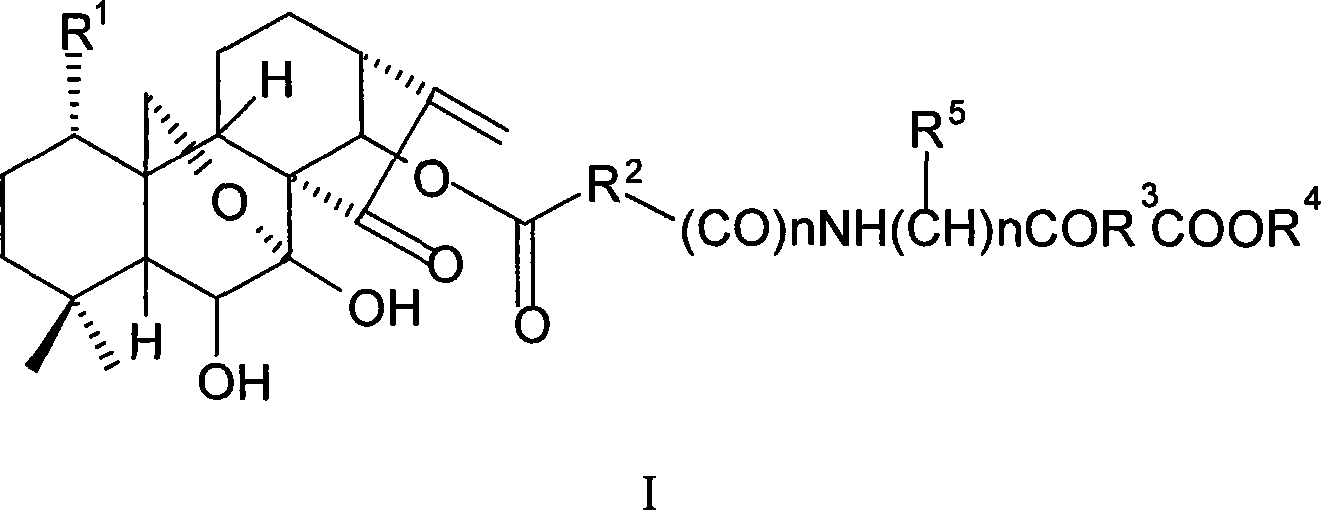
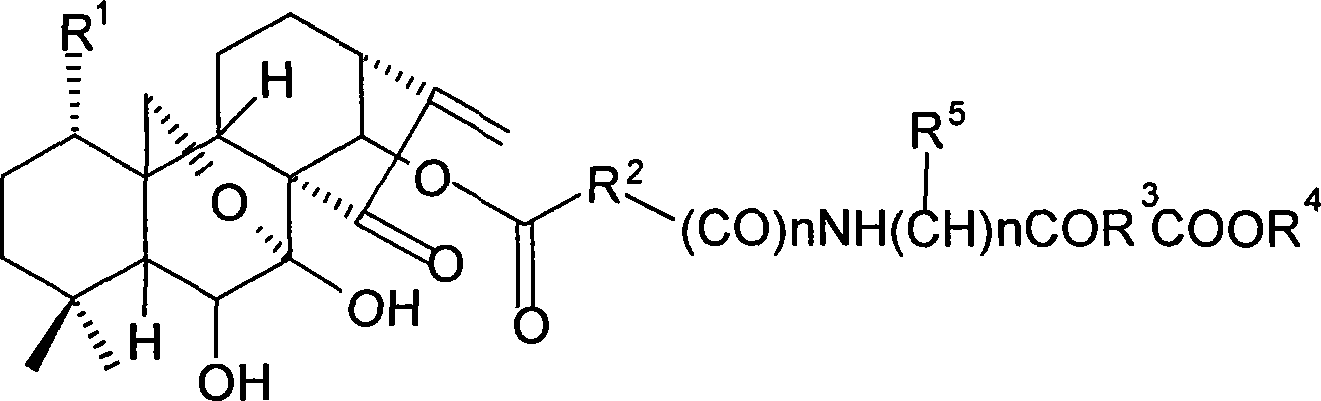
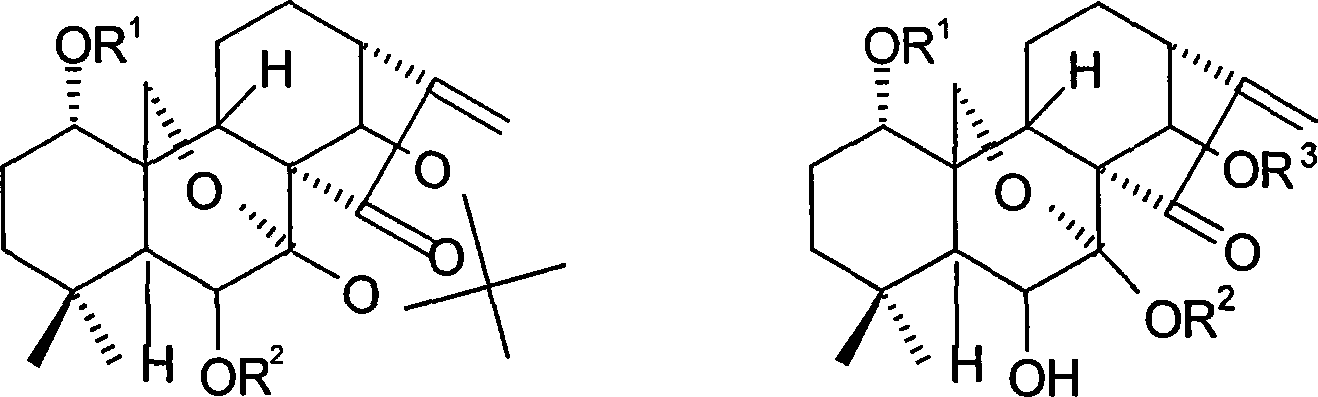Oridonin derivative, preparation method and uses thereof
A technology of oridonin A and its derivatives, which is applied in the field of natural medicine and medicinal chemistry, and can solve the problems of antitumor activity, insufficient water solubility, and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] 7,14-Acetonid oridonine A
[0119] Dissolve oridonin (100mg, 0.274mmol) in 3ml of anhydrous acetone, add 0.4ml of 2,2-dimethoxypropane and a catalytic amount of p-toluenesulfonic acid, nitrogen protection, reflux reaction for 1h, cool to room temperature, add saturated NaHCO 3 solution with CHCl 3 Extracted 3 times, then backwashed 2 times with water, the organic layer was washed with anhydrous MgSO 4 Drying, evaporation to dryness, column chromatography (petroleum ether: acetone = 3:1 ~ 1:1), yielded 94.1 mg of white solid, yield: 85%, m.p.219-221°C.
[0120] MS-ESI (m / z): 427[M+Na] + , 405[M+1] + , 403[M-1] - .
Embodiment 2
[0122] 1-O-acetyl-7,14-acetonid oridonin
[0123] Dissolve the compound (80 mg, 0.20 mmol) prepared in Example 1 in 2 ml of pyridine, add 0.3 ml of acetic anhydride, stir at room temperature for 14 h, add saturated NaHCO 3 The solution was stirred until no bubbles were produced, extracted 3 times with ethyl acetate, the organic layer was washed 4 times with water, and anhydrous MgSO 4 Dry overnight and evaporate to dryness under reduced pressure. used directly in the next reaction.
[0124] MS-ESI(m / z): 469[M+Na] + , 447[M+1] + .
Embodiment 3
[0126] 1-O-acetyl oridonin A
[0127] The compound (80 mg, 0.2 mmol) prepared in Example 2 was dissolved in 2 ml of 80% acetic acid solution, stirred at room temperature for 10 h, evaporated to dryness under reduced pressure (adding toluene to bring out acetic acid and water), the reaction was carried out quantitatively, and the obtained crude product was subjected to Purified by column chromatography (petroleum ether: acetone = 2:1) to obtain 63.7 mg of white solid, yield: 78.5%. m.p.196-198°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com