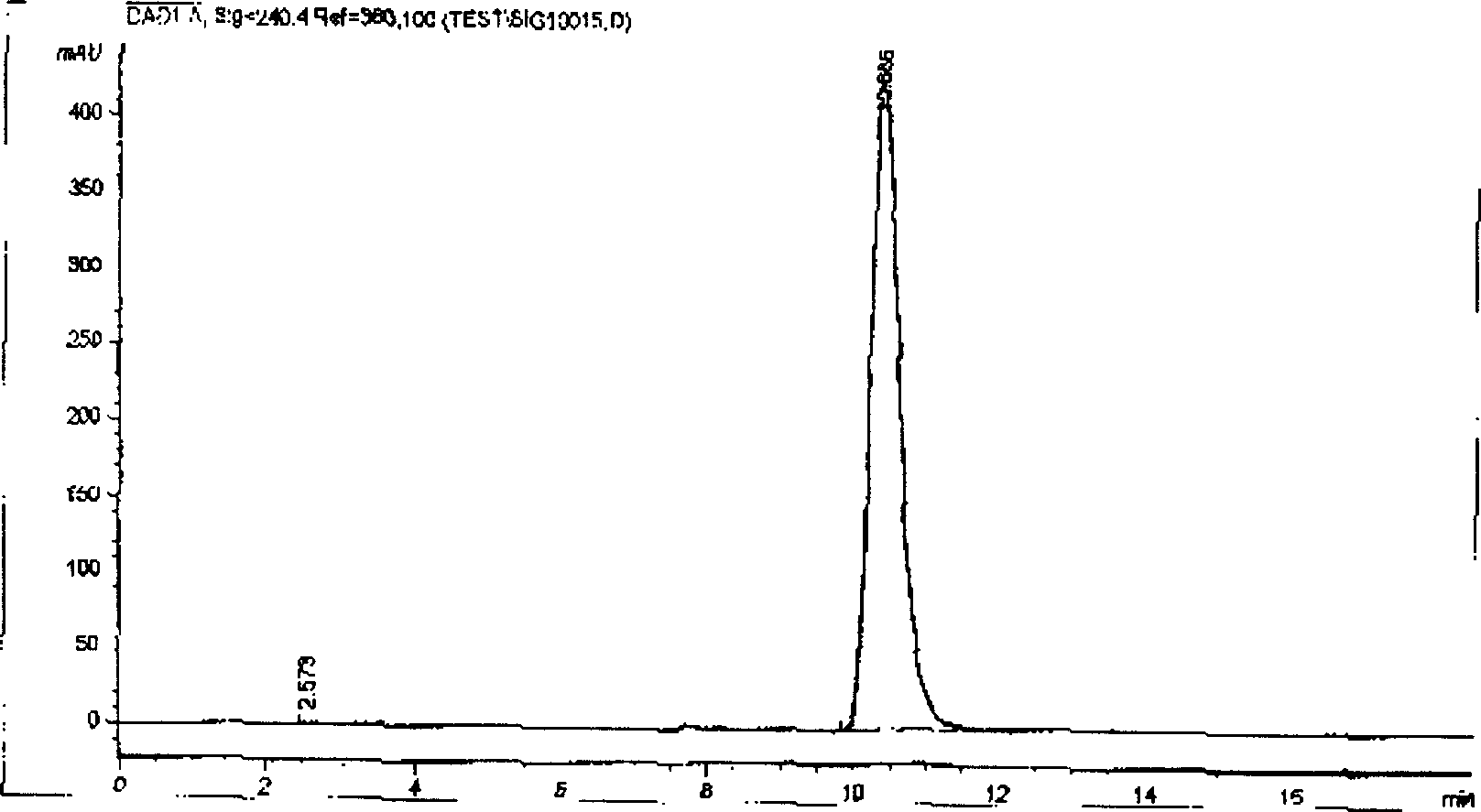
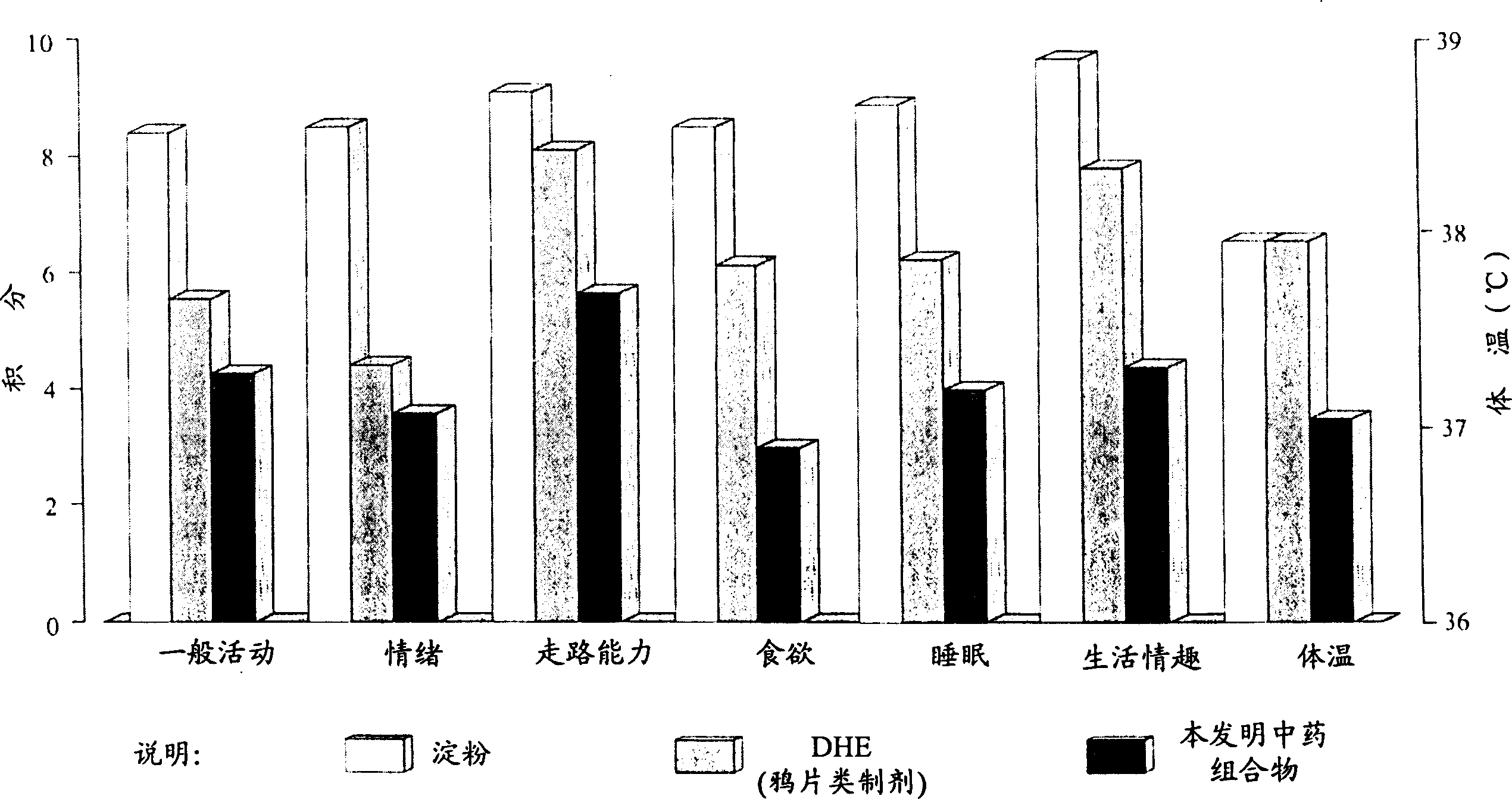
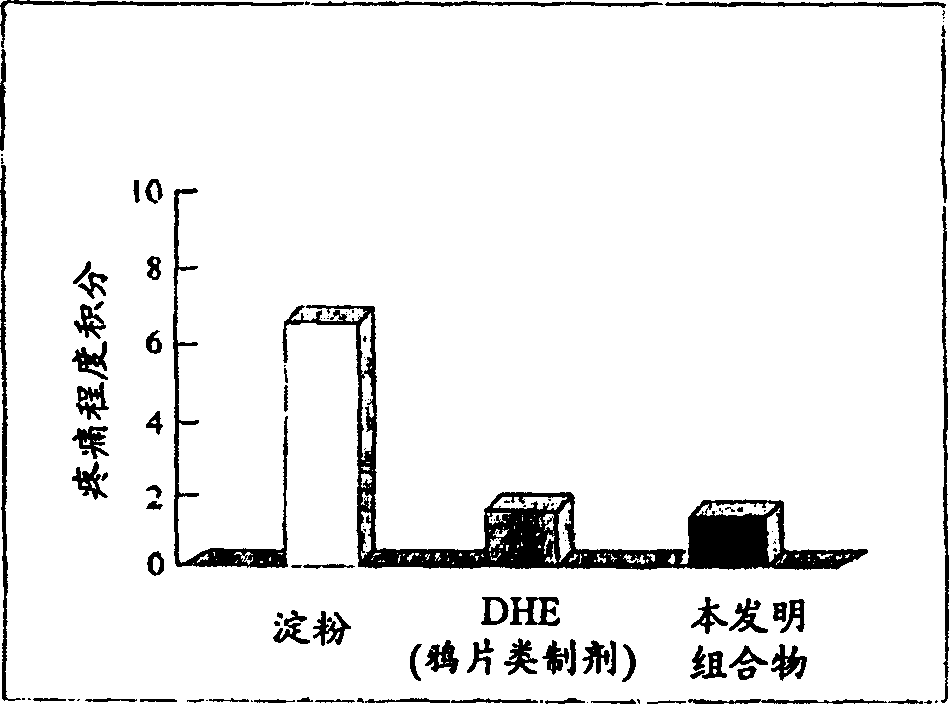
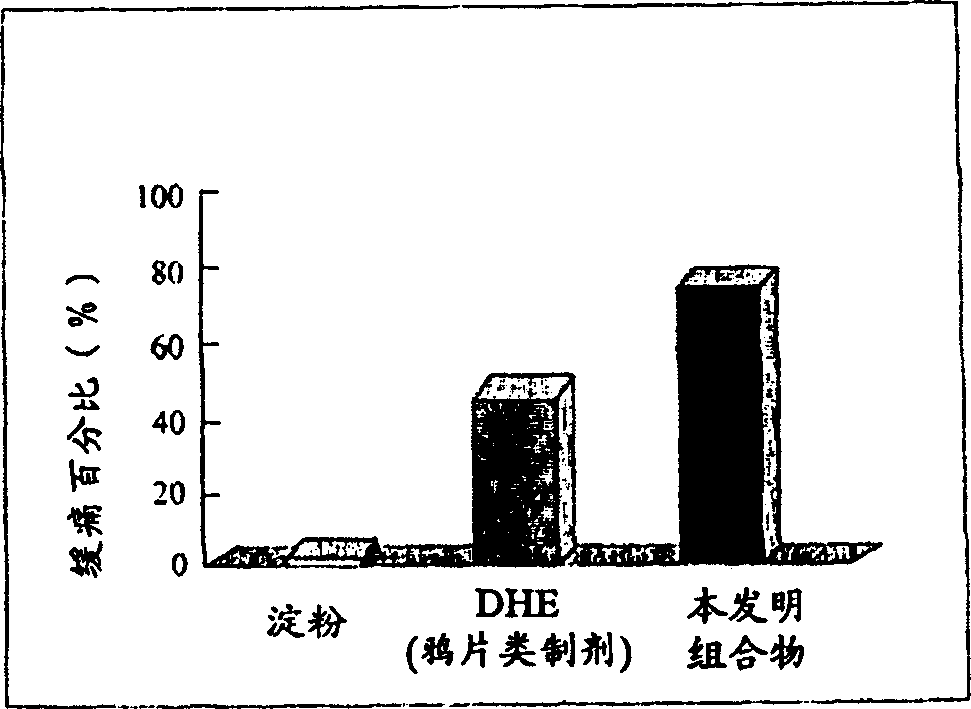
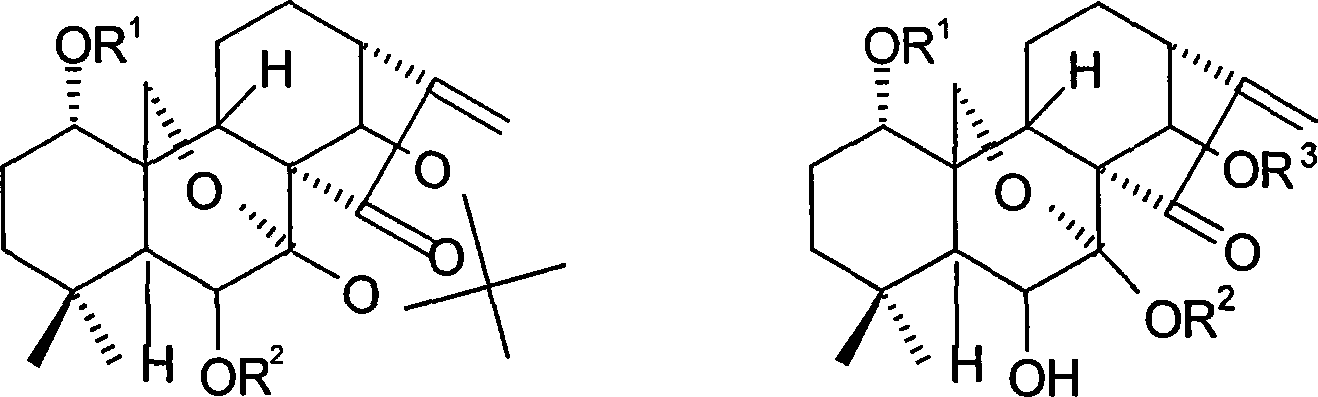Patents
Literature
157 results about "Rubescensin S" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
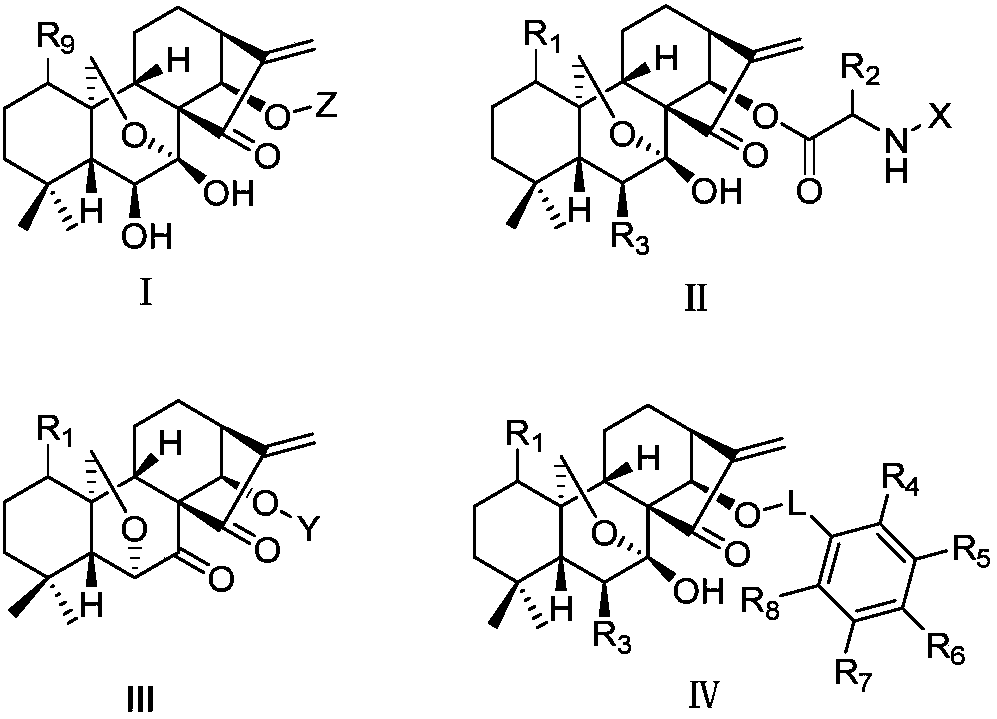
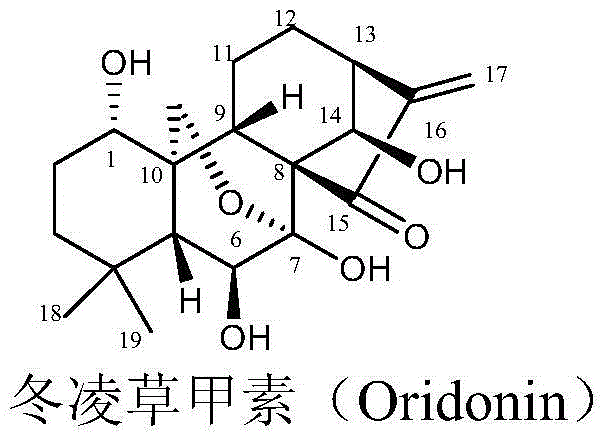
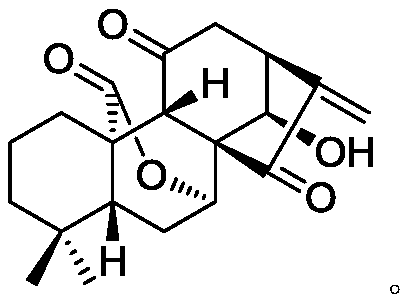
Oridonin derivative, preparation method and uses thereof
InactiveCN101139350AGrowth inhibitionOrganic active ingredientsOrganic chemistryMedicinal chemistryRubescensin S
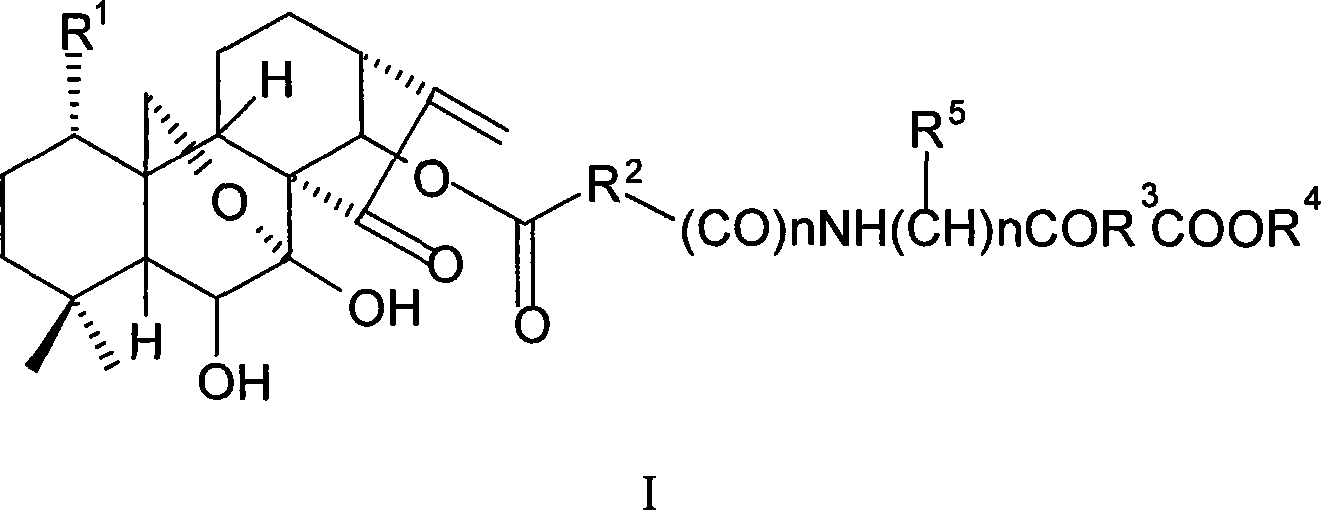
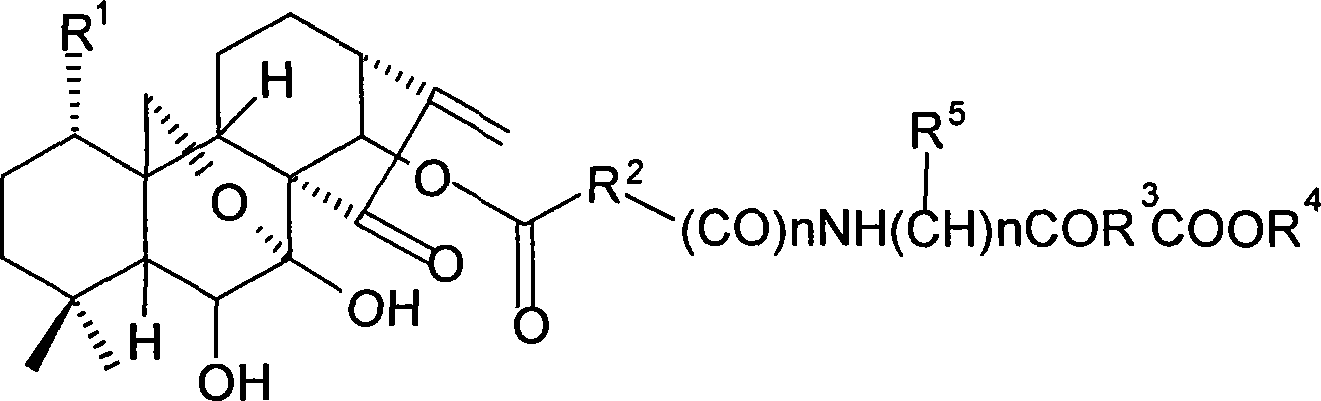
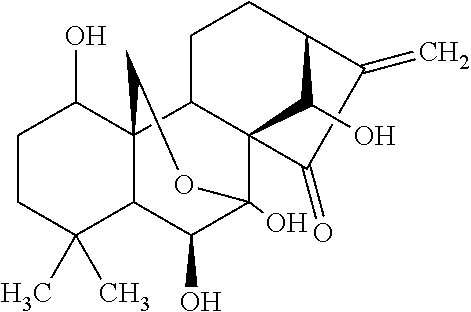
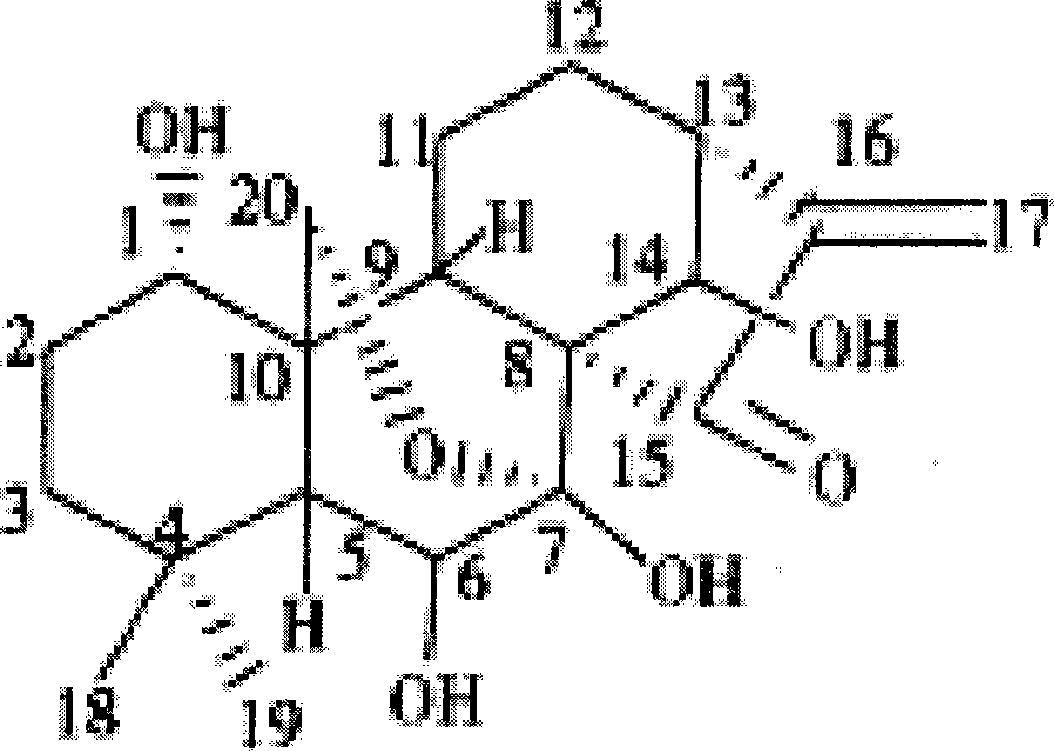
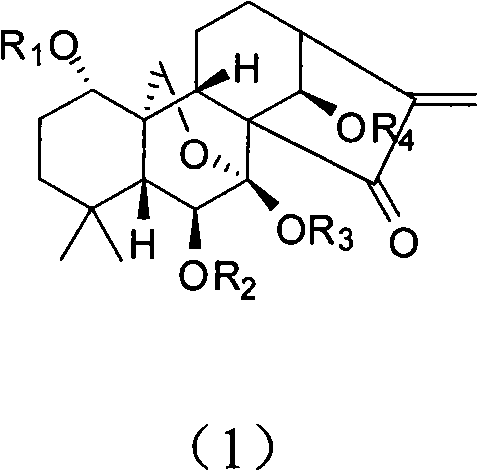
The present invention relates to a natural drug and the chemical field of drugs, specifically relating to an oridonin derivative (I); the derivative is the product after the structural transformation of C1 and C14 of the oridonin. The present invention also discloses the preparation method of the oridonin derivatives and the application of the novel oridonin derivative in the anti-tumor field.
Owner:CHINA PHARM UNIV
Rubescensine A having antitumor activity and fluorine-containing derivatives of 6,7-cyclobebescensine A, preparation method and use
InactiveCN102295649AOrganic active ingredientsOrganic chemistryFluorinated derivativesAntitumor activity
The invention relates to the fields of natural medicine and medicinal chemistry, in particular to a class of oridonin and ent-6,7-ring-opened kaurene-type oridonin fluorine-containing derivatives with antitumor activity. The invention also discloses the preparation method of these oridonin A and ent-6,7-opening ring oridonin A fluorine-containing derivatives, the pharmaceutical composition containing the compound and the use of the compound in the treatment of tumor diseases Applications.
Owner:CHINA PHARM UNIV
Ent-6,7-open-cycle kaurene type rubescensine a derivative with Anti-tumor activity and preparation method and use thereof
The invention relates to the fields of natural medicaments and medicinal chemistry, in particular to a 14-position modified ent-6,7-open-cycle kaurene type rubescensine A derivative with anti-tumor activity. The invention also discloses a method for preparing the 14-position modified ent-6,7-open-cycle rubescensine A derivative and application of a medicinal composition comprising the compound and the compound for treating tumor diseases.
