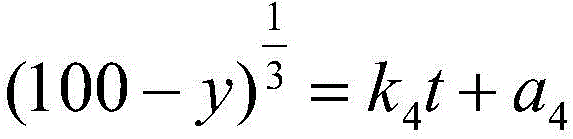Oridonin cubic liquid crystal nanoparticle and preparation method thereof
A technology of oridonin A and nanoparticles is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Achieving the problems of therapeutic concentration and low bioavailability, and achieving the effect of improving oral relative bioavailability, good application prospects and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of Rubescensin A Cubic Liquid Crystal Nanoparticles and Investigation of Different Liquid Crystal Systems
[0054] The preparation method of the Rubescensin A cubic liquid crystal nanoparticles of the present embodiment comprises the following steps:
[0055] 20mg Rubescensin A was dissolved in propylene glycol to obtain a drug solution; according to each prescription in Table 1 (all by mass percentage), take glycerol monooleate or phytantriol and stabilizer F127 (poloxamer 407) in a 50mL plastic centrifuge tube, melted in a water bath at 60°C, then slowly added it to the above drug solution, vortexed and mixed thoroughly for 5 minutes, then added the prescribed amount of ultrapure water, vortexed for 5 minutes, and the obtained system was cooled at room temperature After equilibrating in the dark for 48 hours, a transparent oridonin cubic liquid crystal gel was obtained. Under the condition of ice bath, the obtained oridonin A cubic liquid crysta...
Embodiment 2
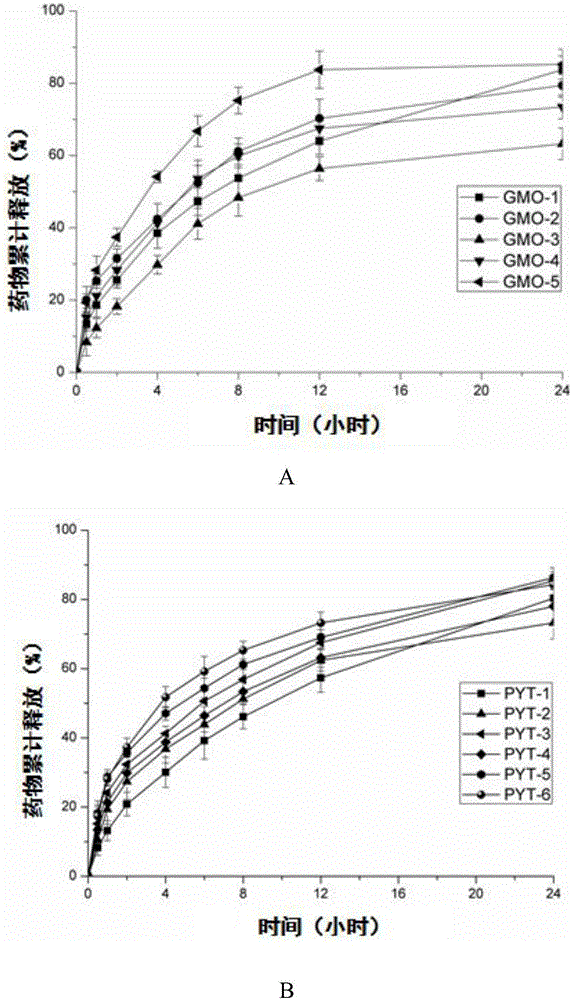
[0061] Example 2 Effects of different mass ratios of F127 and lipids in oridonin A cubic liquid crystal nanoparticles on liquid crystal phase state and drug release behavior
[0062] Only after the oridonin liquid crystal is further prepared into a nanoparticle solution can it be taken orally, and it can pass through the cells of the gastrointestinal tract and be absorbed by the human body; The particles are easy to aggregate and precipitate, adding a stabilizer can support and maintain the network structure of the liquid crystal lattice unit, prevent the aggregation and precipitation of the nanoparticles during the high-pressure homogenization process, and maintain the stability of the liquid crystal nanoparticles during the preparation and storage process.
[0063] (1) The present embodiment investigates the oridonin cubic liquid crystal nanoparticle samples of F127 and lipid (GMO or PYT) with different mass ratios (0%, 9%, 12%, 15%, 20%), Its preparation method is the same ...
Embodiment 3
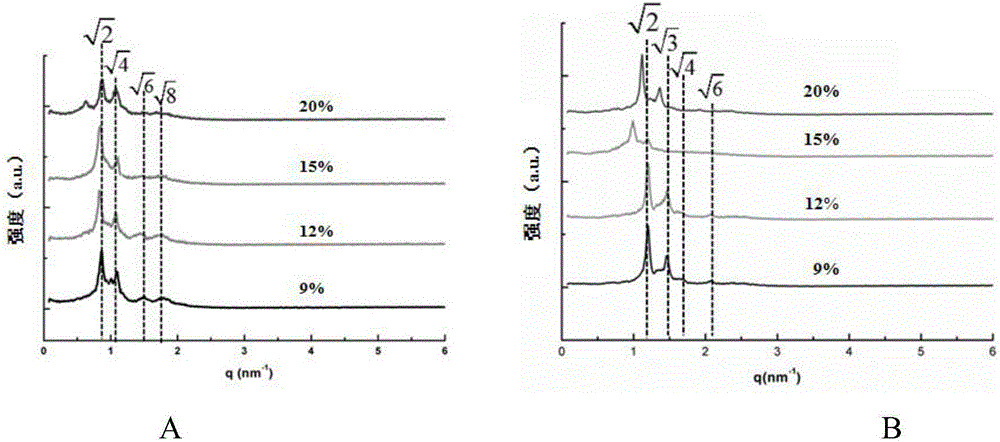
[0071] Example 3: Effect of drug loading in cubic liquid crystal nanoparticles of oridonin on particle size of cubic liquid crystal nanoparticles and drug encapsulation efficiency
[0072] Drugs with different structures and different contents will affect the internal structure of cubic liquid crystal nanoparticles. Therefore, this embodiment prepares cubic liquid crystal nanoparticles of oridonin A with different drug loadings (raw material prescriptions are shown in Table 2), and the preparation method is as follows: Shown in embodiment 1.
[0073] Table 2 Raw Material Prescription of Oridonin A Cubic Liquid Crystal Nanoparticles
[0074]
[0075]
[0076] The small-angle X-ray scattering patterns (test parameters are the same as those in Example 2), particle size distribution, and encapsulation efficiency of Rubescensin A cubic liquid crystal nanoparticles with different drug loadings in this example were measured.
[0077] The particle size of oridonin cubic liquid cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com