Owner:CHINA PHARM UNIV
Cosmetic compositions comprising oridonin and new cosmetic uses
ActiveUS20120028916A1Improve skin appearanceReduce discontinuityBiocideCosmetic preparationsRubescensin SDarutigenol
Owner:SEDERMA SA
Oridonin derivative and preparation method thereof
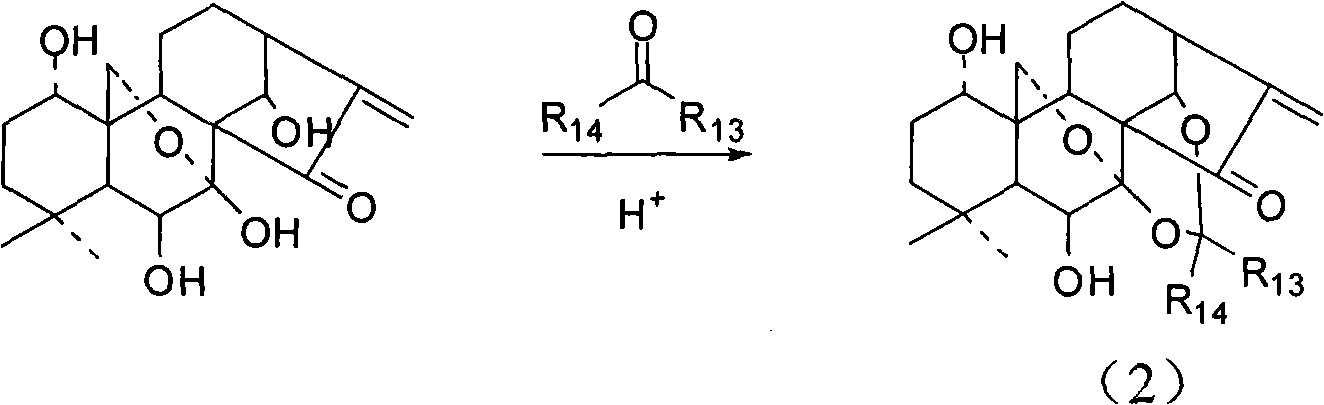
The invention provides an oridonin derivative as expressed in the general formula by changing substituent groups in a first position, a fourth position and a seventeenth position on the premise of not destroying the active center of oridonin. The oridonin derivative has higher anti-tumor cell activity. Meanwhile, the invention also provides a preparation method of the compound.
Owner:YANTAI TARGET DRUG RES
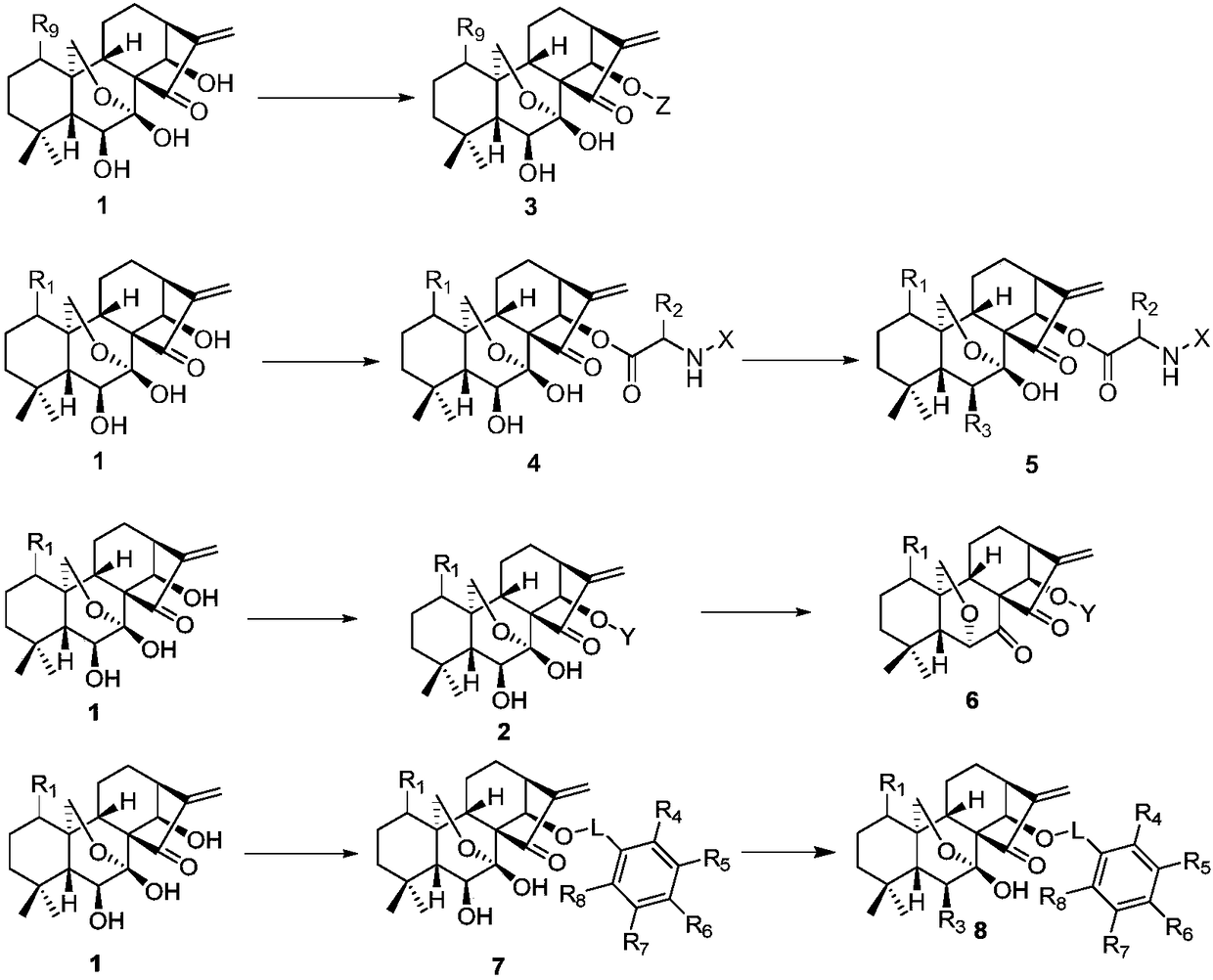
Rubescensin A derivatives and preparation method and applications thereof
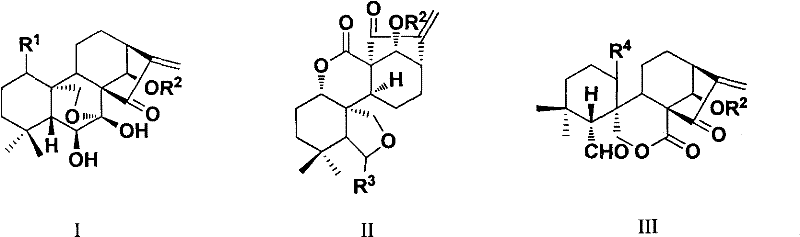
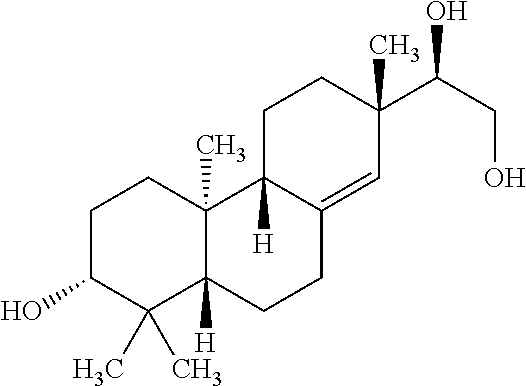
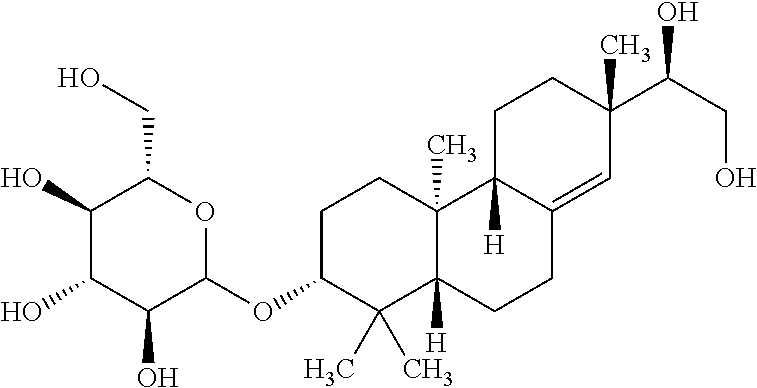
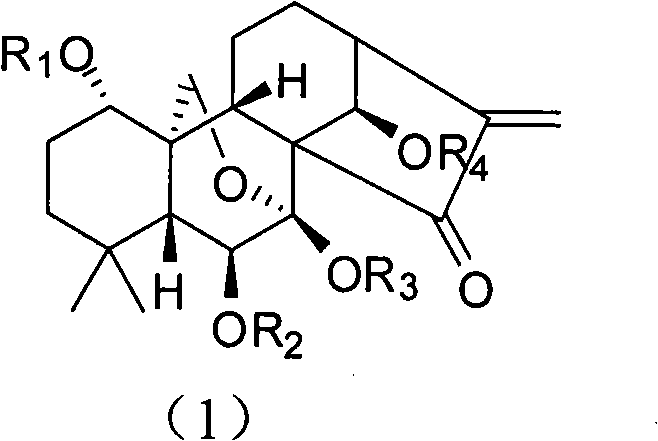
The invention discloses rubescensin A derivatives and a preparation method and applications thereof. The rubescensin A derivatives have structures represented by formulas (I), (II), (III), or (IV). Onthe premise that the active center of rubescensin A is not destroyed, the hydroxyl groups on the 1st, 6th, and 14th positions or the hydroxyl groups on the 6th and 7th positions are subjected to ringchange modification; and obtained rubescensin A derivatives have higher antitumor activity, higher intercellular selectivity, lower dosage, and lower toxicity. Compared with that of rubescensin A, the performance of rubescensin A derivatives on killing cancer cells of human liver cancer, human multiple myeloma, human lung cancer, and the like, is enhanced by 3 to 5 times; the optimal performancecan be strengthened by 11 times; and the rubescensin A derivatives can be used to prepare antitumor drugs for treating liver cancer, rubescensin A derivatives, lung cancer, and the like.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD +1
Oridonin polymer micelle administration preparation and preparation method thereof
InactiveCN101422615AProlong circulation time in the bodyPromote enrichmentOrganic active ingredientsPharmaceutical delivery mechanismChemical reactionPolymer science
The invention belongs to the medicine technology field, which provides an oridonin A polymer micelle drug delivery preparation and a preparation method thereof. The polymer is diblock copolymer, and the hydrophilic section is polyethylene glycol monomethyl ether while the hydrophobic section can be chosen from biodegradable poly (D, L-lactic acid), poly (L-lactic acid), poly (lactide-glycolide), caprolactone, PHDCA or the mixture thereof. The polymer medicament micelle traditional Chinese medicine is enveloped in one or two polymers by a physics form or prepared to be the micelle after being covalent bonded with the polymer by a chemical reaction. The prepared micelle exists in the state of aqueous dispersion or freeze-dried powder. The particle size distribution range of the oridonin A polymer micelle is 5 to 500nm while the drug loading is 0.01 to 40 percent. The oridonin A polymer micelle prolongs the circulation time of the medicament in bodies, and AUC0-infinite value is 2.19 times of that of common injections, thus leading the medicament to enrich in tumor parts easier, improving the efficacy and reducing the toxicity.
Owner:SHENYANG PHARMA UNIVERSITY
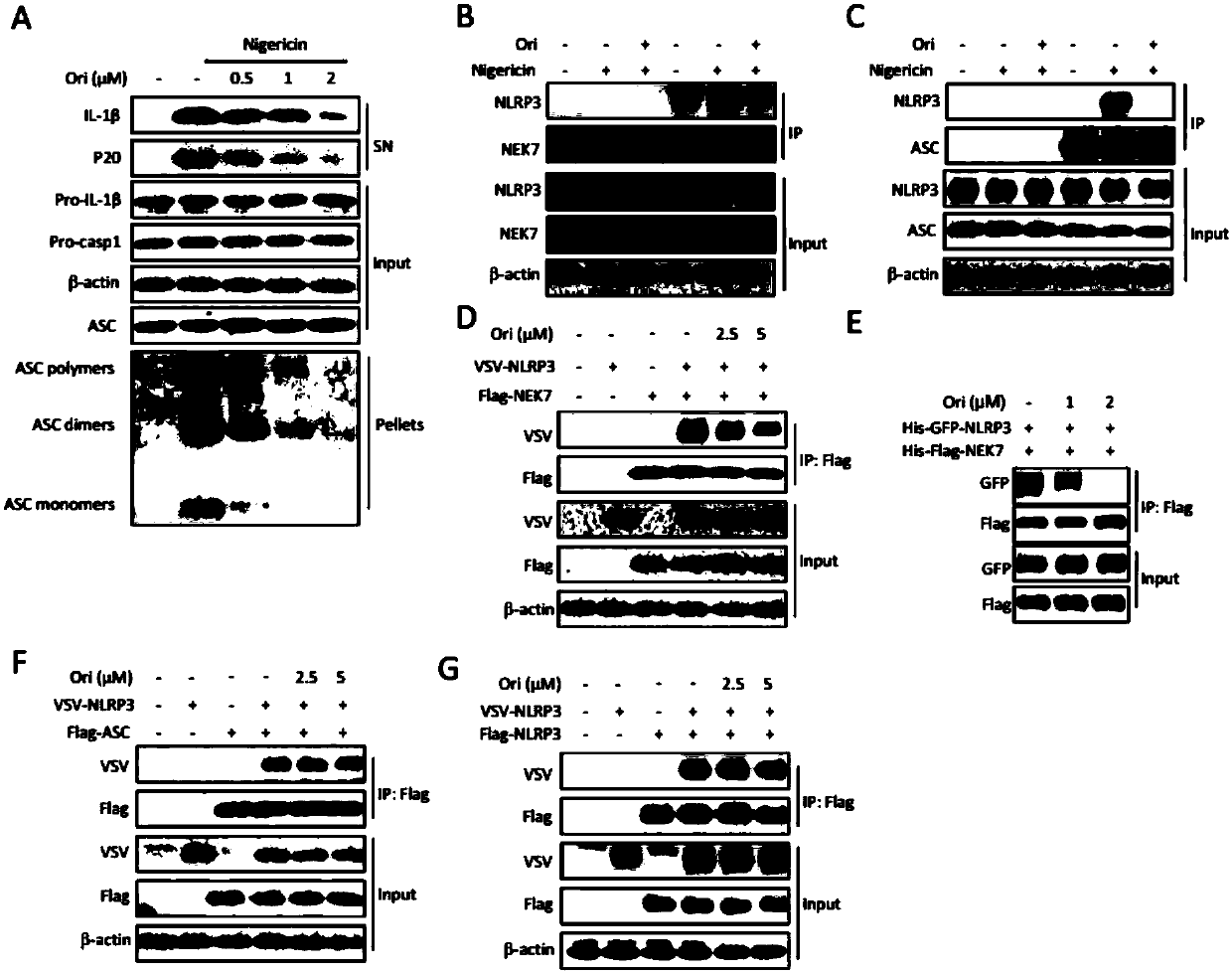
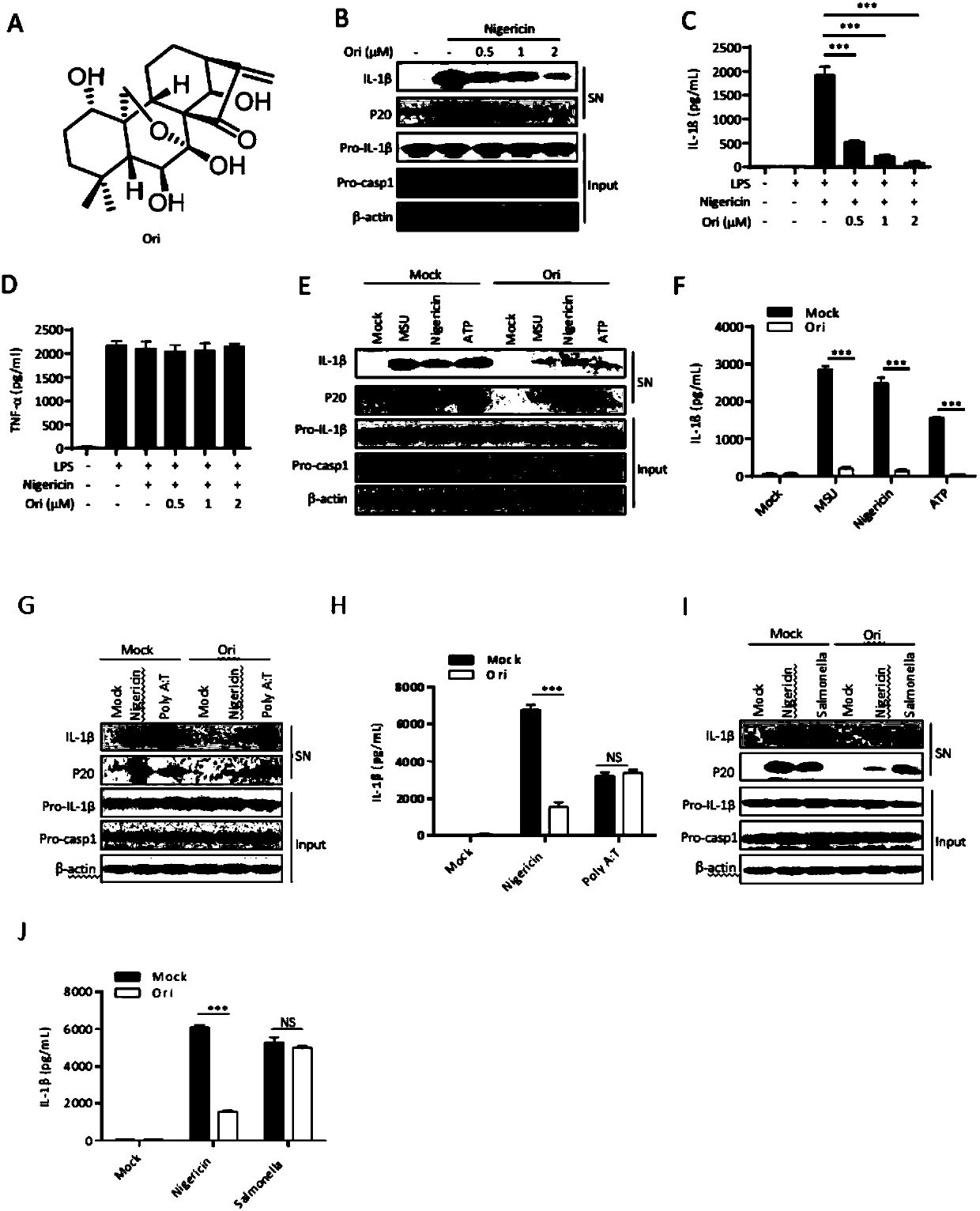
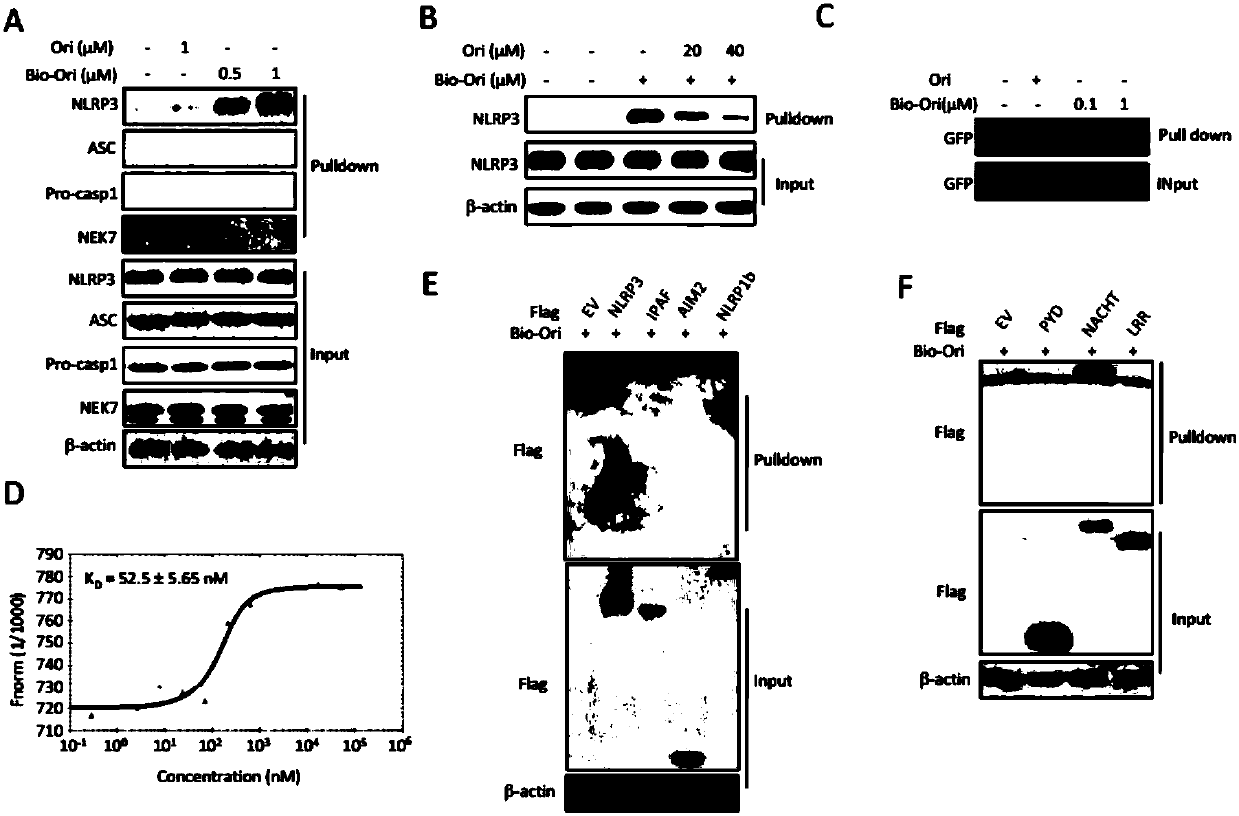
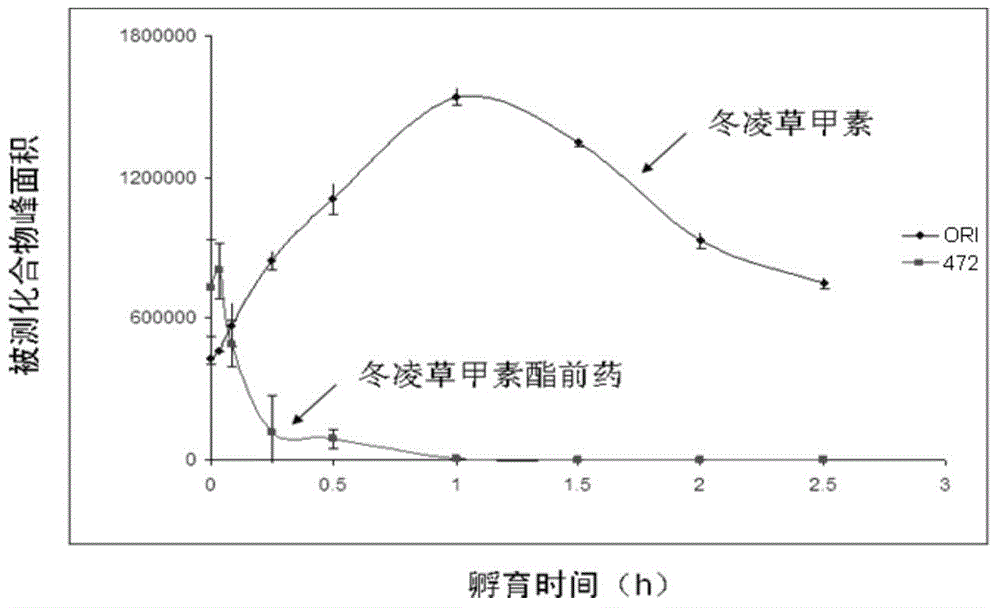
Application of oridonin in preparing drugs for preventing or treating NLRP3 inflammasome-related diseases
ActiveCN110151749AEnhance pharmacological effectsInhibition of activationOrganic active ingredientsNervous disorderDiseaseIn vivo
The invention relates to application of oridonin in preparing drugs for preventing or treating NLRP3 inflammasome-related diseases. The oridonin has a strong pharmacological effect, is safe and innoxious, and indicates a good medicinal prospect. The oridonin can inhibit the activation of an NLRP3 inflammasome in vitro, and can treat pathological conditions of NLRP3 inflammasome-related disease model in vivo. The oridonin can be directly combined with NLRP3 through covalent bonds, thereby inhibiting the interaction of the NLRP3 and NEK7, inhibiting the assembly of the inflammasome, and achieving the purpose of inhibiting the activation of the inflammasome.
Owner:UNIV OF SCI & TECH OF CHINA
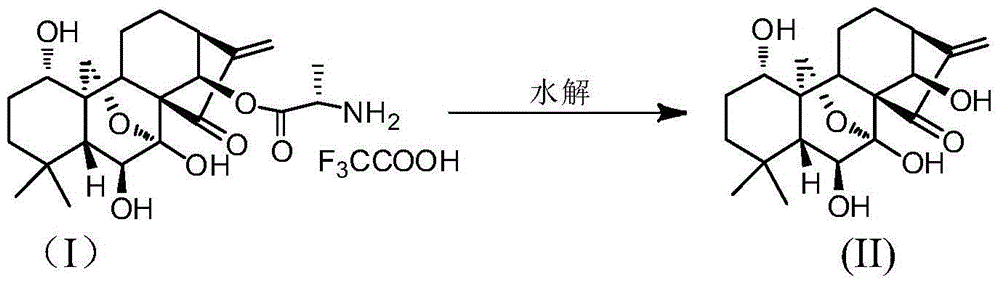
L-alanine-(14-oridonin) ester trifluoroacetate as well as preparation method and application thereof
ActiveCN104017000AGood water solubilityHigh activityOrganic active ingredientsOrganic chemistryEsophagus CancersLeukemia
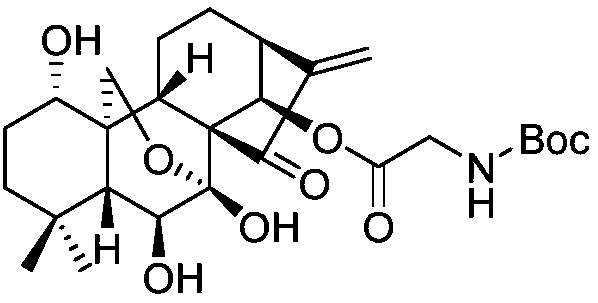
The invention relates to L-alanine-(14-oridonin) ester trifluoroacetate as well as a preparation method and application thereof. The preparation method of the compound comprises the following steps of: by taking oridonin (II) as a starting material, generating 14-position esterification reaction with N-BOC-L-alanine in the presence of DCC to obtain N-BOC-L-alanine oridonin ester (III), removing a BOC protective group in trifluoroacetic acid, and salt-forming to obtain L-alanine-(14-oridonin) ester trifluoroacetate (I). The compound can be applied to clinic very well, and is used for treating esophagus cancer, gastric cancer, primary liver cancer, pancreatic cancer, cardia cancer, colorectal cancer, bladder cancer, breast cancer, acute myelogenous leukemia, and the like.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Technical method for extracting oridinin from rabdosia rubescens
The invention advances a new technique proper to separate and purify oridonin. It adopts alcohol solution extraction, then makes water float reflux treatment, where the water float liquor directly passes through the column of macroporous resin (like HZ-841 macroporous absorbing resin) polymerized of styrene and divinylbenzene as monomers to eliminate sugar, pigment, etc, adopts silica gel and magnesium chloride mixed column chromatography to separate and elute, and finally recrystallize to obtain higher purity oridonin. It is safe, low-cost, and applied to industrialized production.
Owner:EAST CHINA UNIV OF SCI & TECH
Rabdosia rubescens A microglobule medicinal agent and its preparation method
A medicine for intravenous injection includes the powder injection of rebescensine A liposome prepared from medicine, coating material, film stabilizer, antioxidizing agent and water-soluble carrier, and the lipid nanoparticles of rebescensine A prepared from medicine, lipid, emulsifier, emulsifying aid and water-soluble carrier. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Chinese medicinal composition for treating prostate cancer, its preparation method and its application in the preparation of medicine for treating prostate cancer
A Chinese medicine for treating prostatic cancer is preapred from 7 Chinese-medicinal materials including ganoderma, chrysanthemum flower, liquorice root, notoginseng, etc through extracting their active components. Its advantages are high curative effect and low by-effect.
Owner:SHANGHAI ZHONGYAO BIO TECH CO LTD
Chinese medicinal composition for anti-cancer and analgesia, its preparation method and its application in the preparation of product and medicine for treating cancer and analgesia
An anticancer and antalgic Chinese medicine is prepared from 7 Chinese-medicinal materials including ganoderma, cordalis tuber, notoginseng, liquorice root, etc through extracting their active components. Its advatnages are high curative effect and low by-effect.
Owner:SHANGHAI ZHONGYAO BIO TECH CO LTD
Dry powder inhaler with constituent rubescensin A as well as preparation method and application thereof
InactiveCN102058566APromote absorptionNo first pass effectPowder deliveryOrganic active ingredientsOral medicationAdditive ingredient
The invention relates to a dry powder inhaler with a constituent rubescensin A as well as a preparation method and application thereof. The dry powder inhaler is a medicinal composition formed by mixing the constituent rubescensin A as an active constituent with pharmaceutically acceptable excipients. Compared with orally administrated medicines, the dry powder inhaler developed by using the technical scheme provided by the invention greatly improves the effect of the constituent rubescensin A on treating esophagus cancers, lung tumors and leukemia.
Owner:CHINA PHARM UNIV
Water soluble oridonin derivative and preparation method thereof
ActiveCN101525338AGood water solubilityEnhance drug propertiesGroup 5/15 element organic compoundsBulk chemical productionSolubilityWater soluble
The invention discloses a water soluble oridonin derivative shown as a general formula (1) and preparation method thereof. Water soluble groups such as succinate, sulphosuccinic acid ester, sulphonic acid ester, and the like are added through the structure so as to greatly improve the water solubility and the medicinal property of the water soluble oridonin derivative.
Owner:SHENZHEN JYMED TECH
Oridonin cubic liquid crystal nanoparticle and preparation method thereof
ActiveCN106727336AHigh encapsulation efficiencyOvercome the disadvantage of low oral bioavailabilityPowder deliveryOrganic active ingredientsMass ratioSolvent
The invention relates to an oridonin cubic liquid crystal nanoparticle and a preparation method thereof. The oridonin cubic liquid crystal nanoparticle is prepared from the following raw materials in percentage by weight: 0.1 to 0.5 percent of oridonin, 7 to 64 percent of amphiphilic lipid material, 6 to 29 percent of solvent, 1 to 8 percent of stabilizer, and 25 to 65 percent of water, wherein the amphiphilic lipid material is glycerol mono-oleate or phytould likeriol; the mass ratio of a liquid crystal material to the stabilizer is 1 to (0.01 to 0.30). The oridonin cubic liquid crystal nanoparticle provided by the invention is smaller in particle size, good in uniformity, and beneficial to endocytosis and transfer of enterocyte; by utilizing a unique structure of cubic liquid crystal, a dissolution barrier and a permeation barrier of the oridonin during an oral absorption process are effectively overcome, the oral relative bioavailability of the oridonin is remarkably improved, and meanwhile, the oridonin can be slowly released.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Method for extracting oridonin from rabdosia rubescens and purifying oridonin
The invention discloses a novel process for extracting oridonin from rabdosia rubescens and purifying the oridonin. In the process, extraction is performed in 60 to 70 percent ethanol for 2 to 3 times, active carbon is used for decolorization, the extract is condensed under reduced pressure till the concentration of the methanol is 20 to 30 percent, macroporous resin absorption is performed, 75 to 85 percent methanol is used for elution, condensation is performed, extraction is performed step by step by using petroleum ether and ethyl acetate, ethyl acetate extracting solution is recovered, a small amount of petroleum ether is added, the solution is stood for crystallization, and refluxing and saturated dissolution and crystallization in 70 percent methanol and 90 percent methanol are performed in turn. The process is simple and is easy in operation.
Owner:NANJING ZELANG MEDICAL TECH
Application of oridonin powder aerosol in treatment of acute lung injury
The invention discloses application of oridonin powder aerosol in treatment of acute lung injury. The oridonin powder aerosol is prepared by an oridonin nano-drug delivery system, wherein the dosage form of the nano-drug delivery system is selected from lipidosome, nanoparticles, nanosuspension, nanoemulsion and micro-emulsion. The medicines in the oridonin powder aerosol are stable, easily enter the deep parts of lung tissues, are positioned in a targeted mode and are convenient to carry and use, and the treatment of acute lung injury is promoted. The oridonin powder aerosol is used for treating the acute lung injury caused by infection, shock, smoking, trauma, toxin poisoning, inhalation of irritant gases, radiation, high oxygen and low oxygen, wherein the inhaled irritant gases comprise phosgene, diphosgene, triphosgene, chlorine, nitric oxides, formaldehyde, dimethyl sulfate, hydrogen chloride, hydrogen bromide, hydrogen fluoride, ammonia, ozone and sulfur dioxide.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Self microemulsion preparation of Rubescensin A, and preparation method
InactiveCN101091696APromote absorptionHigh drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsTG - TriglycerideOil phase
The present invention discloses a rabdosia rubescens extract microemulsion preparation and its preparation method. It is formed from rabdosia rubescens extract, oil phase, surfactant and assistant of surfactant. The described oil phase is one kind selected from transacylated corn oil or midchain fatty triglyceride or their mixture; the described surfactant is one kind selected from polyoxyethylenated castor oil, polyoxyethylene hydrogenated castor oil, polyglycol-8-glycerocaprylic acid / caprate or their mixture, and the assistant of surfactant is one kind selected from dicarboxyl mono-ethyl ether ester, 1-2-propylene glycol and polyethylene glycol 400 or their mixture.
Owner:SHANGHAI UNIV OF T C M
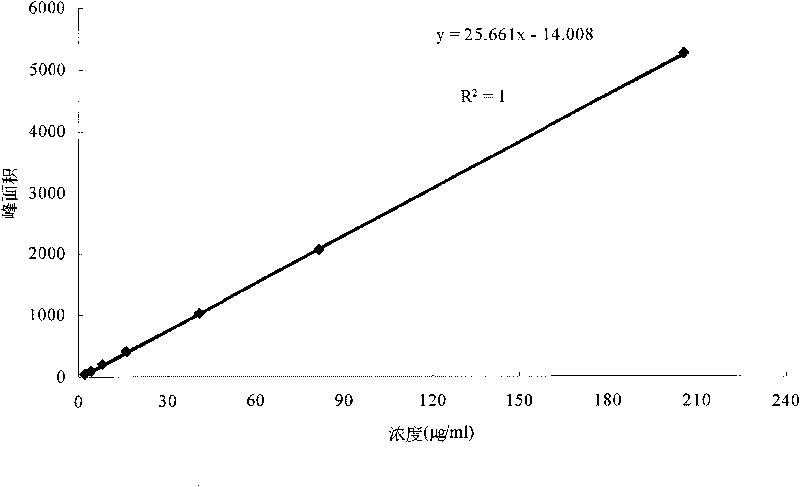
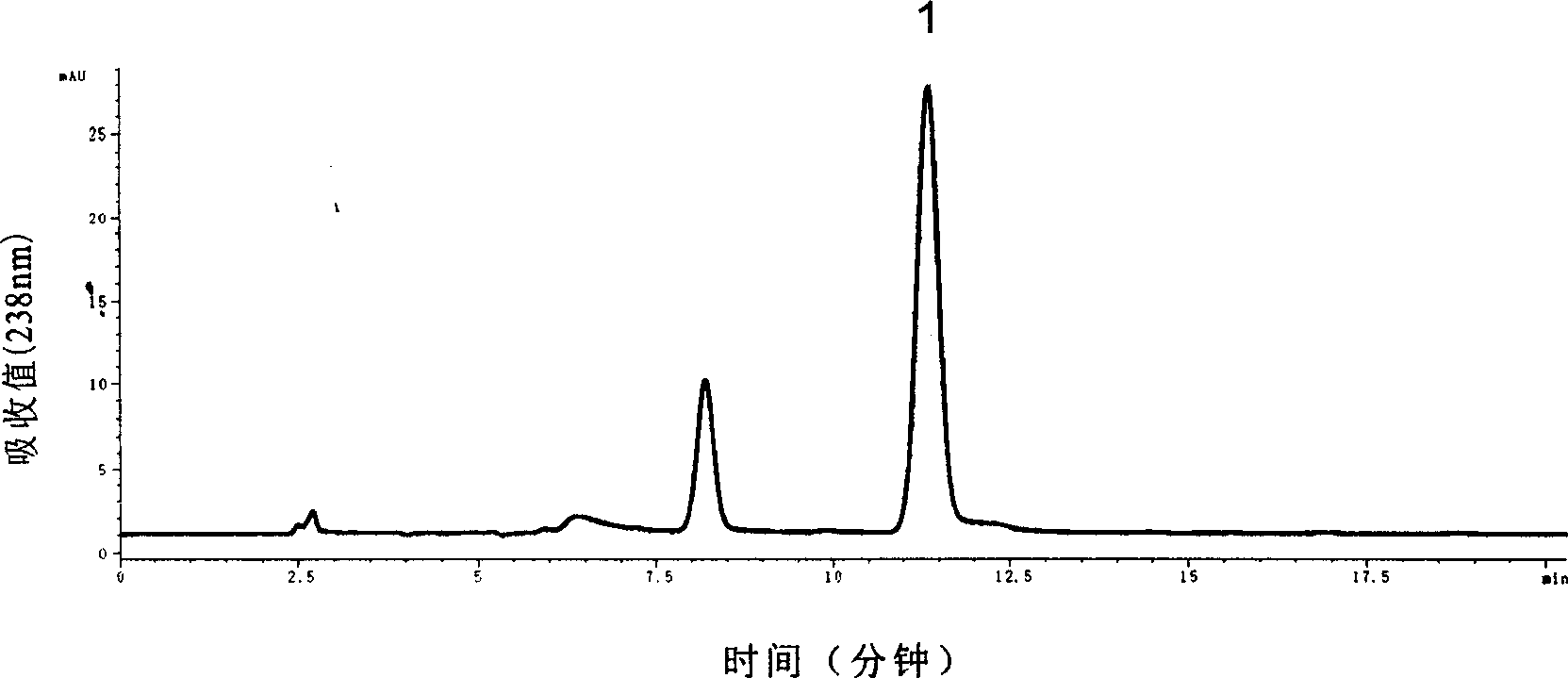
Method for determining blushred rabdosis A and rosmarinic acid contents in blushred rabdosis and its preparation
InactiveCN1645131AAccurate measurementAccurate contentComponent separationTest sampleAnalytical chemistry
A method using liquid chromatograph to measure content of Rabdosia rubescens A element and rosemary acid in high efficiency includes preparing conditions for test, preparing control sample solution, preparing tested sample solution and carrying out measurement.
Owner:张海
Preparation process and products of oridonin fine particles
InactiveCN101798308AGood compressibilityEasy to controlOrganic chemistryAntineoplastic agentsAnti solventCurative effect
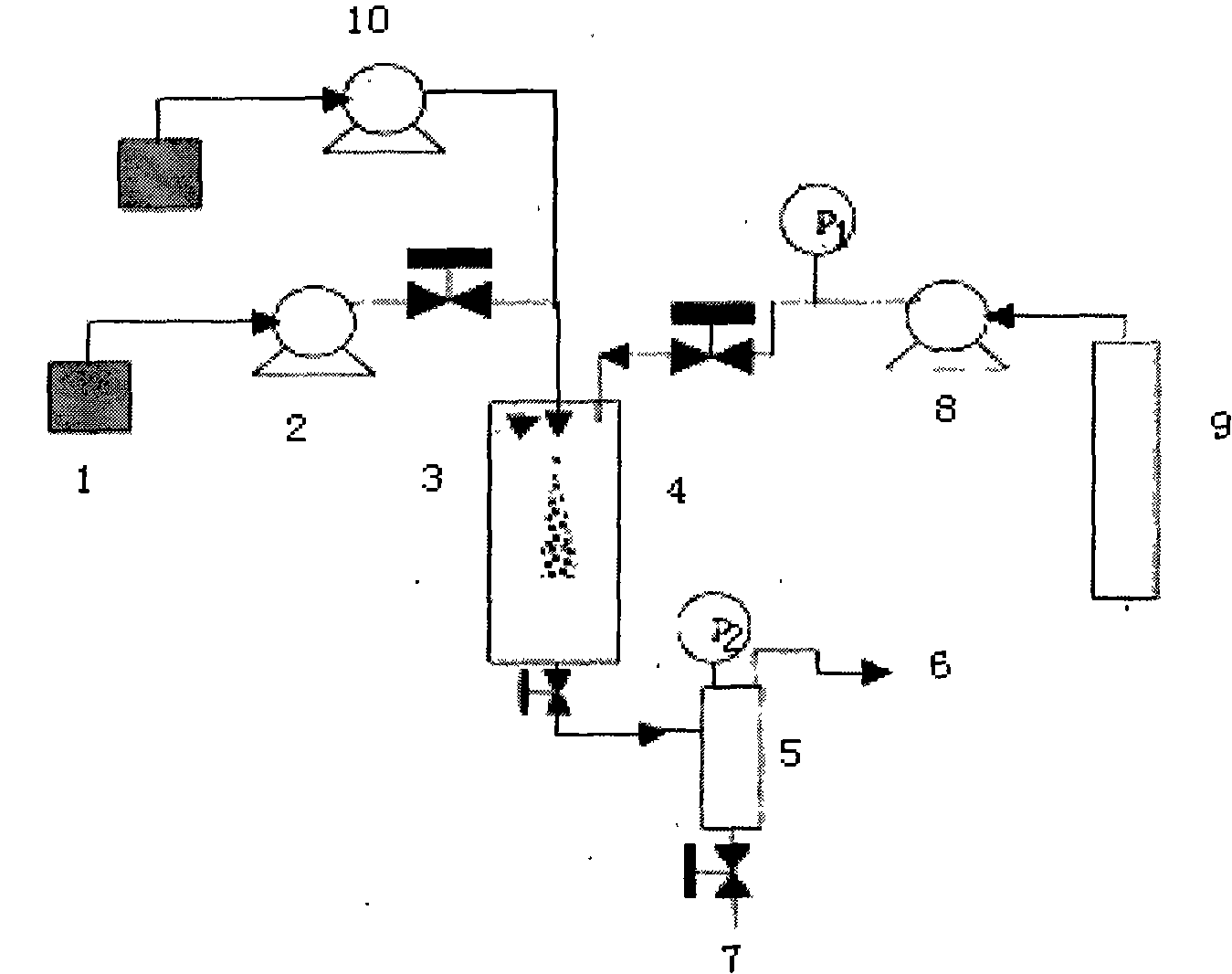
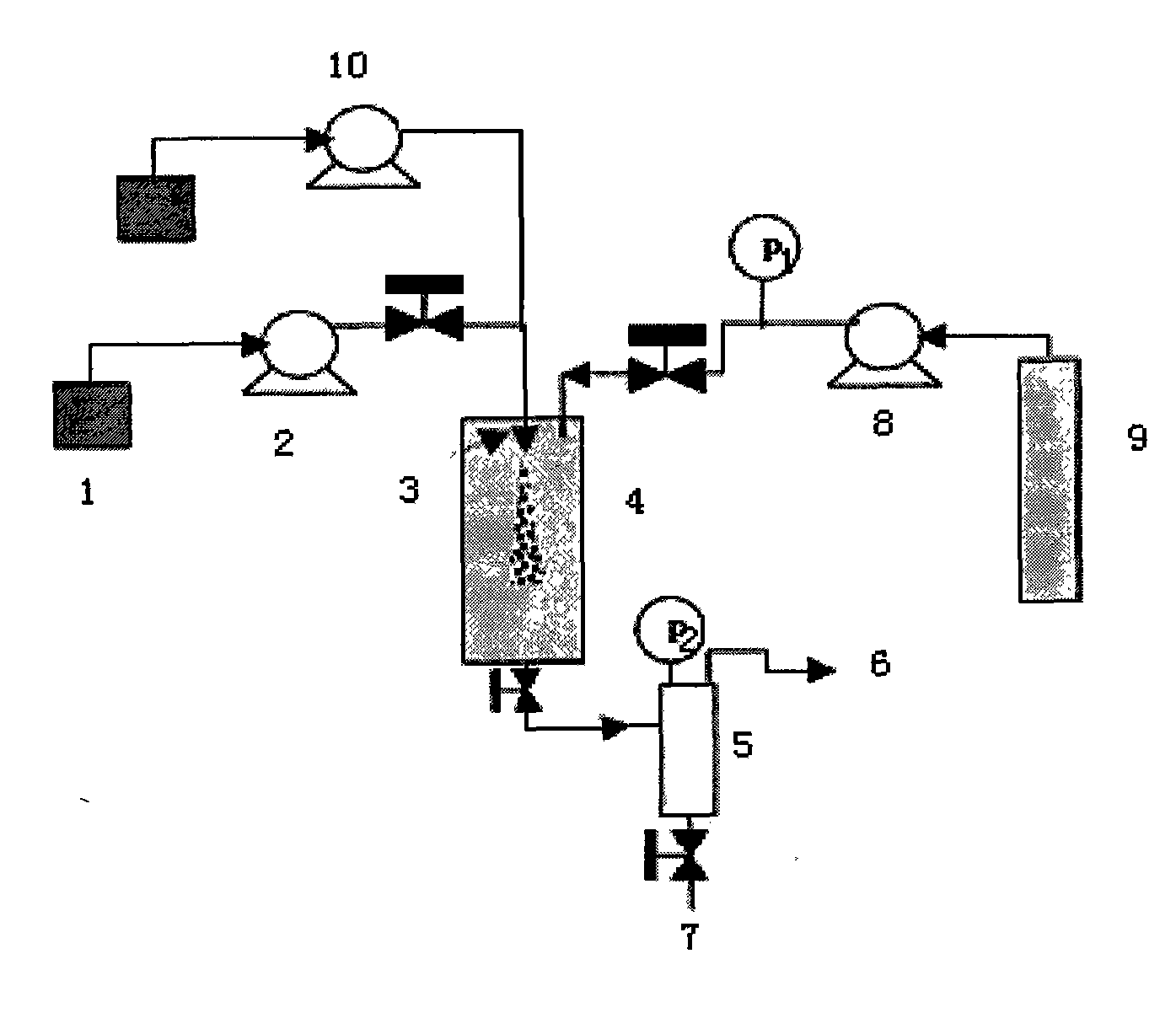
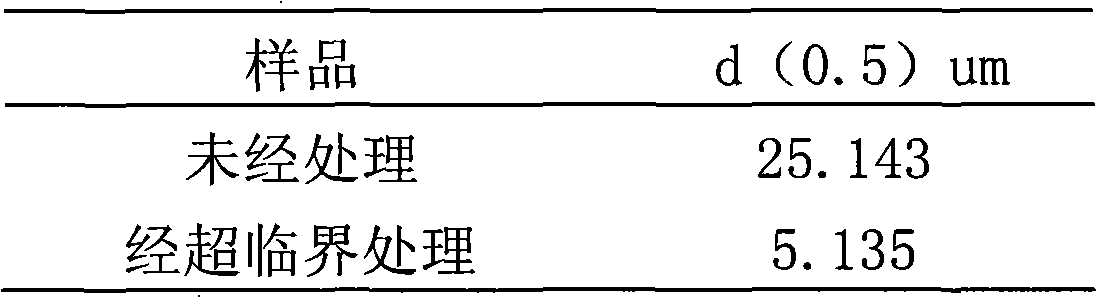
A preparation process of oridonin fine particles is characterized by comprising the following steps: (1) preparing oridonin solution; (2) connecting the oridonin solution prepared in the step (1) with a solution pump; (3) charging carbon dioxide; (4) jetting the oridonin solution into a crystallization reactor by a nozzle in a supercritical fluid anti-solvent equipment system through the solution pump; and (5) separating out the oridonin crystals. The oridonin fine particles prepared by the process are characterized in that the particles are loose amorphous white superfine powder; and the particle size d (0.5) is 5.135um. The process is suitable for the superfine oridonin powder, improves the medicine dissolution and the curative effects and is used for preparing the oridonin fine particles by applying the supercritical fluid crystallization technology.
Owner:CHINA NAT ACAD NANOTECH & ENG
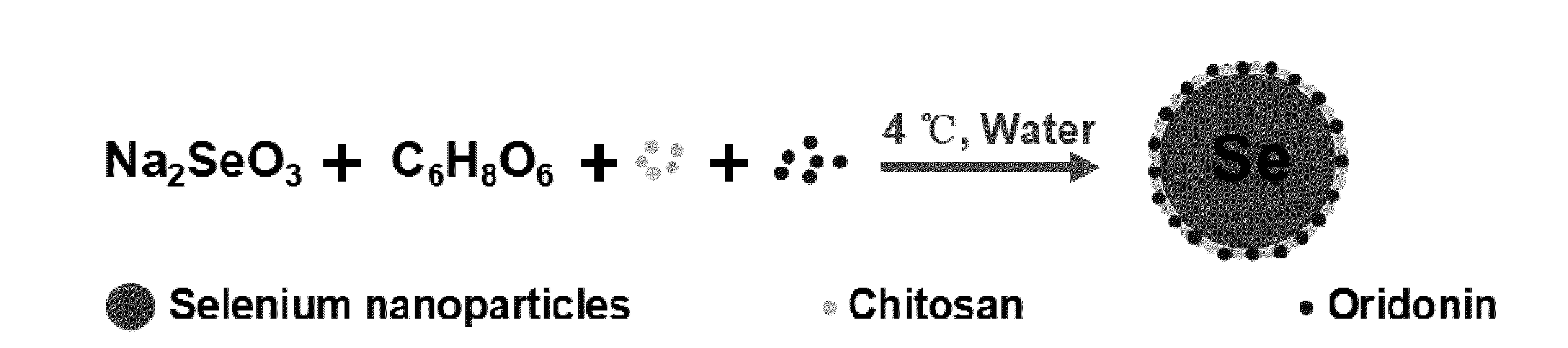
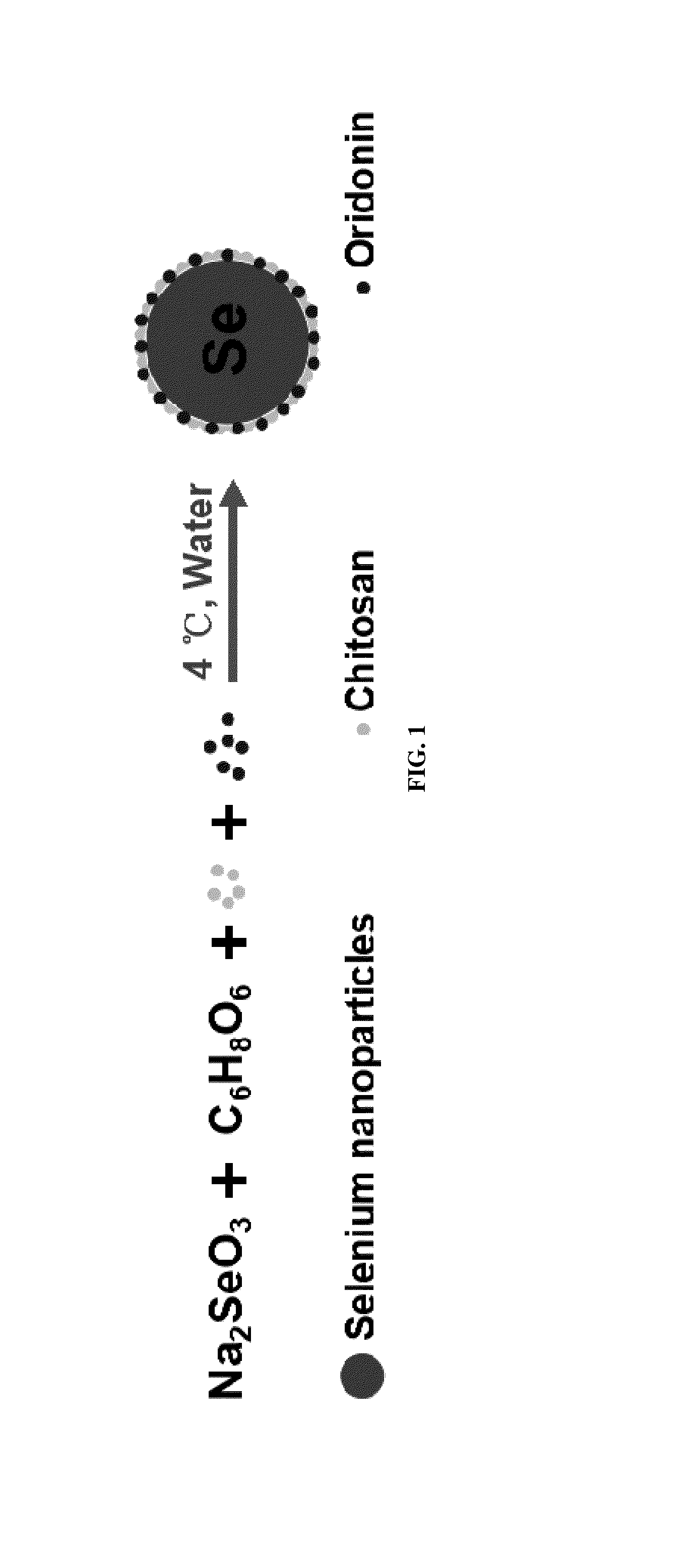
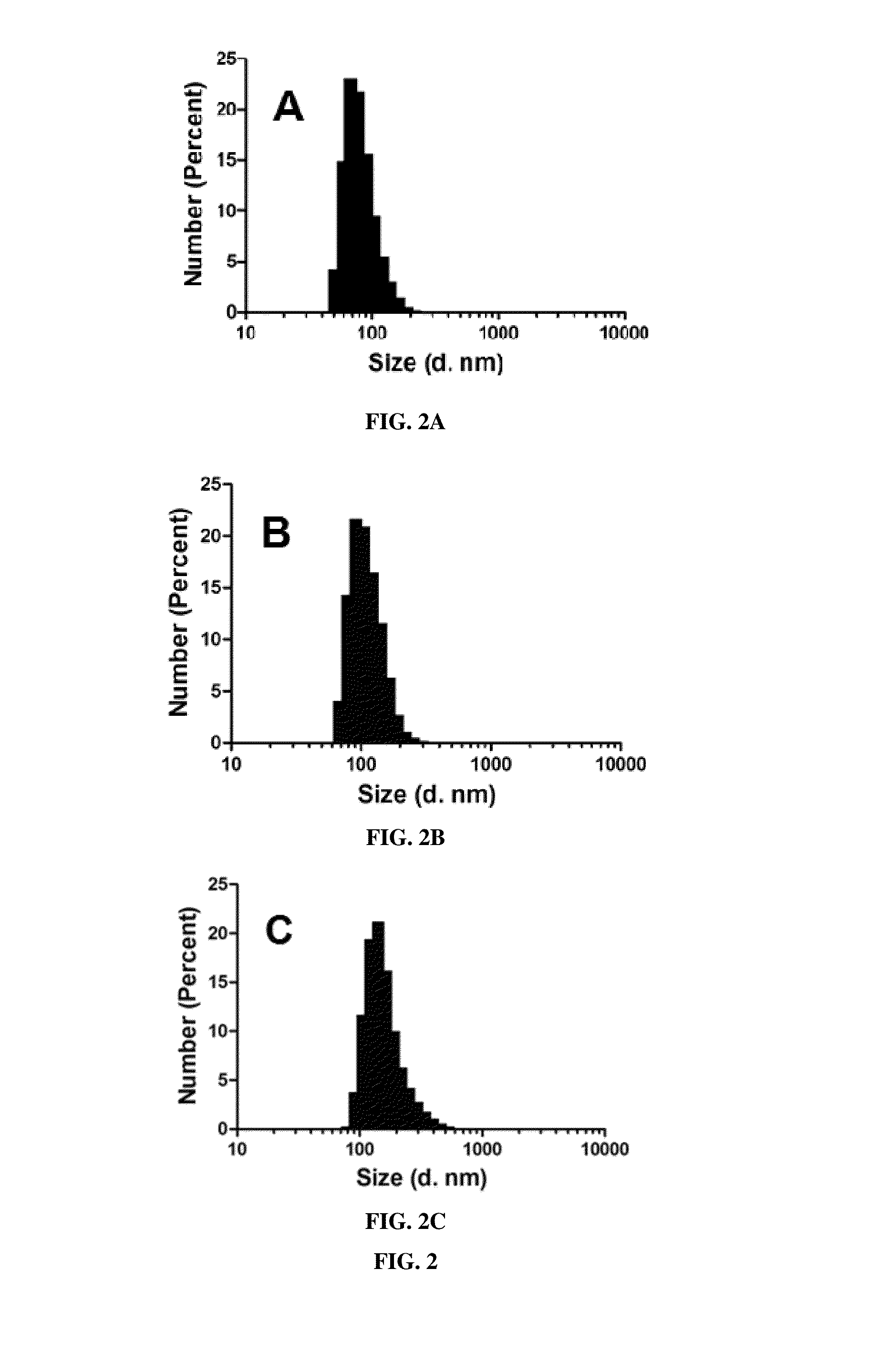
Oridonin functionalized selenium nanoparticles and method of preparation thereof
ActiveUS20160257694A1Good water solubilitySafety managementOrganic chemistryAntipyreticNanoparticleWater soluble
Owner:MACAU UNIV OF SCI & TECH
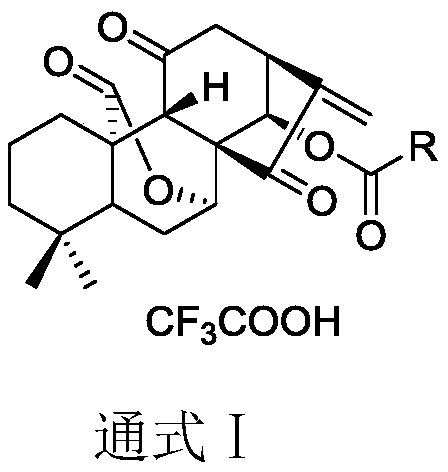
11, 20-dicarbonyl Jiyuan rubescensin a and L-amino acid-14-ester trifluoroacetate
ActiveCN110229168ASimple manufacturing methodNo allergiesOrganic chemistryAntineoplastic agentsWilms' tumorLeukemia
Belonging to the field of pharmaceutical compounds, the invention relates to 11, 20-dicarbonyl Jiyuan rubescensin a and L-amino acid-14-ester trifluoroacetate thereof, and a preparation method and usethereof. The preparation method includes: adopting Jiyuan rubescensin a as the raw material and performing oxidation with a Jones reagent, thus obtaining 11, 20-dicarbonyl Jiyuan rubescensin a, the structure of which is shown as the specification; then carrying out 14-position esterification reaction with N-BOC-L-amino acid to obtain N-BOC-L-amino acid-11, 20-dicarbonyl Jiyuan rubescensin a ester(III), then removing BOC protective group in trifluoroacetic acid, and then conducting salt forming to obtain L-amino acid-14-(11, 20-dicarbonyl Jiyuan rubescensin a)ester trifluoroacetate, which hasthe following general formula. The compound has good stability and anti-tumor activity, and provides the basis for screening antitumor drugs against esophageal cancer, gastric cancer, primary liver cancer, pancreatic cancer, cardiac carcinoma, colorectal cancer, bladder cancer, breast cancer, acute myelogenous leukemia and the like.
Owner:ZHENGZHOU UNIV
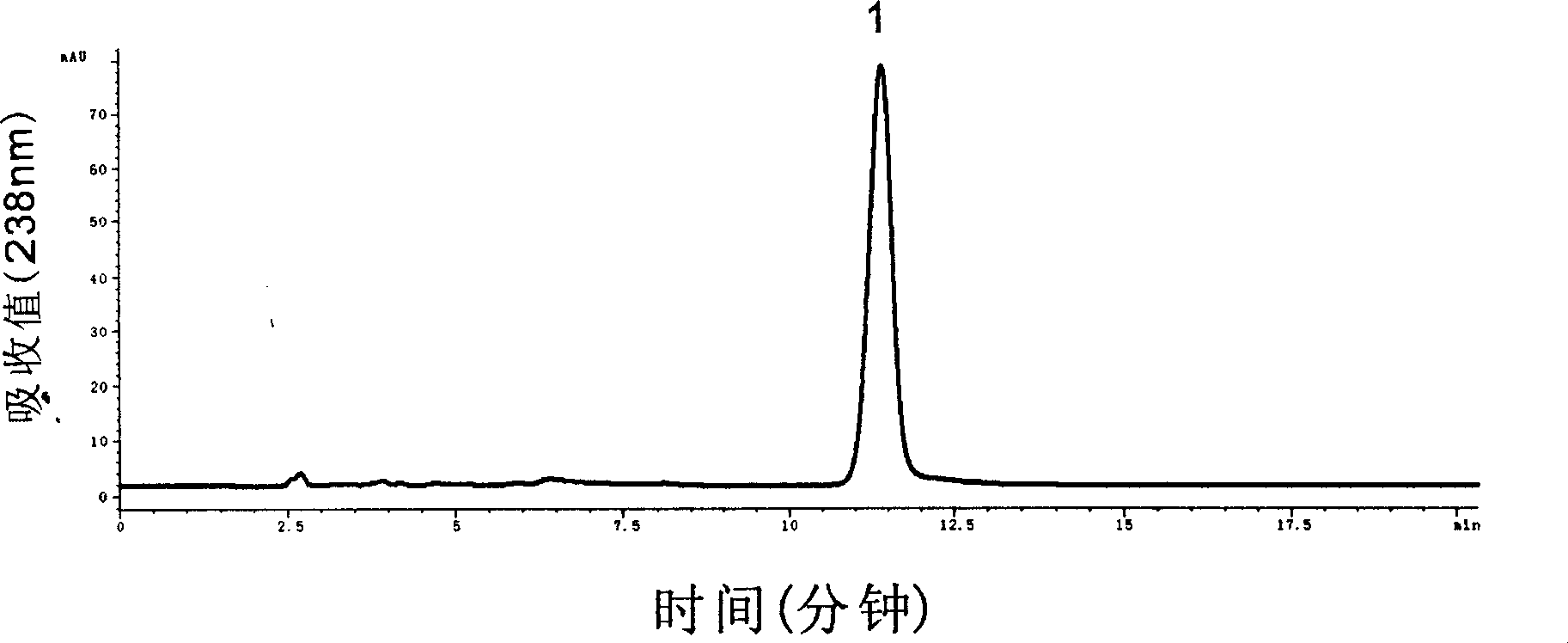
Method for extracting purified oridonin from rabdosia rubescens
InactiveCN103232468AReduce dosageHigh yieldOrganic chemistryChemical recyclingUltraviolet detectorsPhysical chemistry
The invention discloses a method for preparing oridonin from rabdosia rubescens. The method comprises the following steps of: (1), crushing rabdosia rubescens material, adding 60%-90% methanol solution for ultrasonically extracting for two to three times with each time being 20-50 minutes, combining and filtering, adding an ultra-filtration membrane for ultra-filtration, concentrating permeate in vacuum under reduced pressure to obtain a concentrated solution; (2), adding the concentrated solution to macroporous resin for adsorbing and evaporating moisture and packing, using a mixed solution of dichloromethane and acetone for eluting, collecting fraction components, and concentrating under reduced pressure; and extracting the concentrated solution by an ethyl acetate solution and collecting the extracting solution; (3), and purifying the concentrated extracting solution by adopting high-speed counter-current chromatography, carrying out online monitoring by an ultraviolet detector, collecting target components according to a figure, recycling a reagent and drying under reduced pressure to obtain the oridonin. The method for preparing the oridonin is simple to operate, high in product yield, high in purity, capable of recycling the reagent and low in production cost.
Owner:NANJING ZELANG MEDICAL TECH
Novel preparation technology of rabdosia rubescens tablet
ActiveCN102755379AIncrease contentReduce lossesDigestive systemPill deliveryPolymer scienceBULK ACTIVE INGREDIENT
The invention belongs to the field of the production and storage of Chinese patent medicine, and particularly discloses a novel preparation technology of rabdosia rubescens tablet. The technology comprises the following steps of: cutting the rabdosia rubescens into sections or grinding into coarse powder; adding ethanol and extracting for 1 minute to 4 hours; filtering the extraction liquid; collecting the filtrate; adding beta-cyclodextrin which is 1-15 times by weight as much as the filtrate into the filtrate and preparing into an inclusion compound by the cyclodextrin inclusion technology; drying; adding accessories and mixing uniformly; and granulating and tabletting to obtain the rabdosia rubescens tablet, wherein the extraction mode is CO2 supercritical extraction. The terpene components such as oridonin are unstable when heated. The extraction method disclosed by the invention can completely extract the active ingredients and can reduce or avoid the use of heat sources; and through the supermolecular technology and the barrel structure of beta-cyclodextrin, the terpene components such as oridonin can form a supermolecule body (inclusion compound) through hydrogen bond, Van der Waals' force and the like, and the damage to the terpene components such as oridonin is avoided.
Owner:HENAN UNIVERSITY
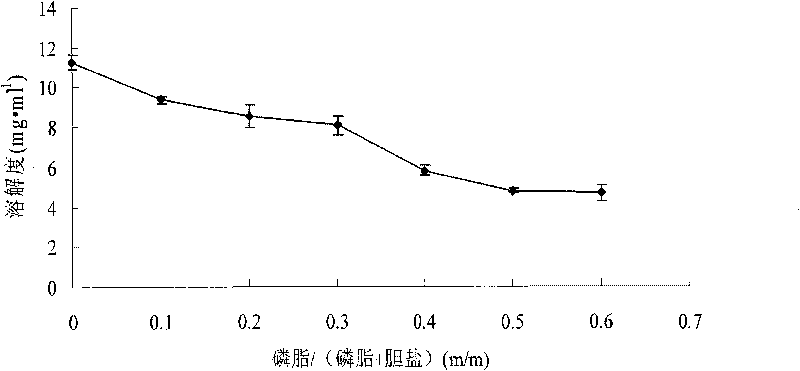
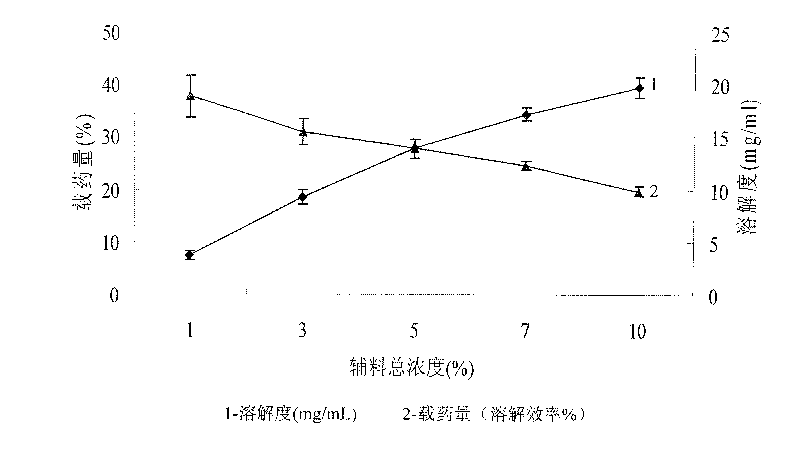
Oridonin-loaded micelle preparation and preparation method thereof
InactiveCN101708154AGood water solubilityImprove stabilityOrganic active ingredientsPowder deliverySolubilitySide effect
The invention discloses an oridonin-loaded micelle preparation which comprises the raw materials of oridonin, cholate and phosphatide. An associated structure of the cholate and the phosphatide is used as a carrier of the oridonin, the water solubility of the oridonin is greatly improved through reasonably setting the proportion of the components, and the stability of the preparation is good. The micelle preparation has high drug-loading rate and easy control; the raw materials have not side and toxic effects, safety and reliability, wide source and low cost, and thus the micelle preparation is very suitable for wide clinical application. The preparation method has simple process, easy implementation, good repeatability, and suitability of large-scale production; and the liquid micelle preparation can be frozen to form frozen powder, thereby being more convenient for storage and transportation.
Owner:ARMY MEDICAL UNIV
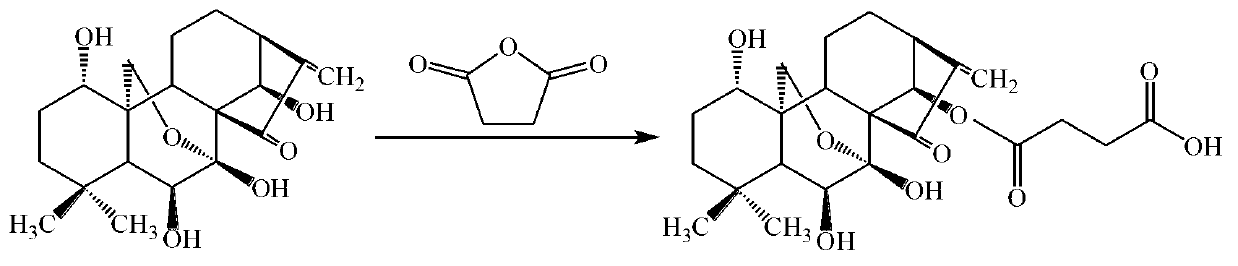
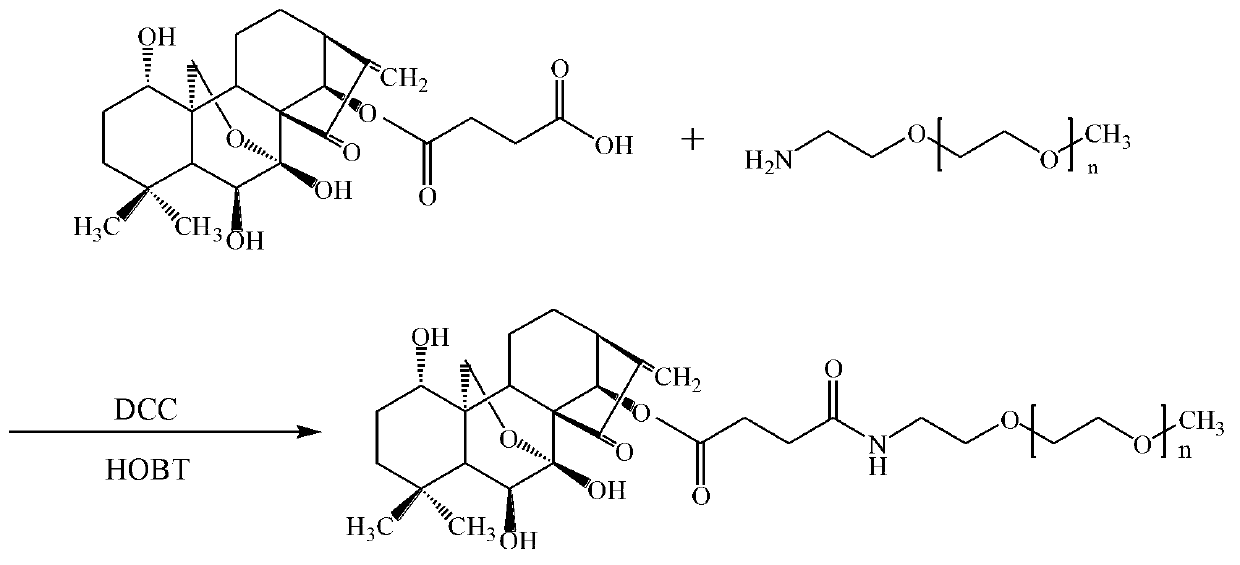
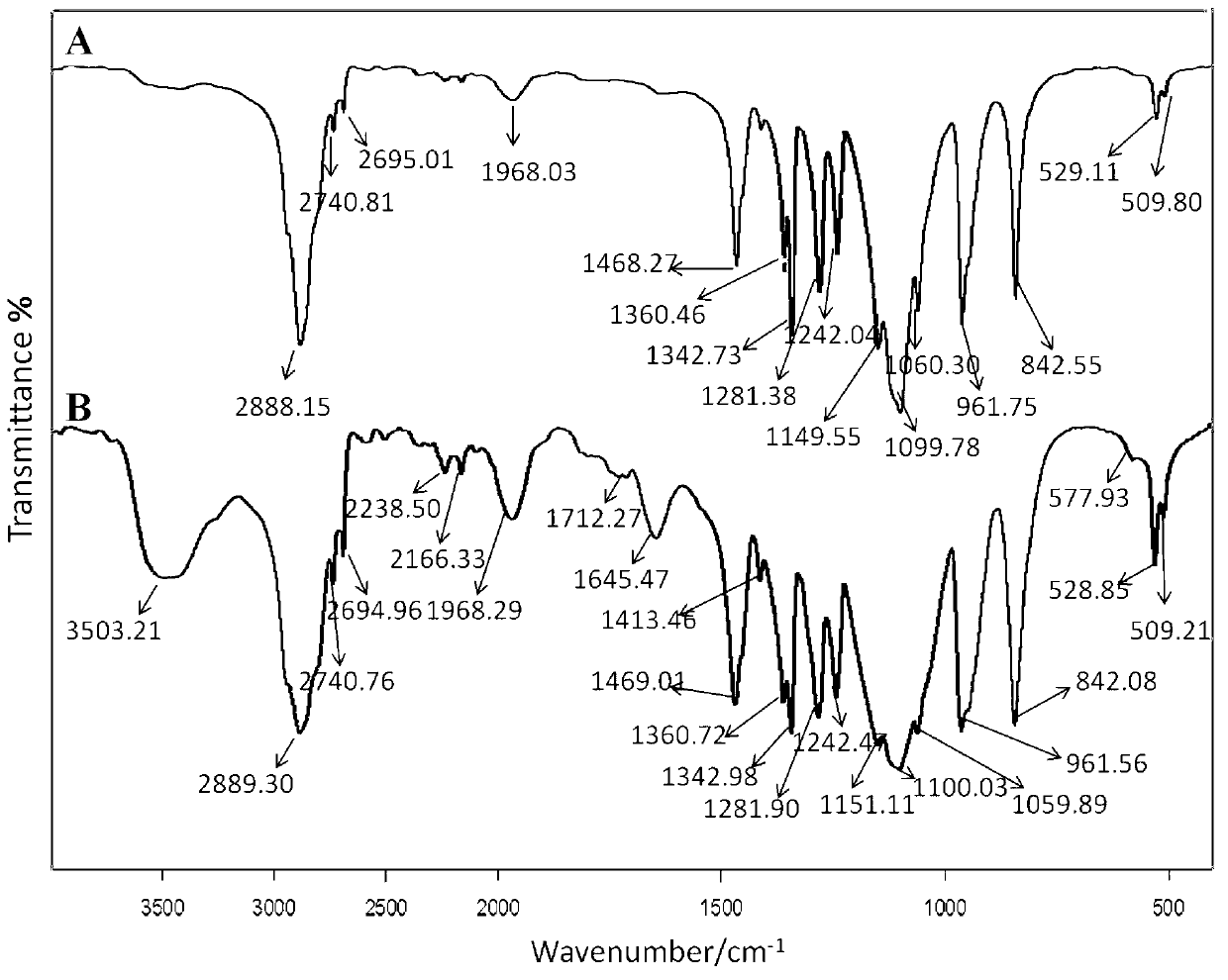
Prodrug of oridonin with polyethylene glycol serving as vector and preparation method thereof
ActiveCN102847166AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMonomethoxypolyethylene glycol
The invention discloses a prodrug of oridonin with polyethylene glycol serving as a vector. The prodrug has the structural formula shown in the specification, wherein n is an integer from 110 to 910. The preparation method comprises the following steps: (1) preparing carboxylation oridonin: performing reaction to oridonin and succinic anhydride, thus obtaining carboxylation oridonin; and (2) synthesizing prodrug of oridonin modified via methoxypolyethylene glycol amine: reacting the mono-methoxypolyethylene glycol amine with carboxylation oridonin, thus obtaining the prodrug of oridonin which is in form of white solid. According to the prodrug of oridonin with polyethylene glycol serving as the vector disclosed by the invention, the succinic acid serves as a joint arm for combining the oridonin with the hydrophilic polyethylene glycol derivative, so that the dissolubility of the oridonin is improved, the performance of the medicine is enhanced and improved, and the stability of the oridonin is improved; and the prodrug can be decomposed and fallen at a proper environment, so as to release the oridonin, so that the acting time in the organism can be increased, and the purpose of long circulation can be achieved.
Owner:山东希力药业有限公司
Oridonin clathrate compound, and medicinal prepn. thereof
InactiveCN1947714AEasy to understandOrganic active ingredientsSolution deliveryActive componentPharmaceutical formulation
An inclusion compound of rebescensine A is prepared from the rebescensine A as active component and cyclodextrin as including material in the weight ratio of 1: (0.5-50). A medicine containing said inclusion compound is also disclosed.
Owner:SINOPHARM A THINK PHARMA
Rubescensin preparation and preparation method and use of same
InactiveCN101536980AIncrease surface areaConducive to sublimationEmulsion deliveryAntineoplastic agentsMedicinePharmaceutical formulation
The invention generally relates to a diterpene pharmaceutical preparation and preparation method and use of the same. Particularly, the invention discloses a pharmaceutical preparation, which comprises diterpenes such as the rubescensin and amphiphilic surfactants, and any other pharmaceutically acceptable carriers and / or excipients. In addition, the invention also relates to the preparation method and the use of the pharmaceutical preparation in disease treatment.
Owner:SHANGHAI JIAO TONG UNIV
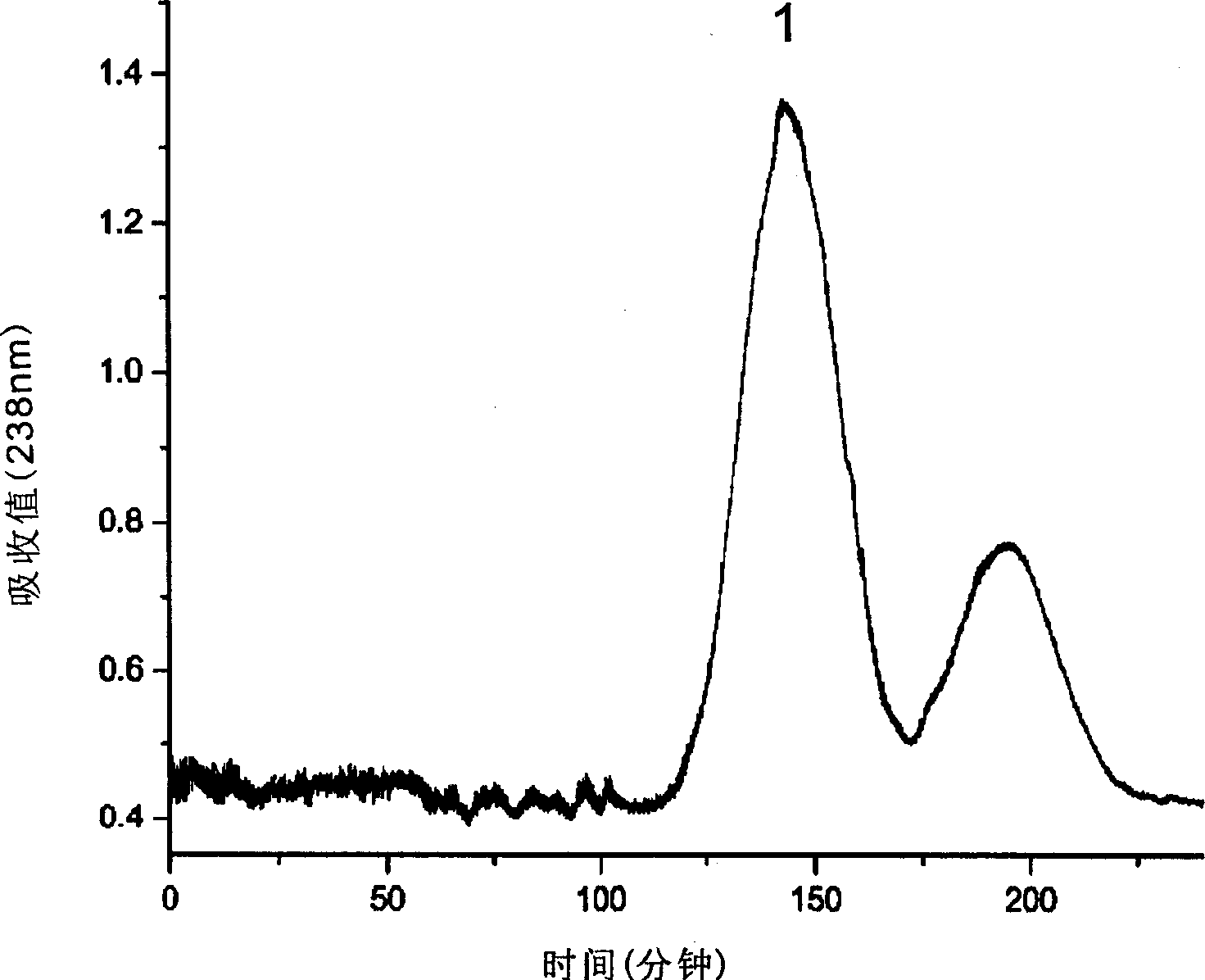
Method for separating rubescensinA from rabdosia
The method of separating oridonin from rabdosia is one continuous liquid-liquid distributing adverse current chromatographic method without needing of any solid support and thus caused adsorption, loss, property change and other troubles. The method has high peak form resolution, great separation amount, no sample loss, high recovering rate, mild separating circumstance and low solvent consumption, and may be used in separating great amount of coarse oridonin product to reach purity over 97 %. In addition, the present invention adopts relatively simple and cheap adverse current chromatograph and economic mixture solvent comprising two or more of normal alkane, halohydrocarbon, fatty alcohol, aliphatic ketone, aliphatic ester, ether and water.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com